Abstract
Sensory feedback plays a major role in the regulation of the spinal neural locomotor circuitry in cats. The present study investigated whether sensory feedback also plays an important role during walking in 20 healthy human subjects, by arresting or unloading the ankle extensors 6 deg for 210 ms in the stance phase of gait.
During the stance phase of walking, unloading of the ankle extensors significantly (P < 0·05) reduced the soleus activity by 50 % in early and mid-stance at an average onset latency of 64 ms.
The onset and amplitude of the decrease in soleus activity produced by the unloading were unchanged when the common peroneal nerve, which innervates the ankle dorsiflexors, was reversibly blocked by local injection of lidocaine (n = 3). This demonstrated that the effect could not be caused by a peripherally mediated reciprocal inhibition from afferents in the antagonist nerves.
The onset and amplitude of the decrease in soleus activity produced by the unloading were also unchanged when ischaemia was induced in the leg by inflating a cuff placed around the thigh. At the same time, the group Ia-mediated short latency stretch reflex was completely abolished. This demonstrated that group Ia afferents were probably not responsible for the decrease of soleus activity produced by the unloading.
The findings demonstrate that afferent feedback from ankle extensors is of significant importance for the activation of these muscles in the stance phase of human walking. Group II and/or group Ib afferents are suggested to constitute an important part of this sensory feedback.
In the cat, the basic walking pattern is generated by a spinal network that is under the control of supraspinal structures (Grillner, 1981; Armstrong, 1988). In addition, sensory feedback from the skin and the moving muscles has repeatedly been shown to play a significant role in the regulation of the network activity and the locomotor movements. Graham Brown (1914) demonstrated that cats with supracollicular lesions of the brainstem are able to adjust their locomotion to the speed and slope of the treadmill at which they are walking. Furthermore, Grillner & Rossignol (1978) demonstrated in spinalised cats that changes in the hip position have a significant effect on the timing and amplitude of the locomotor bursts. Several recent studies have demonstrated a resetting of the locomotion movements in the cat by stimulation of different afferents such as group Ib afferents (Conway et al. 1987) and the afferents involved in flexor reflexes (FRA afferents; Schomburg et al. 1998). This indicates that the sensory feedback mediated by these afferents is closely integrated into the activity of the spinal network generating the locomotion. Several studies have demonstrated a positive contribution of feedback activity in group I afferents to the locomotor activity, either relatively directly or via an effect on the spinal locomotor network (Gossard et al. 1994; McCrea, 1998; Pearson et al. 1998; Hiebert & Pearson, 1999). Other studies have shown that the basic muscle activation profile in the stance phase of walking is not substantially altered by short latency afferent input in the very early part of stance whereas a significant sensory contribution later in stance was argued (Gorassini et al. 1994). Group II afferents also activate a group of interneurones in the midlumbar region, which are believed to be closely integrated into the spinal locomotor circuitry and thereby to add significantly to the generation of the locomotor activity (Edgley et al. 1988).
It is still unclear to what extent a spinal network is involved in the generation of human walking and whether sensory feedback to the spinal cord plays a similarly significant role in human muscle activation as in the cat. In large parts of the stance phase of human walking, the ankle extensors undergo eccentric contractions, which make the large and fast conducting muscle afferents from these muscles increase their discharge strongly (e.g. Prochazka, 1995). Through monosynaptic or polysynaptic projections, muscle afferents might, therefore, be expected to make a significant contribution to the activation of the muscle ankle extensors in the stance phase of walking. Consequently, several studies have attempted to evaluate the contribution of muscle afferent feedback to the EMG activity during walking by measuring stretch reflex activity in different phases of the walking cycle (Yang et al. 1991; Sinkjær et al. 1996, 1999). However, such data only provide evidence of the significance of the muscle afferents in the generation of the correction of possible unexpected external perturbations of the gait, but do not reveal much about the involvement of the muscle afferent activity in generating the EMG activity during unperturbed movements.
In the present study, a portable device was used to unload the ankle extensors in the stance phase of walking (Andersen & Sinkjær, 1995). The idea was that if the muscle afferents contributed significantly to the background EMG, unloading of the ankle extensors would diminish the firing of the muscle afferents from the ankle extensors and thereby reduce the background EMG. Unloading of the ankle extensors did indeed reduce the soleus EMG at a latency of around 64 ms. The reduction of EMG activity was still present when transmission in Ia afferents was blocked by ischaemia and when transmission in the antagonist nerve was blocked by local anaesthesia. On this basis group Ib and/or group II afferents are suggested to make a significant contribution to the extensor EMG activity in the stance phase of human walking.
METHODS
Twenty healthy subjects (six females and 14 males, age 26–40 years) were examined. The experiment was repeated in two subjects to ensure reproducibility of the results. All subjects gave their consent to participate. The experimental procedure was approved by the local ethics committee and conducted in conformity with the Declaration of Helsinki.
Experimental set-up
Bipolar EMG electrodes were placed 2 cm apart on the soleus and the tibialis anterior muscles of the left leg. A ground electrode was placed under the knee. The EMG signals were amplified and filtered from 20–1000 Hz (DISA, model 15C01). Angular position and EMG signals were fed to a data collection module at a sampling rate of 2 kHz (Data Acquisition Card PCL718; 486–66 MHz IBM compatible PC). The EMGs were digitally rectified and filtered from 0 to 20 Hz.
