Abstract
A role for P2 purinoceptors in the chemosensory response of respiratory neurones localised in the ventrolateral medulla to changes in arterial CO2 levels was investigated in the anaesthetised rat. Extracellular recordings were made from different classes of respiratory neurone and the effects of P2 receptor blockade on CO2-evoked changes in activity investigated.
Increasing inspired CO2 excited 85 % of inspiratory neurones in the pre-Bötzinger complex. In all cases, CO2-evoked excitation was blocked by ionophoretic application of the P2receptor antagonists suramin (0·02 M) and pyridoxal-phosphate-6-azophenyl-2′,4′-disulphonic acid (PPADS; 100 μM), but not the adenosine receptor antagonist 8-phenyltheophylline (8-PT; 100 μM). Suramin and PPADS often reduced ongoing activity, and blocked the excitatory effects of ATP. Inspiratory neurones were also excited by the P2X receptor agonist αβ-methyleneATP, suggesting a specific role for P2X receptors.
Sixty-six per cent of pre-inspiratory neurones were also excited by CO2. This effect was reduced or abolished by prior application of P2 receptor antagonists. Although post-inspiratory and expiratory neurones were excited by increasing levels of CO2, and also by ionophoretically applied ATP, the CO2-evoked effects were unaffected by P2 receptor blockade.
We suggest that ATP, possibly acting via P2X purinoceptors localised within the ventral respiratory group, is involved in central chemoreception. Specifically, these distinctive CO2-P2X-mediated actions were observed only in inspiratory neurones (incrementing inspiratory neurones and pre-inspiratory neurones), which appear to have purinoceptors with pH sensitivity that can account for the actions of CO2 in modifying ventilatory activity.
It is a century since Haldane described that minute ventilation in man is exquisitely sensitive to changes in the partial pressure of carbon dioxide (PCO2) in the air breathed. The increase in minute ventilation evoked by increasing inspired levels of CO2 results in a relatively stable PCO2 in the alveolar air, and therefore arterial blood and brain extracellular fluid (Haldane & Priestley, 1935). The level of PCO2 in arterial blood (Pa,CO2) provides one of the principal drives to ventilation. Pa,CO2 is monitored by two sets of chemoreceptors – the peripheral chemoreceptors and central chemosensitive elements in the medulla oblongata (Loeschcke, 1982; Nattie, 1995; Daly, 1997; Wang et al. 1998). Despite the fact that the central ‘chemoreceptors’ provide tonic excitatory drive to the central respiratory network, to date the cellular mechanisms mediating an increase in central respiratory drive in response to high levels of CO2 remain unresolved. It is widely accepted that CO2 itself does not have a direct effect on breathing, rather that the changes in H+ concentration, which accompany changes in Pa,CO2, mediate the central chemosensory mechanism. It has been suggested that changes in extracellular pH that follow alterations in Pa,CO2 are important in this mechanism (Cherniack, 1993), although this is controversial since evidence also exists to indicate a role for changes in intracellular pH in chemoreception (Ritucci et al. 1998). Whilst the number of regions of the lower brainstem reputed to be important in central chemoreception continues to increase, and now includes the nucleus tractus solitarii, locus coeruleus, medullary raphe and the ventrolateral medulla (VLM) (Loeschcke, 1982; Nattie, 1995; Wang et al. 1998; Oyamada et al. 1998), it is clear that the ventrolateral part of the brainstem (VLM) is particularly significant in mediating CO2-evoked respiratory responses. The VLM contains an extensive network of respiratory neurones, the ventral respiratory group, involving the pre-Bötzinger and Bötzinger complexes (Richter, 1996). Recent in vitro studies provide compelling evidence that the respiratory neurones in this region of the brainstem are directly affected by changes in CO2 and pH (Kawai et al. 1996). The responses reported were resistant to tetrodotoxin, low Ca2+ and high Mg2+ or Cd2+, implying that these actions are mediated by intrinsic membrane or intracellular mechanisms (Kawai et al. 1996).
