Abstract
In the supraoptic nucleus, taurine, selectively released in an osmodependent manner by glial cells through volume-sensitive anion channels, is likely to inhibit neuronal activity as part of the osmoregulation of vasopressin release. We investigated the involvement of various kinases in the activation of taurine efflux by measuring [3H]taurine release from rat acutely isolated supraoptic nuclei.
The protein tyrosine kinase inhibitors genistein and tyrphostin B44 specifically reduced, but did not suppress, both the basal release of taurine and that evoked by a hypotonic stimulus. Inhibition of tyrosine phosphatase by orthovanadate had the opposite effect.
The tyrosine kinase and phosphatase inhibitors shifted the relationship between taurine release and medium osmolarity in opposite directions, suggesting that tyrosine phosphorylation modulates the osmosensitivity of taurine release, but is not necessary for its activation.
Genistein also increased the amplitude of the decay of the release observed during prolonged hypotonic stimulation. Potentiation of taurine release by tyrosine kinases could serve to maintain a high level of taurine release in spite of cell volume regulation.
Taurine release was unaffected by inhibitors and/or activators of PKA, PKC, MEK and Rho kinase.
Our results demonstrate a unique regulation by protein tyrosine kinase of the osmosensitivity of taurine efflux in supraoptic astrocytes. This points to the presence of specific volume-dependent anion channels in these cells, or to a specific activation mechanism or regulatory properties. This may relate to the particular role of the osmodependent release of taurine in this structure in the osmoregulation of neuronal activity.
Taurine is an abundant sulfonic β-amino acid present intracellularly at high concentration and best known for its active participation in cell volume regulation (Huxtable, 1992; Pasantes-Morales & Schousboe, 1997). Cells exposed to hypotonic medium swell by water incorporation and progressively recover their initial volume despite the lower tonicity of the extracellular medium through a process known as regulatory volume decrease (RVD; Hoffman & Dunham, 1995; Lang et al. 1998). RVD is achieved via the efflux of inorganic ions and organic osmolytes that include taurine. A large body of evidence supports the notion that taurine leaves the cell upon swelling through ubiquitous, broadly permeable volume-sensitive anion channels, referred to as volume-sensitive organic osmolyte and anion channels (VSOACs), volume-regulated anion channels, or outwardly rectifying Cl− channels (Strange et al. 1996; Okada, 1997; Nilius et al. 1997; Kirk, 1997). This conclusion is based on the one hand on the strong similarities between volume-dependent taurine efflux and swelling-induced Cl− currents through VSOACs with regard to their pharmacological properties, their kinetics of activation, and their implication in volume regulation, and on the other hand on the direct taurine permeability of VSOACs (Strange et al. 1996; Basavappa & Ellory, 1996; Pasantes-Morales & Schousboe, 1997; Kirk, 1997; Nilius et al. 1997; Manolopoulos et al. 1997). However, as mentioned by Kirk (1997), the correspondence between swelling-induced taurine efflux and VSOACs is only correlative, and has yet to be proven, and evidence for alternative taurine pathways has been provided in some cell preparations.
VSOACs have been studied in a wide variety of cell preparations, and if these studies agree on several common features of the channels, they also point to different properties depending on the cell model used, notably regarding their activation and regulation. VSOACs are characterised by an outward rectification, an inactivation at positive potentials, a 20–90 pS conductance, a weak selectivity among anions and a high permeability to the organic osmolytes myo-inositol and taurine (Strange et al. 1996; Nilius et al. 1997; Kirk, 1997). The mechanism of activation of VSOACs/taurine efflux upon cell swelling is still poorly understood. It has been argued that membrane stretch is unlikely to directly activate VSOACs (Strange et al. 1996; Okada, 1997; Nilius et al. 1997). Reduction of intracellular ionic strength has been proposed as the initial trigger of channel activation (Voets et al. 1999), although other authors have found that ionic strength regulates the volume sensitivity of the channels (Cannon et al. 1998). In most preparations, activation of VSOACs is independent of changes in intracellular Ca2+ (Strange et al. 1996; Pasantes-Morales & Schousboe, 1997; Okada, 1997). Implication of phosphorylation events is also controversial. Indeed, if VSOAC activation generally requires the presence of intracellular ATP (Strange et al. 1996; Basavappa & Ellory, 1996; Nilius et al. 1997; Crepel et al. 1998; Miley et al. 1999), its hydrolysis is not necessary in many cell preparations as ATP can be replaced by non-hydrolysable analogues (Strange et al. 1996; Okada, 1997; Nilius et al. 1997; Miley et al. 1999; Bond et al. 1999). This observation argues for a lack of involvement of protein kinases in the activation mechanism. On the other hand, ATP hydrolysis appears critical in other cell preparations (Meyer & Korbmacher, 1996; Crepel et al. 1998), and protein tyrosine kinases (PTKs) have been proposed to play a pivotal role in the activation of VSOACs in many cell types including cultured astrocytes (Crepel et al. 1998; Mongin et al. 1999), cardiac myocytes (Sorota, 1995), lymphocytes (Lepple-Wienhues et al. 1998), endothelial (Voets et al. 1998) and epithelial cells (Tilly et al. 1993). In cultured astrocytes, this process requires further activation of the mitogen-activated protein kinases (MAPK) Erk1 and Erk2 (Crepel et al. 1998). However, an inhibitory effect of increased tyrosine phosphorylation has also been reported (Doroshenko, 1998; Thoroed et al. 1999). Conflicting results also exist as to the role of protein kinase A (PKA), protein kinase C (PKC), or calmodulin kinase II depending on the cell preparation (Basavappa & Ellory, 1996; Strange et al. 1996; Kirk, 1997; Nilius et al. 1997; Okada, 1997). In several cell types, volume-sensitive Cl− currents can also be triggered by GTP-binding protein activation (Doroshenko et al. 1991; Nilius et al. 1997, 1999). Moreover, inhibition of either Rho protein (Tilly et al. 1996; Nilius et al. 1999) or Rho kinase (Nilius et al. 1999) affects activation of VSOACs, suggesting the involvement of small GTP-binding proteins of the Rho family in the regulation or the activation of the channel.
