Abstract
Responses to pudendal afferent stimulation and depolarizing intracellular current injection were examined in sacral sphincter motoneurones in decerebrate cats.
In 16 animals examined, 2–10 s trains of electrical stimulation of pudendal afferents evoked sustained sphincter motoneurone activity lasting from 5 to >50 s after stimulation. The sustained response was observed in: 11 animals in the absence of any drugs; two animals after the intravenous administration of 5-hydroxytryptophan (5-HTP; ≤ 20 mg kg−1); one animal in which methoxamine was perfused onto the ventral surface of the exposed spinal cord; and two animals following the administration of intravenous noradrenergic agonists.
Extracellular and intracellular recordings from sphincter motoneurones revealed that the persistent firing evoked by afferent stimulation could be terminated by motoneurone membrane hyperpolarization during micturition or by intracellular current injection.
Intracellular recordings revealed that 22/40 sphincter motoneurones examined displayed a non-linear, steep increase in the membrane potential in response to depolarizing ramp current injection. The mean voltage threshold for this non-linear membrane response was -43 ± 3 mV. Five of the 22 cells displaying the non-linear membrane response were recorded prior to the administration of 5-HTP; 17 after the intravenous administration of 5-HTP (≤ 20 mg kg−1).
It is concluded that sphincter motoneurones have a voltage-sensitive, non-linear membrane response to depolarization that could contribute to sustained sphincter motoneurone firing during continence.
Urinary continence and faecal continence are achieved through an interplay of the sympathetic neurones controlling the smooth muscle of the lower urinary tract, bowel and rectum and the sacral somatic motoneurones controlling the urethral and anal striated sphincter muscles (for reviews see de Groat, 1990; Dubrovsky & Filipini, 1990). The present study focused on the sacral pudendal ventral horn motoneurones that innervate the striated muscles of the urethral and anal sphincters in the adult decerebrate cat. Many studies examining cat sacral sphincter motoneurones have revealed significant differences between these spinal motoneurones and the well-studied cat lumbosacral hindlimb motoneurones (for reviews on hindlimb motoneurones see Burke, 1981; Binder et al. 1996). There is little or very weak monosynaptic afferent excitation of sphincter motoneurones (Jankowska et al. 1978; Mackel, 1979) and the main excitatory segmental afferent inputs to the sphincter motoneurones are polysynaptic pathways from the perineum, urethra, bladder and colon (Bishop et al. 1956; Garry et al. 1959; Mackel, 1979; Fedirchuk et al. 1992). There is evidence of descending brainstem inputs to sphincter motoneurones (Mackel, 1979; Holstege & Tan, 1987), as well as postsynaptic inhibition of urethral sphincter motoneurones during micturition reflexes evoked by bladder distension or electrical stimulation of the pontine micturition centre (PMC; Fedirchuk & Shefchyk, 1993). With respect to the inhibitory inputs to the sphincter motoneurones, GABAergic terminals have been reported on the soma and dendrites of sphincter motoneurones (Ramirez-Leon & Ulfake, 1993) and there is both pharmacological and anatomical evidence for a glycinergic control of these motoneurones (Shefchyk et al. 1998). A variety of passive electrical membrane properties (Hochman et al. 1991; Sasaki, 1991) and several detailed descriptions of the morphology (Beattie et al. 1990; Sasaki, 1994) of urethral and anal sphincter motoneurones have also been published. Several features of these motoneurones that are of particular interest include their low rheobase values (Hochman et al. 1991; Sasaki, 1991) and their lack of recurrent inhibition (Jankowska et al. 1978; Mackel, 1979) in spite of the presence of recurrent axon collaterals (Sasaki, 1994).
In the 1980s our understanding of the translation of synaptic inputs to motoneurone output changed significantly with the description of active non-linear spinal motoneurone membrane properties (i.e. plateau potentials, bistability) in the turtle (Hounsgaard & Kiehn, 1985, 1989) and the cat (Hounsgaard et al. 1984, 1988; Conway et al. 1988; Crone et al. 1988). The presence of bistability or plateau potentials produces an increase in motoneurone excitability that can translate into an amplification and/or prolongation of motoneurone firing in response to membrane depolarization (Eken & Kiehn, 1989; Kiehn & Eken, 1997; Bennett et al. 1998a,b). In turtle motoneurones, the underlying mechanism of the plateau potential is a nifedipine-sensitive calcium current (Hounsgaard & Kiehn, 1989). It is generally accepted that a persistent inward current, perhaps mediated by the L-type calcium conductance as described in turtle motoneurones, is responsible for plateau potentials in cat motoneurones (Lee & Heckman, 1998b).
One of several factors contributing to urinary continence is the tonic activity in urethral sphincter motoneurones upon which phasic reflexes may be superimposed. The role of a non-linear membrane response to depolarization could be to enhance the actions of excitatory synaptic inputs to the sphincter motoneurones and to promote sustained motoneurone output. Such properties may help to maintain optimal firing rates and force generation needed for urethral closure. To date, the role of such intrinsic sphincter motoneurone membrane properties has not been explored and forms the basis of the study described here. Some of the results in this paper have been previously presented in abstract form (Shefchyk & Paroschy, 1998).
METHODS
Intracellular recordings were obtained from 24 decerebrate adult cats of either sex. In eight of these 24 animals only intracellular recordings from sphincter motoneurones during ramp current injection were examined. In the other 16 animals, responses to trains of stimuli delivered to afferent nerves for 2–10 s were recorded in external urethral sphincter (EUS) and external anal sphincter (EAS) electroneurograms (ENGs) along with intracellular recordings from single motoneurones. All surgical and anaesthetic procedures were carried out under the guidelines established by the Canadian Council for Animal Care and approved by the University of Manitoba Animal Research Protocol Review Committee. Surgical anaesthesia was induced and maintained using 2–4 % halothane carried in a mixture of nitrous oxide and oxygen. The level of anaesthesia was monitored with pedal withdrawal reflex testing and systemic arterial blood pressure was monitored via a cannula placed in a femoral artery. The nerves innervating the following muscles on the left side of the animal were cut and placed on bipolar hook electrodes: external urethral sphincter (EUS), external anal sphincter (EAS), which together are referred to as pudendal (Pud). The urethral (urSPud) and cutaneous (cutSPud) branches of the left sensory pudendal nerve were cut and mounted on individual electrodes. If not separated they were together referred to as the sensory pudendal (SPud) nerve. The right (contralateral) sensory pudendal nerve was cut to remove a source of reflex excitation to the sphincter motoneurones (Fedirchuk et al. 1992). This was done to decrease the possibility that the sphincter motoneurones received sufficient ongoing synaptic excitation to elevate them into a region of altered excitability and non-linearity as has been described for hindlimb motoneurones (Bennett et al. 1998a). Likewise, to remove any excitation originating from bladder distension afferents, a cannula was inserted into the dome of the bladder and allowed to drain freely throughout the experiment. The animals had been fasted for at least 12 h prior to the experiment and no cats defecated at any time during the experiment. A laminectomy exposed the caudal lumbar and sacral spinal segments. The animals were mechanically decerebrated at the pre-collicular, post-mammillary level and the cerebral cortices and all tissue rostral to the transection were removed. The anaesthetic was then discontinued and gallamine triethiodide (Flaxedil; 2–3 mg kg−1 h−1) was administered. The animals were artificially ventilated such that expired CO2 was maintained between 3 and 5 %. A femoral venous cannula was used to provide a continuous infusion of a glucose buffer solution and if necessary, a high molecular weight dextran solution was given intravenously to maintain blood pressure levels above 80 mmHg after the decerebration. At the termination of the experiment the animal was killed with an overdose of anaesthetic or an intravenous injection of potassium chloride solution and the artificial ventilation discontinued.
