Abstract
Effects of external pH on the human P2X4 purinoceptor, an ATP-activated ion channel, were studied using the Xenopus oocyte expression system.
Changing the external pH from 7·4 to 6·5 significantly reduced, whilst an increase to pH 8 enhanced, maximum ATP-activated current amplitude, without changing the current- voltage relationship of the ATP-activated current.
Diethyl pyrocarbonate (DEPC; 10 mM) treatment of P2X4-injected oocytes had no effect on the pH sensitivity of the ATP-activated current.
Site-directed mutagenesis of histidine 286 (H286) to alanine completely abolished the pH sensitivity of the P2X4 receptor at all agonist concentrations. ATP potency showed a small (fourfold) leftward shift. Mutagenesis of the other three histidines present in the P2X4 sequence had no effect on pH sensitivity.
The results show that pH modulation of P2X4 in the pathophysiological range is mediated by protonation of H286. This provides direct confirmation that pH sensitivity resides in the P2X4 channel protein rather than the agonist species.
P2X channels may mediate fast excitatory actions of ATP when ATP is co-released with other transmitters in both the central and peripheral nervous systems (see North & Barnard, 1997, for review). Modulators of ATP-gated channels may therefore act as important regulators of synaptic transmission. To date seven isoforms of the P2X receptor have been cloned. These share 35–50 % sequence homology and in in vitro expression systems are capable of forming homomeric channels (Surprenant, 1996). P2X receptors show widespread distribution in neuronal, secretory and muscle tissues with P2X4 (Bo et al. 1995; Buell et al. 1996b; Seguela et al. 1996; Soto et al. 1996) and P2X6 predominating in the CNS (Buell et al. 1996a; Collo et al. 1996) suggesting that these sub-types might have important physiological and pathophysiological roles in the brain.
Neuronal activity (Chesler & Kaila, 1992) as well as a number of pathophysiological conditions give rise to changes in extracellular pH across a range from pH 6.0 to pH 8.0 (Siesjöet al. 1996). In common with a number of other ion channels, ATP-gated channels are sensitive to extracellular pH. P2X receptors native to bullfrog dorsal root ganglion neurons and rat nodose ganglion neurons (Li et al. 1996, 1997), as well as rat P2X2 (rP2X2) receptors expressed in Xenopus oocytes (King et al. 1996), show enhanced ATP-activated currents when extracellular pH is reduced. In contrast, a reduction in extracellular pH decreased ATP-induced currents in human P2X1 (hP2X1), rP2X3 and rP2X4 receptors (Stoop et al. 1997).
The detailed structure of P2X channels and how the structure determines channel function is poorly understood. Analysis of primary sequence data indicates that the channels are composed of intracellular N- and C-termini, and two transmembrane domains joined by a large extracellular loop region (Hansen et al. 1997). This has been supported by glycosylation (Newbolt et al. 1998) and cysteine scanning studies (Rassendren et al. 1997). There is little information on the molecular location of sites that modulate channel gating but this basic topography permits structure-function studies of modulation by extracellular ions to be focused on the single putative extracellular domain.
In this paper we have characterized the sensitivity of the hP2X4 channel to extracellular pH and performed site-directed mutagenesis to identify the site of protonation. We chose to look at this member of the family because it is the most abundant P2X channel expressed centrally and because the rP2X4 channel shows the greatest sensitivity to extracellular pH (Stoop et al. 1997). We show that ATP- gated currents in hP2X4 channels are reduced by lowering extracellular pH in the physiological range. pH sensitivity is abolished by site-directed mutagenesis of histidine (H) 286 to alanine (A) indicating that protonation of this histidine mediates the pH sensitivity of the hP2X4 receptor. These data define the molecular basis of an important regulatory site on this channel. Brief reports of some of this work have appeared elsewhere in abstract form (Clarke et al. 1998).
METHODS
Preparation of hP2X4 histidine mutants
The full-length clone of hP2X4 (GenBank accession number A65875) was used in these studies. This sequence differs from that published by Garcia-Guzman et al. (1997) only in an alanine instead of a serine at position 6. Mutant H172A was introduced using the QuickChange polymerase chain reaction (PCR)-based mutagenesis kit (Stratagene) according to the manufacturer's protocol, using the expression vector, pCDN-P2X4, as the template for mutagenesis. Mutants H140A, H241A and H286A were mutated using a PCR-based method (Li & Shapiro, 1993). The 5′ primers for each mutation (H140AFOR, H241AFOR and H286AFOR) were used with the 3′ primer, pCDNREV, and pCDN-P2X4 as the template, to produce fragments of P2X4 containing each mutation at the 5′ end. These products were then used as the 3′ primer in a second PCR reaction, with pCDNFOR as the 5′ primer and pCDN-P2X4 as the template. The product of this second PCR reaction was a full-length P2X4 gene containing the mutation. The PCR products were then digested with EcoRI and BamHI, and ligated into EcoRI/BamHI-digested pCDN.
