Abstract
The biophysical and pharmacological characteristics of the hyperpolarization activated non- selective cation current (If) were recorded using whole-cell voltage clamp in embryonic stem (ES) cell-derived cardiomyocytes at different stages of development.
The cation current was detected in a large percentage (65 %) of early stage (EDS, differentiated for 7 + 3–4 days) cells at a current density of 11·4 ± 0·6 pA pF−1 (n= 47). In late stage (LDS, differentiated for 7 + 9–12 days) cells the percentage of cells expressing If decreased (45 %), but If densities (15·5 ± 0·9 pA pF−1, n= 20) were increased.
The muscarinic agonist carbachol (CCh, 1 μM) depressed basal If in EDS cells by 45·7 ± 6·5 %, n= 5) and was without effect in LDS cardiomyocytes (n= 4). The β-adrenoceptor agonist isoprenaline (ISO, 1 μM) stimulated If in LDS cells by 33 ± 5·2 % (n= 6) but not in EDS cells (n= 5).
Cell infusion with the catalytic subunit of the cAMP-dependent protein kinase (PKA, 7 μM) stimulated If in EDS cells by 37·0 ± 2·9 %, (n= 4), but subsequent superfusion of 8-bromo-cAMP (200 μM) was without effect. Intracellular perfusion of LDS cardiomyocytes with the highly selective peptide inhibitor of PKA (PKI, 20 μM) completely inhibited the stimulation of the L-type Ca2+ current (ICa,L) as well as of If by ISO (1 μM).
Extracellular superfusion with phosphodiesterase (PDE) inhibitors – IBMX, a non-selective antagonist, Erythro-9-(2-hydoxy-3-nonyl)adenine (EHNA), a PDE2 antagonist and rolipram, a PDE4 antagonist – resulted in stimulation of ICa,L and If in EDS cells. By contrast, milrinone and cilostamide, two PDE3 antagonists, stimulated ICa,L, but not If.
The present work demonstrates that If is functionally expressed during early cardiomyogenesis. Similar to ICa,L, If is regulated during embryonic development by phosphorylation via PKA. In contrast to ICa,L, If is not regulated by PDE3 suggesting different localization of these ion channels with respect to PDE3.
A key feature of the heart is its spontaneous electrical activity, which is under the regulatory control of the autonomic nervous system. The automaticity of the sinus node is due to the diastolic depolarization and the hyperpolarization-activated non-selective cation current If appears to be involved in its generation (DiFrancesco, 1993). The biophysical characteristics of If comprise time-dependent activation kinetics, a linear current-voltage (I–V) relationship and voltage-dependent block upon extracellular application of Cs+. Recently, If has been cloned and shown to display similar structural characteristics to other voltage-gated ion channels (Gauss et al. 1998; Ludwig et al. 1998; Santoro et al. 1998). As also reported for other cyclic nucleotide-gated ion channels, the carboxyl terminal region of If contains a consensus sequence site for cAMP-dependent protein kinases (Ludwig et al. 1998).
The non-selective cation current If has been detected in cells of the cardiac conductive system of different species such as frog sinus venosus (Bois & Lenfant, 1990), rabbit sino-atrial node (Yanagihara & Irisawa, 1980), rabbit atrioventricular node (DiFrancesco et al. 1980), cat atrium (Zhou & Lipsius, 1992a) and rabbit Purkinje fibres (DiFrancesco & Ferroni, 1983). In addition, If is present during embryonic and perinatal development since the current has been observed in chick embryonic ventricle (Satoh & Sperelakis, 1991), in freshly isolated and cultured ventricular cells from newborn rats (Robinson et al. 1997) as well as in dedifferentiated adult rat ventricular cells from primary cultures (Fares et al. 1998). In contrast to early postnatal development, If has been detected in adult mammalian ventricle (Yu et al. 1993, 1995), but only upon applying strongly hyperpolarizing test pulses (negative to −120 mV).
It is well known that the autonomic nervous system regulates the chronotropy of the heart and that If is regulated by sympathetic and parasympathetic neurotransmitters. Indeed, the β-adrenoceptor agonist isoprenaline (ISO) stimulates If by shifting the activation curve to more positive voltages whereas the vagal neurotransmitter acetylcholine (ACh) inhibits If by shifting the activation curve to more negative potentials (DiFrancesco & Tromba, 1988). If regulation clearly involves the modulation of adenylyl cyclase activity (Lindemann & Watanabe, 1990; Pappano, 1990) and both the direct binding of cAMP to f-channels (DiFrancesco & Tortora, 1991) and the phosphorylation of the channels via cAMP-dependent protein kinase A (PKA) (Chang et al. 1991; Yu et al. 1995) contribute to the overall effect on If.
Since pathologically altered cardiac myocytes re-express early embryonic genes (Vikstrom & Leinwand, 1996) and If has been detected in ventricular cardiomyocytes of hypertensive rats (Cerbai et al. 1996) as well as recently in human failing heart (Cerbai et al. 1997; Hoppe & Beuckelmann, 1998), we examined the expression and modulation of If during early cardiomyogenesis. We have shown earlier that the regulation of the L-type Ca2+ channel current (ICa,L) during early stages of cardiomyogenesis differs strongly from that known for terminally differentiated cardiomyocytes. At these early stages intrinsic adenylyl cyclase and phosphodiesterase (PDE) activities are high, whereas β-adrenoceptors are not yet functionally coupled (Ji et al. 1999; Maltsev et al. 1999). Due to the known difficulties of harvesting murine cardiomyocytes from the early murine embryo prior to E11.5 (Davies et al. 1996), we have used in the present study murine embryonic stem (ES) cell-derived cardiomyocytes, known to recapitulate cardiomyogenesis in vitro (Wobus et al. 1991; Maltsev et al. 1994; Hescheler et al. 1997).
We demonstrate that If is expressed early during murine cardiomyogenesis and displays similar biophysical characteristics to those reported for terminally differentiated cardiomyocytes. We further show that during embryonic development If is modulated by phosphorylation and that If and ICa,L are differentially regulated by PDEs.
METHODS
Embryonic stem cell preparation
Murine ES cells of the line D3 were cultured and differentiated into spontaneously beating cardiomyocytes as previously described (Wobus et al. 1991; Maltsev et al. 1994). Briefly, ES cells differentiated within spheroidal aggregates (embryoid bodies) in hanging drops for 2 days. The embryoid bodies were thereafter transferred into suspension for 5 days and finally plated for different periods of time. The EDS cells were kept in suspension for 7 days and thereafter plated for 3–4 days (7 + 3–4) prior to dissociation. The IDS cells were kept for 7 days in suspension and plated for 5–8 days (7 + 5–8). The LDS cells were also kept in suspension for 7 days and plated for 9–12 days (7 + 9–12) in 24 micro-well plates. Single cardiomyocytes were isolated from clusters of spontaneously beating areas by a modification of the procedure described by Isenberg & Klöckner (1982). Beating areas of 15 to 20 embryoid bodies were isolated with a sterile microscalpel and collected in low Ca2+ solution containing (mM): 120 NaCl, 5.4 KCl, 5 MgSO4, 5 sodium pyruvate, 20 glucose, 20 taurine, 10 Hepes (pH 6.9 with NaOH). The tissue was then incubated in enzyme medium (1 mg ml−1 collagenase B, Boehringer, Mannheim, FRG; 30 μM CaCl2) for 20 min at 37°C. Tissue fragments were transferred to a medium containing (mM): 85 KCl, 30 K2HPO4, 5 MgSO4, 1 EGTA, 2 Na2ATP, 5 sodium pyruvate, 5 creatine, 20 taurine, 20 glucose, pH 7.2, where they were kept at room temperature for 1 h, and then resuspended in Dulbecco's modified Eagle's medium (DMEM; Gibco, Eggenstein, FRG) complemented with 20 % fetal calf serum. Isolated cells were plated onto sterile, gelatine-coated glass cover slips and kept in an incubator for 24–48 h. Spontaneously contracting myocytes could be observed within 12 h after cell preparation.
