Abstract
EMG responses evoked in hand muscles by transcranial stimulation over the motor cortex were conditioned by a single motor threshold electrical stimulus to the median nerve at the wrist in a total of ten healthy subjects and in five patients who had electrodes implanted chronically into the cervical epidural space.
The median nerve stimulus suppressed responses evoked by transcranial magnetic stimulation (TMS) in relaxed or active muscle. The minimum interval between the stimuli at which this occurred was 19 ms. A similar effect was seen if electrical stimulation was applied to the digital nerves of the first two fingers.
Median or digital nerve stimulation could suppress the responses evoked in active muscle by transcranial electrical stimulation over the motor cortex, but the effect was much less than with magnetic stimulation.
During contraction without TMS, both types of conditioning stimuli evoked a cutaneomuscular reflex that began with a short period of inhibition. This started about 5 ms after the inhibition of responses evoked by TMS.
Recordings in the patients showed that median nerve stimulation reduced the size and number of descending corticospinal volleys evoked by magnetic stimulation.
We conclude that mixed or cutaneous input from the hand can suppress the excitability of the motor cortex at short latency. This suppression may contribute to the initial inhibition of the cutaneomuscular reflex. Reduced spinal excitability in this period could account for the mild inhibition of responses to electrical brain stimulation.
Several groups have used transcranial magnetic stimulation (TMS) to test how the excitability of the motor cortex is affected by afferent input. Much of the initial work was concerned with testing the concept of excitatory transcortical reflexes. These reflexes are readily obtained in hand muscles after electrical or natural stimulation of cutaneous and/or muscle afferents and have a variety of names, such as LLR II/III, E2, V2, M2 and long-latency stretch reflex (Caccia et al. 1973; Marsden et al. 1976; Jenner & Stephens, 1982; Deuschl et al. 1985). Data from neurological patients very strongly suggests that many of these responses are produced by activity in a transcortical reflex pathway that operates in parallel with spinal systems (Marsden et al. 1977a,b; Jenner & Stephens, 1982; Noth et al. 1985). Experiments with transcranial stimulation gave results that were consistent with this idea. They showed that stimuli capable of eliciting long latency reflexes also increased the excitability of the motor cortex to transcranial magnetic stimulation with a time course consistent with traffic in a transcortical loop (Day et al. 1991).
In contrast with these reports, some studies have indicated that peripheral input can suppress the excitability of motor cortex. In a short note, Delwaide & Olivier (1990) reported that stimulation of the median nerve at the wrist could profoundly suppress EMG responses evoked in relaxed hand muscles by transcranial magnetic stimulation of the cortex 18–21 ms later. Similar effects could be seen after stimulation of the cutaneous nerves of the index finger. Since H-reflexes in forearm muscles were unaffected Delwaide & Olivier (1990) suggested that the effect occurred at the cortical rather than the spinal level. Maertens de Noordhout et al. (1992) investigated the sequence of excitatory and inhibitory reflexes (Caccia et al. 1973) in the first dorsal interosseous muscle evoked by electrical stimulation of digital nerves. They used transcranial magnetic and electrical stimulation to show that motor cortical excitability was reduced by electrical stimulation of the digital nerves at a time corresponding to the transition between the initial inhibition and subsequent facilitation of the cutaneomuscular reflex. Palmer & Ashby (1992) reported the same result. Most recently, Bertolasi et al. (1998) found that stimulation of probable muscle afferents in the median nerve could suppress the excitability of cortical projections to forearm extensor muscles whilst radial stimulation suppressed the excitability of cortical projections to forearm flexor muscles. Stimulation of cutaneous afferents in digital nerves failed to have any effect. They suggested that the effect from muscle afferents was a cortical analogue of spinal reciprocal inhibition.
The purpose of the present experiments was to extend the original observations of Delwaide & Olivier (1990). They confirm the presence of this early, striking period of inhibition, and show that it is a cortical phenomenon. We also speculate that it is related to and may even be responsible for the initial period of inhibition evident in cutaneo-muscular reflexes of the hand.
METHODS
Subjects
Experiments were conducted on 10 healthy volunteers (aged 25–46 years), and on five patients (aged 44–58 years) who had a spinal cord stimulator implanted for treatment of intractable dorso-lumbar pain or for peripheral vascular disease. Most of the healthy subjects took part in more than one experiment. Each patient was studied on a single occasion 2–3 days after implantation of the electrode (Model Quad 3487A, Medtronic, Minneapolis, MN, USA) at the C1-C2 level during the trial screening period when the electrode connections were externalised. All gave their oral informed consent, and the local ethical committee approved the procedures.
Transcranial brain stimulation
Test responses in the target muscles were evoked by transcranial magnetic (Magstim 200 stimulator with 10 cm diameter figure of eight coil; Magstim Co, Whitland, Dyfed, Wales) or electrical (Digitimer D180 stimulator with anode 6 cm lateral and cathode on scalp vertex; Digitimer Ltd, Welwyn Garden City, Herts, UK) stimulation. In all experiments, the magnetic coil was held to induce electrical currents that flowed perpendicular to the presumed line of the central sulcus in a posterior-anterior direction. Electrical stimuli had a time constant of 50 μs and were delivered through surface Ag-AgCl 9 mm diameter electrodes attached to the scalp with collodion. Unless otherwise stated, the intensity of stimulation was such as to evoke a test response in the first dorsal interosseous (FDI) muscle of 0.5-2 mV peak-to-peak amplitude. Stimuli were applied at irregular intervals every 4–5.5 s. In experiments comparing the effect of conditioning stimuli on magnetic and electrically evoked responses, the two types of stimuli were randomly applied within each trial block, and their intensity was adjusted to produce control responses of equal amplitude.
Conditioning stimuli
Conditioning stimuli were single pulses (200 μs) of electrical stimulation applied through bipolar electrodes to the median nerve at the wrist or through ring electrodes (cathode proximal) to the digital nerves of the index and middle fingers. The intensity of the former was set at just over motor threshold for evoking a visible twitch of the thenar muscles; the latter was 2–3 times perceptual threshold.
In the patients we also recorded the somatosensory potentials evoked on the scalp by conditioning stimuli to the left median nerve. The active electrode was attached 3 cm posterior to C3 (10–20 system) and the reference electrode was 3 cm posterior to C4. Five hundred responses were averaged to identify the latency of the N20 peak.
EMG and spinal cord recording
EMGs were recorded from 9 mm diameter Ag-AgCl surface electrodes over the muscle of interest. For the first dorsal interosseous and abductor pollicis brevis (APB), the reference electrode was placed on the metacarpo-phalangeal joint, and the active electrode over the motor point; for the flexor carpi radialis the reference electrode was placed on the elbow and the active electrode over the motor point.