Walking protocol
A portable stretch device capable of rotating the human ankle joint during walking on a treadmill elicited perturbations of the ankle joint. The system consisted of a mechanical joint, which was mounted coaxially with the ankle joint pivot. The mechanical joint was connected to a powerful actuator system by means of two flexible Bowden cables. Leg casts were made in polypropylene to give a form-fitting interface from the mechanical joint to the subject's ankle. The joint comprised two coaxial actuators that were aligned on a fixed axle with six independent slide bearings. The first joint was attached to the foot and the calf and acted about the ankle joint. The second joint was connected to the Bowden mechanism and also acted about the ankle joint, but with a slip of ±3.5 deg. The slip was imposed to compensate for the elasticity of the Bowden wires and to give the motor controller a proper input to act upon (details in Andersen & Sinkjær, 1995). When a position feedback from the joint was used, the motor was regulated in such a way that it followed the movement of the ankle joint without influencing the pattern of gait. At any desired time in the gait cycle, a perturbation about the ankle could be applied by rotating the ankle joint with a displacement of up to 10 deg within 40 ms. This could be achieved in subjects of up to 80 kg body weight. For further details on the system, see Andersen & Sinkjær (1995).
During the experiment, the mechanical joint was strapped to the subject's left leg. A heel contact was placed in the subject's left shoe, and an insole of the same dimensions was put in the right shoe to equalize the heights above groundlevel in both shoes. The subject walked with a natural cadence at 3.5-4 km h−1. After an adaptation period of 5 min, an averaged EMG profile, triggered from heel contact, was obtained from the left soleus and tibialis anterior muscles. The stance phase was approximately 500 ms long in most subjects and never shorter than 400 ms. Within the stance phase two different kinds of input were delivered to the ankle joint at various times, 100, 200, 300, 400, or 500 ms, after heel contact. In experiment 1, a 6 deg unloading of the ankle extensors was imposed with a velocity of 330 deg s−1 and a hold phase of 210 ms. In experiment 2, a 0–3 deg hold input was imposed for 210 ms, causing an ankle joint velocity of 0 deg s−1 within 5–10 ms after onset of the ‘hold’ input. For each experiment, the time lags before perturbations were imposed were randomly assigned at 4–6 step intervals until 8–10 stretches at each lag time had been accomplished. The procedure was then repeated for the other experiment. The order in which the two experiments were applied shifted from subject to subject. Eleven subjects participated in this protocol.
In three subjects, the kinematics consequences of the unload perturbation during walking were measured around the knee and the hip joint by use of goniometers (Penny & Giles M180). In early and mid-stance phases, the perturbation was followed by a knee extension of 2–4 deg with an onset around 80 ms after perturbation onset. No clear responses related to the perturbation were observed around the hip joint.
Reversible block of the common peroneal nerve
Part of the walking experiments was repeated in three subjects after the common peroneal nerve (CPN) was blocked by injecting 5–15 ml lidocaine (5 mg ml−1) in doses of up to 5 ml per injection. Lidocaine was injected around the common peroneal nerve just distal to the head of the fibula. The block was considered complete when the subject was unable to produce any measurable voluntary contraction in the dorsiflexors, judged from the tibialis anterior EMG. To test whether lidocaine had spread to the nerves of the ankle extensors, the maximal M-wave in the EMG of the soleus muscle was elicited by surface stimulation of the tibial nerve in the popliteal fossa before and after the injection. No change in the maximal M-wave (Mmax) was seen in two of the subjects. In one subject, a decrease of approximately 15 % of Mmax in the soleus M-wave was observed. The results from this subject did not differ from those in which the maximal M-wave was unchanged after the CPN block. The completeness of the block was also judged from the subject's inability to dorsiflex the foot.
Ischaemia of the lower leg
In nine subjects, the left leg was compressed by using tourniquets to perform an ischaemic nerve block by occlusion. The cuff was placed around the thigh approximately 10 cm above the patella and was inflated to 240 mmHg to block the blood flow completely during walking. At regular intervals after the onset of the inflation, the H-reflex (in three subjects) and stretch reflex of soleus were elicited in the sitting subject. To evoke H-reflexes, a spring-loaded ball electrode was placed in the popliteal fossa with a reference electrode located above the patella. The H-reflex experiment was performed without removing the portable stretch apparatus. After the H-reflex and the short latency stretch reflex had diminished to less than 10 % of the initial values, the subject was asked to walk with a similar walking pattern as before ischaemia, and part of the walking experiments was repeated. The experiment was terminated when the M-wave began to decrease in amplitude.
Data analysis
The data were analysed off-line on a personal computer. The EMG activities of the soleus and tibialis anterior muscles were averaged, rectified, and low-pass filtered at 20 Hz (1st order). If not otherwise stated, the values show averaged data based on 8–10 trials per average.
Statistics
The data were tested by use of the Wilcoxon matched-pair test with a level of significance of P < 0.05.
RESULTS
In all subjects, the averaged EMGs and position recordings from the control steps were superimposed on the averaged recordings with perturbation. Only trials in which the EMGs and position signals in the control and perturbed steps were matched in the period prior to the perturbation were included in the analysis.
Unloading of ankle extensors produces a depression of soleus EMG in the stance phase of walking
Figure 1 shows responses from two different subjects in whom the ankle joint was either perturbed by a 6 deg unloading of the ankle extensors in early stance (‘Unload’, Fig. 1 left) or arrested by applying a ‘hold’ input to the ankle 200 ms after heel contact (‘Hold’, Fig. 1 right). In each graph, the EMGs and ankle joint position recorded in the control situation (thick lines) and with the hold or unloading (thin lines) are shown superimposed. At time zero, the steps, in which a displacement was imposed, deflected from the position of the control steps (Fig. 1A; 0 deg equals standing position). When the ankle was released and returned to the control trajectory, the ankle returned within 150 ms to the position in the control step (Fig. 1A). In the subject shown to the left in Fig. 1, soleus EMG prior to the unloading matched the soleus EMG activity in the control steps until approximately 60 ms after stretch onset (Fig. 1B, left). A marked decrease in the soleus EMG activity was present at this time and until approximately 180 ms after unloading onset. This was followed by an increase in the soleus EMG activity. When the unloading was returned, it was followed by a small peak with an onset at 40 ms. Most probably this peak reflects the short latency stretch reflex. In the subject to the left in Fig. 1, the perturbation, which stretched the passive dorsiflexors, was followed by a distinct burst in the tibialis anterior EMG with an onset latency of 75 ms (Fig. 1C, left). Part of this response appears to be mediated by a transcortical reflex loop (Petersen et al. 1998; Christensen et al. 1999). Not all subjects had this late tibialis anterior (TA) response (see e.g. Fig. 3).
