Abstract
The well-developed cytoarchitecture of rat organotypic spinal cord culture makes it a suitable model to explore how persistent suppression of certain synaptic inputs might be compensated by increased synaptic efficacy (homeostatic plasticity).
Spontaneous or electrically evoked synaptic transmission of patch-clamped ventral horn interneurons was studied in control solution after blocking, for the second week in culture, AMPA/kainate receptors with CNQX or glycine and GABAA receptors with strychnine and bicuculline, or indiscriminately removing inputs with tetrodotoxin (TTX).
In untreated cells, spontaneous postsynaptic currents (PSCs) had fast (τ < 5 ms) or slow (τ > 10 ms) decay. A similar separation was observed when recording miniature currents (mPSCs). Slow decay PSCs were suppressed by strychnine plus bicuculline while fast decay events were eliminated by CNQX.
After chronic CNQX treatment the frequency of spontaneous, fast PSCs (of larger amplitude) or mPSCs was almost doubled with respect to control. These events were blocked by acutely applied CNQX, which unmasked slow PSCs.
After chronic TTX treatment neither the frequency nor the amplitude of spontaneous events was changed.
After chronic strychnine and bicuculline treatment the frequency and amplitude of all PSCs was decreased in most cells. mPSCs were also decreased in frequency. Spontaneous or electrically evoked currents acquired a larger component mediated by NMDA receptor activity.
The developing spinal network thus operated distinct homeostatic processes which led to strong enhancement in glutamatergic transmission after CNQX block or to broad downregulation of synaptic activity following chronic exposure to strychnine and bicuculline.
Extensive deprivation of excitatory inputs to a central network like the mammalian visual cortex largely influences its growth and function (Shatz, 1990). It is, however, less clear if a more discrete, albeit long-lasting, suppression of a certain transmitter system can be compensated, through homeostatic mechanisms, by a neuronal network. This issue raises the question of whether nerve cells can establish a functionally coherent pattern of signalling despite removal of discrete inputs: to examine this aspect, chronic block of either excitatory or inhibitory synaptic transmission has been employed as an experimental model. As a consequence of such treatment, a novel form of synaptic modification which has been termed homeostatic plasticity (Turrigiano, 1999) emerges, whereby neurons respond to sustained increases or decreases in their excitatory inputs by changing the properties, distribution and/or composition of certain transmitter postsynaptic receptors (Craig, 1998; Turrigiano, 1999). In particular, block of GABA and/or glycine receptors leads to downregulation of spontaneous glutamatergic transmission, while chronic block of glutamate receptors boosts on-going postsynaptic glutamate receptor-mediated activity. Such phenomena have been recently described in cultured mammalian neurons from various brain areas (Bessho et al. 1994; Rao & Craig, 1997; Kirsch & Betz, 1998; Levi et al. 1998; Lissin et al. 1998; O'Brien et al. 1998; Turrigiano et al. 1998) and, in addition to receptor changes, may include alterations in membrane excitability and consequently in firing activity (Desai et al. 1999). These observations are compatible with a recent hypothesis (originated from studies of the chick embryo spinal cord; Chub & O'Donovan, 1998) that the electrical behaviour of a network is governed by homeostatic mechanisms, that is, any long-lasting reduction in excitatory activity would be counteracted by increasing the efficacy of distinct excitatory transmitter systems. Whether homeostatic plasticity is a paradigm for network remodelling during neuronal development in mammals remains, however, an intriguing and scarcely explored possibility. During development, qualitative and quantitative reshaping of afferent projections may normally occur, thus leading to variations in excitatory input (Crair, 1999). Furthermore, the onset of axonal sprouting and de novo synaptogenesis is influenced by synaptic activity itself (Cline & Constantine-Paton, 1990), suggesting that activity regulates the synaptic targeting of each receptor type in a complex way (Craig, 1998).
To explore the presence of homeostatic plasticity and its role in regulating network activity during development, we wished to use a culture system which generates a considerable degree of network organization. For this purpose we employed organotypic spinal cord cultures from rat embryos (Spenger et al. 1991; Streit et al. 1991; Ballerini & Galante, 1998; Ballerini et al. 1999), as this preparation maintains the basic cytoarchitecture of a spinal segment, and enables direct recording from visually identified neurons during in vitro development (Ballerini & Galante, 1998; Ballerini et al. 1999). Activity-dependent modulation of synaptic transmission was investigated by recording postsynaptic currents (PSCs) and miniature PSCs (mPSCs) from ventral horn interneurons at 14 days in vitro (DIV) in cultures incubated for the entire second week in the presence of various receptor blockers and comparing these responses with those of control cultures. Although these interneurons have not been individually characterized in terms of their excitatory or inhibitory function, it is likely that the large population sampled in the present study included excitatory as well as inhibitory interneurons since a strong, mixed input is normally recorded from nearby motoneurons (Streit, 1993).
We adopted three experimental strategies: (1) chronic block of AMPA/kainate glutamate receptors with CNQX to remove the main, spontaneous excitatory input to these cells, since NMDA receptors play a rather minor role in excitatory transmission on these cells (Ballerini & Galante 1998; Ballerini et al. 1999); (2) chronic block of Na+-dependent action potential mechanisms in an attempt to mimic a more systematic suppression of afferent inputs, like one might expect following chronic lesions; (3) chronic block of GABAA and glycine receptors with bicuculline and strychnine to evoke sustained bursting activity (Ballerini & Galante, 1998).
METHODS
Experimental procedure and drug solutions
The experiments were performed on organotypic slice cultures grown 13–14 DIV according to amply reported methods (Ballerini & Galante, 1998; Ballerini et al. 1999). Pregnant rats were deeply anaesthetized with chloral hydrate (10.5 %, 0.4 ml (100 g)−1i.m.) and killed by intracardiac injection (2 ml) of chloral hydrate. This procedure is in accordance with the regulations of the Italian Animal Welfare Act and is approved by the local authority veterinary service.
For experiments involving chronic treatments, cultures were incubated at day 6 with a medium containing 10 μM CNQX, 1 μM TTX or 1 μM strychnine plus 20 μM bicuculline. At day 13 (after 7 days of incubation), the medium was replaced with a fresh one without these blockers for 2–24 h prior to electrophysiological recording. Control cultures were subjected to the same medium changes without addition of blockers. Preliminary experiments were performed to check the chemical stability of these blockers by diluting them at the appropriate final concentration in control extracellular solution and storing them for 6 days at 24°C. These solutions were subsequently applied to control cultures and checked for their ability to block excitatory synaptic transmission in the case of CNQX, to block spike activity in the case of TTX, or to induce spontaneous bursting in the case of strychnine and bicuculline. In each case the solutions retained their pharmacological properties. Furthermore, we investigated the persistence of observed synaptic changes induced by chronic treatments by washing cells for a 1–3 day period in control medium: we found that these changes in synaptic transmission disappeared after 3 days (L. Ballerini & M. Galante, unpublished observation). This test also confirmed that the 2–24 h wash period which elapsed after washing out the chronically applied blockers did not introduce significant changes to the effects induced by chronic treatments.
For electrophysiological recording a coverslip with overlying culture was mounted on an inverted microscope and superfused with Krebs solution containing (mM): NaCl 156, KCl 4, MgCl2 1, CaCl2 2, Hepes 10, glucose 10. The pH was adjusted to 7.4 using NaOH (osmolarity was 305 mosmol l−1). All the experiments were performed at room temperature (22 ± 2°C). All agents were bath-applied; these included 3-((RS)-2-carboxy-piperazine-4-yl)-propyl-1-phosphonate (CPP; Tocris), 6-cyano-7-nitroquinoxaline-2,3-dione disodium salt (CNQX; Tocris), bicuculline methiodide (Sigma), strychnine nitrate (Sigma) and tetrodotoxin (TTX; Affiniti, UK).