In the patients, recordings were made between the most proximal and distal of the four electrode contacts on the epidural electrode. These had a surface area of 2.54 mm2 and were 30 mm apart. The distal contact was connected to the reference input of the amplifier. EMG and epidural activity was bandpass filtered from 3 Hz-3kHz (Digitimer D150 amplifiers) and each single trial was recorded on computer for later analysis using a CED 1401 A/D converter (Cambridge Electronic Design, Cambridge, UK) and associated software.
The latency of each component of the descending volley was measured to its peak. Amplitudes were measured from the peak to the next trough in order to minimise distortions due to stimulus artefact. Only consistent deflections with a mean amplitude over ten responses of > 2 μV were analysed.
Experimental design and data analysis
Each experimental session was divided up into several blocks separated by ≥ 5 min. Each block typically consisted of 40 trials. The motor cortex was stimulated on every trial; in three-quarters of trials selected at random the cortical stimulus was preceded at one of three different intervals by a conditioning stimulus to peripheral nerve. Within each block we therefore obtained an average (of ten trials each) of the response to a cortical stimulus alone, and when preceded by conditioning stimuli at three different intervals. In some blocks, magnetic and electrical transcranial stimuli or magnetic and H-reflex stimuli were intermixed randomly. In these blocks, only one conditioning interval was studied.
Measurements were made on mean peak-to-peak amplitudes of EMG responses and components in the descending volley, and the size of conditioned responses was expressed as a percentage of the unconditioned response recorded in the same block of trials. Because of time constraints in a clinical setting, we were not always able to record a full set of conditioning-test intervals in every patient. For the descending volley data of Fig. 5, all five patients contributed data to conditioning-test intervals of 1–4 ms, four patients to intervals of 0 and 5 ms, and three patients to the remaining data points.
Figure 5. Summary of the mean (± s.e.m.) data from all five patients showing the effect of a single median nerve stimulus on EMG responses (CMAP) and components of the descending corticospinal volley (I1, I2, I3) elicited by transcranial magnetic stimulation over the motor cortex.
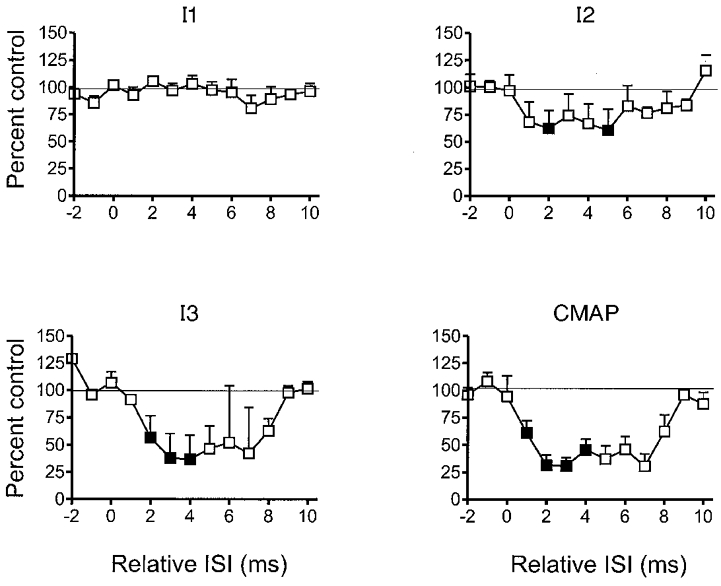
The interval between median nerve and cortical stimuli has been corrected for the latency of the N20 component of the somatosensory evoked potential in each subject (see text). The sizes of the descending volleys and EMG responses are expressed as a percentage of the response to magnetic stimulation given alone. Note the inhibition of the I2 and I3 components of the volley, and the EMG response at +1 to +8 ms. Filled symbols indicate intervals at which the digital nerve shock produced a significant (P < 0.05, Student's paired t test on mean data from each subject with and without median nerve stimulation) effect.
Single factor repeated measures analysis of variance (SPSS general linear model procedure) was used to test the significance of the time course of the conditioning effect. Comparisons between time courses of the conditioning effect on magnetic and electrically evoked responses were made using a two factor repeated measures analysis of variance with time and stimulus type as the main factors. When the number of subjects was seven or less, statistical analysis was performed on the mean data from adjacent time points. Effectively this halved the number of time points entered into the analysis, whilst reducing the total variance. Student's unpaired t test was used to identify individual time points at which the conditioning stimulus had a significant effect on the size of the test responses.
RESULTS
Effect of median nerve stimulation on magnetically evoked EMG responses in the FDI and APB muscle of subjects at rest
Figure 1a illustrates the typical effect of a single motor threshold shock to the median nerve at the wrist on typical EMG responses evoked by magnetic stimulation over the motor cortex. The records are from the FDI muscle and show that the response was inhibited when the median nerve stimulus was given 20 ms before the cortical stimulus, but not when the interval was 18 ms. Figure 1B shows the time course of this effect for the FDI of seven subjects superimposed. In different individuals inhibition occurred when the interstimulus interval (ISI) was 19–21 ms. Figure 1C plots the mean (± s.e.m.) data for both FDI and APB muscles. One-way analysis of variance on the mean data values collapsed across adjacent time points (see Methods) revealed a significant effect of ISI in both the FDI (F(5,35) = 4.25, P < 0.005) and APB (F(5,35) = 4.66, P < 0.005). Individual intervals at which there was a significant effect in the mean data are indicated using filled symbols. Inhibition began at ISI = 19 ms, and was maximal at 21 ms.
Figure 1. Inhibition of EMG responses evoked by transcranial magnetic stimulation of motor cortex by single electrical stimuli to the median nerve at the wrist in relaxed subjects.
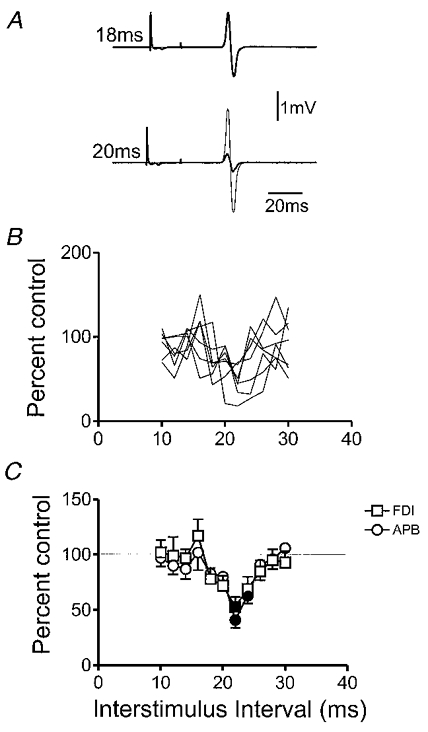
A, examples of average (of 10 trials each) EMG responses evoked in the FDI muscle by TMS over the motor cortex in a healthy subject. Each row consists of two superimposed trials: thin lines indicate the response to cortical stimulation alone; thick lines the response when conditioned by a median nerve stimulus given 18 or 20 ms earlier. B, time course of the median nerve effect in seven subjects. The interval between the median shock and the cortical stimulus is plotted on the x-axis. The size of the response after median nerve stimulation has been expressed on the y-axis as a percentage of the size following cortical stimuli given alone. C, mean ± s.e.m. data from the same subjects as in B. The two lines are for responses evoked in FDI (same data as in B), and APB. Filled symbols indicate intervals at which the median nerve shock produced a significant (P < 0.05, Student's paired t test on mean data from each subject with and without median nerve stimulation) effect on the MEP.
