Abstract
Twenty animals with benign esophageal strictures are presented. Most of the esophageal strictures were thought to be related to gastroesophageal reflux during ovariohysterectomy and were located at the distal portion of the thoracic esophagus (caudal to the base of the heart). For the dilation procedure, the endoscope tip or a balloon catheter was used and the outcome was generally considered to be good. The endoscope tip was an adequate instrument for dilation in some cases.
Introduction
Acquired benign esophageal stricture is not very common in the dog and cat (1,2,3). It usually occurs secondary to severe esophagitis that extends into the muscle layers of the esophageal wall and results in scar tissue formation (1,2,3,4,5,6,7,8). The most common causes of esophagitis include chemical, thermal, traumatic, and infectious agents; persistent vomiting; esophageal foreign bodies; and gastroesophageal reflux, usually occurring during anesthesia (1,2,4,5,9,10).
Stricture may occur at any point along the esophagus (5). Clinical signs depend on the location and the diameter of the stricture and include regurgitation, esophagodynia, hypersalivation, weight loss (3,4,7,8), and respiratory signs (coughing, tachypnea, diffuse crackles) due to aspiration pneumonia (9,10). For diagnosis, both the esophagogram and esophagoscopy can be used to provide complementary information. An esophagogram identifies the number, location, and length of strictures, while esophagoscopy permits the direct visualization of at least the most cranial stricture site and has the benefit of mucosal evaluation (1,3,9).
Therapeutic options include surgical resection and anastomosis or a variety of technically demanding alternative surgical methods (2,11,12), as well as dilation by using balloon catheters, bougies, endotracheal tubes, or the endoscope tip (5,13), with each presenting advantages and disadvantages (3,14).
The purpose of the present study was to evaluate the effects of various predisposing factors on the pathogenesis of esophageal stricture formation and to report on the outcome of the non-surgical therapeutic procedures used in 13 dogs and 7 cats.
Materials and methods
Thirteen dogs and 7 cats with esophageal stricture formation were referred to the Veterinary Teaching Hospital, School of Veterinary Medicine, Aristotle University of Thessaloniki, Greece, from January 1992 to June 1999.
On admission, historical data were collected and a thorough physical examination was carried out in every case, followed by a) complete blood count, b) biochemical screening (serum urea nitrogen, creatinine, alkaline phosphatase, alanine aminotransferase, glucose, creatine kinase, lactate dehydrogenase, and total proteins), c) fecal examination (Baermann, Teleman, Faust), d) thoracic radiography (plain and contrast), and e) esophagoscopy using a flexible endoscope (Olympus type XP-20; Olympus Inc., Hamburg, Germany).
Barium contrast esophagogram and esophagoscopy provided the definitive diagnosis in every case. For estimation of luminal diameter at the site of the stricture the endoscope tip and foley catheters were used.
Dilation was performed using either the endoscope tip or a balloon catheter (Large Omega NV Balloon Dilation Catheter; William Cook Europe A/S, Bjaeverskov, Denmark) passed under endoscopic visualization. Selection of the appropriate method was based upon the size of the stricture. The dilation trial was repeated depending on clinical evaluation. The mucosa was carefully examined for hemorrhage, tears and perforation after every dilation procedure.
In order to manage post dilation esophagitis prednisone (0.5 mg/kg BW, q12h, for dogs, 1 mg/kg BW, q12h, for cats) was given in combination with cisapride (1.0 mg/kg BW, q12h,), slurry of sucralfate (1 g/30 kg/BW, q8h,), cimetidine (10 mg/kg BW, q8h,), and antibiotics. Cisapride is usually used to increase the lower esophageal sphincter and to improve gastric emptying. Feeding with canned food (i/d; Hill's Pet Nutrition, Topeka, Kansas, USA) was instituted within 24 to 48 h of the dilation procedure. One month after successful management of esophageal stricture, the toleration for dry food was tried. Canned food was recommended for the rest of the life of those animals that regurgitated the dry food.
