Abstract
Capsaicin and ATP can activate ligand-gated cation channels in nociceptive rat dorsal root ganglion (DRG) neurones. We have studied cross-desensitization between these two agents in rat isolated DRG neurones using the whole-cell voltage-clamp technique.
ATP (10 μM) activated an inward current in DRG neurones at a holding potential of −60 mV. ATP evoked ‘fast’ responses that underwent rapid activation and desensitization, ‘slow’ responses that activated and desensitized more slowly, or responses that displayed a mixture of these two characteristics. The time course of the response to ATP was not related obviously to capsaicin sensitivity.
Prior application of capsaicin (0·5 μM) increased the proportion of cells displaying only fast responses to ATP (10 μM) suggesting that cross-desensitization had occurred between capsaicin and the slow component of the ATP response. Prior desensitization to ATP had no apparent effect on the inward current response to capsaicin (0·5 μM).
Cross-desensitization between capsaicin and ATP was Ca2+ dependent.
Changing the membrane holding potential (Vh) to +40 mV for a brief period before applying ATP at −60 mV had a similar effect to capsaicin, i.e. the proportion of cells displaying only fast responses to ATP was increased significantly. This effect of depolarization was not Ca2+ dependent.
The heterogenity of responses to ATP is probably due to co-expression of homomeric P2X3 receptors and heteromeric receptors comprising P2X3 subunits with other P2X subunits. We propose that the change in time course of the ATP response produced by prior desensitization to capsaicin is due to selective cross-desensitization with the heteromeric P2X receptors.
ATP can evoke a sensation of pain in humans when it is applied to a blister base (Bleehan & Keele, 1977). The precise cellular mechanism of the painful response has yet to be established but it is known that ATP evokes a Ca2+-permeable, cation-specific inward current in rat sensory neurones (Krishtal et al. 1988; Bean, 1990) and that the receptors mediating this response are of the P2X subtype (Kennedy & Leff, 1995; Burnstock, 1996). Correspondingly, attention has focused on the possibility that ATP acts directly on nociceptive sensory neurones by binding to P2X receptors (Bland-Ward & Humphrey, 1997; Dowd et al. 1998; Hamilton et al. 1999) and P2X receptors have been suggested as potential targets for novel analgesic drugs (Kennedy & Leff, 1995; Burnstock, 1996).
Seven P2X receptor subunits have been identified and cloned to date (Valera et al. 1994; Brake et al. 1994; Suprenant et al. 1996; Soto et al. 1997) and at least six of these are expressed in sensory neurones (Collo et al. 1996; Buell et al. 1996). One particular P2X subunit, P2X3, is of special interest since it is expressed preferentially in small-diameter sensory neurones (Chen et al. 1995; Lewis et al. 1995). In dorsal root ganglion (DRG) the expression of P2X3 is largely restricted to the IB4-positive, mostly non-peptidergic subpopulation of sensory neurones (Bradbury et al. 1998) that also express VR1 (capsaicin) receptors (Guo et al. 1999) and probably function as nociceptors. The P2X receptors in nociceptive sensory neurones are predominantly P2X3-containing oligomers that may be homomers (P2X3) or heteromers (e.g. P2X2/3). Activation of P2X3 produces a rapidly desensitizing transient response while activation of P2X2/3 produces a relatively persistent response (Lewis et al. 1995). The response to ATP in a single nociceptive sensory neurone may be either one or a mixture of these response types depending on the complement of receptors that are available in the cell (Lewis et al. 1995; Cook et al. 1997; Grubb & Evans, 1999). Recently, Ueno et al. (1999) have refined this model still further by suggesting that small-diameter, capsaicin-sensitive (presumed nociceptive) DRG neurones express P2X3 receptors while medium-diameter, capsaicin-insensitive neurones express P2X2/3 receptors.
Given that desensitization to capsaicin can lead to a reduction in the responsiveness of sensory neurones to other noxious stimuli (see Szallasi & Blumberg, 1996) and that ATP may be important in the initiation of pain (see above), the aim of the present study was to determine if prior desensitization to capsaicin affects the response of adult rat isolated DRG neurones to ATP and vice versa. Since desensitization of capsaicin-activated inward currents is triggered by an increase in intracellular Ca2+ levels (Docherty et al. 1996; Koplas et al. 1997) and that is also true of ATP-evoked inward currents, at least for P2X3 + 3 receptors (King et al. 1997), there is a reasonable prospect that heterologous desensitization might occur. Some of the results have already been published in abstract form (Piper & Docherty, 1996).
METHODS
Preparation of rat dorsal root ganglion neurone cultures
Experiments were carried out using dorsal root ganglion (DRG) neurones taken from adult Wistar, or Hooded rats (150−250 g, either sex) maintained in primary culture. The strain and sex of the animals had no obvious influence on the behaviour of the sensory neurones in the context of the present experiments. Animals were killed by asphyxiation in a chamber filled with a slowly rising concentration of CO2 gas. For details of culture methods and recording conditions see Docherty et al. (1996). Briefly, neurones were maintained in culture in the presence of either 50 ng ml−1 (2.5S) or 200 ng ml−1 (7S) of nerve growth factor (NGF) for 1–7 days and replated on p-ornithine-coated glass coverslips 2–6 h prior to an experiment. Replating provided a preparation of spherical neuronal somata that were free of neurites.
Electrophysiological recordings
All recordings were made from neuronal somata using the whole-cell patch-clamp technique with an Axopatch−2B amplifier and pCLAMP software (Axon Instruments). After the whole-cell configuration was achieved a waiting period of at least 5 min was allowed for equilibration of the pipette solution with the contents of the cell. Cell capacitance (5−96 pF) and series resistance (2−8 MΩ) were compensated by 50–80 %.
Capsaicin (0.5 μM) and ATP (10 μM) were applied to cells using a U-tube apparatus. This allowed a complete solution change in the vicinity of the cell in less than 0.7 s. Only one recording was performed on any coverslip of cells to ensure that recordings were not made from cells inadvertently exposed to capsaicin or ATP.
In order to characterize P2X receptor-activated responses ATP (10 μM) was applied to cells for 5 or 10 s, at least twice, 2 min apart. Capsaicin was then applied to these cells for up to 2 min in order to assess their capsaicin sensitivity. In a second set of experiments, capsaicin (0.5 μM) was applied first for up to 2 min and ATP (10 μM) was then applied afterwards in order to determine if prior exposure to capsaicin affected P2X receptor-activated inward current. The holding potential (Vh) was −60 mV throughout unless otherwise indicated.