In the hypothalamic supraoptic nucleus (SON), taurine, which is prominently concentrated in glial cells (Decavel & Hatton, 1995), is released through volume-activated Cl− channels in response to hypotonic swelling (Deleuze et al. 1998). Release of taurine is highly sensitive to even minute, physiological changes in extracellular osmotic pressure (Deleuze et al. 1998). Such small stimuli apparently do not induce RVD, since the resulting release of taurine is sustained as long as the stimulus is applied (Deleuze et al. 1998). Rather, release of glial taurine induced by these weak decreases in osmolarity would contribute to the control of the electrical activity of SON neurones as part of the osmoregulation of vasopressin secretion (Hussy et al. 1997). As an effort to characterise the mechanism of activation of the volume-dependent channel carrying taurine efflux in SON, we studied the influence of tyrosine phosphorylation on the activation and osmosensitivity of taurine release from acutely isolated SON. Our results point to an important regulatory role of tyrosine phosphorylation on the osmosensitivity of volume-activated taurine-permeable Cl− channels, but with no direct implication in the cascade of events leading to activation of the efflux pathway. A preliminary account of these results has appeared in abstract form (Deleuze et al. 1999).
METHODS
Dissection
Adult male Wistar rats (150–250 g) were used in accordance with the European laws for the care and use of experimental animals. SON were isolated as previously described (Deleuze et al. 1998). After decapitation with a guillotine and without anaesthesia, the brain was rapidly removed and placed in oxygenated Locke solution (mM: NaCl, 132; KCl, 5; CaCl2, 2; MgCl2, 2; KH2PO4, 1.2; Hepes, 10; glucose, 10; pH 7.4; osmolarity, 300 mosmol l−1) at 4°C. Two thin strips of tissue located lateral to the optic chiasm, corresponding to the SON, were carefully dissected and freed from residual optic tracts and blood vessels. Tissues were then incubated for 40 min in oxygenated Locke solution supplemented with 400–600 nM [3H]taurine (Amersham) at 35°C and washed three times.
Measurement of taurine release
Tissues were placed into perfusion chambers (250 μl) maintained at 35°C and constantly perfused with oxygenated Locke solution at a rate of 250 μl min−1 using a peristaltic pump (Gilson). During the first 20 min, the perfusate was discarded; samples were then collected every 2 min (LKB sample collector) and the radioactivity in each sample was estimated by scintillation counting. Hyposmotic solutions were Locke solutions from which the appropriate amount of NaCl was omitted. The isotonic solutions were made by adding sucrose to the respective hypotonic media up to the osmolarity of 300 mosmol l−1. Hyperosmotic solutions were obtained by adding sucrose to Locke solution. Thus, differences between iso- hypo- or hyperosmotic solutions resulted solely from changes in the concentration of sucrose. In control (i.e. without drugs) isosmotic conditions, basal release of taurine decreased with a monoexponential time course (Deleuze et al. 1998). A single exponential function was fitted to the baseline and experimental points were divided by the fit to express release relative to this baseline. When drugs were to be used, tissues were first perfused with control isosmotic solution for 15 min before drug application, and perfusate was collected at least 45 min after washout of the drug. This allowed the estimation of the control baseline in the absence of the drug with an exponential fit that was then used to normalise all data points. Thus, unless otherwise stated, all data are expressed as a percentage of the level of release measured in control isosmotic medium, referred to as the control baseline. In each experiment, half of the perfusion chambers were used as control and the effects of applications of the different drugs were systematically compared with these controls.
Drugs
Genistein, phorbol 12-myristate 13-acetate (PMA) and dibutyryl-cAMP (db-cAMP) were purchased from Sigma; tyrphostin A1, tyrphostin B44, sodium orthovanadate, PD-98059, 4α-phorbol and bisindolylmaleimide I (GF-109203X) were purchased from Alexis; daidzein was purchased from RBI and Y-27632 was a gift from Yoshitomi Pharmaceutical Industries. All drugs except orthovanadate and db-cAMP were dissolved in DMSO, with the final concentration of DMSO not exceeding 0.3 %. The lack of effect of the vehicle was attested by the systematic addition of the same amount of DMSO to test and control solutions.
Statistical analysis
Analysis was performed with Origin software (Microcal Software). All results were obtained with at least two different preparations. Results are expressed as means ±s.e.m. In all figures, error bars are shown only when exceeding the size of the symbols.
RESULTS
Modulation of taurine release by tyrosine phosphorylation
Perfusion of isolated SON with a 6.6 % hypotonic medium (280 mosmol l−1) for 14 min induced a rapid increase in [3H]taurine release, an effect that has been previously attributed to swelling of astrocytes in this preparation (Deleuze et al. 1998). The peak of hyposmolarity-evoked release reached 154 ± 2 % of control baseline (n= 52, Fig. 1). PTK inhibitors, pre-applied for 30 min in the isosmotic medium (300 mosmol l−1) and added to the hypotonic solution, strongly reduced the level of release in both osmotic conditions. Genistein (50 μM) reduced release in isosmotic medium by 28 ± 1 % (n= 22) and decreased the peak of the response to a hypotonic stimulus from 152 ± 5 to 107 ± 10 % of control baseline (n= 5, Fig. 1a). Taking into account the reduced basal release, this corresponded to a 29 ± 4 % inhibition of hyposmolarity-evoked release. Similarly, tyrphostin B44 (50 μM) inhibited basal release by 27 ± 5 % (n= 4) and reduced the peak level of efflux in hypotonic medium from 159 ± 7 to 108 ± 3 % of control baseline (n= 4, Fig. 1C), corresponding to an inhibition of 27 ± 5 %. Daidzein (50 μM) and tyrphostin A1 (50 μM), the respective inactive analogues of genistein and tyrphostin B44, were without effect (n= 3 and 4, respectively; Fig. 1B and D). The action of genistein was dose dependent, with negligible effect at 10 μM, and an increasing inhibition of release with concentrations of genistein between 25 and 100 μM, both at 300 and 280 mosmol l−1 (Fig. 2). However, part of the effect obtained with 100 μM genistein appeared non-specific to an action on PTK because the same concentration of daidzein also had a small inhibitory effect (data not shown). Therefore, the concentration of genistein was kept to 50 μM throughout the rest of the study.
Figure 1. Inhibition of protein tyrosine kinase reduces taurine release.
