Abstract
Many processes in mammalian and invertebrate central nervous systems exhibit habituation and/or sensitization of their responses to repetitive stimuli. Here, we studied the adaptive behaviours of the respiratory pattern generator in rat on repetitive vagal-afferent stimulation and compared these behaviours obtained in vivo with the reported effects of such stimuli on synaptic transmission in the corresponding signal pathway in vitro.
Sustained (1 min) electrical pulsed stimulation of the vagus nerve elicited the classic Hering-Breuer (HB) reflex slowing of the respiratory rhythm followed by a bi-exponential recovery, and a post-stimulus rebound (PR). The recovery from the HB reflex satisfied the classic criteria of habituation.
The fast component of the recovery and the PR were abolished by systemic administration of an NMDA receptor antagonist or electrolytic lesioning of the pontine Kölliker-Fuse nucleus. The characteristics of the fast recovery and PR suggest a vagally induced desensitization of the NMDA receptor-dependent pontine input to the respiratory pattern generator.
The slow component of recovery persisted after both experimental interventions and accounted for the habituation to the vagal input. The characteristics of the slow recovery in vivo were reminiscent of the reported synaptic accommodation in vitro in the medullary region where vagal afferents terminate.
The habituation of vagal input and desensitization of pontine input act in concert to offset the HB reflex. Such simultaneous habituation-desensitization in parallel neural pathways with differing sensitivities to NMDA receptor activation represent a hitherto unknown pairing of dual non-associative learning processes in the mammalian brain.
Habituation and sensitization are two common forms of non-associative learning exhibited in many neuronal structures of mammalian and invertebrate nervous systems (Thompson & Spencer, 1966; Groves & Thompson, 1970; Poon, 1996; Cohen et al. 1997). According to the classic Dual-Process Theory of non-associative learning (Groves & Thompson, 1970), an evoked physiological response is decreased (habituated) continually with repetitive application of the same stimulus, and increased (sensitized) by the same or other stimuli along a separate neural pathway (state system) in parallel with the primary stimulus-response (S-R) pathway. Habituation (with or without sensitization) is ubiquitous in many parts of the mammalian brain such as the motor (Thompson & Spencer, 1966; Egger, 1978), visual (Peterzell, 1993), auditory (Davis et al. 1982; Pilz & Schnitzler, 1996), olfactory (Wilson, 1998), appetitive (Bassareo & Di Chiara, 1997), vestibular (Cohen et al. 1992), visceral (Siniaia et al. 1992) and autonomic systems (Siniaia & Yazykov, 1981; Groome et al. 1994). Similar effects have also been reported at the cellular level in certain reduced preparations in vitro (Teyler & Alger, 1976; Platt & Withington, 1997). The mechanisms underlying such adaptive behaviours are not fully understood and are likely to vary with differing brain structures. In simple invertebrate nervous systems, habituation and sensitization have been shown to result from activity-dependent phasic depression and enhancement of synaptic efficacy in corresponding S-R pathways, respectively (Kandel, 1978; Cohen et al. 1997).
Recently, similar activity-dependent phasic depression (accommodation) of synaptic transmission has been found in vitro (Miles, 1986; Zhou et al. 1997; Zhou & Poon, 2000) and in vivo (Mifflin & Felder, 1990) in mammalian brainstem nucleus tractus solitarius (NTS) – a gateway for a multitude of primary afferents from cardiovascular, respiratory and visceral sensory receptors. The synaptic accommodation in NTS was dependent on stimulus frequency (Miles, 1986; Zhou & Poon, 2000) and independent of NMDA receptor activity (Zhou et al. 1997). These findings suggest the possible existence of (and a cellular mechanism for) S-R habituation in corresponding sensory systems.
As many respiratory (Karczewski & Romaniuk, 1980; Younes & Polacheck, 1985; Bisgard & Neubauer, 1995; Poon, 1996) and cardiovascular reflexes (Mifflin & Felder, 1990; Heesch & Barron, 1992) exhibit activity-dependent phasic adaptation upon sustained afferent stimulation, we wondered if such reflex adaptations were manifestations of habituation at the behavioural level and synaptic accommodation at the cellular level. In this investigation, we sought to obtain a physiological correlation of synaptic accommodation in NTS by studying the temporal characteristics of the classic Hering-Breuer (HB) respiratory reflex (Breuer, 1868; Hering, 1868) elicited by sustained vagal input in rats. We found strong evidence of habituation in the HB reflex and showed that it had similar characteristics to the synaptic accommodation in NTS neurons. Surprisingly, the NMDA receptor-independent habituation of the vagal pathway was accompanied by an NMDA receptor-dependent desensitization (rather than sensitization) of a parallel pathway in the pontine ‘pneumotaxic centre.’ Such coupled habituation- desensitization of parallel signal pathways with differing sensitivities to NMDA receptor activation represent a novel and demonstrable pairing of dual non-associative learning processes in the mammalian brain.
METHODS
Animal preparation
The experimental protocols were reviewed and approved by the Institute's Committee on Animal Care in accordance with published guidelines. Studies were conducted on 25 adult Sprague-Dawley rats of either sex weighing 250–470 g and anaesthetized with urethane (1.5 g kg−1i.p.), with supplemental doses (0.15-0.3 g kg−1i.v.) given periodically throughout the experiment as necessary. Adequacy of anaesthesia was assessed continuously in all rats by monitoring changes in arterial blood pressure (BP) and/or respiration rate. A tracheostomy was performed and atropine sulphate (0.05 mg kg−1i.p.) was injected to minimize tracheal secretions. A carotid artery and femoral vein were cannulated for BP monitoring and for drug infusion, respectively. Animals were paralysed with pancuronium bromide (Sigma; initial dose 1 mg kg−1i.v. supplemented every hour at 40–50 % of the initial dosage) and artificially ventilated with medical-grade air by using a servo-ventilator (CWE, Model AVS-1) which maintained expired CO2 constant at 4.6-4.8 % as determined by an infrared CO2 analyser (CWE, CapStar-100). Rectal temperature was maintained at 37.5 ± 0.2°C with a temperature controller (CWE, TC-831).
Stimulation and recording
A phrenic nerve (Phr) was dissected at the C5 level by a ventral approach and mounted on custom-made bipolar silver wire electrodes. Phrenic activity was amplified (Axon Instruments, CyberAmp 380) and time averaged with a leaky integrator (time constant, 15 ms), and the amplitude of integrated phrenic nerve activity (∫Phr) and respiratory frequency (f) were measured. Both vagus nerves were isolated, cut distally and put on silver hook electrodes for stimulation. All severed nerves were kept in warm mineral oil pools.
Electrical stimulation of the vagus nerve elicits the HB reflex by activating the afferent fibres from slowly adapting pulmonary stretch receptors (SARs). We defined the stimulation threshold at any stimulation frequency as the lowest stimulus current that produced an appreciable inhibitory effect on phrenic nerve discharges during a 5 s period of repetitive stimulation. For the purpose of the present experiments the stimulus current used was kept at a minimal range of 1.5-2 × threshold (T), or approximately 15–40 μA, unless stated otherwise. The relatively low stimulation intensity minimized the possibility of vagus nerve fatigue (Stanley et al. 1975) or the activation of vagal C-fibres and rapidly adapting stretch receptor (RAR) fibres which have relatively high activation thresholds (Bergren & Peterson, 1993).
After a 1 h stabilization period one of the vagus nerves (contralateral or ipsilateral to the phrenic nerve) was stimulated repetitively (at 40 or 80 Hz, pulse duration 0.1 ms) for 60 s. The initiation of the stimulation period was triggered by the onset of phrenic discharges. Phrenic activity was recorded continuously during the vagal stimulation period and for a 2 min control period both before and after stimulation. In some experiments varying vagal stimulation protocols were used to study the effect of habituation to sustained vagal inputs.
