Abstract
In the dog, the elevation of the ribs during inspiration results from the combined actions of the parasternal and external intercostal muscles. In the present studies, the hypothesis was tested that co-ordinated activity among these two sets of muscles reduces the distortion of the rib cage.
During spontaneous inspiration before or after section of the phrenic nerves, the ribs moved cranially and outward in the same way as they did during passive inflation. However, whereas the sternum moved cranially during passive inflation, it was displaced caudally during spontaneous inspiration.
When the parasternal intercostal muscles were selectively denervated, both the sternum and the ribs moved cranially, but the rib outward displacement was markedly reduced. In contrast, when the external intercostals were excised and the parasternal intercostals were left intact, the sternum continued to move caudally and the outward displacement of the ribs was augmented relative to their cranial displacement.
These observations establish that the external intercostal muscles drive the ribs primarily in the cranial direction, whereas the parasternal intercostals drive the ribs both cranially and outward. They also indicate, in agreement with the hypothesis, that co-ordinated activity among these two sets of muscles displaces the ribs on their relaxation curve.
However, this co-ordinated activity also displaces the sternum caudally. Although this distortion requires an additional energy expenditure, it enhances the outward component of rib displacement which is more effective with respect to lung expansion.
Elevation of the ribs and expansion of the rib cage are prominent features of the inspiratory phase of the breathing cycle. In the dog, this phenomenon is well known to result from the actions of three groups of intercostal muscles, namely the internal intercostals of the parasternal area (the so-called parasternal intercostals), the external intercostals, and the levator costae (De Troyer & Kelly, 1982; De Troyer & Farkas, 1989; De Troyer, 1991). Recent studies, however, have shown significant heterogeneities in the mechanical advantage of these muscle groups. Specifically, the parasternal intercostals have, by and large, a definite inspiratory mechanical advantage, but this advantage decreases progressively from the third to the seventh interspace (De Troyer et al. 1996). The mechanical advantage of the parasternal intercostal in any given interspace also decreases from the sternum toward the chondrocostal junction (De Troyer & Legrand, 1995). Furthermore, although the inspiratory mechanical advantage of the external intercostal muscle in the dorsal portion of the second interspace is about as large as that of the medial bundles of the parasternal intercostal in this interspace, this advantage decreases rapidly toward the base of the rib cage and toward the costochondral junctions (De Troyer et al. 1999). The external intercostals, as a whole, have therefore a lower inspiratory mechanical advantage than the parasternal intercostals.
Electromyographic recordings from the canine intercostal muscles have also shown that the distribution of inspiratory activity among the parasternal and external intercostals during breathing mirrors the distribution of mechanical advantage. Thus inspiratory activity in a given parasternal intercostal is predominant in the bundles situated near the sternum and decreases progressively toward the chondrocostal junction (De Troyer & Legrand, 1995). Inspiratory activity in the medial parasternal intercostal bundles is also greatest in the third interspace and smallest in the seventh (Legrand et al. 1996). Similarly, the canine external intercostal muscles display greater inspiratory activity in the rostral than the caudal interspaces and greater inspiratory activity in the dorsal than the ventral aspect of the rib cage (Legrand & De Troyer, 1999); a similar distribution of external intercostal inspiratory activity has been found in the cat (Kirkwood et al. 1982; Greer & Martin, 1990). This close matching between the topographic distribution of inspiratory intercostal activity during breathing and the topographic distribution of inspiratory mechanical advantage might suggest that the inspiratory muscle pump operates with optimum effectiveness, yet activating muscles with a low mechanical advantage should be less effective with respect to lung volume than activating exclusively the muscles with the greatest mechanical advantage. In other words, why are the parasternal and external intercostals in the caudal interspaces ever active during inspiration, or, alternatively, why is it that intercostal inspiratory activity is not entirely concentrated in the medial portion of the parasternal intercostals in the rostral interspaces?
In the process of answering this question, we reasoned that these muscles operate to expand both the lung and the chest wall, rather than the lung alone. We also reasoned that the configuration of the chest wall is a key determinant of the work of breathing. Thus, for a given increase in lung volume, the elastic work of breathing is lowest when the chest wall is displaced along its relaxed configuration (Mead, 1974), and this led to the idea that the distribution of activity among the inspiratory intercostal muscles might help in expanding the rib cage compartment of the chest wall with minimum distortion. The present studies were therefore undertaken to test the related hypothesis that breathing with either the parasternal intercostals alone or the external intercostals alone causes larger rib cage distortion than breathing with the two muscle groups together.