All mutations were confirmed by sequencing.
The primer sequences are as follows.
H140AFOR:
H172AFOR:
H241AFOR:
H286AFOR:
pCDNFOR:
pCDNREV:
Base changes introducing the mutations are in bold type and underlined.
Oocyte preparation and injection
Surgery was carried out in accordance with the United Kingdom Animals (Scientific Procedures) Act (1986) and conformed to SmithKline Beecham ethical standards. Oocytes were removed from each mature female Xenopus laevis on two separate occasions. For the first surgery the Xenopus laevis frog was completely anaesthetized by immersion in 0.2 % MS-222. The level of anaesthesia was assessed firstly by checking if the frog could right itself when tipped over in the anaesthetic, it was then removed from the anaesthetic and reflexes tested by both pinching and pricking the toes with a pin. Once the frog was unresponsive a small incision was made in the abdomen and oocytes removed. The incision was closed in two layers and the frog was allowed to recover in a small freshwater tank whilst being observed before being returned to the main holding tank. At least 1 week after the first removal of oocytes the frog was completely anaesthetized by immersion in 0.4 % MS-222 and then decapitated before the remaining oocytes were removed. Upon removal oocytes were placed into a sterile modified Barth's solution (MBS) containing (mM): NaCl 88, KCl 1, NaHCO3 2.4, Hepes 1.5, MgSO4 0.82, Ca(NO3)2 0.33, CaCl2 0.41; buffered to pH 7.4 with NaOH. The tissue was disaggregated into small clumps and agitated in calcium-free Barth's solution (direct replacement of calcium with magnesium) containing 1–2 % collagenase A (Boehringer Mannheim) for 1–3 h at room temperature. Injections of cDNA for either human P2X4 receptors or mutants (in pCDN vector) were made into the nuclei of defolliculated Stage 5–6 Xenopus oocytes (1.5 ng cDNA per oocyte or 5 ng cDNA per oocyte for H286A). The oocytes were then incubated in filtered MBS supplemented with 0.1 mg ml−1 gentamicin at 19–22°C. Diethyl pyrocarbonate (DEPC) results were obtained by adding 10 mM DEPC to this solution and incubating for 24 h prior to recording.
Recording
Oocytes were placed in a recording chamber 1–3 days after injection and continuously perfused (14 ml min−1) with MBS solution (adjusted to the appropriate pH with 1 M NaOH or 5 M HCl). The solution was applied using large bore tubing (internal diameter 1.5 mm) which facilitated rapid solution exchange (half-time 350–1000 ms). Recordings were obtained using the two-electrode voltage clamp technique, holding potential -80 mV (unless otherwise stated). Electrodes used were low resistance (0.8-2 MΩ) when filled with 3 M KCl. Current responses, digitized at 2 kHz and filtered at 1 kHz, were stored for later analysis using chart (CED) software.
External solution pH was equilibrated for 1 min before applying ATP, buffered to the relevant pH, for 3–5 s. This length of application of ATP allowed the current to reach maximal amplitude before washout. Reproducible responses to 100 μM ATP at pH 7.4 were recorded at the start of each experiment. These could be obtained with a washout interval of 3–5 min. Concentration- response curves to ATP were obtained at extracellular pH values of 6.5, 7.4 and 8.0 over a range of concentrations from 30 nM to 3 mM. Data were normalized to the current evoked by 100 μM ATP at pH 7.4 and curves fitted to the logistic equation using Origin software. The concentration of ATP that gave 20 % of the maximum response (EC20) at each pH was estimated from these concentration-response curves for use in later experiments.
For the experiments investigating the effect of pH on the ATP current-voltage relationship, the oocyte was bathed in the test pH solution and currents activated by ATP EC20 whilst ramp tests from -100 to +50 mV were performed using pCLAMP software (Axon Instruments).