Is the inhibition cortical or spinal?
H-reflex testing
The simplest way of testing whether the inhibition produced by the median nerve stimulus is of cortical or spinal origin is to compare its effect on motor evoked potentials (MEPs) and H-reflexes. However, because H-reflexes are difficult to observe in intrinsic hand muscles at rest this was not possible in the present experiments. In one subject experiments were conducted on the flexor carpi radialis (FCR) muscle rather than a hand muscle. In this subject, responses evoked by magnetic stimulation of the motor cortex were inhibited (to 46 ± 10 % of control size) by a median nerve shock when ISI = 22 ms, whereas the H-reflex was slightly facilitated (to 118 ± 12 % of control size). This would be compatible with a cortical origin for the inhibition. However, it should be noted that inhibition of FCR occurred at only one of the intervals tested. In a previous study, Bertolasi et al. (1998) stimulated a different nerve (the digital nerves of fingers 1 and 2) and did not find any inhibition of magnetically evoked responses in forearm muscles in any of the nine subjects tested.
Transcranial electrical stimulation
To obtain more direct evidence for the origin of the inhibition in hand muscles we used transcranial electrical stimulation. At threshold, transcranial electrical stimulation activates the axons of corticospinal fibres in the white matter, whereas magnetic stimulation activates the same fibres trans-synaptically (Di Lazzaro et al. 1998a). Thus electrically evoked responses are not as sensitive to changes in cortical excitability as those evoked by magnetic stimulation. The experiments were performed during active contraction, rather than at rest (as in the experiments above), to ensure that threshold stimuli evoked responses of measurable size. Figure 2 compares the effect of median nerve stimulation on magnetically and electrically evoked EMG responses in the FDI of five subjects. ISIs for electrical stimulation were adjusted for the earlier latency of EMG responses evoked by electrical stimulation (see Fig. 2 legend). Analysis of variance on the mean data in Fig. 2B (combining data from adjacent intervals to reduce the number of degrees of freedom in the interval term) revealed a significant effect of both main factors, interval (F(2,8) = 28.8; P < 0.001) and stimulus type (F(1,4) = 26.4; P < 0.01). The interaction term was not significant. The implication is that the median nerve stimulus had a greater effect on the responses evoked by magnetic than electrical stimulation. Comparisons at individual time points showed that median nerve stimulation inhibited magnetically evoked responses from ISI = 20 ms onwards, whereas electrically evoked responses were suppressed only at ISI = 26 and 30 ms.
Figure 2. Comparison of the effect of median nerve stimulation on EMG responses evoked in active FDI muscle by electrical or magnetic transcranial stimulation of the motor cortex.
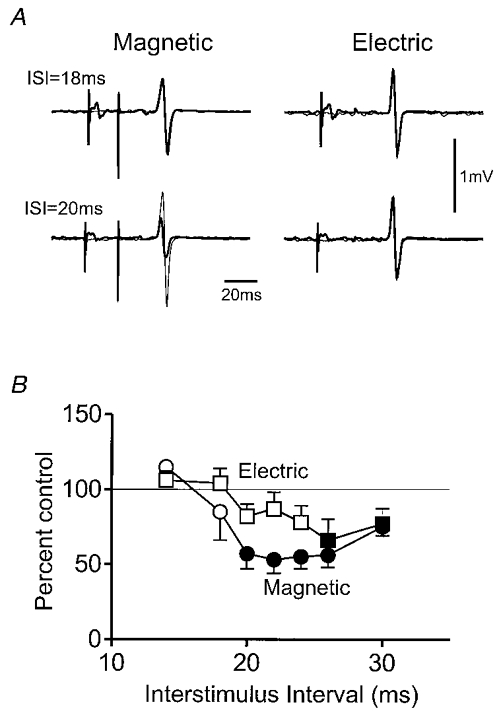
A, examples of average (of 10 trials each) EMG responses evoked in the FDI muscle by transcranial magnetic or electrical stimulation over the motor cortex in a healthy subject. In order to use minimal intensities of electrical stimulation all experiments were conducted whilst the subject contracted the FDI by 5 % maximum. Averages of responses in the absence (thick lines) and presence (thin lines) of median nerve stimulation are superimposed at conditioning test intervals of 18 and 20 ms. Note that magnetically but not electrically evoked responses are inhibited at 20 ms. B, mean (± s.e.m.) time course of the median nerve effect in five subjects. Note that the interval between median nerve and cortical stimuli has been adjusted for the difference in onset latency of EMG responses to electrical and magnetic stimuli in each subject. For example, the electrically evoked responses might have had an onset 2 ms earlier than those evoked by magnetic stimulation. If the actual interval between median nerve stimulation and electrical brain stimulation was 20 ms, it is plotted in this graph as 18 ms. No adjustment has been made for the interval between magnetic and median nerve stimulation. For explanation of symbols, see Fig. 1.
The implication from these results was that the major part of the median nerve inhibition, at least in actively contracting muscles, is primarily of cortical origin. However, since there was also some inhibition of responses to electrical stimulation, there may be a coexistent spinal component.
Digital nerve conditioning stimuli
A disadvantage of using median nerve conditioning stimuli is that threshold stimulation invariably evokes a small M-wave in the thenar muscles. During voluntary contraction there may even be an H-reflex. Both of these responses can contaminate EMGs recorded from surface electrodes over the FDI, the muscle that was the main focus of the present experiments. Because of these potential problems we explored digital nerve conditioning stimuli as Delwaide & Olivier (1990) had shown this to be almost equally effective as median nerve stimulation in inhibiting magnetically evoked responses.