METHODS
The experiments were carried out on eight adult mongrel dogs (14-28 kg) as approved by the Animal Ethics and Welfare Committee of the Brussels School of Medicine. The animals were anaesthetized with pentobarbitone sodium (initial dose, 30 mg kg−1i.v.), placed in the supine posture and intubated with a cuffed endotracheal tube. A venous catheter was inserted in the forelimb to give maintenance doses of anaesthetic. The rib cage and intercostal muscles were then exposed on both sides of the chest from the first to tenth rib by deflection of the skin and underlying muscle layers, including the scalene, serratus ventralis, serratus dorsalis cranialis and iliocostalis muscles. In addition, a mid-line incision of the neck was performed to isolate bilaterally the C5, C6 and C7 phrenic nerve roots, and loose ligatures were placed around them so that they could be identified easily later.
The animals were maintained at a constant, rather deep level of anaesthesia throughout the study. Specifically, they had no pupillary light reflex and no flexor withdrawal of the forelimbs; the corneal reflex was also abolished. Rectal temperature was maintained constant between 36 and 38°C with infrared lamps. At the end of the study, the animal was given an overdose of anaesthetic (30-40 mg kg−1).
Measurements
In each animal, we measured the craniocaudal (axial) displacements of the ribs and sternum by using linear displacement transducers (Schaevitz Eng., Pennsauken, NJ, USA). This technique has been described in detail previously (De Troyer & Kelly, 1982). Hooks were thus screwed into the fourth rib in the midaxillary line and into the sternum, and a long inextensible thread was attached to each screw and led caudally over a pulley to a displacement transducer positioned at the foot of the table; the cranial and caudal displacements of the rib and sternum were therefore translated into up-and-down motions of the cores of the transducers. An additional thread was attached to the screw in the rib and led laterally, perpendicular to the midsagittal plane of the body, to measure the lateral displacements of the rib.
In each animal, we also measured airflow at the endotracheal tube with a heated Fleisch pneumotachograph connected to a differential pressure transducer (± 2 cmH2O), integrated the flow signal to assess the changes in lung volume, and recorded the electromyograms of the parasternal intercostal and external intercostal muscles in the third right interspace with pairs of silver hook electrodes spaced 3–4 mm apart. Each pair was placed in parallel fibres. The parasternal intercostal electrodes were implanted in the bundles situated near the sternum, and the external intercostal electrodes were implanted in the dorsal portion of the muscle, 1–2 cm ventral to the rib angle. The two electromyographic (EMG) signals were processed with amplifiers (model 830/1; CWE Inc., Ardmore, PA, USA), bandpass filtered below 100 and above 2000 Hz, and rectified before their passage through leaky integrators with a time constant of 0.2 s.
Protocol
The animal was allowed to recover for 30 min after instrumentation, after which it was connected to a ventilator (Harvard pump, model 613 A; Chicago, IL, USA) and hyperventilated until the disappearance of the parasternal intercostal and external intercostal inspiratory EMG activity. The transducers were then calibrated, and measurements of rib and sternum motion during inflation of the relaxed chest wall were obtained. After completion of these measurements, mechanical ventilation was stopped, and the relaxation position of the rib and sternum was determined. Spontaneous breathing was then allowed to resume for 10–15 min until stabilization of the breathing pattern, at which point tidal volume, rib and sternum motion, and EMG activity were measured. Two runs of resting, room air breathing alternating with periods of mechanical ventilation were obtained in each animal. The C5, C6, and C7 phrenic nerve roots were subsequently infiltrated with 2 % lidocaine (lignocaine) and sectioned to induce a complete paralysis of the diaphragm, and the entire procedure was repeated. Thus, after a new calibration of the transducers, measurements of rib and sternum motion were obtained first during passive inflation, then during spontaneous, room air breathing. As in the control condition, two runs of spontaneous breathing alternating with mechanical ventilation were obtained.
Two experimental protocols were then followed. In four animals (dogs 1-4), the internal intercostal nerves in all interspaces from the first to the eighth were exposed at the chondrocostal junctions on both sides of the chest, and the nerves were sectioned to induce complete paralysis of the parasternal intercostal muscles. Inactivation of the muscle in each interspace was demonstrated by abolition of the inspiratory EMG activity. In the other four animals (dogs 5-8), the internal intercostal nerves were left intact but the external intercostal muscles on both sides of the chest were sectioned in the first to eighth interspaces from the costochondral junctions to the spine. The section included the levator costae muscles, and postmortem examination of the rib cage in these animals confirmed that both the external intercostal and the levator costae muscle in each interspace was indeed severed entirely. A last set of measurements, including mechanical ventilation, was obtained after a recovery period of 10–15 min.