Electrophysiology
For patch clamp recordings only spontaneously beating, single cardiomyocytes were selected, using the whole-cell configuration of the patch-clamp technique (Hamill et al. 1981). The cells were held in the voltage-clamp mode using an Axopatch 200-A (Axon Instruments, Foster City, USA) or an EPC-9 (Heka, Lambrecht, FRG) amplifier. For If measurements, cardiomyocytes were clamped at a holding potential of −35 mV for 200 ms to obtain inactivation of Na+ currents and hyperpolarizing pulses to −110 mV (2–5 s duration) were used. For recording of the steady-state activation curves, hyperpolarizing voltage steps (2 s) from −40 to −160 mV in 10 mV intervals were applied (holding potential −35 mV) and tail currents measured at 15 mV. The current-voltage (I–V) relationship for If was determined by measuring the tail currents (2 s) 15 ms after voltage steps to potentials ranging from −120 to 50 mV in 10 mV step intervals following a 2 s hyperpolarizing voltage step to −115 mV (holding potential (Vh) −35 mV). The regulation of If was investigated by applying hyperpolarizing pulses to −110 mV (holding potential −35 mV). Due to the slow activation kinetics of If, voltage steps were applied at low frequency (maximum rate, 0.1 Hz). The protocol for the simultaneous recording of ICa,L and If consisted of depolarizing steps from a holding potential of −50 mV to 0 mV (200 ms duration, activating preferentially for ICa,L) followed by a hyperpolarization to −110 mV for 2 s for the recording of If. The hyperpolarizing step to −110 mV was followed by a depolarization to −35 mV for 50 ms to inactivate ICa,L prior to returning to a holding potential of −50 mV. Cell membrane capacity was determined on-line using the ISO2 (MFK, Frankfurt, FRG) or Pulse (Heka) acquisition software program. Data were acquired at a sampling rate of 10 kHz, filtered at 1 kHz, stored on hard disk and analysed off-line using the ISO2 or the Pulsefit (Heka) analysis software package. The glass cover slips containing the cells were placed into a temperature-controlled (37°C) recording chamber and perfused continuously with extracellular solution by gravity at a rate of 1 ml min−1. Substances were applied by exchanging the solution in the chamber; a 90 % volume exchange was achieved within approximately 20 s. Patch pipettes (2–4 MΩ resistance) were pulled from Clark borosilicate glass (Electromedical Instruments, Reading, UK) using a Zeitz puller (DMZ, Munich, FRG). The solutions used for voltage-clamp recordings had the following composition (mM): internal solution for measurement of If: NaCl 10, potassium aspartate 130, Na2ATP 2, Na2GTP 0.1, MgCl2 2, EGTA 1, Hepes 10, pH 7.2 (adjusted with KOH). TEA (10 mM) was added to the If internal solution for the simultaneous recording of If and ICa,L. External solution for measurement of If (mM): NaCl 140, NaOH 2.3, MgCl2 1, KCl 5.4, CaCl2 1.8, Hepes 5, glucose 10, CdCl2 0.5, BaCl2 1,4-aminopyridine 2, pH 7.4 (adjusted with NaOH). In the experiments showing a simultaneous recording of both ICa,L and If, 0.5 mM CdCl2 was omitted from the If external solution.
Analysis of data
The amplitude of If was measured as the difference between the instantaneous current at the beginning of the voltage step and the steady-state current recorded at the end of the voltage step (Cerbai et al. 1994). Currents were normalized to cell membrane capacity where indicated. For the analysis of steady-state activation curves, the amplitude of the tail currents was determined as the difference current between peak and sustained currents and Boltzmann distributions fitted to the normalized values. For I–V relations, the amplitude and the reversal potential of currents were analysed from the tail currents measured 15 ms after application of the voltage step in order to avoid contamination by Na+ or capacitance currents (Hoppe & Beuckelmann, 1998). In the time course diagrams the time point '0′ indicates the beginning of recordings shortly after establishment of the whole-cell configuration. When Cs+ or carbachol (CCh) were applied, the instantaneous current amplitude in the presence of Cs+ or CCh was taken as the reference value for the estimation of If. For the estimation of If densities data from all EDS cells used in these experiments were included, whereas in order to increase the number of IDS and LDS cells, data from experiments not reported here were included. Averaged data are expressed as means ±s.e.m. Statistical analysis was performed using Student's paired and unpaired t tests and a P value of < 0.05 was considered significant. Substances were applied only after establishment of stable current amplitudes.
Reagents
The catalytic subunit of PKA was obtained from Promega (Heidelberg, FRG) and the protein kinase A inhibitor (PKI, fragment 6–22 amide) was purchased from Sigma (Deisenhofen, FRG). PKI was dissolved in intracellular solution and stored frozen at −20°C. The aliquots were thawed immediately prior to use and diluted with pipette solution to the desired concentration. Cilostamide was obtained from Calbiochem (Meudon, France). All other substances were purchased from Sigma (Deisenhofen, FRG) and freshly prepared by dissolving in extracellular solution.
RESULTS
Biophysical and pharmacological characteristics of If in EDS cells
The whole-cell configuration of the patch-clamp technique was used for the recording of If in isolated ES cell-derived cardiomyocytes of different developmental stages. Figure 1a illustrates a representative experiment where the steady-state current-activation curve of If was recorded in a spontaneously contracting EDS cardiomyocyte. It can be seen that the threshold of activation of If was close to −50 mV. However, a precise determination could not be made since applying much longer voltage steps was not tolerated by the cells. The activation curve was determined by measuring tail currents at +15 mV upon fully activating If by applying hyperpolarizing voltage steps from −30 to −160 mV (left panel). Peak amplitudes of tail currents were measured in three representative EDS and/or IDS cardiomyocytes, normalized and fitted with a Boltzman function yielding a Vh of −98.1 ± 1.1 mV (Fig. 1a, right panel). Next we determined the current-voltage (I–V) relationship by measuring the tail currents at different potentials after a prepulse (2 s) to −115 mV (Fig. 1B). As can be seen in Fig. 1B (right panel) the I–V relation is linear with a reversal potential close to −30 mV. Thus, the slow activation kinetics (left panel), the relatively linear I–V relationship with an average reversal potential of −30.9 ± 1.1 mV (n= 4) and the activation threshold close to −50 mV were similar to those reported for If in sino-atrial node cells or Purkinje fibres from adult hearts (for review see DiFrancesco, 1993). It was reported for Purkinje (Callewaert et al. 1984) and sino-atrial (SA) node cells (DiFrancesco, 1986) that Cs+ exerts a voltage-dependent block on f-channels at potentials negative to the reversal potential of If. Similarly, extracellular application of Cs+ (2 mM) in ES cell-derived cardiomyocytes strongly inhibited If (Fig. 1C, n= 10). The time course of a representative experiment, where each data point represents the amplitude of If evoked by a voltage step to −110 mV (holding potential −35 mV, see original traces in the inset, left panel) demonstrates an almost complete block of the inward current by Cs+ (Fig. 1C, left panel) and the reversibility of the block upon washout in an EDS cell. The right panel of Fig. 1C shows the I–V curve recorded in another cardiomyocyte where the voltage dependence of the inhibition of If by Cs+ was tested. In the presence of Cs+ (filled squares) If is blocked at potentials negative to the reversal potential, whereas at potentials positive to the reversal potential the amplitude of If evoked by depolarizing voltage steps is unaltered. Our results demonstrate that the overall biophysical and pharmacological characteristics of If in ES cell-derived cardiomyocytes are similar to those reported in adult cardiomyocytes.
Figure 1. Biophysical and pharmacological characterization of If in ES cell-derived cardiomyocytes.