Figure 3a and B confirm two of the main findings seen using median nerve stimulation. First, digital nerve stimulation inhibits magnetically evoked EMG responses in the FDI muscle whether relaxed or active (Fig. 3A; 5 subjects). The onset latency of the inhibition was about 2–3 ms longer than when conditioning stimuli were applied to the median nerve, corresponding to the conduction time in cutaneous afferents across the palm of the hand. The second confirmatory finding in three subjects was that, as with median nerve input, digital nerve conditioning produced clear early suppression of responses evoked by magnetic stimulation but this was not prominent with electrical stimulation (Fig. 3B).
Figure 3. Summary of the effect of digital nerve stimulation on responses to transcranial stimulation evoked in the FDI muscle.
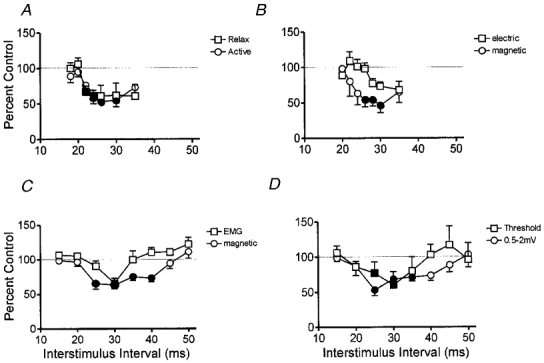
In all graphs, single electrical stimuli at 2–3 times perceptual threshold were applied to the digital nerves of the index and middle finger. The size of responses in the FDI muscle evoked by cortical stimulation at different times after the digital nerve shock is expressed as a percentage of the size of responses evoked by cortical stimulation alone. All points are means ± s.e.m. Filled symbols indicate intervals at which the digital nerve shock produced a significant (P < 0.05, Student's paired t test on the mean data from each subject with and without median nerve stimulation) effect. A, time course of the digital nerve effect on responses elicited by magnetic stimulation in relaxed or active muscle in five subjects. The intensity of stimulation was adjusted so that the control responses were the same amplitude in each situation. B compares the effect of digital nerve stimulation on responses evoked by electrical or magnetic stimulation in three subjects during 5 % maximum contraction of the FDI. The interstimulus interval has been corrected for the difference in latency of the control responses to electrical and magnetic stimulation. C compares the time course of the digital nerve reflex with that of the digital nerve effect on magnetically evoked EMG responses in active (5 % max) muscle. Nine subjects were studied, and the interstimulus interval has been corrected for the conduction time from cortex to muscle as described in the text. D shows how digital nerve stimulation affects responses evoked by small (evoking control responses of up to 0.5 mV) and medium sized (responses from 0.5-2.0 mV) magnetic stimuli in the relaxed FDI of four subjects.
The experiment on the FDI muscle in Fig. 3C compares the time course of the reflex response to digital nerve stimulation (the cutaneomuscular reflex) with its effect on the amplitude of magnetically evoked EMG responses. The experiment was conducted on nine subjects during active contraction of the muscle. In order to illustrate the effects graphically, the time course of the reflex response in this figure has been shifted 20 ms to the left in order to compensate for the conduction time between cortex and muscle. Effectively, this shows what the mean EMG in the muscle would have looked like at the time the MEP was recorded. The digital nerve stimulus produces an initial period of inhibition in the ongoing EMG. However, inhibition of the magnetically evoked response lasts for longer, and starts earlier. This difference in time course was confirmed by a significant interaction term interval × response type in the analysis of variance (F(7,56) = 2.2, P < 0.05). Post hoc analysis showed that the amount of inhibition was significantly different between EMG and MEP at ISI = 25, 35 and 40 ms (Student's paired t test, P < 0.01). The fact that the MEP is inhibited for longer than the EMG is discussed by Maertens de Noordhout et al. (1992).
The final experiment using digital nerve conditioning stimuli examined whether the time course of inhibition in relaxed muscle was affected by the size of the response evoked by transcranial magnetic stimulation. Figure 3D plots the mean data from four subjects in whom the intensity of magnetic stimulation was adjusted to evoke either responses of 0.5 mV or less (threshold responses), or responses with an amplitude between 0.5 and 2 mV. Although suprathreshold responses seem to be inhibited for slightly longer than threshold responses, the inhibition was not significant in this small number of subjects (no significant interaction term interval × response size in the analysis of variance).
Descending volleys recorded in conscious patients
Data in normal subjects suggested that the major part of the median nerve effect was due to short latency inhibition at the cortex. Direct recordings of descending corticospinal volleys from the patients confirmed this. In order to combine data from patients of different height and arm length, we subtracted the latency of the N20 component of the SEP from the ISI between median nerve and cortical stimulation. This corrects for any differences in conduction delay between wrist and cortex between patients. Positive values indicate that the magnetic stimulus was given after the arrival of afferent input at the cortex. All recordings were performed with the patients relaxed.
Figure 4 illustrates the average data from one patient. MEPs were smaller than control values when the magnetic stimulus was given 1–8 ms after the N20 from the median nerve stimulus (relative ISI = 1–8 ms). The mean time course of MEP suppression is shown in Fig. 5 (bottom right panel).
Figure 4. Average data from one patient with an implanted cervical epidural stimulator showing the effect of median nerve stimulation on descending volleys (left) and EMG responses (right) evoked by transcranial magnetic stimulation.
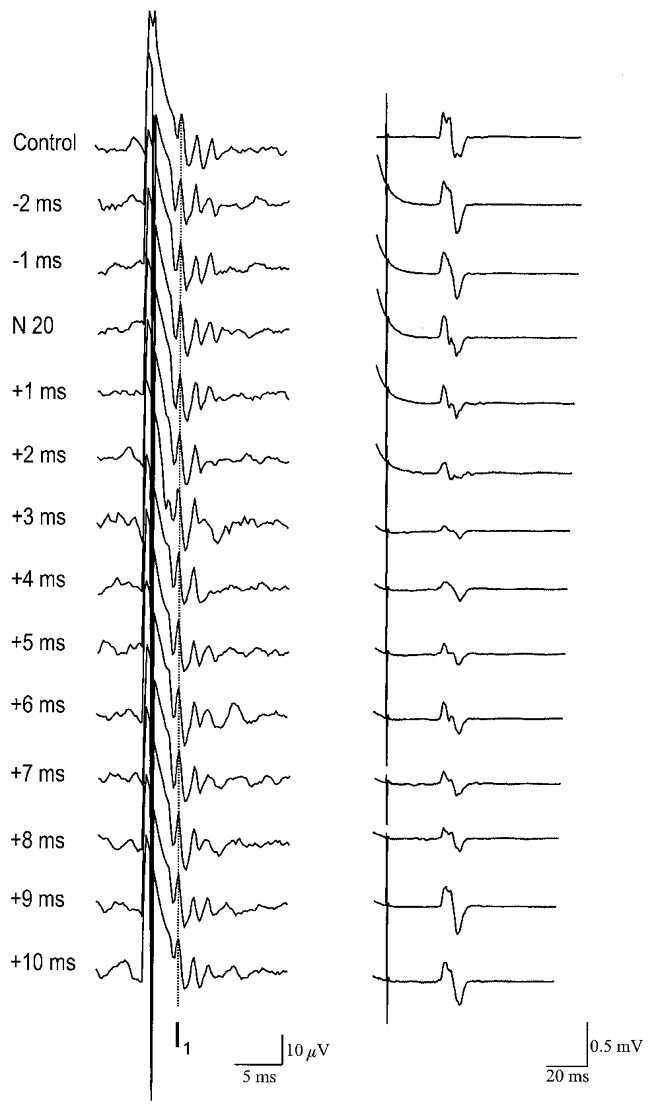
Each trace is the average of 10 trials during relaxation. The latency of the N20 somatosensory evoked potential has been subtracted from the interval between median nerve shock and cortical stimulus. A positive interval indicates that the cortical stimulus was applied after the presumed arrival of sensory input at the cortex. Note the inhibition of EMG responses at +1 to +8 ms. The I1 component of the descending epidural volleys has been indicated by the vertical dotted line.