Drug administration
We studied the effect of the non-competitive NMDA receptor antagonist dizocilpine (MK-801; Research Biochemicals International, Natick, MA, USA) on the habituation to vagal stimulation. The drug was dissolved in saline and a single dose (1–1.5 mg kg−1) was administered intravenously. This dosage has been reported to elicit increases in inspiratory duration and an apneustic breathing pattern in Sprague-Dawley rats (Connelly et al. 1992). The effect of MK-801 on the habituation process was analysed before and after drug administration at time intervals of up to 1 h. At the end of each experiment the animal was killed by pentobarbitone overdose.
Pontine lesion
In five animals specific regions in the rostral pons including the medial and lateral parabrachialis (PB) and Kölliker-Fuse (KF) nuclei were lesioned electrolytically. The skull was drilled and insulated tungsten microelectrodes (125 μm in diameter, AM system) were inserted stereotaxically (Paxinos & Watson, 1986) at PB (8.7-9.1 mm caudal to bregma and 2.0-2.6 mm lateral to the midline, at a depth of 7.0-7.8 mm from bregma) or KF (8.7-9.0 mm caudal to bregma and 2.6-2.7 mm lateral to midline, at a depth of 7.6-7.9 mm). Lesions were induced by applying a small positive DC current (50–70 μA) over 1 min.
Histology
At the end of each lesion experiment, the animal was perfused through the carotid artery with 2 % paraformaldehyde. The brain was then removed, placed in 4 % paraformaldehyde and stored for at least 24 h. Sections were cut in the frontal plane (75–100 μm), examined microscopically and the extent of the lesion was mapped according to standard atlas (Paxinos & Watson, 1986). Some slices were stained with thionin.
Data
All results are presented as means ± s.e.m. unless otherwise quoted. Statistical tests used were Student's t test and F tests. Significance was set at P < 0.05.
RESULTS
Habituation and desensitization of Hering-Breuer reflex
Repetitive stimulation of the vagus nerve elicited a characteristic inhibition of phrenic discharges (Fig. 1) with shortening of inspiratory time (TI), prolongation of expiratory time (TE) as well as corresponding decreases in f and ∫Phr similar to the well-known HB reflex. However, the response in f recovered gradually in the face of sustained vagal stimulation (for 1 min) and rebounded transiently above the control level upon removal of the vagal stimulus. The biphasic response in f was obtained despite unchanging chemoreceptor inputs as reflected by the stability of expired CO2 levels throughout the experimental period (Fig. 1, bottom). Eventually, the breathing pattern (in terms of TI, TE, f and ∫Phr) returned to normal in the steady state after vagal stimulation. (For simplicity, the responses in f and ∫Phr were used as a proxy of the HB reflex in this investigation unless stated otherwise.)
Figure 1. Effects of repetitive electrical stimulation (at 80 Hz) of vagus nerve over 1 min (horizontal bar) on phrenic nerve activity in a vagotomized rat.
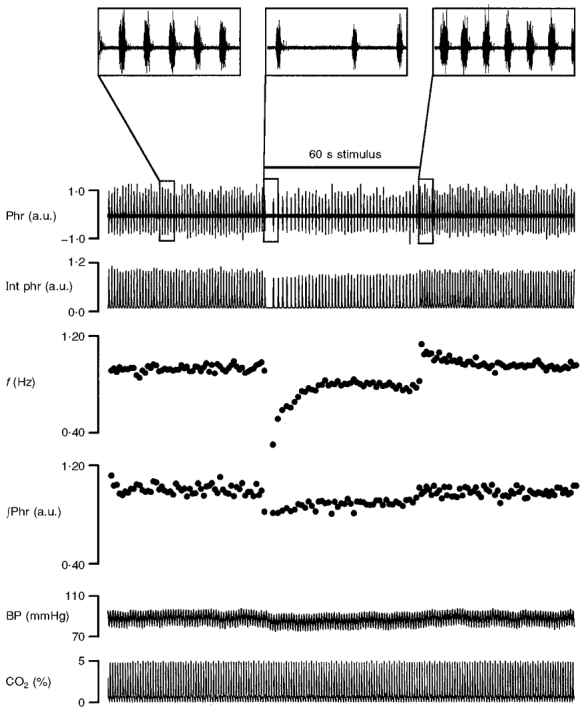
From top to bottom: phrenic discharges in arbitrary units (a.u.) (Phr); integrated phrenic activity (Int phr); respiratory frequency (f); amplitude of integrated phrenic activity (∫Phr); arterial blood pressure (BP); percentage CO2 level in respired air (CO2 (%)). Each point in the f and ∫Phr traces corresponds to one breath. Insets at the top show expanded views of phrenic discharges in the control period and at the onset and offset of vagal stimulation.
In Fig. 2, the mean response (n = 5) to 1 min vagal stimulation at two frequencies (40 and 80 Hz) revealed a frequency-dependent inhibition and subsequent recovery of the response in f and – to a lesser extent – in ∫Phr. At low stimulation frequency (40 Hz) the response in f returned to the baseline level within 1 min of stimulation, whereas at a higher stimulation frequency (80 Hz) – with greater initial inhibition – the recovery was incomplete and f remained lower than the baseline level at the end of the stimulation period. The recovery of the response in f during vagal stimulation may be fitted by two exponential functions (single exponential models were rejected by F test analysis, P < 0.05): a fast component, with time constant τ1 = 1.6 ± 0.9 s and 2.9 ± 1.1 s for a stimulus frequency of 40 Hz and 80 Hz, respectively; and a slow component, with time constant τ2 = 36.0 ± 6.4 s and 31.1 ± 7.4 s, for 40 Hz and 80 Hz, respectively. The post-stimulus rebound (PR) had a single time constant, τ3 = 11.8 ± 3.5 s and 13.4 ± 2.5 s, for 40 Hz and 80 Hz, respectively. The time constants were not significantly different for differing stimulation frequencies (P > 0.1, Student's t test); the pooled estimates were: τ1 = 2.4 ± 0.7 s; τ2 = 33.1 ± 4.9 s; τ3 = 12.7 ± 2.0 s (all significantly different from zero at 5 % level, t test). The response in ∫Phr did not demonstrate fast recovery or PR behaviour.
Figure 2. Adaptation of Hering-Breuer reflex.
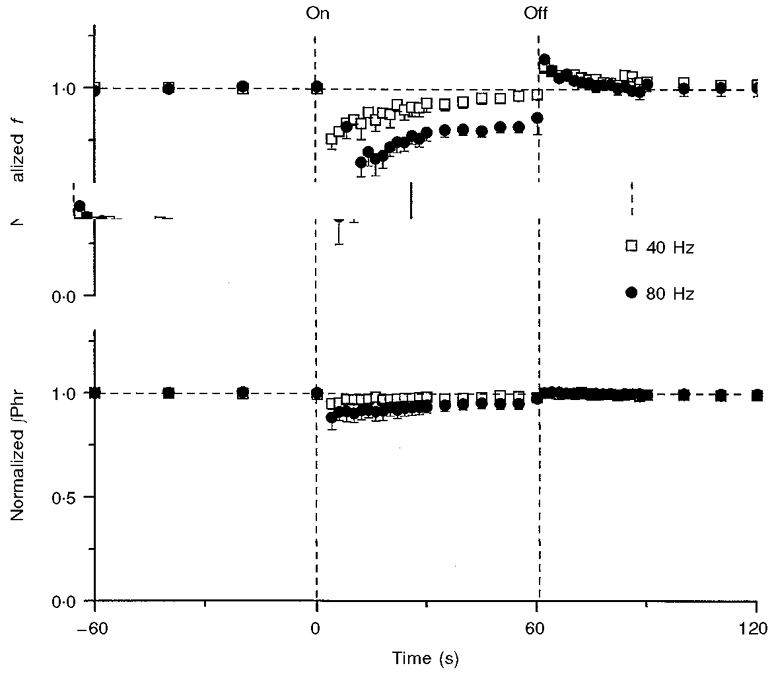
Mean responses (means ± s.e.m.; n = 5) in respiratory frequency (f) and amplitude of integrated phrenic activity (∫Phr) before, during and after 1 min vagal stimulation (demarcated by vertical dashed lines) at two different stimulation frequencies. Note fast recovery and post-stimulus rebound of the response in f but not in ∫Phr. The data from each rat were averaged over several breaths in variable time windows that ranged from 1 s (at the onset of vagal stimulation) to 20 s (in the control period), in accordance with the relative speed of response. Responses in each rat were normalized with respect to the corresponding mean responses in the control period before the data from all rats were pooled.