RESULTS
Rapid bath application of 300 μM ATP onto Xenopus oocytes expressing the hP2X4 receptor, held under voltage clamp at -80 mV, resulted in rapidly rising inward currents. Maximal responses recorded reached up to 5 μA at 300 μM (pH 8) and showed rapid desensitization (Fig. 1A). With washout periods of 3–5 min, reproducible responses could be obtained. ATP-gated currents were extremely sensitive to extracellular pH. Figure 1A shows responses to 300 μM ATP from one oocyte over a range of extracellular pH. Peak amplitude of the inward current declined from 1.5 to 0.25 μA as pH was reduced from 8.0 to 6.5. Full concentration-response curves to ATP (30 nM to 3 mM) at pH 6.5, 7.4 and 8 show EC50 values of 14.5, 2.6 and 1.9 μM, respectively (Fig. 1C). Maximal responses at each of the pH values tested were obtained at 300 μM ATP and relative to 100 μM ATP at pH 7.4 were: 0.53 ± 0.11 (n = 7) at pH 6.5, 1.02 ± 0.07 (n = 6) at pH 7.4 and 1.3 ± 0.12 (n = 6) at pH 8 indicating that the maximal amplitude of response to ATP decreased with decreasing pH as well as a rightward shift in the concentration-response curve. This differed to the findings for the rP2X4 receptor in which pH 6.5 produced a rightward shift in the concentration-response curve to ATP but had no effect on the maximal response obtained (Stoop et al. 1997).
Figure 1. Effect of pH on the hP2X4 receptor.
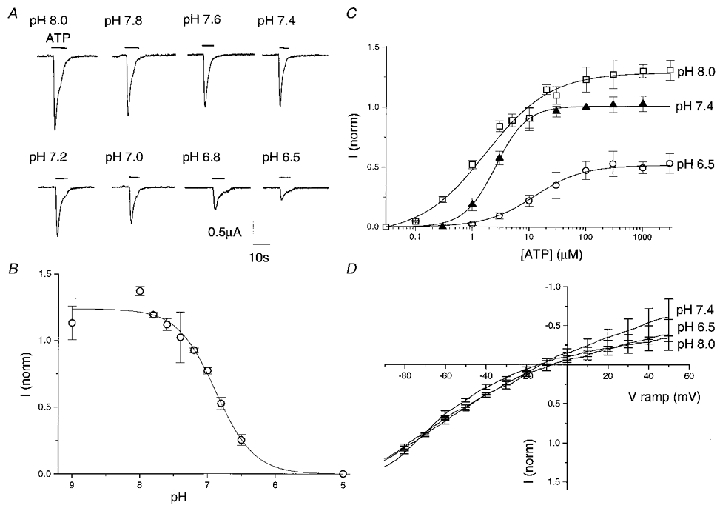
A, example of responses of the hP2X4 receptor to 300 μM ATP over a pH range from 8.0 to 6.5 recorded from a single oocyte. B, pH concentration-response curve. Responses evoked at 300 μM ATP over a range of pH from 9 to 5 were normalized to the response recorded in the same oocyte at 100 μM ATP, pH 7.4. Data plotted are means ±s.e.m. (n = 4). C, ATP concentration-response curves for the hP2X4 receptor at pH 6.5 (^), 7.4 (▴) and 8.0 (□). Responses were evoked at a range of ATP concentrations from 0.03 μM to 3 mM and normalized to the response recorded in the same oocyte at 100 μM ATP, pH 7.4. Values plotted are means ±s.e.m. (n ≥ 5). D, current-voltage relationship of the hP2X4 receptor at pH 8.0, 7.4 and 6.5, with ATP EC20. Currents were obtained by voltage ramps from -90 to +50 mV and normalized to the response obtained at -80 mV for each test pH. Data are shown as means ±s.e.m. (n = 4).
The best fitting pH titration curve to this data at 300 μM ATP yielded an apparent pKa (negative log of the acid dissociation constant) of 6.8 and a slope of 1.65 (Fig. 1B). This pKa suggests that an imidazole side chain on an extracellular histidine is most likely to be the proton binding site (Lehninger, 1975).
The rapid onset of pH-induced modulation suggests a direct effect of pH change by an extracellular site of action. To examine this further we analysed the effect of pH on the ATP-gated currents at a range of potentials. Figure 1D shows voltage ramp data generated at pH 8.0, 7.4 and 6.5 with EC20 ATP concentrations and scaled to the current at -80 mV. The ramp currents do not differ significantly, showing that the pH sensitivity is not affected by membrane potential between -80 and +50 mV. This again indicates that the pH-sensitive site is outside the membrane field.