Electrophysiological recordings
Voltage-clamp recordings in the whole cell configuration were obtained from ventrally located spinal neurons using pipettes filled with a solution of the following composition (mM): potassium gluconate 120, KCl 20, Hepes 10, EGTA 10, MgCl2 2, Na2ATP 2 (pH 7.35, adjusted with KOH). Ventral interneurons were identified on the basis of criteria previously described (Ballerini & Galante, 1998; Ballerini et al. 1999). Values of membrane potentials were systematically corrected for liquid junction potentials and other offset potentials (see Marty & Neher 1983). Responses were amplified, stored on videotape for further analysis, digitized at 10–20 kHz with the pCLAMP software (Axon Instruments; version 6.2), and displayed on a chart recorder. Events which appeared as summated responses or were superimposed on a large event were discarded. Single spontaneous synaptic events were detected by use of the AxoGraph 3.5.5 (Axon Instruments) event detection program on a MacIntosh computer (Clements & Bekkers, 1997). On average 200–600 PSCs and 50–100 mPSCs were analysed from each cell in order to obtain mean kinetic and amplitude parameters. From the average of these events we measured the rise time, calculated from 10 to 90 % peak amplitude, half-width, calculated at half-amplitude of the event peak, and the value of decay (expressed as τ) by fitting a mono-exponential function. Extracellular electrical stimulation of the dorsal root ganglion (DRG) cells was performed with a low-resistance patch pipette containing external bath solution. Short voltage pulses (100 μs) of amplitude ranging between 5 and 50 V (eliciting 80 % of maximal response) were delivered by a stimulator at a low pace (0.05 Hz). Evoked PSC area was obtained by integrating the average tracings of 5–10 successive currents.
Statistical analysis
All electrophysiological values from batches of cultures subjected to the same experimental protocols were pooled together and expressed as mean ±s.d., with n indicating the number of cells. For statistical comparison data from chronically treated cells were tested against a general pool of control, aged-matched cultures or against data obtained from untreated cells from the same embryo culture series (termed sister cultures). Statistical analysis of mean values was carried out using Student's t test or ANOVA and differences were considered significant at the P < 0.005 level, unless stated otherwise. The significance of any difference between cumulative probability plots was established using a Kolmogoroff-Smirnov test (level of significance≤ 0.0001).
RESULTS
The database for the present study comprised 185 ventral interneurons (from 80 culture series prepared weekly) always recorded at 13–14 DIV and voltage clamped at a holding potential (Vh) of -56 mV. In each case we compared synaptic activity of neurons in control cultures with that of analogous cells from sister cultures which had been incubated for 7 days (see Methods) with 10 μM CNQX, 1 μM TTX or 1 μM strychnine and 20 μM bicuculline. These pharmacological blockers were washed out prior to recording.
The effects of these chronic manipulations on interneuronal activity were assessed by recording spontaneous and miniature PSCs plus the current responses evoked on the same cells by electrical stimulation of DRG cells. This combined assay measures three aspects of network synaptic function. In particular, spontaneous PSCs, which are generated by action potential-dependent as well as spontaneous quantal release, should mainly reflect random firing of local neurons and thus provide an index of how changes in network activity can shape the function of a single interneuron. mPSCs measurements are independent of network function and should primarily help to localize observed changes in synaptic transmission to pre- and/or postsynaptic level. Finally, evoked DRG activity should assay mono- and polysynaptic projections to the recorded cell.
Properties of synaptic activity in control cultures
PSCs were recorded from 47 ventral interneurons in different preparations under control conditions: an example is shown on the left of Fig. 1A, in which PSCs are inward currents (13.1 Hz frequency) of fluctuating amplitude. On average, PSC frequency was 12.6 ± 3.5 Hz, with a mean amplitude of -19 ± 5 pA (n = 30). Control PSCs of the majority of these interneurons (70 %) decayed either rapidly (τ = 2.1 ± 0.5 ms) or slowly (τ = 13.1 ± 5.6 ms), a difference which was statistically significant despite the similar rise times (0.9 ± 0.2 and 1.3 ± 0.3 ms, respectively). Note that there was no correlation between rise time and half-width of these events (Fig. 1D), suggesting that different decay values were unlikely to be due to electrotonic filtering. The mean amplitude of fast events was analogous to that of slow ones (-18.3 ± 5.2 and -21.1 ± 8.4 pA, respectively), with fast events ranging from 21 to 73 % of all events in each cell. The right panel of Fig. 1A shows average amplitudes of fast and slow events (fast and slow decay responses correspond to top and bottom records, respectively) while the corresponding raw data are in the middle panel. In a minority (30 %) of cells, fast events only (τ = 3.4 ± 0.7 ms) were recorded (rise time = 1.0 ± 0.3 ms).
Figure 1. Spontaneous and evoked activity from a ventral interneuron in an untreated culture.
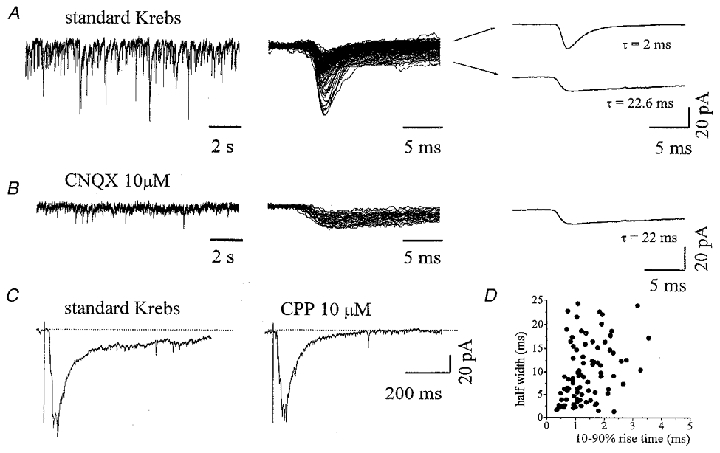
A, spontaneous synaptic activity from a ventral interneuron is composed by inward currents of variable amplitude (left); single PSCs comprise events with fast or slow decay times (middle, superimposed tracings). Rise time and decay time are measured from the fast PSC average (top right: rise time = 0.8 ms, τ = 2 ms) and the slow PSC average (bottom right: rise time = 1.1 ms, τ = 22.6 ms). B, application of CNQX (left, same cell as in A) completely blocks all fast events and leaves only slow ones (middle, superimposed traces). Kinetics are measured from the average of slow decay events (right, rise time = 1.5 ms, τ = 22 ms). C, synaptic currents evoked in the same interneuron as in A and B by DRG stimulation are shown in standard Krebs (left) and in the presence of CPP (right); each panel represents the average of 7 consecutive evoked PSCs. Note that the application of CPP reduces the area subtended by the evoked PSC by 38 %. Dotted lines indicate the baseline. D, scatter plot of rise time versus half-width (same cell as in A); regression analysis reveals no linear relationship between these two parameters (r2 = 0.006, Pearson correlation coefficient = 0.07).
The NMDA receptor antagonist CPP (10 μM, 15 min) had no effect on event frequency (4/10 cells), or slightly reduced it (15.6 ± 6.1 % reduction in 6/10 cells) without altering the proportion between fast and slow τ events. Mean amplitude was always unchanged (-19 ± 4 pA) like onset and decay of PSCs. As exemplified in Fig. 1B (same cell as in Fig. 1A), the AMPA/kainate receptor antagonist CNQX (10 μM, 10 min) fully suppressed fast τ PSCs, but did not suppress slow τ events (19.3 ± 8.3 ms; rise time = 1.9 ± 0.2 ms; mean amplitude = 12.8 ± 1.1 pA), the frequency of which was reduced from 1.8 ± 1.5 Hz to 1.1 ± 0.5 Hz (n = 5). Further application of CPP did not affect these events, while, after washout of CPP, co-application of strychnine (1 μM) and bicuculline (20 μM) eliminated all events (n = 3, not shown).
In cells bathed in standard Krebs solution, strychnine and bicuculline invariably induced repetitive bursts (Ballerini & Galante, 1998; Ballerini et al. 1999): during interburst intervals only fast decay events could be detected (n = 3). In those cells in which only fast events could be identified in Krebs solution, application of CNQX fully abolished any spontaneous activity (n = 3).