Results
History, therapeutic procedure and outcome of all the cases are shown in Table I. Nine of the 20 animals (45%) had a history of being subjected to routine elective ovariohysterectomy, in which the stage of ovarian cycle could not be determined; 2 (10%) had been ovariohysterectomized due to pyometra; one (5%) had a cesarian section (c-section); one (5%) had septic peritonitis due to bite wounds, which was managed surgically; 3 (15%) had an esophageal foreign body that had been removed endoscopically (Figure 1); and 1 (5%) was suffering from non-specific gastroenteritis. In the remaining 3 animals (15%), no indication of the causative agent was retrieved from the history. Eighteen of the 20 animals were female (90%) and the rest male. The age of the animals ranged in age from 4 mo to 13 y (mean, 4.75 y; median, 3 y). Clinical signs of esophageal stricture were evident by 8 to 21 d following the exposure of the animals to the suspected causative agent. Clinical signs included hypersalivation, dysphagia, esophagodynia and progressively worsening regurgitation of solid food.
Table I.
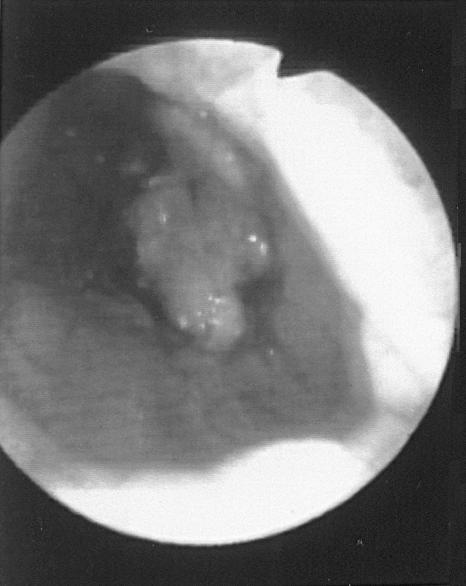
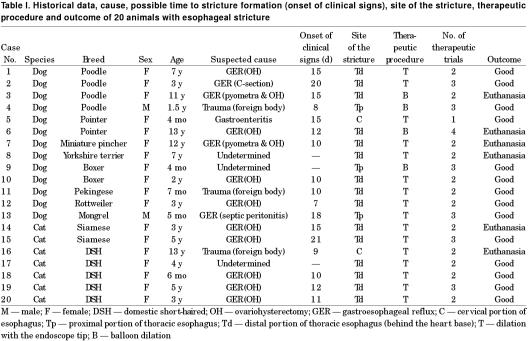
Figure 1. Endoscopic photo of a bone lodged in the esophagus of a dog (case 11).
The anesthetic regimen that had been used in the animals previously subjected to a surgical procedure included xylazine hydrochloride (Rompun; Veterin, Athens, Greece), atropine sulphate (Atropine; Demo, Athens, Greece) and ketamine hydrochloride (Ketaset; Ceva, Athens, Greece) all given intramuscularly (IM) in the cats, while in the dogs included propionylpromazine (Combelen; Veterin), given IM, and thiopentone sodium (Pentothal; Abbott, Athens, Greece) given IV. For the maintenance of a surgical plane of anesthesia, halothane (Halothan; Hoechst, Athens, Greece) in oxygen was administered, in all dogs and cats, after endotracheal intubation had been performed.
All the animals had only one stricture, which was located at the cervical portion of the esophagus in 2 animals (10%), at the proximal portion of the thoracic esophagus (cranial to the base of the heart) in 3 animals (15%), or at the distal portion of the thoracic esophagus (caudal to the base of the heart) in 15 (75%). All the 12 animals that had been ovariohysterectomized or had a c-section were found to have the stricture located in the distal portion of the thoracic esophagus (Figure 2).