Solutions
Cells were superfused with a solution of the following composition (mM): NaCl, 130; CaCl2, 1; MgCl2, 1; KCl, 3; Hepes, 5; glucose, 11; 0.5 % dimethylsulphoxide (DMSO); pH 7.4. For experiments in which external Ca2+ was removed the external solution had the following composition (mM): NaCl, 130; MgCl2, 1; KCl, 3; Hepes, 5; EGTA, 0.1; glucose, 11; 0.5 % DMSO; pH 7.4. The pipette solution was made up as follows (mM): NaCl, 100; CaCl2, 1; MgCl2, 1; EGTA, 10 or BAPTA 10; Hepes, 10; pH 7.4. The pH of both superfusate and pipette solution was adjusted to 7.4 by addition of NaOH thus increasing the sodium concentration in the extracellular solution by 2–3 mM and in the pipette solution by 27–29 mM. Chemicals were prepared as concentrated stock solutions in either distilled water or DMSO and diluted to the final concentration using extracellular or pipette solution as appropriate. Capsaicin, ATP, EGTA and BAPTA were supplied by Sigma Chemical Co., NGF by Promega or Alomone Laboratories.
Data analysis
The kinetics of ATP-induced inward currents were determined as follows. The 10–90 % rise time was calculated as the time taken for current to rise from 10 % to 90 % of the peak (rise time10−90 %). The decay time was calculated as the time taken for current to fall from 90 % to 10 % of the peak (decay time90−10 %). For cells that displayed more than one peak the rise time to the first peak was calculated and the decay time from the last peak determined. No attempt was made to fit exponential functions to the decay phase of the P2X receptor-activated current. A high proportion of cells demonstrated biphasic responses with multiple inactivation phases and occasionally more than one peak. We felt under these circumstances that the fitting of exponential functions to these data was inappropriate. In the text data are expressed as means ± standard error of the mean. Data were compared by Student's two-tailed unpaired t tests or χ2 tests as indicated (applied using Microsoft Excel software).
RESULTS
In total, 100/189 (53 %) of the adult rat dorsal root ganglion neurones tested in this study responded to ATP (10 μM applied for 5 or 10 s either before or after exposure to capsaicin) with an inward current. Of these, 24 % (45/100) responded to capsaicin (0.5 μM) (i.e. 45 % of the ATP-sensitive population) while 29 % (55/100) were capsaicin insensitive (55 % of the ATP-sensitive population). Of the remaining cells that did not respond to ATP 19/89 responded to capsaicin (21 % of ATP-insensitive population) while 70/89 responded to neither agonist (79 % of ATP-insensitive population).
ATP (10 μM) was applied at least twice at 2 min intervals before capsaicin (0.5 μM) and in the absence of any other conditioning stimulus or treatment to 25 neurones. In agreement with others we found that P2X receptor-mediated responses to ATP (irrespective of capsaicin sensitivity) often consisted of more than one kinetic component. We categorized individual responses as ‘fast’ (fast rise and fast decay), ‘slow’ (slow rise and slow decay) or ‘intermediate’ (fast rise and slow decay) according to the following criteria (see Table 1). Fast responses had a rise time10−90 % of less than 100 ms and a decay time90−10 % of less than 500 ms. Intermediate responses also had a rise time less than 100 ms but had a decay time greater than 500 ms. Slow responses had a rise time greater than 100 ms and a decay time greater than 500 ms. An example of each type is shown in Fig. 1. When we compared the distribution of response types in the capsaicin-sensitive and capsaicin-insensitive groups we found that there was a tendency for capsaicin-sensitive neurones to fall within the intermediate group but there was no statistical difference in the distributions of response types (comparing rows 1 and 2 of Table 1, χ2 test, P = 0.19).
Table 1.
Pooled data for responses to ATP obtained under different conditions and in different classes of cells
| Row no. | Experimental conditions | Order of drug application | Sensitive to Caps? | n | Amplitude (nA) | Rise time(10–90%)(ms) | Decay time(90–10%)(s) | Fast | IM | Slow |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Capsaicin applied in | ATP first | Yes | 9 | 0.77 ± 0.28 | 163 ± 73 | 7.2 ± 1.7 | 0 | 6 | 3 |
| 2 | normal extracellular | ATP first | No | 16 | 1.01 ± 0.19 | 221 ± 78 | 5.8 ± 1.5 | 4 | 5 | 7 |
| 3 | solution | ATP after Caps | Yes | 15 | 1.17 ± 0.17 | 43.7 ± 11.1 | 1.2 ± 0.5 | 10 | 3 | 2 |
| 4 | ATP after Caps | No | 8 | 0.81 ± 0.20 | 304 ± 152 | 5.1 ± 2.3 | 4 | 1 | 3 |
| 5 | Capsaicin applied in | ATP after caps | Yes | 7 | 0.52 ± 0.14 | 608 ± 458 | 5.5 ± 2.5 | 0 | 3 | 4 |
| 6 | Ca2+-free solution | ATP after Caps | No | 5 | 1.95 ± 0.88 | 90.4 ± 54.4 | 5.4 ± 2.9 | 2 | 2 | 1 |
| 7 | BAPTA replacing | ATP first | Yes | 6 | 1.08 ± 0.26 | 57.3 ± 28.5 | 3.1 ± 1.8 | 3 | 2 | 1 |
| 8 | EGTA in pipette | ATP first | No | 5 | 0.68 ± 0.12 | 15.6 ± 6.9 | 3.8 ± 2.8 | 2 | 3 | 0 |
| 9 | solution | ATP after Caps | Yes | 8 | 0.79 ± 0.27 | 54.0 ± 18.8 | 5.8 ± 2.7 | 4 | 3 | 1 |
| 10 | ATP after Caps | No | 8 | 1.62 ± 0.42 | 216 ± 206 | 4.3 ± 2.4 | 4 | 3 | 1 |
| 11 | Prior step to +40 mV | ATP first | — | 9 | 1.68 ± 0.40 | 19.4 ± 7.1 | 0.7 ± 0.3 | 7 | 2 | 0 |
| 12 | Prior step to +40 mV in ‘Ca2+ free’ | ATP first | — | 5 | 3.27 ± 0.25 | 32.0 ± 12.0 | 0.7 ± 0.5 | 4 | 1 | 0 |
Each row is numbered for easy identification from the text and has a brief description of the experimental conditions, indicates whether ATP was applied to the cell before or after capsaicin (Caps), whether the cells in that group were capsaicin sensitive or insensitive, the total number (n) of cells in the group, the mean value (mean ±s.e.m.) of the amplitude, rise time10–90% and decay time90–10% and how many cells in the group could be classified as having either ‘fast’, ‘intermediate’ (IM) or ‘slow’ responses.
Figure 1. Heterogeneity of the time course of inward current responses to ATP (10 μM) recorded in individual DRG neurones at a Vh of −60 mV.