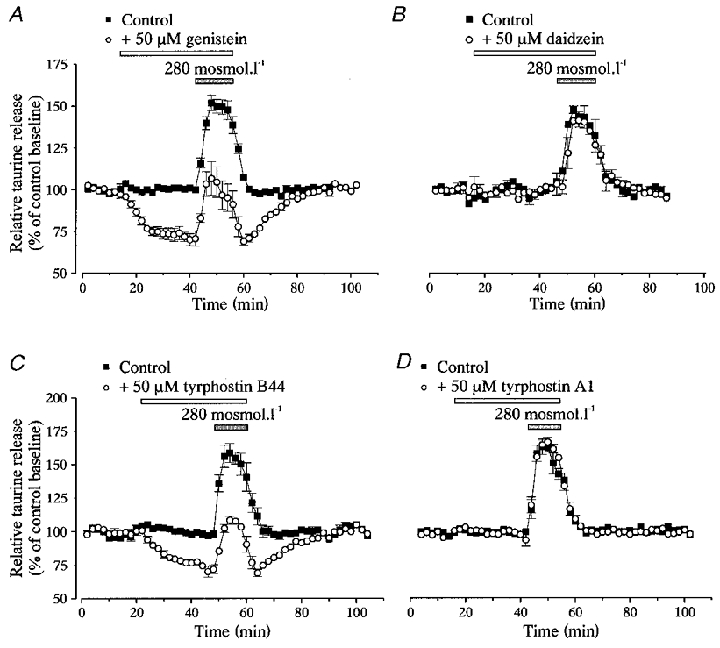
Taurine release from isolated SON perfused with control (300 mosmol l−1) and hyposmotic (280 mosmol l−1, grey bar) solutions. Release is expressed as percentage of control baseline. A, application of the PTK inhibitor genistein (50 μM), for the duration indicated by the open bar, decreases taurine release in both osmotic conditions (n= 5). B, the genistein inactive analogue daidzein has no effect (n= 3). C, the other PTK inhibitor tyrphostin B44 (50 μM) similarly reduces the osmodependent release of taurine (n= 4). D, tyrphostin A1, the tyrphostin inactive analogue, does not affect release (n= 4).
Figure 2. Dose dependency of genistein inhibitory effect.
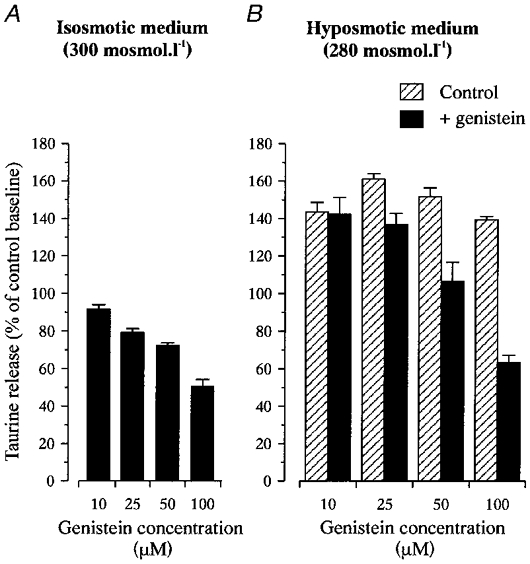
Inhibition of taurine release in isosmotic (A) and hyposmotic media (measured at the peak of the response to a stimulus of 280 mosmol l−1; B) by the different concentrations of genistein indicated. Data are expressed as percentage of control baseline. Number of observations is 3–5, except for inhibition of basal release by 50 μM genistein for which n= 22.
Orthovanadate (100 μM), an inhibitor of tyrosine phosphatase, potentiated basal release by 22 ± 1 % (n= 14) and increased the peak of release in hyposmotic solution from 153 ± 4 to 192 ± 4 % of control baseline (n= 4, Fig. 3). This corresponded to a 25 ± 3 % potentiation.
Figure 3. Orthovanadate potentiates taurine efflux.
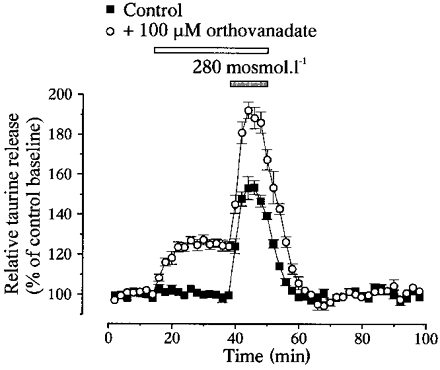
Inhibition of tyrosine phosphatase by 100 μM orthovanadate potentiates both basal taurine release in isosmotic medium and that during a hypotonic stimulus of 280 mosmol l−1 (n= 4).
Regulation of taurine release by tyrosine phosphorylation is osmodependent
To check whether interfering with tyrosine phosphorylation affected the activation of taurine efflux or its sensitivity to volume changes, we measured the effects of genistein (50 μM) and orthovanadate (100 μM) on responses to hyposmotic stimuli of various intensities (280–235 mosmol l−1) as well as to a hyperosmotic stimulus (330 mosmol l−1, Fig. 4a and B). Interestingly, the magnitude of the effect of the inhibitors was not similar at the different osmolarities. This is noticeable on the graph showing the osmodependence of taurine efflux, obtained by plotting the peak amplitude of the release as a function of the medium osmolarity, in the absence and the presence of genistein or orthovanadate (Fig. 4C). Genistein shifted the curve to the left whereas orthovanadate shifted it to the right, respectively decreasing and increasing the osmosensitivity of the taurine release mechanism. Therefore, the degree of phosphorylation on tyrosine residues appears important in the determination of the set point of the osmotic activation of taurine efflux from SON astrocytes.
Figure 4. The level of tyrosine phosphorylation determines the osmosensitivity of taurine release.