In summary then, in control cultures (14 DIV), spontaneous synaptic activity comprised in most cases both fast and slow τ PSCs. Fast events were abolished by CNQX, while slower ones (resistant to CNQX) were suppressed by co-application of antagonists of GABAA and glycine receptors.
To investigate further the contribution of glutamate receptors to network activity, we performed experiments (n = 14) in which evoked polysynaptic currents were elicited by focal stimulation of the homolateral dorsal root ganglion (DRG; Fig. 1C, left trace). Evoked PSCs (n = 3) were completely inward at a Vh =−56 mV, biphasic (early inward current followed by an outward component) at Vh =−20 mV, and fully outward at Vh = 0 mV. In all cells tested (n = 5), application of CNQX completely removed any evoked event, which recovered upon 20 min washout (not shown). On average, CPP reduced the area of the evoked currents by 34 ± 11 %, as shown in the example of Fig. 1C.
Properties of synaptic activity in cultures chronically treated with CNQX
Spontaneous synaptic activity was recorded in 47 ventral interneurons from various cultures after 7 days of chronic incubation with CNQX (see Methods). In all cells analysed (n = 25) a strong and significant increase (61 % rise; compared to control cultures) in spontaneous PSC frequency was detected (20.3 ± 4.1 Hz). This phenomenon was also characterized by a qualitative change in spontaneous synaptic transmission, as virtually all cells possessed fast PSCs only (τ = 3.6 ± 1.3 ms; rise time = 0.97 ± 0.27 ms). Figure 2A shows sample records from two interneurons from sister cultures, one in control conditions (top traces) and one which had been chronically treated with CNQX (bottom traces). Figure 2B shows the corresponding cumulative plots of PSCs where peak amplitudes for the chronically treated interneuron were distributed to the right of those of the control sister cell (a statistically significant difference). There was again no linear relation between peak amplitude and rise time values for these currents (Fig. 2C). Similar results were obtained from three different culture series. Note that if data were not compared between sister cultures, pooling together all responses from CNQX-treated cells yielded a mean peak amplitude (-24.8 ± 8 pA) which was not significantly different from that of pooled controls.
Figure 2. PSC amplitudes from control cultures or cultures chronically treated with CNQX.
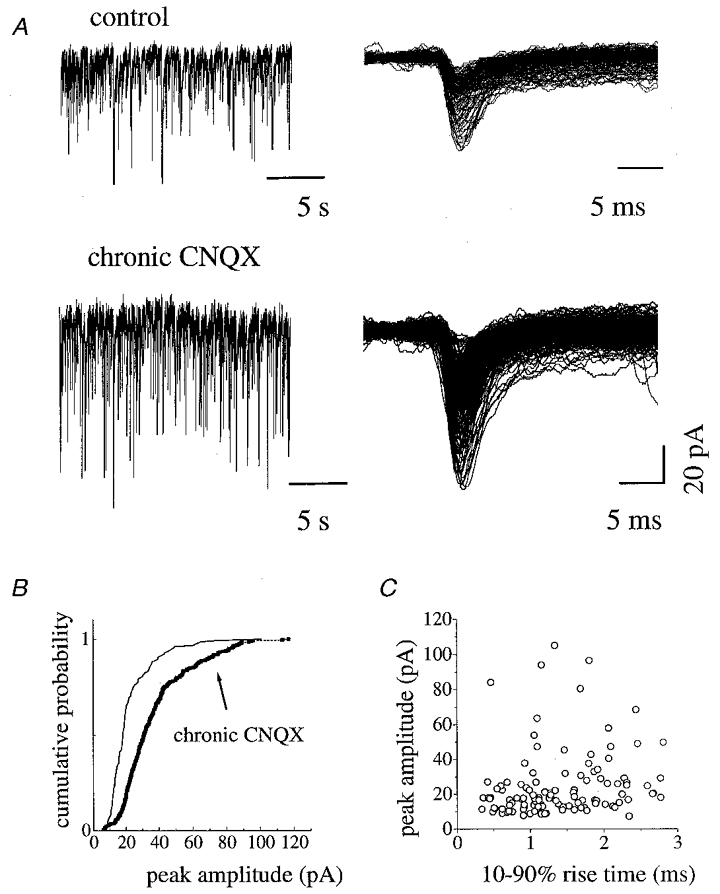
A, spontaneous synaptic currents in standard Krebs solution are shown in both control culture (top tracings, left) and chronically CNQX-treated sister culture (bottom tracings, left). Single PSCs are shown to the right, note that both fast and slow decay events are present in the control interneuron (top right) while in that from the chronically CNQX-treated culture only fast PSCs are detected (bottom right). B, cumulative amplitude plot for PSCs recorded in control and chronically CNQX-treated sister cultures; the entire distribution of PSC amplitudes includes larger values for CNQX-treated interneurons (see arrow). C, scatter plot of rise time against peak amplitude in control culture (same cell as in A). Note the lack of linear relationship between these parameters (r2 = 0.00003, Pearson correlation coefficient = 0.006).
In 5/8 interneurons, CPP (15 min) reduced spontaneous synaptic event frequency by 24 ± 7 %, with a small reduction in mean amplitude (-17.3 ± 6.6 pA, not shown). Unlike the relatively slight or even absent action of CPP, in all 13 neurons tested the effect of acute application of CNQX was characterized by a large change in synaptic transmission: in fact, while fast τ events disappeared, they were replaced by slow τ PSCs occurring at variable frequency (7.3 ± 4.3 Hz). This result is clearly illustrated by comparing data in Fig. 3A and B. On average, in the presence of CNQX, τ for slow decay was 27.2 ± 9 ms (PSCs had rise time of 2.22 ± 0.38 ms and a mean amplitude of -14.4 ± 4.1 pA). Further application of CPP did not affect these events (n = 4), while, after washout of CPP, co-application of strychnine and bicuculline fully blocked slow τ PSCs (n = 4). In three cells displaying slow decay events after acute application of CNQX, subsequent addition of TTX evoked a dramatic reduction in the occurrence of spontaneous slow events which became very rare (0.03 Hz in two cells) or disappeared altogether in one cell.
Figure 3. Spontaneous and evoked activity from ventral interneurons in cultures chronically treated with CNQX.
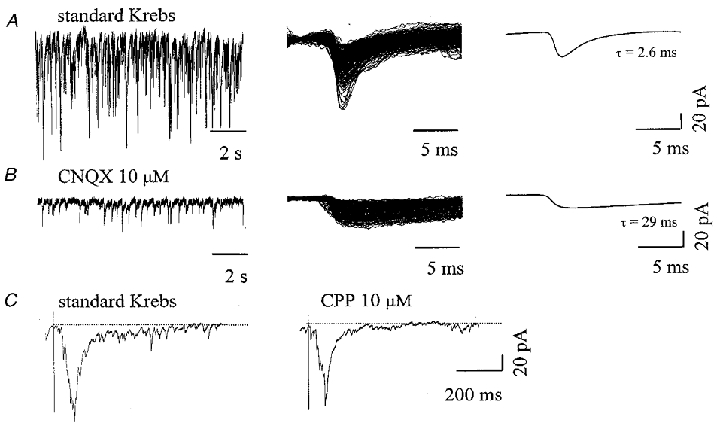
A, recordings from a ventral interneuron in CNQX-treated culture (left) show very intense spontaneous activity; PSCs comprise only fast decay events (middle, superimposed traces; right, average response), with τ of 2.6 ms and a rise time of 0.8 ms, measured as in Fig. 1 B,. block of AMPA/kainate receptors by CNQX (left, same cell as in A) fully abolished fast PSCs and unmasked slow τ events; middle, single events are superimposed, and kinetics are measured from the average PSC (right, τ = 29 ms, rise time = 1.6 ms). C, DRG stimulations evoke polysynaptic currents in the patched ventral interneuron. Evoked currents in standard Krebs solution (left) appear reduced (by 41 %) in area after the application of CPP (right); each trace (left and right) represents the average of 5 consecutive evoked PSCs (different cell from A and B).
