Abstract
Local inhibition in the deep layers of the superior colliculus plays a crucial role in sensorimotor integration. Using intracellular and extracellular recording techniques, we studied the organization of inhibitory circuits in the deep layers of the superior colliculus in anaesthetized rabbits.
We identified a new cell type in the deep superior colliculus that showed a characteristic burst response to stimulation of both the predorsal bundle and optic chiasm. The response had a jittering latency and failed to follow high frequency stimuli, indicating trans-synaptic (orthodromic) events. Moreover, the predorsal bundle stimulation-evoked orthodromic response could be made to collide with the response to a preceding stimulation of the optic chiasm, suggesting that burst-firing cells received excitatory inputs from the axonal collaterals of predorsal bundle-projecting cells.
Stimulation of the predorsal bundle could evoke an IPSP in predorsal bundle-projecting cells. The latency of the IPSP was 0·5-1·0 ms longer than the orthodromic response in burst-firing cells. Simultaneous recordings showed that the IPSP in predorsal bundle-projecting cells was preceded by a burst of extracellular spikes from burst-firing cells with short latency (≈0·9 ms), indicating an inhibitory monosynaptic connection from burst-firing cells to predorsal bundle-projecting cells.
Burst-firing cells exhibited a prolonged depression after the predorsal bundle or optic chiasm stimulation due to an inhibitory postsynaptic potential. Latency analysis implies that burst-firing cells may form mutual inhibitory connections.
Together our results suggest that burst-firing cells and predorsal bundle-projecting cells form reciprocal excitatory and inhibitory connections and burst-firing cells may function as the recurrent inhibitory interneurons in the deep layers of the rabbit superior colliculus.
The deep layers of the superior colliculus (SC) form a centre for sensorimotor integration in mammals (Sparks, 1986; Redgrave et al. 1993). Collicular neurons within these deep layers integrate multiple sensory inputs (e.g. visual, somatosensory and auditory), transform them into motor commands, and then convey the commands to various brain structures to initiate approach and avoidance responses (for review see Redgrave et al. 1993). In humans, monkeys, cats and rabbits, the movements of the eyes, head and ears (in some animals) are highly co-ordinated during orienting approach movements towards novel stimuli (Bartz, 1966; Bizzi et al. 1971; Collewijn, 1977; Blakemore & Donaghy, 1980). The motor commands that control these types of orienting responses are sent out by efferent neurons in SC via the predorsal bundle (PDB) and the interactions between these efferent neurons are crucial for generating co-ordinated movements (Sparks, 1986; Redgrave et al. 1993). A network of local inhibition in the deep layers of SC has been heavily implicated in t∼he interactions of efferent neurons in SC (Behan & Kime, 1996; Kadunce et al. 1997; Wang & Redgrave, 1997; Aizawa & Wurtz, 1998; Meredith & Ramoa, 1998; Munoz & Istvan, 1998). It is hypothesized that local inhibition not only moulds the discharge patterns of efferent neurons, but also co-ordinates the activity in the bilateral SC.
Although there is good anatomical and physiological evidence for the existence of local inhibitory interneurons in the deep layers of SC (Mize et al. 1994; Meredith & Ramoa, 1998; Munoz & Istvan, 1998), the exact organization of the local inhibitory circuitry remains unclear. This is due in part to the small size of interneurons, which has so far obstructed direct recording from these cells (Mize et al. 1991; Munoz & Istvan, 1998). In this study, we succeeded in recording directly from inhibitory interneurons in the deep layers of SC. We provide evidence that these cells receive excitatory inputs from the axonal collaterals of PDB-projecting cells and that they form inhibitory synapses onto both PDB-projecting neurons and themselves. The results illustrate the organization of the recurrent inhibitory circuitry of PDB-projecting neurons of the rabbit.
METHODS
Male New Zealand White rabbits weighing 2.3-3.0 kg were anaesthetized by an intravenous injection of sodium pentobarbital (Nembutal, 40 mg kg−1). All experiments were carried out according to the guidelines laid down by the Animal Care Committee of the Chinese Academy of Sciences. Supplemental doses (10 mg kg−1) were given as needed to maintain the animals in a state in which the foot withdrawal reflex was absent and/or in a state of slow-wave sleep, as determined by monitoring the cortical EEG. All pressure points and incisions were infiltrated with lidocaine. During recording, the rabbits were also immobilized by intermittent administration of gallamine triethiodide (Flaxedil, 3–5 mg kg−1 h−1) and breathing was maintained artificially. The end-tidal CO2 of the animals was kept in the range of 3.8-4.5%. Body temperature was maintained within the normal range (37.3 ± 0.3°C).
Four silver electrodes were fixed on the surface of the primary visual cortex so that we could record EEG and evoked potentials from stimulation of the optic chiasm (OX). Insulated stainless steel electrodes, with an exposed tip of 60 μm in diameter, were stereotaxically inserted into the ipsilateral central lateral nucleus of the thalamus (co-ordinates: A2, L3, D7.5), OX (co-ordinates: A3, L1, D13-15), contralateral SC (co-ordinates: P11, L3, D7) and PDB (co-ordinates: P13.5, L1.5, D11.5-13.5) according to the atlas of Sawyer et al. (1954). The final electrode tip position for OX stimulation was ascertained by finding the position at which the threshold of evoked cortical potentials was less than 50 μA. The final electrode tip positions for stimulating the central lateral nucleus, SC and PDB were fixed where visible eye movements (e.g. nystagmus and vertical saccadic eye movements; cf. Schaefer, 1970; Collewijn, 1975) were evoked at the lowest stimulating current. The current ranged from 40 to 70 μA for three-pulse stimulation at 300 Hz and ranged from 150 to 220 μA for single-pulse stimulation. This task was performed before the recording and injection of Flaxedil, and when the animals were in a lighter anaesthetized state (i.e. EEG began to show a tendency of increasing frequency and decreasing amplitude). The rabbits did not appear disturbed during the task, which typically took only a few minutes. Supplementary Nembutal was given directly following the task. The positions of the steel stimulating electrode tips were confirmed by Prussian Blue reaction at the end of the experiments. Stimulating electrode tracks were reconstructed after conventional histological processing. The test pulse was typically composed of a negative pulse (200 μs, 5–250 μA) passing through the stimulating electrodes.