Diethyl pyrocarbonate (DEPC) carbethoxylates the imidazole ring of histidine and the side groups of arginine, cysteine and tyrosine (Leonard et al. 1970). DEPC treatment of the hP2X4-injected oocytes was carried out in an attempt to alter the pH sensitivity of the hP2X4 receptor. We tried prolonged exposure to high concentrations of DEPC as no effect of short pre-exposure to DEPC was found on rP2X4 by Stoop et al. (1997). Responses to 300 μM ATP were recorded at pH 6.5, 7.4 and 8.0 in control oocytes and those incubated overnight in 10 mM DEPC. Data were normalized to 100 μM ATP, pH 7.4. No significant differences could be found between the two groups at any of the test pH values (n = 4 for each test pH, data not shown).
The results with DEPC are not inconsistent with pH sensitivity being mediated through histidine protonation. A histidine imidazole might be accessible to protons but not the much larger DEPC molecule. To test this hypothesis, we continued with a mutagenesis approach. As the hP2X4 amino acid sequence only contains four histidines, all predicted to be extracellular, site-directed mutagenesis of each of the histidines to alanines was carried out to form mutants H140A, H172A, H241A and H286A (Fig. 2A). Each of the mutants and wild-type P2X4 were injected into Xenopus oocytes and 1–2 days later responses to a maximal concentration of 300 μM ATP were tested at pH 6.5, 7.4 and 8.0 (Fig. 2B). While data for H140A, H172A and H241A overlaid wild-type responses, wild-type pH sensitivity was completely lost in mutant H286A. Responses to 300 μM ATP at pH 6.5 were similar in amplitude to those at pH 8.0 for H286A. ATP-induced responses of H286A, like wild-type channel, were rapidly desensitizing (Fig. 3A), but maximal currents were much smaller than those seen in wild-type channel (0.45 ± 0.17 and 2.78 ± 0.38 μA, respectively, n = 4). We also noted that expression was less efficient with H286A. Expression rates for H286A were improved by injecting 5 ng of cDNA for H286A rather than 1.5 ng but were still only 20–30 % compared to 80–90 % of oocytes responding when injected with all other mutant types and wild-type receptor. To further characterize the change in pH sensitivity in the H286A mutant, full concentration- response curves to ATP were carried out at pH 6.5, 7.4 and 8.0 (Fig. 3B). In contrast to wild-type (c.f. Fig. 1C) concentration-response curves for H286A superimpose showing that the pH sensitivity of the hP2X4 receptor at all ATP concentrations had been lost.
Figure 2. Effects of histidine mutagenesis on pH sensitivity of maximal ATP responses.
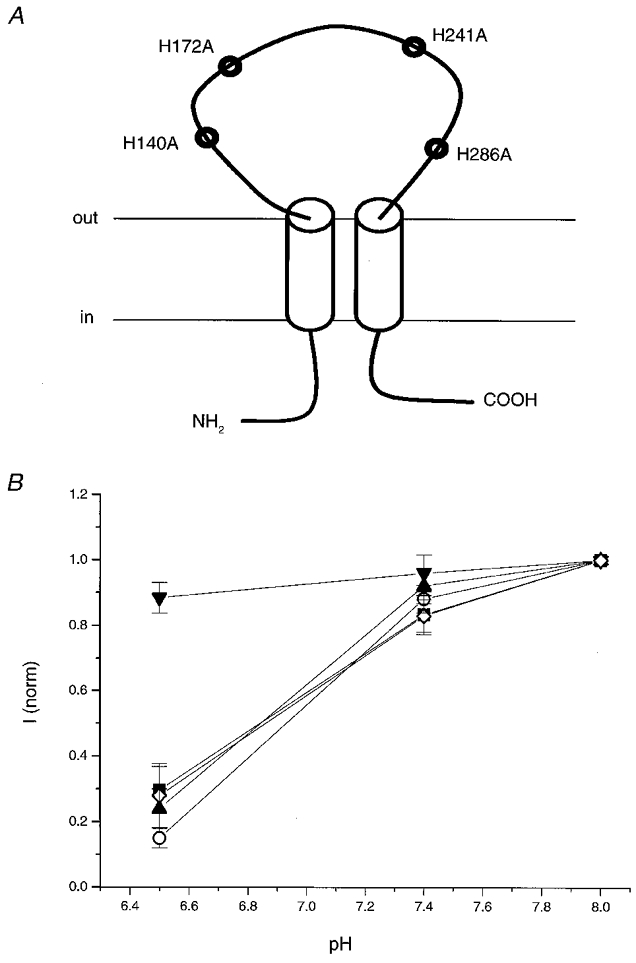
A, diagram showing the four extracellular histidines present in the hP2X4 amino acid sequence that were mutated to alanines. B, graph showing the responses of H140A (▪), H172A (^), H241A (▴), H286A (▾) and wild-type hP2X4 (⋄) to 300 μM ATP at pH 6.5, 7.4 and 8.0. Responses have been normalized to the response obtained at pH 8.0. The actual values at pH 8 were: H140A, 3.05 ± 0.46 μA; H172A, 2.42 ± 1.03 μA; H241A, 3.3 ± 0.42 μA; H286A, 0.45 ± 0.17 μA and wild-type, 2.78 ± 0.38 μA. Values plotted and actual values are means ±s.e.m. (n = 4).