The initial inhibition and subsequent exponential recovery of phrenic activity with sustained vagal stimulation is analogous to a habituation process. To examine whether the recovery of the HB reflex conformed to the classical definition of habituation, we employed the following criteria set forth by Thompson and coworkers (Thompson & Spencer, 1966; Groves & Thompson, 1970) which have been applied variously to the study of habituation in certain brain structures (Teyler & Alger, 1976; Kandel, 1978; Siniaia et al. 1992).
Inverse-dependence on stimulus intensity
At a given stimulation frequency (80 Hz) the response in f recovered more readily with a weak stimulus current than a stronger one (Fig. 3a). Thus, the rate of recovery of phrenic activity was inversely dependent on stimulus intensity.
Figure 3. Criteria for habituation of Hering-Breuer reflex in terms of the recovery pattern of respiratory frequency (f) with varying vagal stimulation protocols (indicated by different horizontal bars; stimulation frequency, 80 Hz).
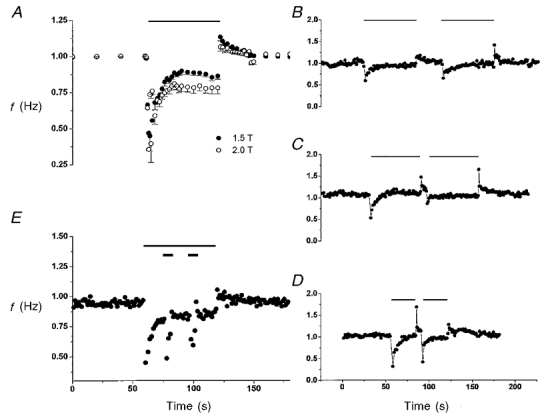
A, rate of recovery was inversely dependent on stimulation intensity. Data show mean responses (n = 3) to 1 min vagal stimulation at two different stimulation intensities (1.5 and 2.0 × T). B, recovery patterns were similar in consecutive 1 min vagal stimulation episodes separated by a 30 s intermission period in one rat. However, the group data in three rats showed that the magnitude of the initial inhibitory response at onset of the second episode was slightly lower (-15 ± 1.15 %) than that of the first episode, suggesting some residual effect of the habituation from the first episode. C, inhibitory effect of vagal stimulus was diminished in the second stimulation episode when the intermission period was shortened to 5 s. The magnitude of the initial inhibitory response was markedly decreased (-45 ± 10 %, n = 3) in the second episode compared with the first episode, suggesting that the residual effect from the first episode (see B) represents a time-dependent short-term memory. D, the short-term memory of habituation became less pronounced when the duration of the first vagal stimulation episode was shortened by half. The initial inhibitory response in the second episode was not significantly different (-8.0 ± 7.7 %, n = 3; P > 0.1, t test) from the first one. E, dishabituation and habituation of dishabituation. Thin and thick bars indicate the stimulation of the first and second vagus nerves (at a stimulation intensity of 1.5T and 4T, respectively). Note that strong stimulation of the second vagus nerve reversed (‘dishabituated’) the habituation of the response to stimulation of the first vagus nerve as evidenced by the pronounced response evoked by the second vagal stimulus. However, the response to the second vagal stimulus was diminished (i.e. habituated) when the stimulus was applied a second time. Thus, the dishabituation effect of the second vagal stimulus was itself habituated.
Short-term memory
As shown in Fig. 3B, when the vagal stimulus was applied a second time after an intermission period of 30 s, a similar response pattern as in the first episode ensued. With a shorter intermission period (5 s), the habituation effect carried over to the second episode such that the inhibitory effect of the second vagal stimulus was habituated throughout (Fig. 3C). This behaviour suggests the presence of a short-term memory of the habituation. The memory effect was lessened when the first vagal stimulation period was shortened (Fig. 3D). Similar short-term memory effects as shown in Fig. 3B–D were observed when the vagus nerves were stimulated at 80 Hz (n = 3) or 40 Hz (n = 3).
Dishabituation and habituation of dishabituation
In many nervous system structures, a habituated system may be ‘dishabituated’ (i.e. reversing the effect of habituation) by novel and/or strong stimuli (Groves & Thompson, 1970; Hawkins et al. 1998). Figure 3E shows that the habituation to the vagal stimulus was dishabituated by a second, strong stimulus (4 × T) applied to the vagus nerve contralateral to the first one, which provided a novel and strong input by activating additional nerve fibres (and perhaps also other fibre types). This dishabituating vagal input was again habituated on repeated application of this input.
Desensitization
According to the classic Dual-Process Theory of habituation (Groves & Thompson, 1970), an afferent input would elicit habituation in the S-R pathway as well as sensitization in a separate ‘state’ system which interacts with the S-R pathway to yield the resultant response. The existence of such a state system in the HB reflex is indicated by the presence of PR, which decayed to the baseline level in ∼30 s after the vagal stimulus was removed (Fig. 2).
The PR is comprised of at least two components. First, removal of the vagal input would result in an abrupt termination of the (habituated) HB reflex in the S-R pathway, bringing the response back to the control (pre-stimulation) level. Second, memory in the state system would account for any response above the control level. The initial PR's in f at stimulation frequency of 40 Hz and 80 Hz were 12.6 ± 2.9 % (P < 0.01) and 16.3 ± 3.1 % (P < 0.01, t test) above control level, respectively, which corresponded to 43.8 and 24.0 % of total adaptation to the HB reflex.
The PR above the control level indicates that the adaptation in the state system pathway complemented (rather than counteracted) the habituation in the vagal pathway. This implies the state system pathway was depressed, or desensitized (rather than sensitized), during vagal stimulation. (Here, ‘desensitization’ refers to a depression in neurotransmission within the state system, rather than the desensitization of AMPA receptors in the NTS (Zhou et al. 1997).) The desensitization persisted briefly in the post-stimulation period when signal transmission in the state system was gradually restored (or re-sensitized). The habituation-desensitization in the S-R and state system pathways is in contradistinction to the habituation- sensitization pairing predicted by the Dual-Process Theory (see Discussion).
Effects of NMDA receptor antagonist
In a different group of rats (n = 7) the habituation and desensitization processes were compared before and after intravenous injection of MK-801. As shown in Fig. 4a, MK-801 resulted in a tachypnoeic respiratory rhythm in a vagotomized animal with prolongation of TI (63.0 ± 15.0 % from control) and shortening of TE (-56.4 ± 4.2 %). The effects of MK-801 were dose dependent: with a higher dose an apneusis-like respiratory pattern ensued (Connelly et al. 1994).
Figure 4. Effects of intravenous injection of MK-801 on habituation and desensitization of Hering-Breuer reflex in a vagotomized rat.