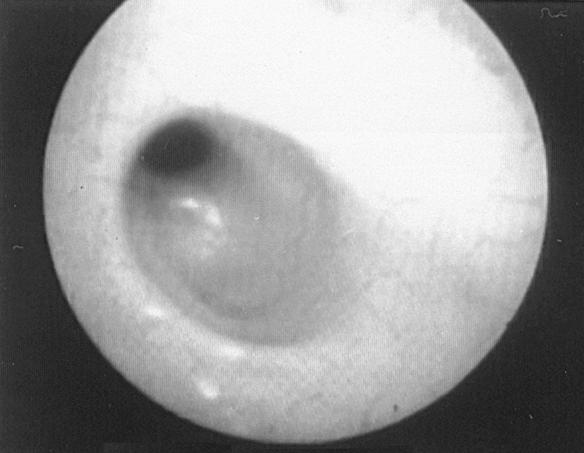
Figure 2. Endoscopic view of an esophageal stricture, demonstrating small diameter of the esophageal lumen just cranial to lower esophageal sphincter.
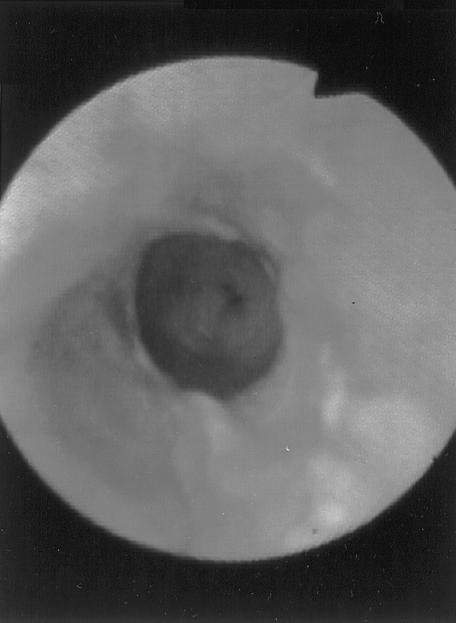
The diameter of the esophageal lumen at the stricture site was estimated using the endoscope tip and visual comparison with the known diameters of foley catheters. Luminal diameter was estimated roughly to be 5 to 7 mm when the endoscope tip could be passed, even with some difficulty, and below 5 mm as indicated by comparison with foley catheters. During endoscopic examination of the esophageal mucosa evidence of active esophagitis was detected in 3 animals (dogs 1, 7, and 11) (Figure 3). The endoscope tip was used as a dilator in cases with wider strictures while a balloon catheter was used in those with the narrower strictures (Figure 4). The number of repeat dilations ranged from one to 4 and the intervals between them were individually estimated and ranged from 4 to 7 d (Figure 5). Successful outcome required one dilation procedure in one (5%) animal, 2 in 12 animals (60%), 3 in 6 animals (30%), and 4 in one animal (5%).
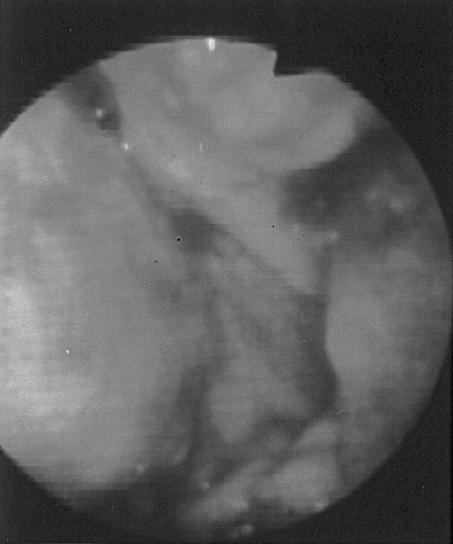
Figure 3. Endoscopic view of the esophageal mucosa after bone retrieval (case 11). Note the extensive erosions and necrosis of the esophageal mucosa.