Extracellular and intracellular recordings were made in the deep layers of SC. Recording microelectrodes were filled with 3 M NaCl, 3 M potassium acetate or 2% Pontamine Sky Blue in 0.5 M potassium acetate solution. The resistance of recording micropipettes was 15–35 MΩ (for PDB-projecting cells) or 25–45 MΩ (for burst-firing cells). Intracellular recordings were analysed if the recorded cells had a resting membrane potential more negative than -55 mV and overshooting action potentials. The intracellular recordings from PDB-projecting cells were very stable and the recordings lasted typically for 10–35 min. However, the intracellular recordings from burst-firing cells were much shorter lasting. Usually only 2–5 min stable recording was achieved before the cells were lost. During extracellular recordings, trans-synaptic stimulations induced orthodromic spikes with jittering latencies. We took the shortest latency of the jittering spikes as the response latency. During intracellular recordings, the latency of IPSPs induced by antidromic stimulation and EPSPs was measured at the resting membrane potential, while that of IPSPs induced by orthodromic stimulation was estimated at a depolarized membrane potential to minimize the effect of the initial EPSP. The depolarization was achieved by injecting a continuous depolarizing current (1.5-4 nA) through the recording electrode. A bridge balance circuit was used to compensate the access resistance. After recordings, Pontamine Sky Blue was injected by passing negative current (5-10 μA, 5–10 min) to localize the positions of recorded neurons, and the animals were then killed by an overdose injection of Nembutal. Recording electrode tracks were reconstructed after conventional histological processing. Each record trace contained typically three to five superimposed sweeps.
RESULTS
We recorded the extracellular and intracellular responses from 149 neurons in the deep layers of SC. Of these cells, 112 cells were identified as PDB-projecting cells because they were antidromically activated by stimulation of PDB, and two of the PDB targets, the contralateral SC and central lateral nucleus in the thalamus. The properties of this type of cell have been previously characterized (Zhu & Lo, 1995). In this study, we focused on the remaining 37 cells that responded to PDB stimulation with a characteristic burst of two to four spikes. Reconstruction of the recording electrode tracks indicated that these burst-firing cells were located in either stratum griseum intermediale or stratum griseum profundum of SC (n = 22; Fig. 1A), the same layers in which PDB-projecting cells were located (Zhu & Lo, 1995).
Figure 1. Responses and locations of burst-firing neurons in the deep layers of the superior colliculus.
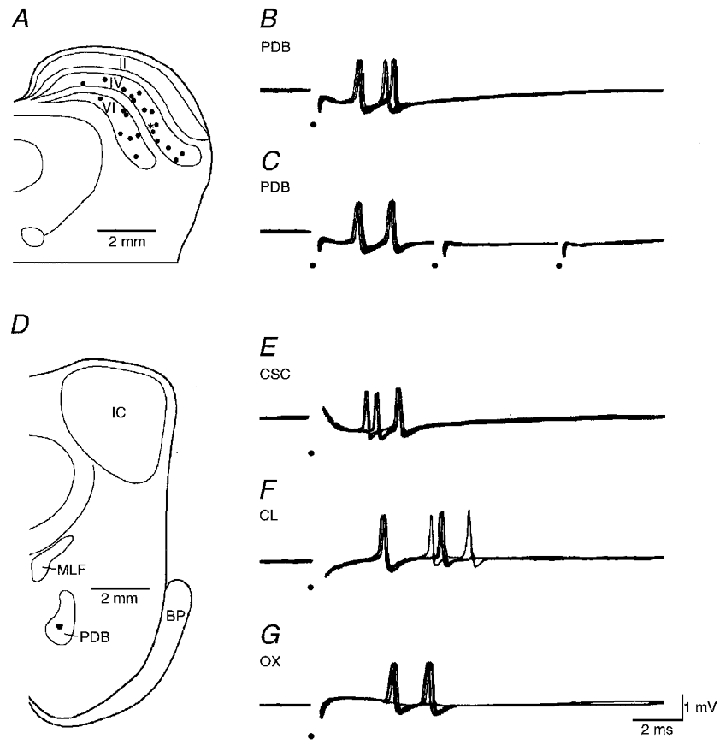
A, locations of 22 burst-firing neurons. B and C, responses of a burst-firing neuron to a single pulse (B) and a short train (C) of PDB stimulation. D, the filled spot in PDB indicates the location of the tip of PDB stimulating electrode. E-G, responses of the same neuron to single contralateral superior colliculus (CSC), central lateral nucleus (CL) and OX stimulation. Electrical stimuli are marked by dots. II, stratum griseum superficiale of the superior colliculus; IV, stratum griseum intermediale; VI, stratum griseum profundum; BP, brachium pontis; IC, inferior colliculus; MLF, medial longitudinal fasciculus; PDB, predorsal bundle.
Monosynaptic activation of burst-firing cells by axonal collaterals of PDB-projecting cells
The extracellular responses of burst-firing cells to PDB stimulation had a jittering latency (n = 37; Fig. 1B) and failed to follow high frequency stimuli (200 Hz, n = 32; Fig. 1C), suggesting involvement of synaptic transmission. The threshold for the responses ranged from 11 to 85 μA. The stimulating electrode tracks were reconstructed and the stimulating sites were all located in PDB (Fig. 1D). Similar trans-synaptic responses were obtained from the stimulation of the contralateral SC or ipsilateral central lateral nucleus (Fig. 1E and F). The mean latencies from these stimuli were 2.14 ± 1.34 ms (mean ±s.d.; n = 37), 1.60 ± 0.83 ms (n = 21) and 3.16 ± 1.47 ms (n = 25), respectively. The shortest latencies were 0.69, 0.69 and 1.07 ms, respectively (Fig. 2A–C). Both the mean and shortest latencies were 0.5-1.0 ms longer than the antidromic responses in PDB-projecting neurons (Zhu & Lo, 1995), suggesting an extra synaptic delay for the activation of burst-firing cells. Because there is no anatomical evidence showing projections from PDB and central lateral nucleus to the deep layers of SC (Holstege & Collewijn, 1982; Wells et al. 1989), burst-firing cells most likely receive monosynaptic excitatory inputs from the axonal collaterals of PDB-projecting neurons. Like PDB-projecting cells, burst-firing cells also responded to OX stimulation (n = 35; Fig. 1G). The latency of the responses ranged from 1.37 to 9.16 ms, with a mean of 3.88 ± 1.97 ms (Fig. 2D), similar to that of PDB-projecting cells (Zhu & Lo, 1995).
Figure 2. Latency distributions of burst-firing neuron responses.