For several years it has been known that ATP can act as a neurotransmitter in the central nervous system (Edwards et al. 1992). Recent work from our laboratory demonstrated that exogenously applied ATP has a marked modulatory action on the control of central respiratory activity within the ventral respiratory group of neurones (Ralevic et al. 1998). Further, blockade of P2 receptors localised in this region had remarkable effects on respiratory drive, markedly reducing the sensitivity of the central respiratory network to changes in CO2 (Thomas et al. 1999). In this context, a total of seven P2X receptor subtypes have now been cloned (Ralevic & Burnstock, 1998), and it is known that certain members of this family are highly sensitive to changes in pH (King et al. 1996; Stoop et al. 1997). Therefore, we hypothesised that CO2-evoked changes in breathing are mediated by actions that can be attributed to pH-sensitive P2 receptors located within the ventral respiratory group. We have addressed this possibility in this study using in vivo recordings from single respiratory neurones in this region of the brainstem and ionophoretic techniques.
METHODS
General
Twenty-six male Sprague-Dawley rats (270–310 g) were prepared for extracellular recordings from the medulla oblongata as described previously (Thomas & Spyer, 1998). Briefly, anaesthesia was induced and maintained with pentobarbitone sodium (Sagatal, 60 mg kg −1i.p., then 10 mg kg−1 h−1i.v.). The depth of anaesthesia was assessed by the stability of blood pressure, heart rate and phrenic neurogram, and the minimal effects on cardiovascular variables of a pinch to the paw. Arterial blood pressure was recorded via a catheter placed in the femoral artery and the femoral vein was cannulated for the administration of anaesthetic. The animal was then placed in a stereotaxic frame and ventilated artificially (after paralysis with gallamine triethiodide, 10 mg kg−1i.v., then 1–2 mg kg−1 h−1i.v.) with O2-enriched air using a positive pressure ventilator (Harvard rodent ventilator, model 683) with a tidal volume of 1.2-1.5 ml and a ventilator frequency similar to spontaneous frequency. During artificial ventilation, the depth of anaesthesia was assessed by the stability of cardiovascular and respiratory variables. End-tidal levels of CO2 were monitored on-line using a fast-response CO2 analyser (Analytical Development, Herts, UK) and maintained at 3–5 % by altering tidal volume and ventilator frequency. Arterial partial pressures of CO2 and pH were monitored regularly using a Corning pH/blood gas analyser (model 283) to ensure they remained within physiological limits (except where the protocol dictated). However, levels of arterial PO2 were kept > 120 mmHg in order to ensure that changes in peripheral chemoreceptor drive were minimal. In addition, in six animals, the vagi and aortic nerves were cut, and the carotid sinus nerves sectioned at the junction with the glossopharyngeal trunk. Phrenic nerve activity was recorded as an indication of central respiratory drive. Activity was amplified (×10000), filtered (500–1500 Hz) and rectified and smoothed (τ= 100 ms). In several animals, rectal temperature was maintained at 37–38°C. An occipital craniotomy was performed and the cerebellum aspirated to expose the dorsal surface of the medulla oblongata.
Protocol
Extracellular single-unit recordings and ionophoretic application of drugs were made using 5-barrelled microelectrodes (tip diameter, 1–2 μm, 2–10 MΩ), as described previously (Thomas & Spyer, 1998). The recording barrel contained 4 M sodium chloride and others contained L-glutamate (0.1 M), ATP (0.02 M) or αβ-methyleneATP (0.002 M), and suramin (0.02 M) or pyridoxal-phosphate-6-azophenyl-2′,4′-disulphonic acid (PPADS; 100 μM) or 8-phenyltheophylline (8-PT; 100 μM), and Pontamine Sky Blue dye. When not ejecting drug, a retaining current of 20–30 nA was applied to each barrel. Recordings were amplified (× 5000; Neurolog system NL104, Digitimer) and filtered (0.5-1.5 kHz). We recorded neurones from a location approximately 2.0-2.5 mm rostral to the obex, 1.5-2.5 mm lateral to the mid-line, and 2.5-3.0 mm ventral to the dorsal surface.