Epidural volleys were recorded in all patients. The characteristics of the responses evoked by magnetic (all 5 patients) and anodal electrical (2 patients) stimulation given alone are summarised in Table 1. The earliest volleys evoked by electrical stimulation had a latency of 2.4 and 2.6 ms, and were presumed to be D-waves. The earliest magnetic volleys had latencies of 3.4-3.8 ms and were therefore called I1 waves. Later volleys are numbered according to their order of appearance. Figure 4 illustrates the volleys from one patient. When the magnetic stimulus was given 1–8 ms after the median nerve N20 (relative ISI = 1–8 ms), descending volleys were smaller than control. In particular it seemed as if the later waves were much more affected than the earlier volleys. The mean data from all patients is shown separately for all I-wave volleys in Fig. 5. The most prominent effect was on the I2 and I3 waves, whilst the I1 was relatively spared. The time course of suppression mirrors the time course of EMG inhibition very well.
Table 1.
Mean latency (ms) of the different descending volleys recorded in patients
| Volley | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 |
|---|---|---|---|---|---|
| D* | 2.4 | 2.6 | — | — | — |
| I1 | 3.8 | 3.6 | 3.4 | 3.6 | 3.6 |
| I2 | 5.2 | 5.2 | 4.8 | 5.2 | 5.0 |
| I3 | 6.6 | 6.6 | — | 6.6 | 6.4 |
| I4 | 8.0 | — | — | — | — |
Evoked by anodal electrical stimulation
As described in Methods, only conditioning-test intervals of 1–4 ms contain data from all five patients. The remaining data is from three or four patients only hence the time points at ISI = 5–7 ms in Fig. 5 (bottom left panel) and at ISI = 5 ms in Fig. 5 (bottom right panel) are not significant, whereas earlier points with similar error bars may be.
DISCUSSION
The present study has confirmed the original observations of Delwaide & Olivier (1990), that stimulation of the median nerve at the wrist, or the digital nerves of the fingers can inhibit EMG responses evoked in the FDI and APB muscles by transcranial magnetic stimulation of the motor cortex. Two new lines of evidence very strongly suggest that this is due to a reduction of cortical excitability. First, median nerve stimulation at an appropriate ISI can reduce the amplitude and number of descending corticospinal volleys evoked by magnetic stimulation. Second, it has a differential effect on magnetically and electrically evoked EMG responses. We conclude that cutaneous or mixed afferent input from the hand can inhibit motor cortex at remarkably short latency.
The results also address the question of the early period of inhibition in cutaneomuscular reflexes. Given the relative timing of cortical inhibition, we speculate that at least part of this inhibition is due to withdrawal of ongoing corticospinal input to the spinal cord. Note that this part of the cutaneomuscular reflex is often called I1 inhibition. However, to avoid any confusion with the nomenclature of descending corticospinal volleys, we will refer to it throughout as ‘early’ cutaneomuscular inhibition.
Cortical inhibition
The most direct evidence that the median nerve and cutaneous input can reduce the excitability of the motor cortex comes from the recordings of corticospinal volleys in patients with implanted electrodes in the cervical epidural space. These show that the I2 and I3 waves are smaller when the magnetic stimulus is given at an appropriate interval after the conditioning peripheral nerve shock. Although we cannot say with certainty that the recorded volleys are destined for hand muscles, it seems likely that reduced corticospinal output is the cause of the reduced MEPs that we recorded in the healthy subjects. It would also be consistent with the relative lack of effect on responses evoked by transcranial electrical stimulation. At threshold in active muscle, electrical stimulation produces preferential D-wave activation that is less affected by changes in cortical excitability than the I-waves (Rothwell, 1997).
One puzzling feature of the results was the lack of an effect at any ISI on the I1 wave evoked by magnetic stimulation. In the present results it is possible that the amplitude of the I1 wave was saturated, and was therefore insensitive to a reduction in cortical excitability (a ‘ceiling’ effect). However, the same differential effect on I-waves has been noted twice before. The I1 wave is not affected by cortico-cortical inhibition as tested by paired magnetic pulses in the Kujirai (Kujirai et al. 1993; Di Lazzaro et al. 1998c) paradigm, nor is it affected by transcallosal inhibition as tested by the Ferbert (Ferbert et al. 1992; Di Lazzaro et al. 1999a) paradigm. Taken together these results seem to suggest that these various forms of inhibition may share some similarities. In addition, they imply that the mechanism of the first and later I-waves may differ.
Until more is known about the processes involved in generating I-waves, we can only speculate on circuits responsible for this effect. At the present time all we can say is that it is unlikely that inhibition was directed at the cell body of the pyramidal neurones. Inhibition at that point ought to affect all synaptic inputs, and therefore all I-waves equally.
A preferential effect on later I-waves has to be taken into account when estimating the central delay of the present inhibition. In different individuals, inhibition began when the interval between median and cortical stimuli was between 19 and 21 ms. Measurements in the patients showed that this was 1 ms or so longer than the latency of the N20 component of the median nerve SEP, which is conventionally taken as the time at which impulses in median nerve afferents arrive at the somatosensory cortex. If we imagine that the magnetic stimulus evoked up to three I-waves and that all of them contribute to the amplitude of the MEP evoked in relaxed muscle, then the MEP could be suppressed even if inhibition arrived only in time to affect the I3 wave. Since the I3 wave in these patients had a latency that was some 4 ms longer than that of the D-wave, inhibition may actually start up to 1 + 4 = 5 ms after arrival of median nerve input at the cortex. Whether afferent input travels first to the sensory cortex and then via cortico-cortical connections to the motor cortex, or whether the input reaches the motor cortex directly is impossible to say. Whatever the pathway, it must be relatively direct.