Figure 1. Example of averaged recorded data during control steps and steps with unloading or a hold input to the ankle extensors in the stance phase.

Control steps, thick lines; steps with an unloading, thin lines, left; steps during a hold input, thin lines, right. A, ankle angle positions. B, rectified and filtered soleus muscle EMG. The black, white and shaded areas represent the different time windows used to characterise the response. C, rectified and filtered tibialis anterior muscle EMG. 0 deg, standing position. Positive values represent plantar movement directions.
Figure 3. Example of unloading responses in the early stance phase of walking before (left) and after (right) reversible lidocaine block of the common peroneal nerve (CPN).
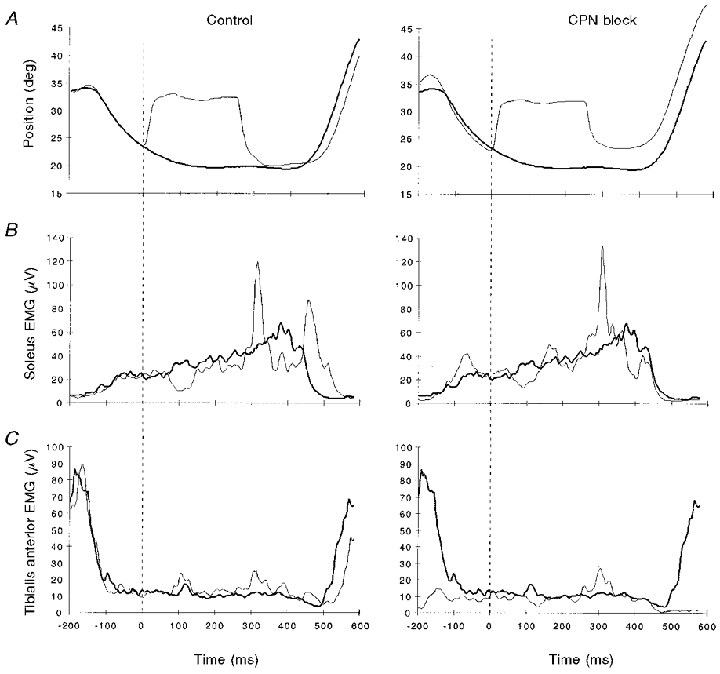
Thick lines are control steps and thin lines are perturbed steps. A, ankle angle positions. B, rectified and filtered soleus muscle EMG. C, rectified and filtered tibialis anterior muscle EMG. After CPN block, no TA EMG activity was present except crosstalk from other non-blocked muscles. Positive values represent plantar flexion movement direction. One subject.
Figure 1 (right) shows an example from a subject, in whom the ankle movement was arrested instead of unloaded, i.e. the ankle extensors (instead of being unloaded) and the ankle flexors (instead of being stretched) were both being kept constant in muscle length for 210 ms. In this subject, the onset of the EMG depression was seen at a latency of 53 ms, and it lasted until 42 ms after the release of the hold when a marked short latency response occurred. In this subject, no response was seen in TA. Due to the actuator properties of the perturbation system, the release after the ‘hold’ input caused a < 3.5 deg stretch of the ankle extensors (see Methods). Most probably, this explains the marked short latency stretch reflex in the soleus at this time. The variation in the EMG responses by ‘re-stretch’ at the end of the 210 ms perturbation may be related to a change in the subjects’‘set’ (e.g. ‘fusimotor set’, see Prochazka et al. 1985) triggered by the unload perturbation, or the response to this second ‘re-stretch’ perturbation being mixed with adaptive responses to the first.
Modulation of the unloading induced depression of soleus EMG in the stance phase of walking
The decrease in soleus EMG following an unloading of the ankle extensors was calculated as the difference between the soleus EMG during the control steps and the perturbed steps. It was then expressed as a percentage of the soleus EMG at the corresponding time in the unperturbed record. For each of the five perturbation onset times (100, 200, 300, 400 and 500 ms from the onset of the stance phase; Fig. 2), no difference in the decrease in soleus EMG was present for perturbations induced 50, 100 and 150 ms following the onset of the decrease in soleus EMG (Fig. 2A). The maximal decrease of 51 ± 7 % was seen at 100 ms after heel contact (Fig. 2, left). The differences at 100 ms during ‘hold’ input (Fig. 2, right) were probably caused by the fact that fewer data points were collected here because four subjects did not receive a hold input at this time due to technical problems.
Figure 2. The effect of unloading (left) and holding (right) the ankle extensor movement 100, 200, 300, 400 and 500 ms into the stance phase of walking.

A, percentage decreases in soleus EMG 50 ms (▪), 200 ms (□) and 150 ms (×) from onset of the EMG response. B, onset latency of the unloading (left) and hold (right) responses. C, velocity of the ankle joint during the control steps. The decrease in background soleus EMG following unloading or a hold of the ankle extensors was calculated as the difference between the soleus EMG during the control steps and the perturbed steps, expressed as a percentage of the soleus EMG during the control steps. Responses are means ± 1 s.d.
The ‘hold’ input might not always have prevented the ankle joint from being stretched during the 210 ms hold, which would result in a low estimate for the decrease in soleus EMG in Fig. 2A (right). In contrast to this, the unloading input always ensured a distinct shorting of the ankle extensors.
