Abstract
We examined an epileptic focus by electroencephalography (EEG) by using an international 10-20 electrode system in 11 Shetland sheep dogs affected with familial idiopathic epilepsy. We also performed an evaluation of the amino acids in the cerebrospinal fluid (CSF) and a pathologic examination of the brains of 8 dogs that died from status epilepticus. Continuous electroencephalography demonstrated that an epileptic focus was initially detected in the frontal lobe, particularly the internal area, and that paroxysmal foci developed diffusely in other lobes of affected dogs with recurrent convulsions. The EEG analyses indicated spike and sharp wave complexes, which were considered to be paroxysmal discharges. An increased value for glutamate or aspartate was found in the CSF of some epileptic dogs. Histologically, acute neuronal necrosis and astrocytosis were distributed predominantly in the cingulate cortex and internal area of frontal cortex, less frequently in other areas of the cerebrum. The results of this study suggest that, initially, the dogs have an epileptic focus in the frontal lobe, and that the focus extends gradually to other areas of the cerebrum. Based on the distribution of neuronal necrosis and astrocytosis, acute neuronal damage may be related to the superexcitation of neurons following epilepsy.
Introduction
Epilepsy is one of the most common and significant disorders of the central nervous system (CNS), and has been classified into 2 etiologic categories in humans and dogs: 1) idiopathic epilepsy, which includes those convulsive disorders that cannot be associated with observable structural pathology, and 2) symptomatic epilepsy, which includes those disorders arising from definable injury, metabolic disease, or neurostructural abnormality (1,2,3). Although inheritance has been suggested as a possible etiology of idiopathic epilepsy in dogs and humans, the pathophysiology of idiopathic epilepsy is not fully understood (4,5,6,7,8).
There are some reports on the electroencephalographic (9,10,11) and pathologic findings of idiopathic epilepsy in dogs (12,13); however, the relationship between neurophysiologic events and neuropathologic findings has not been clearly documented. Previous reports on some familial epileptic dogs have described acute neuronal necrosis in the hippocampal pyramidal cells and other cerebral cortices, without an electrophysiologic examination (12,13). In humans, an international 10-20 electrode system was used to detect the epileptic focus in detail (14,15) though this method has not been applied to the localization of the epileptic focus in animals (10,11).
Therefore, this study was designed to determine where the epileptic focus was initially evoked in the brain, by using a 10-20 electrode system, and to estimate the electroencephalography (EEG) records and neuropathologic findings. To the authors' knowledge, this is the first report of neurophysiologic and pathologic examination in naturally occurring, genetically epileptic dogs.
Materials and methods
Dogs
The parents and first- to fifth-generation offspring in a single family of Shetland sheepdogs were studied (Figure 1). The female parent dog produced 6 litters, for a total of 28 puppies. At the time when 3 litters had been produced, a 2-year-old female from the second litter (dog 1) suddenly showed a generalized seizure and was referred to a veterinary clinic for clinical diagnosis. She did not show any abnormalities upon examination of the urine and the blood. She showed generalized epilepsy for 3 y. She died after status epilepticus at age of 5 y, and then was submitted to our laboratory for pathologic diagnosis. Most dogs in the first 4 litters of first-, second-, and third-generation offspring were not available for evaluation, as the puppies had been given away without obtaining information about the new owner. Dogs from a litter of first-generation (litter no. 6) and fifth-generation (litter no. 17) puppies, which were produced for this study, were evaluated propectively. A conventional vaccination schedule was followed, and a monthly heartworm preventive treatment was administered. Appropriate veterinary care was provided if a generalized seizure occurred. This study was carried out under the control of the Animal Research Committee, in accordance with the guidelines for animal experimentation of the Faculty of Agriculture, Tottori University.
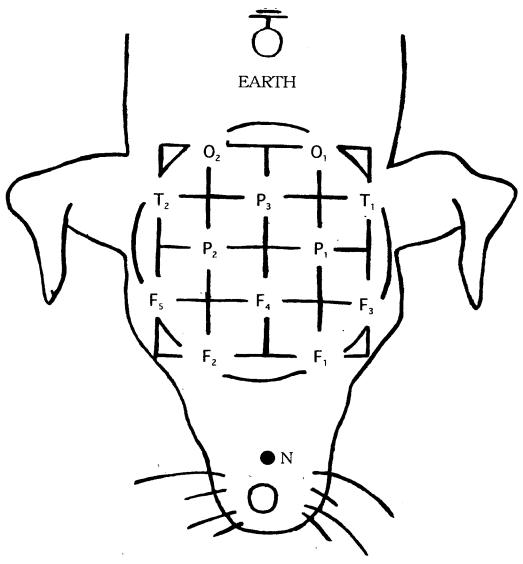
Figure 1. Placement of the subcutaneous electrodes for electroencephalographic recording in epileptic and control dogs. F1 and F3 — left frontal; F2 and F5 — right frontal; central frontal (F4), left parietal (P1); right parietal (P2); central parietal (P3); left temporal (T1); right temporal (T2); left occipital (O1); right occipital (O2); N — reference electrode; earth — ground electrode.