Comparison with previous TMS studies of transcortical reflex pathways
Several authors have used transcranial magnetic stimulation as a tool to probe the effect of afferent input on the excitability of motor cortex (Day et al. 1991; Taylor et al. 1995; Rossini et al. 1996). As outlined in Introduction, several of these have produced results that are compatible with the notion of excitatory transcortical reflex pathways. For example, natural stimulation of muscle, joint and cutaneous receptors in the hand and forearm by passive rotation of joints increases the excitability of projections to the stretched muscle 25–30 ms after the onset of movement, at the time expected from transmission in a transcortical stretch reflex loop (Day et al. 1991). However, experiments using electrically elicited analogues of these natural reflexes have proved more difficult to interpret. Three groups have investigated the excitatory (E2) component (Caccia et al. 1973), of long latency reflexes elicited by electrical stimulation of digital or mixed nerves in the hand or forearm. Two of them failed to find evidence for increased cortical excitability at an appropriate time after the stimulus (Maertens de Noordhout et al. 1992; Palmer & Ashby, 1992). The third found increased excitability when electrical stimuli were applied to the superficial radial nerve but not to the median nerve, even though both types of stimulation evoked long latency EMG responses that had a latency compatible with a transcortical pathway (Deuschl et al. 1991).
It is difficult to interpret these results with electrical stimulation. Recent work on mirror movement patients has provided virtually conclusive evidence that long latency excitatory cutaneomuscular reflexes (E2 responses) do indeed travel through a transcortical pathway (Matthews et al. 1990), so that a failure to detect clear facilitation of the responses to TMS is puzzling. One possibility is that so much of the outflow is recruited in the reflex response that there is no subliminal fringe of neurones remaining to be activated by transcranial stimulation. However this seems unlikely since long latency reflexes to muscle stretch are the same size or larger than cutaneomuscular reflexes, yet produce clear facilitation of responses evoked by transcranial magnetic stimulation. Probably the best explanation is that electrical stimulation of peripheral afferents has a mixed effect on cortical excitability, inhibiting some circuits and exciting others. Volitional input may be influenced preferentially by the facilitatory effect and results in an excitatory (E2) transcortical reflex. In contrast, transcortical stimulation may access a different set of neurones in which facilitation is not so prominent. In this system, perhaps the net initial effect, at least in the hand area of motor cortex, is primarily inhibitory.
Mechanisms of the early inhibition
Many experiments have confirmed that the motor cortex, particularly the hand area, receives short latency input from peripheral receptors (Porter & Lemon, 1993). Rosen & Asanuma (1972) first suggested in anaesthetised animals that there was a discrete input-output organisation of cortical columns in the motor cortex such that sensory input from cutaneous fields likely to be activated by the movement represented in that column would provide excitatory input whereas other fields would be inhibitory. However, this simple arrangement was not confirmed in many subsequent experiments (Porter & Lemon, 1993). Unfortunately for the present purposes, many of these tended to focus attention on excitatory rather than inhibitory effects. This was partly because inhibition can only be seen in the presence of ongoing activity, and in many cases this was absent either because the animal was at rest, or because it was anaesthetised. In fact, inhibitory effects are probably quite common. Lemon (1981a, b) made recordings from the motor cortex of awake monkeys and found that tonically active motor cortex cells that were responsive to passive rotation of a joint were usually excited by rotation in one direction, and inhibited by rotation in the opposite direction. Indeed many cells that showed an excitatory input at rest were suppressed by the same input if encountered during active movement. However, because natural inputs were used, the effects could not be timed with any accuracy.
Relationship with the early period of inhibition in cutaneomuscular reflexes
Stimulation of the digital nerves evokes cutaneomuscular reflexes in contracting FDI and other hand muscles (Caccia et al. 1973). The reflex begins with a small (often absent) E1 excitation that is spinal in origin. This is followed by a period of inhibition that peaks about 50 ms after the stimulus (the I1 inhibition). The largest component is usually the later E2 excitation that starts about 55–60 ms after the stimulus. Jenner & Stephens (1982) originally showed that the E2 was absent in patients with lesions of the dorsal columns, sensorimotor cortex or pyramidal tract, and hence suggested that it was a transcortical reflex. However, the best evidence for the transcortical nature of the response came from observations in patients with mirror movements (Matthews et al. 1990; Farmer et al. 1990; Mayston et al. 1997). Some of these individuals have corticospinal axons that branch abnormally to innervate homologous hand muscles on both sides of the body. In these patients, stimulation of one hand resulted in a bilateral E2 response, whilst spinal reflexes were strictly unilateral. Again, a transcortical E2 was the most likely explanation of the results.
The mechanism of the early inhibition (I1 component) is not so clear. Jenner & Stephens (1982) thought it was a spinal response and that its excitability was controlled by supraspinal input. However, the present results suggest that there is sufficient time for transcortical mechanisms to be involved. The minimum interval at which a digital nerve stimulus can suppress EMG responses evoked by transcranial magnetic stimulation is about 22 ms. Since it takes a further 20–22 ms for impulses to be conducted from cortex to the FDI muscle, the effect of a digital nerve shock could be seen in muscle as early as 40–42 ms after it is applied. This is before the onset of the inhibitiory component of the reflex as confirmed in Fig. 3C. If the digital nerve input can suppress not only the excitability of systems involved in the response to magnetic stimulation but also the neurones involved in sustaining a voluntary contraction, then the early reflex inhibition could well be a transcortical phenomenon.
The slightly later onset of inhibition during voluntary contraction compared with inhibition of responses to magnetic stimulation is probably more apparent than real. Surface action potentials from motor units in muscle have a finite duration and this can make the onset of inhibition in EMG appear to be later than the time at which the units stop firing. For example, if action potentials have a duration of 5 ms and motor units cease firing at 40 ms after a conditioning stimulus, EMG silence will not occur until 45 ms because of continuing activity from action potentials that fired just before onset of inhibition (Widmer & Lund, 1989). The time of motor unit inhibition may well correspond to the time at which magnetically evoked responses begin to be inhibited. There are two other possible contributing factors to the time difference between magnetically stimulated inhibition and EMG inhibition. (1) Slower conducting corticospinal axons being involved in voluntary contraction compared with the response to magnetic stimulation. (2) The propensity of inhibition to favour cortical pathways involved in later I-waves rather than the I1 wave. This means that MEPs can be reduced in amplitude even if the cortical stimulus is given before the onset of inhibition.