The onset latency in the unloading experiments did not differ for any of the five perturbation onset times in the stance phase. It was on average 63.6 ± 3.5 ms. Excluded from this average were measurements at 500 ms after heel contact. The very small changes in soleus EMG at this time made it difficult to carry out a reliable onset measurement.
As the ankle joint velocity decreased during the control step (Fig. 2C), a decrease in soleus EMG responses to unloading was observed (Fig. 2A).
Block of the common peroneal nerve (CPN) has no effect on the decrease in background soleus EMG after unloading
When ankle extensors are shortened, the dorsiflexors are stretched at the same time. This stretch might possibly excite the muscle afferents of the dorsiflexors to such an extent that it results in reciprocal inhibition of the soleus muscle (Crone et al. 1987), which could explain the observed decrease in soleus EMG. Therefore, the soleus EMG activity was compared in three subjects before and after the neural transmission in the common peroneal nerve (CPN) was blocked by local injection of lidocaine (see Methods). The CPN block caused a ‘foot-drop’ in the subjects, but they maintained the same walking pattern in most of the stance phase. The left panel in Fig. 3 shows the control situation with an unloading input applied to the ankle extensors in early stance. The right panel in Fig. 3 displays the responses after a complete block of the CPN nerve. Before block, the TA muscle was nearly silent in stance and active in swing (Fig. 3C, left). After block, no activity was recorded in the TA muscle, except for a 10 μV increase during the stance phase and a more distinct response at 300–400 ms, which we believe that is cross-talk from other muscles, including pick-up from the large responses in the ankle extensors (Fig. 3C). Unloading caused a marked decrease in the soleus EMG both before and after block (Fig. 3B) and a stretch reflex as the ankle extensors were stretched at the end of the unloading.
In the three subjects investigated the decrease of soleus EMG induced by unloading had the same onset latency and size before and after the CPN block. This suggested that the block of the CPN affected the decrease in the soleus EMG to a very limited extent.
Ischaemia has no effect on the initial decrease in soleus EMG activity after unloading
The average onset latency of 64 ms (Fig. 2B) of the soleus EMG unloading response was too slow to suggest that the response was mediated via a short latency group Ia afferent pathway from the perturbed muscles (Sinkjær et al. 1996). To test if group Ia afferents in the lower leg had an effect on the response through long latency neural pathways, we made the lower leg of nine subjects ischaemic by inflating a cuff positioned around the thigh to 240 mmHg. After 15–25 min of ischaemia, the short latency soleus EMG stretch reflex, elicited with the actuator in the relaxed sitting subject, was reduced to below 10 % of the initial value providing evidence of block of transmission in Ia afferents. Figure 4A gives an example of the amplitude and onset latency of the short latency stretch reflex in a sitting subject. In this case, the stretch reflex was abolished after 18 min, and the subject was then asked to walk. At selected times during the stance phase, a 6 deg unloading was imposed to the ankle extensors. The maximal M-wave was monitored approximately every minute during walking, and the experiment was discontinued when the M-wave started to decrease. At this time, we assumed that the motor nerve fibres began to develop block, and the soleus and TA EMG activity during locomotion started decreasing in amplitude. The cuff was deflated, and within less than a minute, the M-wave was restored to its normal value. Figure 4B–E gives two examples of the effect of the ischaemia at 200 ms after heel contact. Before (Fig. 4C and E, left) and after (Fig. 4C and E, right) ischaemia, a distinct decrease was observed in the soleus EMG. When measured over the first 50 ms after unloading onset, the soleus EMG activity decreased on average 49 ± 5 % before and 45 ± 5 % during ischaemia (Fig. 5, left); the onset latencies were on average 59.7 ± 3.5 and 63.5 ± 2.6 ms, respectively (Fig. 5, right). Values for soleus EMG activity in the control steps before and during ischaemia were 25 ± 4 and 36 ± 6 μV, respectively. No significant difference was found when comparing the measurements before and during ischaemia in the nine subjects, except that the subjects had a larger background EMG after ischaemia (P = 0.002).
Figure 4. Stretch and unloading responses before and after applying ischaemia to the thigh of the investigated leg.

A shows the amplitude (left) and onset latency (right) of the short latency stretch reflex in a sitting subject. Time 0 is the onset of the ischaemia. B-E, examples of the ankle position (B and D) and soleus EMG (C and E) before (right) and during ischaemia (left) in two walking subjects. Thick lines are control steps and thin lines are perturbed steps.
Figure 5. The effect of ischaemia on the unloading responses during walking.

The upper panel shows the change in soleus EMG over a 50 ms time window, and the lower panel illustrates the onset latency in response to unloading before (control) and during ischaemia. The unloading effect is calculated as in Fig. 2. Responses are means ± 1 s.d. from nine subjects.
DISCUSSION
This study demonstrates that sensory feedback makes an important contribution to the centrally generated motor commands during human locomotion.
The responses to the perturbations applied in this study are the consequences of many kinds of sensory input, some of which are probably inhibitory and some excitatory on the motoneurones of the investigated muscles. The interpretation of such complex sensory input is discussed in Gorassini et al. (1994). One important issue in relation to the present experiments is whether the leg cast causes pressure to be applied to skin and deeper tissue at the shank, which might influence the responses during the perturbation? We do not believe this for the reasons given below.
Firstly, the casts were customised to the subject's leg and padded to produce a form-fitting interface that evenly distributed the forces applied by the perturbation. No subjects reported that the perturbation caused abnormal unpleasant sensory input from the shank. Secondly, the responses, which we observed with the portable device applied in this study, behave very similarly to the reflex responses, which we obtained in other stationary devices where an ankle rotation can be applied without any strapping to the shank (Sinkjær et al. 1988).