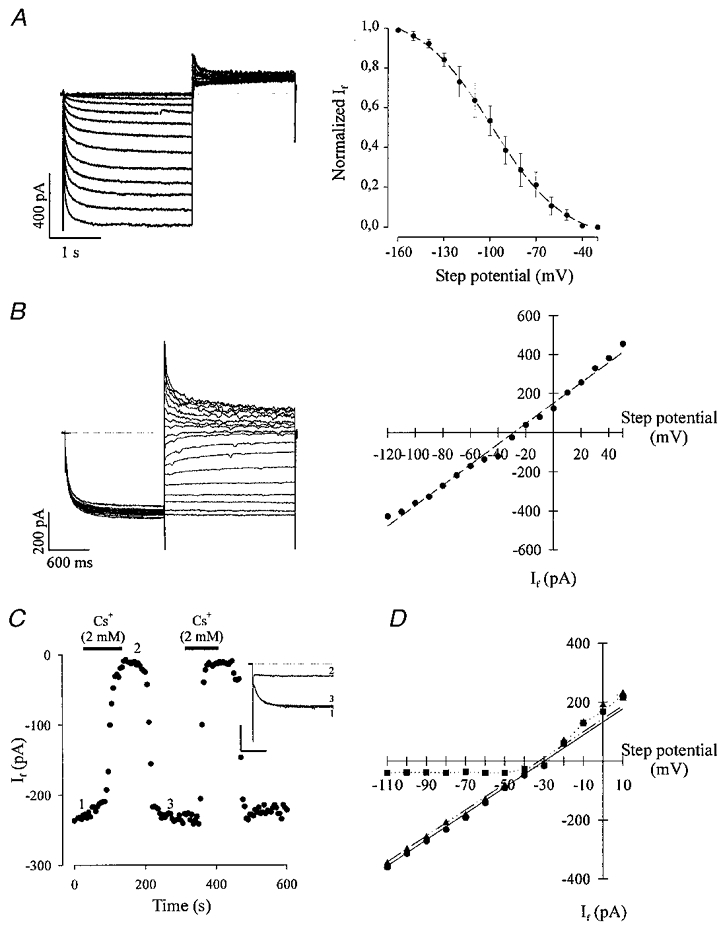
A, left panel, IDS cardiomyocyte displaying the steady-state activation characteristics tested by 2 s lasting hyperpolarizing voltage steps from −40 to −160 mV in 10 mV intervals and subsequent 2 s depolarization to +15 mV (holding potential −35 mV, the dotted line indicates the zero current level). The typical slow activation kinetics for If was observed, the threshold potential proved close to −50 mV. The right panel shows the steady-state activation characteristics of 3 representative experiments. Data points were obtained by measuring the peak of the deactivating tail currents, normalized and fitted with a Boltzmann curve yielding a Vh of −98.2 ± 1.1 mV. B, a representative I–V relationship of an EDS cardiomyocyte obtained by applying 2 s lasting prepulses to −115 mV (holding potential −35 mV) and subsequent 2 s lasting voltage steps ranging from −120 to +50 mV in 10 mV intervals. The I–V relationship (right panel) was determined by measuring If 15 ms after applying the depolarizing voltage steps. The linear regression of the data point yielded a reversal potential of −29 mV. C, extracellular application of Cs+ (2 mM) resulted in a complete block of If (holding potential −35 mV, step potential −110 mV) in an EDS cardiomyocyte; this could be reversed by washout (left panel). The numbers in the time course diagram indicate when the current traces displayed in the inset (calibration bars correspond to 300 ms and 200 pA, respectively) were recorded (the current in the presence of Cs+ was taken as reference value for the estimation of If amplitude). The right panel shows the I–V relation of If in absence and presence of extracellular Cs+ (2 mM) in an EDS cardiomyocyte. Cs2+ almost completely blocked If at potentials negative to the reversal potential (the linear regression yielded −30 mV prior to application of Cs+ and −32.5 mV after washout of Cs+) whereas positive to −30 mV no effect of Cs+ on If was observed (•, control; ▪, in the presence of 2 mM CsCl; ▴, after washout of CsCl).
Figure 2a shows the percentage of cells expressing If at different stages of cardiomyogenesis. If was detected in a large percentage (65 %, n= 18) of EDS cells (7 + 3–4) days, whereas at the IDS (7 + 5–8) day stage, If was detected in fewer ES cell-derived cardiomyocytes (50 %, n= 10). Moreover, the percentage of cells expressing If was even less (45 %, n= 8) in LDS cells (7 + 9–12) days. The If densities were 11.4 ± 0.6 pA pF−1 (n= 47) and 11.9 ± 1.7 pA pF−1 (n= 11) in EDS and IDS cells, respectively. LDS cells displayed a significantly higher current density of 15.5 ± 0.9 pA pF−1 (n= 20, P < 0.05) as compared with EDS and IDS cells (Fig. 2B).
Figure 2. Functional expression of If in ES cell-derived cardiomyocytes at different stages of development.
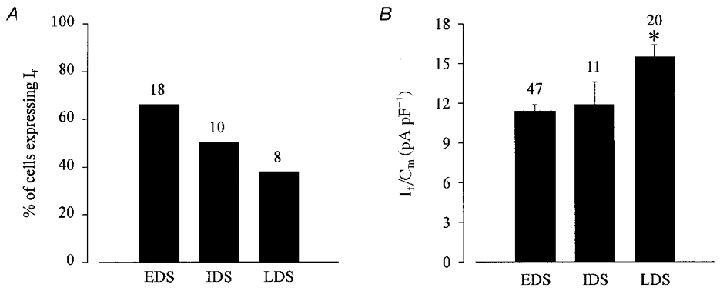
A, the percentage of cells expressing If declined during cardiomyogenesis. B, at later stages of development an increase in the current density (If/Cm, where Cm is membrane capacitance) of If was observed. If was evoked by applying a 2 s hyperpolarizing voltage step from a holding potential of −35 to −110 mV (*statistically significantly different to EDS and IDS at the P < 0.05 level using Student's unpaired t test). Numbers above bars are numbers of replicates.
Effects of β-adrenergic and muscarinic agonists on If in EDS and LDS cells
It is well established that β-adrenergic and muscarinic agonists stimulate and inhibit If, respectively (DiFrancesco & Tromba, 1987; Zhou & Lipsius, 1992b; DiFrancesco, 1995a; Renaudon et al. 1997). Since the steady-state activation curves yielded a relative negative Vh and modulation of If is known to work through a shift in the activation curve on the voltage axis, If was first evoked by depolarizing steps to −110 mV. In line with our previous findings on the development-dependent establishment of the regulation of ICa,L (Maltsev et al. 1999; Ji et al. 1999), CCh did not depress basal If in LDS cells (Fig. 3B, If control density: 12.7 ± 1.9 pA pF−1; If density in presence of CCh: 12.5 ± 1.9 pA pF−1, n= 4) whereas ISO did not stimulate If in EDS cells (Fig. 4a, control If density: 11.9 ± 2 pA pF−1; If density upon application of ISO: 11.2 ± 2.2 pA pF−1, n= 5). Figure 3a shows the current traces (left panel) and corresponding time course (right panel) of the effect of CCh (1 μM) on If in EDS cells. Similar to what was reported for ICa,L, CCh application resulted in a strong depression of If (45.7 ± 6.5 %, n= 5) and this effect could be completely reversed upon washout of CCh. A summary (Fig. 3a, histogram) of If densities obtained under control condition (12.5 ± 1.5 pA pF−1), in the presence of CCh (1 μM) (6.7 ± 1 pA pF−1) and upon washout (11.1 ± 1.2 pA pF−1) is shown.
Figure 3. Carbachol (CCh) strongly depresses the amplitude of basal If in EDS cardiomyocytes.
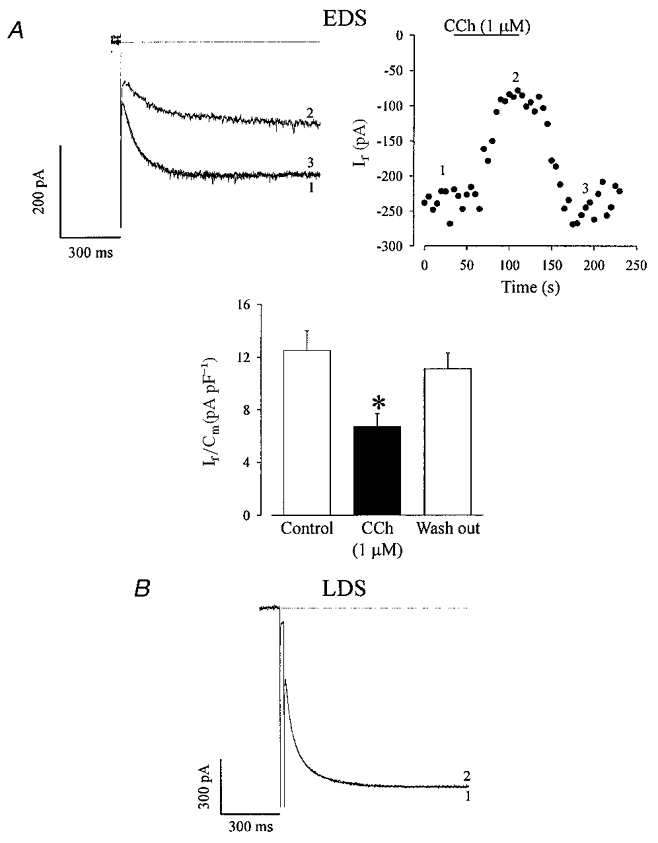
A, CCh depressed basal If in a representative EDS cardiomyocyte. The effect was completely reversed upon washout (left panel). This is also seen in the time course of this experiment (right panel), the numbers indicate the traces displayed in A (the instantaneous current in presence of CCh was taken as reference for the estimation of the If amplitude). The effect of CCh on If in EDS cells (n= 5) is summarized in the histogram. *Statistically significantly different to control at the P < 0.05 level (paired t test). B, in LDS cardiomyocytes CCh did not depress basal If. (Voltage protocol identical to Fig. 2). 1, control; 2, CCh (1 μM).
Figure 4. Isoprenaline (ISO) stimulates If in LDS cardiomyocytes.