Intracellular recordings were obtained from antidromically activated EUS and EAS motoneurones within the ventral horn of the first sacral spinal segment. Recordings were made using sharp microelectrodes filled with either a 2 M potassium acetate solution or a 2 M potassium acetate solution with the lidocaine derivative QX-314 (50 mM). Lidocaine derivatives such as QX-314 have been shown to block sodium action potentials in motoneurones and although they may have actions on other ionic currents (Engberg et al. 1984; Nathan et al. 1990; Talbot & Sayer, 1996), QX-314 has been used previously to examine non-linear membrane properties in cat spinal motoneurones (see Brownstone et al. 1994; Bennett et al. 1998a,b). Discontinuous current clamp (DCC; 3–6 kHz sampling rate; Axoclamp 2A) was used to record membrane potential while delivering continuous DC bias current, triangular ramp (ascending arm of ramp normally 5 s long) or square-wave depolarizing current injections of varying durations. The ability to inject current via the microelectrode with the selected DCC parameters was continually monitored during the intracellular recording and confirmed in the extracellular fluid outside the cell. Trials in which the electrode appeared to block during current injection were discarded.
The cutSPud and/or urSPud branch of the left sensory pudendal nerve was stimulated at 5 or 10 times threshold (T; defined as the lowest stimulus strength that produced a response on the cord dorsum recording). Short (< 5 s) or longer (5-10 s) trains of stimuli (2-10 Hz) were used. In six animals, micturition was evoked by distension of the bladder with saline at room temperature infused at a rate of 2 ml min−1. The pontine micturition centre (PMC) was electrically stimulated in one animal using a monopolar steel electrode with stimulation parameters of 150–200 μA (0.5 or 1.0 ms square-wave pulses) at 20 Hz (for details refer to Fedirchuk & Shefchyk, 1991, 1993). The electroneurograms (high pass 300 Hz and low pass 10 kHz) were rectified and integrated (cut-off frequency 1–30 Hz) and both the raw and integrated ENG (Int ENG) records were digitized (10-15 kHz and 5 kHz, respectively). The microelectrode signal (10 kHz), cord dorsum (5 kHz), microelectrode current monitor (5 kHz), and stimulus markers (5 kHz) were digitized and stored on a Pentium PC running QNIX for later analysis using software developed within the Winnipeg Spinal Cord Research Centre. In some animals the serotonin precursor 5-hydroxytryptophan (5-HTP; 2.5-20 mg kg−1) was administered intravenously to facilitate the expression of non-linear properties (Bennett et al. 1998a,b). In one animal a solution of 50 mM methoxamine was delivered to the ventral surface of the spinal cord via a cannula (PE10 silicone tubing) threaded under the cord (see Lee & Heckman, 1998a,b). In two animals in which the systemic blood pressure was below 80 mmHg, a dilute solution of noradrenaline (2 mg in 100 ml saline) and etilefrine hydrochloride (Effortil, Beohringer Ingelheim, 10 mg in 20 ml saline) was added to the 80–100 ml reservoir of glucose buffer and infused to elevate the blood pressure. Results are presented as means and standard deviations (±s.d.). Comparisons were made using a Mann-Whitney rank sum test or Student's t test (paired or unpaired) as indicated in the text.
RESULTS
Afferent-evoked sustained sphincter responses
With the perineal and pudendal nerves cut and an empty bladder, there was little or no tonic activity in the EUS and EAS ENGs in the absence of electrical stimulation of peripheral nerves in this sample of decerebrate cats. Electrical stimulation of urethral and/or cutaneous sensory pudendal afferents at 5T or 10T evoked a stimulus-locked response in the EUS and EAS ENGs that was followed by a period of sustained efferent activity (examples in Figs 1, 2, 3, 6 and 7). Figure 1A shows the EUS ENG recording from one animal in which neither 5-HTP nor monoaminergic drugs were administered. In this example, a number of efferent units remained active for ∼4 s after termination of ipsilateral urSPud nerve stimulation. In the present sample of 16 animals, stimulus trains lasting < 5 s evoked activity persisting for 3–30 s beyond the end of the stimulus train. Longer duration stimulation (5-10 s) produced 15 to >50 s of sustained activity. This sustained efferent activity was observed in: 11 animals without administration of 5-HTP or monoaminergic drugs; two animals after the administration of 2.5-20 mg kg−1 5-HTP 1 h before recording; one animal after methoxamine had been infused onto the spinal cord; and two animals in which a dilute solution of noradrenaline and/or Effortil had been administered intravenously throughout the experiment to elevate a depressed blood pressure. Figure 1B shows that when several trains of stimuli were repeated at intervals of <10 s, the duration of the sustained activity following a later train was longer (20-60 s) compared with that following a single train of stimuli. This was seen in all six animals examined suggesting a ‘warm-up’ phenomenon similar to that described by Bennett and coworkers (Bennett et al. 1998b; also see Svirskis & Hounsgaard, 1998). Two of the six animals received 5-HTP prior to testing while the other four had not received 5-HTP or monoaminergic drugs.
Figure 1. Stimulus-locked and sustained EUS ENG activity evoked by stimulation of pudendal afferents.
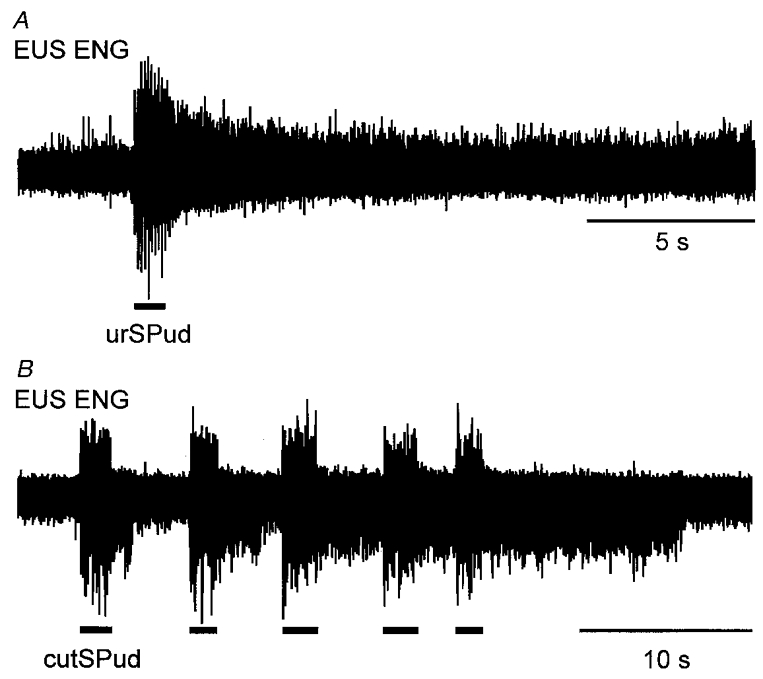
A, a 10 Hz train of stimuli (5T) to the urSPud nerve evoked multi-unit ENG activity during the period of stimulation (indicated by bar below the ENG) that persisted for seconds after the stimulation. B, response in another animal in which repeated trains (10 Hz, 5T) of stimulation of the cutSPud nerve evoked sustained responses that increased in duration with subsequent stimulation. Neither animal had been given 5-HTP, methoxamine or noradrenaline.
Figure 2. Sustained responses and increased firing frequency in EUS units following afferent stimulation.