In eight ventral interneurons, PSCs elicited by DRG stimulation were inward at -56 mV (Fig. 3C), became biphasic at Vh =−20 mV and were fully outward at Vh = 0 mV, as in conttrolsister cells (not shown). Acute application of CNQX completely and reversibly abolished evoked PSCs (n = 3). Evoked PSCs were partly reduced by CPP (see Fig. 3C), a depression which on average amounted to 44.4 ± 10.4 % (n = 5).
Properties of synaptic activity in cultures chronically treated with TTX
Spontaneous events recorded from ventral interneurons (n = 24) after chronic TTX treatment had a mean frequency (15 ± 4 Hz) comparable (19 % rise) to that observed in control cells and an average amplitude of -19.9 ± 4.8 pA, again close to the control amplitude. In 60 % of recorded interneurons, only fast τ PSCs were detected (for an example, see Fig. 4A, middle and right; the cumulative amplitude plot for this cell, together with a sister culture control, is shown in Fig. 4D). On average, τ values for fast decay were 3.8 ± 0.6 ms, with a rise time of 0.9 ± 0.3 ms. In 40 % of observed interneurons, spontaneous PSCs were mixed, with fast (τ = 2.7 ± 0.7 ms) and slow (τ = 12 ± 4 ms) decay values (rise times, 0.9 ± 0.2 ms and 1.3 ± 0.2 ms, respectively) equally distributed in terms of occurrence (as in untreated cells). Superfusion with CPP reduced (by 37 ± 7 %) the frequency of all events in 4/6 cells. Application of CNQX (n = 3) suppressed fast events, leaving no residual activity in those cells in which only fast events were detected (example in Fig. 4B). In interneurons with fast and slow decay events, application of CNQX left only slow decay events, as reported for control cultures (not shown).
Figure 4. Spontaneous and evoked activity from ventral interneurons in cultures chronically treated with TTX.
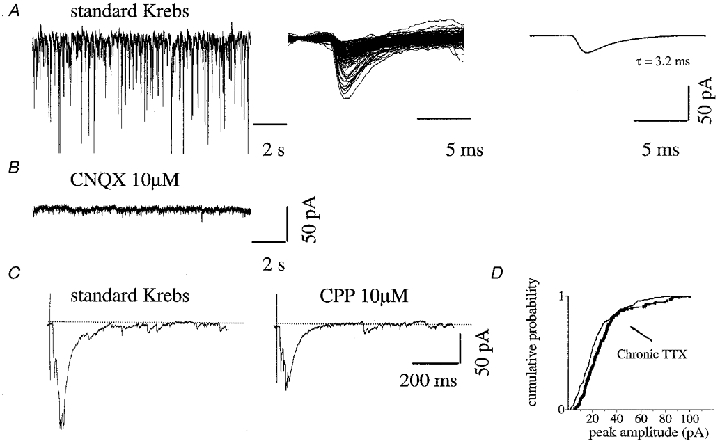
A, left, example of spontaneous activity recorded from an interneuron in a chronically TTX-treated culture; middle, superimposed events: only fast decay events are detected; right, from their average, rise time (0.9 ms) and τ (3.2 ms) values are measured. B, addition of CNQX blocked all fast events leaving no residual activity (same cell as in A C,). evoked PSCs elicited by DRG stimulations are shown in standard Krebs solution (left) and in the presence of CPP (right); dotted line represents the baseline current. CPP produced a 44 % reduction in the of the evoked PSC. Each panel represents the average of 5 consecutive evoked PSCs (different cell from A and B). D, cumulative distribution plot for PSC data obtained after chronic TTX treatment or in control shows no statistically significant difference.
The left trace in Fig. 4C shows the average synaptic current evoked by DRG stimulation. As in control or chronically CNQX-treated cultures, evoked currents were inward at Vh =-56 mV, biphasic at Vh =−20 mV and fully outward at Vh = 0 mV (n = 3). The right trace in Fig. 4C shows that the area of the evoked PSC was reduced by 44 % in the presence of CPP. In five interneurons, the reduction in evoked PSCs brought about by CPP applications was 50.6 ± 13.7 %.
Properties of synaptic activity in cultures chronically treated with strychnine plus bicuculline
After a 7 day incubation in solution containing strychnine and bicuculline (see Methods), 14/30 interneurons displayed spontaneous, irregular bursting activity (an example of these inward burst currents is shown in Fig. 5A, left). In the majority of cells (n = 18) the frequency of background synaptic activity (measured either during the quiescent period between bursts or at rest in those cells without spontaneous bursting) was significantly lower (8.2 ± 3.0 Hz) than in control cultures (35 % decrease). Usually, both fast (τ = 3.1 ± 1.0 ms) and slow (τ = 11 ± 3 ms) decay events could be detected, with similar rise times (0.8 ± 0.2 ms and 1.0 ± 0.4 ms, respectively), as exemplified in Fig. 5A (middle and right). Fast events were on average 70 ± 12 % of all measured events. Mean peak amplitude was smaller (-14.4 ± 3.4 pA) than in control cultures. In Fig. 5D, the cumulative amplitude distribution plot shows that data from a treated cell are significantly different from analogous data from control cultures and lie to the left of the standard curve. Similar results were obtained in three different culture series.
Figure 5. Spontaneous and evoked activity from ventral interneurons in cultures chronically treated with strychnine plus bicuculline.
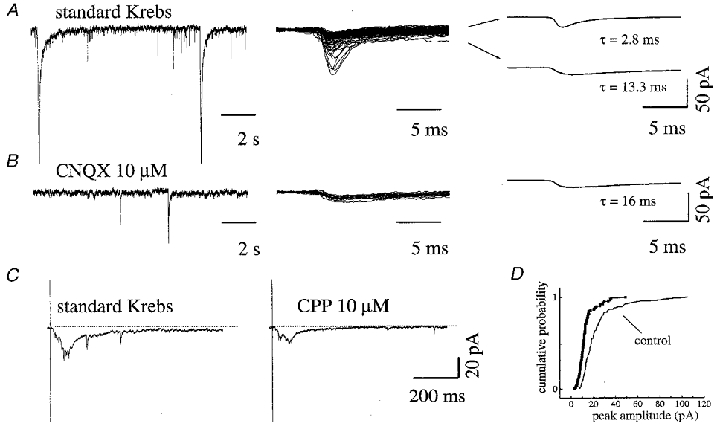
A, spontaneous synaptic activity from a ventral interneuron appears as bursts of inward currents (left) followed by quiescent periods during which both fast and slow PSCs (middle, superimposed traces) are detected; kinetics are measured from the average of PSCs showing both fast decay (top right: rise time = 0.9 ms, τ = 2.8 ms) and slow decay (bottom right: rise time = 1.5 ms, τ = 13.3 ms). B, application of CNQX (same cell as in A) abolished spontaneous bursting (left) and completely removed fast decay events; only slow decay events survived (middle, superimposed traces), with kinetics (right) similar to those normally found in standard Krebs solution. C, evoked synaptic currents induced by stimulation of DRG (left) appear strongly reduced in area (by 60 %) during the application of CPP (right). Each trace (left and right) is the average of 8 subsequent responses. D, cumulative probability plot of PSC amplitudes from a ventral interneuron (different cells) in control (see arrow) and in a sister culture chronically treated with strychnine plus bicuculline: the entire amplitude distribution for PSCs deviates towards smaller values for the treated interneuron.
CPP significantly decreased the PSC frequency (41.1 ± 9.3 %, n = 9; not shown) while CNQX (Fig. 5B) completely suppressed fast events (as reported for control cultures), leaving only slow events (τ = 15.6 ± 6.2 ms; rise time = 1.6 ± 0.4 ms; n = 5), the frequency of which was also reduced, from 0.50 ± 0.35 to 0.23 ± 0.15 Hz.