There is one argument against the idea that the early cutaneomuscular inhibition is transcortical. In their study of cutaneous reflexes in the patient with mirror movements, Farmer et al. (1990) found that digital nerve stimulation evoked a bilateral E2 response, but that the inhibition only occurred in muscles ipsilateral to the stimulus. However a more recent study in a larger number of patients suggests that this is not always the case (Mayston et al. 1997): some patients with mirror movements and bilateral corticospinal projections to hand muscles have bilateral E2 and inhibitory responses. The reason for these discrepancies probably lies in the difference between inhibiting ongoing EMG activity and exciting additional EMG activity. Kanouchi et al. (1997) asked patients with mirror movements to make an intentional contraction of one hand. This was accompanied by an unintended (mirror) contraction of the other hand. Transcranial magnetic stimulation of the motor cortex contralateral to the intended contraction evoked bilateral MEPs that were followed by a post-excitatory silent period. This silence is usually ascribed to withdrawal of cortical drive to spinal cord (Fuhr et al. 1991). The fact that it occurred in both hands indicates that this hemisphere was providing descending drive to the contraction in both intended and mirroring hands. The authors then stimulated the hemisphere ipsilateral to the intended movement. This evoked bilateral MEPs but there was no silent period. The authors concluded that this hemisphere was not contributing voluntary drive to the movement. This was confirmed in PET activation studies, which showed that intended movement of one hand was accompanied by a preferential increase in blood flow in the contralateral cortex.
If we apply these arguments to the cutaneomuscular reflex, we can see that even if both responses rely on a transcortical reflex pathway only excitation is guaranteed to be bilateral whereas inhibition may remain unilateral. Imagine the mirror movement patient making an intended movement of both hands and that each hemisphere only provides volitional drive to the contralateral hand. Stimuli applied to the fingers of the right hand will produce input to the left motor cortex and any initial inhibition of its output would be seen only in the right hand. However, later excitation (E2) would be conducted from the left hemisphere to both hands and evoke a bilateral E2. The crux of the matter depends on how much of the drive to a contracting muscle in these patients comes from each hemisphere. If it is asymmetrical then the early cutaneomuscular inhibition need not be bilateral.
Finally it should be mentioned that there may be more than one variety of early reflex inhibition. Pierrot-Deseilligny and colleagues (Burke et al. 1994) showed that stimulation of the superficial radial nerve at the wrist could produce a short latency inhibition in the ongoing EMG of forearm extensor muscles. The onset of this inhibition was shorter than the usual cutaneomuscular inhibition, and the authors concluded that it was caused by inhibition of propriospinal-like neurones in the cervical cord that mediated the voluntary command to move. Such a mechanism is unlikely to contribute to the early cutaneomuscular inhibition studied here because (1) the latency of the effect is not appropriate, and (2) there is no evidence that propriospinal-like inputs are involved in transmitting voluntary commands to intrinsic hand muscles.
Inhibition of EMG responses evoked by magnetic versus electrical transcranial stimulation
Responses to electrical stimulation were inhibited significantly less than those evoked by magnetic stimulation. The most likely explanation for this is that since the comparison between the two forms of stimulation was performed during active contraction, the effect on the electrically elicited response was due to changes in the level of background EMG activity in the early period of reflex inhibition. Effects at a cortical level are unlikely in view of the recent demonstration that the D-wave evoked by transcranial electrical stimulation in man is the same whether subjects are at rest or actively contracting (Di Lazzaro et al. 1999b). Since I-waves are enhanced during contraction (Di Lazzaro et al. 1998b), the implication is that the electrical D wave in man is relatively insensitive to levels of cortical excitability.
Conclusion
These results show that electrical stimulation of mixed or cutaneous nerves from the hand can suppress the excitability of cortical projections to hand muscles. The onset latency of the effect is only some 5 ms or so after the arrival of afferent input at the cortex, and implies a relatively direct pathway from sensory input to motor output. The mechanism is unclear, but could involve either direct inhibition of motor cortex from fast conducting afferents, or withdrawal of tonic facilitation from other structures, such as thalamus. We speculate that the effect is related to and even responsible for the early period of inhibition in cutaneomuscular reflexes, and that the technique may be a useful way of probing sensorimotor interaction in man.
Acknowledgments
Part of this work was funded by a European Union Large Scale Facility Grant to the Human Movement and Balance Unit in London.
References
- Bertolasi L, Priori A, Tinazzi M, Bertasi V, Rothwell JC. Inhibitory action of forearm flexor muscle afferents on corticospinal outputs to antagonist muscles in humans. The Journal of Physiology. 1998;511:947–956. doi: 10.1111/j.1469-7793.1998.947bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D, Gracies JM, Mazevet D, Meunier S, Pierrot Deseilligny E. Non-monosynaptic transmission of the cortical command for voluntary movement in man. The Journal of Physiology. 1994;480:191–202. doi: 10.1113/jphysiol.1994.sp020352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caccia MR, Mccomas AJ, Upton AR, Blogg T. Cutaneous reflexes in small muscles of the hand. Journal of Neurology, Neurosurgery and Psychiatry. 1973;36:960–977. doi: 10.1136/jnnp.36.6.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day BL, Riescher H, Struppler A, Rothwell JC, Marsden CD. Changes in the response to magnetic and electrical stimulation of the motor cortex following muscle stretch in man. The Journal of Physiology. 1991;433:41–57. doi: 10.1113/jphysiol.1991.sp018413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delwaide PJ, Olivier E. Conditioning transcranial cortical stimulation (TCCS) by exteroceptive stimulation in parkinsonian patients. Advances in Neurology. 1990;53:175–181. [PubMed] [Google Scholar]
- Deuschl G, Michels R, Berardelli A, Schenck E, Inghilleri M, Lucking CH. Effects of electric and magnetic transcranial stimulation on long latency reflexes. Experimental Brain Research. 1991;83:403–410. doi: 10.1007/BF00231165. [DOI] [PubMed] [Google Scholar]