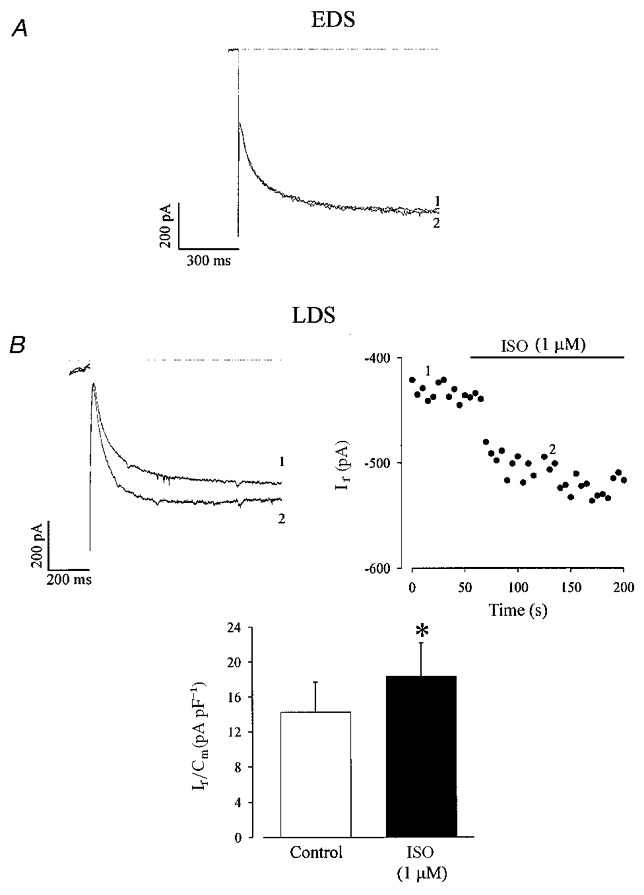
A, ISO (1 μM) did not stimulate If in EDS cardiomyocytes, 1, control; 2, ISO (1 μM). B, ISO stimulated If in LDS cardiomyocytes (left panel: original recordings; right panel: time course of this experiment, voltage protocol identical to Fig. 2). *Difference in current density between the absence and the presence of ISO was statistically significant (B, histogram, n= 6; P < 0.05, paired t test).
In Fig. 4B current traces (left panel) and corresponding time course (right panel) of a typical experiment in an LDS cardiomyocyte are displayed, where If (recorded at −110 mV) was stimulated upon extracellular application of ISO (1 μM). If current densities in control condition and in the presence of ISO were 14.2 ± 3.4 pA pF−1 (n= 6) and 18.2 ± 3.9 pA pF−1 (n= 6), respectively (Fig. 4B, histogram); thus, the stimulation amounted to 33.0 ± 5.2 %. It should be noted that the stimulation of If by ISO in LDS cells was mimicked by extracellular application of forskolin (1 μM), a direct activator of adenylyl cyclase. Forskolin stimulated If by 31.0 ± 2.0 % (n= 4, data not shown).
We conclude that CCh depresses basal If in EDS but not in LDS cells, whereas ISO stimulates If in LDS but not in EDS cells. Furthermore, these results clearly show that the regulation of If parallels that of ICa,L during the course of cardiomyogenesis.
If is stimulated by phosphorylation via PKA in EDS cells
Since If was shown to be stimulated both by a direct interaction with cAMP, as reported for the sinus node (DiFrancesco, 1995a; Bois et al. 1997; DiFrancesco, 1999), and by cAMP-dependent phosphorylation via PKA, as found in Purkinje fibres (Chang et al. 1991; Yu et al. 1993) we examined the effect of the catalytic subunit of PKA (Fig. 5, left panels, C-subunit) as well as of cAMP analogues (Fig. 5, right panels) on If during early and late developmental stages of cardiomyogenesis. Figure 5a (left panel) shows a representative experiment where intracellular application of the catalytic subunit of PKA (7 μM) increased the amplitude of If in an EDS cardiomyocyte. The time course of this experiment displays a pronounced stimulation of the amplitude of If with a maximal effect obtained around 3 min after establishment of the whole-cell configuration (Fig. 5B, left panel), as expected for the diffusion kinetics of PKA through the opening of the patch pipette. On average, PKA stimulated If by 37.0 ± 2.9 % (n= 4) in all EDS cells tested. If densities under control conditions (immediately after break in) and after 5 min of cell dialysis with the C-subunit of PKA were 10.9 ± 1.6 and 14.8 ± 2.0 pA pF−1 (n= 4), respectively (Fig. 5C, left panel). Similarly, a stimulation of If amplitude was observed upon extracellular application of 8 bromo-cAMP (200 μM), a membrane-permeable analogue of cAMP (Fig. 5a and B, right panels). If densities under control conditions and after extracellular application of 8 Br-cAMP were 9.7 ± 1.0 (n= 5) and 12.8 ± 1.4 pA pF−1 (n= 5), respectively (Fig. 5C, right panel). In order to evaluate PKA-mediated stimulation of Ifvs. direct cAMP binding further, two sets of additional experiments were performed. First, we tested whether If could be additionally increased by 8-bromo-cAMP (200 μM) in the presence of the catalytic subunit of PKA (7 μM) in the pipette solution. As can be seen in Fig. 5D 8-bromo-cAMP did not have any additional stimulatory effect on If under such conditions (n= 4), demonstrating that, if present, a direct cAMP stimulation of If is not additive with a maximal indirect stimulation of the current via PKA. Second, the effect of 8-bromo-cAMP was examined in the presence of PKI, a highly selective peptide inhibitor of PKA (Cheng et al. 1986; Skeberdis et al. 1997). Under intracellular perfusion with PKI (20 μM, intracellular solution) in EDS cells, a strong depression of If was observed demonstrating a high constitutive PKA activity in these cells. However, 8-bromo-cAMP had no effect on If under these conditions (n= 2, data not shown). These results demonstrate that If is stimulated by phosphorylation via the cAMP-dependent PKA in EDS cells.
Figure 5. The catalytic subunit of PKA (C-subunit of PKA, 7 μM) and 8-bromo-cAMP (200 μM) stimulate If in EDS cardiomyocytes.
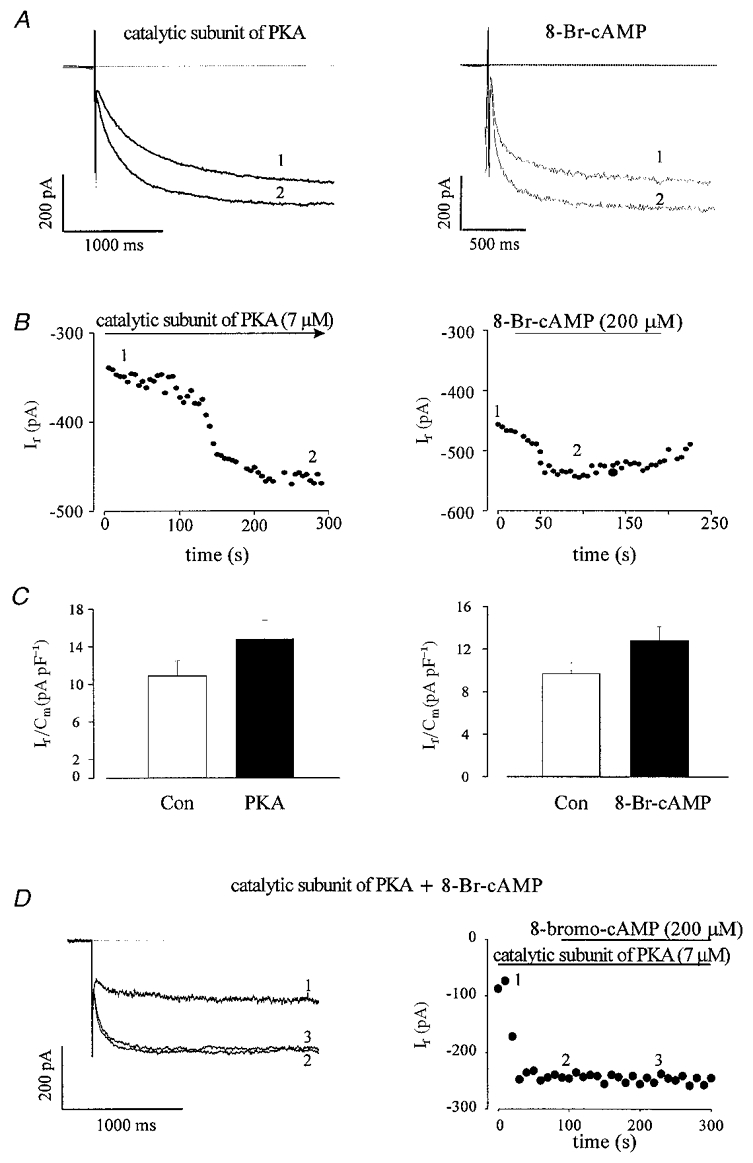
A, the C-subunit of PKA (7 μM through pipette, left panel) as well as 8-bromo-cAMP (8-Br-cAMP 200 μM, extracellular application, right panel) stimulated If in EDS cells. The corresponding time courses are shown in B. The effects of C-subunit of PKA (n= 4) and 8-Br-cAMP (n= 5) on If are summarized in the left and right panels of C, respectively. *Statistically significantly different to control (Con) at the P < 0.05 level (paired t test). D shows that 8-Br-cAMP has no additional effect on If after activation of the current by the C-subunit of PKA (7 μM): left panel, current traces (1 control, 2 C-subunit of PKA, 3 C-subunit of PKA + 8-bromo-cAMP); right panel, corresponding time course.