Synaptic currents evoked by DRG stimulation appeared fully inward at Vh =−56 mV (Fig. 5C), became biphasic at Vh =−20 mV and outward at Vh = 0 mV. CPP significantly reduced (by 60 ± 20 %, n = 5) the PSC area, as shown in Fig. 5C (right). Application of CNQX fully blocked the evoked PSCs in 2/5 cells only; in three cells a residual evoked current (11 ± 8 % of control area) remained.
In 12/30 cells the spontaneous events had a frequency significantly higher (22.3 ± 10.1 Hz) than control and comprised only fast τ events (τ = 3.7 ± 1.1 ms; rise time = 1.0 ± 0.3 ms; mean amplitude =−23.7 ± 12.1 pA; n = 10; not shown) which were abolished by CNQX. CPP did not affect event frequency in three cells and reduced it by 40 ± 23 % in two cells. The probability of observing either an enhancement or a fall in the frequency of background synaptic activity bore no reference to the presence of irregular bursting.
mPSCs in control and treated cultures
In control cultures, acute application of TTX revealed mPSCs which could be separated into two groups on the basis of their decay values (2.6 ± 1.1 ms and 11.5 ± 4.5 ms, respectively), although they all possessed similar rise times (1.0 ± 0.2 and 1.6 ± 0.1 ms for fast and slow τ events, respectively). As shown by the example of Fig. 6A, a cell with fast PSCs only generated exclusively fast τ mPSCs (in a sample of 4 cells, τ = 3.9 ± 0.3 ms; rise time = 1 ± 0.1 ms). The cumulative inter-event frequency plot for this cell is shown in Fig. 6E (right, ^) while its mPSC amplitude distribution is plotted in Fig. 6E (left, ^). In a sample of 9 cells, the mean frequency of mPSCs was 0.52 ± 0.12 Hz. The mean amplitude of mPSCs was -12.2 ± 3 pA (n = 9) and was not significantly changed by co-application of strychnine and bicuculline (106 ± 35 %; n = 3), while their frequency was reduced (60 ± 14 %), confirming that in organotypic spinal culture neurons mPSCs are mediated by glutamate, GABA or glycine (Streit & Luscher, 1992).
Figure 6. Spontaneous mPSCs in control and chronically treated cultures.
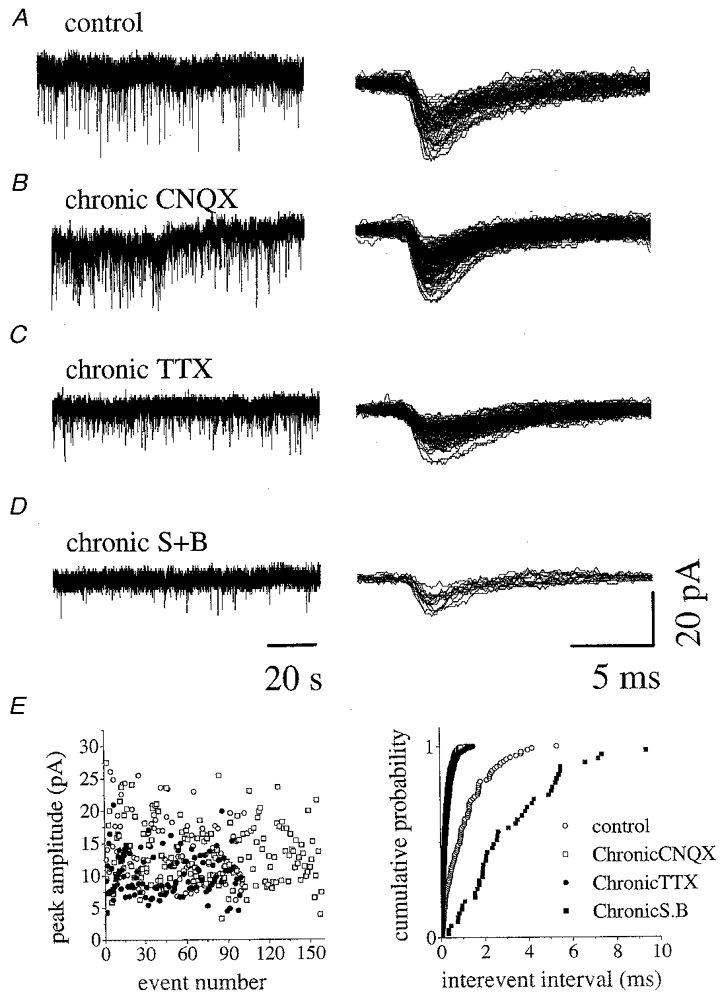
A, recordings from a ventral interneuron in the presence of TTX from a control culture (left): events are superimposed at faster speed on the right. B, miniature currents in an interneuron from a chronically CNQX-treated culture (left): note the higher frequency of mPSCs (superimposed traces, right) compared to control culture. C, spontaneous mPSCs from a chronically TTX-treated culture; note the higher frequency of mPSCs (superimposed traces, right) compared to control. D, miniature currents recorded from an interneuron in a culture chronically treated with strychnine plus bicuculline (S+B, left); note very low frequency of the miniature events (superimposed traces, right), compared to the other culture conditions (A-C). E, distribution of mPSC amplitude (left) and mPSC inter-event distribution plot (right) from the cells shown in A–D. In the righthand panel the plot for cells chronically treated with CNQX, TTX and strychnine plus bicuculline (S.B) are significantly different from control. Note almost complete overlap of CNQX data with TTX data.
Figure 6B shows a sample of mPSCs after applying TTX to a chronically CNQX-treated interneuron. With this protocol, mPSCs had a frequency significantly larger than in control (1.1 ± 0.3 Hz; see example of raw data in Fig. 6B and cumulative inter-event interval plot in Fig. 6E, right, □) and comprised exclusively fast τ events (τ = 4.1 ± 0.9 ms; rise time = 1.0 ± 0.1 ms) with amplitudes comparable to control mPSCs (see Fig. 6E, left, ^), which on average was -13.6 ± 3.5 pA (n = 11). Subsequent application of CNQX did not unmask slow mPSCs but simply blocked all activity (n = 7). As in the case of the events recorded in control conditions, co-application of strychnine and bicuculline did not change the amplitude of mPSCs (-11.3 ± 2.0 pA; n = 4), but it reduced their frequency (46 ± 27 %).
In cultures previously subjected to chronic TTX treatment, subsequent acute application of TTX disclosed mPSCs occurring at high frequency (1.0 ± 0.4 Hz; P < 0.05 versus control; n = 4; mean peak amplitude =−13.5 ± 3.6 pA). Figure 6C shows raw data from a cell chronically treated with TTX and Fig. 6E shows the corresponding inter-event frequency plot (right, •; note complete overlap with CNQX treatment data) and amplitude distribution (left, •), which are similar to control values. In terms of shape, these mPSCs reproduced the situation observed with standard, untreated cells, namely that they displayed only fast τ events (τ = 3.9 ± 0.9 ms; rise time = 1.0 ± 0.2 ms) if in control solution a single PSC population was present, or that they displayed both fast and slow τ events (τ values of 2.0 ± 0.2 and 12 ± 2.3 ms) whenever two PSC populations existed before adding TTX.
Figure 6D shows mPSCs after chronic treatment with strychnine and bicuculline: in this example the interneuron was representative of the majority group in which PSCs occurred at low frequency before TTX application. The inter-event frequency plot (Fig. 6E, right, ▪) was found to the right of the control plot, indicating a lower event frequency, while the amplitude scatter plot showed that the range was between -5 and -15 pA (Fig. 6E, left, ▪). In a sample of four such cells, mPSC frequency was found to be very low (0.09 ± 0.01 Hz; P < 0.05; mean peak amplitude =−9.1 ± 2.8 pA). Events with both fast (τ = 4.2 ± 1.3 ms) and slow (τ = 16 ± 4 ms) values were recorded (rise times, 0.9 ± 0.1 and 1.5 ± 0.2 ms, respectively). The low frequency of these responses precluded their systematic pharmacological analysis. However, in two cases it was possible to observe a further reduction (by 53 ± 18 %) in mPSC frequency after applying strychnine plus bicuculline.