Examination and clinicopathologic evaluations
Six dogs from 2 litters (nos. 6 and 17), which were produced for this study, were evaluated every 1 to 2 mo by EEG, beginning at 8 mo of age and continuing until they developed a generalized seizure. One dog (dog 3; litter 5) from the first-generation puppies was evaluated 4 times by EEG at 6, 9, 13, and 15 mo of age. Three dogs (litter 13) from the fourth-generation puppies were evaluated 4 times by EEG, at 9, 12, 16, and 18 mo of age. One dog (dog 7; litter 15) from the fifth-generation puppies was evaluated twice by EEG, at 6 and 12 mo of age. Electoencephalographic and histopathologic examinations were performed on 6 dogs (dogs 3–7, 10). Two dogs (dogs 1 and 2) were examined by histopathology only, and 4 other dogs from 2 litters (litters 6 and 17) were analyzed only by EEG.
Hematologic examination was performed on all the dogs mentioned above. Complete blood counts were determined by using standard methods. Serum electrolyte and biochemical analyses (albumin, creatine phosphokinase, blood urea nitrogen, cholesterol, total calcium, chloride, sodium, potassium, inorganic phosphorus, glucose, and total protein) were performed by using 2 autobiochemical analyzers (Dry-Chem 800 and Dry-Chem 3500I; Fyji Photo Film, Tokyo, Japan).
Electroencephalogram recording
Electroencephalographic examination was performed every 1 to 3 mo. The EEG was recorded when the dogs were under sedation by IM injection of xylazine (Celactal; Bayer, Leverkussen, Germany), 1.0 mg/kg body weight (BW). Before recording the EEG, each dog was placed in a dimly lighted, sound-attenuated room. Four female age-matched Shetland sheepdogs without seizure history, from 1 to 2 y of age, were used as control cases.
All the dogs were submitted to a single recording using a standard 10-20 surface electrodes, as used in humans (14,15) (Figure 1). Before insertion of the electrodes, local subcutaneous infiltration anesthesia with 1% lidocaine hydrochloride (Xylocaine; Fujisawa Pharmaceuticals, Tokyo, Japan) was administered around the placement sites. The electrodes (stainless-steel needles) were inserted subcutaneously, bilaterally over the left frontal, right frontal, central frontal, left parietal, right parietal, central parietal, left temporal, right temporal , left occipital, right occipital, and vertex areas. Reference and ground electrodes were also inserted just behind the tip of the nose and at the caudal edge of the external occipital protuberance, respectively. Connections to the recording devices were made via flexible cable. A 12-channel electroencephalograph was used for recording the EEG, using a polygraph (No. 1A52; NEC San-ei, Tokyo, Japan) with a time constant of 0.3 s, amplification sensitivity of 50 μV/5 mm, and high-frequency filter of 60 Hz (16). The EEG signals were recorded on paper for visual analysis (paper speed, 3 cm/s).
Cerebrospinal fluid collection
Cerebomedullary cisternal cerebrospinal fluid (CSF) was collected from 6 epileptic dogs that were clinically normal, from 1 dog that had died after status epilepticus, and from 4 female, age-matched control Shetland sheepdogs. In all epileptic dogs, collection of CSF was done at 1 wk (or longer) after the last observed seizure. All dogs were anesthetized by IM injections of medetomidine hydrochloride (Domitor; Meiji Seika, Tokyo, Japan), 30 μg/kg BW; midazolam (Dormicum; Yamanouchi Pharmaceutical, Tokyo, Japan), 0.2 mg/kg BW; and ketamine hydrochloride (Ketalal; Sankyo Pharmaceutical, Tokyo, Japan), 5.0 mg/kg BW. After induction of anesthesia, the dogs were intubated and the hair over a 4-cm × 6-cm area, from the occipital protuberance to the mid-body of the second cervical vertebra, was shaved, and the skin was prepared for CSF collection. The CSF was collected aseptically by using a 22-gauge, 2-inch spinal needle and the samples were immediately frozen in liquid nitrogen. The samples were then transferred to a refrigerator where they were kept at −80°C until analysis.