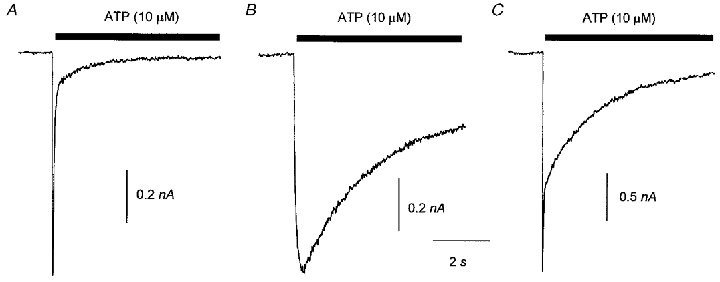
A, an example of a ‘fast’ response to ATP that had a rise time10−90 % of less than 100 ms and a decay time10−90 % of less than 500 ms. B, a ‘slow’ response to ATP that had a rise time10−90 % of more than 100 ms and a decay time10−90 % of more than 500 ms. C, an example of an ‘intermediate’ response to ATP that had a rise time10−90 % of less than 100 ms and a decay time10−90 % of more than 500 ms. The data in B were from a cell that was subsequently shown to be capsaicin sensitive and the data in A and C were from capsaicin-insensitive cells.
Figure 2A shows the average of currents evoked by ATP and recorded in cells that were subsequently shown to be either capsaicin sensitive or capsaicin insensitive. Figure 2B shows the data expressed as current density (i.e. normalized to cell capacitance). The mean peak inward current of the ATP response in capsaicin-sensitive cells was 0.77 ± 0.28 nA (40.0 ± 16.8 pA pF−1, n = 9), and in capsaicin-insensitive cells was 1.01 ± 0.19 nA (56.3 ± 10.3 pA pF−1, n = 16). There was no significant difference between these values (P = 0.50 comparing peak current, P = 0.29 comparing peak current density, unpaired two-tailed t test). Note that the value of the mean peak current is less than the peak value of the average current shown in Fig. 2 since the peak does not always occur at the same point in time.
Figure 2. Averaged inward current responses to ATP (10 μM) recorded in rat isolated DRG neurones at a Vh of −60 mV.
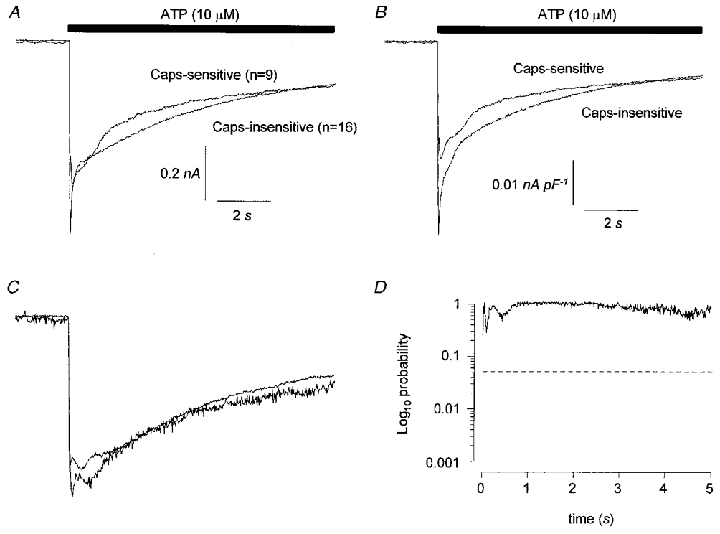
Data in A are superimposed averages of current records from capsaicin-sensitive and capsaicin-insensitive cells and B shows the same data expressed as current density. In C, current traces for individual cells have been normalized to the peak of the inward current and averaged. The resultant averaged, normalized current traces for capsaicin-sensitive and capsaicin-insensitive cells are superimposed. In D the averaged, normalized data are compared at 10 ms intervals by two-tailed, unpaired t tests and the probability that the data are different is plotted on a log10 scale. The horizontal dashed line in D indicates a value of P = 0.05.
There was no difference in the kinetics of P2X receptor-activated current in capsaicin-sensitive or capsaicin-insensitive cells. Figure 2C shows data from individual cells that have been normalized to the peak inward current and the results averaged so that each cell has a similar impact on the comparison irrespective of current amplitude or density. The probability that the responses differ has been calculated throughout the time course of the response at 10 ms intervals using unpaired, two-tailed t tests and plotted against time (Fig. 2D). There was no statistically significant difference in the time courses of responses in the capsaicin-sensitive and capsaicin-insensitive groups. In keeping with this, there was no significant difference between the mean rise times10−90 % or the decay times90−10 % of ATP-induced currents (see Table 1) in capsaicin-sensitive and capsaicin-insensitive cells.
There was little recovery from desensitization of the P2X receptor-induced inward current after 2 min irrespective of the kinetics of the response during drug application. When ATP (10 μM) was reapplied the peak inward current was reduced by 70 ± 6 % (n = 25) compared to the first application of ATP. There was no difference in the degree of this desensitization whether ATP was applied for 5 s or 10 s (data not shown). The characteristics of homologous desensitization were not studied further.
Prior desensitization of DRG neurones to ATP (5 or 10 s application of 10 μM applied at least twice) had no obvious effect on capsaicin-activated currents (0.5 μM) evoked 2 min after the application of ATP. In cells where capsaicin was applied for 2 min at Vh = −60 mV after ATP (n = 5, Fig. 3A) then the capsaicin-induced current peaked after 30.6 ± 11.8 s, at a maximum of 0.77 ± 0.35 nA and decayed by 60.5 ± 14.1 % by the end of 2 min. When capsaicin was applied for 2 min before ATP (n = 14, Fig. 3B) then the current induced by capsaicin peaked after 21.1 ± 2.8 s to a maximum of 1.04 ± 0.34 nA and then decayed by 55.5 ± 8.7 %. There was no significant difference in the parameters describing the capsaicin responses obtained in either situation.
Figure 3. Prior desensitization to ATP had no effect on the time course of capsaicin-evoked inward current.