β-adrenergic regulation of If is due to phosphorylation via PKA in LDS cells
The effect of β-adrenergic agonists on ICa,L is related to an increase in adenylyl cyclase activity, a rise in cAMP levels and phosphorylation via PKA in LDS cells (Ji et al. 1999; Maltsev et al. 1999). Since in LDS cells β-adrenoceptors were functionally coupled resulting in the stimulation of both If and ICa,L by ISO (1 μM), we next examined whether the effect on If was mediated through a direct cAMP binding and/or an indirect phosphorylation via cAMP-dependent PKA at this developmental stage. In order to differentiate between these two modulatory pathways, we tested again the effect of an intracellular perfusion with PKI (Fig. 6). The simultaneous recording of ICa,L and If allowed us to test the efficacy of PKI in blocking the stimulation of ICa,L by ISO. The current traces of ICa,L and If (Fig. 6a) as well as the time course of this experiment (Fig. 6B) demonstrate that intracellular infusion with PKI (20 μM) prevented the stimulatory effects of ISO (1 μM) on both If and ICa,L. A summary of these data are shown in Fig. 6C, where If densities under control conditions and after dialysis with PKI and in presence of ISO (1 μM) amounted to 11.5 ± 3.2 pA pF−1 (n= 6) and 10.5 ± 2.6 pA pF−1 (n= 6), respectively. For ICa,L, the current densities were 12.6 ± 2.7 pA pF−1 for control condition (after dialysis with PKI) and 6.9 ± 1.9 pA pF−1 (n= 4) after superfusion of the cell with ISO (1 μM) (Fig. 6D). The decrease of ICa,L density is due to current run down.
Figure 6. Effect of a selective peptide inhibitor of cAMP-dependent protein kinase A (PKI, 20 μM) on the ISO-stimulated If and L-type Ca2+ current (ICa,L) in LDS cells.
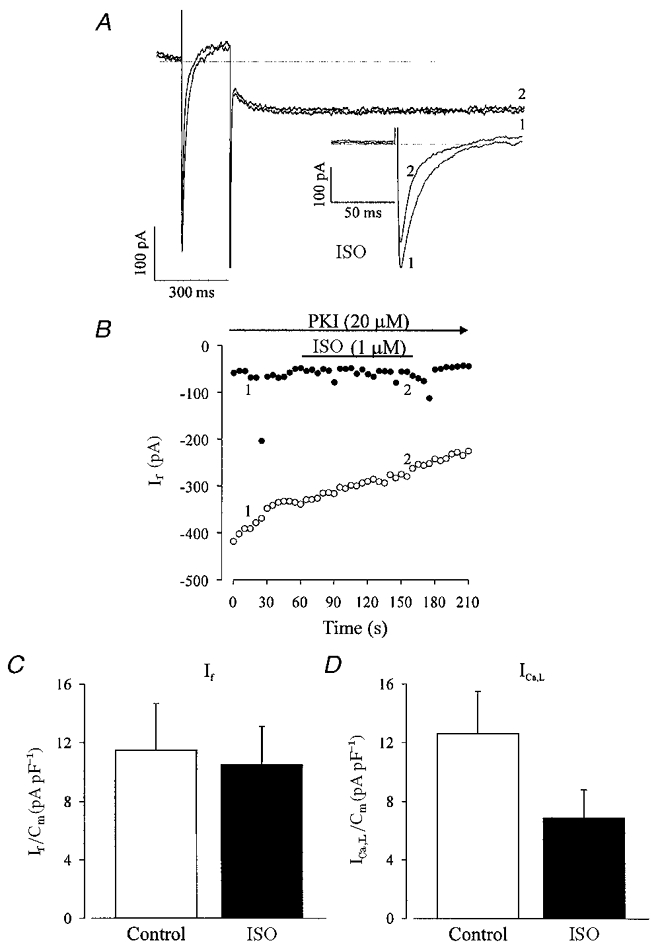
A, ISO neither stimulated If nor ICa,L in the presence of PKI (1 PKI, 2 PKI and ISO). The inset in A displays ICa,L at a more extended time scale. Simultaneous recording of ICa,L and If consisted of a depolarizing step from a holding potential of −50 to 0 mV for 200 ms and a subsequent hyperpolarization to −110 mV for 2 s. B, time course of the experiment displayed in A. The If (•) amplitude remained unaltered upon addition of ISO whereas the pronounced decrease in ICa,L (^) was due to run down. A statistical summary of these data demonstrates that neither If (C; n= 6) nor ICa,L (D; n= 4) was stimulated by ISO in the presence of PKI.
Altogether, these experiments demonstrate that during early and late stages of cardiomyogenesis If is stimulated by phosphorylation via cAMP-dependent protein kinase.
Effects of PDE inhibitors on If in EDS cells
In our previous study investigating the regulation of ICa,L by cAMP-dependent pathways we showed that the high intrinsic basal adenylyl cyclase activity in EDS cells is counterbalanced by a high intrinsic PDE activity due to PDE2-4 subtypes (Maltsev et al. 1999). Thus in the present study, we have investigated which particular PDE subtype regulates If and whether there are any differences in the regulation of ICa,L and If by PDEs. Simultaneous recordings of ICa,L and If were performed. Figure 7a illustrates the effects of extracellular application of IBMX (200 μM), a non-selective PDE inhibitor on If and ICa,L in EDS cells. As can be seen, IBMX stimulates ICa,L as well as If (the inset shows on a more extended time scale the stimulation of ICa,L by IBMX). On average, IBMX (200 μM) stimulated If in three out of four cells tested (Fig. 7D) by 39.3 ± 18.1 % and ICa,L by 48.8 ± 13.8 % (n= 3). The If densities in control conditions and in presence of IBMX amounted to 10.1 ± 1.0 and 13.8 ± 0.9 pA pF−1 (n= 3), respectively (Fig. 7E). This clearly demonstrates that If, like ICa,L, is controlled by an intrinsic PDE activity in EDS cells. In order to determine which type(s) of PDEs regulate(s) If in EDS cardiomyocytes, we examined the effects of specific inhibitors of the PDE2-4 isoforms. Figure 7B and C show that EHNA (30 μM), a selective PDE2 antagonist (Méry et al. 1995), and rolipram (10 μM), a selective PDE4 antagonist (Fischmeister & Hartzell, 1991), increased the ICa,L as well as the If amplitude in EDS cells. On average, EHNA (30 μM) and rolipram (10 μM) stimulated If by 45.7 ± 17.0 (n= 4) and 25.4 ± 4.6 % (n= 5), respectively (Fig. 7D), whereas they stimulated ICa,L by 45.4 ± 22.0 and 54.0 ± 29.0 %, respectively. If densities in control conditions and in the presence of EHNA were 11.3 ± 2.9 and 15.7 ± 4.0 pA pF−1 (n= 4) and for control vs. rolipram they were 11.5 ± 1.4 and 14.2 ± 1.2 pA pF−1 (n= 5), respectively (Fig. 7E).
Figure 7. The non-selective phosphodiesterase (PDE) inhibitor IBMX as well as PDE2 and PDE4 antagonists stimulate If and ICa,L in EDS cardiomyocytes.