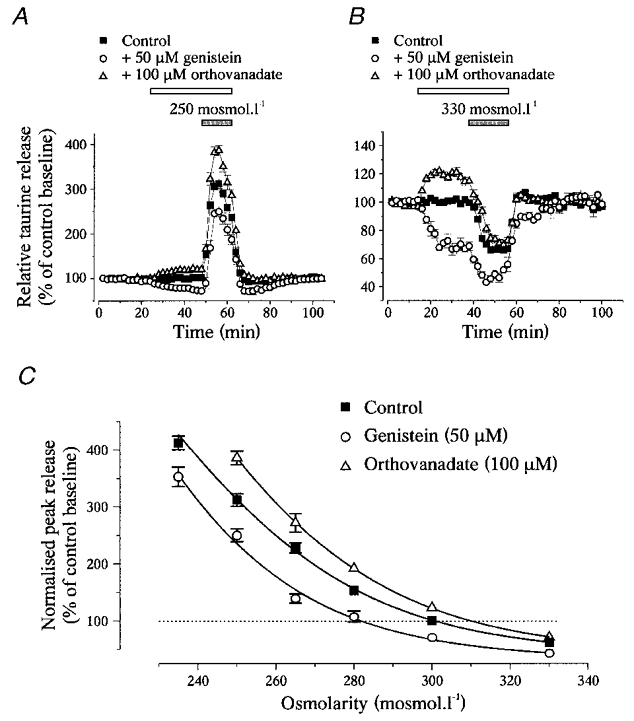
A and B, effects of genistein and orthovanadate on taurine release during osmotic stimulation with a medium of 250 mosmol l−1 (A) and 330 mosmol l−1 (B). Note the different scales on the Y-axis in A and B. C, relationships between the peak amplitude of taurine release and medium osmolarity in the absence (▪) and presence of genistein (^) or orthovanadate (▵). Peak release is expressed as percentage of control baseline measured at 300 mosmol l−1 in the absence of the drug. Relationships were modelled with sigmoid curves (Boltzmann equation, continuous lines). Genistein and orthovanadate shift the relationship to the left and to the right, respectively. Number of observations is 3 or 4 for orthovanadate (except at 300 mosmol l−1 where it is 14), 4 or 5 for genistein (except at 300 mosmol l−1 where it is 22), and 4–10 for the control.
Inhibition of PTK affects the time course of taurine release
To assess the impact of tyrosine phosphorylation on the kinetics of the hyposmolarity-evoked efflux of taurine, we applied hypotonic solutions (280 and 250 mosmol l−1) for long duration (46 min) in the absence or the presence of 50 μM genistein. In the latter case, genistein was present in both iso- and hyposmotic media for the whole duration of the experiment. To compare the time course of the release in the different experimental conditions, the basal level of release was subtracted from the data, which were subsequently normalised to the peak value of release. The two hypotonic stimuli induced responses that peaked within 6–8 min and then decreased in spite of the continuous perfusion with hyposmotic media (Fig. 5). The response could be separated into a decay component that could be fitted with a monoexponential function, and a sustained component, the amplitude of which was estimated by extrapolation of the exponential fit. The time constant of the decay was similar for the two hypotonic stimuli, with mean values of 12.1 ± 4.9 min for 280 mosmol l−1 (n= 9) and 15.8 ± 1.2 min for 250 mosmol l−1 (n= 10). However, the stronger the hypotonic stimulus, the larger the relative amplitude of the decay component, which represented 29 ± 4 and 55 ± 1 % of the maximal responses to stimuli of 280 and 250 mosmol l−1, respectively. Genistein increased the proportion of this decay component to 50 ± 3 % (280 mosmol l−1, n= 9) and 75 ± 1 % (250 mosmol l−1, n= 8), without changing its time constant (13.6 ± 2.5 min for 280 mosmol l−1 and 13.6 ± 0.9 min for 250 mosmol l−1).
Figure 5. Genistein modifies the decay kinetics of taurine release.
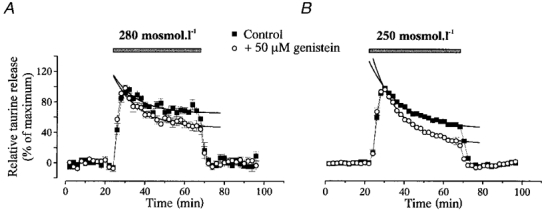
Release of taurine induced by long duration (46 min) stimuli with hyposmotic solutions of 280 (A) and 250 mosmol l−1 (B) in the absence or continuous presence of 50 μM genistein. The responses display a decay component during the hypotonic stimulus. For comparison purposes, basal release is subtracted from the data and evoked release is normalised to the peak amplitude. The decay component is fitted with a monoexponential function (continuous lines). Genistein specifically increases the relative amplitude of this decay component of taurine release with no change in its time constant.
Other families of kinases are not involved in the regulation of taurine release
To test the involvement of the MAPK Erk-1 and Erk-2 in the modulation of taurine release from SON glial cells, we used the specific MAPK kinase (MEK) inhibitor PD-98059 (50 μM). PD-98059 did not affect the basal release of taurine and did not inhibit taurine release induced by hypotonic solution (280 mosmol l−1). In fact, PD-98059 slightly increased the latter from 159 ± 3 to 182 ± 7 % of control baseline (n= 4, Fig. 6). This shows that Erk1 and Erk2 are not implicated in the regulation of taurine efflux by tyrosine kinases. To determine whether the Rho family of small GTP-binding proteins participates in this regulation, we used the specific blocker of Rho kinase Y-27632 (Uehata et al. 1997; Nilius et al. 1999). Application of this compound (25 μM) did not affect taurine release either in isosmotic or hyposmotic medium (280 mosmol l−1, n= 4, Fig. 6).
Figure 6. Other kinases are not involved in the regulation of taurine release.
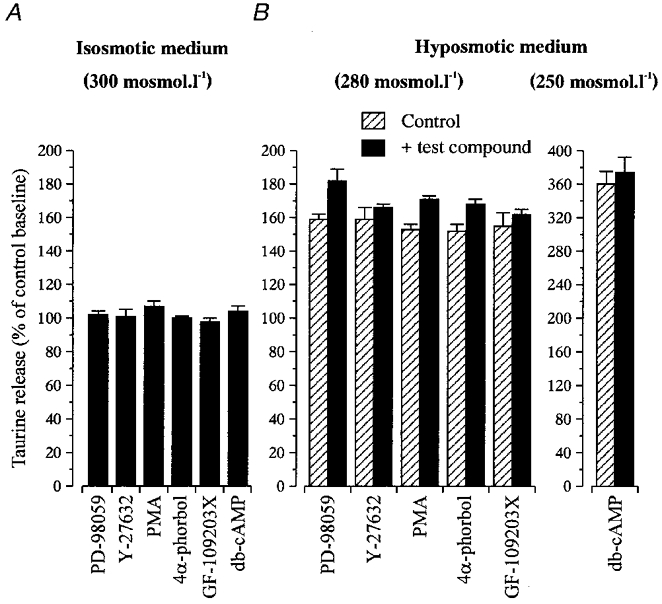
Bar graphs summarising the effects of activators and inhibitors of various other protein kinases on release of taurine in isosmotic conditions (A) and at the peak of the response to a hyposmotic stimulus (B). All compounds were tested with a stimulus of 280 mosmol l−1, except db-cAMP, which was tested with a stimulus of 250 mosmol l−1. Data (mean of 4–6 observations) are expressed as percentage of the control baseline. Compounds include inhibitors of MEK (PD-98059, 50 μM), and Rho kinase (Y-27632, 25 μM), an activator of PKC (PMA, 100 nM) and its inactive analogue 4α-phorbol (100 nM), an inhibitor of PKC (GF-109203X, 500 nM), and an activator of PKA (db-cAMP, 1 mM).