The effects of ATP applied in close proximity to recorded units using ionophoresis (Neurophore, Medical Systems) were determined. Once a steady firing rate was obtained after the removal of ATP, systemic hypercapnia was induced by increasing levels of inspired CO2 step-wise, up to a level of 8–10 % end-tidal CO2, for 1–2 min. The level of inspired CO2 was then returned to normal to re-establish physiological levels of arterial gases and pH, and once a steady firing rate had resumed suramin, PPADS or 8-PT was applied ionophoretically. The effects of ATP were then re-tested in the continued presence of the antagonist. Inspired levels of CO2 were increased to the same level as that obtained before application of the antagonist. In all cases the automatic current balancing on the Neurophore limited current artefacts, although in some experiments the minimal effects of vehicle ejection at the same pH as the applied drug were confirmed. Recording sites were marked by ionophoretic deposition of Pontamine Sky Blue dye (300 nA for 5–10 min). The animals were killed by an overdose of anaesthetic, the brain was removed, frozen, sectioned serially (40 μm) and counterstained with Neutral Red. The locations of the recording sites were then examined by light microscopy and mapped using a stereotaxic atlas (Paxinos & Watson, 1998).
Characterisation of respiratory neurones
Four classes of respiratory neurone were characterised according to established criteria in the adult rat (Schwarzacher et al. 1991). (1) Inspiratory neurones exhibited a steady increase in firing rate during the inspiratory phase of phrenic nerve activity. (2) Phase spanning expiratory-inspiratory, or pre-inspiratory, neurones showed discharge starting during stage 2 expiration that then increased steadily during inspiration. (3) Post-inspiratory neurones discharged with a rapid onset immediately at the end of the inspiratory phase and then showed a decrement in firing during the post-inspiratory phase. (4) Expiratory neurones had a delayed onset in their discharge after the post-inspiratory phase of the phrenic neurogram, and increasing activity during the expiratory phase.
Data analysis
Records of neuronal activity, phrenic nerve activity and end-tidal CO2 were analysed using Spike 2 software (Cambridge Electronic Design (CED), UK). Single-unit activity is represented as raw data or rate histograms, after discrimination of activity with a window discriminator (Digitimer D130). Peri-stimulus triggered histograms were derived using the peak of the phrenic nerve cycle as the stimulus over a period of ten to thirty-five cycles of the phrenic nerve. A change in baseline levels of > 10 % was considered significant.
Drugs
Drugs were dissolved freshly in either 1 mM saline or distilled water, and pH was adjusted by addition of 0.05 M NaOH or 0.5 M HCl. L-Glutamic acid (0.1 M, pH 8.0), ATP (0.02 M, pH 8.0) and αβ-methyleneATP (0.002 M, pH 8.0) were dissolved in distilled water. Suramin (0.02 M, pH 8.0), PPADS (100 μM, pH 4.0) and 8-PT (100 μM, pH 8.0) were dissolved in saline. Pontamine Sky Blue was dissolved in 0.5 M sodium acetate and distilled water.
RESULTS
We obtained data from 72 neurones, which showed rhythmic related respiratory activity in the Bötzinger and pre-Bötzinger complexes, localised caudal to the retrofacial nucleus in the medulla oblongata (Richter, 1996; Paxinos & Watson, 1998). Ventilation of the animals with a gas mixture containing an increased level of CO2 (2–4 %) resulted in an increase in end-tidal CO2 levels from 2.5-4.5 to 5–10 %. The evoked increase in central respiratory drive during hypercapnia was reflected by an increase in the amplitude and/or frequency of phrenic nerve bursts (Fig. 1, Fig. 2 and Fig. 3).
Figure 1. Extracellular recordings from inspiratory neurones in the pre-Bötzinger complex.