The long latency EMG responses in tibialis anterior muscle
The unloading response in soleus was often followed by a long latency facilitation in the TA at a latency of approximately 80 ms (Fig. 1, right). This facilitation, which may be part of a transcortical reflex loop (Petersen et al. 1998), was rarely present at the ‘hold’ input (Fig. 2). Qualitatively the TA responses seemed to be dependent on the time within the stance phase at which the unloading was given, and the amplitude of unloading (Christensen et al. 1999). The combination of soleus unloading followed by a TA facilitation might reflect properties of a complex compensatory reflex mechanism with spinal and transcortical reflex pathways. As long latency TA responses were not observed in standing subjects (unreported results from four subjects instructed to stand with a precontracted soleus muscle receiving a 6 deg unloading input), we find it unlikely that the EMG responses reported during walking simply reflected long latency postural reflex patterns (Nashner, 1976; Diener et al. 1983). Independently of the presence of a TA response, the unloading of the ankle extensors always caused a decrease in the background soleus EMG, even when the CPN nerve was blocked.
Which afferents contribute to the soleus EMG unloading response?
The late onset of the unloading response (64 ms; Fig. 3B) does not exclude a monosynaptic Ia contribution. For example, Nichols & Houk (1976) showed that Ia short latency reflexes were active and did contribute to the unloading responses. The responses were smaller and had a longer latency than the corresponding Ia responses to stretch. Nichols & Houk (1976) attributed this to the known non-linearity of the muscle spindle Ia afferents. The inability of the muscle fibres, bearing the muscle spindles, to keep up with the rate of origin-to-insertion shortening imposed by the perturbation will further delay the onset latency of the unloading response compared with a stretch response. Involvement of a non-monosynaptic Ia pathway is also possible (Jankowska & Lundberg, 1981). However, both of these possibilities seem to be of minor importance, since a pronounced unloading response was still present when conduction in Ia afferents was blocked during ischaemia, as evidenced by the disappearance of the short latency Ia-mediated stretch reflex (Fig. 4A). In a few subjects the onset of the unloading response was delayed during ischaemia (cf. Fig. 4E), which could suggest a group Ia contribution to the initial part of the unloading response, but for the majority of subjects this was not the case, and by far the main part of the response was preserved during ischaemia in all subjects. The ischaemia data thus do not preclude a contribution from Ia afferents, but merely demonstrate that afferents other than Ia afferents make the most important contribution to the unloading response. This implies that, as previously suggested by Dietz et al. (1985, 1987), sensory feedback from other afferents than group Ia afferents plays an important role in the generation of the soleus EMG background activity during human gait.
Why would the Ia short latency reflex pathway contribute significantly when the muscle is unexpectedly lengthened during walking, as demonstrated by Yang et al. (1991) and Sinkjær et al. (1996), but less when it is unexpectedly shortened? We would like to propose the following explanation, although we realise that the evidence to support this is still rather weak. There is good evidence to suggest that the monosynaptic input onto the motoneurones is depressed by presynaptic inhibition during normal human walking (e.g. Stein & Capaday, 1988; Faist et al. 1996). This may at least provide some of the explanation for why the Ia afferent feedback generated during normal walking seems to make only a minor contribution to the soleus EMG activity. If a strong external force perturbs the ankle during the stance phase of walking, however, the Ia afferents will respond with discharges at a very high rate. As shown by Morita et al. (1998), presynaptic inhibition seems to be very ineffective in depressing the synaptic input to the spinal motoneurones from Ia afferents discharging at this high rate, and a stretch reflex may therefore be elicited in spite of presynaptic inhibition. In other words, the strength of the presynaptic inhibition of the monosynaptic Ia afferent input onto the motoneurones may result in the Ia afferents making little or no contribution to the background EMG during normal walking, as reflected in the unchanged unloading response after block of the Ia afferents (Fig. 5). It is, however, too weak to prevent a mechanically strong compensatory short latency stretch reflex from acting in response to a large disturbance.
Another possible explanation of the unloading response could be reciprocal inhibition of soleus motoneurones following the stretch of the ankle dorsiflexors, which accompanies the unloading of the ankle extensors (Nichols & Koffler-Smulevitz, 1991; Sinkjær et al. 1995). However, the unloading response could still be seen when transmission in the common peroneal nerve was blocked, and this possibility may therefore be discarded. Incidentally, the lack of change in the effect of stretching the dorsiflexors on the soleus EMG activity with and without block of the common peroneal nerve is also in line with the suggestion made by Petersen et al. (1999). They suggested a functional depression of the reciprocal inhibition from ankle dorsiflexors to ankle extensors in the stance phase of walking.
It is likely that cutaneous afferents contributed to the EMG responses that we observed. However, during the ischaemia experiments, all subjects reported anaesthesia or at least decreased sensation in the leg distal to the inflated cuff. This implies that at least large cutaneous afferents were blocked and therefore did not contribute significantly to the unloading response.