Neurotransmitter analysis
Cerebrospinal fluid from all dogs was analyzed for levels of aspartate, glutamate, asparagine, glutamine, arginine, taurine, and gamma-aminobutyric acid (GABA). Continuous sample analysis was done by high performance liquid chromatography with electrochemical detection (HPLC-ECD) (BAA-300; Eicom, Kyoto, Japan). Samples from epileptic and control dogs were randomly interspersed during analysis.
Necropsy and histopathology
Complete postmortem examinations were performed on 7 affected dogs. Systemic organs were fixed by immersion in 10% neutral buffered formalin, routinely processed into paraffin, and sectioned at 4 μm. Sections were stained with hematoxylin and eosin, combined luxol-fast-blue-hematoxylin-eosin, Klüver-Barrera, modified Bielschowsky, and Gallyas-Braak stains.
Immunohistochemistry
Immunohistochemical analysis was performed using an anti-human glial fibrillary acid protein (GFAP) mouse monoclonal antiserum (DAKO, Glostrup, Denmark) in an indirect immunoperoxidase staining procedure. Briefly, after blocking endogeneous peroxidase activity with 3% hydrogen peroxide, sections were preincubated with 10% normal goat serum for 30 min at room temperature, incubated with the primary antibody (1:100 dilution) at 4°C overnight, and then incubated with biotinylated goat anti-mouse IgG antiserum (1:400; DAKO) at room temperature, followed by peroxidase-conjugated streptavidin (1:400; DAKO) for 30 min. After the first and second incubations, the sections were given 5-minute washes in phosphate-buffered saline (PBS), and the immunoreaction was developed in substrate solution containing 0.05% 3,3-diaminobenzidine tetrahydrochloride and 0.003% hydrogen peroxide. Sections were counterstained lightly with hematoxylin. Specificity controls were carried out by replacing the primary antibody with PBS. Samples from 2 beagles, 3 mongrels, and 2 Shetland sheepdogs that had died of non-neurological disorders served as controls.
Results
Pedigree
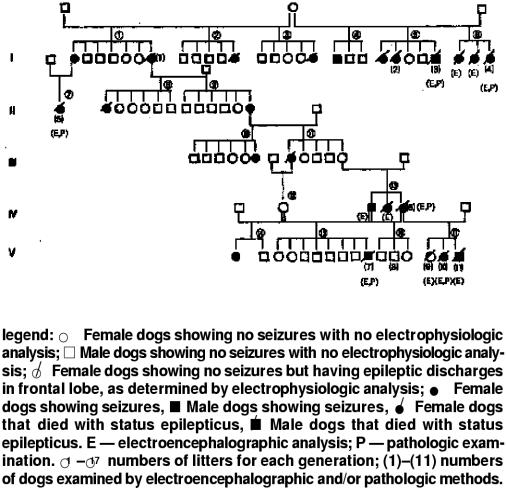
An epilepsy that was determined to be idiopathic epilepsy was histopathologically diagnosed in 2 male and 6 female offspring. Affected males and females were identified. Females were over- represented, constituting 19 of 24 (79.2%) affected dogs (Figure 2). Male-to-male transmission occurred in one parent and son, thus excluding mitochondrial or sex-linked inheritance as a possibility for a cause of the epilepsy. It seems, from the pedigree analysis, that the disease process is multifactorial.
Figure 2. Pedigree of the family of dogs used in the epilepsy study
Clinical history and clinicopathologic evaluations
The Shetland sheep dogs used in the present study were obtained from a single family. The family had bred about 20 dogs. Many of them showed generalized seizures as puppies. Seizure onset was usually at 1 to 1.5 y of age, or later, and the ictal period ranged from 1 to 4 min (average: 1 to 2 min). The frequency of the seizures varied from one seizure every week to one every 6 mo, although the dogs were not watched continuously. The owner of this colony had observed convulsion activity mostly at night. When seizures occurred during the daylight hours, they occurred when the dogs were in a drowsy state (with eyes half closed, lying with head down). Eight offspring from the epileptic colony were obtained, some of which were observed continuously using a monitor (VM-H720, D.S.P.III; Hitachi, Tokyo, Japan). Approximately two-thirds of the dogs had a prodromal phase, characterized by restlessness and depression. Some dogs always exhibited their seizures right after leaving their cage; these dogs soon came to expect their spasms upon exiting the cage. The ictal phase was dominated by lateral recumbency, tonic-clonic contraction of muscles, excessive salivation, urination, repetitive jaw movements, opening and closing of the mouth, and staring. Loss of consciousness was observed in almost all cases. The postictal phase was characterized by fatigue, disorientation, hunger, and thirst, which usually lasted for a few to 30 min.