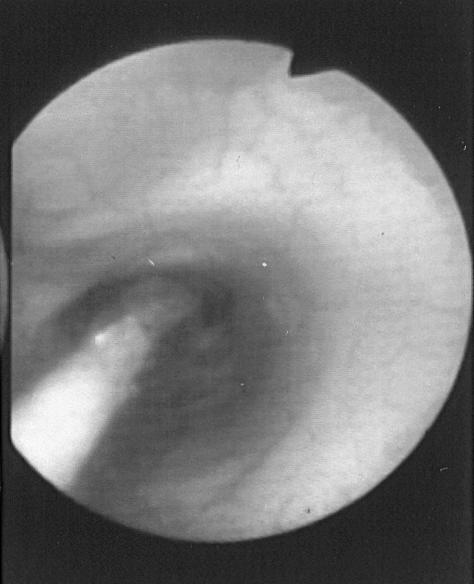
Figure 4. Endoscopic view of a balloon dilator in an esophageal stricture.
Figure 5. A dilated esophageal stricture (2nd procedure).
The outcome was considered to be good when complete resolution of clinical signs was noted or when they were occurring much less frequently (regurgitation of dry food only) and the animals were in an acceptable clinical condition, as assessed by their owners. Fourteen (70%) dogs and cats had a good outcome, while 6 (30%) were euthanized at the owner's request because of poor response or poor prognosis.
The endoscope tip was used for dilation in 16 animals. Among them, 12 (75%) animals had a good outcome and 4 (25%) were euthanized. Dog no. 7 was euthanized because of esophageal perforation during dilation procedure and poor prognosis, while the remaining 3 animals were euthanized because their owners were not persuaded that there was a possibility for a good outcome. A balloon catheter was used for dilation in 4 animals. Among them, 2 animals had a good outcome, and 2 were euthanized at the owner's request because of the expense of repeat therapy.
Discussion
According to the history, the etiology of stricture formation in most of the 20 animals included in the present study was esophagitis secondary to gastroesophageal reflux occurring during anesthesia (65%). It is known that anesthesia-related reflux is the most common cause of esophageal stricture and it can occur after relatively short or long surgical procedures (3). Many preanesthetic agents and the most commonly used induction agents reduce lower esophageal sphincter pressure, predisposing the animal to gastroesophageal reflux (4,5,7,15,16,17). Atropine, xylazine, and to a lesser extent, propionylpromazine, which were used as premedicants in our cases, have been reported to increase the incidence of gastroesophageal reflux in dogs (6). On the contrary, thiopentone has been associated with a rather low incidence of reflux in dogs (12,16), and ketamine has been reported to decrease lower esophageal sphincter pressure in cats less than other commonly used anesthetic agents (15). However, although the incidence of anesthesia related reflux has been reported to be high enough in the dog (6,17,18), very few animals develop postanesthetic esophageal stricture (17,18), suggesting that other factors (abnormal esophageal clearance, altered mucosa resistance, modified esophageal mucosal cell junctions) than the mere presence of gastric contents in the esophagus may have to be present for mucosal damage to develop.
The incidence of reflux was significantly higher in dogs that had undergone abdominal surgery than in dogs subjected to non-abdominal operations and it was speculated that intra-abdominal manipulations during surgery might account for this difference (18). The head-down position selected by several surgeons may predispose to reflux esophagitis (4,7). However, body position (sternal, dorsal, and left or right lateral) and the tilt of the body during surgery (horizontal or tilted to an 8° head-up or head-down position) had no influence on the incidence of gastroesophageal reflux (18). Seemingly, although body position reduced lower esophageal sphincter pressure, the risk of reflux was also found to be minimal (19). It is surprising that the majority of the animals in the literature developed esophageal stricture after ovariohysterectomy rather than any other surgical procedure (1,2,5,11,14,20,21,22). Similarly, esophageal stricture developed following obstetric surgery (ovariohysterectomy or c-section) in the majority of the animals of the present study (12 of 20, 60%), percentage which was higher among the animals that had previously undergone a surgical procedure (12 of 13, 92.3%). The high incidence of esophageal stricture formation following obstetric surgery in small animals may simply reflect the fact that ovariohysterectomy in particular is one of the most common surgical procedures requiring intra-abdominal manipulations. However, other factors may also be responsible. During pregnancy in human beings, the likelihood of reflux is increased as a result of the elevated progesterone levels, which decrease lower esophageal sphincter pressure, and not by the increased gastric pressure from an enlarging uterus, as it was previously thought (3). Furthermore, from human and experimental studies it was found, that progesterone, in combination with estrogen, reduces basal lower esophageal sphincter pressure, and this may account for the increased incidence of reflux symptoms observed in pregnant women (23,24,25).