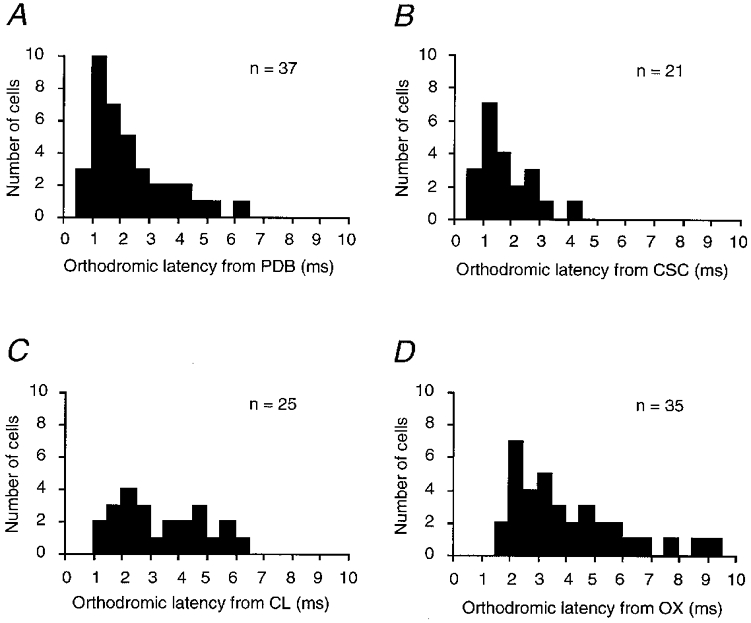
A, B, C and D are latency histograms from the stimulation of PDB, CSC, CL and OX, respectively. n is the total number of cells sampled in each histogram.
We used a modified collision test (Lo & Xie, 1987; Zhu & Lo, 1997) to confirm that burst-firing cells receive excitatory inputs from the axonal collaterals of PDB-projecting cells (n = 6). Figure 3A is a schematic diagram of our postulated recurrent inhibitory circuit. If correct, the orthodromic spike from PDB stimulation will collide with the orthodromic spike evoked by OX stimulation at some place along the axon of the PDB-projecting cell, preventing the burst-firing neuron from responding to PDB stimulation. Figure 3B–E shows the results of a collision test on a burst-firing neuron. This cell was activated orthodromically by both OX and PDB stimulation. PDB stimulation intensity was adjusted so as to induce only a single spike in the cell. When OX stimulation preceded PDB stimulation at an appropriate interval, the response from PDB stimulation was blocked (Fig. 3C). Partial or total recovery could be induced if the interval between OX and PDB stimulation was slightly increased (Fig. 3D and E). Similar results were obtained from five other burst-firing cells.
Figure 3. Collision test showing that burst-firing neurons receive excitatory inputs from the axonal collaterals of PDB-projecting cells.
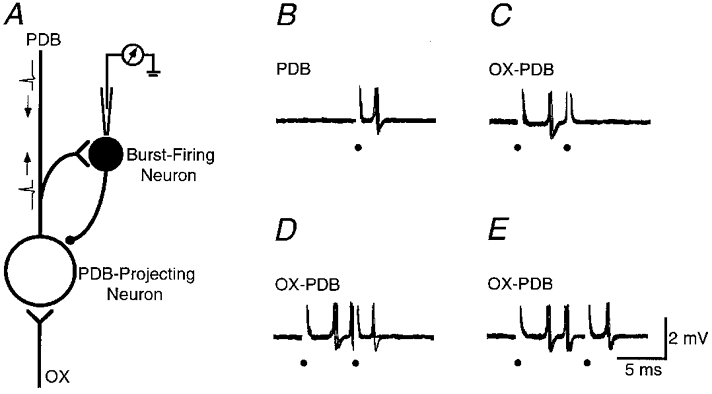
A, a schematic diagram shows the putative recurrent inhibitory circuit in the deep SC. B, responses of a burst-firing neuron to PDB stimulation. C-E, collision test. Note that the response to PDB stimulation is blocked completely (C) or partially (D) by the preceding OX stimulation. Electrical stimuli are marked by small filled circles.
Inhibitory effects of burst-firing cells on PDB-projecting cells
Our previous intracellular recordings showed that PDB stimulation induced an inhibitory postsynaptic potential (IPSP) in PDB-projecting cells (Zhu & Lo, 1995). In this study, we analysed the latency of the IPSP from 22 PDB-projecting cells. The resting membrane potential of these cells was -64.2 ± 4.2 mV (n = 22). The latency of the IPSP ranged from 1.2 to 4.5 ms, with a mean of 3.2 ± 0.8 ms (n = 22). Both the shortest and mean latencies of the response were 0.5-1.0 ms longer than those of responses in burst-firing cells recorded extracellularly, suggesting that the IPSP might result from the activation of burst-firing cells.
Using single recording micropipettes, we could sometimes record simultaneously from two distinguishable neurons in the deep layers of SC (n = 5 pairs; cf. Contreras et al. 1997; Guido et al. 1992). Figure 4A–E shows a simultaneous recording intracellularly from a neuron and extracellularly from another one. The intracellularly recorded neuron was a PDB-projecting cell since it could be antidromically activated by PDB stimulation. The extracellularly recorded neuron was a burst-firing cell that responded to PDB stimulation trans-synaptically with a burst of two to three spikes. A weak OX stimulation evoked a small excitatory postsynaptic potential (EPSP) in the PDB-projecting cell (Fig. 4A). A stronger stimulation induced a larger EPSP in the PDB-projecting cell and a burst of extracellular spikes from the nearby burst-firing cell. These responses were followed by a late IPSP in the PDB-projecting cell (Fig. 4B). A subthreshold PDB stimulation failed to induce any responses (Fig. 4C). A suprathreshold PDB stimulation induced an intracellular antidromic spike in the PDB-projecting cell and a burst of extracellular spikes in the burst-firing cell. These responses were also followed by a late IPSP in the PDB-projecting cell (Fig. 4D). The duration of the IPSPs was about 125 ms. Since the IPSPs were always preceded by a burst of extracellular spikes, it is likely that they resulted from the activation of the burst-firing cell. Similar results were obtained in the other four simultaneous recordings.
Figure 4. Simultaneous recording of a PDB-projecting neuron intracellularly and a burst-firing neuron extracellularly.
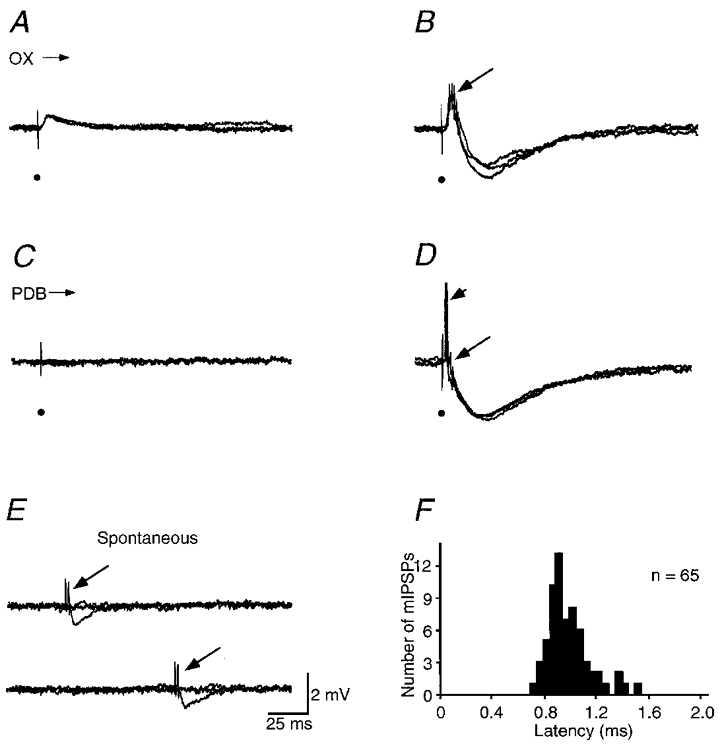
A and B, responses of the two cells to OX stimulation at low and high stimulation intensity. C and D, responses of the two cells to PDB stimulation at low and high stimulation intensity. Note that the antidromic spikes are truncated in D. E, spontaneous bursts in the burst-firing neuron evoked an inhibitory postsynaptic potential in the PDB-projecting cell. Short arrow and long arrows indicate the antidromic spikes in the PDB-projecting cells and bursts in the burst-firing neuron, respectively. The scale bar in E applies to A-E. Electrical stimuli are marked by filled circles. F, latency histogram of the miniature IPSPs recorded in PDB-projecting cells after the spontaneous bursts in burst-firing cells. Resting membrane potential of the PDB-projecting cell was -59 mV.