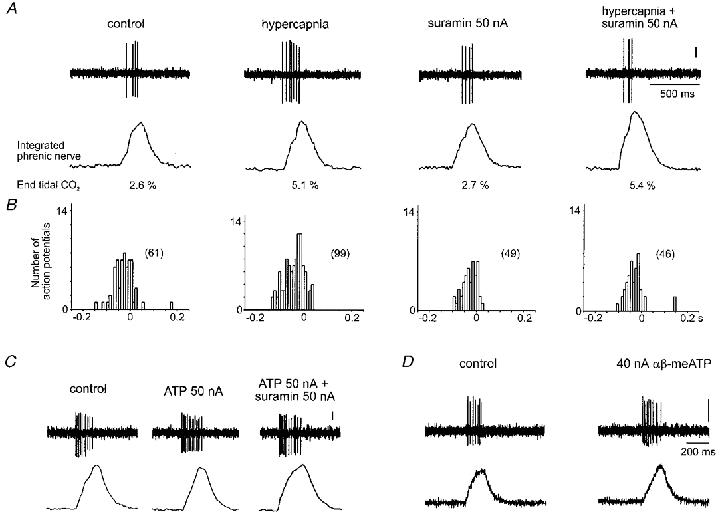
A, raw data illustrating the neuronal response to increased levels of inspired CO2 in the absence, and presence, of the P2 receptor antagonist suramin, applied using the currents shown. B, phrenic-triggered histograms taken from the neurone represented in A indicating responses to hypercapnia during control conditions and in the presence of suramin over 15 respiratory cycles (bin size = 0.01 s). The total number of action potentials is given in parentheses. C, response of a second inspiratory neurone to application of ATP and blockade of this by suramin. D, response of an inspiratory neurone to the application of αβ-methyleneATP (αβ-meATP). In all cases the vertical scale bar represents 100 μV.
Figure 2. Raw data illustrating that CO2-evoked excitation of inspiratory neurones is blocked by the P2 receptor antagonist PPADS.
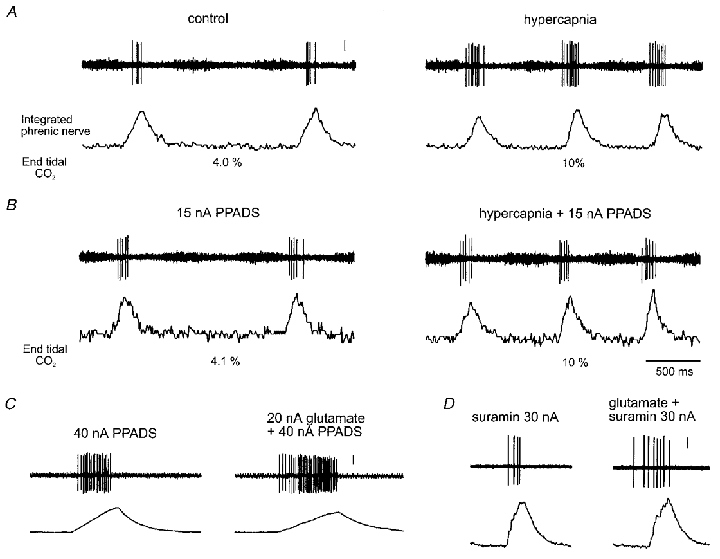
A and B, raw data illustrating the neuronal response to hypercapnia in the absence, and presence, of PPADS applied ionophoretically at the currents shown. C, response to L-glutamate in the presence of PPADS. D, response to L-glutamate in the presence of suramin (same neurone as in Fig. 1A). Vertical scale bars represent 100 μV.
Figure 3. CO2-evoked excitation of inspiratory neurones does not involve P1 purinoceptors.