Besides gastroesophageal reflux during anesthesia, other causes of esophageal strictures are chemical burns, foreign body trauma, persistent vomiting, and hiatal hernia (3). In our study, esophageal stricture was also observed after endoscopic removal of a lodged esophageal foreign body (3 animals) and after an incidence of acute gastroenteritis (1 animal), while in 3 animals the etiology remained undetermined in spite of the thorough questioning of the owners about the possible causes.
According to the results of our study, females (18/20, 90%) seem to be at greater risk of developing benign esophageal stricture because of ovariohysterectomy. Prevalence in females was found also in other studies (2,5,11,14,15,18,20,21,22) and, as already mentioned, it is mainly related to the fact that stricture formation was observed commonly after ovariohysterectomy.
In the present study, clinical signs were evident by 8 to 21 d following the exposure of the animals to the suspected causative agent. It has been recorded that a mature stricture may take up to a week to form (2,4,8), although clinical signs attributable to stricture formation can develop within 2 d of the insult (21). Occasionally, significant clinical signs are not evident until 4 to 6 wk later (3).
Esophagogram may detect long or multiple strictures, which cannot be found if an endoscope cannot pass through the most proximal stricture (4,8,9). However, Pearson et al (21), found that the radiographic appearance did not always correspond exactly to the length of the stricture. On the other hand, the diameter and the activity of the stricture can be judged more accurately endoscopically than radiographically (9,26). Moreover, in the majority of cases a solitary, fibrous band is noted (4). In our study, the confirmation of the presence of the sole esophageal stricture was done by an esophagogram and the condition of the esophageal mucosa was evaluated endoscopically.
Strictures in the dog and cat may occur at any point along the esophagus (4,5). In our study the majority of strictures was observed at the distal portion of the thoracic esophagus (caudal to the heart base, 75%) and in a lesser frequency at the cervical (10%) and at the proximal portion of the thoracic esophagus (15%). The causes of the strictures cranial to the heart base were variable (removal of lodged foreign bodies, gastroenteritis, surgical management of septic peritonitis, and undetermined). It is interesting that in all the animals, which had undergone obstetric surgery, the strictures were located at the distal portion of the thoracic esophagus. In some previous reports the findings were variable; although there seems to be a rather equal distribution along the entire length of the esophagus (1,5,9,21,26), some authors report a high incidence of stricture formation at or around the thoracic inlet perhaps as a result of gastric fluid pooling in this region (11,14,20,22). These contradictory findings may be related to the animal's position during surgery and the volume of gastric content (5). The head-down position, possibly in association with a large volume of gastric content, partially determines the volume of the refluxate and may predispose to pooling of gastric fluid in the more cranial segments of the esophagus whenever the anti-reflux mechanism becomes incompetent (5,18). On the other hand, a horizontal position and a small volume of gastric content may account for strictures located at the distal portion of the thoracic esophagus. All the animals in the present study had been kept in a horizontal position during surgery.
The results of treatment for esophageal strictures have been less than completely satisfactory (1). The described methods have included dilation by using the endoscope tip (5,13), bougies, or balloon catheters, as well as surgical resection and anastomosis or a variety of technically demanding alternative surgical methods (2,3,11,12). Endotracheal tubes may be used as dilators for cervical esophageal strictures in small dogs and cats (14). Human studies comparing bougienage and balloon catheter dilation techniques suggest that they are equally effective and safe (8,10,27). Although the incidence of side effects and complications may be largely operator-dependent, balloon dilation might be safer when performed by experienced operators (10). In this study, dilation of strictures was performed using the endoscope tip or a balloon catheter, depending on the estimated diameter of the esophageal lumen. It seems that when the passage of the endoscope tip through the stricture is possible, even with some difficulty, the prognosis is good in most of the cases. This may indicate that the wider esophageal lumen has a better ability to dilate, has to be widened less or that such a stricture is generally less severe than narrower lessions, for the animal to be symptom free. Furthermore, having in mind the cost, special equipment and skill needed for the balloon dilation (8,9,14) the use of the endoscope tip as a dilator may be judged advantageous in certain cases. The esophageal perforation of the dog no. 7 was attributed to the severe degree of esophagitis, which was extended into the muscle layers, according to the histopathology performed at the time of necropsy.