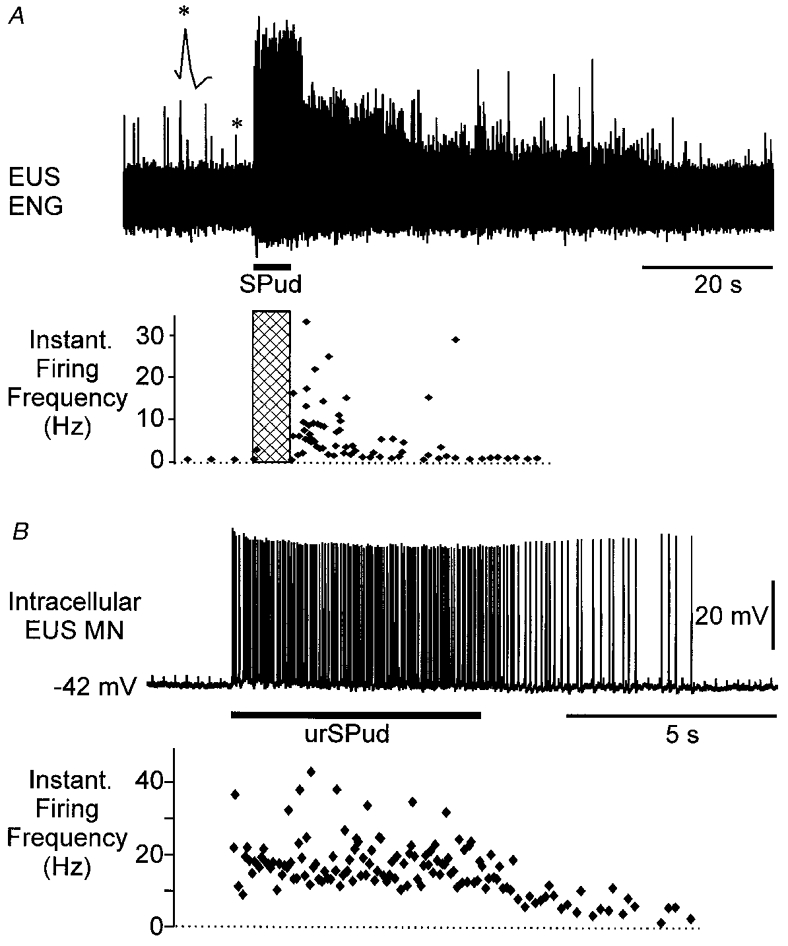
A, a single unit (*, enlarged above trace) discriminated from the population ENG (upper panel) and the instantaneous firing frequency of the unit following stimulation of the SPud nerve at 10 Hz (5T, hatched bar) (lower panel). No drugs had been given to this animal. B, intracellular recording from an EUS motoneurone (MN, upper panel, resting membrane potential (Vm) -42 mV) revealed that this cell fired at ≈20 Hz during stimulation of the urSPud nerve (5T, 4 Hz) and that the firing continued at ≈8 Hz for almost 5 s after stimulation. In this animal, intravenous noradrenaline had been given to elevate the blood pressure.
Figure 3. Effect of membrane potential on afferent-evoked persistent firing in an EAS motoneurone.
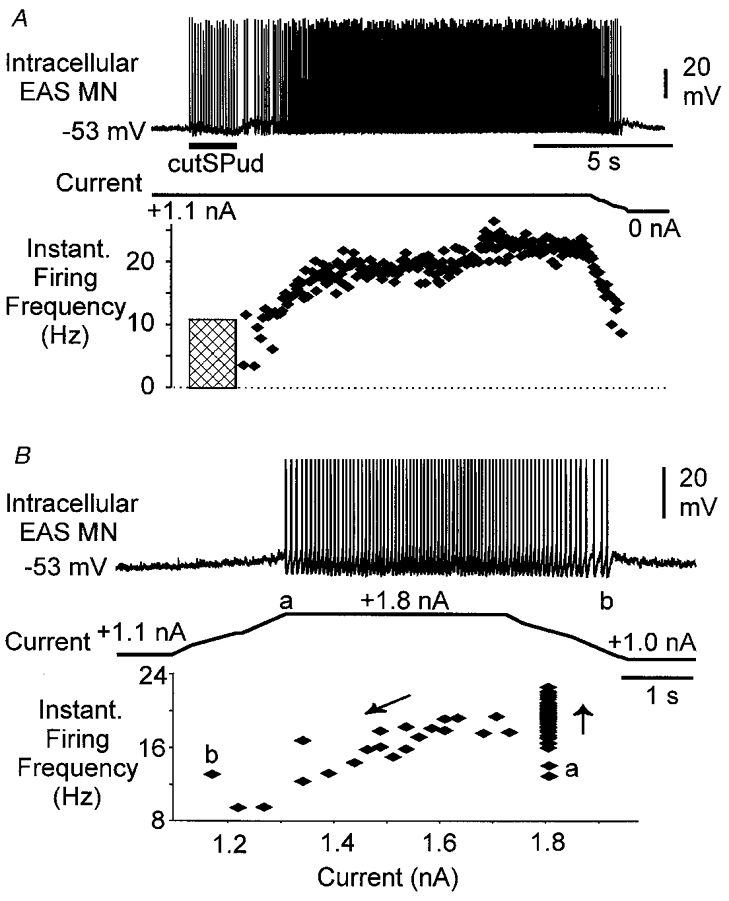
A, intracellular EAS motoneurone recording (upper panel, resting Vm -57 mV) shows that when the cell was depolarized to -53 mV with 1.1 nA of bias current, persistent firing was evoked by cutSPud afferent stimulation (10T, 10 Hz). A plot of the instantaneous firing frequency of the motoneurone (lower panel) revealed the persistent firing (≈18 Hz) lasted several seconds then increased to 21 Hz. Reducing the depolarizing bias current resulted in a drop off in the persistent firing of the cell. The hatched region represents the period of stimulation when doublet and triplet firing was evoked by each stimulus but was not included in this plot of instantaneous firing frequency. B, in this same cell, current injection alone produced persistent 18 Hz firing that terminated when the membrane was hyperpolarized (i.e. depolarizing bias current removed). Spikes have been truncated for illustration purposes. The plot of the instantaneous firing versus current (lower panel) shows that firing started at 1.8 nA of current (a), accelerated (upward arrow) then persisted as the current was decreased (downward arrow) to below (b) the initial current (a) that activated the cell. This animal had been given 16 mg kg−1 5-HTP 2.5 h prior to the recording.
Figure 6. Effects of synaptically evoked membrane hyperpolarization on persistent motoneurone firing.
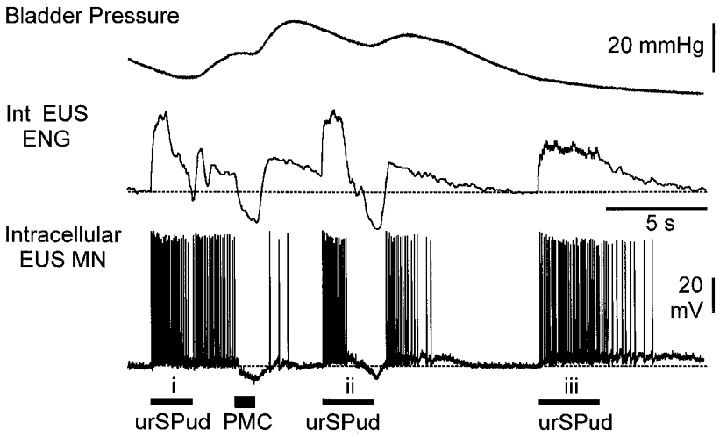
Bladder pressure, integrated (Int) EUS ENG and intracellular EUS motoneurone records (resting Vm -58 mV) during stimulation of the urSPud nerve and pontine micturition centre (PMC) are shown. Trains of stimuli to the urSPud nerve (i; 10T, 20 Hz) evoked EUS firing that persisted until the motoneurone membrane was hyperpolarized by PMC stimulation (50 Hz, 200 μA). The second train of urethral stimulation (ii) evoked stimulus-locked firing that stopped when the membrane hyperpolarized and resumed after the stimulation and hyperpolarization was terminated. The final train (iii) of urSPud stimulation (10T, 10 Hz) evoked firing that persisted for several seconds before stopping spontaneously. The dashed line indicates the level of ENG activity and the motoneurone membrane potential prior to any stimulation. This animal had received 8.4 mg kg−1 5-HTP 30 min before the recording.
Figure 7. Suppression of afferent-evoked sustained efferent responses during distension-evoked micturition.