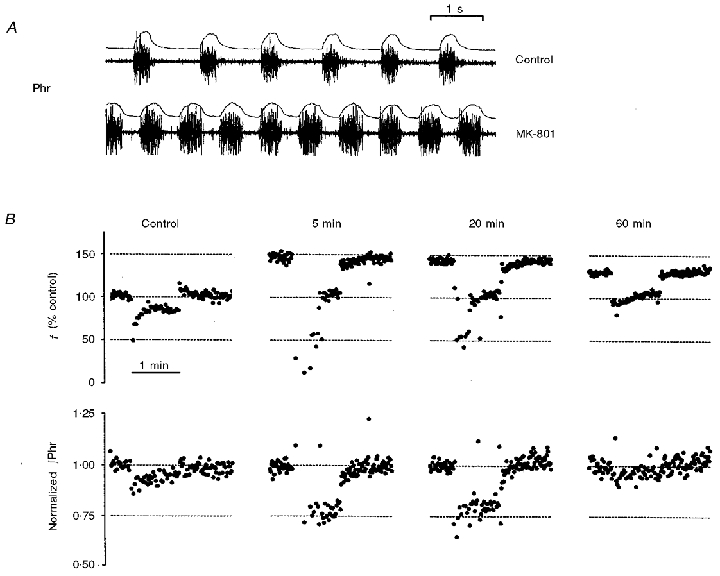
A, phrenic discharges and integrated phrenic activity before and after MK-801 administration. B, responses in respiratory frequency (f) and normalized phrenic amplitude (∫Phr) to 80 Hz vagal stimulation before (control) and 5, 20 and 60 min after MK-801 injection. Note that post-stimulus rebound in f remained absent throughout the period after drug administration despite variations in the magnitudes of the HB reflex and its habituation. The f plots were normalized by the quiescent level in the control period before MK-801 injection while the ∫Phr plot at each time instant was normalized independently by the corresponding mean level in the control period prior to vagal stimulation. Horizontal dotted lines indicate reference levels at various experimental states.
The transient effects of MK-801 on habituation- desensitization of the HB reflex are demonstrated in Fig. 4B. Within 5 min after MK-801 administration both processes were altered dramatically. Vagal stimulation at 80 Hz elicited abrupt and marked decreases in both f and ∫Phr from the baseline levels (after MK-801) without any recovery initially. Approximately 20–30 s after the onset of stimulation there was an abrupt, partial recovery of the response in f. Upon cessation of the vagal stimulus both f and ∫Phr returned slowly to the corresponding baseline levels without any rebound. In addition, there were time-dependent changes in these response patterns following MK-801 administration. The inhibitory response in ∫Phr vanished 60 min after MK-801 administration while the baseline level in f recovered partially. The initial non-adaptation period (with no recovery in f) during vagal stimulation became shorter 20 min after MK-801 administration (compared with that after 5 min) and eventually vanished after 60 min, when a recovery phase for f during vagal stimulation became clearly visible. Nevertheless, there remained no indications of a PR in f up to 60 min after MK-801 administration.
Figure 5 shows the mean responses in control (n = 7) and in the steady state (60 min) after MK-801 administration (n = 5). The initial HB reflex in f was followed by an exponential recovery similar to a habituation response. A bi-exponential model (τ1 = 2.0 ± 1.1 s; τ2 = 44.7 ± 3.1 s) did not provide a better curve fit (according to F test) to the recovery response than a mono-exponential fit (τ = 13.1 ± 7.4 s) and was rejected on the basis of parsimony as determined by the Akaike information criterion (Akaike, 1974). Again, there was no PR in any of the animals 60 min after MK-801 treatment.
Figure 5. Effect of NMDA receptor blockade on adaptation of Hering-Breuer reflex.
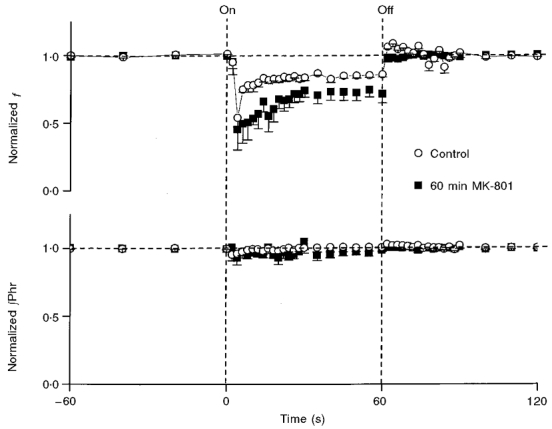
Mean responses in normalized respiratory frequency (f) and phrenic amplitude (∫Phr) to repetitive vagal stimulation at 80 Hz before (control) and 60 min after MK-801. Post-stimulus rebound in f was absent (not significantly different from zero; P > 0.1) after MK-801. See Fig. 2 legend for notations.
Effects of pontine pneumotaxic lesion
To examine the possible role of NMDA receptor-dependent pontine pneumotaxic input (Foutz et al. 1989; Ling et al. 1994) in the desensitization of the HB reflex, we repeated the above experiment in five rats after lesioning the rostrolateral pons in the vicinity of the PB and KF nuclei which receive projections from vagal SAR relay neurons in the NTS (Ezure et al. 1998). Figure 6 is a composite diagram showing the sites of lesion and corresponding histological examples. In the first group of animals we lesioned bilaterally (n = 2) or unilaterally (n = 1) only the KF nuclei. In the second group (n = 2) we lesioned KF bilaterally and PB unilaterally.
Figure 6. Schematic diagram of transverse sections of rat brainstem at three levels showing sites of lesion in the rostrolateral pons encompassing the PB and KF nuclei in 5 rats.
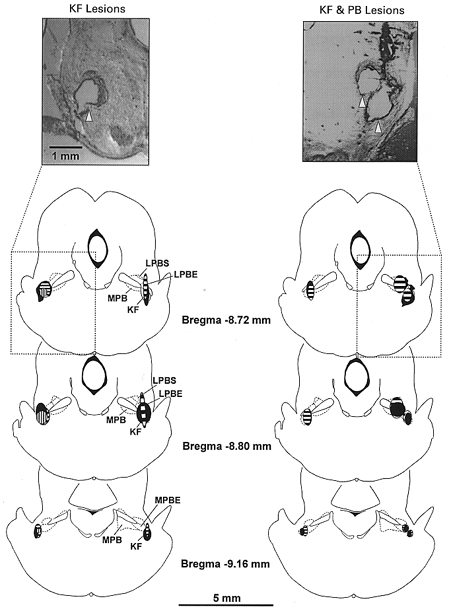
Left, KF only lesions, bilaterally in 2 rats (areas filled or with horizontal shading) and unilaterally in one rat (vertical shading). Right, sites of unilateral PB lesion and bilateral KF lesions in two rats (areas filled or with horizontal shading). Insets are photomicrographs illustrating the lesions (indicated by arrows) in two rats. Abbreviations: PB, parabrachialis nucleus; LPBS, lateral parabrachial nucleus, sup; LPBE, lateral parabrachial nucleus, ext; MPB, medial parabrachial nucleus; KF, Kölliker-Fuse nucleus.
Bilateral KF lesioning had little effect on breathing pattern in the vagi-intact animal but produced an apneustic breathing pattern in the vagotomized animal (Fig. 7a), as reported previously (Caille et al. 1981; Wang et al. 1993). As with the MK-801-treated animals, the fast component of recovery and PR of the HB reflex were absent (Fig. 7B). These behaviours were observed in all rats with either unilateral or bilateral lesioning of KF, with or without PB lesioning.
Figure 7. Effects of bilateral Kölliker-Fuse nucleus (KF) lesions on habituation and post-stimulus rebound of Hering-Breuer reflex.