Some strictures may require multiple dilation procedures (from 2 to more than 10) (3,7,8). It is not possible to predict the number of dilation procedures that will be required for each patient (8). In our cases the therapeutic procedures ranged from one to 4 and it seems that the animals with a good outcome did not need more than 3.
After the dilation trial, medical management is used to optimize chances for a successful outcome (8). Goals of the medication administered are to decrease ongoing reflux, resolve present inflammation and prevent stricture reformation (7,8). H2-antagonists or proton-pump inhibitors, prokinetic agents and mucosal protectives, as well as diet modification in a consistency (slurry or gruel) that can be swallowed and retained have been suggested (8). The use of corticosteroids in anti-inflammatory dosages in order to decrease fibroblastic activity and prevent further stricture formation is controversial (8), and some studies do not support their value (3,7). In the present cases, the administration of corticosteroids in combination with cisapride, sucralfate, cimetidine, antibiotics, and dietary measures seemed to be beneficial.
In conclusion, esophageal stricture in dogs and cats is usually associated with obstetric surgery. Strictures are often located at the distal part of the thoracic esophagus (caudal to the heart base) and, dilation with the endoscope tip may be effective in less severe cases.
Footnotes
Address correspondence and reprint requests to Dr. Katerina K. Adamama-Moraitou, tel: +30 31 994502, fax: +30 31 994470, e-mail: kadamama@vet.auth.gr
Received July 3, 2001. Accepted October 22, 2001.
References
- 1.Burk RL, Zawie DA, Garvey MS. Balloon catheter dilation of intramural esophageal strictures in the dog and cat: a description of the procedure and a report of six cases. Semin Vet Med Surg (Small Anim) 1987;2:241–247. [PubMed]
- 2.Johnson KA, Maddison JE, Allan GS. Correction of cervical esophageal stricture in a dog by creation of a traction diverticulum. J Am Vet Med Assoc 1992;201:1045–1048. [PubMed]
- 3.Tams TR. Diseases of the esophagus. In: Handbook of Small Animal Gastroenterology. Philadelphia: WB Saunders, 1996:163–216.
- 4.Zawie DA. Esophageal strictures. In: Kirk RW, Bonagura JD, eds. Current Veterinary Therapy X. Small Animal Practice. Philadelphia: WB Saunders, 1989:904–906.
- 5.Galatos AD, Rallis T, Raptopoulos D. Post anaesthetic oesophageal stricture formation in three cats. J Small Anim Pract 1994;35:638–642.
- 6.Galatos AD, Raptopoulos D. Gastro-oesophageal reflux during anaesthesia in the dog: the effect of preoperative fasting and premedication. Vet Rec 1995;137:479–483. [DOI] [PubMed]
- 7.Guilford WG, Strombeck DR. Diseases of swallowing. In: Guilford WG, Center CA, Strombeck DR, Williams DA, Meyer DJ, eds. Small Animal Gastroenterology. 3rd ed. Philadelphia: WB Saunders, 1996:211–238.
- 8.Weyrauch EA, Willard MD. Esophagitis and benign esophageal strictures. Compend Contin Educ Pract Vet 1998;20:203–211.