In the resting situation, activity in Ib afferents exerts a weakly negative feedback on homonymous motoneurones (Nichols & Houk, 1976), but during locomotion in the cat this inhibition is replaced by a positive feedback effect (Conway et al. 1987; Gossard et al. 1994; Pearson et al. 1998; McCrea, 1998). Stephens & Yang (1999) adduced evidence for a reduction in what they believed to be Ib inhibition during walking. They suggested that a similar shift may take place from negative to positive feedback control as in the cat. The unloading response that we have found here could thus be due to removal of Ib-mediated excitatory input to soleus motoneurones. In the cat, Ib afferents exert an excitatory effect on the spinal motoneurones during locomotion either via a disynaptic pathway converging with group Ia afferents on common non-reciprocal interneurones or via a long latency pathway. This pathway is closely integrated with the extensor part of the rhythm-generating network in the spinal cord (McCrea, 1998). From the latency of the unloading response observed, involvement of a disynaptic pathway seems unlikely. Bergego et al. (1981) estimated that a volley in Ib afferents evoked by stimulation at knee level would arrive at the spinal cord level only 6 ms later than a volley in Ia afferents. Even with an extra synaptic delay (0.5-1.0 ms), this is much shorter than the observed difference in the latency between the Ia-mediated monosynaptic stretch reflex (40 ms, Sinkjær et al. 1996) and the unloading response of 64 ms found in this study. The later excitatory effects of Ib afferents, which are closely integrated into the locomotor network in the cat, have a central latency of 3.5-4.5 ms (Hultborn et al. 1998). Central processing of the Ib input through a possible locomotor network in the human spinal cord may require a longer time than in the cat. Therefore, the long latency of the response does not necessarily argue against this possibility. The lack of change in the unloading response during ischaemia also does not exclude an involvement of Ib afferents, as their diameter and thus their sensitivity to ischaemia are overlapping with those of the Ia afferents on one hand and motor axons on the other. Therefore, it is difficult to say whether Ib afferents were or were not blocked with the duration of ischaemia that we used (sufficiently long to block transmission in Ia afferents, but insufficiently long to block transmission in motor axons). However, if the unloading response reflects removal of a facilitatory Ib afferent-mediated input onto the soleus motoneurones, this response would be expected to increase as the forces on the ankle extensors increased during the stance phase. In contrast to this, the unloading response decreased as the forces on the ankle extensors built up during the stance phase. In fact, the pattern of the afferent-mediated soleus EMG during the stance phase of walking (Fig. 2C) suggests rather that it is mediated by receptors carrying information about muscle displacement such as the muscle spindle afferents
A final possibility is that the unloading response is mediated by group II afferents. In support of this idea, the unloading response and the M2 stretch reflex seem to share several features. The onset latency of the unloading response was within the same range as the onset time of the long latency M2 stretch reflex seen during walking (Dietz et al. 1987; Sinkjær et al. 1999) as well as in sitting subjects (Gotlieb & Agarwall 1979, 1980; Toft et al. 1991). Furthermore, the response observed by Dietz et al. (1987) was preserved during walking when ischaemia was applied to the leg, as we have also observed in the case of the unloading response (Figs 4 and 5). It now seems fairly well established that the soleus M2 stretch reflex is mediated by stretch-sensitive group II afferents during walking (Berger et al. 1984; Dietz et al. 1987) and standing (Corna et al. 1995; Nardone et al. 1996). Therefore, it seems possible that the unloading response is also group II mediated.
Based on acute animal studies, the monosynaptic excitation from group II afferents to homonymous motoneurones is weak compared with that of group Ia (Lundberg et al. 1977). Therefore, it seems unlikely that such projections should be involved in the unloading response observed here. During human walking, several authors (e.g. Dietz et al. 1987; Yang et al. 1991) have suggested that strong central effects of group II muscle afferents are mediated via a complex neural pathway influenced by supraspinal input and peripheral input. In the cat, flexor group II afferents have been found to project to a group of mid-lumbar interneurones, which are closely integrated into the spinal neural locomotor circuitry (e.g. Edgley et al. 1988; Perreault et al. 1995). Our results are consistent with such a strong group muscle II pathway, but as no animal data, to our knowledge, are available on extensor group II afferent projections to homonymous motoneurones via the locomotor spinal neural circuitry, we lack firm evidence for such a pathway at present. One way of obtaining such evidence could be to test the effect of α2-adrenergic receptor agonists (such as tizanidine) on the unloading response, since both animal (Bras et al. 1989) and human data (Corna et al. 1995) have suggested that these drugs induce a decrease in transmission in spinal group II pathways. However, in a preliminary study on three subjects (each receiving approximately 150 μg kg−1 tizanidine orally, as in Corna et al. 1995), we failed to find any effect of tizanidine on the soleus EMG unloading response or for that matter on the medium latency M2 stretch reflex. This does not necessarily suggest that the unloading response is not mediated by a group II pathway, as Corna et al. (1995) were only able to demonstrate a depression of the medium latency stretch response in the ankle dorsiflexors, but not in the soleus muscle. More probable explanations would be that the dose was too low and/or that group II pathways from flexors and extensors have different properties.
Regardless of which afferents and which pathways are responsible for the unloading response, our data signify that sensory feedback from ankle extensors makes an important contribution to ongoing muscle activity during human walking. One possibility is that it provides a background input to spinal interneurones and motoneurones, which may be used by central motor commands to achieve an optimal activation of the muscles adjusted to the properties of the supporting surface.
Acknowledgments
The Danish National Research Foundation is acknowledged for financial support for the studies reported in this paper. Lars O. D. Christensen was supported by a grant from the Danish Research Council.