Figure 3. The H286A mutant is pH insensitive.
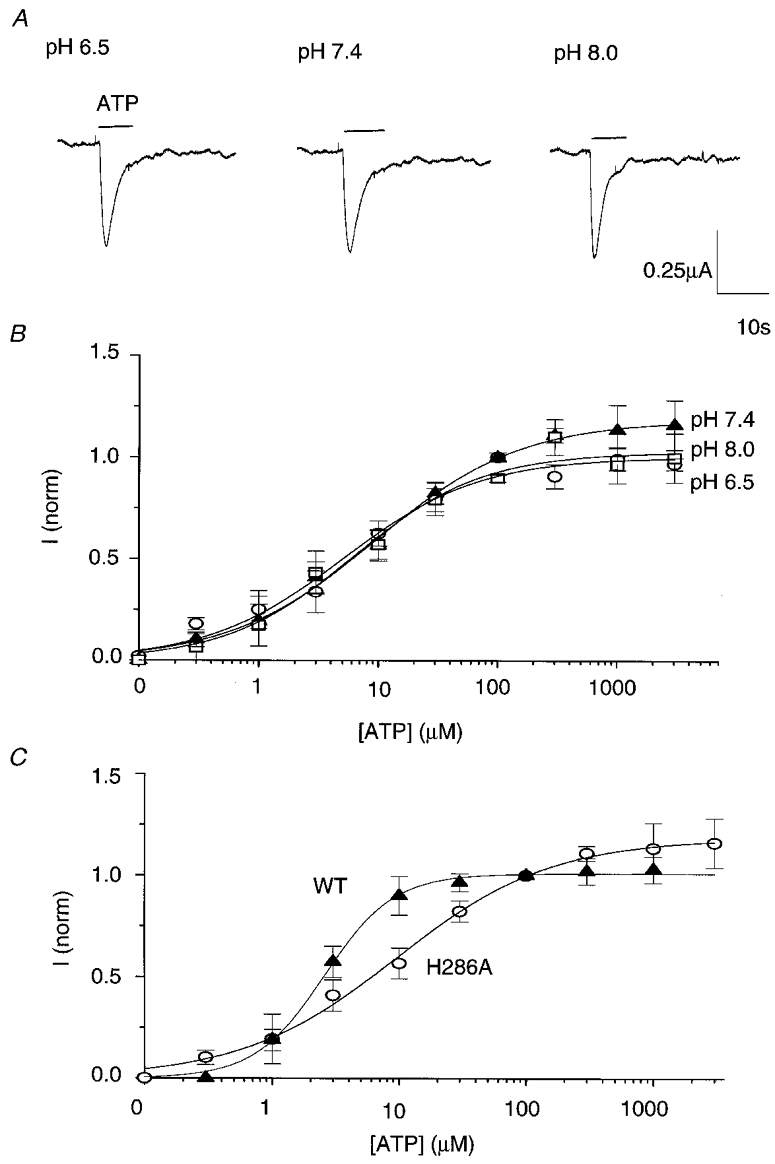
A, example of responses of mutant H286A at pH 6.5, 7.4 and 8.0. Recordings were taken from a single oocyte held at -80 mV. B, ATP concentration-response curves for the H286A mutant at pH 6.5 (^), 7.4 (▴) and 8.0 (□). Responses were evoked at a range of ATP concentrations from 0.03 μM to 3 mM and normalized to the response recorded in the same oocyte at 100 μM ATP, pH 7.4. Data plotted are means ±s.e.m. (n ≥ 5). C, plots of the concentration-response curves for ATP at pH 7.4 for wild-type (▴) and mutant H286A (^). Values were normalized to responses at 100 μM ATP, pH 7.4. The curve for mutant H286A is shifted approximately fourfold to the right compared with wild-type hP2X4. ATP EC50 values are 2.56 and 9.5 μM, respectively. Data are means ±s.e.m. (n ≥ 4).
Comparison of the ATP concentration-response curves for both wild-type and mutant H286A at pH 7.4 (Fig. 3C) revealed that the mutation caused a fourfold decrease in EC50 from 2.6 to 9.5 μM. The Hill slope for the H286A concentration-response curve at pH 7.4 was reduced from 1.58 to 0.71. These data suggest that in addition to being responsible for pH sensitivity H286 has a minor effect on ligand binding and/or gating.