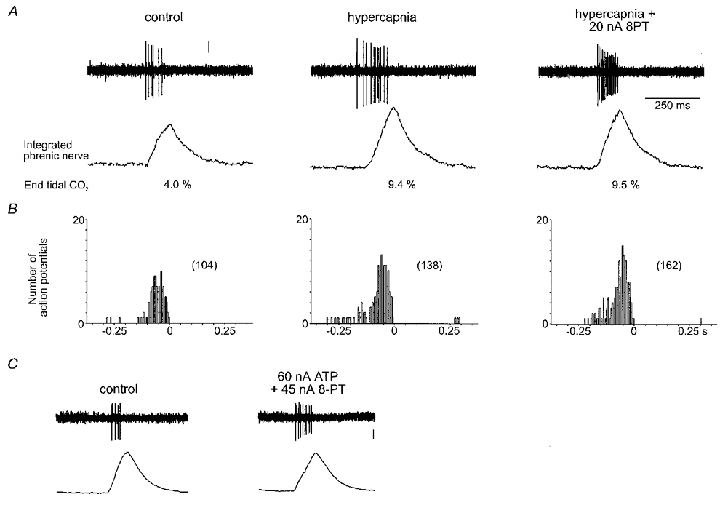
A, raw data illustrating the neuronal response to increased levels of inspired CO2 in the absence, and presence, of the adenosine receptor antagonist 8-PT applied using the current shown. B, phrenic-triggered histograms taken from the neurone represented in A indicating responses to hypercapnia during control conditions and in the presence of 8-PT over 12 respiratory cycles (bin size = 0.01 s). The total number of action potentials is given in parentheses. C, data illustrating that the excitatory responses to ATP were unaffected by blockade of P1 purinoceptors by 8-PT. Vertical scale bars represent 100 μV. The time calibration in A also applies to C.
Inspiratory neurones
Incrementing inspiratory neurones showed a marked sensitivity to changes in inspired CO2, with 85 % of these neurones responding rapidly to raised levels of inspired CO2 with an increase in their firing rate synchronous with the phrenic nerve burst (n= 28 neurones; Figs 1–3). This parallel increase in neuronal activity, and in the activity of the whole phrenic nerve, often persisted for several minutes after normocapnia had been resumed. Fifteen per cent of inspiratory neurones were either unaffected by changes in inspired CO2, or their firing rate fell slightly. In 100 % of cases tested, prior ionophoretic application of either of the P2 receptor antagonists suramin (20–90 nA) or PPADS (15–40 nA) markedly reduced, or abolished, the excitatory effects of CO2 on the activity of inspiratory neurones (n= 12). Examples of these effects are shown in Figs 1 and 2. The responses observed in peripherally denervated animals did not differ from those obtained from intact animals (data not shown; see also Thomas et al. 1999).
Ionophoretic application of suramin reduced baseline firing in six of nine neurones tested (-28 ± 7 %, mean ±s.e.m.). In the other three neurones baseline firing either showed no change or a slight increase. PPADS also reduced the baseline firing rate in four of six inspiratory neurones tested (-25 ± 10 %) and produced no change in the other two neurones. The doses of suramin and PPADS used were sufficient to antagonise the excitatory effects of ATP (20–60 nA) which we observed in 55 % of inspiratory neurones recorded (Fig. 1C). Wherever tested, the P2X receptor agonist αβ-methyleneATP also evoked excitation of inspiratory neurones (Fig. 1D). However, in 20 % of inspiratory neurones discharge fell on application of ATP. The adenosine receptor antagonist 8-PT (20–45 nA) had no effect on the CO2-evoked changes in the activity of inspiratory neurones (n= 5; Fig. 3). In fact, blockade of adenosine receptors appeared to augment the excitatory effects of CO2 on firing rate (Fig. 3).
Because suramin is relatively unselective, affecting NMDA-type glutamate receptors in addition to its action at P2 purinoceptors (Balcar et al. 1995), we tested the effects of suramin at the doses used on the excitatory effects of ionophoretic application of L-glutamate on a proportion of neurones recorded. Suramin and PPADS did not affect the excitatory effect of glutamate on any neurone recorded (52 ± 26 % increase in activity compared with 37 ± 20 % before application of suramin; P > 0.05, Student's paired t test, n= 4; see Fig. 2C and D).
Pre-inspiratory neurones
Sixty-six per cent of pre-inspiratory neurones were also excited by increasing levels of inspired CO2 (n= 8). Interestingly, the excitation appeared to be more marked during the expiratory, compared with the inspiratory, phase of activity. Both suramin and PPADS were able to reduce or abolish this CO2-evoked excitation, as described above for inspiratory neurones (n= 4). ATP excited a similar proportion of these neurones, and P2 receptor antagonists could antagonise this effect (data not shown).