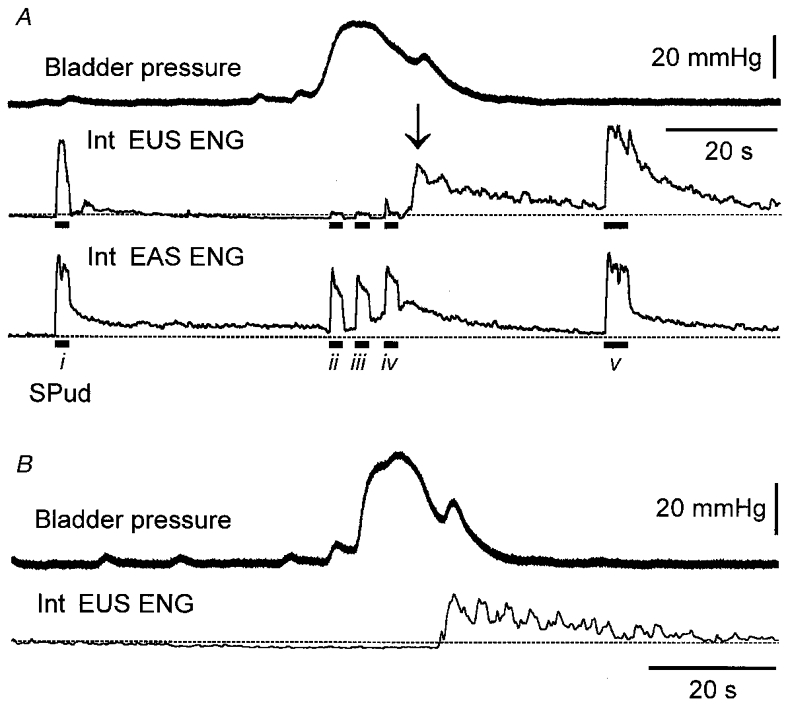
A, distension of the bladder evoked a micturition reflex characterized by an increase in bladder pressure (upper trace) during which time there was a suppression of EUS ENG activity (second trace, 1 Hz integrated) that was not seen in the EAS ENG activity (third trace). The dashed line indicates the pre-void level of ENG activity. SPud nerve stimulation (5T, 10 Hz; indicated by bar below trace) evoked sustained EUS activity prior to (i) and after (v) the void. During micturition (ii-iv), both stimulus-locked and sustained EUS ENG responses evoked by afferent stimulation were attenuated while the evoked EAS ENG responses were largely unchanged. As the bladder contraction ended, an increase in EUS ENG activity (arrow) was evident. B, in this same animal, the post-void increase in EUS activity was also evident in the absence of peripheral nerve stimulation.
The firing frequency of single sphincter efferents was examined in six animals using either the ENG record or microelectrode recordings from single motoneurones. Figure 2A shows the instantaneous firing frequency of a unit (indicated by * and enlarged) discriminated from the population ENG. It was not possible to isolate the unit during the period of stimulation (hatched bar) because of recruitment of units of similar amplitude. Immediately after the train of afferent stimuli this unit fired at an average rate of 13 Hz for 10 s, then at ∼4 Hz for an additional 10 s before returning to the pre-stimulus baseline firing rate of 1 Hz. Figure 2B shows the firing of an intracellularly recorded EUS motoneurone which fired at ∼20 Hz during afferent stimulation and then maintained an average firing rate of ∼8 Hz for almost 5 s after the stimulation. In the present sample of eight units, the firing frequencies during steady, sustained firing occurring within the first 10 s after the end of the stimulus train included 20 Hz (1 EAS, 3 EUS), 13 Hz (2 EUS) and 6–8 Hz (2 EUS).
Intracellular recordings from three motoneurones showed that persistent firing evoked by trains of pudendal afferent stimulation could be terminated by intracellular hyperpolarizing current injection. This is illustrated in Fig. 3 where a small sub-threshold depolarizing current was applied before the stimulation. In Fig. 3A, afferent stimulation evoked persistent firing (18-20 Hz) that terminated when the membrane potential was hyperpolarized by removal of the intracellular current injection. Figure 3B shows that in this same motoneurone, constant depolarizing current injection initiated firing that showed no evidence of adaptation; the cell fired at around 18 Hz until the membrane potential was sufficiently hyperpolarized. A plot of the instantaneous firing frequency versus injected current (Fig. 3B, lower panel) shows a relationship similar to the counter-clockwise hysteresis characteristic of bistable firing properties (Hounsgaard et al. 1984, 1988; Bennett et al. 1998a,b; Gorassini et al. 1999).
Sphincter motoneurone responses to depolarizing current injection
Persistent sphincter efferent discharge evoked by afferent stimulation could result from continued activity of polysynaptic pathways or the activation and maintenance of depolarizing conductances in the motoneurones. To address the latter possibility we examined the responses of sphincter motoneurones to intracellular ramp depolarizing current injections. Other studies have examined persistent inward currents (Lee & Heckman, 1998b) or plateau potentials and bistability (see Introduction) and in the present study we document non-linear responses defined by deviations in the linear trajectory of the membrane voltage in response to linear ramp depolarizing currents (refer to Fig. 4).
Figure 4. Non-linear changes in membrane potential in response to current injection.
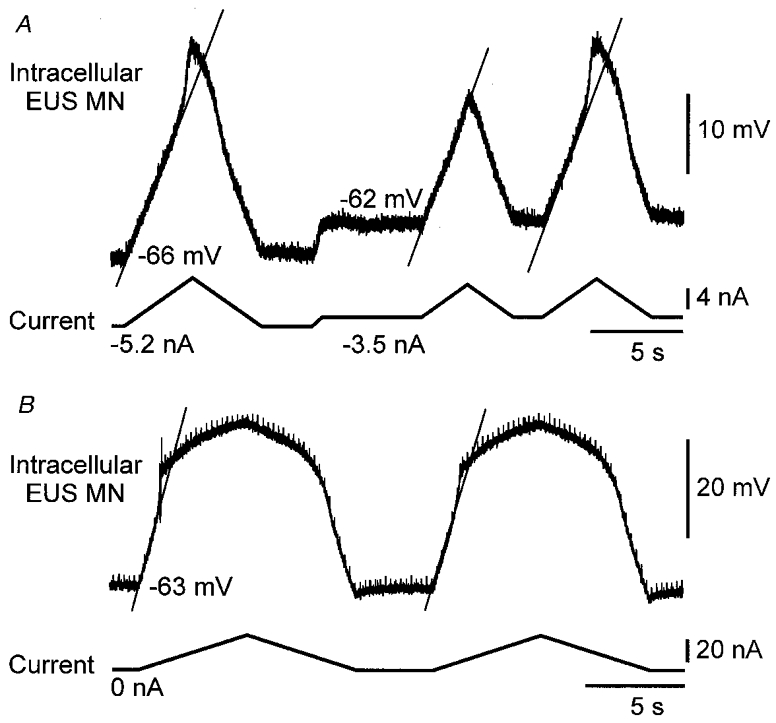
A, changes in membrane potential (upper trace) in response to ramp current injections of varying sizes (lower trace) are shown in EUS motoneurones recorded with QX-314 in the micropipette. Depolarizing current sufficient to bring the Vm to approximately -47 mV resulted in an abrupt change in the slope of the membrane trajectory (indicated by continuous diagonal line). The middle current ramp was not of sufficient size to evoke this non-linear response. This animal had been given 4 mg kg−1 5-HTP prior to the recording. B, another EUS motoneurone displayed a non-linear membrane response with longer ramp injections; no 5-HTP had been administered in this animal. In A, the motoneurone resting Vm was -55 mV; in B it was -61 mV.
Depolarizing intracellular ramp currents were injected in 40 sphincter motoneurones. In 24 motoneurones the microelectrode recording solution included QX-314. Figure 4A shows two non-linear responses to current injection. Note the sudden depolarization of the membrane potential that occurs at about -47 mV in response to the first and third current ramps illustrated.
In the presence of QX-314 it was possible to examine the motoneurone membrane response in the absence of spiking and thus identify the voltage threshold of observed non-linear responses. The voltage threshold for the non-linear response was determined by adjusting the intracellular DC bias current and the magnitude of the depolarizing ramp current. There was no systematic evaluation of the effect of varying the interval between the ramps on possible ‘warm-up’ of the non-linear response with the intracellular recordings. Seventeen out of 24 motoneurones recorded with QX-314 displayed a non-linear membrane response to depolarizing ramp current injection. Because QX-314 can have actions on currents other than the sodium current (see Methods), the possibility existed that the presence of QX-314 could influence the ability to record non-linear responses. There was no difference (P = 0.9, Mann-Whitney rank sum test) in the resting Vm in motoneurones recorded with QX-314 included in the micropipette and those recorded with a potassium acetate solution alone. In a sample of six motoneurones in which QX-314 was used, the membrane input resistance was 3.1 ± 1.2 MΩ; in seven motoneurones recorded with only potassium acetate, it was 2.1 ± 0.7 MΩ. These values were within the range previously reported in pudendal motoneurones in decerebrate (Hochman et al. 1991) and anaesthetized cats (Sasaki, 1991) using conventional microelectrode solutions.