DISCUSSION
Our results show that hP2X4 receptor ATP-activated currents are reduced by reducing extracellular pH and that this pH sensitivity is completely abolished by mutation of histidine 286 to alanine. Thus, protonation of a single histidine seems to confer pH sensitivity on this channel. The rP2X4 is also pH sensitive but unlike the findings of Stoop et al. (1997) we found that the maximal response to ATP in hP2X4 was also affected by pH changes. Recently published experiments on rP2X4 extending to lower pH show a reduction in maximal ATP response indicating that there is not a major difference in the behaviour of the two isoforms (Wildman et al. 1999). Our mutagenesis data provide direct support for the hypothesis that pH sensitivity is due to protonation of the channel rather than protonation of the agonist ATP (Wildman et al. 1999).
Comparison of the sequences of rat and human P2X4 (Rassendren et al. 1997) shows that H286 is conserved suggesting that H286 might also mediate the pH sensitivity in the rat isoform. The observation that the rP2X4 shows different pH sensitivity permitting full agonist responses to ATP at pH 6.3, might be explained by the subtly altered consequences for gating, dependent on other non-conserved amino acids. hP2X1 and rP2X3 channels also show similar but less dramatic pH sensitivity and have ten and two extracellular histidines, respectively. Pairwise comparisons show that none of these are precisely homologous to H286 on hP2X4. Interestingly, rP2X5 does have an analogous histidine (Collo et al. 1996), but the pH sensitivity of this channel has not been explored.
Knowledge of the structure of the P2X family of channels is to date limited. Hydrophobicity plots suggest that each subunit has two transmembrane (TM) regions and the lack of a signal peptide sequence suggests a structure with intracellular N- and C-terminals and a large extracellular loop between the two TM regions. This basic motif is supported by studies of N-glycosylation sites (Newbolt et al. 1998; Torres et al. 1998a,b). Cysteine mutagenesis of the second TM domain of P2X2 suggests that hydrophilic residues on this helix form part of the pore lining and that the N-terminal end of this region (positions 328-336) define part of the outer vestibule (Rassendren et al. 1997; Egan et al. 1998). The large extracellular loop codes for the ATP binding site and by analogy to other ion channels may contain a pore-forming loop that provides further structural elements of the pore (Brake et al. 1994). Thus the location of H286 relative to the pore cannot be defined at present. The absence of voltage dependence of pH sensitivity (Fig. 1D) indicates a site extracellular to the membrane field, consistent with the location of H286 in the extracellular loop. The lack of voltage sensitivity is also clear evidence that the pH effect is not mediated by proton block of the permeation pathway. The lack of effect of DEPC might be explained by lack of accessibility to the H286 protonation site suggesting that the H286 imidazole projects into a restricted steric space on the extracellular surface. Based on this limited data it seems most likely that the pH modulation is mediated by an allosteric conformational change that affects gating rather than by a direct interaction with the channel gate itself.
Many ion channels are modulated by pH but for relatively few has the molecular basis been determined. The H452Q mutant of the Kv1.5 channel shows about 50 % reduction in sensitivity to low extracellular pH (Steidl & Yool, 1999). Extracellular histidines also affect the pH sensitivity of the inward rectifier potassium channel, HIR (Coulter et al. 1995). However, for other channels regulated by pH, glutamate residues have been shown to be important such as the L-type voltage-gated Ca2+ channel where several glutamate residues that line the pore affect pH sensitivity (Chen et al. 1996), and CNG (cyclic nucleotide gated) channels (Rho & Park, 1998).
Human P2X4 mRNA shows widespread distribution; it was detected in many tissues analysed by Garcia-Guzman et al. (1997), and it is the predominant P2X receptor, along with P2X6 receptor in the CNS (Buell et al. 1996a). The CNS is exposed to both transient shifts in pH with normal neuronal activity (Chesler & Kaila, 1992), and more sustained acidosis with ischaemia and hypoxia in the brain (Siesjöet al. 1996). ATP may be co-released as a transmitter during normal CNS function and in greater amounts during ischaemia. The pH sensitivity of P2X4 channels may naturally limit signalling and toxicity mediated by this pathway during acidosis and might, together with the pH sensitivity of NMDA-gated channels (Traynelis & Cull-Candy, 1990), explain why increases in extracellular alkalinity have been shown to exacerbate injury of cultured cortical neurons subjected to both glutamate neuronal toxicity and oxygen- glucose deprivation (Giffard et al. 1992). In this paper we have shown that ATP-gated hP2X4 channels are acutely sensitive to extracellular pH and that the basis for this sensitivity is protonation of histidine 286.