The involvement of PKC in the activation of taurine release from SON glial cells was tested using the phorbol ester PMA, an activator of PKC. Addition of 100 nM PMA to both control (14 min pre-application) and hypotonic medium (280 mosmol l−1) induced a small increase in taurine release in both osmotic conditions (107 ± 2 % of control baseline at 300 mosmol l−1, and from 153 ± 3 to 171 ± 2 % of control baseline at 280 mosmol l−1, n= 6, Fig. 6). However, this effect may not result from a specific activation of PKC since a similar potentiation of the response to hypotonic stimulus (from 152 ± 4 to 168 ± 3 % of control baseline, n= 4, Fig. 6) was also observed with the inactive phorbol ester analogue 4α-phorbol (100 nM). Moreover, application of 500 nM GF-109203X, an inhibitor of PKC, did not affect taurine release in either osmotic condition (n= 4, Fig. 6). Similarly, activation of PKA with 1 mM db-cAMP, a membrane-permeant analogue of cAMP, applied 10 min before and during a hypotonic stimulus of 250 mosmol l−1, had no effect on taurine release (n= 4, Fig. 6). These data show the lack of implication of PKA and PKC in the activation and regulation of taurine-permeable Cl− channels in SON astrocytes.
DISCUSSION
Protein tyrosine kinases modulate the osmosensitivity of taurine release in the SON
We report here the effects of interfering with tyrosine phosphorylation on the osmodependent taurine release from isolated rat SON. In this preparation, we previously demonstrated that [3H]taurine is selectively taken up by glial cells and released through volume-sensitive anion channels (Deleuze et al. 1998). This acute preparation thus allows the study of the in situ properties of taurine efflux from a homogeneous cell population. We found that inhibition of PTK specifically and dose-dependently decreases the osmodependent release of taurine whereas inhibition of tyrosine phosphatase increases it. The opposite actions of tyrosine kinase and tyrosine phosphatase inhibitors, the similar effects of genistein and tyrphostin B44 that act on different sites to inhibit PTK, as well as the lack of effect of their inactive analogues, strongly argue that these observations result from specific alterations in the level of tyrosine phosphorylation.
Strikingly, the magnitude of the effects of the inhibitors on taurine release depended on the intensity of the hyposmotic stimulus. This reflected an alteration of the relationship between taurine efflux and medium osmolarity, rather than a simple inhibition and activation of the taurine-permeable channels. Indeed, genistein and orthovanadate shifted this relationship in opposite directions, modifying, therefore, the osmosensitivity of the release mechanism. Inhibition of tyrosine kinase decreased, whereas that of tyrosine phosphatase increased, the osmosensitivity of taurine release. These data suggest that the degree of tyrosine phosphorylation determines the osmotic set point of the activation of taurine efflux, and therefore plays a key role in the modulation of the sensitivity of taurine-permeable channels to volume changes. On the other hand, because the effect of inhibiting or potentiating tyrosine phosphorylation could always be compensated by further decreasing or increasing the magnitude of the osmotic stimulus, activation of PTK does not appear as a required step in the activation cascade of volume-dependent, taurine-permeable channels in SON astrocytes.
This is at variance with the many reports in various cell types (see above), including rat cultured astrocytes (Crepel et al. 1998), where phosphorylation by PTK has been shown as an essential step in the swelling-induced activation of VSOACs. Activation of these channels has been reported to further involve the MAPK Erk1 and Erk2 in cultured astrocytes (Crepel et al. 1998), and Rho kinases in endothelial cells (Nilius et al. 1999). This is clearly different from our present results, where the Erk kinase (MEK) inhibitor PD-98059 and the Rho kinase inhibitor Y-27632 both failed to inhibit taurine efflux, indicating that these kinases are not involved in the regulation of taurine release through volume-sensitive Cl− channels from SON glial cells in situ. Our results also differ from those found in bovine chromaffin cells and mouse fibroblasts, in which tyrosine phosphorylation appears to inhibit VSOACs (Doroshenko, 1998; Thoroed et al. 1999).
Finally, we found no effect of activation or inhibition of PKA and PKC on taurine release in SON glial cells. The implication of these two pathways in the activation or modulation of VSOACs and taurine efflux has been controversial. According to the cell type, activation of PKA inhibits (Du & Sorota, 1997), potentiates (Strange et al. 1996; Meng & Weinman, 1996), or does not affect swelling-activated taurine efflux and Cl− current (Pasantes-Morales et al. 1990; Manolopoulos et al. 1997). Interestingly, the potentiation of VSOACs by the cAMP-PKA pathway in rat hepatocytes results from a modification of the osmotic set point of activation (Meng & Weinman, 1996). Similarly, regulation of VSOACs by PKC in various cells has been reported to be inhibitory (Duan et al. 1995; Dick et al. 1998; von Weikersthal et al. 1999), potentiating (Strange et al. 1996; Du & Sorota, 1999), or absent (Manolopoulos et al. 1997).
Do SON glial cells express a particular taurine-permeable channel?