Similar non-linear membrane responses were observed in 5/16 cells recorded with a potassium acetate solution alone. In all but one cell the action potentials evoked by depolarization failed at, or near, the top of the depolarizing ramp making it impossible to examine the relationship between firing frequency and current injection. It should be noted that in almost all of these motoneurones subsequent membrane hyperpolarization restored spiking in the cell.
Table 1 summarizes the sample of EUS, EAS and pudendal motoneurones that displayed a linear or non-linear response to depolarizing ramp current injection, the resting membrane potentials of the motoneurones, and the voltage thresholds for the non-linear response. The voltage threshold for the non-linear response was 1–19 mV more depolarized than the resting membrane potential. In several motoneurones in which the Vm was more depolarized than -40 mV, continuous hyperpolarizing current injection upon which large depolarizing ramp current injections were superimposed failed to reveal a non-linear response. We considered it likely that these motoneurones were injured by the microelectrode impalement and were excluded from Table 1. Examination of the motoneurones included in the sample failed to reveal a significant difference (P = 0.9, unpaired t test) between the resting membrane potentials in the motoneurones that displayed a non-linear response to ramp current injection (resting Vm -54 ± 6 mV; n = 22) and those that did not (resting Vm -54 ± 9 mV; n = 18). Both EUS and EAS motoneurones displayed a non-linear membrane response and there was no difference in the voltage threshold for the non-linear response between the two populations (P = 0.9, unpaired t test). In five motoneurones (resting Vm -56 ± 10 mV), short hyperpolarizing pulses (1-2 nA, 20 ms) revealed a 56 ± 10 % reduction in the membrane input resistance (1.3 ± 0.5 MΩ) during the non-linear membrane response compared with the resting membrane input resistance (3.0 ± 0.7 MΩ; P < 0.001, paired t test). In addition, during the non-linear response an increase in synaptic activity was evident in most of the 22 cells.
Table 1.
Summary of incidence, resting Vm and nonlinear voltage thresholds in sphincter motoneurones examined with triangular ramp current injection
| Resting Vm of motoneurones | |||
|---|---|---|---|
| Motoneurone identity | with linear response(mV) | with non-linear response(mV) | Voltage threshold for non-linear membrane response(mV) |
| EUS(17) | −54 ± 9(7) | −52 ± 6(10) | −43 ± 3 |
| EAS(11) | −52 ± 6(8) | −58 ± 8(3) | −45 ± 4 |
| Pudendal(12) | −60 ± 13(3) | −54 ± 7(9) | −43 ± 3 |
The number of neurones is given in parentheses. Pudendal, EUS and EAS stimulated together.
A persistent depolarization of the membrane potential was observed after depolarizing square-wave pulse injections in three motoneurones, one of which is shown in Fig. 5. This EUS motoneurone responded to depolarizing current steps with a sustained 2–3 mV depolarization that could be turned off by hyperpolarizing current pulses longer than 200 ms (*). In each of these three motoneurones, when a continuous bias current was used to depolarize the membrane potential to a level more positive than the voltage threshold for the non-linear response, additional depolarizing pulses failed to produce a further depolarization of the membrane potential (not shown).
Figure 5. Persistent membrane depolarization in response to depolarizing square wave pulses.
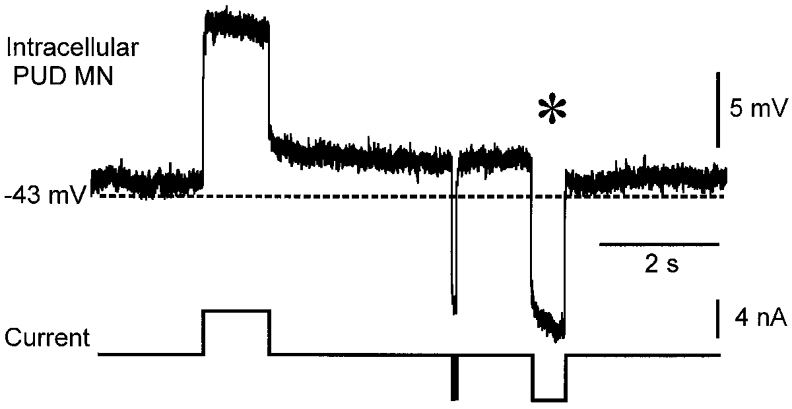
In this pudendal motoneurone (resting Vm -43 mV), depolarizing pulses produced a persistent membrane depolarization of several millivolts. The depolarization was terminated by a hyperpolarizing current injection longer than 200 ms (*) but not by a very brief pulse of the same amplitude. QX-314 (50 mM) was included in the recording micropipette solution. This animal had received 8 mg kg−1 5-HTP ≈40 min prior to the recording.
While the intravenous administration of 5-HTP increased the likelihood of non-linear membrane responses in the motoneurones, non-linear responses were observed in five motoneurones in the absence of 5-HTP or methoxamine. Furthermore, 11 motoneurones with resting membrane potentials between -50 and -69 mV failed to display non-linear responses during ramp injections (depolarization to -30 mV) after the administration of between 2.5 and 20 mg kg−1 of 5-HTP. Thus, the administration of 5-HTP was not necessary for observing this non-linear membrane response in all motoneurones nor did its presence at the given doses ensure non-linear responses in all sphincter motoneurones. Sustained afferent-evoked activity in the ENGs and non-linear motoneurone membrane responses were observed in one animal following application of a solution of methoxamine to the ventral surface of the exposed spinal cord. Due to the nature of this delivery method (see Methods), the concentration of the drug within the ventral spinal cord is unknown.
Suppression of sustained EUS activity during micturition
Figure 3 shows that intracellular injection of hyperpolarizing current could terminate persistent firing in sphincter motoneurones. We wished to determine if synaptically mediated membrane hyperpolarization could also terminate persistent firing in these motoneurones. Unfortunately, sphincter motoneurones do not receive significant segmental reflex inhibition at rest (see Introduction). However, they are phasically hyperpolarized during micturition evoked by bladder distension, electrical stimulation of the pontine micturition centre (Shimoda et al. 1992; Fedirchuk & Shefchyk, 1993) or by stimulation of urethral afferents under certain conditions (Shefchyk & Buss, 1998). Thus, in six experiments, EUS motoneurones were transiently hyperpolarized by activation of the micturition circuitry and afferent-evoked persistent firing in these motoneurones was examined.
In one experiment, intracellular recordings from two EUS motoneurones were obtained during synaptically evoked motoneurone membrane hyperpolarization. In both cells, hyperpolarization of the motoneurone membrane interrupted afferent-evoked persistent firing in the motoneurone which then resumed as the membrane repolarized. This is illustrated in Fig. 6 in which both EUS ENG and intracellular motoneurone recordings are shown. Urethral stimulation (Fig. 6, i) evoked stimulus-locked action potentials in the motoneurone. This firing persisted after the afferent stimulation until PMC stimulation resulted in a 6 mV hyperpolarization of the membrane. As the membrane repolarized, several spikes reappeared spontaneously. The second train of urethral stimulation (Fig. 6, ii) initially evoked stimulus-locked firing which was replaced by a membrane hyperpolarization that again stopped the motoneurone firing. The membrane hyperpolarization evoked by the urethral stimulation reflects the activation of the micturition circuitry by the urethral afferent stimulation when the bladder volume and pressure is close to, or at, the micturition threshold (Shefchyk & Buss, 1998). Following urethral nerve stimulation and repolarization of the membrane potential, the motoneurone firing resumed for several seconds before stopping spontaneously. It would appear that the excitatory stimuli depolarized the motoneurone sufficiently to recruit the mechanisms underlying the persistent firing while micturition-related inhibitory pathways to the EUS motoneurones were able to suppress these responses. Interestingly, changes in the membrane potential of this motoneurone are similarly reflected in the integrated ENG record from the EUS motoneurone population (Fig. 6).