Although denervation of the parasternal intercostals and section of the external intercostals and levator costae was proved, respectively, by electromyography and autopsy, these procedures might have elicited inspiratory contraction of several neck muscles, such as the sternohyoid, the sternothyroid, and the sternomastoid, which are not used during breathing under normal circumstances. To evaluate the potential influence of this confounding factor on the respiratory displacement of the ribs and sternum, the electromyogram of these muscles was examined in each animal. No EMG activity was ever recorded. We can ensure, therefore, that the external intercostals (and levator costae) were the only muscles active during inspiration in dogs 1–4, and that the parasternal intercostals were the only muscles active during inspiration in dogs 5–8.
Data analysis
The inspiratory displacements of the ribs and sternum in each condition were measured relative to their relaxation position, as determined during hyperventilation-induced apnoea. The cranial and outward rib motion and the caudal sternum motion that relaxation of the triangularis sterni and other expiratory muscles induces at the end of the expiratory pause in the dog (De Troyer & Ninane, 1986) were therefore not included in these measurements and were not taken into account. The peak inspiratory displacements thus obtained were averaged over ten consecutive breaths from each run in each condition and expressed in millimetres; by convention, positive motions indicate inspiratory displacements in the cranial or outward direction, and negative motions indicate inspiratory displacements in the caudal or inward direction. In addition, to gain further insight into the pattern of rib motion, the axial and lateral displacements of the ribs during spontaneous inspiration were also displayed as X–Y plots on a storage oscilloscope (model 5111; Tektronix, Beaverton, OR, USA) and compared to the displacements induced by passive inflation. The curve thus obtained during passive inflation will be referred to here as the relaxation curve of the ribs.
Phasic inspiratory electrical activity in the parasternal intercostal and external intercostal muscles during breathing was averaged over the same consecutive ten breaths and quantified by measuring the peak height of the integrated EMG signal in arbitrary units. To allow comparison between the different animals, EMG activity was subsequently expressed as a percentage of the activity recorded during breathing before phrenicotomy (control).
Data of rib and sternum displacement, EMG activity and tidal volume were finally averaged for the animal group, and they are presented as means ±s.e.m. Statistical assessments of the effects of phrenicotomy, parasternal intercostal denervation, and external intercostal section were made using Student's paired t tests; the criterion for statistical significance was taken as P < 0.05.
RESULTS
Baseline data
When breathing in the control condition, the eight animals studied had phasic inspiratory EMG activity in the parasternal and external intercostal muscles and a tidal volume of 383 ± 54 ml, and the pattern of rib and sternum motion is illustrated by the records of a representative animal in Fig. 1. In agreement with our previous investigations (De Troyer & Kelly, 1982; De Troyer & Wilson, 1993), the sternum moved caudally during inspiration, whereas it moved cranially during passive inflation; for the animal group, the sternum displacement at the peak of inspiration averaged -1.34 ± 0.54 mm. However, the ribs moved cranially and outward during spontaneous inspiration as well as during passive inflation. The motion of the ribs during inspiration was, in fact, very close to their relaxation curve or superimposed on it, as shown in Fig. 2 (left).
Figure 1. Respiratory displacements of the sternum and 4th rib during mechanical ventilation (A) and spontaneous breathing (B) in a representative animal.
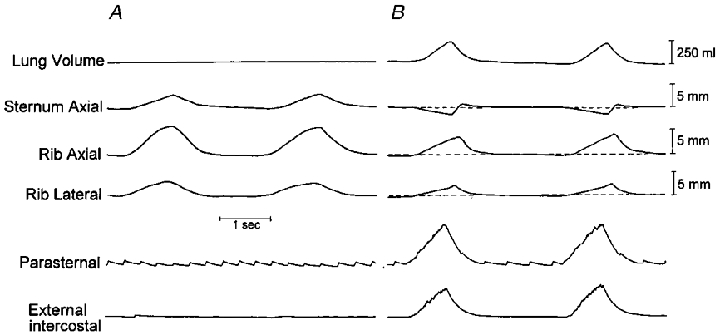
The horizontal dashed lines indicate the relaxation position of the rib and sternum, and upward deflections indicate cranial or outward displacements. The traces of lung volume and EMG activity (integrated signals) of the parasternal intercostal and external intercostal muscles in the third interspace are also shown, although lung volume was not recorded during mechanical ventilation. Note that during mechanical ventilation, inflation caused the sternum to move cranially and the rib to move cranially and outward. The rib also moved cranially and outward during inspiration, but the sternum moved caudally.
Figure 2. Pattern of rib displacement during inspiration.