Our results do not support a mechanism of activation of taurine efflux involving a necessary phosphorylation step, which is in agreement with the phosphorylation-independent activation of VSOACs reported in several preparations (Strange et al. 1996; Okada, 1997; Miley et al. 1999; Bond et al. 1999). All these differences in the involvement of protein kinases in the activation or modulation of VSOACs in various cell types suggest the presence of multiple VSOAC types, multiple activation pathways, or various indirect effects of kinases on the channel function. In SON glial cells, the channel carrying taurine efflux displays a unique regulation of its osmosensitivity by PTK, a property that has not been described so far in any other cell type (although regulation of osmosensitivity of VSOACs by PKA was described in hepatocytes; Meng & Weinman, 1996). This points to the specificity of the volume-sensitive taurine efflux pathway in these hypothalamic glial cells, a peculiarity which may be related to their functional specialisation with regard to the regulation of the osmolarity of extracellular fluids (Wells, 1998; Hatton, 1999). One possibility is that SON glial cells express a particular subtype of VSOAC, or a different, non-VSOAC type of taurine-permeable volume-sensitive channel. It is of interest that in Ehrlich ascites tumour cells and HeLa cells, swelling-activated taurine and Cl− efflux appear to involve different channels (Lambert & Hoffmann, 1994; Stutzin et al. 1997). Besides VSOACs, the molecular nature of which has not been established, other swelling-activated Cl− channels could potentially carry taurine efflux. These include the large conductance anion channel described in several cell types including astrocytes (Strange et al. 1996; Kirk, 1997; Kirk & Strange, 1998), and which presents strong similarities with the voltage-dependent anion channel (VDAC) found in mitochondrial outer membrane (Guibert et al. 1998). Whether these channels have a significant permeability to taurine is not established, but it is notable that mitochondrial VDACs are known to provide the pathway for the transport of various anionic metabolites (Kirk & Strange, 1998). Another candidate is phospholemman, a small membrane protein isolated from cardiac muscle cells that, when reconstituted in lipid bilayers, forms an anionic channel highly permeable to taurine (Ptaurine/PCl= 70; Moorman et al. 1995). The volume dependence of its activation has yet to be investigated.
Functional significance of the regulation of taurine release by tyrosine phosphorylation
Interestingly, we observed that inhibition of PTK by genistein modified the time course of the taurine release induced by long duration hypotonic stimuli, by selectively increasing the relative amplitude of the decay component without changing its time constant. Such decay of the response in the continuous presence of the hypotonic stimulus is commonly believed to result from RVD (Pasantes-Morales & Schousboe, 1997). The increased magnitude of this decay by genistein may suggest an increased magnitude of RVD in the absence of tyrosine phosphorylation, albeit with unchanged kinetics. Alternatively, this effect could result from a decreased osmosensitivity of taurine release. Indeed, PTKs are known to be activated within a few minutes upon hypotonic stimulation (Tilly et al. 1993; Sadoshima et al. 1996; Crepel et al. 1998), and this is likely to lead to an enhancement of taurine efflux relative to the level of cell swelling, thus decreasing the impact of RVD on the release. SON astrocytes would then naturally undergo a stronger RVD than initially thought based on the time course of taurine release (Deleuze et al. 1998), RVD that would be truly revealed after blockade of tyrosine phosphorylation. This interpretation could point to the functional significance of the upregulation of the osmosensitivity of swelling-dependent taurine efflux by PTK in these astrocytes. This potentiating influence could be a way to maintain a high level of taurine release during changes in osmotic pressure in spite of the developing RVD. As taurine probably represents a determinant factor in the osmotic inhibition of the activity of vasopressin neurones and therefore of the release of this antidiuretic hormone (Hussy et al. 1997), a sustained level of extracellular taurine in the SON appears important as long as the decreased osmolarity of the extracellular fluids persists.
Regulation of the osmosensitivity of taurine release by PTK could also be the target of external modulatory agents. Growth factors are well known for triggering tyrosine kinase activity, and could therefore serve as such modulators of taurine efflux. The expression of their receptors has been widely documented in brain glial cells (Labourdette & Sensenbrenner, 1995), and acute modulation of ionic channel function by the activation of growth factor receptors has been recently reported (Hilborn et al. 1998). Regulation of taurine release could also potentially occur through activation of neurotransmitter receptors, since activation of PTK by glutamate or acetylcholine has been recently shown in neurones (Boxall & Lancaster, 1998; Hayashi et al. 1999), and the receptors of these neurotransmitters are expressed in many astrocytes (Kimelberg, 1995). A regulatory mechanism involving activation of protein tyrosine phosphatases to decrease the osmosensitivity of taurine release is also conceivable (Neel & Tonks, 1997). Whatever the factors activating or inhibiting tyrosine phosphorylation, it should endow the system with the capacity to finely tune the responsiveness of glial cells to osmotic changes. This would add to the already impressive list of modulatory factors present in the hypothalamo-neurohypophysial complex that regulate the activity of neurosecretory cells, therefore participating in the optimal adaptation of the release of the neurohormones vasopressin and oxytocin to the various physiological states of the animal (Hatton, 1999).
Acknowledgments
We are grateful to Michel G. Desarménien, Govindan Dayanithi and Philippe Richard for critical reading of the manuscript. This work was supported in part by the Centre National d'Etudes Spatiales (CNES no. 98/7346/793).