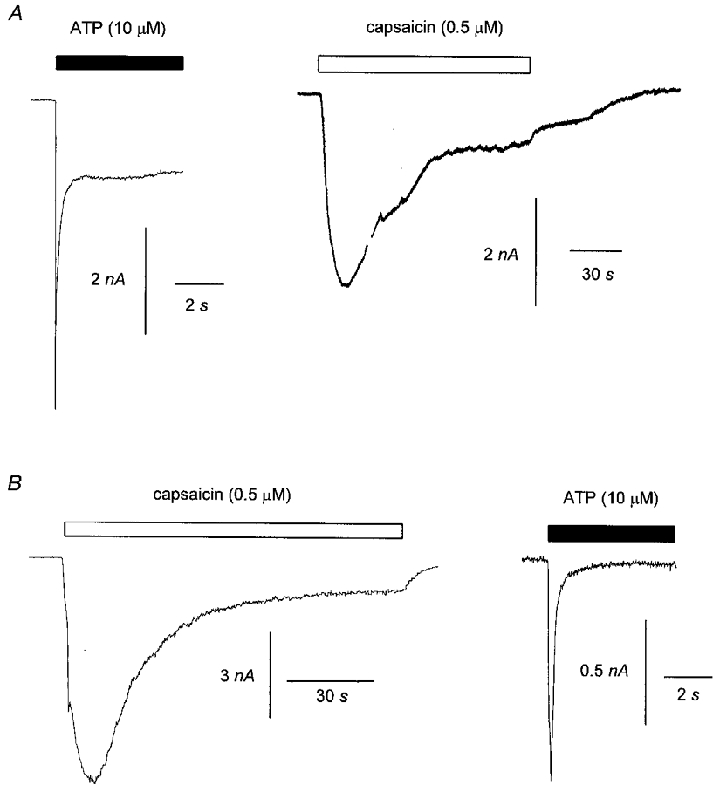
The data in A show inward currents in response to ATP (10 μM) and capsaicin (0.5 μM) in a single DRG neurone. ATP was applied before capsaicin. The data in B show inward currents in response to capsaicin (0.5 μM) and ATP (10 μM) in a second cell. Capsaicin was applied before ATP. Vh was −60 mV throughout.
Prior application of capsaicin (0.5 μM for 2 min) changed the kinetics of ATP responses in capsaicin-sensitive cells. Responses to ATP (10 μM) were obtained from 15 capsaicin-sensitive neurones after the neurones had been exposed to capsaicin and from 9 capsaicin-sensitive neurones before the neurones had been exposed to capsaicin. The mean amplitude of responses to ATP were not significantly different in the two groups (P = 0.20, two-tailed t test, comparing data in rows 1 and 3 in Table 1). Figure 4A shows averaged responses to ATP in capsaicin-sensitive neurones obtained before or after capsaicin application. The data from individual cells have been normalized to the peak of the ATP-induced current and then averaged. There is a clear and significant increase in the rate of decay of the ATP response in the cells that had been exposed previously to capsaicin (Fig. 4B). ATP tended to produce mostly fast-type responses after exposure to capsaicin. In keeping with this the distribution of response types is significantly different in the two groups (comparing data in rows 1 and 3 in Table 1, P = 0.002, χ2 test). Both the rise time10−90 % and the decay time90−10 % of ATP responses were significantly faster (0.05 and < 0.001, respectively, by unpaired, two-tailed t test) in cells that had previously responded to capsaicin. By contrast, in capsaicin-insensitive cells there was no significant difference in the ATP-induced inward current whether ATP was applied before or after capsaicin (Fig. 4C and D). The distribution of response types in the capsaicin-insensitive cells was similar whether ATP was applied before or after capsaicin (i.e. comparing data in rows 2 and 4 in Table 1) and there was no significant difference in the rise time10−90 % or the decay time90−10 % of the response.
Figure 4. Prior desensitization to capsaicin (0.5 μM) increases the desensitization rate of ATP-evoked inward current.
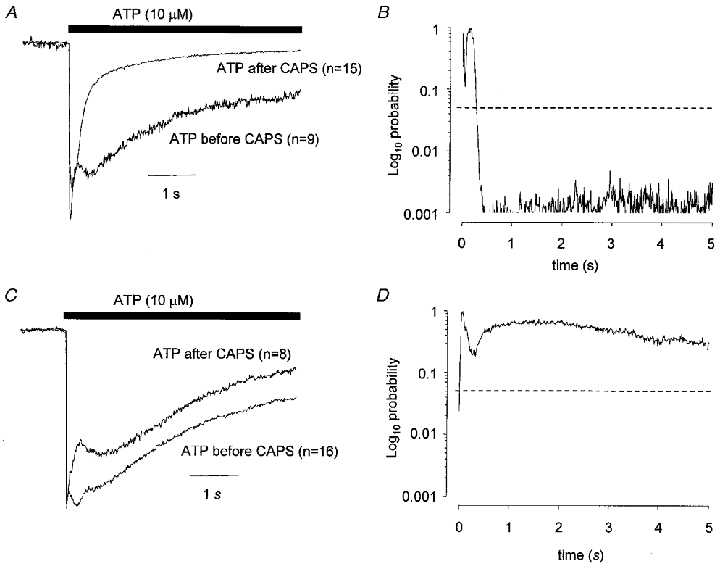
The data shown in A are superimposed averaged normalized currents to ATP (10 μM) when ATP was applied before or after capsaicin. The data in B are plots of probability values obtained from unpaired t tests applied at 10 ms intervals comparing the currents evoked when ATP was applied before or after capsaicin and plotted against time. The data in A and B were obtained in capsaicin-sensitive cells. Data shown in C and D are equivalent to that in A and B but were obtained from capsaicin-insensitive cells. Vh was −60 mV.
Removal of extracellular Ca2+ eliminated the effect of capsaicin on the kinetics of subsequent ATP responses. Experiments where ATP was applied to cells after capsaicin were repeated but Ca2+ was removed from the extracellular solution during the time that the capsaicin was applied. When the data obtained after capsaicin + Ca2+-free application were compared to responses obtained under the same conditions from capsaicin-insensitive cells (Fig. 5E and F and comparing data in rows 5 and 6, Table 1) or in capsaicin-sensitive cells before capsaicin application (Fig. 5C and D and comparing data in rows 1 and 5, Table 1) there was no significant difference in any measured parameter. The ATP response obtained after capsaicin + Ca2+-free application was significantly different from that obtained when capsaicin was applied in Ca2+-containing solution (Fig. 5A and B; and comparing data in rows 3 and 5 in Table 1). There was no significant difference in the rise times10−90 % but the decay times90−10 % of ATP responses were significantly slower (P = 0.02, two-tailed t test) if Ca2+ was absent when capsaicin was applied. The distribution of response types was significantly different (P = 0.018, χ2 test). Taken together, these data suggest that the increase in the desensitization rate of the ATP response after prior desensitization to capsaicin is a Ca2+-dependent phenomenon.
Figure 5. Cross-desensitization between ATP and capsaicin in DRG neurones is Ca2+ dependent.