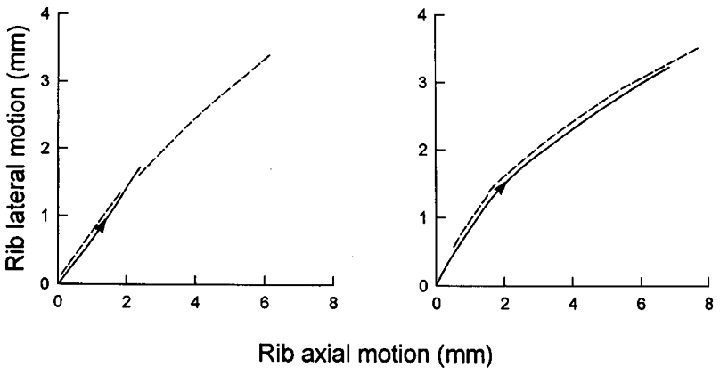
Records obtained in a representative animal in the control condition (left panel) and after section of the phrenic nerves in the neck (right panel). The continuous line in each panel corresponds to spontaneous inspiration, and the dashed line corresponds to mechanical ventilation. Note that the pattern of rib motion during spontaneous inspiration was very close to that seen during mechanical ventilation both before and after phrenic nerve section.
Effects of phrenicotomy
Sectioning the phrenic nerve roots caused an increased inspiratory EMG activity in the parasternal intercostal and external intercostal muscles to 141.4 ± 15.4 and 138.0 ± 12.7 % of the control activity, respectively, and the inspiratory displacements of the ribs and sternum were augmented as well. For the eight animals, the caudal displacement of the sternum at peak inspiration thus increased from -1.34 ± 0.54 to -1.97 ± 0.62 mm (P < 0.05), and the cranial displacement of the ribs increased from 4.69 ± 0.84 to 8.99 ± 1.16 mm (P < 0.001). However, the outward displacement of the ribs was equally augmented from 2.45 ± 0.46 to 4.57 ± 0.64 mm (P < 0.001). As a result, the motion of the ribs was still superimposed on their relaxation curve (Fig. 2, right). In spite of this increased rib displacement, tidal volume was reduced to 271 ± 37 ml (P < 0.005).
Effects of parasternal intercostal denervation
Figure 3 shows the records obtained during spontaneous breathing and during mechanical ventilation in a representative animal after denervation of the parasternal intercostals in interspaces 1–8. Denervating the parasternal intercostals elicited a marked increase in peak external intercostal inspiratory EMG activity in the four animals from 121.7 ± 18.7 to 240.0 ± 55.2 % of the control value (P = 0.05) and reversed the inspiratory caudal displacement of the sternum into a large inspiratory cranial displacement (before: -2.20 ± 0.71; after: +8.21 ± 1.55 mm; P < 0.01). The inspiratory displacement of the ribs in the cranial direction was unchanged (before: +10.36 ± 2.10 mm; after: +10.51 ± 2.48 mm; n.s.). However, the inspiratory outward displacement of the ribs was markedly reduced in three animals and virtually suppressed in one animal (P < 0.01), such that the pattern of rib motion was consistently displaced to the right of the relaxation curve (Fig. 4). Tidal volume in the animal group was concomitantly reduced from 283 ± 71 to 168 ± 54 ml (P < 0.01).
Figure 3. Respiratory displacements of the sternum and 4th rib during mechanical ventilation (A) and spontaneous breathing (B) after denervation of the parasternal intercostal muscles in a representative animal.
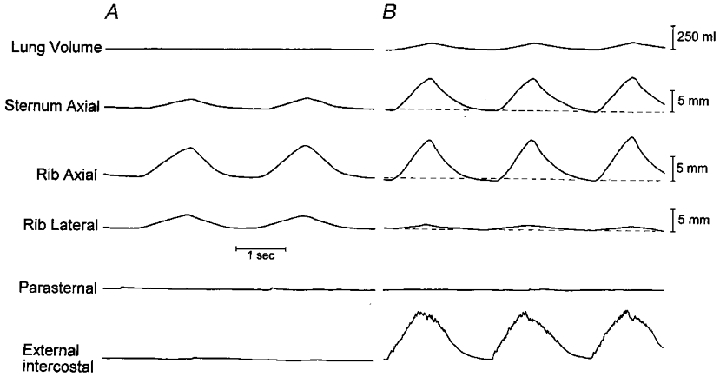
Same animal and same conventions as in Fig. 1. Note that the inspiratory cranial displacement of the rib during spontaneous breathing was similar to that during mechanical ventilation, but the lateral displacement of the rib was smaller and the cranial displacement of the sternum was larger. Note also the large amount of postinspiratory EMG activity in the external intercostal muscle; this strong postinspiratory activity, which was seen in the four animals studied, can probably be attributed to the greater chemical respiratory drive (i.e. the increase in arterial PCO2) combined with an increase in spindle afferent activity (Easton et al. 1999).