Post-inspiratory and expiratory neurones
Fifty per cent of post-inspiratory (n= 18) and 55 % of expiratory neurones (n= 18) showed an increase in activity during hypercapnia. However, these effects were not antagonised by application of suramin or PPADS. An example of this is illustrated in Fig. 4. ATP evoked a marked excitation in expiratory neurones (Fig. 4C) and post-inspiratory neurones (not shown). These effects were blocked by prior application of suramin or PPADS (n= 3), but not the adenosine receptor antagonist 8-PT (n= 3) (data not shown).
Figure 4. CO2-evoked increases in activity of expiratory neurones recorded in the Bötzinger complex are not affected by blockade of P2 purinoceptors.
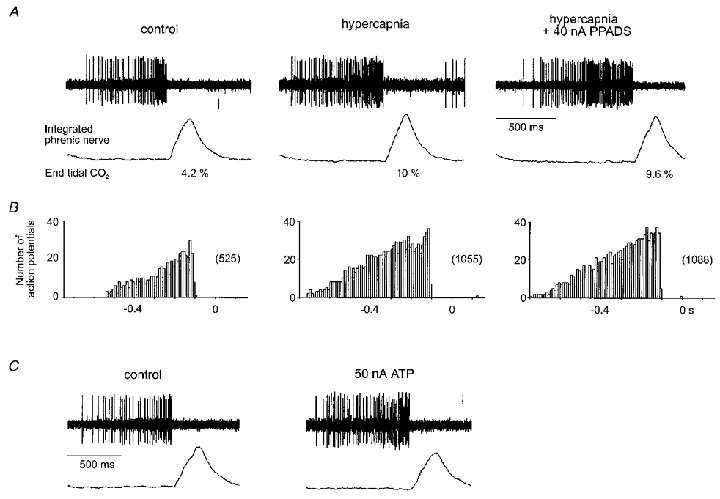
A, raw data illustrating an increase in discharge during hypercapnia under control conditions and during application of PPADS at the current shown. B, phrenic-triggered histograms derived from the neurone illustrated in A for 12 respiratory cycles (bin size = 0.01 s). Note different time scale. C, excitatory response of the same excitatory neurone to application of ATP. Vertical scale bars represent 100 μV.
DISCUSSION
We have provided evidence consistent with the view that a limited group of inspiratory neurones within the retrofacial area of the VLM represent a principal site of action through which CO2-evoked changes in respiration are mediated. All classes of respiratory neurone appear to have P2 receptors associated with them, since all types were excited, albeit differentially, by the application of ATP. However, our data imply that only purinoceptors on the neurones responsible for driving inspiratory activity appear to have a pH sensitivity that can account for CO2 actions. These distinctive CO2-P2X-mediated actions on neurones associated with inspiration were not observed in post-inspiratory or stage 2 expiratory neurones, which had varying sensitivities to increases in inspired CO2. This is unlikely to be due to the lack of P2 receptors associated with these neurones since the post-inspiratory and expiratory neurones studied were extremely sensitive to ionophoretic application of ATP, often responding with an excitation much greater than that observed in the inspiratory neurones. The excitatory effects of ATP on respiratory neurones might be mediated through several actions. For example, ATP acting at P2 purinoceptors inhibits a persistent K+ current and activates a Ca2+-sensitive Na+ current and Ca2+-sensitive non-selective cationic channels in locus coeruleus neurones (Harms et al. 1992). P2 purinoceptors also activate a suramin-sensitive inward current via increased conductance in hypoglossal motoneurones (Funk et al. 1997). The mechanism for excitation of respiratory neurones by ATP that we describe here is unclear.