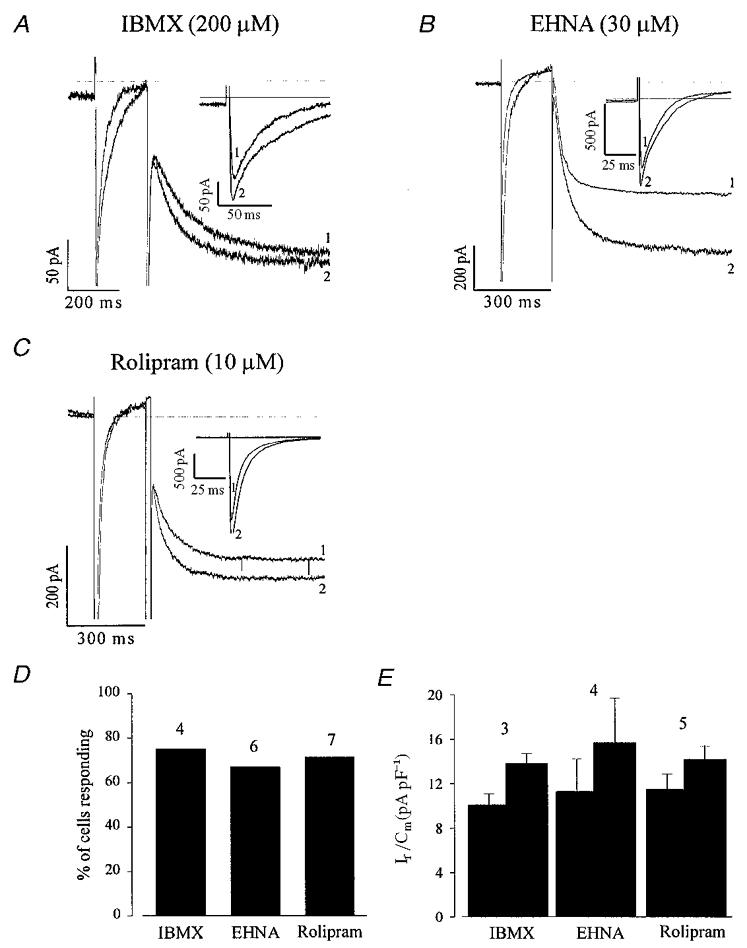
A–C, the non-selective antagonist IBMX (200 μM; A), the PDE2 selective antagonist EHNA (30 μM; B) and the PDE4 antagonist rolipram (10 μM; C) stimulate both If and ICa,L in EDS cardiomyocytes. The same voltage protocol as in Fig. 6 was used. The insets display ICa,L at a more extended time scale. Due to the slight delay in the maximal response of If as compared with ICa,L and the fast run down of the latter, the ICa,L traces displayed in B and C were recorded prior to If. D shows that most of the EDS cardiomyocytes tested responded to PDE antagonist application with a stimulation of If and ICa,L. E shows that an increase in If density was also observed under these conditions.
Unlike IBMX and PDE2 and/or PDE4 inhibitors, the PDE3 selective antagonists milrinone (10 μM, Fig. 8a) and cilostamide (300 nM, Fig. 8B) stimulated ICa,L but not If in the EDS cells. Milrinone (100 %, n= 11) as well as cilostamide (82 %, n= 11) had no stimulatory effect on If (Fig. 8C), whereas they stimulated ICa,L by 36.0 ± 5.4 and 39.0 ± 5.8 %, respectively. In the case of cilostamide, a small (9.9 ± 3.9 %) stimulation of If was observed in 2 out of 11 cells (Fig. 8D). These data confirm that under basal conditions, PDE2 and PDE4, but not PDE3 regulate If, whereas all three PDE subtypes regulate ICa,L.
Figure 8. The PDE3 antagonists milrinone and cilostamide stimulate ICa,L but not If in EDS cardiomyocytes.
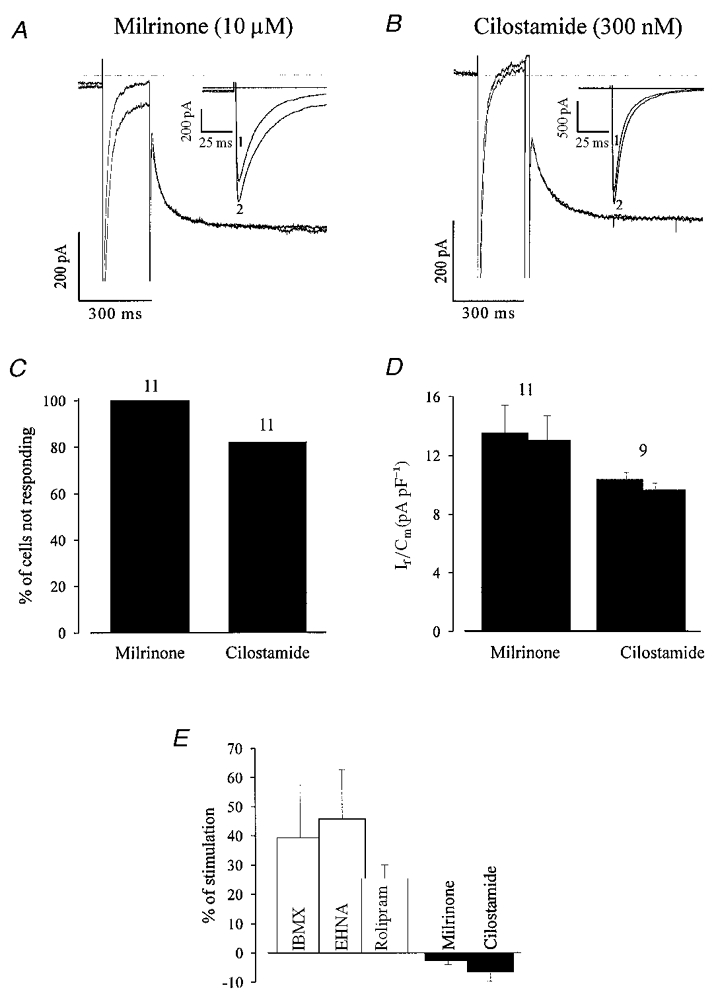
A and B, milrinone and cilostamide stimulated ICa,L (see also inset for a more extended time scale), but If remains unaltered (voltage protocol identical to Fig. 6). C, in the large majority of cells If is not stimulated upon PDE3 antagonist application. D, the current density of the non-responding cells decreased slightly in the presence of PDE inhibitors indicating a small run down. E, a quantitative comparison of the percentage of If density stimulation demonstrates that If was stimulated by PDE2 and 4 antagonists, but not by PDE3 antagonists.
DISCUSSION
Until now little has been known about the biophysical and pharmacological properties of If during heart development. If was shown to be expressed in 3-day-old embryonic chick ventricular myocytes with a current density decreasing at day 10 and becoming almost undetectable at day 17 (Satoh & Sperelakis, 1991, 1993). Furthermore, If was found to be stimulated by ISO and depressed by CCh in young embryonic chick ventricular myocytes (Satoh & Sperelakis, 1993). A recent report (Liu et al. 1996) showed that most of the characteristics of If in cultured sino-atrial node cells were similar to those measured in freshly isolated pacemaker cells. Here, we report, that the percentage of spontaneously beating cells expressing If during early stages of cardiomyogenesis is high, indicating that this ion channel may play an important role at this developmental stage. With further cellular specialization, atrial as well as ventricular cardiomyocytes obviously downregulate If, whereas in cells of the conduction system current density increases. The fact that in ventricular cardiomyocytes of patients with failing hearts an increased current density of If is detected (Cerbai et al. 1997; Hoppe & Beuckelmann, 1998) suggests that these cells may dedifferentiate and recover their embryonic phenotype.