At times, burst-firing cells generated spontaneous bursts. The spontaneous bursts were reliably followed by a smaller IPSP in PDB-projecting cells (65 out of 68 spontaneous events recorded from 5 paired cells; Fig. 4E), indicating that the miniature IPSP in PDB-projecting cells was caused by the activation of burst-firing cells. The high release probability is consistent with the burst firing in presynaptic cells (Lisman, 1997). The latency of the miniature IPSP after the burst ranged from 0.68 to 1.47 ms, with a mean of 0.94 ± 0.16 ms (n = 65 from 5 pairs of cells; Fig. 4F). These results suggest that burst-firing cells form an inhibitory monosynaptic connection on PDB-projecting cells.
Mutual inhibition of recurrent inhibitory interneurons
Burst-firing cells exhibited a depression after an initial excitation. This was revealed by a paired stimulation test (Fig. 5A). The probability of discharges from these cells after OX stimulation was taken as an index. Without any conditioning stimulation, the probability of discharges evoked by a test OX stimulation was set at 100%. After a conditioning stimulation of OX, the probability of discharges dropped remarkably at certain conditioning-test intervals. The time course of the depression could be obtained by plotting the probabilities of responses against conditioning- test intervals. The depression after OX stimulation lasted for about 125 ms and peaked at about 40–60 ms (n = 7). A very similar depression was found after PDB stimulation (n = 7; Fig. 5B). The similarity of the depression curves suggests that the depressions after OX or PDB stimulation may be mediated by the same neuronal circuit.
Figure 5. Depression of a burst-firing neuron after OX or PDB stimulation.
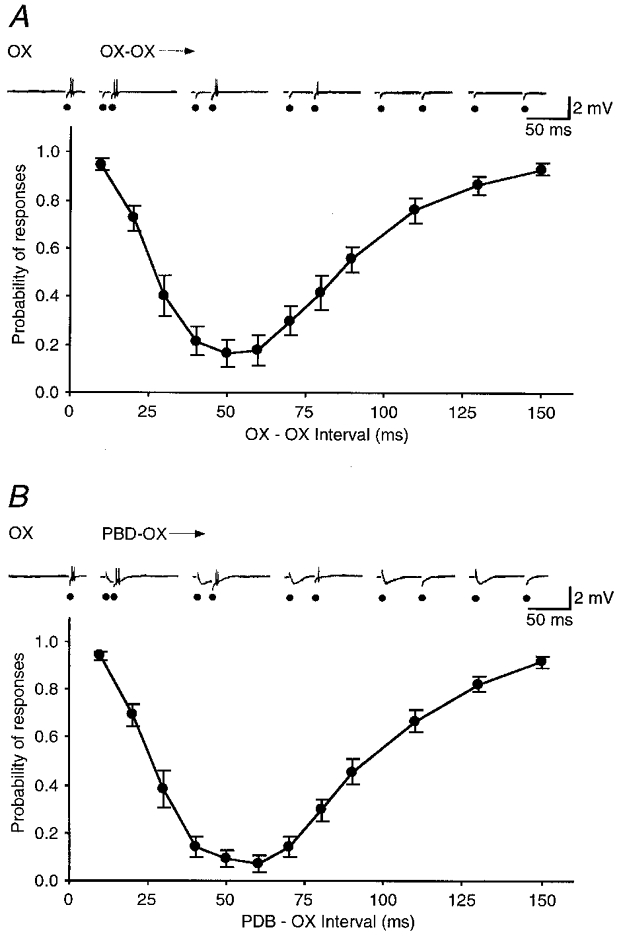
The ordinate shows the probability of response to a test OX stimulation after a sub-threshold conditioning OX (A) or PDB (B) stimulation at different intervals. The abscissa shows the interval between the conditioning and test stimulation. The data points are averaged from 7 cells and error bars show the standard errors of the mean. The recording traces on the top exemplify responses at five points on the curves recorded from one cell.
To confirm that the same neuronal circuit is responsible for the depressions after OX or PDB stimulation, we tested whether the summation of the weak conditioning OX and PDB stimulations could induce the depression (Fig. 6). In this experiment, the intensity of OX and PDB stimulation was set at a low level so that they alone did not affect the discharge probability induced by test OX stimulation. When these weak OX and PDB stimulations were applied together, they produced a prominent suppression of the test responses (n = 4; Fig. 6B). The time course of the depression was similar to the depression induced by OX or PDB stimulation (Fig. 5). The result thus supports the idea that the depressions after OX or PDB stimulation share a similar circuit.
Figure 6. Depression of a burst-firing neuron after OX and PDB stimulation.