Acknowledgments
Our thanks to Dr Andy Randall for help with the analysis of the voltage ramp data.
References
- Bo X, Zhang Y, Nassar M, Burnstock G, Schoepfer R. A P2X purinoceptor cDNA conferring a novel pharmacological profile. FEBS Letters. 1995;375:129–133. doi: 10.1016/0014-5793(95)01203-q. [DOI] [PubMed] [Google Scholar]
- Brake AJ, Wagenbach MJ, Julius D. New structural motif for ligand-gated ion channels defined by an ionotropic ATP receptor. Nature. 1994;371:519–523. doi: 10.1038/371519a0. [DOI] [PubMed] [Google Scholar]
- Buell G, Collo G, Rassendren F. P2X receptors: An emerging channel family. European Journal of Neuroscience. 1996a;8:2221–2228. doi: 10.1111/j.1460-9568.1996.tb00745.x. [DOI] [PubMed] [Google Scholar]
- Buell G, Lewis C, Collo G, North RA, Surprenant A. An anatagonist insensitive P2X receptor expressed in epithelia and brain. EMBO Journal. 1996b;15:55–62. [PMC free article] [PubMed] [Google Scholar]
- Chen X-H, Bezprozvanny I, Tsien RW. Molecular basis of proton block of L-type Ca2+ channels. Journal of General Physiology. 1996;108:363–374. doi: 10.1085/jgp.108.5.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesler M, Kaila K. Modulation of pH by neuronal activity. Trends in Neurosciences. 1992;15:396–402. doi: 10.1016/0166-2236(92)90191-a. [DOI] [PubMed] [Google Scholar]
- Clarke CE, Meadows HJ, Tomlinson WJ, Carpenter D, Sanger GJ, Benham CD. Extracellular acidification inhibits current flow through human P2X4 receptors expressed in Xenopus oocytes. The Journal of Physiology. 1998;506.P:44P. [Google Scholar]
- Collo G, North A, Kawashima E, Merlo-Pich M, Neidhart S, Surprenant A. Cloning of P2X5 and P2X6 receptors and the distribution and properties of an extended family of ATP-gated ion channels. Journal of Neuroscience. 1996;16:2495–2507. doi: 10.1523/JNEUROSCI.16-08-02495.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulter KL, Perrier F, Raked CM, Vandenburg CA. Identification and molecular localization of a pH-sensing domain for the inward rectifier potassium channel HIR. Neuron. 1995;15:1157–1168. doi: 10.1016/0896-6273(95)90103-5. [DOI] [PubMed] [Google Scholar]
- Egan TM, Haynes WR, Voigt MM. A domain contributing to the ion channel of ATP gated P2X2 receptors identified by the substituted cysteine accessibility method. Journal of Neuroscience. 1998;18:2350–2359. doi: 10.1523/JNEUROSCI.18-07-02350.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Guzman M, Soto F, Gomez-Hernandez JM, Lund P, Stühmer W. Characterisation of recombinant human P2X4 receptor reveals pharmacological differences to the rat homologue. Molecular Pharmacology. 1997;51:109–118. doi: 10.1124/mol.51.1.109. [DOI] [PubMed] [Google Scholar]
- Giffard RG, Weiss JH, Choi DW. Extracellular alkalinity exacerbates injury of cultured cortical neurons. Stroke. 1992;23:1817–1821. doi: 10.1161/01.str.23.12.1817. [DOI] [PubMed] [Google Scholar]
- Hansen MA, Barden JA, Balcar VJ, Keay KA, Bennett MR. Structural motif and characteristics of the extracellular domain of P2X receptors. Biochemical and Biophysical Research Communications. 1997;236:670–675. doi: 10.1006/bbrc.1997.6815. [DOI] [PubMed] [Google Scholar]
- King BF, Ziganshina LE, Pintor J, Burnstock G. Full sensitivity of P2X2 purinoceptor to ATP revealed by changing extracellular pH. British Journal of Pharmacology. 1996;117:1371–1373. doi: 10.1111/j.1476-5381.1996.tb15293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehninger AL. Biochemistry. New York: Worth Publishers; 1975. The amino acid building blocks of proteins; pp. 71–80. [Google Scholar]