As indicated above, a smaller sample (12/30) of cells chronically treated with strychnine and bicuculline displayed higher frequency PSCs. This enhanced occurrence was also observed when the same cells were subsequently exposed to TTX: in fact, their mPSC frequency (n = 4) was 1.1 ± 0.2 Hz and was partly reduced (by 27 ± 18 %) by acutely applied strychnine and bicuculline.
DISCUSSION
The principal finding of the present report is that long-term application of blockers for AMPA/kainate or GABAA and glycine receptors induced profound and contrasting changes in the synaptic activity of developing spinal networks in vitro. Inhibition of AMPA/kainate receptors resulted in an increase in PSC and mPSC frequency. Inhibition of Cl−-mediated synaptic activity by GABAA and glycine receptor antagonists led to decrease in PSC and mPSC frequency. Inhibition of spike-mediated activity with TTX could not mimic any of these effects on PSCs, thus suggesting that as long as spontaneous quantal events of a composite nature were present, they represented a signal sufficient for stabilisation of synaptic activity.
Synaptic activity at network or single cell level
The organotypic slice cultures of the rat spinal cord rely on various chemical transmitters to signal network activity. Glutamate, GABA and glycine (Ballerini & Galante 1998; Ballerini et al. 1999) are thought to be the main transmitters in this system. The use of pharmacological tools to identify the neurotransmitter relative contribution to the observed PSCs remains of questionable value as block of GABAA and glycine receptors is accompanied by strong, sustained bursting (Ballerini & Galante, 1998), which biases excitatory transmission operation. Likewise, the activity of GABA- or glycine-releasing neurons is often dependent on the tonic and/or phasic drive exerted by other cells liberating glutamate (or possibly even GABA or glycine) onto them. This situation is, for instance, made apparent by the fall in the frequency of GABAergic and glycinergic PSCs after application of CNQX (as observed in the present study). In the present investigation, we studied the effects of chronic pharmacological treatments on the amplitude and frequency of spontaneous PSCs, as the latter are a reliable index of synaptic activity at network level. Under these conditions, acute application of pharmacological blockers allowed assessment of the extent and/or nature of the network drive contributing to the PSCs rather than identification of the transmitter mediating these events. Recordings of mPSCs were used to clarify if any observed change in synaptic transmission had a pre- or postsynaptic origin. Pharmacological isolation of mPSCs was attempted when their frequency made it possible to collect a sufficient number of events for further analysis. This was hardly feasible, for example, in the case of chronic strychnine and bicuculline treatment.
Synaptic activity in control cultures
The majority of recorded ventral interneurons possessed spontaneous synaptic activity manifested as inward currents (in our recording conditions), made up of a mixed population of PSCs mediated by different receptors (Ballerini & Galante, 1998; Ballerini et al. 1999). In control medium, PSCs in most ventral interneurons were heterogeneous in their kinetics, as they included events with fast (τ < 5 ms) or slow (τ > 10 ms) decay. In a smaller sample of cells (30 %), PSCs comprised fast events only. This differentiation was preserved when recording mPSCs from the same cells.
The slowly decaying currents always disappeared following treatment with strychnine and bicuculline, and persisted, although reduced in frequency, in the presence of CNQX. Thus, events with intrinsically slower ‘off’ kinetics appeared to be mainly Cl− mediated via activation of GABAA or glycine receptors. Their frequency was partly sensitive to CNQX, presumably indicating that a few of these cells required a glutamatergic drive at network level.
All fast τ events were fully blocked by CNQX and presumably represented a mixture of glutamate-mediated synaptic activity plus Cl−-mediated events (with fast kinetics) driven by AMPA receptor activation at network level. In fact, in spinal neurons Cl−-mediated inhibitory events recorded as potentials (Curtis & Eccles, 1959) or currents (Singer & Berger, 1999) can be as fast as (or even faster than) excitatory signals. It is, however, difficult to differentiate which Cl−-mediated events (with fast or slow kinetics) are due to GABA or glycine (or both). In cultured rat spinal neurons, inhibitory receptors have been proposed to be heterogeneous, since mIPSCs display τ values ranging from 5 to 40 ms and they are all decreased in frequency by strychnine or bicuculline (Lewis & Faber, 1996).
Fast PSCs displayed a τ value consistent with AMPA receptor activation (Hestrin, 1993; Wyllie et al. 1994), and always lacked any biexponential decay, suggestive of a second, NMDA receptor-mediated component. In fact, application of CPP did not affect the amplitude or the kinetics of any spontaneous (fast or slow) events. This could be due to the presence, in the extracellular solution, of 1 mM Mg2+, together with the negative value of the holding potential (Mayer & Westbrook 1987). Furthermore, if NMDA receptors were largely contributing to spontaneous, network-mediated activity, a clear reduction in event frequency should have been observed with CPP. Nevertheless, since CPP reduced the area of PSCs induced by electrical stimulation of DRG cells, it appears that when a strong excitatory input synchronized the network to elicit a polysynaptic interneuronal response, an NMDA receptor-mediated component was unmasked. Evoked PSCs had composite reversal potentials (Ballerini et al. 1999), suggesting that, even with this type of synaptic transmission, the heterogeneous contribution by different neurotransmitters found with spontaneous events was present.
It is noteworthy that different τ values were presumably not simply related to different synaptic locations, as suggested by the similar mean rise time values of all detected events (Hestrin, 1993; Wyllie et al. 1994; Nusser et al. 1997). Furthermore, the lack of a linear relationship between rise time and half-width values, or between rise time and peak current amplitude (Wyllie et al. 1994; Nusser et al. 1997) is also not consistent with decays mainly shaped by electrotonic filtering.
Synaptic activity in chronically treated cultures
CNQX-treated cultures
In the present investigation we pharmacologically simulated a selective reduction in the main excitatory synaptic input, which during the first week in vitro is chiefly mediated by AMPA/kainate receptor activity (Ballerini & Galante 1998). After 1 week of chronic CNQX treatment (starting at 6 DIV) all cells recorded in control solution displayed a significant and very large increase in the frequency of synaptic currents which homogeneously had fast decay only, with τ values and pharmacology similar to the control fast events. Network activity was thus changed to express large amplitude, rapid responses at a very high rate.
When cumulative amplitude plots of PSCs from treated cultures were compared with those from control sister cultures, a significant shift of the curve towards larger values was detected. Summation of larger excitatory signals within a network with extensive connectivity might have been responsible for the increased frequency of spontaneous synaptic activity. Should this hold true, one can eliminate the contribution by NMDA receptor upregulation, since CPP had minimal effects on spontaneous events. Furthermore, this possibility contrasts with the fact that electrically evoked synaptic transmission appeared to be similar to that found in control conditions. This observation suggests that large and indiscriminate rearrangement of synaptic contacts and/or fibre projections had not taken place. It is noteworthy that, despite the increased frequency of mPSCs, their mean amplitude was not changed, suggesting a presynaptic locus of action, like for example, enhanced activity of synaptic boutons at certain strategic sites which are not necessarily recruited to play a role in electrically evoked transmission. A complementary hypothesis would be that chronic treatment with CNQX led to the activation of previously silent synapses, again presumably located at certain network sites. Future experiments based on molecular biology or immunocytochemical characterization of synapses will be necessary to solve this issue. It is interesting that in organotypic cultures of the hippocampus analogous electrophysiological effects are induced by block of NMDA receptors and not by antagonism of AMPA/kainate receptors (McKinney et al. 1999).