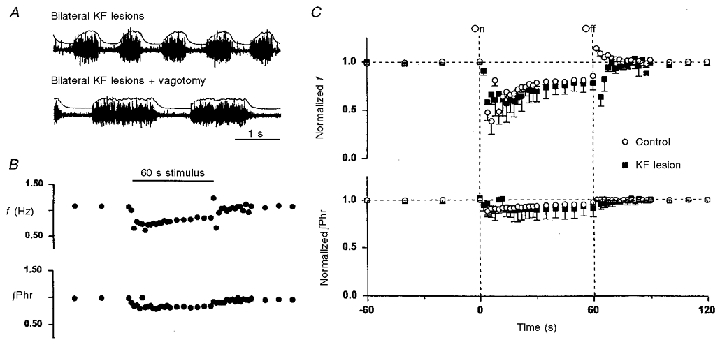
A, raw and integrated phrenic activity post-KF lesioning before and after vagotomy in one rat. B, responses in respiratory frequency (f) and phrenic amplitude (∫Phr) before, during and after 1 min vagal stimulation at 80 Hz (indicated by horizontal bar) in a KF-lesioned rat. C, mean responses (n = 3) in f and ∫Phr before and after KF lesions. Post-stimulus rebound in f was abolished after KF lesion (P > 0.1). See Fig. 2 legend for notations. Similar responses were obtained in animals (n = 2) after bilateral KF and unilateral PB lesions.
Figure 7C shows the mean responses in f and ∫Phr in all KF-lesioned animals during and after vagal stimulation. The response patterns were similar to those in the MK-801 group (60 min after drug administration) although the initial decrease in f was greater in the MK-801 group than the KF-lesioned group. The recovery of the HB reflex in f was fitted equally well (F test) by a mono-exponential (τ = 19.9 ± 6.9 s) and bi-exponential model (τ1 = 13.8 ± 5.3 s; τ2 = 50.7 ± 3.2 s), with the latter being rejected by the Akaike test (Akaike, 1974). These findings confirm that PR (and perhaps also the fast component of HB recovery) responses were mediated by the input from KF nucleus to the respiratory pattern generator in the medulla.
DISCUSSION
Validity of results
For simplicity, our analysis was focused on the adaptation of the responses in f and ∫Phr during and after vagal stimulation although strictly speaking the HB reflex is concerned primarily with the modulation of TI and TE by vagal SAR input. For the present purpose, however, changes in f always correlated inversely with changes in TE (see Figs 1, 4A and 7A).
Although the resting respiratory rhythm was altered by NMDA receptor blockade and pontine lesion after vagotomy, such changes in the breathing pattern are unlikely to have any direct influence on the desensitization response. Neither MK-801 administration nor pontine lesion abolished the HB reflex or its habituation, and there is no reason to expect that a change in ventilatory pattern induced by these interventions per se would selectively affect PR. Indeed, MK-801 and pontine lesion had opposite effects on respiratory frequency (cf. Fig. 4a and 7A) and yet PR was abolished in both cases.
Although non-associative learning is generally defined on the basis of whole-animal behaviour irrespective of the specific afferent fibres (Thompson & Spencer, 1966; Groves & Thompson, 1970; Siniaia & Silakov, 1990; Poon, 1996), from a functional perspective it is of interest to distinguish the afferent contributions to such behaviour. The relatively low stimulation intensity used in this study precluded the activation of pulmonary C-fibres, which have significantly higher activation thresholds than SAR fibres in the rat (Bergren & Peterson, 1993). Although the ranges of activation threshold for SAR and RAR fibres overlap to some extent, the latter are rare in the rat (Bergren & Peterson, 1993). Indeed, vagal stimulation in the rat at similar intensities as those used in the present study was found to elicit a response in phrenic discharge that was similar to the HB reflex resulting from lung inflation (Hayashi & McCrimmon, 1996). The reported effect of vagal stimulation on phrenic activity was consistent with the activation of SAR fibres rather than RAR or C-fibres. Although expiratory-related activity in the pharyngeal branches of the vagus nerve may be activated by vagal stimulation (Hayashi & McCrimmon, 1996), these fibres innervate mainly the pharyngeal constrictors and are concerned primarily with physiological and behavioural functions of the upper airway such as swallowing, vomiting, coughing and vocalization (Grélot et al. 1989; Siniaia & Miller, 1996), which are unrelated to the HB reflex. Most importantly, similar biphasic adaptation patterns were observed when the HB reflex was elicited by lung inflation instead of electrical vagal stimulation (Stanley et al. 1975). Thus, it may be concluded that the HB reflex and the associated habituation-desensitization of the reflex in central sites were induced primarily by activation of SAR fibres instead of RAR or C-fibres.
Vagal habituation and pontine desensitization in HB reflex
The recovery of the HB reflex and the PR response presently observed in rats confirm previous reports of similar adaptations of the respiratory rhythm on sustained vagal stimulation or lung inflation in dogs (Stanley et al. 1975), cats (Younes & Polacheck, 1985) and rabbits (Karczewski & Romaniuk, 1980). In addition, our results showed that vagal stimulation evoked adaptive changes simultaneously in the vagal and pontine pathways that act to mitigate the HB reflex. Since pontine stimulation and vagal stimulation elicited similar direct and adaptive responses in the respiratory rhythm (Younes et al. 1987), the vagal and pontine components of the adaptation in HB reflex can be ascribed to similar decreases in the efficacy of neurotransmission in the vagal and pontine pathways, namely, habituation and desensitization, respectively. It should be noted, however, that the pontine desensitization of HB reflex could also result from enhancement of neurotransmission in other pontine pathways that counteract the HB reflex, such as those reported recently (Mutolo et al. 1998). A hypothetical model showing the adaptation of vagal HB reflex due to vagal habituation and pontine desensitization is depicted in Fig. 8.
Figure 8. Schematic diagram showing hypothetical model of central adaptations to vagal Hering-Breuer reflex.
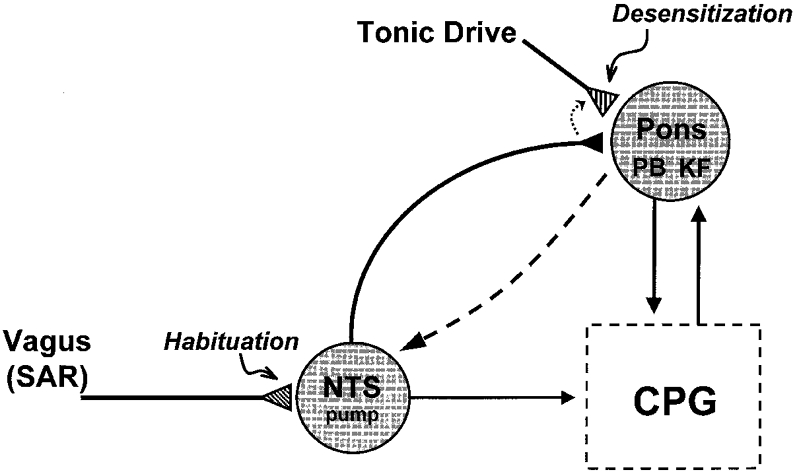
Repetitive vagal stimulation elicits synaptic accommodation of glutamatergic (mainly non-NMDA receptor mediated) neurotransmission in NTS pump cells (Zhou et al. 1997; Zhou & Poon, 2000), resulting in habituation of HB reflex. This process may be influenced by pontine modulation of neurotransmission in NTS (long dashed arrow) (Mifflin & Felder, 1990). The vagal input in turn desensitizes pontine neurotransmission (mainly NMDA receptor mediated) via neuronal projection of pump cells to pontine pneumotaxic centre including the parabrachialis (PB) and Kölliker-Fuse (KF) nuclei (Ezure et al. 1998). Upon removal of vagal stimulus the pontine pathway is re-sensitized and the absence of vagal volume-related input from slowly adapting receptors (SAR) is compensated for by a tonic pontine drive that ensures proper inspiratory-expiratory phase switching in the respiratory central pattern generator (CPG). Thus, pontine pathway serves as a backup or fail-safe surrogate mechanism for the vagal pathway and will ‘kick in’ only when vagal volume feedback is suppressed. At the cellular level, desensitization of the pontine pathway could result from presynaptic disfacilitation or heterosynaptic depression (short dotted arrow) (Poon et al. 1999) of an excitatory tonic pontine drive (as shown). Similar desensitization effects could also result from presynaptic facilitation or heterosynaptic potentiation of an inhibitory drive (not shown). In this event, the output of the adapting pontine neuron must be inverted by another inhibitory interneuron in order to preserve the excitatory throughput of the pontine pathway that effects the inspiratory-expiratory off-switch. Alternatively, pontine desensitization of the HB reflex may also result from vagally induced enhancement of neurotransmission in other pontine pathways that counteract the HB reflex, such as those reported recently (Mutolo et al. 1998).