- 9.Harai BH, Johnson SE, Sherding RG. Endoscopically guided balloon dilatation of benign esophageal strictures in 6 cats and 7 dogs. J Vet Intern Med 1995;9:332–335. [DOI] [PubMed]
- 10.Willard MD, Weyrauch EA. Esophagitis. In: Bonagura JD, ed. Kirk's Current Veterinary Therapy XIII. Small Animal Practice. Philadelphia: WB Saunders, 2000:607–610.
- 11.Gregory CR, Gourley IM, Bruyette DS, Schultz LJ. Free jejunal segment for treatment of cervical esophageal stricture in a dog. J Am Vet Med Assoc 1988;193:230–232. [PubMed]
- 12.Fingeroth JM. Surgical techniques for oesophageal disease. In: Slatter DJ, ed. Textbook of Small Animal Surgery. 2nd ed. Philadelphia: WB Saunders, 1993:549–561.
- 13.Papazoglou LG, Patsikas M, Rallis T, Galatos A, Adamama-Moraitou K. Hiatal hernia with esophageal stricture in a cat. Feline Pract 2000;28:10–14.
- 14.Hardie EM, Greene RT, Ford RB, Davidson MG, Herring M. Balloon dilatation for treatment of esophageal stricture: a case report. J Am Anim Hosp Assoc 1987;23:547–550.
- 15.Hashim MA, Waterman AE. Effects of thiopentone, propofol, alphaxalone-alphadolone, ketamine and xylazine-ketamine on lower oesophageal sphincter pressure and barrier pressure in cats. Vet Rec 1991;129:137–139. [DOI] [PubMed]
- 16.Waterman AE, Hashim MA. Effects of thiopentone and propofol on lower oesophageal sphincter and barrier pressure in the dog. J Small Anim Pract 1992;33:530–533.
- 17.Raptopoulos D, Galatos AD. Gastro-oesophageal reflux during anaesthesia induced with either thiopentone of propofol in the dog. J Vet Anaesth 1997;24:20–22.
- 18.Galatos AD, Raptopoulos D. Gastro-oesophageal reflux during anaesthesia in the dog: the effect of age, positioning and type of surgical procedure. Vet Rec 1995;137:513–516. [DOI] [PubMed]
- 19.Waterman AE, Hashim MA, Pearson H. Effect of body position on oesophageal and gastric pressures in the anaesthetised dog. J Small Anim Pract 1995;36:196–200. [DOI] [PubMed]
- 20.Harvey HJ. Iatrogenic esophageal stricture in the dog. J Am Vet Med Assoc 1975;166:1100–1102. [PubMed]
- 21.Pearson H, Darke PGG, Gibbs C, Kelly DF, Orr CM. Reflux oesophagitis and stricture formation after anaesthesia: a review of seven cases in dogs and cats. J Small Anim Pract 1978;19: 507–519. [DOI] [PubMed]
- 22.Harvey CE, Chiapella AM, Dubielzig RR. Palato-pharyngeal fusion, laryngeal necrosis and esophageal stricture by gastric reflux in a dog: a case report. J Am Anim Hosp Assoc 1981;17: 213–217.
- 23.Nebel OT, Fornes MF, Castell DO. Symptomatic gastroesophageal reflux: Incidence and precipitating factors. Am J Dig Dis 1976;21:953–956. [DOI] [PubMed]
- 24.Van Thiel DH, Gavaler JS, Stremple JL. Lower esophageal sphincter pressure in women using sequential oral contaceptives. Gastroenterol 1976;71:232–234. [PubMed]
- 25.Schulze K, Christensen J. Lower sphincter of the Opossum esophagus in pseudopregnancy. Gastroenterol 1977;73: 1082–1085. [PubMed]
- 26.Sullivan M, Miller A. Endoscopy (fibreoptic) of the oesophagus and stomach in the dog with persistent regurgitation or vomiting. J Small Anim Pract 1985;26:369–379.
- 27.Scolapio JS, Pasha TM, Gostout CJ, Mahoney DW, Ott BJ, Lindor KD. A randomized prospective study comparing rigid to balloon dilators for benign esophageal strictures and ring. Gastrointest Endosc 1999;50:13–17. [DOI] [PubMed]