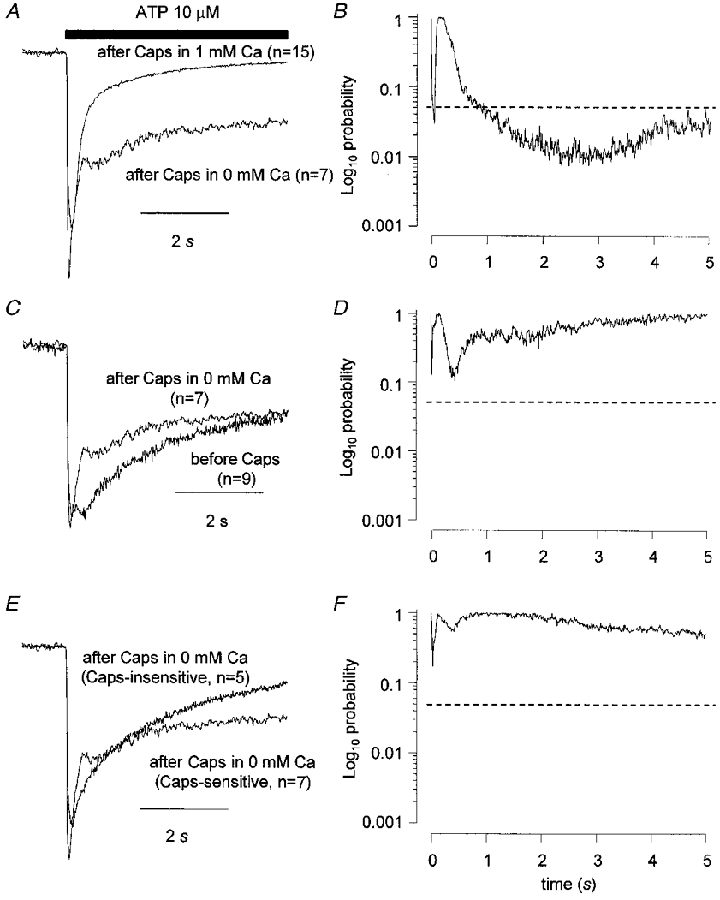
The records in A, C and E show the averaged, normalized current to ATP (10 μM) obtained when ATP was applied to capsaicin-sensitive cells after capsaicin but capsaicin was applied in Ca2+-free solution. In A the data are compared to the equivalent data from cells where capsaicin was applied in normal Ca2+-containing solution. In C the data are compared to the ATP response obtained before capsaicin was applied in capsaicin-sensitive cells. In E the data are compared to the ATP response obtained after capsaicin was applied in the absence of Ca2+ but to capsaicin-insensitive cells. The data in B, D and F are plots of probability values obtained from unpaired t tests applied at 10 ms intervals comparing the current traces in A, C and E, respectively. The horizontal, dashed lines in B, D and F indicate a value of P = 0.05. Vh was −60 mV.
Replacing EGTA in the recording pipette with BAPTA reduced the size of the slow component of the ATP response and eliminated the effect of capsaicin on the kinetics of ATP responses. When BAPTA replaced EGTA in the recording pipette there was a tendency for ATP to produce more fast responses especially in the capsaicin-sensitive group (P = 0.023, χ2 test comparing the data in rows 1 and 7, Table 1) and the slow component of the ATP response was smaller (Fig. 6A and B). However, there was no significant difference between the peak amplitudes of ATP responses or in the rise times10−90 % or decay times90−10 % of BAPTA-loaded compared to EGTA-loaded cells. When ATP was applied either before or after capsaicin in BAPTA-loaded capsaicin-sensitive or capsaicin-insensitive cells there were no significant differences in the time course (Fig. 6C, D, E and F), the rise time10−90 % or the decay time90−10 % of the response or in the distributions of response types for different groups (Table 1).
Figure 6. Replacing EGTA in the recording pipette with BAPTA reduced the size of the slow component of the ATP response and eliminated the effect of capsaicin on the kinetics of ATP responses.
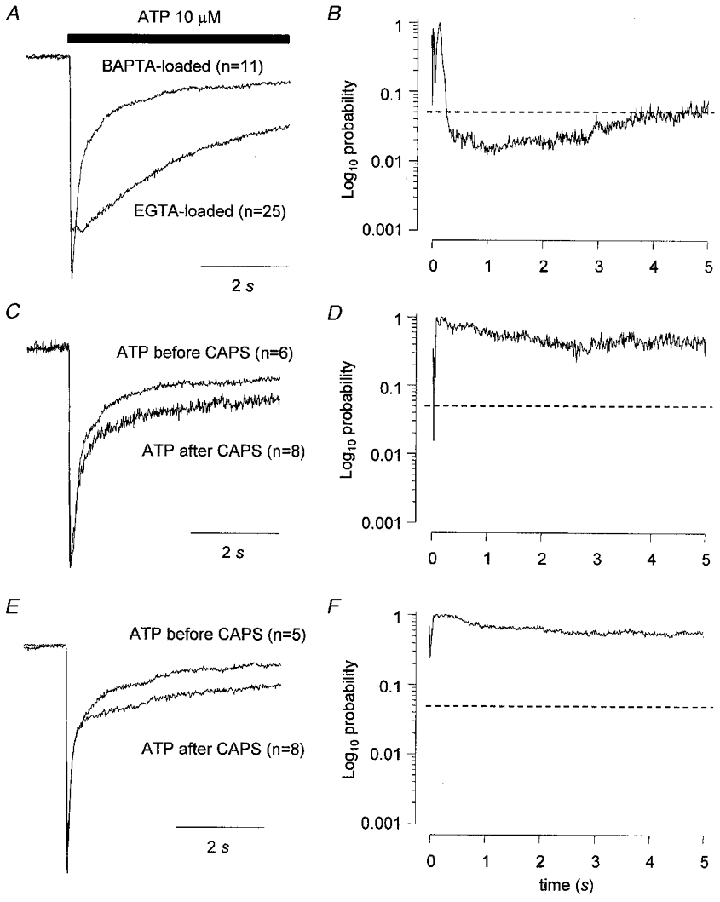
The records in A show the averaged, normalized current to ATP (10 μM) obtained when ATP was applied to cells (irrespective of capsaicin sensitivity) using an EGTA-based solution in the recording pipette compared to data obtained using a BAPTA-based solution. The data in C and E were obtained using a BAPTA-based recording solution and compare ATP responses obtained either before or after capsaicin application. Data in C are from capsaicin-sensitive cells and data in E are from capsaicin-insensitive cells. The data in B, D and F are plots of probability values obtained from unpaired t tests applied at 10 ms intervals comparing the current traces in A, C and E, respectively. Vh was −60 mV. The horizontal dashed lines in B, D and F indicate a value of P = 0.05.