Figure 7 shows the firing in the EUS motoneurone population during micturition evoked by continuous infusion of fluid into the bladder. Afferent-evoked sustained responses in the EUS ENG were abolished as fluid was expelled during the micturition reflex. As voiding ended and the bladder pressure began to decrease, the sustained afferent-evoked EUS ENG responses reappeared in each animal. In contrast, the afferent-evoked sustained response in the EAS ENGs could be evoked throughout the void. In 5/6 animals, the afferent stimulus-locked responses recorded in EUS efferents were also attenuated during voiding (to < 10 % of pre-void control values; Fig. 7A; also see Fedirchuk & Shefchyk, 1993; Shefchyk & Buss, 1998). An increase in EUS ENG activity after the void occurred spontaneously in 2/6 animals (Fig. 7B). Regardless of whether trains of afferent stimulation were delivered (Fig. 7A) or not (Fig. 7B), the suppression of EUS activity during the period when the motoneurones are inhibited was temporary. We suggest that during voiding the micturition-mediated motoneurone inhibition illustrated in Fig. 6 interfered with the mechanism(s) underlying the tonic activity in the population of EUS motoneurones (Fig. 7).
DISCUSSION
Non-linear membrane properties in sphincter motoneurones
Using intracellular triangular-wave current injections, a non-linear membrane response to depolarization was revealed in over half of the sample of sacral sphincter motoneurones examined in unanaesthetized decerebrate cats. The threshold for this non-linear membrane response was around -43 mV; during the non-linear response the motoneurone membrane conductance nearly doubled. We propose that a non-linear membrane property in sacral sphincter motoneurones contributes to the persistent activity of these motoneurones during continence and following brief trains of perineal afferent stimulation. Given the present observations and the lack of evidence to indicate otherwise at this time, we propose that a non-linear membrane response similar to the plateau potentials or bistability described in brainstem (Hsiao et al. 1998) and spinal limb motoneurones (see Introduction) is present in sacral sphincter motoneurones.
Plateau potentials and bistability can amplify and/or prolong hindlimb motoneurone reflex output in decerebrate (for example Bennett et al. 1998a,b; Crone et al. 1988) and awake animals (Eken & Kiehn, 1989; Gorassini et al. 1999), as well as in humans (Kiehn & Eken, 1997). Studies examining turtle and cat motoneurones suggest that serotonergic and adrenergic agonists are necessary for the production of motoneurone plateau potentials. Sphincter motoneurones receive a descending serotonergic input (Kojima & Sano, 1983) and recently 5-HTP has been shown to enhance pudendal efferent reflex output via unidentified spinal mechanisms (Espey et al. 1998). We have shown that the administration of 5-HTP or adrenergic agonists increased the likelihood of observing a non-linear membrane response in sphincter motoneurones. The decerebrate preparation used in the present study may have variable levels of activity in the descending pathways that release serotonin and noradrenaline within the sacral spinal cord. In the present study, this may have been reflected in the fact that non-linear membrane responses and persistent motoneurone firing were observed in the absence of 5-HTP or adrenergic agonists in some animals while in other animals, modest doses of 5-HTP or adrenergic agonists were required to facilitate these responses (see current results and also Bennett et al. 1998a,b). Acetylcholine has also been shown to facilitate plateau potentials (Svirskis & Hounsgaard, 1998) and may contribute to the expression of a non-linear response in sphincter motoneurones. Interestingly, a potential cholinergic input to sphincter motoneurones may come from their own axon collaterals (Sasaki, 1994). Recordings from sphincter efferents have demonstrated that there may be a ‘warm-up’ phenomenon similar to that reported in both turtle and cat limb motoneurones. In a few sphincter motoneurones examined in the current study, non-linear membrane responses were not observed during initial current ramps but appeared with subsequent ramps even though the baseline membrane potential, bias currents and ramp sizes were unchanged. In several other motoneurones, the voltage threshold for the non-linear response shifted by 1–3 mV to a more hyperpolarized value after several current ramps were injected at intervals of <10 s (also see Bennett et al. 1998b). Our use of inter-ramp intervals of >10 s in the majority of cells precluded an examination of possible warm-up phenomenon during intracellular current ramps; however, population EUS and EAS ENG recordings did reveal a facilitation of afferent-evoked sustained responses when trains of afferent stimulation were repeated in rapid succession. Unfortunately, like Bennett and coworkers (1998b), we can offer no judgement as to the mechanism responsible for the possible ‘warm-up’ observed.
The increase in membrane conductance observed in a sample of motoneurones examined in the present study may be a reflection of the persistent inward currents that Lee & Heckman (1998b) have described in cat hindlimb motoneurones. Furthermore, the mean voltage threshold for the non-linear membrane response in the sphincter motoneurones sampled was -43 mV. This voltage is similar to the voltage reported by Lee & Heckman (1998b) for the onset of the persistent inward current in what they called partially bistable motoneurones, and is slightly more depolarized than the onset voltage in fully bistable cells (about -50 mV).
In several motoneurones the injection of depolarizing square-wave pulses produced a persistent membrane depolarization after the pulse. Depending upon the membrane holding potential, the prolonged depolarization was brief (1 s) or seconds long. Similar observations have been made on stretch-activated plateaus in cat hindlimb motoneurones (Fig. 6 in Bennett et al. 1998a). Because the sphincter motoneurone membrane time constant is <12 ms (Hochman et al. 1991; Sasaki, 1991), it is unlikely that the time constant of the cell could explain the persistent membrane depolarization. A more likely explanation is that variations in the duration of the prolonged sphincter membrane depolarization reflect varying degrees of activation of the voltage-sensitive current underlying the response.
There was a higher incidence of non-linear responses to ramp injections in the sample of motoneurones recorded with QX-314 included in the micropipette. One can suggest that the actions of QX-314, perhaps by reducing potassium currents, facilitated the expression of the non-linear responses (however, see Lee & Heckman, 1999). Since we did not measure the input resistance of all the cells recorded with the QX-314 solution versus potassium acetate alone, we cannot exclude differences in the health of the cells as an explanation for the difference in the incidence of non-linear responses.
Implications for urethral sphincter function during continence and micturition
In the present study we began by removing the major sources of segmental excitation to the sphincter motoneurones. This was achieved by cutting perineal afferents bilaterally, ipsilateral leg afferents and maintaining the bladder empty between micturition trials. Under these conditions, little (1-3 Hz) or no activity was seen in the sphincter efferents and this is similar to the 1–2 Hz firing reported in human sphincter EMG units when the bladder is empty (Blaivas et al. 1977). Tonic activity in sphincter efferents has been described in both humans (Blaivas et al. 1977) and animals (Krier, 1985; S. J. Shefchyk, unpublished observations) and it is generally accepted that polysynaptic pathways originating with viscera and perineal afferents are largely responsible for producing and maintaining sphincter activity (Bishop et al. 1956; Garry et al. 1959; Mackel, 1979; Fedirchuk et al. 1992; Buss & Shefchyk, 1999). EUS efferent firing rates of 20 Hz have been reported in the cat when the bladder is distended just prior to micturition (Shimoda et al. 1992) and 8–35 Hz firing rates have been documented in cat anal sphincter units in response to electrical or mechanical stimulation of the rectum and perineum (Krier, 1985). These firing rates are similar to the 6–21 Hz observed in urethral and anal sphincter efferents during the persistent firing evoked by afferent stimulation in the present study. A discussion of afferent-evoked tonic activity in the sphincter efferents must acknowledge the potential contribution of the interneurones interposed in these pathways. However, until these interneurones are identified and examined, their contribution to sustained motoneurone output remains unknown.