References
- Basavappa S, Ellory JC. The role of swelling-induced anion channels during neuronal volume regulation. Molecular Neurobiology. 1996;13:137–153. doi: 10.1007/BF02740638. [DOI] [PubMed] [Google Scholar]
- Bond T, Basavappa S, Christensen M, Strange K. ATP dependence of the ICl,swell channel varies with rate of cell swelling. Evidence for two modes of channel activation. Journal of General Physiology. 1999;113:441–456. doi: 10.1085/jgp.113.3.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxall AR, Lancaster B. Tyrosine kinases and synaptic transmission. European Journal of Neuroscience. 1998;10:2–7. doi: 10.1046/j.1460-9568.1998.00009.x. [DOI] [PubMed] [Google Scholar]
- Cannon CL, Basavappa S, Strange K. Intracellular ionic strength regulates the volume sensitivity of a swelling-activated anion channel. American Journal of Physiology. 1998;275:C416–422. doi: 10.1152/ajpcell.1998.275.2.C416. [DOI] [PubMed] [Google Scholar]
- Crepel V, Panenka W, Kelly MEM, Macvicar BA. Mitogen-activated protein and tyrosine kinases in the activation of astrocyte volume-activated chloride current. Journal of Neuroscience. 1998;18:1196–1206. doi: 10.1523/JNEUROSCI.18-04-01196.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decavel C, Hatton GI. Taurine immunoreactivity in the rat supraoptic nucleus: prominent localization in glial cells. Journal of Comparative Neurology. 1995;354:13–26. doi: 10.1002/cne.903540103. [DOI] [PubMed] [Google Scholar]
- Deleuze C, Duvoid A, Hussy N. Properties and glial origin of osmotic-dependent release of taurine from the rat supraoptic nucleus. The Journal of Physiology. 1998;507:463–471. doi: 10.1111/j.1469-7793.1998.463bt.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deleuze C, Duvoid A, Hussy N. Tyrosine phosphorylation modulates the osmosensitivity of taurine efflux in glial cells of the rat supraoptic nucleus. The Journal of Physiology. 1999;517.P:96. doi: 10.1111/j.1469-7793.2000.t01-2-00291.x. P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick GM, Bradley KK, Horowitz B, Hume JR, Sanders KM. Functional and molecular identification of a novel chloride conductance in canine colonic smooth muscle. American Journal of Physiology. 1998;275:C940–950. doi: 10.1152/ajpcell.1998.275.4.C940. [DOI] [PubMed] [Google Scholar]
- Doroshenko P. Pervanadate inhibits volume-sensitive chloride current in bovine chromaffin cells. Pflügers Archiv. 1998;435:303–309. doi: 10.1007/s004240050516. [DOI] [PubMed] [Google Scholar]
- Doroshenko P, Penner R, Neher E. Novel chloride conductance in the membrane of bovine chromaffin cells activated by intracellular GTPγS. The Journal of Physiology. 1991;436:711–724. doi: 10.1113/jphysiol.1991.sp018575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X-Y, Sorota S. Modulation of dog atrial swelling-induced chloride current by cAMP: protein kinase A-dependent and -independent pathways. The Journal of Physiology. 1997;500:111–122. doi: 10.1113/jphysiol.1997.sp022003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X-Y, Sorota S. Protein kinase C stimulates swelling-induced chloride current in canine atrial cells. Pflügers Archiv. 1999;437:227–234. doi: 10.1007/s004240050773. [DOI] [PubMed] [Google Scholar]
- Duan D, Fermini B, Nattel S. Alpha-adrenergic control of volume-regulated Cl− currents in rabbit atrial myocytes. Characterization of a novel ionic regulatory mechanism. Circulation Research. 1995;77:379–393. doi: 10.1161/01.res.77.2.379. [DOI] [PubMed] [Google Scholar]
- Guibert B, Dermietzel R, Siemen D. Large conductance channel in plasma membranes of astrocytic cells is functionally related to mitochondrial VDAC-channels. International Journal of Biochemistry and Cell Biology. 1998;30:379–391. doi: 10.1016/s1357-2725(97)00137-4. [DOI] [PubMed] [Google Scholar]
- Hatton GI. Astroglial modulation of neurotransmitter/peptide release from the neurohypophysis: present status. Journal of Chemical Neuroanatomy. 1999;16:203–222. doi: 10.1016/s0891-0618(98)00067-2. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Umemori H, Mishina M, Yamamoto T. The AMPA receptor interacts with and signals through the protein tyrosine kinase Lyn. Nature. 1999;397:72–76. doi: 10.1038/16269. [DOI] [PubMed] [Google Scholar]
- Hilborn MD, Vaillancourt RR, Rane SG. Growth factor receptor tyrosine kinases acutely regulate neuronal sodium channels through the src signaling pathway. Journal of Neuroscience. 1998;18:590–600. doi: 10.1523/JNEUROSCI.18-02-00590.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann EK, Dunham PB. Membrane mechanisms and intracellular signalling in cell volume regulation. International Review of Cytology. 1995;161:173–262. doi: 10.1016/s0074-7696(08)62498-5. [DOI] [PubMed] [Google Scholar]
- Hussy N, Deleuze C, Pantaloni A, Desarménien MG, Moos F. Agonist action of taurine on glycine receptors in rat supraoptic magnocellular neurones: possible role in osmoregulation. The Journal of Physiology. 1997;502:609–621. doi: 10.1111/j.1469-7793.1997.609bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxtable RJ. Physiological actions of taurine. Physiological Reviews. 1992;72:101–163. doi: 10.1152/physrev.1992.72.1.101. [DOI] [PubMed] [Google Scholar]
- Kimelberg HK. Receptors on astrocytes – what possible functions? Neurochemistry International. 1995;26:27–40. doi: 10.1016/0197-0186(94)00118-e. [DOI] [PubMed] [Google Scholar]
- Kirk K. Swelling-activated organic osmolyte channels. Journal of Membrane Biology. 1997;158:1–16. doi: 10.1007/s002329900239. [DOI] [PubMed] [Google Scholar]
- Kirk K, Strange K. Functional properties and physiological roles of organic solute channels. Annual Review of Physiology. 1998;60:719–739. doi: 10.1146/annurev.physiol.60.1.719. [DOI] [PubMed] [Google Scholar]
- Labourdette G, Sensenbrenner M. Growth factors and their receptors in the central nervous system. In: Kettenmann H, Ransom BR, editors. Neuroglia. NY, USA: Oxford University Press; 1995. pp. 441–459. [Google Scholar]
- Lambert IH, Hoffmann EK. Cell swelling activates separate taurine and chloride channels in Ehrlich mouse ascites tumor cells. Journal of Membrane Biology. 1994;142:289–298. doi: 10.1007/BF00233436. [DOI] [PubMed] [Google Scholar]
- Lang F, Busch GL, Ritter M, Völkl H, Weldegger S, Gulbins E, Häussinger D. Functional significance of cell volume regulatory mechanisms. Physiological Reviews. 1998;78:247–306. doi: 10.1152/physrev.1998.78.1.247. [DOI] [PubMed] [Google Scholar]