Changing the holding potential at which capsaicin is applied to +40 mV decreases the apparent amount of homologous desensitization of capsaicin responses that occurs (Piper & Docherty, 1999). We wanted to determine whether a similar change in holding potential whilst capsaicin was being applied would reduce the effect of capsaicin on the kinetics of the subsequent ATP response. However, we found whilst performing the controls for these experiments that changing the holding potential from −60 mV to +40 mV for 2 to 5 min before applying ATP was sufficient of itself to abolish the slow component of the response (Fig. 7A). In other words, a 2–5 min depolarizing step to +40 mV had a very similar effect to capsaicin on responses to ATP. The response to ATP, after a depolarizing step, was larger (P = 0.04, two-tailed t test), the rise time10−90 % was not significantly different but the decay time90−10 % was significantly faster (P < 0.01, two-tailed t test). In keeping with this, responses were more likely to be of the fast type (P < 0.01, χ2 test) and the time course of the averaged, normalized response was dramatically different to controls (Fig. 7A and B). This experiment was repeated when Ca2+ was removed from the extracellular solution during the depolarizing conditioning step but the response to ATP was very similar to that obtained to ATP following a depolarizing step in Ca2+-containing solution (Fig. 7C and D). The response was significantly larger (P < 0.01, two-tailed t test), the rise time10−90 % was not significantly different but the decay time90−10 % was significantly faster (P = 0.03, two-tailed t test) and the responses were more likely to be of the fast type (P < 0.01, χ2 test).
Figure 7. Strong depolarization changed the kinetics of ATP responses by a mechanism that was not dependent on extracellular Ca2+.
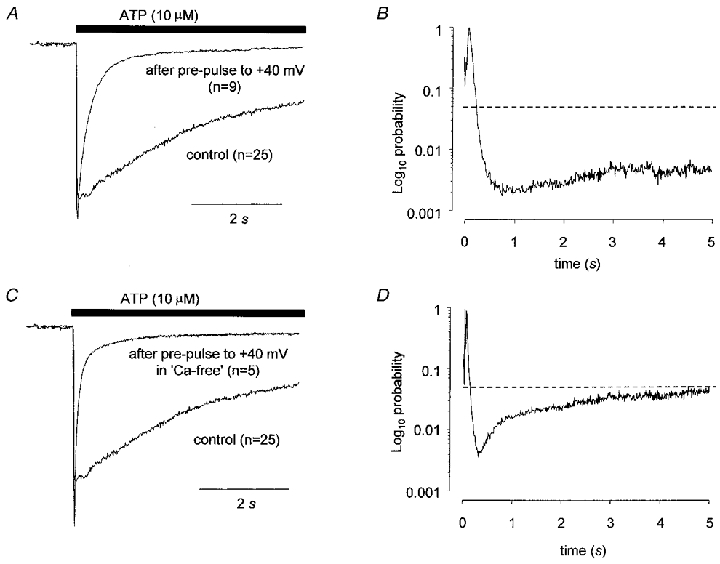
The data shown in A are the averaged, normalized current to ATP (10 μM) obtained when ATP was applied to cells (irrespective of capsaicin sensitivity) in the absence of any other treatment compared to the current obtained in cells that had been exposed to a 2–5 min conditioning step to +40 mV and then repolarized to −60 mV 2 min prior to ATP application. The data in C are equivalent to the data in A except that the conditioning pre-pulse was applied in the absence of extracellular Ca2+. The data in B and D are plots of probability values obtained from unpaired t tests applied at 10 ms intervals comparing the current traces in A and C, respectively. Vh was −60 mV. The horizontal dashed lines in B and D indicate a value of P = 0.05.
DISCUSSION
The data presented above suggest that one-way cross-desensitization can occur between capsaicin and ATP in rat isolated dorsal root ganglion neurones. Responses to capsaicin are not affected by prior exposure to ATP but exposure of cells to capsaicin before application of ATP significantly increases the desensitization rate of ATP responses. Cross-desensitization appeared to be mediated by Ca2+ as it was abolished when capsaicin was applied in the absence of Ca2+. The effect could be mimicked by a strong depolarizing conditioning pulse whether or not extracellular Ca2+ was present.
We found that overall, 53 % of DRG neurones tested in this study responded to ATP. Previous studies have shown that the proportion of ATP-sensitive rat DRG neurones range from 43 % (Bean, 1990) to 96 % (Grubb & Evans, 1999). In each case these values are higher than might be expected from in situ hybridization studies (Chen et al. 1995; Bradbury et al. 1998) of P2X3 receptor distribution, which show that about 25–35 % of DRG neurones express P2X3 (Chen et al. 1995; Bradbury et al. 1998). While this discrepancy could indicate that P2X responses in isolated cells may arise from receptors that do not contain P2X3 subunits it may simply be due to a sampling bias in favour of smaller cells on the part of the electrophysiologists. We found that 45 % of ATP-sensitive neurones respond to capsaicin and 70 % of capsaicin-sensitive neurones respond to ATP which is in good agreement with Ueno et al. (1999) who reported values of 48 % and 70 %, respectively, and also agrees well with in situ hybridization data which show that 75 % of VR1-positive neurones also express P2X3 subunits (Guo et al. 1999). Although we have not included a pharmacological characterization of responses in the present study it is reasonable to conclude that the P2X receptor-mediated responses that we have observed in the present study are due predominantly to activation of P2X3-containing P2X receptors.
The range of response types that we have observed (i.e. fast, intermediate and slow) is in good agreement with others (Robertson et al. 1996; Cook et al. 1997; Cook & McCleskey, 1997; Grubb & Evans, 1999; Ueno et al. 1999) although the incidence of the response types is quite different. In our study, in cells that had no prior exposure to a conditioning stimulus, we observed a much higher proportion of responses that contained a slowly inactivating component (intermediate or slow) than has been reported by most others (but see Bean, 1990). In particular, we found that responses with a slowly inactivating component were as likely to occur in capsaicin-sensitive neurones as in other neurones. Only when neurones were first exposed to capsaicin, or to a prolonged depolarizing stimulus, was there an increased occurrence of transient, rapidly inactivating responses. In contrast to the results of Ueno et al. (1999), our data suggest that nociceptive DRG neurones, like nociceptive trigeminal neurones (Cook & McCleskey, 1997; Cook et al. 1997), can display both transient and persistent responses to ATP.