The sphincter motoneurones possess a number of characteristics that may predispose them to tonic activity. Membrane input resistance and rheobase values reported for sphincter motoneurones are low (Hochman et al. 1991; Sasaki, 1991), akin to slow hindlimb motoneurones. However, the duration of their after-hyperpolarizations are shorter than those reported in slow hindlimb motoneurones (for review of hindlimb properties see Burke, 1981). Furthermore, the presence of dendritic bundling (Beattie et al. 1990; Ramirez-Leon & Ulfake, 1993) and sphincter motor axon collaterals terminating within the area of the sphincter motor nucleus (Sasaki, 1994) suggests the possibility of electrical coupling and recurrent excitation between sphincter motoneurones. The presence of a non-linear membrane property such as plateau potentials would compliment these features and help to amplify and maintain output from the motoneurones. We suggest that the lack of recurrent and reciprocal inhibition, together with these other motoneurone features and the presence of a non-linear response to membrane depolarization would produce a pool of motoneurones recruited easily, synchronously, and tonically – a set of characteristics suitable for activation of sphincter motor units as demanded by continence.
The present study, as well as many previous observations in human and cat models, show that tonic activity and afferent-evoked sustained responses in EUS efferents are suppressed during micturition. The suppression of continence mechanisms (see Shefchyk, 1998) is vital to efficient bladder emptying in humans and cats. It has been shown previously that cat urethral sphincter motoneurones undergo membrane hyperpolarization during micturition (Shimoda et al. 1992; Fedirchuk & Shefchyk, 1993; Fedirchuk et al. 1994). This hyperpolarization could prevent the motoneurone membrane from reaching the critical voltage threshold for the non-linear response described in the present work, regardless of whether the plateau potential is generated in the soma or the dendrites of the neurone (Bennett et al. 1998a,b).
In the absence of pathology, the lower urinary tract remains in continence mode for much of the time and we propose that a non-linear motoneurone membrane property such as described here may be expressed most of the time in EUS motoneurones. Periodic voids would then require the central micturition circuitry to transiently prevent expression of this membrane response. Furthermore, the micturition-related postsynaptic inhibition of sphincter motoneurones may be self-limiting due to the presence of anomalous rectifying currents in the motoneurone that would counteract any membrane hyperpolarization (for discussion see Sasaki, 1991). These rectifying currents could function to re-establish conditions in the motoneurone that would be conducive for the expression of a non-linear membrane response and sustained motoneurone activity. We have shown that both EUS and EAS motoneurones display similar afferent-evoked sustained responses and non-linear membrane responses to depolarizing current injection. The central micturition circuitry, with its selective inhibitory action on EUS motoneurones, may exert a unique control over EUS motoneurone properties. Presumably, the neural circuitry coordinating defecation would have a similar control over anal sphincter motoneurone properties. Although we cannot as yet define the mechanism(s) underlying the non-linear membrane response to depolarization reported in the present study, this should not detract from the assertion that the presence of this property in sphincter motoneurones plays a role in the initiation and maintenance of sphincter activity during continence.
Previous discussions about abnormal sphincter function have focused on the descending and segmental reflex control of sphincter motoneurones. The potential contribution of intrinsic sphincter motoneurone membrane properties to abnormal activity has not been addressed. Enhancement in the expression of non-linear membrane responses in sphincter motoneurones could contribute to the increased tonic sphincter activity while at the other extreme, the loss of plateau potentials or similar non-linear membrane responses in sphincter motoneurones might contribute to diminished motoneurone output and the striated sphincter muscle weakness that is observed in some forms of incontinence. Thus, the observations reported in the present study introduce a new factor to be considered in the constellation of elements that could contribute to altered striated sphincter muscle function.
Acknowledgments
This work was funded by the Medical Research Council of Canada. The authors wish to thank S. Deschamps for her excellent technical assistance and Drs B. Fedirchuk and D. A. McCrea for their valuable comments.
References
- Beattie MS, Li Q, Leedy MG, Bresnahan JC. Motoneurons innervating the external anal and urethral sphincter of the female cat have different patterns of dendritic arborisation. Neuroscience Letters. 1990;111:69–74. doi: 10.1016/0304-3940(90)90346-b. [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Hultborn H, Fedirchuk B, Gorassini M. Synaptic activation of plateaus in hind limb motoneurones of decerebrate cats. Journal of Neurophysiology. 1998a;80:2023–2037. doi: 10.1152/jn.1998.80.4.2023. [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Hultborn H, Fedirchuk B, Gorassini M. Short-term plasticity in hind limb motoneurones of decerebrate cat. Journal of Neurophysiology. 1998b;80:2038–2045. doi: 10.1152/jn.1998.80.4.2038. [DOI] [PubMed] [Google Scholar]
- Binder MD, Heckman CJ, Powers RK. The physiological control of motoneuron activity. In: Rowell LB, Shepherd JT, Smith J, editors. Handbook of Physiology, Neural Control of Movement. Vol. 1. Oxford: Oxford University Press; 1996. pp. 3–53. assoc. [Google Scholar]
- Bishop B, Garry RC, Roberts TDM, Todd JK. Control of the external sphincter of the anus of the cat. The Journal of Physiology. 1956;134:229–240. doi: 10.1113/jphysiol.1956.sp005639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaivas JG, Labib KB, Bauyer SB, Retik AB. A new approach to electromyography of the external urethral sphincter. Journal of Urology. 1977;117:773–777. doi: 10.1016/s0022-5347(17)58622-x. [DOI] [PubMed] [Google Scholar]
- Brownstone RM, Gossard J-P, Hultborn H. Voltage-dependent excitation of motoneurones from spinal locomotor centres in the cat. Experimental Brain Research. 1994;102:34–44. doi: 10.1007/BF00232436. [DOI] [PubMed] [Google Scholar]
- Burke RE. Handbook of Physiology, section 1, The Nervous System, Motor Control. II. Bethesda, Maryland: American Physiological Society; 1981. Motor units: anatomy, physiology and functional organization; pp. 345–422. part 2. [Google Scholar]
- Buss RR, Shefchyk SJ. Excitability changes in sacral afferents innervating the urethra, perineum and hindlimb skin of the cat during micturition. The Journal of Physiology. 1999;514:593–607. doi: 10.1111/j.1469-7793.1999.593ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway BA, Hultborn H, Kiehn O, Mintz I. Plateau potentials in alpha-motoneurones induced by intravenous injection of L-DOPA and clonidine in the spinal cat. The Journal of Physiology. 1988;405:369–384. doi: 10.1113/jphysiol.1988.sp017337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone C, Hultborn H, Kiehn O, Mazieres L, Wigstrom H. Maintained changes in motoneuronal excitability by short-lasting synaptic inputs in the decerebrate cat. The Journal of Physiology. 1988;405:321–343. doi: 10.1113/jphysiol.1988.sp017335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groat WC. Neurobiology of Incontinence. Toronto: John Wiley & Sons; 1990. Central neural control of the lower urinary tract; pp. 27–56. Ciba Foundation Symposium 151. [DOI] [PubMed] [Google Scholar]
- Dubrovsky B, Filipini D. Neurobiological aspects of the pelvic floor muscles involved in defecation. Neuroscience and Biobehavioral Reviews. 1990;14:157–168. doi: 10.1016/s0149-7634(05)80216-7. [DOI] [PubMed] [Google Scholar]
- Eken T, Kiehn O. Bistable firing properties of soleus motor units in unrestrained rats. Acta Physiologica Scandinavica. 1989;136:383–394. doi: 10.1111/j.1748-1716.1989.tb08679.x. [DOI] [PubMed] [Google Scholar]