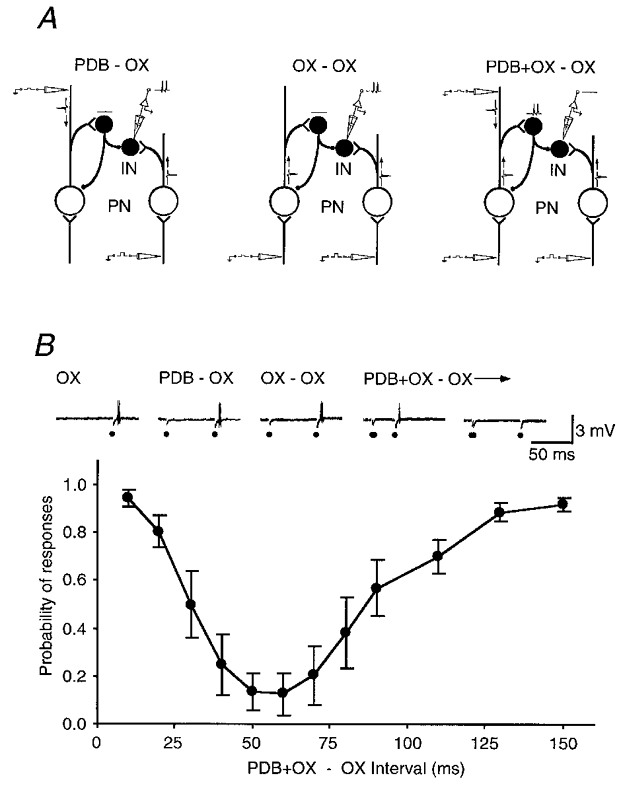
A, a schematic diagram shows the experimental design. Note that neither OX nor PDB stimulation alone at low intensity will block the suprathreshold OX stimulation-evoked responses, but together they will. IN, inhibitory burst-firing neuron; PN, PDB-projecting neuron. B, the ordinate shows the probability of a response to a test OX stimulation after a combination of conditioning OX and PDB stimulation at different intervals. The abscissa shows the interval between the conditioning and test stimulation. The data points are averaged from 4 cells and error bars show the standard errors of the mean. The recording traces on the top exemplify responses at two points on the curve recorded from one cell.
To reveal the underlying mechanism of the depression, we impaled some burst-firing cells after extracellular recording (Fig. 7). The resting membrane potential of these cells was -63.6 ± 4.0 mV (n = 5). Both OX and PDB stimulation induced an EPSP, consistent with the notion that burst-firing cells were activated trans-synaptically by OX and PDB stimulation (Fig. 7C and D). The EPSP was followed by a prolonged IPSP. The IPSP induced by OX stimulation had a duration of 114 ± 9 ms (n = 5), while that induced by PDB stimulation had a duration of 120 ± 13 ms (n = 5). The latencies of these IPSPs were estimated by holding cells at a depolarized membrane potential by injecting a depolarizing current. They were 0.8-1.1 ms longer than the latencies of extracellular spikes recorded in the same cells from the corresponding stimuli, implying an extrasynaptic delay for the IPSPs (Fig. 7E and F). The similarity of the IPSPs evoked by either OX or PDB stimulation is consistent with the view that the same inhibitory circuit is involved in generating the IPSPs.
Figure 7. Extracellular and intracellular recordings from a burst-firing neuron.
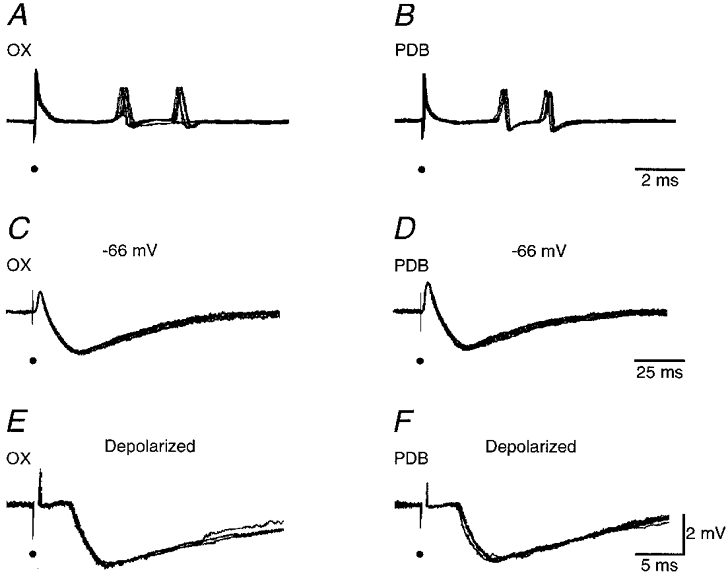
A and B, extracellular responses of the interneuron to OX and PDB stimulation, respectively. C and D, intracellular responses of the same burst-firing neuron to OX and PDB stimulation at a lower intensity to avoid action potentials. E and F, intracellular responses of the same neuron to OX and PDB stimulation at a depolarized holding potential by injecting 1.2 nA depolarizing current. Electrical stimuli are marked by small filled circles. Resting membrane potential of the cell was -66 mV.
DISCUSSION
In this study, we identified a group of burst-firing cells in the deep layers of SC of the rabbit. These neurons receive excitatory inputs from the axonal collaterals of PDB-projecting cells, and in turn inhibit PDB-projecting cells, as well as themselves. These data suggest that burst-firing cells in the SC function as the interneurons mediating recurrent inhibition for PDB-projecting cells.
Synaptic organization in the deep SC
Axonal patterns of PDB-projecting cells have been examined in rats (Tokunaga & Otani, 1976), cats (Grantyn & Grantyn, 1982; Moschovakis & Karabelas, 1985), hamsters (Rhoades et al. 1987) and monkeys (Moschovakis et al. 1988). These studies have shown that some PDB-projecting cells in SC send recurrent collaterals back into SC. It has been speculated that these recurrent collaterals may be involved in generating recurrent inhibition and/or excitation (e.g. Keller & Edelman, 1994; Munoz & Istvan, 1998; Pettit et al. 1999). However, since local circuit neurons had not been identified, it was still an open question whether recurrent inhibitory circuits exist.
In this study, we recorded from a population of neurons in SC that responded to PDB stimulation with an EPSP and a burst of orthodromic spikes. Since the stimulating current spread was limited within PDB and its vicinity (∼400 μm in diameter, giving the current a threshold of ∼85 μA; Zhu & Lo, 1995, 1996), where no afferent was found to project to the deep layers of SC (Holstege & Collewijn, 1982; Wells et al. 1989), the responses probably resulted from the activation of the recurrent bcollaterals of PDB. This hypothesis was supported by latency analysis of the responses in burst-firing cells and in PDB-projecting neurons, and confirmed by the modified collision test. Because the PDB stimulation-evoked IPSP in PDB-projecting cells was one synaptic delay longer than the response in burst-firing cells, the IPSP might result from the activation of burst-firing cells. Indeed, we found that PDB and OX stimulation induced a burst of spikes in burst-firing cells and an IPSP in PDB-projecting cells during simultaneous recordings. Moreover, the spontaneous bursts in burst-firing cells induced a small IPSP in PDB-projecting cells with high reliability. This indicates that the IPSP is due to the activation of burst-firing cells. Dual recordings from synaptically connected PDB-projecting and burst-firing cells (Contreras et al. 1997; Lampl et al. 1999) should further confirm this result. Together, our results suggest that PDB-projecting cells and burst-firing cells in SC form reciprocal excitatory and inhibitory connections and burst-firing cells serve as the recurrent inhibitory interneurons for PDB-projecting neurons. This notion is consistent with the findings that both antidromic and orthodromic stimuli can induce an IPSP in PDB-projecting cells (Moschovakis et al. 1988; Zhu & Lo, 1995).