Our hypothesis that this mechanism involves P2X receptors is strengthened by the fact that the P2X receptor agonist αβ-methyleneATP excited these inspiratory neurones. The effects of ATP that we describe are unlikely to involve modulation of glutamatergic transmission since the excitatory effects of ionophoretically applied glutamate were unaffected by P2 receptor antagonists. We are as yet unable to comment confidently on the types of P2 receptor subtypes mediating these responses, and it is likely that a number of receptor proteins combine to form a heteromeric receptor associated with these respiratory neurones. However, it is known that certain P2X receptors are highly sensitive to changes in pH (King et al. 1996; Stoop et al. 1997). For example, the affinity for ATP at the P2X2 receptor subtype is selectively enhanced during acidic conditions (King et al. 1996; Wildman et al. 1997). This particular P2X subunit, amongst others, is present in the ventrolateral region of the medulla (Tuyau et al. 1997; Kanjhan et al. 1999). Our previous experiments indicated that end-tidal levels of CO2 in the range 8–10 % obtained during hypercapnia lead to an arterial pH of around 7.2 (see Thomas et al. 1999). Since the brain has a high buffering capacity this might correlate to a pH in the cerebrospinal fluid of around 7.1. Studies on recombinant P2X2 receptors indicate that the responses to ATP are increased 2- to 3-fold at this level of pH compared with those at 7.45 (King et al. 1996; Wildman et al. 1997).
The fall in discharge observed in response to adenosine in a proportion of inspiratory neurones can be attributed to the rapid extracellular breakdown of ATP by ecto-nucleotidases to adenosine, which is known to depress the activity of inspiratory neurones in the ventrolateral medulla (Herlenius et al. 1997). Moreover, since blockade of adenosine receptors augmented the excitatory effects of CO2 on firing rate we suggest that adenosine might function to modulate the degree of excitation of inspiratory neurones during hypercapnia.
In conclusion, we have provided evidence of a potential physiological role for purinoceptors in the central nervous system In addition, this study provides a description of a novel mechanism through which changes in carbon dioxide in the air breathed bring about changes in respiratory activity.
Acknowledgments
This work was supported by the British Heart Foundation. Facilities were supported by the Wellcome Trust.
References
- Balcar VJ, Dias LS, Li Y, Bennett MR. Inhibition of [3H] CGP39653 binding to NMDA receptors by a P2 antagonist, suramin. NeuroReport. 1995;7:69–72. [PubMed] [Google Scholar]
- Cherniack NS. Physiological roles of central chemoreceptors. In: Speck DF, Dekin MS, Revellette WR, Frazier DT, editors. Respiratory Control; Central and Peripheral Mechanisms. University of Kentucky; 1993. pp. 138–146. [Google Scholar]
- de Burgh Daly, M. Peripheral Arterial Chemoreceptors and Respiratory-Cardiovascular Integration. Oxford: Oxford University Press; 1997. Monograph for The Physiological Society. [Google Scholar]
- Edwards FA, Gibb AJ, Colquhoun D. ATP receptor-mediated synaptic currents in the central nervous system. Nature. 1992;359:144–147. doi: 10.1038/359144a0. [DOI] [PubMed] [Google Scholar]
- Funk GD, Kanjhan R, Walsh C, Lipski J, Comer AM, Parkis MA, Housley GD. P2 receptor excitation of rodent hypoglossal motoneuron activity in vitro and in vivo: a molecular physiological analysis. Journal of Neuroscience. 1997;17:6325–6337. doi: 10.1523/JNEUROSCI.17-16-06325.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldane JS, Priestley JC. Respiration. New Haven: Yale University Press; 1935. [Google Scholar]
- Harms L, Finta EP, Tschopl M, Illes P. Depolarization of rat locus coeruleus neurones by adenosine 5′-triphosphate. Neuroscience. 1992;48:941–952. doi: 10.1016/0306-4522(92)90282-7. [DOI] [PubMed] [Google Scholar]
- Herlenius E, Lagercrantz H, Yamamoto Y. Adenosine modulates inspiratory neurons and the respiratory pattern in the brainstem of neonatal rats. Pediatric Research. 1997;42:46–53. doi: 10.1203/00006450-199707000-00008. [DOI] [PubMed] [Google Scholar]