The animals did not show any abnormalities upon examination of the urine and the blood. However, some dogs that died after status epilepticus had mildly elevated values of creatine kinase, ranging from 380 to 600 (IU/L).
Electroencephalogram
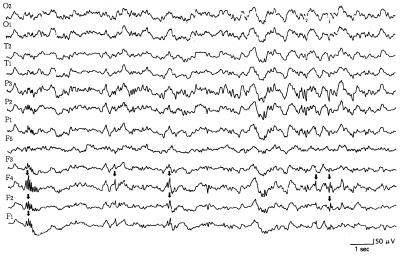
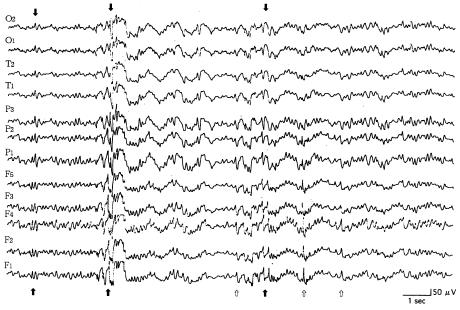
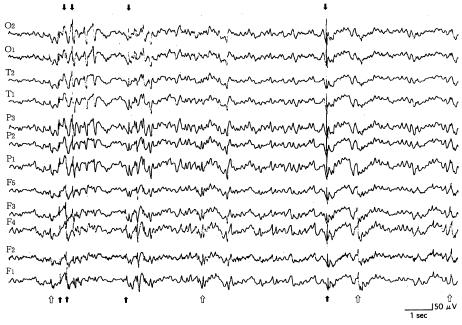
The puppies were monitored continuously from a young age, during the clinical normal state. In all young dogs lacking clinical epileptic phenomena, the paroxysmal discharges were localized in the frontal lobe, especially in the internal area (F4 lead). In those young dogs with clinical signs (generalized tonic-clonic seizure, with or without loss of consciousness), the interictal EEG records consisted of sharp waves (range 50 to 150 μV) (Figure 3). A short period (about 4 to 10 mo) after the onset of the clinical signs, the interictal EEG records consisted of paroxysmal discharges in all leads. Frontal leads frequently showed spikes and sharp waves, whereas parietal and temporal leads only occasionally showed sharp waves (Figure 4). The dogs showed generalized seizures at irregular intervals. In the stage of life where there were recurrent convulsions (from 10 to 30 mo of age), the interictal EEG records consisted of paroxysmal discharges in all leads. Parietal and occipital leads occasionally showed spikes and sharp waves (range 50 to 180 μV) more often than the frontal leads, but only the frontal leads showed these high amplitude waves frequently (Figure 5). Spike and sharp wave complexes were usually seen in the EEG tracings after 30 min. The background EEG activities consisted of waves with a frequency of 1 to 4 Hz and an amplitude of 10 to 30 μV. No paroxysmal discharges were found on the EEG tracings for the 4 control dogs.
Figure 3. Reference monopolar interictal recording of paroxysmal discharges (arrows) in leads 10, 11, and 12 (frontal lobe). This 14-month-old, male dog (dog 3) had tonic-motor seizures without loss of consciousness once a month. Calibration marks indicate 50 μV and 1 s. See Figure 1 for legend for electrodes.
Figure 4. Reference monopolar interictal recording of paroxysmal discharges (arrows) in all leads. Frontal leads frequently showed the discharges (open arrows); parietal and temporal ones also showed discharges. This 18-month-old, female dog (dog 10) had generalized seizures at irregular intervals during the previous 2 wk. Calibration marks indicate 50 μV and 1 s. See Figure 1 for legend for electrodes.
Figure 5. Reference monopolar interictal recording of paroxysmal discharges (arrows) in all leads. Parietal and occipital leads sometimes showed high amplitude, and frontal leads frequently showed low to high amplitude (open arrows). This 26-month-old, male dog (dog 3) had generalized seizures at irregular intervals during the previous 3 wks. Calibration marks indicate 50 μV and 1 s. See Figure 1 for legend for electrodes.