The latency of recurrent inhibitory interneurons from OX stimulation was 3.88 ms, which was about 0.7 ms longer than that for neurons in the superficial layers of SC (Zhu & Lo, 1994, 1995). This result indicates that the main visual inputs to interneurons come from an indirect pathway, presumably via the ventral lateral geniculate nucleus and/or superficial layers of SC (e.g. Takahashi et al. 1977; Conley & Friederich-Ecsy, 1993; Isa et al. 1998). However, the shortest OX latency for interneurons (1.37 ms) was not longer, but rather shorter than that for superficial layer cells (1.78 ms; Zhu & Lo, 1994). Therefore, like PDB-projecting neurons (Zhu & Lo, 1995), some interneurons may also receive a direct retinal input. These results are in the line with the previous observations on the monkey, cat and rat (Berson & McIlwain, 1982; Beckstead & Frankfurter, 1983).
Recurrent interneurons responded to OX and PDB stimulation with an initial excitation followed by a prolonged depression. Intracellular recording revealed that a sequence of EPSP-IPSP was the underlying mechanism for the biphasic responses in recurrent interneurons. The depressions or IPSPs induced by OX and PDB stimulation had a similar time course, suggesting the involvement of the same inhibitory circuit. This was confirmed by the depression induced by the combination of weak OX and PDB stimulations, which alone produced no depression. Because the latency of the IPSPs had an extra synaptic delay, the IPSPs are likely to be mediated by the mutual inhibitory connections between recurrent interneurons.
The putative inhibitory circuitry in the deep layers of SC is shown in Fig. 8, which reveals a striking similarity between this recurrent inhibitory system and several other recurrent inhibitory systems in the CNS, for instance, those in the lateral geniculate nucleus (e.g. Ahlsén et al. 1984, 1985; Lo & Xie, 1987; Zhu & Lo, 1999), lateral posterior-pulvinar complex (e.g. Zhu & Lo, 1997, 1998), and the well-known ventral spinal horn (e.g. Eccles et al. 1954; Ryall, 1970; Hultborn et al. 1971). This raises the intriguing possibility that similar neuronal circuitry is used by the CNS to process both ascending sensory and descending motor information.
Figure 8.
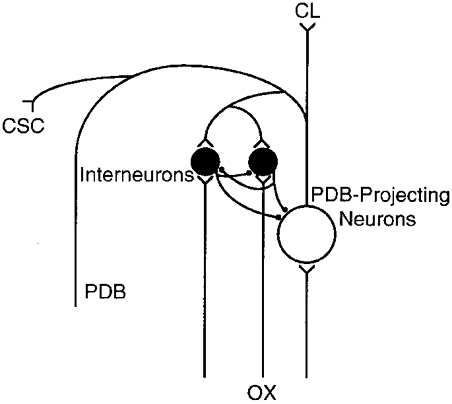
Inhibitory circuitry in the deep layers of the superior colliculus.
Functional significance of recurrent inhibitory circuitry in SC
Inhibition plays an important role in integrating inputs and moulding ouputs (e.g. Ferster & Jagadeesh, 1992; Binns & Salt, 1997; Larkum et al. 1999). Since some PDB-projecting cells send recurrent collaterals back to the area near to their somata (Grantyn & Grantyn, 1982; Moschovakis & Karabelas, 1985) and PDB-projecting cells can be inhibited by their neighbouring interneurons (this study), it is possible that interneurons may provide feedback inhibition to the PDB-projecting cells which provide them with excitation. This type of ‘true’ recurrent inhibition (Lo & Sherman, 1994) is useful to regulate the output gain of efferent neurons (Hultborn et al. 1979). In addition, some recurrent axonal collaterals of PDB-projecting cells travel over a long distance in the ipsilateral SC and give rise to commissural collaterals projecting to the contralateral SC (Grantyn & Grantyn, 1982; Moschovakis & Karabelas, 1985), they may mediate lateral inhibition (Hultborn et al. 1971). The lateral inhibition is believed to be important for PDB-projecting cells to integrate different modalities of sensory inputs and co-ordinate the outputs from the different parts of the ipsilateral and contralateral SC (Rhoades et al. 1987; Kadunce et al. 1997; Munoz & Istvan, 1998).
PDB-projecting neurons in SC are also involved in an inhibitory pathway from SC to the thalamus, which may provide a visual suppression during saccadic eye movements (Lo, 1988; Zhu & Lo, 1996, 1998). When PDB-projecting neurons in the deep layers of SC initiate saccadic eye movement, some of them also feed excitation, via the central lateral nucleus in the thalamus, to recurrent inhibitory interneurons for the lateral geniculate nucleus and lateral posterior-pulvinar complex in the thalamus. Activation of these recurrent interneurons in the thalamic reticular nucleus will modulate the ascending visual transmission in the thalamus during saccadic eye movements by generating a prolonged IPSP in thalamocortical cells. It is important for the visual suppression to be released after the eyes stop moving and focus on a new target. Previously, we have suggested the rebound excitation in thalamocortical cells and mutual inhibition in reticular cells serve this purpose. In addition, the recurrent inhibitory circuitry in SC may also help to terminate the suppression by preventing PDB-projecting cells from sending continuous excitatory signals to the inhibitory pathway. Thus, multiple synaptic and intrinsic properties are employed by the inhibitory pathway to ensure that saccadic suppression is released following saccadic eye movements.
Acknowledgments
The authors thank Drs Troy Margrie, Paul Heggelund, Michael Brecht, Ted Weyand, José Esteban and Sarah Lodwich for their helpful comments, and Dr Y. Qin and G.-Y. Xie for expert technical assistance. The major part of this and other related studies was completed by J. J. Zhu as a partial fulfilment of the requirements for a degree of Master of Science awarded by the Chinese Academy of Sciences. This work was supported by the Chinese Academy of Sciences, the National Natural Science Foundation of China and the Max-Planck Gesellschaft.