Figure 4. Pattern of rib displacement after denervation of the parasternal intercostal muscles in dogs 1-4.
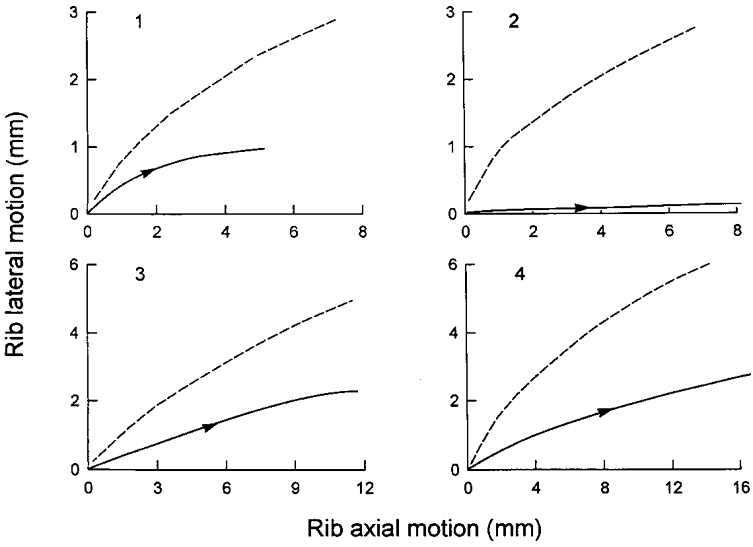
The continuous line in each panel corresponds to spontaneous inspiration, and the dashed line corresponds to mechanical ventilation. For a given cranial displacement, the outward displacement of the ribs in each animal was smaller during inspiration than during mechanical ventilation.
Effects of external intercostal section
Sectioning the external intercostal and levator costae muscles in interspaces 1–8 did not affect tidal volume (before: 258 ± 37 ml; after: 252 ± 46 ml; n.s.) or the inspiratory EMG activity recorded from the parasternal intercostals, and the sternum continued to move caudally during inspiration (before: -1.74 ± 1.13 mm; after: -2.89 ± 1.11 mm; n.s.). The ribs also continued to move both cranially (before: +7.63 ± 0.81 mm; after: +6.56 ± 0.23 mm) and outward (before: +4.29 ± 0.84 mm; after: +4.52 ± 0.80 mm). Because of the small number of animals, none of these changes reached the level of statistical significance. However, since the displacement of the ribs tended to be smaller in the cranial direction but larger outwards, the pattern of rib motion was invariably displaced to the left of the relaxation curve (Fig. 5).
Figure 5. Pattern of rib displacement after section of the external intercostal muscles in dogs 5-8.
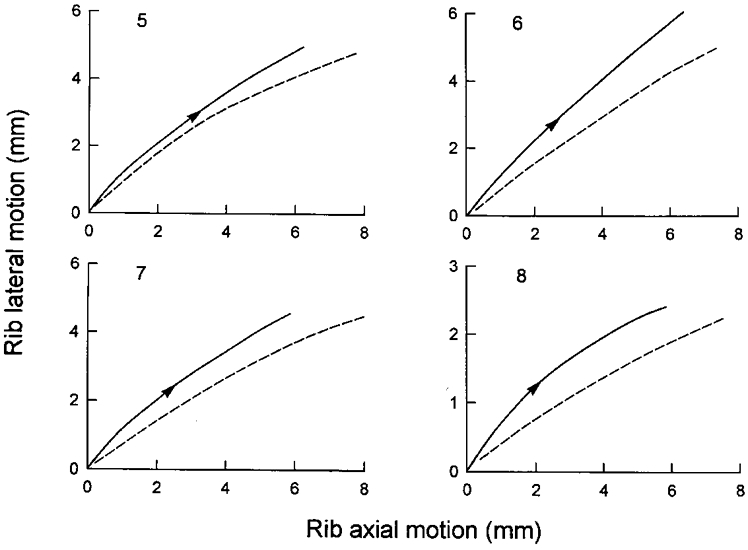
The continuous line in each panel corresponds to spontaneous inspiration, and the dashed line corresponds to mechanical ventilation. For a given cranial displacement, the outward displacement of the ribs in these animals was larger during inspiration than during mechanical ventilation.