Cerebrospinal fluid amino acid values
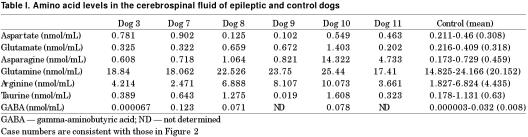
A high concentration of aspartate was found in the CSF of 3 epileptic dogs (dogs 3, 7, and 10), and a high concentration of glutamate was evident in the CSF from 3 epileptic dogs (dogs 8, 9, and 10), as compared with the control group (Table I). The CSF levels of glycine, glutamine, and arginine showed a tendency to be high, but the values did not significantly differ from those of the control group.
Table I.
Pathologic findings
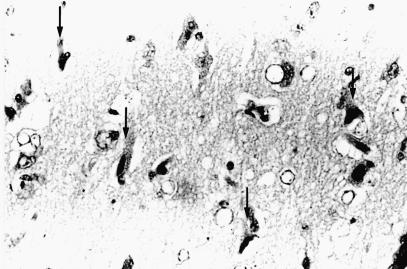
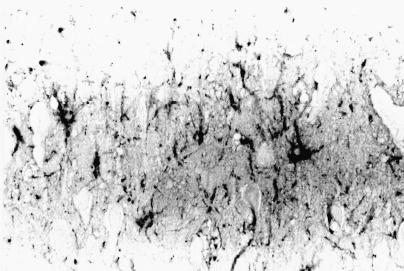
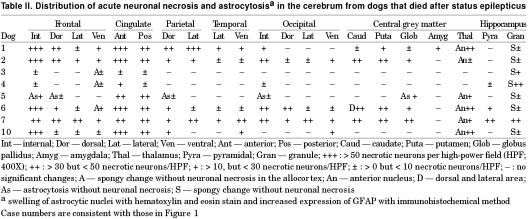
Grossly, in 3 affected dogs, the internal portion of the cerebral frontal lobe and cingulate cortex showed mild whitish discoloration. On histological examination of all cases, acute nerve cell change, with occasional perineuronal basophilic incrustaion, swelling of astrocytic nuclei, and mild vascular endothelial swelling were found in the cerebral frontal cortex, especially in the internal area, and anterior to posterior portions of the cingulate cortex (Figure 6). Acute nerve cell change consisted of a shrinkage of nerve cell bodies with eosinophilic and homogeneous cytoplasm and atrophic nuclei. These necrotic neurons were found randomly dispersed among neurons with normal cell bodies. Immunohistochemistry, using anti-GFAP antiserum, showed hypertrophic astrocytic processes in the cerebral cortex (Figure 7). Similar findings were observed, though to a lesser extent, in the parietal and occipital cortices and central grey matters (caudate nucleus, putamen, globus pallidus, thalamus). In 6 cases, various degrees of spongy change were evident in the granule cell layer of the hippocampus. Few acute neuronal changes were found in the pyramidal cell layer, except for in one dog (Table II). There were no inflammatory or neoplastic lesions or any storage disease or glial/neuronoglial malformative lesions (cerebral cortical dysplasia, microdysgenesis). Pulmonary congestive edema was found in 5 of 7 cases. In all cases, scattered deposition of lipofuscin was found in the tubular epithelial cells of the kidney.
Figure 6. Photomicrograph of the anterior portion of the cingulate cortex in the right cerebral frontal lobe of dog 6. Notice acute nerve cell change (arrows), swelling of astrocytic nuclei, and mild vascular endothelial swelling. Acute nerve cell change consists of a shrinkage of nerve cell bodies with eosinophilic and homogeneous cytoplasm and atrophic nuclei. Hematoxylin and eosin stain; magnification: 340X.
Figure 7. Immunohistochemical microscopy of the cingulate cortex of dog 6, stained with glial fibrillary acid protein antibody. Notice some hypertrophic astrocytic processes positive for GFAP. Light hematoxylin counterstain; magnification: 340X.
Table II.