References
- Andersen JB, Sinkjær T. An actuator system for investigating electrophysiological and biomechanical features around the human ankle joint during gait. Transaction on Rehabilitation Engineering. 1995;3(4):299–306. [Google Scholar]
- Armstrong DM. The supraspinal control of mammalian locomotion. The Journal of Physiology. 1988;405:1–37. doi: 10.1113/jphysiol.1988.sp017319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergego C, Pierrot Deseilligny E, Mazieres L. Facilitation of transmission in Ib pathways by cutaneous afferents from the contralateral foot sole in man. Neuroscience Letters. 1981;27:297–301. doi: 10.1016/0304-3940(81)90446-8. [DOI] [PubMed] [Google Scholar]
- Berger W, Dietz V, Quintern J. Corrective reactions to stumbling in man: neuronal coordination of bilateral leg muscle activity during gait. The Journal of Physiology. 1984;357:109–125. doi: 10.1113/jphysiol.1984.sp015492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bras H, Cavallari P, Jankowska E, McCrea D. Comparison effects of monoamines on transmission in spinal pathways from group I and group II muscle afferents in the cat. Experimental Brain Research. 1989;76:27–37. doi: 10.1007/BF00253620. [DOI] [PubMed] [Google Scholar]
- Brown TG. On the nature of the fundamental activity of the nervous centres; together with an analysis of the conditioning of rhythmic activity in progression, and a theory of the evolution of function in the nervous system. The Journal of Physiology. 1914;48:18–46. doi: 10.1113/jphysiol.1914.sp001646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen LOD, Morita H, Petersen N. Evidence suggesting that a transcortical reflex pathway contributes to cutaneous reflexes in the tibialis anterior muscle during walking in man. Experimental Brain Research. 1999;124:59–68. doi: 10.1007/s002210050600. [DOI] [PubMed] [Google Scholar]
- Conway BA, Hultborn H, Kiehn O. Proprioceptive input resets central locomotor rhythm in the spinal cat. Experimental Brain Research. 1987;68:643–656. doi: 10.1007/BF00249807. [DOI] [PubMed] [Google Scholar]
- Corna S, Grasso M, Nardone A, Schienppati M. Selective depression of medium latency leg and foot muscle responses to stretch by an alpha 2-agonist in humans. The Journal of Physiology. 1995;484:803–809. doi: 10.1113/jphysiol.1995.sp020705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone C, Hultborn H, Jespersen B, Nielsen J. Reciprocal Ia inhibition between ankle flexors and extensors in man. The Journal of Physiology. 1987;389:163–85. doi: 10.1113/jphysiol.1987.sp016652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diener H-C, Bootz F, Dichgans J, Bruzek W. Variability of postural reflexes in humans. Experimental Brain Research. 1983;52:423–428. doi: 10.1007/BF00238035. [DOI] [PubMed] [Google Scholar]
- Dietz V, Quintern J, Berger W. Afferent control of human stance and gait: evidence for blocking of group I afferents during gait. Experimental Brain Research. 1985;61:153–163. doi: 10.1007/BF00235630. [DOI] [PubMed] [Google Scholar]
- Dietz V, Quintern J, Sillem M. Stumbling reactions in man: Significance of proprioceptive and pre-programmed mechanisms. The Journal of Physiology. 1987;368:149–163. doi: 10.1113/jphysiol.1987.sp016527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley SA, Jankowska E, Shefchyk S. Evidence that mid-lumbar neurones in reflex pathways from group II afferents are involved in locomotion in the cat. The Journal of Physiology. 1988;403:57–71. doi: 10.1113/jphysiol.1988.sp017238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faist M, Dietz V, Pierrot-Deseilligny E. Modulation, probably presynaptic in origin, of monosynaptic Ia excitation during human gait. Experimental Brain Research. 1996;109:441–449. doi: 10.1007/BF00229628. [DOI] [PubMed] [Google Scholar]
- Gorassini MA, Prochazka A, Hiebert GW, Gauthier MJ. Corrective responses to loss of ground support during walking. I. Intact cats. Journal of Neurophysiology. 1994;71:603–10. doi: 10.1152/jn.1994.71.2.603. [DOI] [PubMed] [Google Scholar]
- Gossard J-P, Brownstone RM, Barajon I, Hultborn H. Transmission in a locomotor-related group Ib pathway from hindlimb extensor muscles in the cat. Experimental Brain Research. 1994;98:213–228. doi: 10.1007/BF00228410. [DOI] [PubMed] [Google Scholar]
- Gottlieb GL, Agarwall GC. Response to sudden torques about ankle in man: myotatic reflex. Journal of Neurophysiology. 1979;42:91–106. doi: 10.1152/jn.1979.42.1.91. [DOI] [PubMed] [Google Scholar]
- Gotlieb GL, Agarwall GC. Response to sudden torques about the ankle in man: II-post-myotatic reactions. Journal of Neurophysiology. 1980;43:86–101. doi: 10.1152/jn.1980.43.1.86. [DOI] [PubMed] [Google Scholar]
- Grillner S. Control of locomotion in bipeds, tetrapeds and fish. In: Brooks VB, editor. Handbook of Physiology, The Nervous System. Vol. 2. Bethesda: American Physiologial Society; 1981. pp. 1179–1236. parts 1 and 2. [Google Scholar]
- Grillner S, Rossignol S. On the initiation of the swing phase of locomotion in chronic spinal cats. Brain Research. 1978;146:269–277. doi: 10.1016/0006-8993(78)90973-3. [DOI] [PubMed] [Google Scholar]
- Hiebert GW, Pearson KG. Contribution of sensory feedback to the generation of extensor activity during walking in the decerebrate cat. Journal of Neurophysiology. 1999;81:758–770. doi: 10.1152/jn.1999.81.2.758. [DOI] [PubMed] [Google Scholar]