Extracellular recording studies revealed that neurons in the pontine pneumotaxic centre exhibit spontaneous firing in synchrony with the respiratory rhythm but the activity is suppressed by vagal SAR input (Feldman & Gautier, 1976; Wang et al. 1993; Dick et al. 1994). This observation is consistent with the present study which demonstrated that vagal stimulation may desensitize neural transmission in the pontine pathway. On the other hand, pontine input may modulate neural transmission of certain peripheral inputs (particularly baroreceptor input) within NTS (Mifflin & Felder, 1990). However, this mechanism will take effect only when the primary afferent pathway is active. Because PR persisted after the vagal input was removed, it was not simply the result of direct vagal inhibition of pontine respiratory-related neurons or pontine enhancement of vagal neurotransmission in NTS. Nevertheless, it is possible that pontine modulation of NTS neurotransmission may contribute to the habituation of HB reflex elicited by vagal SAR input. Further study is necessary to elucidate the interaction of vagal and pontine pathways and their specific contributions to the adaptation of the HB reflex.
The role of NMDA receptors in respiratory rhythmogenesis has received considerable attention. Because an apneustic respiratory pattern similar to that resulting from pontine pneumotaxic lesion may be produced by systemic administration of MK-801 (Foutz et al. 1989; Connelly et al. 1992) or microinjection of NMDA-receptor antagonists in the pontine pneumotaxic centre (Fung et al. 1994; Ling et al. 1994), it has been suggested that the NMDA receptor mediates the pontine pneumotaxic input to the respiratory pattern generator (Foutz et al. 1989; Borday et al. 1998). In agreement with these previous findings, the present study showed that MK-801 altered the respiratory rhythm in bivagotomized rats within 5 min of its systemic administration. However, despite a similar tendency toward an apneustic pattern the respiratory rhythms and the initial responses to vagal stimulation were not exactly the same after MK-801 administration and pontine pneumotaxic lesion (cf. Fig. 4a and 7A) in the vagotomized rat. Thus, MK-801 may exert influence not only on the pontine pathway but perhaps other targets in the respiratory pattern generator such as peripheral chemoreceptor input to NTS (Aylwin et al. 1997) or certain respiratory-related neurons in the medulla (Pierrefiche et al. 1991; Takeda & Matsumoto, 1998).
In addition, our results showed that the effect of MK-801 on the respiratory pattern was accompanied by an abolishment of the PR in the HB reflex – an effect that was also obtained after pontine pneumotaxic lesion. Therefore, desensitization in the pontine pathway is dependent on NMDA receptors whereas habituation in the vagal pathway is not. The latter result is consistent with the NMDA receptor-independent synaptic accommodation found in type I cells of rat NTS in vitro (Zhou et al. 1997; Zhou & Poon, 2000). However, MK-801 also resulted in profound, time-dependent changes in the adaptations of the responses in f and ∫Phr during vagal stimulation and a steady state was not established until 60 min after MK-801 administration. The delayed effects suggest that the recovery of the HB reflex is influenced by some slowly adapting processes in the respiratory pattern generator that are NMDA receptor dependent.
Habituation and desensitization in mammalian CNS
Our results demonstrate a novel pairing of dual non-associative learning processes – habituation and desensitization – that coexist in the S-R and parallel state system signal pathways in the mammalian brain. The habituation (slow component of recovery) of the HB reflex conformed to the classic criteria of habituation set forth by Thompson and colleagues (Thompson & Spencer, 1966; Groves & Thompson, 1970), and its characteristics manifested in vivo (in terms of response time constant and its insensitivity to NMDA receptor blockade) conformed with the synaptic accommodation in the corresponding vagal-NTS pathway revealed in vitro. Although habituation and, to a lesser extent, sensitization are commonly found in many sensory or sensorimotor pathways of the mammalian and invertebrate nervous systems, the present findings give the first demonstrable example of habituation- desensitization dual processes in the mammalian brain. In particular, it is possible that activity-dependent phasic adaptations in other cardiorespiratory reflexes – such as baroreflex resetting (Heesch & Barron, 1992) and hypoxic respiratory depression (Bisgard & Neubauer, 1995) – are all similar manifestations of habituation in corresponding signal pathways engendered by synaptic accommodation in NTS or other brainstem neurons, but demonstration of such correlations at the behavioural and cellular levels is currently lacking. Likewise, it is possible that the pontine-mediated (ventrolateral pons) decline of respiratory frequency following sustained hypoxia or carotid sinus nerve stimulation (Hayashi et al. 1993; Coles & Dick, 1996) may represent a similar manifestation of desensitization, although it is not clear whether such post-stimulus decline is secondary to (or acts independently of) habituation in the primary signal pathway.
The differing forms of dual non-associative learning processes (habituation-sensitization vs. habituation-desensitization) in parallel signal pathways may have important functional significance. In mammalian or invertebrate nervous systems, habituation and sensitization are usually associated with innocuous and noxious stimuli, respectively. Examples of the latter are the startle reflex in the mammalian brain (Davis et al. 1982) and the gill- and siphon-withdrawal reflexes elicited by tail or mantle shocks in the invertebrate Aplysia (Hawkins et al. 1998). Thus, habituation may alleviate the animal of the burden of a mundane physiological signal, whereas sensitization may alert the animal of the impending onslaught of a non-physiological challenge. By contrast, vagal SAR input and pontine pneumotaxic input are two complementary signalling pathways that contribute importantly to the regulation of the normal respiratory rhythm (for review, see Bianchi et al. 1995). Indeed, simultaneous interruption of vagal and pontine inputs may lead to apneusis (Fig. 7a) whereas excessive vagal stimulation may cause apnoea via the HB reflex (Fig. 1) - both events being potentially lethal. In this regard, the pontine pneumotaxic pathway may be viewed as a ‘back-up’ system that provides a fail-safe mechanism for respiratory rhythmogenesis by averting apneusis in the event of a breakdown of vagal SAR feedback. Likewise, a coupled habituation-desensitization mechanism in parallel vagal-pontine pathways may protect the animal against the possibility of respiratory arrest in the presence of an abnormally large vagal input. Such complementary dual learning processes in redundant signal pathways may therefore contribute importantly to the maintenance of normal respiratory rhythm over a wide dynamic range of vagal SAR input. This notion is consistent with the proposition that synaptic depression (accommodation) may be an important rhythmogenic mechanism in oscillatory neural networks (O'Donovan & Rinzel, 1997).
Although it is generally accepted that the habituation response in invertebrates is mediated primarily by synaptic depression (Stopfer & Carew, 1996; Cohen et al. 1997), the mechanism of habituation in the mammalian central nervous system (CNS) is not certain. The present results provide some evidence that habituation in one mammalian CNS structure may be linked to synaptic accommodation in a corresponding sensory pathway. On the other hand, the cellular mechanism of desensitization is less clear. Since pontine desensitization persisted in the form of a PR after the vagal input was terminated, its underlying mechanism must be different from that of habituation in the vagal pathway. A more plausible mechanism for the desensitization response is a vagally induced heterosynaptic disfacilitation of pontine input – which is analogous to the heterosynaptic facilitation found in some invertebrates (Stopfer & Carew, 1996). Further studies are necessary to elucidate the cellular basis of such coupled habituation-desensitization processes in the mammalian brain.
Acknowledgments
D.L.Y. is recipient of a National Science Foundation pre-doctoral Fellowship. This work was supported in part by National Heart, Lung and Blood Institute grants HL52925 and HL60064 and Office of Naval Research grants N00014-95-1-0414 and N00014-95-0863.