DISCUSSION
The present studies have confirmed that both the parasternal intercostal muscles and the external intercostal muscles (including the levator costae) elevate the ribs when they contract (De Troyer & Kelly, 1982; De Troyer & Farkas, 1989), but more importantly, they have established that the rib displacements induced by these two sets of muscles are different. Specifically, in agreement with the prediction of Loring & Woodbridge (1991), the external intercostals cause the ribs to move primarily in the cranial direction (Fig. 4), whereas the parasternal intercostals drive the ribs both cranially and outward (Fig. 5). And yet, when the parasternal and external intercostals were activated together during breathing, including after bilateral phrenicotomy, the ribs moved on their relaxation curve (Fig. 2). These results therefore support our hypothesis that inspiratory activity among these two sets of muscles is co-ordinated to displace the ribs with minimum distortion.
Co-ordinated parasternal and external intercostal activity, however, also caused the sternum to move caudally; i.e. in the opposite direction to passive inflation (Fig. 1). This inspiratory caudal displacement of the sternum has been previously observed in cats (Da Silva et al. 1977) and in dogs (De Troyer & Kelly, 1982), and direct experimental evidence has been provided indicating that it is due to the action of the parasternal intercostals. When the canine parasternal intercostal muscles in one or two interspaces are selectively activated by electrical stimulation, the sternum moves caudally (De Troyer & Kelly, 1982). On the other hand, when the external intercostal or the levator costae muscle in one interspace is selectively activated, or when the parasternal intercostals in all interspaces and the diaphragm are denervated such that the external intercostals and levator costae are the only muscles active during inspiration, the sternum moves cranially (De Troyer & Farkas, 1989). The sternum was also observed to move cranially after denervation of the parasternal intercostals in the animals of this study (Fig. 3). The hypothesis could be raised, therefore, that a greater activation of the external intercostals, relative to the parasternal intercostals, would be required to displace the sternum cranially during inspiration so as to drive the entire rib cage on its relaxed configuration.
This hypothesis is examined in Fig. 6. In this figure, the ratio of lateral to axial rib displacements is plotted against the ratio of the axial displacements of the sternum and the ribs for the different conditions of the study. The filled circles correspond to the mean data obtained during spontaneous breathing in the control condition, after bilateral phrenicotomy, after denervation of the parasternal intercostals (‘External’), and after section of the external intercostals (‘Parasternal’), and the open circle corresponds to the data obtained during passive inflation. There was a close linear relationship (coefficient of correlation, r = 0.98) between all data obtained during spontaneous breathing, such that for a given cranial rib displacement, a larger caudal displacement of the sternum yielded a larger outward displacement of the ribs. However, the data obtained during passive inflation lay well above this relationship, thus indicating that the rib cage cannot be displaced on its relaxed configuration by any combination of parasternal and external intercostal activity. In other words, if inspiratory activity in the external intercostals were increased relative to the parasternal intercostals so as to drive the sternum in the cranial direction, the ribs would move outward less and they would depart from their relaxation curve with a trajectory similar to that shown in Fig. 4.
Figure 6. Pattern of rib cage displacement during spontaneous breathing and during mechanical ventilation.
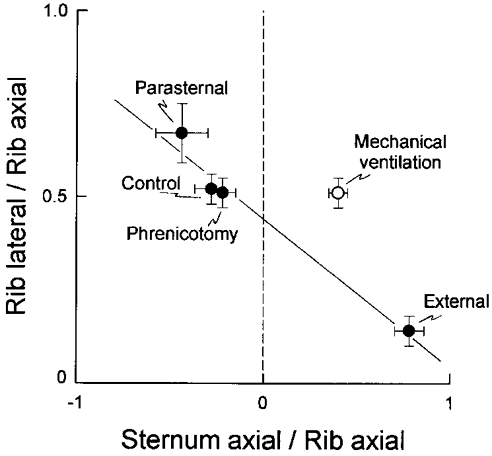
The ordinate is the ratio of lateral to axial rib displacement, and the abscissa is the ratio of the axial displacement of the sternum to the axial displacement of the ribs; a negative value indicates a sternum displacement in the caudal direction. The filled circles correspond to the mean (±s.e.m.) data obtained during spontaneous breathing in the control condition (eight animals), after phrenicotomy (eight animals), after phrenicotomy and denervation of the parasternal intercostals (‘External’, four animals), and after phrenicotomy and excision of the external intercostals (‘Parasternal’, four animals). The open circle corresponds to the mean (±s.e.m.) data obtained during passive inflation in the eight animals. A linear relationship with a negative slope fits all data well during spontaneous breathing. However, the data obtained during passive inflation lies well above this relationship.