The absence of slowly decaying PSCs following chronic CNQX application is intriguing. Some possibilities seem unlikely to account for this observation. In particular, (1) a functional lack of some GABA or glycine receptors; (2) a change in location of slow event synapses. The first possibility is unlikely since it appears that neurons were apparently still capable of expressing GABA or glycine receptor-mediated slow responses, which were uncovered after acute block of AMPA/kainate receptors. The second hypothesis also seems improbable since these slow events, which suddenly emerged after reapplying CNQX acutely, had standard amplitudes and rise times. The lack of slow events might then require a different interpretation. In fact, their detection could have been obscured by the superimposed dramatic rise in network-mediated fast synaptic events and was made possible only after CNQX-induced block of such fast events. The slow events presumably required a network drive (independent of AMPA/kainate receptors), in view of the very low releasing probability of their synapses (compare rare event occurrence in TTX solution). A variant of this interpretation would be that, after chronic treatment with CNQX, a considerable change in circuit operation took place whereby fast synaptic transmission was so strongly upregulated that somehow it even inhibited slow synaptic events which could be detected only after blocking AMPA/kainate receptor activity. While the present study could not distinguish between these possibilities, it seems clear that chronic CNQX treatment transformed synapses mediating slow events into a low releasing probability mode which became apparent when the network drive was absent.
TTX-treated cultures
The effects on synaptic activity obtained by excitatory receptor block were mimicked only in part by long-term treatment with TTX. Although interneurons treated chronically with TTX could express fast and slow events, as in control conditions, the majority of them now generated fast events only (with unchanged frequency or kinetic and pharmacological properties, including sensitivity to acute application of TTX). Note that, by analogy with the data obtained following chronic CNQX treatment, there was a significant increase in frequency of mPSCs without a parallel change in peak amplitude. These observations suggest that, as far as the elementary activity of synaptic transmission (studied as mPSCs) is concerned, chronic TTX and CNQX treatment shared similar presynaptic effects manifested as increased event frequency. Nevertheless, at network level (studied in terms of PSCs), long-term block of all types of propagated activity (dependent on Na+ action potentials) was apparently compensated by mechanisms which must have affected excitation as well inhibition so as to leave the fundamental properties of circuit operation unscathed. This condition clearly differed from the effect of chronic removal of AMPA/kainate inputs.
Strychnine plus bicuculline treated cultures
GABAA and glycine receptors were blocked by chronic incubation of slices in strychnine and bicuculline. In this case, cultures exhibited two opposite behaviours. (1) In the majority of cells a generalized decrease in the frequency of synaptic activity was present (together with a decrease in mPSC frequency) without overall change in spontaneous event kinetics. The relative contribution by glutamate or glycine and GABA to mPSC activity was apparently the same as observed in control cultures. In these cultures depressant effects by CPP on spontaneous and evoked synaptic activity were clearly observed, implying a larger contribution of NMDA receptors to synaptic activity. (2) In a smaller group of cells, a strong increase in spontaneous synaptic activity (of both PSCs and mPSCs) was present and usually included fast events only (as was observed in chronically CNQX-treated cells), although slow events were never detected even after acute CNQX application. The infrequent occurrence of this pattern of activity prevented its systematic investigation. Nevertheless, the sensitivity of such mPSCs to strychnine and bicuculline was not significantly different from that observed in any other chronically treated culture.
It is unclear whether the different pattern of synaptic activity induced by strychnine and bicuculline reflects the existence of distinct classes of interneuron or the differential sensitivity to bicuculline treatment previously observed at 8 DIV (Ballerini & Galante, 1998) when the chronic treatment was started. The non-normal distribution of PSC frequency across the entire neuronal population justified grouping them into two separate classes although their functional significance remains uncertain.
Thus, in most cells, the effect on the network operation, in terms of frequency of spontaneous events, of incubation with blockers of GABAA and glycine receptors was opposite to that observed with chronic CNQX application. In addition, in these cells, the role of NMDA receptors in mediating electrically evoked synaptic transmission was enhanced by chronic treatment with strychnine plus bicuculline. It is noteworthy that the present experimental protocol did not allow discrimination between effects resulting from mere block of GABAA and glycine receptors and the functional consequences of sustained bursting activity (accompanied by intense firing; Bracci et al. 1996), which is invariably associated with such treatment. Future experiments will be necessary to elucidate this issue.
Homeostatic plasticity and spinal networks
Recent investigations have addressed the role of neuronal activity in the homeostatic regulation of neuronal excitability, synaptic strength and synapse stabilisation (Rao & Craig, 1997; Kirsch & Betz, 1998; Levi et al. 1998; Lissin et al. 1998; O'Brien et al. 1998; Turrigiano et al. 1998). In particular, using different culture models chronically treated with various blockers of synaptic transmission in isolation or combination (hippocampus, Rao & Craig, 1997; Lissin et al. 1998; visual cortex, Turrigiano et al. 1998; spinal cord, O'Brien et al. 1998), it has been shown that glutamate receptors undergo a form of long-term activity-dependent regulation primarily based on postsynaptic changes. Note, however, that, even within the same brain area, different types of neuron appear to respond differently to the same chronic protocol (Rao & Craig, 1997; Turrigiano et al. 1998; Turrigiano, 1999).
The present study has provided little evidence for postsynaptic increase or decrease in AMPA/kainate receptors, since the mPSC amplitude remained essentially unchanged after various chronic treatments. There were, however, consistent variations (enhancement or depression) in the rate of spontaneous release estimated from the frequency of mPSCs and PSCs, lending support to the possibility that, in this model, homeostatic plasticity was based on presynaptic mechanisms within the spinal network, perhaps including receptors for glutamate, GABA and/or glycine. A postsynaptic increase in synaptic efficacy could, nevertheless, be manifested as a straightforward increase in mPSC frequency if it were caused by activation of silent synapses. In the latter case, one would, however, expect to observe a larger synaptic response to electrical stimuli unless the formerly silent receptors were totally inaccessible to evoked transmitter release, a possibility which so far has no experimental support.
The issue of whether homeostatic plasticity might be developed within a network and how it may influence network operation has not been previously investigated in mammalian central neurons. The present study has used a simple index of this phenomenon, namely the frequency of activity-dependent synaptic currents, which was found to be drastically changed. It is interesting to consider why organotypic spinal cultures appear to have extensively used this process of synaptic plasticity. In a well-organized network, generation of rhythmic activity appears at a very early stage and is developmentally regulated via a series of homeostatic mechanisms which tend to preserve network excitability (Feller, 1999). For example, in the chick embryonic spinal cord, qualitatively similar rhythmic patterns can eventually be generated by distinct neurotransmitter systems, even when one of them is blocked so as to preserve its function (Chub & O'Donovan, 1998; Milner & Landmesser, 1999). The present investigation indicates that in a structurally well-preserved mammalian spinal cord culture which can express network rhythmicity (Ballerini & Galante, 1998; Ballerini et al. 1999), homeostatic plasticity was not simply due to a series of postsynaptic changes in glutamate receptor activity occurring at single cell level, but included a complex rearrangement of network operation which also involved other neurotransmitters like GABA and glycine.
Acknowledgments
This work was supported by grants from Ministero per l'Università e la Ricerca Scientifica e Tecnologica (cofinanziamento) and Istituto Nazionale di Fisica della Materia. The financial support of Telethon-Italy is gratefully acknowledged (Grant no. 1184 to L.B.). We thank Dr Micaela Grandolfo for her assistance with tissue cultures.