If has been proposed to play an important role in the regulation of the heart rhythmicity modulated by the autonomous nervous system (DiFrancesco, 1995a). While there is general agreement that the modulation is mediated by adenylyl cyclase activity, it is still a matter of debate whether direct nucleotide (cAMP) binding or phosphorylation by PKA is the main mechanism involved. Phosphorylation of f-channels via cAMP-dependent PKA has been reported in Purkinje fibres (Chang et al. 1991; Yu et al. 1995), whereas a direct action of cAMP on f-channels has been proposed in the sinus node (DiFrancesco & Tortora, 1991; DiFrancesco, 1995b; Fares et al. 1998) as well as for heterologously expressed f-channels (Ludwig et al. 1999). We have therefore examined the regulation of If during various stages of cardiomyogenesis. Since in common with the early murine heart (E11.5) (An et al. 1996) the β-adrenoceptor response is absent at the EDS cell stage, we investigated in these cardiomyocytes the action of the catalytic subunit of PKA on If. Our data clearly demonstrate a stimulation of If by PKA suggesting that at this stage phosphorylation via cAMP-dependent PKA is involved. Interestingly, our negative results obtained with 8-bromo-cAMP, used either after a maximal PKA activation or after a complete block of PKA by PKI, a selective peptide inhibitor of PKA, does not provide any evidence for a direct regulation of If by cAMP binding to the f-channel in EDS cells. Likewise, at the LDS stage, the reproducible stimulation of ICa,L and If upon β-adrenoceptor stimulation was abolished upon intracellular perfusion with PKI. Thus, during early and late stages of cardiomyogenesis the activation of f-channels appears to be mediated by phosphorylation via PKA rather than by direct cAMP binding. However, a possibility remains that phosphorylation of the channels by PKA is a prerequisite for a direct activation of the channel by cAMP at these developmental stages. In support of this hypothesis is our finding that muscarinic depression (in EDS cells) and β-adrenergic stimulation (in LDS cells) of If were also accompanied by a slowing and acceleration of the activation kinetics, respectively. This may indicate the presence of a small component of direct cAMP modulation acting in concert with a more robust PKA modulation. Furthermore, minor effects of PKI and/or the catalytic subunit of PKA onto cAMP levels, though unlikely, cannot be ruled out completely. Thus, in order to quantitatively assess the possible direct cAMP- versus the PKA-mediated effect in embryonic cardiomyocytes, future experiments should address whether If is modified by shifting the activation curve on the voltage axis or by a change in conductance. The former is observed in the case of direct cAMP binding. It remains to be examined further in future studies whether and when during postnatal development the switch towards an exclusively direct cAMP-mediated stimulation of If occurs and whether this is related to different types of f-channels or the expression of different splice variants. Our previous studies on the regulation of ICa,L during cardiac embryogenesis demonstrated a high intrinsic activity for the PDE2-4 subtypes at the EDS cell stage (Maltsev et al. 1999). This is most likely correlated to the high intrinsic adenylyl cyclase activity. We therefore examined in this study the role of PDE2-4 subtypes in controlling If by using selective PDE antagonists. The simultaneous recording of ICa,L and If allowed us to examine whether any difference exists in the regulation of these two ion channels. IBMX, a non-selective PDE inhibitor, as well as PDE2 and PDE4 antagonists stimulated both ICa,L and If, confirming the role of basal PDE activity in EDS cells. These findings are in line with a previous report in Purkinje fibres (Chang et al. 1991), where IBMX was found to increase the amplitude of If. However, in contrast to ICa,L, If was not stimulated by selective PDE3 inhibitors. This differential regulation of ICa,L and If may indicate that the relative contribution of PDE3 to the total PDE activity is reduced near f-channels as compared with Ca2+ channels, either due to a reduced PDE3 activity or due to an increased PDE2 and/or PDE4 activity. More recently, in rat ventricular cells, we found that neither a PDE3 nor a PDE4 inhibitor when used alone had any effect on basal ICa,L, while when both inhibitors were used together a strong stimulation of ICa,L was observed (Verde et al. 1999). Additional data from this study on basal and stimulated ICa,L led us to conclude that each PDE played a specific role in regulating ICa,L, with the rank order of potency being PDE4 > PDE3 > PDE2 > PDE1. Our present results on If regulation in EDS cells would tend to suggest that PDE subtypes regulate If with a rank order of potency of PDE2 > PDE4 > PDE3. Our previous study in EDS cells would lead to a different order of potency for the regulation of ICa,L: PDE3 > PDE2 = PDE4. This different qualitative and/or quantitative arrangement of PDE isoforms near the two types of channels may lead to different local concentrations of cAMP. A precedent for this type of cAMP compartmentation exists in the case of β-adrenergic regulation of Ca2+ channels in the frog heart. In this preparation cAMP compartmentation is responsible for a local activation of Ca2+ channels by ISO (Jurevicius & Fischmeister, 1996). More recently, a preferential coupling of β2-adrenergic receptors with Ca2+ channels rather than with K+ channels was found in adult transgenic mouse ventricular myocytes (An et al. 1999). Thus, compartmentation and/or colocalization of ion channels and signalling molecules may provide a general mechanism allowing the specific regulation of one type of ion channel without altering the activity of others.
Acknowledgments
We thank Dr A. M. Wobus for providing ES cells of the cell line D3, M. Bickel, C. Boettinger and B. Hops for assistance in cell culture work. The support of the machine and electronic shop is greatly acknowledged. This work was supported by a Procope France-Germany exchange programme.
The authors N. Abi-Gerges and G. J. Ji contributed equally to this manuscript.
References
- An RH, Davies MP, Doevendans PA, Kubalak SW, Bangalore R, Chien KR, Kass RS. Developmental changes in beta-adrenergic modulation of L-type Ca2+ channels in embryonic mouse heart. Circulation Research. 1996;78:371–378. doi: 10.1161/01.res.78.3.371. [DOI] [PubMed] [Google Scholar]
- An RH, Heath BM, Koch W, Lefkowitz RJ, Kass RS. β2-Adrenergic receptor overexpression in the developing mouse heart: evidence for targeted modulation of ion channels. The Journal of Physiology. 1999;516:19–30. doi: 10.1111/j.1469-7793.1999.019aa.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bois P, Lenfant J. Isolated cells of the frog sinus venosus: properties of the inward current activated during hyperpolarization. Pflügers Archiv. 1990;416:339–346. doi: 10.1007/BF00392071. [DOI] [PubMed] [Google Scholar]
- Bois P, Renaudon B, Baruscotti M, Lenfant J, Difrancesco D. Activation of f-channels by cAMP analogues in macropatches from rabbit sino-atrial node myocytes. The Journal of Physiology. 1997;501:565–571. doi: 10.1111/j.1469-7793.1997.565bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callewaert G, Carmeliet E, Vereecke J. Single cardiac Purkinje cells: general electrophysiology and voltage-clamp analysis of the pace-maker current. The Journal of Physiology. 1984;349:643–661. doi: 10.1113/jphysiol.1984.sp015179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerbai E, Barbieri M, Mugelli A. Characterization of the hyperpolarization-activated current, If, in ventricular myocytes isolated from hypertensive rats. The Journal of Physiology. 1994;481:585–591. doi: 10.1113/jphysiol.1994.sp020465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerbai E, Barbieri M, Mugelli A. Occurrence and properties of the hyperpolarization-activated current If in ventricular myocytes from normotensive and hypertensive rats during aging. Circulation. 1996;94:1674–1681. doi: 10.1161/01.cir.94.7.1674. [DOI] [PubMed] [Google Scholar]
- Cerbai E, Pino R, Porciatti F, Sani G, Toscano M, Maccherini M, Giunti G, Mugelli A. Characterization of the hyperpolarization-activated current, I(f), in ventricular myocytes from human failing heart. Circulation. 1997;95:568–571. doi: 10.1161/01.cir.95.3.568. [DOI] [PubMed] [Google Scholar]
- Chang F, Cohen IS, Difrancesco D, Rosen MR, Tromba C. Effects of protein kinase inhibitors on canine Purkinje fibre pacemaker depolarization and the pacemaker current if. The Journal of Physiology. 1991;440:367–384. doi: 10.1113/jphysiol.1991.sp018713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng HC, Kemp BE, Pearson RB, Smith AJ, Misconi L, van Patten SM, Walsh DA. A potent synthetic peptide inhibitor of the cAMP-dependent protein kinase. Journal of Biological Chemistry. 1986;261:989–992. [PubMed] [Google Scholar]
- Davies MP, An RH, Doevendans P, Kubalak S, Chien KR, Kass RS. Developmental changes in ionic channel activity in the embryonic murine heart. Circulation Research. 1996;78:15–25. doi: 10.1161/01.res.78.1.15. [DOI] [PubMed] [Google Scholar]