The results displayed in Fig. 6 are also important because they imply that lateral rib displacement, axial rib displacement and sternal displacement are linked. In a previous report (De Troyer & Wilson, 1993), we showed that in the dog, the inspiratory caudal displacement of the sternum reduces the cranial displacement of the ribs but does not affect the increase in lung volume. Although we could not readily explain this apparent paradox, we hypothesized that it was related to the kinematics of the costal cartilages. Specifically, we reasoned that because these cartilages slope caudally from the sternum and are constant in length, an isolated caudal displacement of the sternum should induce an outward displacement of the costochondral junctions and, with it, an outward motion of the ribs; this motion might, in turn, cancel the effect of the reduced cranial rib motion on lung volume (De Troyer & Wilson, 1993). The present observations are fully consistent with this speculation. Thus, the inspiratory caudal displacement of the sternum that occurs during quiet breathing in the dog and in the cat does enhance the outward displacement of the ribs, and one would therefore expect that this sternal displacement would augment the increase in lung volume.
The relationship between lateral rib displacement, axial rib displacement and lung volume is more specifically examined in Fig. 7. In this figure, the change in lung volume per unit rib cranial displacement (expressed as litres per centimetre displacement) measured after phrenicotomy, after denervation of the parasternal intercostals and after excision of the external intercostals has been plotted against the corresponding rib lateral displacement per unit rib cranial displacement. In supine dogs, a small fraction of the increase in lung volume during resting breathing results from the relaxation of expiratory muscles, rather than the contraction of inspiratory muscles (De Troyer & Ninane, 1986; Bellemare et al. 1991; Schroeder et al. 1991). Therefore, since the axial and lateral displacements of the ribs considered in this study were measured relative to their relaxation position, the calculated values of lung volume per unit rib axial displacement may be slightly overestimated. Nevertheless, the change in lung volume per unit rib axial displacement and the rib lateral displacement per unit rib axial displacement were closely related to each other; the coefficient of correlation (r) thus calculated was 0.999. If lung volume is denoted VL and rib axial and lateral displacements are denoted Xr and Yr, respectively, one can therefore write the following equation:
and rearranging this equation yields
The coefficients A and B in this equation describe the relative effects of axial and lateral rib displacement on lung volume. Since the linear relationship thus calculated yields A = 0.1 l cm−1 and B = 0.4 l cm−1, it may therefore be concluded that the increase in lung volume per unit rib displacement is about four times greater for outward than for cranial displacement. This conclusion seems reasonable in view of the fact that an outward rib displacement lies closer to the normal to the surface of the lung than a cranial rib displacement.
Figure 7. Relationship between rib lateral displacement, rib axial displacement, and lung volume.
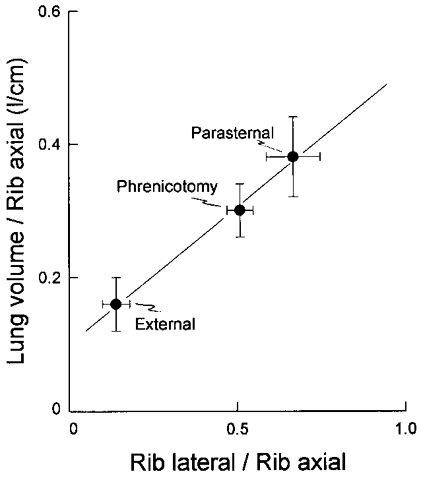
The increase in lung volume per unit rib axial displacement (expressed as litres per centimetre) is shown on the ordinate, and the rib lateral displacement per unit rib axial displacement is shown on the abscissa. Filled circles, same as in Fig. 6. Note the close linear relationship between the data points (r = 0.999); the slope of the relationship is 0.4 l cm−1, and the intercept on the Y axis is 0.1 l cm−1.
The finding that a given rib displacement in the outward direction produces a greater increase in lung volume than the same rib displacement in the cranial direction (Fig. 7), combined with the observation that the ratio of outward to cranial rib displacement during isolated contraction of the parasternal intercostals is greater than during isolated contraction of the external intercostals (Fig. 6), would further suggest that the parasternal intercostals are more effective at increasing lung volume than the external intercostals. And indeed, when the parasternal intercostals in our animals were denervated, expansion of the lung was reduced by ∼40 % even though external intercostal inspiratory activity was increased two- to threefold. On the other hand, when the external intercostals were selectively excised, parasternal intercostal activity was unchanged yet expansion of the lung was maintained. This differential response is unequivocal evidence that in the dog, the parasternal intercostals do contribute a much larger fraction of lung volume expansion than the external intercostals during breathing.
Acknowledgments
This study was supported in part by a grant (HL-45545) from the National Institutes of Health.