Discussion
In this study, the epileptic dogs fulfilled the 3 primary criteria for idiopathic epilepsy. Firstly, the dogs exhibited recurrent seizures. Secondly, electrophysiologic examination disclosed epileptic discharges such as spike and sharp waves. Thirdly, no pathologic findings except for acute brain damage could be identified on neuropathologic examination. Although there have been some neurophysiologic studies on genetically idiopathic canine epilepsy (9,10,11), to our knowledge, no reports have described electrophysiologic estimations by continuously examination of young puppies by using a 10-20 electrode system. This method was used to detect the epileptic focus in great detail in humans (14,15); therefore, we performed this procedure in dogs in order to determine where an epileptic focus is present in the canine cerebrum. We performed the EEG analyses on xylazine-sedated dogs. Xylazine is a non-narcotic analgesic sedative that causes muscle relaxation and generalized CNS depression and that has been reported not to provoke sharp waves and spikes on an EEG (17,18). The results of the electrophysiologic examinations conducted in this study suggest that, initially, all dogs had an epileptic focus in the frontal lobe, especially in the internal area. This focus extended gradually to other areas of the cerebrum, such as parietal and occipital lobes, during the period of recurrent seizures. In the chronic cases, the epileptic focus was always in the frontal lobe. Therefore, the dogs in this study should be considered to belong to the ‘frontal lobe epilepsy’ category; the dogs would then develop secondary generalized seizures (19).
In other epileptic animal models studied, the epileptic discharges initiated at the frontal cortex (such as in photosensitive baboons (20)) or in the parietal cortex (such as in the El mouse (21)); both models developed secondary generalized seizure.
The issue of the heritability of canine idiopathic epilepsy has long been controversial (4,5); however, several forms of evidence suggest that inheritance plays a potentially important role in development of canine idiopathic epilepsy. The dogs in the present study seem to show multifactorial inheritance, though we cannot exclude the possibility that the mode of inheritance is autosomal dominant. Also, the convulsions had frequently occurred at night and during the drowsy state. In humans affected by autosomal dominant nocturnal frontal lobe epilepsy (ADNFLE), seizures occur most commonly as the patients doze (22,23); they exhibit the convulsions very soon after falling sleep, throughout the night, early in the morning, and during a day. Therefore, understanding the factors that contribute to the pathogenesis of the epilepsy examined in this study may provide useful insight into the genetically idiopathic epilepsy of animals and humans, as well as human ADNFLE.
The distinctive pathologic findings of the dogs in this study were acute nerve cell change (ischemic-like change) and astrocytosis. These changes occurred in the cerebral cortex, especially in the fontal lobe, while its absence was noted from the hippocampus. The neuronal change was characterized by a shrinkage of nerve cell bodies with eosinophilic and homogeneous cytoplasm and atrophic nuclei. The morphologic change of the neurons is closely related to that in the cerebrum of human epileptic patients (24,25), as well as animal patients (26,27). Although the pathologic mechanism of acute nerve cell change remains to be elucidated, some reports have described the occurrence of ischemic cell change as a result of the local seizure activity rather than a result of systemic changes modifying local oxygen and glucose supply (28,29).
The neuronal death in status epilepticus has been considered to be a consequence of excessive entry of calcium ions into neurons, through activation of N-methyl-D-aspartate (NMDA) receptors during the seizure activity (30,31). Increases in intracellular calcium can induce activity of various enzymes, such as protease, phospholipase A2, protein kinase, nitric oxide synthetase, and endonuclease, all of which play a significant role of neuronal death (32). Activation of NMDA receptors may be related to excessive release of excitotoxic neurotransmitters, such as glutamate and aspartate. In the present study, the EEGs of the epileptic dogs demonstrated that the epileptic focus was detected in the frontal lobe throughout their entire lives. In addition, pathologic examination demonstrated that acute neuronal necrosis was found exclusively in the frontal lobe. The distribution of abnormal electrophysiologic findings were consistent with that of acute neuronal necrosis.
Analysis of CSF neurotransmitters and their precursors has proven to be a useful tool to explore possible neurochemical changes associated with epilepsy. In human epileptic patients, there have been no significant trends reported for increased or decreased CSF levels of some amino acids, such as glutamate, aspartate, glycine, taurine, and GABA (33,34,35); however, there have been no more than 2 reports describing elevated levels of glutamate (36,37) and aspartate (36) in human epileptic patients. The decreased levels of GABA and increased levels of glutamate in the CSF have been reported in non-familial idiopathic epilepsy in dogs (38). In this study, an increased value of glutamate or aspartate was found in the CSF of some epileptic dogs. Therefore, excitotoxic neurotransmitters, such as glutamate and aspartate, may be a contributing factor for the pathogenesis of the acute brain damage observed in our epileptic dogs. Further studies are needed to assess the association between acute neuronal necrosis and enhanced release of excitotoxic neurotransmitters by using a microdialysis experiment and immunohistochemical study.