- Deuschl G, Schenck E, Lucking CH. Long-latency responses in human thenar muscles mediated by fast conducting muscle and cutaneous afferents. Neuroscience Letters. 1985;55:361–366. doi: 10.1016/0304-3940(85)90462-8. [DOI] [PubMed] [Google Scholar]
- di Lazzaro V, Oliviero A, Profice P, Insola A, Mazzone P, Tonali P, Rothwell JC. Direct demonstration of interhemispheric inhibition of the human motor cortex produced by transcranial magnetic stimulation. Experimental Brain Research. 1999a;124:520–524. doi: 10.1007/s002210050648. [DOI] [PubMed] [Google Scholar]
- di Lazzaro V, Oliviero A, Profice P, Insola A, Mazzone P, Tonali P, Rothwell JC. Effects of voluntary contraction on descending volleys evoked by transcranial electrical stimulation over the motor cortex hand area in conscious humans. Experimental Brain Research. 1999b;124:525–528. doi: 10.1007/s002210050649. [DOI] [PubMed] [Google Scholar]
- di Lazzaro V, Oliviero A, Profice P, Saturno E, Pilato F, Insola A, Mazzone P, Tonali P, Rothwell JC. Comparison of descending volleys evoked by transcranial magnetic and electric stimulation in conscious humans. Electroencephalography and Clinical Neurophysiology. 1998a;109:397–401. doi: 10.1016/s0924-980x(98)00038-1. [DOI] [PubMed] [Google Scholar]
- di Lazzaro V, Restuccia D, Oliviero A, Profice P, Ferrara L, Insola A, Mazzone P, Tonali P, Rothwell JC. Effects of voluntary contraction on descending volleys evoked by transcranial stimulation in conscious humans. The Journal of Physiology. 1998b;508:625–634. doi: 10.1111/j.1469-7793.1998.625bq.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Lazzaro V, Restuccia D, Oliviero A, Profice P, Ferrara L, Insola A, Mazzone P, Tonali P, Rothwell JC. Magnetic transcranial stimulation at intensities below active motor threshold activates intracortical inhibitory circuits. Experimental Brain Research. 1998c;119:265–268. doi: 10.1007/s002210050341. [DOI] [PubMed] [Google Scholar]
- Farmer SF, Ingram DA, Stephens JA. Mirror movements studied in a patient with Klippel-Feil syndrome. The Journal of Physiology. 1990;428:467–484. doi: 10.1113/jphysiol.1990.sp018222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferbert A, Priori A, Rothwell JC, Day BL, Colebatch JG, Marsden CD. Interhemispheric inhibition of the human motor cortex. The Journal of Physiology. 1992;453:525–546. doi: 10.1113/jphysiol.1992.sp019243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhr P, Agostino R, Hallett M. Spinal motor neuron excitability during the silent period after cortical stimulation. Electroencephalography and Clinical Neurophysiology. 1991;81:257–262. doi: 10.1016/0168-5597(91)90011-l. [DOI] [PubMed] [Google Scholar]
- Jenner JR, Stephens JA. Cutaneous reflex responses and their central nervous pathways studied in man. The Journal of Physiology. 1982;333:405–419. doi: 10.1113/jphysiol.1982.sp014461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanouchi T, Yokota T, Isa F, Ishii K, Senda M. Role of the ipsilateral motor cortex in mirror movements. Journal of Neurology, Neurosurgery and Psychiatry. 1997;62:629–632. doi: 10.1136/jnnp.62.6.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD. Corticocortical inhibition in human motor cortex. The Journal of Physiology. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon RN. Functional properties of monkey motor cortex neurones receiving afferent input from the hand and fingers. The Journal of Physiology. 1981a;311:497–519. doi: 10.1113/jphysiol.1981.sp013601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon RN. Variety of functional organisation within the monkey motor cortex. The Journal of Physiology. 1981b;311:521–540. doi: 10.1113/jphysiol.1981.sp013602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maertens de Noordhout A, Rothwell JC, Day BL, Dressler D, Nakashima K, Thompson PD, Marsden CD. Effect of digital nerve stimuli on responses to electrical or magnetic stimulation of the human brain. The Journal of Physiology. 1992;447:535–548. doi: 10.1113/jphysiol.1992.sp019016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden CD, Merton PA, Morton HB. Servo action in the human thumb. The Journal of Physiology. 1976;257:1–44. doi: 10.1113/jphysiol.1976.sp011354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden CD, Merton PA, Morton HB, Adam J. The effect of posterior column lesions on servo responses from the human long thumb flexor. Brain. 1977a;100:185–200. doi: 10.1093/brain/100.1.185. [DOI] [PubMed] [Google Scholar]
- Marsden CD, Merton PA, Morton HB, Adam J. The effect of lesions of the sensorimotor cortex and the capsular pathways on servo responses from the human long thumb flexor. Brain. 1977b;100:503–526. doi: 10.1093/brain/100.3.503. [DOI] [PubMed] [Google Scholar]
- Matthews PB, Farmer SF, Ingram DA. On the localization of the stretch reflex of intrinsic hand muscles in a patient with mirror movements. The Journal of Physiology. 1990;428:561–577. doi: 10.1113/jphysiol.1990.sp018228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayston MJ, Harrison LM, Quinton R, Stephens JA, Krams M, Bouloux PG. Mirror movements in X-linked Kallmann's syndrome. 1. A neurophysiological study. Brain. 1997;120:1199–1216. doi: 10.1093/brain/120.7.1199. [DOI] [PubMed] [Google Scholar]
- Noth J, Podoll K, Friedemann HH. Long-loop reflexes in small hand muscles studied in normal subjects and in patients with Huntington's disease. Brain. 1985;108:65–80. doi: 10.1093/brain/108.1.65. [DOI] [PubMed] [Google Scholar]
- Palmer E, Ashby P. The transcortical nature of the late reflex responses in human small hand muscle to digital nerve stimulation. Experimental Brain Research. 1992;91:320–326. doi: 10.1007/BF00231665. [DOI] [PubMed] [Google Scholar]
- Porter R, Lemon RN. Corticospinal Function and Voluntary Movement. Oxford: Oxford University Press; 1993. [Google Scholar]
- Rosen I, Asanuma H. Peripheral afferent inputs to the forelimb area of the monkey motor cortex: input-output relations. Experimental Brain Research. 1972;14:257–273. doi: 10.1007/BF00816162. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Tecchio F, Sabato A, Finazzi AA, Pasqualetti P, Rossi S. The role of cutaneous inputs during magnetic transcranial stimulation. Muscle and Nerve. 1996;19:1302–1309. doi: 10.1002/(SICI)1097-4598(199610)19:10<1302::AID-MUS7>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Rothwell JC. Techniques and mechanisms of action of transcranial stimulation of the human motor cortex. Journal of Neuroscience Methods. 1997;74:113–122. doi: 10.1016/s0165-0270(97)02242-5. [DOI] [PubMed] [Google Scholar]
- Taylor JL, Fogel W, Day BL, Rothwell JC. Ipsilateral cortical stimulation inhibited the long-latency response to stretch in the long finger flexors in humans. The Journal of Physiology. 1995;488:821–831. doi: 10.1113/jphysiol.1995.sp021014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widmer CG, Lund JP. Evidence that peaks in EMG averages can sometimes be caused by inhibition of motoneurons. Journal of Neurophysiology. 1989;62:212–219. doi: 10.1152/jn.1989.62.1.212. [DOI] [PubMed] [Google Scholar]