- Engberg I, Flatman JA, Lambert JDC. The response of cat spinal motoneurones to the intracellular application of agents with local anaesthetic action. British Journal of Pharmacology. 1984;81:215–224. doi: 10.1111/j.1476-5381.1984.tb10763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espey MJ, Du H-J, Downie JW. Serotonergic modulation of spinal ascending activity and sacral reflex activity evoked by pelvic nerve stimulation in cats. Brain Research. 1998;798:101–108. doi: 10.1016/s0006-8993(98)00401-6. [DOI] [PubMed] [Google Scholar]
- Fedirchuk B, Downie JW, Shefchyk SJ. Reduction of perineal evoked excitatory postsynaptic potentials in cat lumbar and sacral motoneurons during micturition. Journal of Neuroscience. 1994;14:6153–6159. doi: 10.1523/JNEUROSCI.14-10-06153.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedirchuk B, Hochman S, Shefchyk SJ. An intracellular study of perineal and hindlimb afferent inputs onto sphincter motoneurons in the decerebrate cat. Experimental Brain Research. 1992;89:511–516. doi: 10.1007/BF00229875. [DOI] [PubMed] [Google Scholar]
- Fedirchuk B, Shefchyk SJ. Effects of electrical stimulation of the thoracic spinal cord on bladder and external urethral sphincter activity in the decerebrate cat. Experimental Brain Research. 1991;84:635–642. doi: 10.1007/BF00230976. [DOI] [PubMed] [Google Scholar]
- Fedirchuk B, Shefchyk SJ. Membrane potential changes in sphincter motoneurons during micturition in the decerebrate cat. Journal of Neuroscience. 1993;13:3090–3094. doi: 10.1523/JNEUROSCI.13-07-03090.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garry RC, Roberts TDM, Todd JK. Reflexes involving the external urethral sphincter in the cat. The Journal of Physiology. 1959;149:653–665. doi: 10.1113/jphysiol.1959.sp006366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorassini M, Bennett DJ, Kiehn O, Eken T, Hultborn H. Activation patterns of hindlimb motor units in the awake rat and their relation to motoneuron intrinsic properties. Journal of Neurophysiology. 1999;82:709–717. doi: 10.1152/jn.1999.82.2.709. [DOI] [PubMed] [Google Scholar]
- Hochman S, Fedirchuk B, Shefchyk SJ. Membrane electrical properties of external urethral and external anal sphincter somatic motoneurons in the decerebrate cat. Neuroscience Letters. 1991;127:87–90. doi: 10.1016/0304-3940(91)90901-5. [DOI] [PubMed] [Google Scholar]
- Holstege G, Tan J. Supraspinal control of motoneurons innervating the striated muscles of the pelvic floor including urethral and anal sphincter in the cat. Brain. 1987;110:1323–1344. doi: 10.1093/brain/110.5.1323. [DOI] [PubMed] [Google Scholar]
- Hounsgaard J, Hultborn H, Jespersen B, Kiehn O. Intrinsic membrane properties causing a bistable behavior of alpha-motoneurones. Experimental Brain Research. 1984;55:391–394. doi: 10.1007/BF00237290. [DOI] [PubMed] [Google Scholar]
- Hounsgaard J, Hultborn H, Jespersen B, Kiehn O. Bistability of α-motoneurones in the decerebrate cat and in the acute spinal cat after intravenous 5-hydroxytryptophan. The Journal of Physiology. 1988;405:345–367. doi: 10.1113/jphysiol.1988.sp017336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hounsgaard J, Kiehn O. Ca2+ dependent bistability induced by serotonin in spinal motoneurons. Experimental Brain Research. 1985;57:422–425. doi: 10.1007/BF00236551. [DOI] [PubMed] [Google Scholar]
- Hounsgaard J, Kiehn O. Serotonin-induced bistability of turtle motoneurones caused by a nifedipine-sensitive calcium plateau potential. The Journal of Physiology. 1989;414:265–282. doi: 10.1113/jphysiol.1989.sp017687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao C-F, Del Negro CA, Trueblood PR, Chandler SH. The ionic basis for serotonin-induced bistable membrane properties in guinea pig trigeminal motoneurons. Journal of Neurophysiology. 1998;79:2847–2924. doi: 10.1152/jn.1998.79.6.2847. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Padel Y, Zarzecki P. Crossed disynaptic inhibition of sacral motoneurones. The Journal of Physiology. 1978;285:425–444. doi: 10.1113/jphysiol.1978.sp012580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehn O, Eken T. Prolonged firing in motor units: evidence of plateau potentials in human motoneurons? Journal of Neurophysiology. 1997;78:3061–3068. doi: 10.1152/jn.1997.78.6.3061. [DOI] [PubMed] [Google Scholar]
- Kojima M, Sano Y. The organization of serotonin fibers in the anterior column of the mammalian spinal cord. Anatomy and Embryology. 1983;167:1–11. doi: 10.1007/BF00304597. [DOI] [PubMed] [Google Scholar]
- Krier J. Discharge patterns of pudendal efferent fibres innervating the external anal sphincter of the cat. The Journal of Physiology. 1985;368:471–480. doi: 10.1113/jphysiol.1985.sp015869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Bistability in spinal motoneurons in vivo: systematic variations in rhythmic firing patterns. Journal of Neurophysiology. 1998a;80:572–582. doi: 10.1152/jn.1998.80.2.572. [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Bistability in spinal motoneurons in vivo: systematic variations in persistent inward currents. Journal of Neurophysiology. 1998b;80:583–593. doi: 10.1152/jn.1998.80.2.583. [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Paradoxical effect of QX-314 on persistent inward currents and bistable behavior in spinal motoneurones in vivo. Journal of Neurophysiology. 1999;82:2518–2527. doi: 10.1152/jn.1999.82.5.2518. [DOI] [PubMed] [Google Scholar]
- Mackel R. Segmental and descending control of the external urethral and anal sphincter in the cat. The Journal of Physiology. 1979;294:105–122. doi: 10.1113/jphysiol.1979.sp012918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan T, Jensen MS, Lambert JDC. The slow inhibitory postsynaptic potential in the rat hippocampal CA1 neurons is blocked by intracellular injection of QX-314. Neuroscience Letters. 1990;110:309–313. doi: 10.1016/0304-3940(90)90865-7. [DOI] [PubMed] [Google Scholar]
- Ramirez-Leon V, Ulfake G. GABA-like immunoreactive innervation and dendro-dendritic contacts in the ventrolateral dendritic bundle in the cat S1 spinal cord segment: an electron microscope study. Experimental Brain Research. 1993;97:1–12. doi: 10.1007/BF00228812. [DOI] [PubMed] [Google Scholar]
- Sasaki M. Membrane properties of external urethral and external anal sphincter motoneurones in the cat. The Journal of Physiology. 1991;440:345–366. doi: 10.1113/jphysiol.1991.sp018712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki M. Morphological analysis of external urethral and external anal sphincter motoneurones of cat. Journal of Comparative Neurology. 1994;349:269–287. doi: 10.1002/cne.903490209. [DOI] [PubMed] [Google Scholar]
- Shefchyk SJ. Modulation of excitatory perineal reflexes and sacral striated sphincter motoneurones during micturition in the cat. In: Rudomin P, Romo R, Mendell L, editors. Presynaptic Inhibition and Neural Control. New York: Oxford Press; 1998. pp. 398–406. [Google Scholar]
- Shefchyk SJ, Buss RR. Urethral pudendal afferent-evoked bladder and sphincter reflexes in decerebrate and acute spinal cats. Neuroscience Letters. 1998;224:137–140. doi: 10.1016/s0304-3940(98)00155-4. [DOI] [PubMed] [Google Scholar]
- Shefchyk SJ, Espey M-J, Carr P, Nance D, Sawchuk M, Buss R. Evidence for a strychnine-sensitive mechanism and glycine receptors involved in the control of urethral sphincter activity during micturition in the cat. Experimental Brain Research. 1998;119:297–306. doi: 10.1007/s002210050345. [DOI] [PubMed] [Google Scholar]
- Shefchyk SJ, Paroschy KL. Non-linear membrane properties in sacral sphincter motoneurons in the cat. Society for Neuroscience Abstracts. 1998;24:360.4. [Google Scholar]
- Shimoda N, Takakusaki K, Nishizawa O, Tsuchida S, Mori S. The changes in the activity of pudendal motoneurons in relation to reflex micturition evoked in decerebrate cats. Neuroscience Letters. 1992;135:175–178. doi: 10.1016/0304-3940(92)90430-f. [DOI] [PubMed] [Google Scholar]
- Svirskis A, Hounsgaard J. Depolarization-induced facilitation of plateau generating currents in ventral horn neurons in the turtle spinal cord. Journal of Neurophysiology. 1998;79:45–50. doi: 10.1152/jn.1997.78.3.1740. [DOI] [PubMed] [Google Scholar]
- Talbot MJ, Sayer RJ. Intracellular QX-314 inhibits calcium currents in hippocampal CA1 pyramidal neurons. Journal of Neurophysiology. 1996;76:2120–2124. doi: 10.1152/jn.1996.76.3.2120. [DOI] [PubMed] [Google Scholar]