References
- Bellemare F, Bono D, D'Angelo E. Electrical and mechanical output of the expiratory muscles in anesthetized dogs. Respiration Physiology. 1991;84:171–183. doi: 10.1016/0034-5687(91)90115-y. [DOI] [PubMed] [Google Scholar]
- Da Silva KMC, Sayers BM, Sears TA, Stagg DT. The changes in configuration of the rib cage and abdomen during breathing in the anaesthetized cat. The Journal of Physiology. 1977;266:499–521. doi: 10.1113/jphysiol.1977.sp011779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Troyer A. Inspiratory elevation of the ribs in the dog: primary role of the parasternals. Journal of Applied Physiology. 1991;70:1447–1455. doi: 10.1152/jappl.1991.70.4.1447. [DOI] [PubMed] [Google Scholar]
- De Troyer A, Farkas GA. Inspiratory function of the levator costae and external intercostal muscles in the dog. Journal of Applied Physiology. 1989;67:2614–2621. doi: 10.1152/jappl.1989.67.6.2614. [DOI] [PubMed] [Google Scholar]
- De Troyer A, Kelly S. Chest wall mechanics in dogs with acute diaphragm paralysis. Journal of Applied Physiology. 1982;53:373–379. doi: 10.1152/jappl.1982.53.2.373. [DOI] [PubMed] [Google Scholar]
- De Troyer A, Legrand A. Inhomogeneous activation of the parasternal intercostals during breathing. Journal of Applied Physiology. 1995;79:55–62. doi: 10.1152/jappl.1995.79.1.55. [DOI] [PubMed] [Google Scholar]
- De Troyer A, Legrand A, Wilson TA. Rostrocaudal gradient of mechanical advantage in the parasternal intercostal muscles of the dog. The Journal of Physiology. 1996;495:239–246. doi: 10.1113/jphysiol.1996.sp021588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Troyer A, Legrand A, Wilson TA. Respiratory mechanical advantage of the canine external and internal intercostal muscles. The Journal of Physiology. 1999;518:283–289. doi: 10.1111/j.1469-7793.1999.0283r.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Troyer A, Ninane V. Triangularis sterni: a primary muscle of breathing in the dog. Journal of Applied Physiology. 1986;60:14–21. doi: 10.1152/jappl.1986.60.1.14. [DOI] [PubMed] [Google Scholar]
- De Troyer A, Wilson TA. Sternum dependence of rib displacement during breathing. Journal of Applied Physiology. 1993;75:334–340. doi: 10.1152/jappl.1993.75.1.334. [DOI] [PubMed] [Google Scholar]
- Easton PA, Hawes HG, Rothwell B, De Troyer A. Postinspiratory activity of the parasternal and external intercostal muscles in awake canines. Journal of Applied Physiology. 1999;87:1097–1101. doi: 10.1152/jappl.1999.87.3.1097. [DOI] [PubMed] [Google Scholar]
- Greer JJ, Martin TP. Distribution of muscle fiber types and EMG activity in cat intercostal muscle. Journal of Applied Physiology. 1990;69:1208–1211. doi: 10.1152/jappl.1990.69.4.1208. [DOI] [PubMed] [Google Scholar]
- Kirkwood PA, Sears TA, Stagg D, Westgaard RH. The spatial distribution of synchronization of intercostal motoneurones in the cat. The Journal of Physiology. 1982;327:137–155. doi: 10.1113/jphysiol.1982.sp014224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legrand A, Brancatisano A, Decramer M, De Troyer A. Rostrocaudal gradient of electrical activation in the parasternal intercostal muscles of the dog. The Journal of Physiology. 1996;495:247–254. doi: 10.1113/jphysiol.1996.sp021589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legrand A, De Troyer A. Spatial distribution of external and internal intercostal activity in dogs. The Journal of Physiology. 1999;518:291–300. doi: 10.1111/j.1469-7793.1999.0291r.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loring SH, Woodbridge JA. Intercostal muscle action inferred from finite-element analysis. Journal of Applied Physiology. 1991;70:2712–2718. doi: 10.1152/jappl.1991.70.6.2712. [DOI] [PubMed] [Google Scholar]
- Mead J. Mechanics of the chest wall. In: Pengelly LD, Rebuck AS, Stys EJM, editors. Loaded Breathing. Canada: Longmann; 1974. pp. 35–49. [Google Scholar]
- Schroeder MA, Tao HY, Farkas GA. Mechanical role of expiratory muscle recruitment during eupnea in supine anesthetized dogs. Journal of Applied Physiology. 1991;70:2025–2031. doi: 10.1152/jappl.1991.70.5.2025. [DOI] [PubMed] [Google Scholar]