References
- Ballerini L, Galante M. Network bursting by organotypic spinal slice cultures in the presence of bicuculline and/or strychnine is developmentally regulated. European Journal of Neuroscience. 1998;10:2871–2879. doi: 10.1111/j.1460-9568.1998.00296.x. [DOI] [PubMed] [Google Scholar]
- Ballerini L, Galante M, Grandolfo M, Nistri A. Generation of rhythmic patterns of activity by ventral interneurons in rat organotypic spinal slice culture. The Journal of Physiology. 1999;517:459–475. doi: 10.1111/j.1469-7793.1999.0459t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessho Y, Nawa H, Nakanishi S. Selective upregulation of an NMDA receptor subunit mRNA in culture cerebellar granule cells by K+-induced depolarization and NMDA treatment. Neuron. 1994;12:87–95. doi: 10.1016/0896-6273(94)90154-6. [DOI] [PubMed] [Google Scholar]
- Bracci E, Ballerini L, Nistri A. Spontaneous rhythmic bursts induced by pharmacological block of inhibition in lumbar motoneurons of the neonatal rat spinal cord. Journal of Neurophysiology. 1996;75:640–647. doi: 10.1152/jn.1996.75.2.640. [DOI] [PubMed] [Google Scholar]
- Clements JD, Bekkers JM. Detection of spontaneous synaptic events with an optimally scaled template. Biophysical Journal. 1997;73:220–229. doi: 10.1016/S0006-3495(97)78062-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AM. Activity and synaptic receptor targeting: the long view. Neuron. 1998;21:459–462. doi: 10.1016/s0896-6273(00)80555-3. [DOI] [PubMed] [Google Scholar]
- Crair MC. Neuronal activity during development: permissive or instructive? Current Opinion in Neurobiology. 1999;9:88–93. doi: 10.1016/s0959-4388(99)80011-7. [DOI] [PubMed] [Google Scholar]
- Chub N, O'Donovan MJ. Blockade and recovery of spontaneous rhythmic activity after application of neurotransmitter antagonists to spinal networks of the chick embryo. Journal of Neuroscience. 1998;18:294–306. doi: 10.1523/JNEUROSCI.18-01-00294.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline HT, Constantine-Paton M. NMDA receptor agonists and antagonists alter retinal ganglion cell arbour structure in the developing frog retinotectal projection. Journal of Neuroscience. 1990;10:1197–1216. doi: 10.1523/JNEUROSCI.10-04-01197.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis DR, Eccles JC. The time courses of excitatory and inhibitory synaptic actions. The Journal of Physiology. 1959;145:529–546. doi: 10.1113/jphysiol.1959.sp006159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai NS, Rutherford LC, Turrigiano GG. Plasticity in the intrinsic excitability of cortical pyramidal neurons. Nature Neuroscience. 1999;2:515–520. doi: 10.1038/9165. [DOI] [PubMed] [Google Scholar]
- Feller MB. Spontaneous correlated activity in developing neural circuits. Neuron. 1999;22:653–656. doi: 10.1016/s0896-6273(00)80724-2. [DOI] [PubMed] [Google Scholar]
- Hestrin S. Different glutamate receptor channels mediate fast excitatory synaptic currents in inhibitory and excitatory cortical neurons. Neuron. 1993;11:1083–1091. doi: 10.1016/0896-6273(93)90221-c. [DOI] [PubMed] [Google Scholar]
- Kirsch J, Betz H. Glycine-receptor activation is required for receptor clustering in spinal neurons. Nature. 1998;392:717–720. doi: 10.1038/33694. [DOI] [PubMed] [Google Scholar]
- Levi S, Vannier C, Triller A. Strychnine-sensitive stabilization of postsynaptic glycine receptor clusters. Journal of Cell Science. 1998;111:335–345. doi: 10.1242/jcs.111.3.335. [DOI] [PubMed] [Google Scholar]
- Lewis CA, Faber DS. Properties of spontaneous inhibitory synaptic currents in cultured rat spinal cord and medullary neurons. Journal of Neurophysiology. 1996;76:448–460. doi: 10.1152/jn.1996.76.1.448. [DOI] [PubMed] [Google Scholar]
- Lissin DV, Gomperts SN, Carrol RC, Christine CW, Kalman D, Kitamura M, Hardy S, Nicoll RA, Malenka RC, von Zastrow M. Activity differentially regulates the surface expression of synaptic AMPA and NMDA glutamate receptors. Proceedings of the National Academy of Sciences of the USA. 1998;95:7097–7102. doi: 10.1073/pnas.95.12.7097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney RA, Lüthi A, Bandtlow CE, Gahwiler BH, Thompson SC. Selective glutamate receptor antagonists can induce or prevent axonal sprouting in rat hippocampal slice cultures. Proceedings of the National Academy of Sciences of the USA. 1999;96:11631–11636. doi: 10.1073/pnas.96.20.11631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty A, Neher E. Tight-seal whole-cell recording. In: Sakmann B, Neher E, editors. Single-channel Recording. New York and London: Plenum Press; 1983. pp. 107–122. [Google Scholar]
- Mayer ML, Westbrook GL. The physiology of excitatory amino acids in vertebrate central nervous system. Progress in Neurobiology. 1987;28:197–276. doi: 10.1016/0301-0082(87)90011-6. [DOI] [PubMed] [Google Scholar]
- Milner LD, Landmesser LT. Cholinergic and GABAergic inputs drive patterned spontaneous motoneuron activity before target contact. Journal of Neuroscience. 1999;19:3007–302. doi: 10.1523/JNEUROSCI.19-08-03007.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusser Z, Cull-Candy S, Farrant M. Differences in synaptic GABAA receptor number underlie variation in GABA mini amplitude. Neuron. 1997;19:697–709. doi: 10.1016/s0896-6273(00)80382-7. [DOI] [PubMed] [Google Scholar]
- O'Brien JR, Kamboj S, Ehlers MD, Rosen RK, Fischbach GD, Huganir RL. Activity-dependent modulation of synaptic AMPA receptor accumulation. Neuron. 1998;21:1067–1078. doi: 10.1016/s0896-6273(00)80624-8. [DOI] [PubMed] [Google Scholar]
- Rao A, Craig AM. Activity regulates the synaptic localization of the NMDA receptor in hippocampal neurons. Neuron. 1997;19:801–812. doi: 10.1016/s0896-6273(00)80962-9. [DOI] [PubMed] [Google Scholar]
- Shatz CJ. Impulse activity and the patterning of connections during CNS development. Neuron. 1990;5:745–756. doi: 10.1016/0896-6273(90)90333-b. [DOI] [PubMed] [Google Scholar]
- Singer JH, Berger AJ. Contribution of single-channel properties to the time course and amplitude variance of quantal glycine currents recorded in rat motoneurons. Journal of Neurophysiology. 1999;81:1608–1616. doi: 10.1152/jn.1999.81.4.1608. [DOI] [PubMed] [Google Scholar]
- Spenger C, Braschler UF, Streit J, Lüscher HR. An organotypic spinal cord-dorsal root-ganglion skeletal muscle coculture of embryonic rat. I. The morphological correlates of the spinal reflex arc. European Journal of Neuroscience. 1991;3:1037–1053. doi: 10.1111/j.1460-9568.1991.tb00041.x. [DOI] [PubMed] [Google Scholar]
- Streit J. Regular oscillations of synaptic activity in spinal networks in vitro. Journal of Neurophysiology. 1993;70:871–878. doi: 10.1152/jn.1993.70.3.871. [DOI] [PubMed] [Google Scholar]
- Streit J, Lüscher HR. Miniature excitatory postsynaptic potentials in embryonic motoneurons grown in slice cultures of spinal cord, dorsal root ganglia and skeletal muscle. Experimental Brain Research. 1992;89:453–458. doi: 10.1007/BF00228262. [DOI] [PubMed] [Google Scholar]
- Streit J, Spenger C, Lüscher HR. An organotypic spinal cord-dorsal root-ganglion skeletal muscle coculture of embryonic rat. II. Functional evidence for the formation of spinal reflex arcs in vitro. European Journal of Neuroscience. 1991;3:1054–1068. doi: 10.1111/j.1460-9568.1991.tb00042.x. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG. Homeostatic plasticity in neuronal networks: the more things change the more, they stay the same. Trends in Neurosciences. 1999;22:221–227. doi: 10.1016/s0166-2236(98)01341-1. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG, Leslie KR, Desai NS, Rutherford LC, Nelson SB. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature. 1998;391:892–896. doi: 10.1038/36103. [DOI] [PubMed] [Google Scholar]
- Wyllie DJA, Manabe T, Nicoll RA. A rise in postsynaptic Ca2+ potentiates miniature excitatory postsynaptic currents and AMPA responses in hippocampal neurons. Neuron. 1994;12:127–138. doi: 10.1016/0896-6273(94)90158-9. [DOI] [PubMed] [Google Scholar]