- Hultborn H, Conway BA, Gossard JP, Brownstone R, Fedirchuk B, Schomburg ED, Enriquez-Denton M, Perreault MC. How do we approach the locomotor network in the mammalian spinal cord? Annals of the New York Academy of Sciences. 1998;860:70–82. doi: 10.1111/j.1749-6632.1998.tb09039.x. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Lundberg A. Interneurones in the spinal cord. Trends in Neurosciences. 1981;4:230–233. [Google Scholar]
- Lundberg A, Malmgren K, Schomburg ED. Comments on reflex actions evoked by electrical stimulation of group II muscle afferents. Brain Research. 1977;122:551–555. doi: 10.1016/0006-8993(77)90466-8. [DOI] [PubMed] [Google Scholar]
- McCrea DA. Neuronal basis of afferent-evoked enhancement of locomotor activity. Annals of the New York Academy of Sciences. 1998;860:216–25. doi: 10.1111/j.1749-6632.1998.tb09051.x. [DOI] [PubMed] [Google Scholar]
- Morita H, Petersen N, Christensen LOD, Sinkjær T, Nielsen J. Sensitivity of H-reflexes and stretch reflexes to presynaptic inhibition in man. Journal of Neurophysiology. 1998;80:610–620. doi: 10.1152/jn.1998.80.2.610. [DOI] [PubMed] [Google Scholar]
- Nardone A, Grasso M, Giordano A, Schiepatti M. Different effect of height on latency of leg and foot short and medium-latency EMG responses to pertubation of stance in humans. Neuroscience Letters. 1996;206:89–92. doi: 10.1016/s0304-3940(96)12430-7. [DOI] [PubMed] [Google Scholar]
- Nashner LM. Adapting reflexes controlling the human posture. Experimental Brain Research. 1976;26:59–72. doi: 10.1007/BF00235249. [DOI] [PubMed] [Google Scholar]
- Nichols TR, Houk J. Improvement in linearity and regulation of stiffness that results from action of stretch reflex. Journal of Neurophysiology. 1976;39:119–142. doi: 10.1152/jn.1976.39.1.119. [DOI] [PubMed] [Google Scholar]
- Nichols TR, Koffler-Smulevitz D. Mechanical analysis of heterogenic inhibition between soleus muscle and the pretibial flexors in the cat. Journal of Neurophysiology. 1991;66:1139–1155. doi: 10.1152/jn.1991.66.4.1139. [DOI] [PubMed] [Google Scholar]
- Pearson KG, Misiaszek JE, Fouad K. Enhancement and resetting of locomotor activity by muscle afferents. Annals of the New York Academy of Sciences. 1998;860:203–215. doi: 10.1111/j.1749-6632.1998.tb09050.x. [DOI] [PubMed] [Google Scholar]
- Perreault MC, Angel MJ, Guertin P, McCrea DA. Effects of stimulation of hindlimb flexor group II afferents during fictive locomotion in the cat. The Journal of Physiology. 1995;487:211–220. doi: 10.1113/jphysiol.1995.sp020872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen N, Christensen LOD, Sinkjær T, Morita H, Nielsen J. Evidence suggesting a transcortical pathway from muscle afferents to tibialis anterior motoneurones in man. The Journal of Physiology. 1998;512:267–276. doi: 10.1111/j.1469-7793.1998.267bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen N, Morita H, Nielsen J. Modulation of reciprocal inhibition between ankle extensors and flexors during walking in man. The Journal of Physiology. 1999;520:605–619. doi: 10.1111/j.1469-7793.1999.00605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochazka A. ‘Fusimotor set’: New evidence for alpha-independent control of gamma-motoneurones during movement in the awake cat. Brain Research. 1985;339:136–140. doi: 10.1016/0006-8993(85)90632-8. [DOI] [PubMed] [Google Scholar]
- Prochazka A. Proprioceptive feedback and movement regulation. In: Rowell L, Shepard J, Smith J, Dempsey J, Johnson J, Wagner P, Terjung R, editors. Integration of Motor, Ciculatory, Respiratory and Metabolic Control during Exercise. American Handbook of Physiology. New York: Oxford University Press; 1995. pp. 1–29. Assoc. [Google Scholar]
- Schomburg ED, Petersen N, Barajon I, Hultborn H. Flexor reflex afferents reset the step cycle during fictive locomotion in the cat. Experimental Brain Research. 1998;122:339–350. doi: 10.1007/s002210050522. [DOI] [PubMed] [Google Scholar]
- Sinkjær T, Andersen JB, Larsen B. Soleus stretch reflex modulation during gait in man. Journal of Neurophysiology. 1996;76:1112–1120. doi: 10.1152/jn.1996.76.2.1112. [DOI] [PubMed] [Google Scholar]
- Sinkjær T, Andersen JB, Nielsen JF, Hansen HJ. Soleus long-latency stretch reflexes during walking in healthy and spastic humans. Clinical Neurophysiology. 1999;110:951–959. doi: 10.1016/s1388-2457(99)00034-6. [DOI] [PubMed] [Google Scholar]
- Sinkjær T, Nielsen J, Toft E. Mechanical and electromyographic analysis of reciprocal inhibition at the human ankle joint. Journal of Neurophysiology. 1995;74:849–855. doi: 10.1152/jn.1995.74.2.849. [DOI] [PubMed] [Google Scholar]
- Sinkjær T, Toft E, Andreassen S, Horneman BC. Muscle stiffness in human ankle dorsiflexors: Intrinsic and reflex components. Journal of Neurophysiology. 1988;60:1110–1121. doi: 10.1152/jn.1988.60.3.1110. [DOI] [PubMed] [Google Scholar]
- Stein RB, Capaday C. The modulation of human reflexes during functional motor tasks. Trends in Neurosciences. 1988;11:328–323. doi: 10.1016/0166-2236(88)90097-5. [DOI] [PubMed] [Google Scholar]
- Stephens MJ, Yang JF. Loading during the stance phase of walking in humans increases the extensor EMG amplitude but does not change the duration of the step cycle. Experimental Brain Research. 1999;124:363–370. doi: 10.1007/s002210050633. [DOI] [PubMed] [Google Scholar]
- Toft E, Sinkjær T, Andreassen S, Larsen K. Mechanical and electromyographic responses to stretch of the human ankle extensors. Journal of Neurophysiology. 1991;60:1110–1121. doi: 10.1152/jn.1991.65.6.1402. [DOI] [PubMed] [Google Scholar]
- Yang JF, Stein RB, James KB. Contribution of peripheral afferents to the activation of the soleus muscle during walking in humans. Experimental Brain Research. 1991;87:679–687. doi: 10.1007/BF00227094. [DOI] [PubMed] [Google Scholar]