- Kanjhan R, Housley GD, Burton LD, Christie DL, Kippenberger A, Thorne PR, Luo L, Ryan AF. Distribution of the P2X2 receptor subunit of the ATP-gated ion channels in the rat central nervous system. Journal of Comparative Neurology. 1999;407:11–32. [PubMed] [Google Scholar]
- Kawai A, Ballantyne D, Mückenhoff K, Scheid P. Chemosensitive medullary neurones in the brainstem-spinal cord preparation of the neonatal rat. The Journal of Physiology. 1996;492:277–292. doi: 10.1113/jphysiol.1996.sp021308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King BF, Zigansha LE, Pintor J, Burnstock G. Full sensitivity of P2X2 purinoceptor to ATP revealed by changing extracellular pH. British Journal of Pharmacology. 1996;117:1371–1373. doi: 10.1111/j.1476-5381.1996.tb15293.x. Special Report. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeschcke HH. Central chemosensitivity and the reaction theory. The Journal of Physiology. 1982;332:1–24. doi: 10.1113/jphysiol.1982.sp014397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nattie EE. Central chemoreception. In: Dempsey JA, Pack AI, editors. Regulation of Breathing. New York: Dekker; 1995. pp. 473–501. [Google Scholar]
- Oyamada Y, Ballantyne D, Mückenhoff K, Scheid P. Respiration-modulated membrane potential and chemosensitivity of locus coeruleus neurones in the in vitro brainstem-spinal cord of the neonatal rat. The Journal of Physiology. 1998;513:381–398. doi: 10.1111/j.1469-7793.1998.381bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Co-ordinates. London: Academic Press; 1998. [Google Scholar]
- Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacological Reviews. 1998;50:413–492. [PubMed] [Google Scholar]
- Ralevic V, Thomas T, Spyer KM. Effects of P2 purine receptor agonists microinjected into the rostral ventrolateral medulla on the cardiovascular and respiratory systems of the anaesthetized rat. The Journal of Physiology. 1998;509:127. P. [Google Scholar]
- Richter DW. Neural regulation of respiration: rhythmogenesis and afferent control. In: Greger R, Windhorst U, editors. Comprehensive Human Physiology. Vol. 2. Berlin: Springer-Verlag; 1996. pp. 2079–2095. [Google Scholar]
- Ritucci NA, Chambers-Kersh L, Dean JB, Putnam RW. Intracellular pH regulation in neurons from chemosensitive and nonchemosensitive areas of the medulla. American Journal of Physiology. 1998;275:R1152–1163. doi: 10.1152/ajpregu.1998.275.4.R1152. [DOI] [PubMed] [Google Scholar]
- Schwarzacher SW, Wilhem Z, Anders K, Richter DW. The medullary respiratory network in the rat. The Journal of Physiology. 1991;435:631–644. doi: 10.1113/jphysiol.1991.sp018529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoop R, Suprenant A, North RA. Different sensitivities to pH of ATP-induced currents at four cloned P2X receptors. Journal of Neurophysiology. 1997;78:1837–1840. doi: 10.1152/jn.1997.78.4.1837. [DOI] [PubMed] [Google Scholar]
- Thomas T, Ralevic V, Gadd CA, Spyer KM. Central CO2 chemoreception: a mechanism involving P2 purinoceptors localized in the ventrolateral medulla of the anaesthetized rat. The Journal of Physiology. 1999;517:899–905. doi: 10.1111/j.1469-7793.1999.00899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas T, Spyer KM. A novel influence of adenosine on ongoing activity in rat rostral ventrolateral medulla. Neuroscience. 1998;88:1213–1223. doi: 10.1016/s0306-4522(98)00296-6. [DOI] [PubMed] [Google Scholar]
- Tuyau M, Hansen MA, Coleman MJ, Dampney RA, Balcar VJ, Bennett MR. Autoradiography of [3H]α,β-methylene-ATP binding sites in medulla oblongata and spinal cord of the rat. Neurochemistry International. 1997;30:159–169. doi: 10.1016/s0197-0186(96)00062-9. [DOI] [PubMed] [Google Scholar]
- Wang W, Pizzonia JH, Richerson GB. Chemosensitivity of rat medullary raphe neurones in primary tissue culture. The Journal of Physiology. 1998;511:433–450. doi: 10.1111/j.1469-7793.1998.433bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildman SS, King BF, Burnstock G. Potentiation of ATP-responses at a recombinant P2X2 receptor by neurotransmitters and related substances. British Journal of Pharmacology. 1997;120:221–224. doi: 10.1038/sj.bjp.0700903. [DOI] [PMC free article] [PubMed] [Google Scholar]