- Leonard NJ, McDonald JJ, Reochmann ME. Reaction of diethyl pyrocarbonate with nucleic acid components. Proceedings of the National Academy of Sciences of the USA. 1970;67:93–98. doi: 10.1073/pnas.67.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Peoples RW, Weight FF. Proton potentiation of ATP-gated ion channel responses to ATP and Zn2+ in rat nodose ganglion neurons. Journal of Neurophysiology. 1996;76:3048–3058. doi: 10.1152/jn.1996.76.5.3048. [DOI] [PubMed] [Google Scholar]
- Li C, Peoples RW, Weight FF. Enhancement of ATP-activated current by protons in dorsal root ganglion neurons. Pflügers Archiv. 1997;433:446–454. doi: 10.1007/s004240050299. [DOI] [PubMed] [Google Scholar]
- Li XM, Shapiro LJ. Three-step PCR mutagenesis for ‘linker scanning’. Nucleic Acids Research. 1993;21:3745–3748. doi: 10.1093/nar/21.16.3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbolt A, Stoop R, Virginio C, Surprenant A, North RA, Buell G, Rassendren F. Membrane topology of an ATP gated ion channel (P2X receptor) Journal of Biological Chemistry. 1998;273:15177–15182. doi: 10.1074/jbc.273.24.15177. [DOI] [PubMed] [Google Scholar]
- North RA, Barnard EA. Nucleotide receptors. Current Opinion in Neurobiology. 1997;7:346–357. doi: 10.1016/s0959-4388(97)80062-1. [DOI] [PubMed] [Google Scholar]
- Rassendren F, Buell G, Newbolt A, North RA, Surprenant A. Identification of amino acid residues contributing to the pore of a P2X receptor. EMBO Journal. 1997;16:3446–3454. doi: 10.1093/emboj/16.12.3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rho S, Park C. Extracellular proton alters the divalent cation binding affinity in a cyclic nucleotide gated channel pore. FEBS Letters. 1998;440:199–202. doi: 10.1016/s0014-5793(98)01353-2. [DOI] [PubMed] [Google Scholar]
- Seguela P, Haghighi A, Soghomonian JJ, Cooper E. A novel neuronal P2X ATP receptor ion channel with widespread distribution in the brain. Journal of Neuroscience. 1996;16:448–455. doi: 10.1523/JNEUROSCI.16-02-00448.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siesjö BK, Katsura K, Kristián T. Acidosis-related damage. Advances in Neurology. 1996;71:209–233. [PubMed] [Google Scholar]
- Soto F, Garcia-Guzman M, Gomez-Hernandez JM, Hollman M, Karschin C, Stühmer W. P2X4: An ATP-activated ionotropic receptor cloned from rat brain. Proceedings of the National Academy of Sciences of the USA. 1996;93:3684–3688. doi: 10.1073/pnas.93.8.3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steidl JV, Yool AJ. Differential sensitivity of voltage-gated potassium channels Kv1.5 and Kv1.2 to acidic pH and molecular identification of pH sensor. Molecular Pharmacology. 1999;55:812–820. [PubMed] [Google Scholar]
- Stoop R, Surprenant A, North A. Different sensitivities to pH of ATP-induced currents at four cloned P2X receptors. Journal of Neurophysiology. 1997;78:1837–1840. doi: 10.1152/jn.1997.78.4.1837. [DOI] [PubMed] [Google Scholar]
- Surprenant A. P2 Purinoreceptors: Localisation, Function and Transduction Mechanisms. Chichester: Wiley; 1996. Functional properties of native and cloned P2X receptors; pp. 208–222. [DOI] [PubMed] [Google Scholar]
- Torres GE, Egan TM, Voigt MM. N-linked glycosylation is essential for functional expression of the recombinant P2X2 receptor. Biochemistry. 1998a;37:14845–14851. doi: 10.1021/bi981209g. [DOI] [PubMed] [Google Scholar]
- Torres GE, Egan TM, Voigt MM. Topological analysis of the ATP-gated ionotropic P2X2 receptor subunit. FEBS Letters. 1998b;425:19–23. doi: 10.1016/s0014-5793(98)00179-3. [DOI] [PubMed] [Google Scholar]
- Traynelis SF, Cull-Candy SG. Proton inhibition of N-methyl-D-aspartate receptors in cerebellar neurons. Nature. 1990;345:347–350. doi: 10.1038/345347a0. [DOI] [PubMed] [Google Scholar]
- Wildman SS, King BF, Burnstock G. Modulation of ATP-responses at recombinant rP2X4 receptors by extracellular pH and zinc. British Journal of Pharmacology. 1999;126:762–768. doi: 10.1038/sj.bjp.0702325. [DOI] [PMC free article] [PubMed] [Google Scholar]