- Difrancesco D. Characterization of single pacemaker channels in cardiac sino-atrial node cells. Nature. 1986;324:470–473. doi: 10.1038/324470a0. [DOI] [PubMed] [Google Scholar]
- Difrancesco D. Pacemaker mechanisms in cardiac tissue. Annual Review of Physiology. 1993;55:455–472. doi: 10.1146/annurev.ph.55.030193.002323. [DOI] [PubMed] [Google Scholar]
- Difrancesco D. The onset and autonomic regulation of cardiac pacemaker activity: relevance of the f current. Cardiovascular Research. 1995a;29:449–456. [PubMed] [Google Scholar]
- Difrancesco D. The pacemaker current (I(f)) plays an important role in regulating SA node pacemaker activity. Cardiovascular Research. 1995b;30:307–308. [PubMed] [Google Scholar]
- Difrancesco D. Dual allosteric modulation of pacemaker (f) channels by cAMP and voltage in rabbit SA node. The Journal of Physiology. 1999;515:367–376. doi: 10.1111/j.1469-7793.1999.367ac.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Difrancesco D, Ferroni A. Delayed activation of the cardiac pacemaker current and its dependence on conditioning pre-hyperpolarizations. Pflügers Archiv. 1983;396:265–267. doi: 10.1007/BF00587866. [DOI] [PubMed] [Google Scholar]
- Difrancesco D, Noma A, Trautwein W. Separation of current induced by potassium accumulation from acetylcholine-induced relaxation current in the rabbit S-A node. Pflügers Archiv. 1980;387:83–90. doi: 10.1007/BF00584257. [DOI] [PubMed] [Google Scholar]
- Difrancesco D, Tortora P. Direct activation of cardiac pacemaker channels by intracellular cyclic AMP. Nature. 1991;351:145–147. doi: 10.1038/351145a0. [DOI] [PubMed] [Google Scholar]
- Difrancesco D, Tromba C. Acetylcholine inhibits activation of the cardiac hyperpolarizing-activated current, if. Pflügers Archiv. 1987;410:139–142. doi: 10.1007/BF00581906. [DOI] [PubMed] [Google Scholar]
- Difrancesco D, Tromba C. Muscarinic control of the hyperpolarization-activated current (if) in rabbit sino-atrial node myocytes. The Journal of Physiology. 1988;405:493–510. doi: 10.1113/jphysiol.1988.sp017344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fares N, Bois P, Lenfant J, Potreau D. Characterization of a hyperpolarization-activated current in dedifferentiated adult rat ventricular cells in primary culture. The Journal of Physiology. 1998;506:73–82. doi: 10.1111/j.1469-7793.1998.073bx.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischmeister R, Hartzell HC. Cyclic AMP phosphodiesterases and Ca2+ current regulation in cardiac cells. Life Science. 1991;48:2365–2376. doi: 10.1016/0024-3205(91)90369-m. [DOI] [PubMed] [Google Scholar]
- Gauss R, Seifert R, Kaupp UB. Molecular identification of a hyperpolarization-activated channel in sea urchin sperm. Nature. 1998;393:583–587. doi: 10.1038/31248. [DOI] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Archiv. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hescheler J, Fleischmann BK, Lentini S, Maltsev VA, Rohwedel J, Wobus AM, Addicks K. Embryonic stem cells: a model to study structural and functional properties in cardiomyogenesis. Cardiovascular Research. 1997;36:149–162. doi: 10.1016/s0008-6363(97)00193-4. [DOI] [PubMed] [Google Scholar]
- Hoppe UC, Beuckelmann DJ. Characterization of the hyperpolarization-activated inward current in isolated human atrial myocytes. Cardiovascular Research. 1998;38:788–801. doi: 10.1016/s0008-6363(98)00047-9. [DOI] [PubMed] [Google Scholar]
- Isenberg G, Klöckner U. Calcium tolerant ventricular myocytes prepared by preincubation in a ‘KB medium’. Pflügers Archiv. 1982;395:6–18. doi: 10.1007/BF00584963. [DOI] [PubMed] [Google Scholar]
- Ji GJ, Fleischmann BK, Bloch W, Feelisch M, Andressen C, Addicks K, Hescheler J. Regulation of the L-type Ca2+-channel during cardiomyogenesis: Switch from NO to adenylyl-cyclase-mediated inhibition. FASEB Journal. 1999;13:313–324. doi: 10.1096/fasebj.13.2.313. [DOI] [PubMed] [Google Scholar]
- Jurevicius J, Fischmeister R. cAMP compartmentation is responsible for a local activation of cardiac Ca2+ channels by beta-adrenergic agonists. Proceedings of the National Academy of Sciences of the USA. 1996;93:295–299. doi: 10.1073/pnas.93.1.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindemann JP, Watanabe AM. Sympathetic control of cardiac electrical activity. In: Zipes DP, Jalife J, editors. Cardiac Electrophysiology: From Cell to Bedside. Philadelphia, PA, USA.: Saunders; 1990. pp. 277–283. [Google Scholar]
- Liu ZW, Zou AR, Demir SS, Clark JW, Nathan RD. Characterization of a hyperpolarization-activated inward current in cultured pacemaker cells from the sinoatrial node. Journal of Molecular and Cellular Cardiology. 1996;28:2523–2535. doi: 10.1006/jmcc.1996.0244. [DOI] [PubMed] [Google Scholar]
- Ludwig A, Zong X, Jeglitsch M, Hofmann F, Biel M. A family of hyperpolarization-activated mammalian cation channels. Nature. 1998;393:587–591. doi: 10.1038/31255. [DOI] [PubMed] [Google Scholar]
- Ludwig A, Zong X, Stieber J, Hullin R, Hofmann F, Biel M. Two pacemaker channels from human heart with profoundly different activation kinetics. EMBO Journal. 1999;18:2323–2329. doi: 10.1093/emboj/18.9.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltsev VA, Ji GJ, Wobus AM, Fleischmann BK, Hescheler J. Establishment of β-adrenergic modulation of L-type calcium current in the early stages of cardiomyocyte development. Circulation Research. 1999;84:136–145. doi: 10.1161/01.res.84.2.136. [DOI] [PubMed] [Google Scholar]
- Maltsev VA, Wobus AM, Rohwedel J, Bader M, Hescheler J. Cardiomyocytes differentiated in vitro from embryonic stem cells developmentally express cardiac-specific genes and ionic currents. Circulation Research. 1994;75:233–244. doi: 10.1161/01.res.75.2.233. [DOI] [PubMed] [Google Scholar]
- Méry P-F, Pavoine C, Pecker F, Fischmeister R. Erythro-9-(2-hydroxy-3-nonyl)adenine inhibits cyclic GMP- stimulated phosphodiesterase in isolated cardiac myocytes. Molecular Pharmacology. 1995;48:121–130. [PubMed] [Google Scholar]
- Pappano A. Parasympathetic control of cardiac electrical activity. In: Zipes DP, Jalife JJ, editors. Cardiac Electrophysiology: From Cell to Bedside. Philadelphia, PA, USA.: Saunders; 1990. pp. 271–276. [Google Scholar]
- Renaudon B, Bois P, Bescond J, Lenfant J. Acetylcholine modulates I(f) and IK(ACh) via different pathways in rabbit sino-atrial node cells. Journal of Molecular and Cellular Cardiology. 1997;29:969–975. doi: 10.1006/jmcc.1996.0340. [DOI] [PubMed] [Google Scholar]
- Robinson RB, Yu H, Chang F, Cohen IS. Developmental change in the voltage-dependence of the pacemaker current, if, in rat ventricle cells. Pflügers Archiv. 1997;433:533–535. doi: 10.1007/s004240050309. [DOI] [PubMed] [Google Scholar]
- Santoro B, Liu DT, Yao H, Bartsch D, Kandel ER, Siegelbaum SA, Tibbs GR. Identification of a gene encoding a hyperpolarization-activated pacemaker channel of brain. Cell. 1998;93:717–729. doi: 10.1016/s0092-8674(00)81434-8. [DOI] [PubMed] [Google Scholar]
- Satoh H, Sperelakis N. Identification of the hyperpolarization-activated inward current in young embryonic chick heart myocytes. Journal of Developmental Physiology. 1991;15:247–252. [PubMed] [Google Scholar]
- Satoh H, Sperelakis N. Hyperpolarization-activated inward current in embryonic chick cardiac myocytes: developmental changes and modulation by isoproterenol and carbachol. European Journal of Pharmacology. 1993;240:283–290. doi: 10.1016/0014-2999(93)90910-a. [DOI] [PubMed] [Google Scholar]
- Skeberdis VA, Jurevicius J, Fischmeister F. β2-Adrenergic activation of L-type Ca2+ current in cardiac myocytes. Journal of Pharmacological and Experimental Therapeutics. 1997;283:452–461. [PubMed] [Google Scholar]
- Verde I, Vandecasteele G, Lezoualc'h F, Fischmeister R. Characterization of the cyclic nucleotide phosphodiesterase subtypes involved in the regulation of the L-type Ca2+ current in rat ventricular myocytes. British Journal of Pharmacology. 1999;127:65–74. doi: 10.1038/sj.bjp.0702506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vikstrom KL, Leinwand LA. Contractile protein mutations and heart disease. Current Opinion in Cell Biology. 1996;8:97–105. doi: 10.1016/s0955-0674(96)80053-6. [DOI] [PubMed] [Google Scholar]
- Wobus AM, Wallukat G, Hescheler J. Pluripotent mouse embryonic stem cells are able to differentiate into cardiomyocytes expressing chronotropic responses to adrenergic and cholinergic agents and Ca2+ channel blockers. Differentiation. 1991;48:173–182. doi: 10.1111/j.1432-0436.1991.tb00255.x. [DOI] [PubMed] [Google Scholar]
- Yanagihara K, Irisawa H. Inward current activated during hyperpolarization in the rabbit sinoatrial node cell. Pflügers Archiv. 1980;385:11–19. doi: 10.1007/BF00583909. [DOI] [PubMed] [Google Scholar]
- Yu H, Chang F, Cohen IS. Pacemaker current exists in ventricular myocytes. Circulation Research. 1993;72:232–236. doi: 10.1161/01.res.72.1.232. [DOI] [PubMed] [Google Scholar]
- Yu H, Chang F, Cohen IS. Pacemaker current if in adult canine cardiac ventricular myocytes. The Journal of Physiology. 1995;485:469–483. doi: 10.1113/jphysiol.1995.sp020743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Lipsius SL. Properties of the pacemaker current (If) in latent pacemaker cells isolated from cat right atrium. The Journal of Physiology. 1992a;453:503–523. doi: 10.1113/jphysiol.1992.sp019242. [DOI] [PMC free article] [PubMed] [Google Scholar]