References
- Akaike H. A new look at the statistical model identification. IEEE Transactions on Automatic Control. 1974;19:716–723. [Google Scholar]
- Aylwin ML, Horowitz JM, Bonham AC. NMDA receptors contribute to primary visceral afferent transmission in the nucleus of the solitary tract. Journal of Neurophysiology. 1997;77:2539–2548. doi: 10.1152/jn.1997.77.5.2539. [DOI] [PubMed] [Google Scholar]
- Bassareo V, Di Chiara G. Differential influence of associative and nonassociative learning mechanisms on the responsiveness of prefrontal and accumbal dopamine transmission to food stimuli in rats fed ad libitum. Journal of Neuroscience. 1997;17:851–861. doi: 10.1523/JNEUROSCI.17-02-00851.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergren DR, Peterson DF. Identification of vagal sensory receptors in the rat lung: are there subtypes of slowly adapting receptors? The Journal of Physiology. 1993;464:681–698. doi: 10.1113/jphysiol.1993.sp019657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi A, Denavit-Saubié M, Champagnat J. Central control of breathing in mammals: circuitry, membrane properties, and neurotransmitters. Physiological Reviews. 1995;75:1–45. doi: 10.1152/physrev.1995.75.1.1. [DOI] [PubMed] [Google Scholar]
- Bisgard GE, Neubauer JA. Peripheral and central effects of hypoxia. In: Dempsey A, Pack AI, editors. Regulation of Breathing, Lung Biology in Health and Disease. 2. II. New York: Dekker; 1995. pp. 617–668. 79. [Google Scholar]
- Borday V, Foutz AS, Nordholm L, Denavit-Saubié M. Respiratory effects of glutamate receptor antagonist in neonate and adult mammals. European Journal of Pharmacology. 1998;348:235–246. doi: 10.1016/s0014-2999(98)00160-5. [DOI] [PubMed] [Google Scholar]
- Breuer J. Self-steering of respiration through the nerves vagus. In: Porter R, editor. Breathing: Hering-Breuer Centenary Symposium, 1970. London: Churchill; 1868. pp. 365–394. [Google Scholar]
- Caille D, Vibert JF, Hugelin A. Apneusis and apnea after parabrachial or Kölliker-Fuse N. lesion; influence of wakefulness. Respiration Physiology. 1981;45:79–95. doi: 10.1016/0034-5687(81)90051-7. [DOI] [PubMed] [Google Scholar]
- Cohen H, Cohen B, Raphan T, Waespe W. Habituation and adaptation of the vestibuloocular reflex: a model of differential control by the vestibulocerebellum. Experimental Brain Research. 1992;90:526–538. doi: 10.1007/BF00230935. [DOI] [PubMed] [Google Scholar]
- Cohen TE, Kaplan SW, Kandel ER, Hawkins RD. A simplified preparation for relating cellular events to behavior: mechanisms contributing to habituation, dishabituation, and sensitization of the Aplysia gill-withdrawal reflex. Journal of Neuroscience. 1997;17:2886–2899. doi: 10.1523/JNEUROSCI.17-08-02886.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles SK, Dick TE. Neurons in the ventrolateral pons are required for post-hypoxic frequency decline in rats. The Journal of Physiology. 1996;497:79–94. doi: 10.1113/jphysiol.1996.sp021751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connelly CA, Otto-Smith MR, Feldman JL. Blockade of NMDA receptor-channels by MK-801 alters breathing in adult rats. Brain Research. 1992;596:99–110. doi: 10.1016/0006-8993(92)91537-o. [DOI] [PubMed] [Google Scholar]
- Davis M, Parisi T, Gendelman DS, Tischler M, Kenhe JH. Habituation and sensitization of startle reflex elicited electrically from the brainstem. Science. 1982;218:688–690. doi: 10.1126/science.7134967. [DOI] [PubMed] [Google Scholar]
- Dick TE, Bellingham MC, Richter DW. Pontine respiratory neurons in anaesthetized cats. Brain Research. 1994;636:259–269. doi: 10.1016/0006-8993(94)91025-1. [DOI] [PubMed] [Google Scholar]
- Egger MD. Sensitization and habituation of dorsal horn cells in cats. The Journal of Physiology. 1978;279:153–166. doi: 10.1113/jphysiol.1978.sp012337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezure K, Tanaka I, Miyazaki M. Pontine projections of pulmonary slowly adapting receptor relay neurons in the cat. NeuroReport. 1998;9:411–414. doi: 10.1097/00001756-199802160-00010. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Gautier H. Interaction of pulmonary afferents and pneumotaxic center in control of respiratory pattern in cats. Journal of Neurophysiology. 1976;39:31–44. doi: 10.1152/jn.1976.39.1.31. [DOI] [PubMed] [Google Scholar]
- Foutz AS, Champagnat J, Denavit-Saubié M. Involvement of N-methyl-D-aspartate (NMDA) receptors in respiratory rhythmogenesis. Brain Research. 1989;500:199–208. doi: 10.1016/0006-8993(89)90314-4. [DOI] [PubMed] [Google Scholar]
- Fung M-L, Wang W, St John WM. Involvement of pontile NMDA receptors in inspiratory termination in rat. Respiration Physiology. 1994;96:177–188. doi: 10.1016/0034-5687(94)90125-2. [DOI] [PubMed] [Google Scholar]
- Grélot M, Barillot JC, Bianchi AL. Pharyngeal motoneurons: respiratory related activity and responses to laryngeal afferents in the decerebrated cat. Experimental Brain Research. 1989;78:336–344. doi: 10.1007/BF00228905. [DOI] [PubMed] [Google Scholar]
- Groome LJ, Watson JE, Dykman RA. Heart rate changes following habituation testing of the motor response in normal human fetuses. Early Human Development. 1994;36:69–77. doi: 10.1016/0378-3782(94)90034-5. [DOI] [PubMed] [Google Scholar]
- Groves PM, Thompson RF. Habituation: a dual-process theory. Physiological Reviews. 1970;77:419–450. doi: 10.1037/h0029810. [DOI] [PubMed] [Google Scholar]
- Hawkins RD, Cohen TE, Greene W, Kandel ER. Relationships between dishabituation, sensitization, and inhibition of the gill- and siphon-withdrawal reflex in Aplysia californica: effects of response measure, test time, and training stimulus. Behavioral Neuroscience. 1998;112:24–38. doi: 10.1037//0735-7044.112.1.24. [DOI] [PubMed] [Google Scholar]
- Hayashi F, Coles SK, Bach KB, Mitchell GS, Mccrimmon DR. Time-dependent phrenic nerve responses to carotid afferent activation; intact vs. decerebellate rats. American Journal of Physiology. 1993;265:R811–819. doi: 10.1152/ajpregu.1993.265.4.R811. [DOI] [PubMed] [Google Scholar]
- Hayashi F, Mccrimmon DR. Respiratory motor responses to cranial nerve stimulation in rats. American Journal of Physiology. 1996;271:R1054–1062. doi: 10.1152/ajpregu.1996.271.4.R1054. [DOI] [PubMed] [Google Scholar]
- Heesch CM, Barron KW. Is there a central nervous system component to acute baroreflex resetting in rats? American Journal of Physiology. 1992;262:H503–510. doi: 10.1152/ajpheart.1992.262.2.H503. [DOI] [PubMed] [Google Scholar]
- Hering E. Self-steering of respiration through the nerves vagus. In: Porter R, editor. Breathing: Hering-Breuer Centenary Symposium, 1970. London: Churchill; 1868. pp. 359–364. [Google Scholar]
- Kandel ER. A Cell-Biological Approach to Learning. Bethesda, MD, USA.: Society for Neuroscience; 1978. [Google Scholar]