References
- Ahlsén G, Lindström S, Lo F-S. Inhibition from the brain stem of inhibitory interneurones of the cat's dorsal lateral geniculate nucleus. The Journal of Physiology. 1984;347:593–609. doi: 10.1113/jphysiol.1984.sp015085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlsén G, Lindström S, Lo F-S. Interaction between inhibitory pathway to principal cells in the lateral geniculate nucleus of the cat. Experimental Brain Research. 1985;58:134–143. doi: 10.1007/BF00238961. [DOI] [PubMed] [Google Scholar]
- Aizawa H, Wurtz RH. Reversible inactivation of monkey superior colliculus. I. Curvature of saccadic trajectory. Journal of Neurophysiology. 1998;79:2082–2096. doi: 10.1152/jn.1998.79.4.2082. [DOI] [PubMed] [Google Scholar]
- Bartz AE. Eye and head movements in peripheral vision: nature of compensatory eye movements. Science. 1966;152:1644–1645. doi: 10.1126/science.152.3729.1644. [DOI] [PubMed] [Google Scholar]
- Beckstead RM, Frankfurter A. A direct projection from the retina to the intermediate gray layer of the superior colliculus demonstrated by anterograde transport of horseradish peroxidase in monkey, cat and rat. Experimental Brain Research. 1983;52:261–268. doi: 10.1007/BF00236635. [DOI] [PubMed] [Google Scholar]
- Behan M, Kime NM. Intrinsic circuitry in the deep layers of the cat superior colliculus. Visual Neuroscience. 1996;13:1031–1042. doi: 10.1017/s0952523800007689. [DOI] [PubMed] [Google Scholar]
- Berson DM, McIlwain JT. Retinal Y-cell activation of deep-layer cells in superior colliculus of the cat. Journal of Neurophysiology. 1982;47:700–714. doi: 10.1152/jn.1982.47.4.700. [DOI] [PubMed] [Google Scholar]
- Binns KE, Salt TE. Different roles for GABAA and GABAB receptors in visual processing in the rat superior colliculus. The Journal of Physiology. 1997;504:629–639. doi: 10.1111/j.1469-7793.1997.629bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizzi E, Kalil RE, Morasso P. Eye-head coordination in monkeys: evidence for centrally patterned organization. Science. 1971;173:452–454. doi: 10.1126/science.173.3995.452. [DOI] [PubMed] [Google Scholar]
- Blakemore C, Donaghy M. Co-ordination of head and eyes in the gaze changing behaviour of cats. The Journal of Physiology. 1980;300:317–335. doi: 10.1113/jphysiol.1980.sp013164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collewijn H. Oculomotor areas in the rabbits midbrain and pretectum. Journal of Neurobiology. 1975;6:3–22. doi: 10.1002/neu.480060106. [DOI] [PubMed] [Google Scholar]
- Collewijn H. Eye and head movements in freely moving rabbits. The Journal of Physiology. 1977;266:471–498. doi: 10.1113/jphysiol.1977.sp011778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley M, Friederich-Ecsy B. Functional organization of the ventral lateral geniculate complex of the tree shrew (Tupaia belangeri): II. Connections with the cortex, thalamus, and brainstem. Journal of Comparative Neurology. 1993;328:21–42. doi: 10.1002/cne.903280103. [DOI] [PubMed] [Google Scholar]
- Contreras D, Dürmüller N, Steriade M. Plateau potentials in cat neocortical association cells in vivo– synaptic control of dendritic excitability. European Journal of Neuroscience. 1997;9:2588–2595. doi: 10.1111/j.1460-9568.1997.tb01688.x. [DOI] [PubMed] [Google Scholar]
- Eccles JC, Fatt P, Koketsu K. Cholinergic and inhibitory synapses in a pathway from motor-axon collaterals to motoneurones. The Journal of Physiology. 1954;126:524–562. doi: 10.1113/jphysiol.1954.sp005226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferster D, Jagadeesh B. EPSP-IPSP interactions in cat visual cortex studied with in vivo whole-cell patch recording. Journal of Neuroscience. 1992;12:1262–1274. doi: 10.1523/JNEUROSCI.12-04-01262.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grantyn A, Grantyn R. Axonal patterns and sites of termination of cat superior colliculus neurons projecting in the tecto-bulbo-spinal tract. Experimental Brain Research. 1982;46:243–256. doi: 10.1007/BF00237182. [DOI] [PubMed] [Google Scholar]
- Guido W, Lu SM, Sherman SM. Relative contributions of burst and tonic responses to the receptive field properties of lateral geniculate neurons in the cat. Journal of Neurophysiology. 1992;68:2199–2211. doi: 10.1152/jn.1992.68.6.2199. [DOI] [PubMed] [Google Scholar]
- Holstege G, Collewijn H. The efferent connections of the nucleus of the optic tract and the superior colliculus in the rabbit. Journal of Comparative Neurology. 1982;209:139–175. doi: 10.1002/cne.902090204. [DOI] [PubMed] [Google Scholar]
- Hultborn H, Jankowska E, Lindström S. Relative contribution from different nerves to recurrent depression of Ia IPSPs in motoneurones. The Journal of Physiology. 1971;215:637–664. doi: 10.1113/jphysiol.1971.sp009489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H, Lindström S, Wigström H. On the function of recurrent inhibition in the spinal cord. Experimental Brain Research. 1979;37:399–403. doi: 10.1007/BF00237722. [DOI] [PubMed] [Google Scholar]
- Isa T, Endo T, Saito Y. The visuo-motor pathway in the local circuit of the rat superior colliculus. Journal of Neuroscience. 1998;18:8496–8504. doi: 10.1523/JNEUROSCI.18-20-08496.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadunce DC, Vaughan JW, Wallace MT, Benedek G, Stein BE. Mechanisms of within- and cross-modality suppression in the superior colliculus. Journal of Neurophysiology. 1997;78:2834–2847. doi: 10.1152/jn.1997.78.6.2834. [DOI] [PubMed] [Google Scholar]
- Keller EL, Edelman JA. Use of interrupted saccade paradigm to study spatial and temporal dynamics of saccadic burst cells in superior colliculus in monkey. Journal of Neurophysiology. 1994;72:2754–2770. doi: 10.1152/jn.1994.72.6.2754. [DOI] [PubMed] [Google Scholar]
- Lampl I, Reichova I, Ferster D. Synchronous membrane potential fluctuations in neurons of the cat visual cortex. Neuron. 1999;22:361–374. doi: 10.1016/s0896-6273(00)81096-x. [DOI] [PubMed] [Google Scholar]
- Larkum ME, Zhu JJ, Sakmann B. A new cellular mechanism for coupling inputs arriving at different cortical layers. Nature. 1999;398:338–341. doi: 10.1038/18686. [DOI] [PubMed] [Google Scholar]
- Lisman JE. Burst as a unit of neural information: making unreliable synapses reliable. Trends in Neurosciences. 1997;20:38–43. doi: 10.1016/S0166-2236(96)10070-9. [DOI] [PubMed] [Google Scholar]