The present study has shown that desensitization of single DRG neurones to ATP before applying capsaicin does not affect the capsaicin-evoked inward current. However, prior desensitization of single DRG neurones to capsaicin before applying ATP causes a significant change in the time course of the P2X receptor-mediated inward current. The average rate of desensitization was increased, as was the proportion of cells responding to ATP with a fast-type current. The mechanism was dependent on the presence of extracellular Ca2+ and did not occur when BAPTA was used instead of EGTA as an intracellular Ca2+ buffer. These data suggest that a transient rise in intracellular Ca2+ can provoke a change in the kinetics of P2X receptor responses. Multi-subunit P2X receptors are stable structures (Radford et al. 1997; Torres et al. 1999) and presumably they do not disassemble and re-assemble dynamically in the membrane. Therefore, the mechanism of the change in the kinetics of the response probably involves modification of a specific P2X receptor subtype. The subtype involved could be a P2X3 homomeric receptor, which would imply that this receptor has more complex kinetics than has been supposed hitherto (see also King et al. 1997), or it could be a P2X3/n receptor. Given the prevailing view that the slowly inactivating component of P2X receptor responses is mediated by P2X2/3 receptors the most likely mechanism is cross-desensitization between capsaicin receptors with P2X2/3 receptors. Interestingly, desensitization of P2X2 receptors expressed in Xenopus oocytes is strongly influenced by the intracellular environment, which may point to a regulatory site on the P2X2 subunit (Zhou et al. 1998). Since Ca2+-dependent homologous desensitization of capsaicin receptors in sensory neurones involves activation of calcineurin (Docherty et al. 1996; Piper & Docherty, 1999) and this enzyme has also been implicated in the desensitization of P2X3 channels (King et al. 1997) we are currently investigating the possibility that cross-desensitization between capsaicin and P2X receptors also involves calcineurin. The fact that the cross-desensitization was one way (prior application of ATP did not appear to cause desensitization of capsaicin receptors) may be a reflection of the relatively high Ca2+ permeability of the capsaicin-gated ion channel.
As well as eliminating the effect of capsaicin BAPTA reduced the size of the slow component of current although it had no significant effect on kinetics. BAPTA is a more rapid chelator of Ca2+ than EGTA, but has a similar binding affinity for Ca2+ (Tsien, 1980). It seems unlikely that BAPTA has a toxic effect that is selective for the slow component of current. A speculative explanation for this result is that the slow component of ATP-gated current is enhanced in cells dialysed with EGTA due to a transient intracellular acidification since EGTA releases protons when it binds Ca2+ but BAPTA does not. The sensitivity of P2X2-containing receptors to ATP is enhanced by lowering extracellular pH (Li et al. 1996; Stoop et al. 1997) but so far as we are aware the effects of intracellular acidification, if any, are not known.
Prior depolarization of neurones to +40 mV for about 2–5 min increased the average rate of desensitization and the proportion of cells responding to ATP with a fast-type current. This occurred whether cells were sensitive to capsaicin or not and was not due to entry of Ca2+ through voltage-gated channels since the phenomenon still occurred in the absence of extracellular Ca2+. This was an unexpected and surprising result and we are not aware of any reports of similar phenomena with which to compare it. Identification of the molecular mechanism underlying the phenomenon will require further study. The importance of the result in the context of this discussion is that it emphasizes the importance of the history of a given cell in dictating the kinetics of P2X receptor responses and suggests that the kinetics of the response, at least in sensory neurones, may not be a reliable guide as to the subunit composition of the receptor involved.
Cross-desensitization between different noxious stimuli may provide a means of modulating the response of nociceptive neurones to those stimuli. In this respect it is interesting that ATP did not affect inward currents to capsaicin. The cloned capsaicin-activated receptor VR1 also responds to heat and it has been postulated that its physiological role may be as a heat sensor (Caterina et al. 1997). The data presented here suggest that the sensing of painful heat stimuli by nociceptive DRG neurones may in turn affect responses to ATP, which is believed to be important in the initiation of pain (Burnstock, 1996). Such an interaction between noxious stimuli could provide a means by which the responses of nociceptive neurones could be modulated in vivo.
Acknowledgments
This work was supported by the Wellcome Trust (grant reference 045941/Z/95/Z/WRE/HA/JAT).
References
- Bean BP. ATP-activated channels in rat and bullfrog sensory neurons: Concentration dependence and kinetics. Journal of Neuroscience. 1990;10:1–10. doi: 10.1523/JNEUROSCI.10-01-00001.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland-Ward PA, Humphrey PP A. Acute nociception mediated by hindpaw P2X receptor activation in the rat. British Journal of Pharmacology. 1997;122:365–371. doi: 10.1038/sj.bjp.0701371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleehen T, Keele CA. Observations on the algogenic actions of adenosine compounds on the human blister base preparation. Pain. 1977;3:367–377. doi: 10.1016/0304-3959(77)90066-5. [DOI] [PubMed] [Google Scholar]
- Bradbury EJ, Burnstock G, McMahon SB. The expression of P2X3 purinoceptors in sensory neurons: effects of axotomy and glial-derived neurotrophic factor. Molecular and Cellular Neurosciences. 1998;12:256–268. doi: 10.1006/mcne.1998.0719. [DOI] [PubMed] [Google Scholar]
- Brake AJ, Wagenbach MJ, Julius D. New structural motif for ligand-gated ion channels defined by ionotropic ATP receptor. Nature. 1994;371:519–523. doi: 10.1038/371519a0. [DOI] [PubMed] [Google Scholar]
- Buell G, Collo G, Rassendren F. P2X receptors: An emerging channel family. European Journal of Neuroscience. 1996;8:2221–2228. doi: 10.1111/j.1460-9568.1996.tb00745.x. [DOI] [PubMed] [Google Scholar]
- Burnstock G. A unifying purinergic hypothesis for the initiation of pain. Lancet. 1996;347:1604–1605. doi: 10.1016/s0140-6736(96)91082-x. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Chen CC, Akopian A, Sivilotti L, Colquhoun D, Burnstock G, Wood JN. A P2X purinoceptor expressed by a subset of sensory neurons. Nature. 1995;377:428–431. doi: 10.1038/377428a0. [DOI] [PubMed] [Google Scholar]