Footnotes
Acknowledgment
This study was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture of Japan (No. 11836005). The authors are grateful to Mr. T. Watari for providing useful information on clinical observations in the epileptic dogs.
Address correspondence and reprint requests to Dr. T. Morita, telephone/fax: +81-857-31-5424, e-mail: morita@muses.tottori-u.ac.jp.
Received May 2, 2001. Accepted October 15, 2001.
References
- 1.Meldrum BS, Bruton CJ. Epilepsy. In: Adams JH, Duchen LW, eds. Greenfield‘s Neuropathology, London: Edward Arnold, 1992:1246–1283.
- 2.Koestner A. Neuropathology of canine epilepsy. Probl Vet Med 1989;1:516–534. [PubMed]
- 3.Summers BA, Cummings JF, de Lahunta A. Degenerative disease. In: Veterinary Neuropathology. St. Louis: Mosby-Year Book, 1995:208–350.
- 4.Cunningham JG, Farnbach GC. Inheritance and idiopathic canine epilepsy. J Am Anim Hosp Assoc 1988;24:421–424.
- 5.Jaggy A, Faissler C, Gaillard. Genetic aspects of idiopathic epilepsy in Labrador retrievers. Am J Vet Res 1998;39:275–280. [DOI] [PubMed]
- 6.Eshenne B, Rivier F, Humbertclaude V, Roubertie A, Cheminal R, Malafosse A. Benign familial infantile convulsions. Arch Pediatr 1999;6:54–58. [DOI] [PubMed]
- 7.Provini F, Plazzi G, Tinuper P, Vandi S. Lugaresi E, Montagna P. Nocturnal frontal lobe epilepsy. A clinical and polygraphic overview of 100 consecutive cases. Brain 1999;122:1017–1031. [DOI] [PubMed]
- 8.Bartolomei F, Gavaret M, Dravet C, Guerrini R. Familial epilepsy with unilateral and bilateral malformations of cortical development. Epilepsia 1999;40:47–51. [DOI] [PubMed]
- 9.Wiederholt WC. Electrophysiologic analysis of epileptic beagles. Neurology 1974;24:149–155. [DOI] [PubMed]
- 10.Srenk P, Jaggy A. Interictal electroencephalographic findings in a family of golden retrievers with idiopathic epilepsy. J Small Anim Pract 1996;37:317–321. [DOI] [PubMed]
- 11.Jaggy A, Bernardini M. Idiopathic epilepsy in 125 dogs: a long-term study. Clinical and electroencephalographic findings. J Small Anim Pract 1998;39:23–29. [DOI] [PubMed]
- 12.Montgomery DL, Lee AC. Brain damage in the epileptic beagle dog. Vet Pathol 1983;20:160–169. [DOI] [PubMed]
- 13.Morita T, Shimada A, Umemura T, Fukuda S. Oligodendroglial vacuolar degeneration in the bilateral motor cortices and astrocytosis in epileptic beagle dogs. J Vet Med Sci 1999;61:107–111. [DOI] [PubMed]
- 14.Morris HH, Luders H, Lesser RP, Dinner DS, Klem GH. The value of closely spaced scalp electrodes in the localization of epileptiform foci: a study of 26 patients with complex partial seizures. Electroencephalogr Clin Neurophysiol 1986;63:107–111. [DOI] [PubMed]
- 15.Steriade M, Amzica F. Dynamic coupling among neocortical neurons during evoked and spontaneous spike-wave seizure activity. J Neurophysiol 1994;72:2051–2069. [DOI] [PubMed]
- 16.Takeuchi T, Sitizyo K, Harada E. Analysis of the electroencephalogram in growing calves by use of power spectrum and cross correlation. Am J Vet Res 1998;59:777–781. [PubMed]
- 17.Hikasa Y, Kojima N, Takase K, Ogasawara S. Effects of thiopental, ketamine, diazepam, xylazine, and nitrous oxide on EEG spike activity and convulsive behavior during enflurane anesthesia in spontaneously breathing atropinized cats: Effect at surgical depth. Vet Surg 1993;22:318–325. [DOI] [PubMed]
- 18.Purohit RC, Mysinger PW, Redding RW. Effects of xylazine and ketamine hydrochloride on the electroencephalogram and the electrocardiogram in the horse. Am J Vet Res 1981;42:615–619. [PubMed]