- Lo F-S. A study of neuronal circuitry mediating the saccadic suppression in the rabbit. Experimental Brain Research. 1988;71:618–622. doi: 10.1007/BF00248755. [DOI] [PubMed] [Google Scholar]
- Lo F-S, Sherman SM. Feedback inhibition in the cat's lateral geniculate nucleus. Experimental Brain Research. 1994;100:365–368. doi: 10.1007/BF00227207. [DOI] [PubMed] [Google Scholar]
- Lo F-S, Xie G-Y. Location of interneurones in the recurrent inhibitory circuit of the rabbit lateral geniculate nucleus. Experimental Brain Research. 1987;66:83–89. doi: 10.1007/BF00236204. [DOI] [PubMed] [Google Scholar]
- Meredith MA, Ramoa AS. Intrinsic circuitry of the superior colliculus: pharmacophysiological identification of horizontally oriented inhibitory interneurons. Journal of Neurophysiology. 1998;79:1597–1602. doi: 10.1152/jn.1998.79.3.1597. [DOI] [PubMed] [Google Scholar]
- Meredith MA, Stein BE. Descending efferents from the superior colliculus relay integrated multisensory information. Science. 1985;227:657–659. doi: 10.1126/science.3969558. [DOI] [PubMed] [Google Scholar]
- Mize RR, Jeon CJ, Hamada OL, Spencer RF. Organization of neurons labeled by antibodies to gamma-aminobutyric acid (GABA) in the superior colliculus of the Rhesus monkey. Visual Neuroscience. 1991;6:75–92. doi: 10.1017/s0952523800000924. [DOI] [PubMed] [Google Scholar]
- Mize RR, Whitworth RH, Nunes-Cardozo B, van der Want J. Ultrastructural organization of GABA in the rabbit superior colliculus revealed by quantitative postembedding immunocytochemistry. Journal of Comparative Neurology. 1994;341:273–287. doi: 10.1002/cne.903410211. [DOI] [PubMed] [Google Scholar]
- Moschovakis AK, Karabelas AB. Observations on the somatodendritic morphology and axonal trajectory of intracellularly HRP-labeled efferent neurons located in the deeper layers of the superior colliculus of the cat. Journal of Comparative Neurology. 1985;239:276–308. doi: 10.1002/cne.902390304. [DOI] [PubMed] [Google Scholar]
- Moschovakis AK, Karabelas AB, Highstein SM. Structure-function relationships in the primate superior colliculus. I. Morphological classification of efferent neurons. Journal of Neurophysiology. 1988;60:232–262. doi: 10.1152/jn.1988.60.1.232. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Istvan PJ. Lateral inhibitory interactions in the intermediate layers of the monkey superior colliculus. Journal of Neurophysiology. 1998;79:1193–1209. doi: 10.1152/jn.1998.79.3.1193. [DOI] [PubMed] [Google Scholar]
- Pettit DL, Helms MC, Lee P, Augustine GJ, Hall WC. Local excitatory circuits in the intermediate gray layer of the superior colliculus. Journal of Neurophysiology. 1999;81:1424–1427. doi: 10.1152/jn.1999.81.3.1424. [DOI] [PubMed] [Google Scholar]
- Redgrave P, Westby GW, Dean P. Functional architecture of rodent superior colliculus: relevance of multiple output channels. Progress in Brain Research. 1993;95:69–77. doi: 10.1016/s0079-6123(08)60358-1. [DOI] [PubMed] [Google Scholar]
- Rhoades RW, Mooney RD, Klein BG, Jacquin MF, Szczepanik AM, Chiaia NL. The structural and functional characteristics of tectospinal neurons in the golden hamster. Journal of Comparative Neurology. 1987;255:451–465. doi: 10.1002/cne.902550311. [DOI] [PubMed] [Google Scholar]
- Ryall RW. Renshaw cell mediated inhibition of Renshaw cells: patterns of excitation and inhibition from impulses in motor axon collaterals. Journal of Neurophysiology. 1970;33:257–270. doi: 10.1152/jn.1970.33.2.257. [DOI] [PubMed] [Google Scholar]
- Sawyer CH, Everett JW, Green JD. The rabbit diencephalon in stereotaxic coordinates. Journal of Comparative Neurology. 1954;101:801–824. doi: 10.1002/cne.901010307. [DOI] [PubMed] [Google Scholar]
- Schaefer KP. Unit analysis and electrical stimulation in the optic tectum of rabbits and cats. Brain, Behavior and Evolution. 1970;3:222–240. doi: 10.1159/000125475. [DOI] [PubMed] [Google Scholar]
- Sparks DL. Translation of sensory signals into commands for control of saccadic eye movements: role of primate superior colliculus. Physiological Reviews. 1986;66:118–171. doi: 10.1152/physrev.1986.66.1.118. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Ogawa T, Takimori T, Kato H. Intracellular studies of rabbit's superior colliculus. Brain Research. 1977;123:170–175. doi: 10.1016/0006-8993(77)90652-7. [DOI] [PubMed] [Google Scholar]
- Tokunaga A, Otani K. Dendritic patterns of neurons in the rat superior colliculus. Experimental Neurology. 1976;52:189–205. doi: 10.1016/0014-4886(76)90164-3. [DOI] [PubMed] [Google Scholar]
- Wang S, Redgrave P. Microinjections of muscimol into lateral superior colliculus disrupt orienting and oral movements in the formalin model of pain. Neuroscience. 1997;81:967–988. doi: 10.1016/s0306-4522(97)00191-7. [DOI] [PubMed] [Google Scholar]
- Wells GR, Hardiman MJ, Yeo CH. Visual projections to the pontine nuclei in the rabbit: orthograde and retrograde tracing studies with WGA-HRP. Journal of Comparative Neurology. 1989;279:629–652. doi: 10.1002/cne.902790410. [DOI] [PubMed] [Google Scholar]
- Zhu JJ, Lo F-S. Physiological properties of neurons projecting to the lateral posterior nucleus-pulvinar in the superior colliculus of the rabbit. Chinese Journal of Physiological Sciences. 1994;10:289–294. [Google Scholar]
- Zhu JJ, Lo F-S. Physiological properties of the output neurons in the deep layers of the superior colliculus of the rabbit. Brain Research Bulletin. 1995;38:495–505. doi: 10.1016/0361-9230(95)02021-i. [DOI] [PubMed] [Google Scholar]
- Zhu JJ, Lo F-S. Time course of inhibition induced by a putative saccadic suppression circuit in the dorsal lateral geniculate nucleus of the rabbit. Brain Research Bulletin. 1996;41:281–291. doi: 10.1016/s0361-9230(96)00201-8. [DOI] [PubMed] [Google Scholar]
- Zhu JJ, Lo F-S. Recurrent inhibitory interneurons of the rabbit's lateral posterior-pulvinar complex. Journal of Neurophysiology. 1997;78:3117–3124. doi: 10.1152/jn.1997.78.6.3117. [DOI] [PubMed] [Google Scholar]
- Zhu JJ, Lo F-S. Control of recurrent inhibition of the lateral posterior-pulvinar complex by afferents from the deep layers of the superior colliculus of the rabbit. Journal of Neurophysiology. 1998;80:1122–1131. doi: 10.1152/jn.1998.80.3.1122. [DOI] [PubMed] [Google Scholar]
- Zhu JJ, Lo F-S. Three GABA receptor-mediated postsynaptic potentials in interneurons in the rat lateral geniculate nucleus. Journal of Neuroscience. 1999;19:5721–5730. doi: 10.1523/JNEUROSCI.19-14-05721.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]