- Collo G, North RA, Kawashima E, Merlo-Pich E, Neidhart S, Suprenant A, Buell G. Cloning of P2X5 and P2X6 receptors and the distribution and properties of an extended family of ATP-gated ion channels. Journal of Neuroscience. 1996;16:2495–2507. doi: 10.1523/JNEUROSCI.16-08-02495.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook SP, McCleskey EW. Desensitization, recovery and Ca2+-dependent modulation of ATP-gated P2X receptors in nociceptors. Neuropharmacology. 1997;36:1303–1308. doi: 10.1016/s0028-3908(97)00132-9. [DOI] [PubMed] [Google Scholar]
- Cook SP, Vulchanova L, Hargreaves KM, Elde R, McCleskey EW. Distinct ATP receptors on pain-sensing and stretch-sensing neurons. Nature. 1997;387:505–508. doi: 10.1038/387505a0. [DOI] [PubMed] [Google Scholar]
- Docherty RJ, Yeats JC, Bevan S, Boddeke HW G M. Inhibition of calcineurin inhibits the desensitization of capsaicin-evoked currents in cultured dorsal root ganglion neurones from adult rats. Pflügers Archiv. 1996;431:828–837. doi: 10.1007/s004240050074. [DOI] [PubMed] [Google Scholar]
- Dowd E, McQueen DS, Chessell IP, Humphrey PP. P2X receptor-mediated excitation of nociceptive afferents in the normal and arthritic knee joint. British Journal of Pharmacology. 1998;125:341–346. doi: 10.1038/sj.bjp.0702080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubb BD, Evans RJ. Characterization of cultured dorsal root ganglion neuron P2X receptors. European Journal of Neuroscience. 1999;11:149–154. doi: 10.1046/j.1460-9568.1999.00426.x. [DOI] [PubMed] [Google Scholar]
- Guo A, Vulchanova L, Wang J, Li X, Elde R. Immunocytochemical localization of the vanilloid receptor 1 (VR1): relationship to neuropeptides, the P2X3 purinoceptor and IB4 binding sites. European Journal of Neuroscience. 1999;11:946–958. doi: 10.1046/j.1460-9568.1999.00503.x. [DOI] [PubMed] [Google Scholar]
- Hamilton SG, Wade A, McMahon SB. The effects of inflammation and inflammatory mediators on nociceptive behaviour induced by ATP analogues in the rat. British Journal of Pharmacology. 1999;126:326–332. doi: 10.1038/sj.bjp.0702258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy C, Leff P. Painful connection for ATP. Nature. 1995;377:385–386. doi: 10.1038/377385a0. [DOI] [PubMed] [Google Scholar]
- Khiroug L, Giniatullin R, Talantova M, Nistri A. Role of intracellular calcium in fast and slow desensitization of P2-receptors in PC12 cells. British Journal of Pharmacology. 1997;120:1552–1560. doi: 10.1038/sj.bjp.0701060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King B, Chen CC, Akopian AH, Burnstock G, Wood JN. A role for calcineurin in the desensitization of the P2X(3) receptor. NeuroReport. 1997;8:1099–1102. doi: 10.1097/00001756-199703240-00007. [DOI] [PubMed] [Google Scholar]
- Koplas PA, Rosenburg RL, Oxford GS. The role of calcium in the desensitization of capsaicin responses in rat dorsal root ganglion neurons. Journal of Neuroscience. 1997;17:3525–3537. doi: 10.1523/JNEUROSCI.17-10-03525.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishtal OA, Marchenko SM, Obukhov AG. Cationic channels activated by extracellular ATP in rat sensory neurons. Neuroscience. 1988;27:995–1000. doi: 10.1016/0306-4522(88)90203-5. [DOI] [PubMed] [Google Scholar]
- Lewis C, Neidhart S, Holy C, North RA, Buell G, Suprenant A. Coexpression of P2X2 and P2X3 receptor subunits can account for ATP-gated currents in sensory neurons. Nature. 1995;377:432–435. doi: 10.1038/377432a0. [DOI] [PubMed] [Google Scholar]
- Li C, Peoples RW, Weight FF. Acid pH augments excitatory action of ATP on a dissociated mammalian sensory neuron. NeuroReport. 1996;7:2151–2154. doi: 10.1097/00001756-199609020-00018. [DOI] [PubMed] [Google Scholar]
- Piper AS, Docherty RJ. Capsaicin increases the desensitization rate of ATP-evoked inward currents in adult rat dorsal root ganglion (DRG) neurons in vitro. British Journal of Pharmacology. 1996;120:227P. [Google Scholar]
- Piper AS, Docherty RJ. A study of the voltage-dependence of capsaicin-activated membrane currents in rat sensory neurones before and after acute desensitization. The Journal of Physiology. 1999;518:721–733. doi: 10.1111/j.1469-7793.1999.0721p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radford KM, Virginio C, Suprenant A, North RA, Kawashima E. Baculovirus expression provides direct evidence for heteromeric assembly of P2X2 and P2X3 receptors. Journal of Neuroscience. 1997;17:6529–6533. doi: 10.1523/JNEUROSCI.17-17-06529.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson SJ, Rae MG, Rowan EG, Kennedy C. Characterization of a P2X-purinoceptor in cultured neurones of the rat dorsal root ganglia. British Journal of Pharmacology. 1996;118:951–956. doi: 10.1111/j.1476-5381.1996.tb15491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto F, Garcia-Guzman M, Stuhmer W. Cloned ligand-gated channels activated by extracellular ATP (P2X receptors) Journal of Membrane Biology. 1997;160:91–100. doi: 10.1007/s002329900298. [DOI] [PubMed] [Google Scholar]
- Stoop R, Suprenant A, North RA. Different sensitivities to pH of ATP-induced currents at four cloned P2X receptors. Journal of Neurophysiology. 1997;78:1837–1840. doi: 10.1152/jn.1997.78.4.1837. [DOI] [PubMed] [Google Scholar]
- Suprenant A, Rassendren F, Kawashima E, North RA, Buell G. The cytosolic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7) Science. 1996;272:735–738. doi: 10.1126/science.272.5262.735. [DOI] [PubMed] [Google Scholar]
- Szallasi A, Blumberg PM. Mechanisms and therapeutic potential of vanilloids (capsaicin-like molecules) Advances in Pharmacology. 1996;24:123–155. doi: 10.1016/s1054-3589(08)60936-9. [DOI] [PubMed] [Google Scholar]
- Torres GE, Egan TM, Voigt MM. Hetero-oligomeric assembly of P2X receptor subunits. Journal of Biological Chemistry. 1999;274:6653–6659. doi: 10.1074/jbc.274.10.6653. [DOI] [PubMed] [Google Scholar]
- Tsien RY. New calcium indicators and buffers with high selectivity against magnesium and protons: design, synthesis and properties of prototype structures. Biochemistry. 1980;19:2396–2404. doi: 10.1021/bi00552a018. [DOI] [PubMed] [Google Scholar]
- Ueno S, Tsuda M, Iwanaga T, Inoue K. Cell type-specific ATP-activated responses in rat dorsal root ganglion neurons. British Journal of Pharmacology. 1999;126:429–436. doi: 10.1038/sj.bjp.0702319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valera S, Hussy N, Evans RJ, Adami N, North RA, Suprenant A, Buell G. A new class of ligand-gated ion channel defined by P2X receptor for extracellular ATP. Nature. 1994;371:516–519. doi: 10.1038/371516a0. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Monsma LR, Hume RI. Identification of a site that modifies desensitization of P2X2 receptors. Biochemical and Biophysical Research Communications. 1998;252:541–545. doi: 10.1006/bbrc.1998.9689. [DOI] [PubMed] [Google Scholar]