- 19.Commision and classification and terminology of the international league against epilepsy: proposal for revised classification of epilepsies and epileptic syndromes. Epilepsia 1989;30:389–399. [DOI] [PubMed]
- 20.Silva-Barrat C, Menini C, Bryere P, Naquet R. Multiunitary activity analysis of cortical and subcortical structures in paroxysmal discharges and grand mal seizures in photosensitive baboons. Electroencephalogr Clin Neurophysiol 1986;64:455–468. [DOI] [PubMed]
- 21.Ishida N, Kasamo K, Nakamoto Y, Suzuki J. Epileptic seizure of El mouse initiates at the parietal cortex: depth EEG observation in freely moving condition using buffer amplifier. Brain Res 1993; 608:52–57. [DOI] [PubMed]
- 22.Scheffer IE, Bhatia KP, Lopes-Cendes I, et al. Autosomal dominant nocturnal frontal lobe epilepsy: A distinctive clinical disorder. Brain 1995;118:61–73. [DOI] [PubMed]
- 23.Oldani A, Zucconi M, Ferini-Strambi L, Bizzozero D, Smirne S. Autosomal dominant nocturnal frontal lobe epilepsy: electroclinical picture. Epilepsia 1996;37:964–976. [DOI] [PubMed]
- 24.Meldrum BS. Epileptic brain damage: a consequence and a cause of seizures. Neuropathol Appl Neurobiol 1997;23:185–202. [PubMed]
- 25.Wasterlain CG, Fujikawa DG, Penix L, Sanker R. Pathophysiological mechanisms of brain damage from status epilepticus. Epilepsia 1993;34:S37–53. [DOI] [PubMed]
- 26.Evans M, Griffiths T, Meldrum BS. Early changes in the rat hippocampus following seizures induced by bicuculline or L-allylglycine: a light and electron microscope study. Neuropathol Appl Neurobiol 1983;9:39–52. [DOI] [PubMed]
- 27.Evans M, Griffiths T, Meldrum BS. Kainic acid seizures and reversibility of calcium loading in vulnerable neurons in the hippocampus. Neuropathol Appl Neurobiol 1984;10:285–302. [DOI] [PubMed]
- 28.Meldrum BS, Brierley JB. Prolonged epileptic seizures in primates: ischemic change and its relation to ictal physiological events. Arch Neurol 1973;28:10–17. [DOI] [PubMed]
- 29.Meldrum BS, Vigoroux RA, Brierley JB. Systemic factors of epileptic brain damage: Prolonged seizures in paralysed artificially ventilated baboons. Arch Neurol 1973;29:82–87. [DOI] [PubMed]
- 30.Scharfman HE, Schwartzkroin PA. Protection of dentate hilar cells from prolonged stimulation by intracellular calcium chelation. Science 1989;246:257–260. [DOI] [PubMed]
- 31.Tanaka S, Sako K, Tanaka T, Yonemasu Y. Regional calcium accumulation and kainic acid (KA)-induced limbic seizure status in rats. Brain Res 1989;478:385–390. [DOI] [PubMed]
- 32.Meldrum BS, Garthwaite J. Excitatory amino acid neurotoxicity and neurodegenerative disease. Trends Pharmacol Sci 1990; 11:379–387. [DOI] [PubMed]
- 33.Pitkanen A, Riekkinen PJ. Amino acid levels in human CSF after generalized seizure. J Neural Transm 1987;70:313–319. [DOI] [PubMed]
- 34.Devinsky O, Emoto S, Nadi NS, Theodore WH. Cerebrospinal fluid levels of neuropeptides, cortisol, and amino acids in patients with epilepsy. Epilepsia 1993;34:255–261. [DOI] [PubMed]
- 35.Loscher W, Siemes H. Cerebrospinal fluid gamma-aminobutyric acid levels in children with different types of epilepsy: effect of anticonvulsant treatment. Epilepsia 1985;26:314–319. [DOI] [PubMed]
- 36.Ince E, Karagol U, Deda G. Excitatory amino acid levels in cerebrospinal fluid of patients with infantile spasms. Acta Paediatr 1997;86:1333–1336. [DOI] [PubMed]
- 37.Hajek M, Do KQ, Duc C, Boesiger P, Wieser HG. Increased excitatory amino acid levels on brain cysts of epileptic patients. Epilepsy Res 1997;28:245–254. [DOI] [PubMed]
- 38.Podell M, Hadjiconstantinou M. Cerebrospinal fluid γ-aminobutyric acid and glutamate values in dogs with epilepsy. Am J Vet Res 1997;58:451–456. [PubMed]