- Lepple-Wienhues A, Szabò I, Laun T, Kaba NK, Gulbins E, Lang F. The tyrosine kinase p56lck mediates activation of swelling-induced chloride channels in lymphocytes. Journal of Cell Biology. 1998;141:281–286. doi: 10.1083/jcb.141.1.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolopoulos VG, Voets T, Declercq PE, Droogmans G, Nilius B. Swelling-activated efflux of taurine and other organic osmolytes in endothelial cells. American Journal of Physiology. 1997;273:C214–222. doi: 10.1152/ajpcell.1997.273.1.C214. [DOI] [PubMed] [Google Scholar]
- Meng X-J, Weinman SA. cAMP- and swelling-activated chloride conductance in rat hepatocytes. American Journal of Physiology. 1996;271:C112–120. doi: 10.1152/ajpcell.1996.271.1.C112. [DOI] [PubMed] [Google Scholar]
- Meyer K, Korbmacher C. Cell swelling activates ATP-dependent voltage-gated chloride channels in M-1 mouse cortical collecting duct cells. Journal of General Physiology. 1996;108:177–193. doi: 10.1085/jgp.108.3.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miley HE, Brown PD, Best L. Regulation of a volume-sensitive anion channel in rat pancreatic β-cells by intracellular adenine nucleotides. The Journal of Physiology. 1999;515:413–417. doi: 10.1111/j.1469-7793.1999.413ac.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongin AA, Reddi JM, Charniga C, Kimelberg HK. [3H]Taurine and D-[3H]aspartate release from astrocyte cultures are differently regulated by tyrosine kinases. American Journal of Physiology. 1999;276:C1226–1230. doi: 10.1152/ajpcell.1999.276.5.C1226. [DOI] [PubMed] [Google Scholar]
- Moorman JR, Ackerman SJ, Kowdley GC, Griffin MP, Mounsey JP, Chen Z, Cala SE, O'brian JJ, Szabo G, Jones LR. Unitary anion currents through phospholemman channel molecules. Nature. 1995;377:737–740. doi: 10.1038/377737a0. [DOI] [PubMed] [Google Scholar]
- Neel BG, Tonks NK. Protein tyrosine phosphatases in signal transduction. Current Opinion in Cell Biology. 1997;9:193–204. doi: 10.1016/s0955-0674(97)80063-4. [DOI] [PubMed] [Google Scholar]
- Nilius B, Eggermont J, Voets T, Buyse G, Manolopoulos V, Droogmans G. Properties of volume-regulated anion channels in mammalian cells. Progress in Biophysics and Molecular Biology. 1997;68:69–119. doi: 10.1016/s0079-6107(97)00021-7. [DOI] [PubMed] [Google Scholar]
- Nilius B, Voets T, Prenen J, Barth H, Aktories K, Kaibuchi K, Droogmans G, Eggermont J. Role of Rho and Rho kinase in the activation of volume-regulated anion channels in bovine endothelial cells. The Journal of Physiology. 1999;516:67–74. doi: 10.1111/j.1469-7793.1999.067aa.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y. Volume expansion-sensing outward-rectifier Cl− channel: fresh start to the molecular identity and volume sensor. American Journal of Physiology. 1997;273:C755–789. doi: 10.1152/ajpcell.1997.273.3.C755. [DOI] [PubMed] [Google Scholar]
- Pasantes-Morales H, Morán J, Schousboe A. Volume-sensitive release of taurine from cultured astrocytes: properties and mechanism. Glia. 1990;3:427–432. doi: 10.1002/glia.440030514. [DOI] [PubMed] [Google Scholar]
- Pasantes-Morales H, Schousboe A. Role of taurine in osmoregulation in brain cells: mechanisms and functional implications. Amino Acids. 1997;12:281–292. [Google Scholar]
- Sadoshima J, Qiu Z, Morgan JP, Izumo S. Tyrosine kinase activation is an immediate and essential step in hypotonic cell swelling-induced ERK activation and c-fos gene expression in cardiac myocytes. EMBO Journal. 1996;15:5535–5546. [PMC free article] [PubMed] [Google Scholar]
- Sorota S. Tyrosine protein kinase inhibitors prevent activation of cardiac swelling-induced chloride current. Pflügers Archiv. 1995;431:178–185. doi: 10.1007/BF00410189. [DOI] [PubMed] [Google Scholar]
- Strange K, Emma F, Jackson PS. Cellular and molecular physiology of volume-sensitive anion channels. American Journal of Physiology. 1996;270:C711–730. doi: 10.1152/ajpcell.1996.270.3.C711. [DOI] [PubMed] [Google Scholar]
- Stutzin A, Eguiguren AL, Pablo Cid L, Sepúlveda FV. Modulation by extracellular Cl− of volume-activated organic osmolyte and halide permeabilities in HeLa cells. American Journal of Physiology. 1997;273:C999–1007. doi: 10.1152/ajpcell.1997.273.3.C999. [DOI] [PubMed] [Google Scholar]
- Thoroed SM, Bryan-Sisneros A, Doroshenko P. Protein phosphotyrosine phosphatase inhibitors suppress regulatory volume decrease and the volume-sensitive Cl− conductance in mouse fibroblasts. Pflügers Archiv. 1999;438:133–140. doi: 10.1007/s004240050890. [DOI] [PubMed] [Google Scholar]
- Tilly BC, Edixhoven MJ, Tertoolen LGJ, Morii N, Saitoh Y, Narumiya S, de Jonge HR. Activation of the osmo-sensitive chloride conductance involves P21rho and is accompanied by a transient reorganization of the F-actin cytoskeleton. Molecular Biology of the Cell. 1996;7:1419–1427. doi: 10.1091/mbc.7.9.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilly BC, van den Berghe N, Tertoolen LGJ, Edixhoven MJ, de Jonge HR. Protein tyrosine phosphorylation is involved in osmoregulation of ionic conductances. Journal of Biological Chemistry. 1993;268:19919–19922. [PubMed] [Google Scholar]
- Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T, Tamakawa H, Yamagami K, Inui J, Maekawa M, Narumiya S. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389:990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- Voets T, Droogmans G, Raskin G, Eggermont J, Nilius B. Reduced intracellular ionic strength as the initial trigger for activation of endothelial volume-regulated anion channels. Proceedings of the National Academy of Sciences of the USA. 1999;96:5298–5303. doi: 10.1073/pnas.96.9.5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voets T, Manolopoulos V, Eggermont J, Ellory C, Droogmans G, Nilius B. Regulation of a swelling-activated chloride current in bovine endothelium by protein tyrosine phosphorylation and G proteins. The Journal of Physiology. 1998;506:341–352. doi: 10.1111/j.1469-7793.1998.341bw.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Weikersthal SF, Barrand MA, Hladky SB. Functional and molecular characterization of a volume-sensitive chloride current in rat brain endothelial cells. The Journal of Physiology. 1999;516:75–84. doi: 10.1111/j.1469-7793.1999.075aa.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells T. Vesicular osmometers, vasopressin secretion and aquaporin-4: A new mechanism for osmoreception? Molecular and Cellular Endocrinology. 1998;136:103–107. doi: 10.1016/s0303-7207(97)00219-0. [DOI] [PubMed] [Google Scholar]