- Karczewski WA, Romaniuk JR. Neural control of breathing and central nervous system plasticity. Acta Physiologica Polonica. 1980;31(suppl. 20):1–10. [PubMed] [Google Scholar]
- Ling L, Karius DR, Speck DF. Role of N-methyl-D-aspartate receptors in the pontine pneumotaxic mechanism in the cat. Journal of Applied Physiology. 1994;76:1138–1143. doi: 10.1152/jappl.1994.76.3.1138. [DOI] [PubMed] [Google Scholar]
- Mifflin SW, Felder RB. Synaptic mechanisms regulating cardiovascular afferent signals to solitary tract nucleus. American Journal of Physiology. 1990;28:H653–661. doi: 10.1152/ajpheart.1990.259.3.H653. [DOI] [PubMed] [Google Scholar]
- Miles R. Frequency dependence of synaptic transmission in nucleus of the solitary tract in vitro. Journal of Neurophysiology. 1986;55:1076–1090. doi: 10.1152/jn.1986.55.5.1076. [DOI] [PubMed] [Google Scholar]
- Mutolo D, Bongianni F, Pantaleo T. Respiratory responses to chemical stimulation of the parabrachial nuclear complex in the rabbit. Brain Research. 1998;807:182–186. doi: 10.1016/s0006-8993(98)00775-6. [DOI] [PubMed] [Google Scholar]
- O'donovan MJ, Rinzel J. Synaptic depression: dynamic regulator of synaptic communication with varied functional roles. Trends in Neurosciences. 1997;20:431–433. doi: 10.1016/s0166-2236(97)01124-7. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Co-ordinates. 2. New York: Academic Press; 1986. [Google Scholar]
- Peterzel DH. Individual differences in the visual attention of human infants: further evidence for separate sensitization and habituation. Developmental Psychobiology. 1993;26:207–218. doi: 10.1002/dev.420260404. [DOI] [PubMed] [Google Scholar]
- Pierrefiche O, Schmid K, Foutz AS, Denavit-Saubié M. Endogenous activation of NMDA and non-NMDA glutamate receptors on respiratory neurons in cat medulla. Neuropharmacology. 1991;30:429–440. doi: 10.1016/0028-3908(91)90003-t. [DOI] [PubMed] [Google Scholar]
- Pilz PKD, Schnitzler HU. Habituation and sensitization of the acoustic startle response in rats: amplitude, threshold, and latency measures. Neurobiology of Learning and Memory. 1996;66:67–79. doi: 10.1006/nlme.1996.0044. [DOI] [PubMed] [Google Scholar]
- Platt B, Withington DJ. Response habituation in the superficial layers of the guinea-pig superior colliculus in vitro. Neuroscience Letters. 1997;221:153–156. doi: 10.1016/s0304-3940(96)13314-0. [DOI] [PubMed] [Google Scholar]
- Poon C-S. Synaptic plasticity and respiratory control. In: Khoo MCK, editor. Bioengineering Approaches to Pulmonary Physiology and Medicine. New York: Plenum; 1996. pp. 93–113. chap. 6. [Google Scholar]
- Poon C-S, Siniaia M, Young DL, Eldridge FL. Short-term potentiation of carotid chemoreflex: an NMDAR-dependent neural integrator. NeuroReport. 1999;10:2261–2265. doi: 10.1097/00001756-199908020-00007. [DOI] [PubMed] [Google Scholar]
- Siniaia MS, Miller AD. Vestibular effects on upper airway musculature. Brain Research. 1996;736:160–164. doi: 10.1016/0006-8993(96)00674-9. [DOI] [PubMed] [Google Scholar]
- Siniaia MS, Pinchuck DY, Bogdanov OV, Tobias TV. Compensatory plasticity of the brain under conditions of its injury. Neuroscience and Behavioral Physiology. 1992;22:543–549. doi: 10.1007/BF01185446. [DOI] [PubMed] [Google Scholar]
- Siniaia MS, Silakov VL. Plasticity of the Visceral Analyzer. Leningrad, Russia: Nayka; 1990. [Google Scholar]
- Siniaia MS, Yazykov VV. Habituation of the blood pressure and heart rate frequency on prolonged stimulation of afferent visceral nerves. The Journal of Physiology of the USSR. 1981;67:1541–1548. [PubMed] [Google Scholar]
- Stanley NN, Altose MD, Cherniack NS, Fishman AP. Changes in strength of lung inflation during prolonged inflation. Journal of Applied Physiology. 1975;38:474–480. doi: 10.1152/jappl.1975.38.3.474. [DOI] [PubMed] [Google Scholar]
- Stopfer M, Carew TJ. Heterosynaptic facilitation of tail sensory neuron synaptic transmission during habituation in tail-induced tail and siphon withdrawal reflexes of Aplysia. Journal of Neuroscience. 1996;16:4933–4948. doi: 10.1523/JNEUROSCI.16-16-04933.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda M, Matsumoto S. Effects of NMDA and non-NMDA receptor antagonists on the medullary inspiratory neuronal activity during spontaneous augmented breaths in anesthetized rats. Brain Research. 1998;781:194–201. doi: 10.1016/s0006-8993(97)01249-3. [DOI] [PubMed] [Google Scholar]
- Teyler TJ, Alger BE. Monosynaptic habituation in vertebrate forebrain: the dentate gyrus examined in vitro. Brain Research. 1976;115:414–425. doi: 10.1016/0006-8993(76)90358-9. [DOI] [PubMed] [Google Scholar]
- Thompson RF, Spencer WA. Habituation: A model phenomenon for the study of neuronal substrates of behavior. Psychological Review. 1966;73:16–46. doi: 10.1037/h0022681. [DOI] [PubMed] [Google Scholar]
- Vardhan A, Kachroo A, Sapru HN. Excitatory amino acid receptors in commissural nucleus of the NTS mediate carotid chemoreceptor responses. American Journal of Physiology. 1993;264:R41–50. doi: 10.1152/ajpregu.1993.264.1.R41. [DOI] [PubMed] [Google Scholar]
- Wang W, Fung M-L, St John WM. Pontile regulation of ventilatory activity in the adult rat. Journal of Applied Physiology. 1993;74:2801–2811. doi: 10.1152/jappl.1993.74.6.2801. [DOI] [PubMed] [Google Scholar]
- Wilson DA. Habituation of odor responses in the rat anterior piriform cortex. Journal of Neurophysiology. 1998;79:1425–1440. doi: 10.1152/jn.1998.79.3.1425. [DOI] [PubMed] [Google Scholar]
- Younes M, Baker J, Remmers JE. Temporal changes in effectiveness of an inspiratory inhibitory electrical pontine stimulus. Journal of Applied Physiology. 1987;62:1502–1512. doi: 10.1152/jappl.1987.62.4.1502. [DOI] [PubMed] [Google Scholar]
- Younes M, Polacheck J. Central adaptation to inspiratory-inhibiting expiratory prolonging vagal input. Journal of Applied Physiology. 1985;59:1072–1084. doi: 10.1152/jappl.1985.59.4.1072. [DOI] [PubMed] [Google Scholar]
- Zhang W, Mifflin S. Excitatory amino acid receptors within NTS mediate arterial chemoreceptor reflexes in the rat. American Journal of Physiology. 1993;265:H770–773. doi: 10.1152/ajpheart.1993.265.2.H770. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Champagnat J, Poon C-S. Phasic and long-term depression in brainstem nucleus tractus solitarius neurons: differing roles of AMPA receptors desensitization. Journal of Neuroscience. 1997;17:5349–5356. doi: 10.1523/JNEUROSCI.17-14-05349.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Poon C-S. Field potential analysis of synaptic transmission in spiking neurons in a sparse and irregular neuronal structure in vitro. Journal of Neuroscience Methods. 2000;94:193–203. doi: 10.1016/s0165-0270(99)00144-2. [DOI] [PubMed] [Google Scholar]


