Abstract
The mechanisms involved in contraction in guinea-pig myometrium were compared at mid- and late pregnancy. Tension was recorded simultaneously with either membrane potential or cytoplasmic calcium ([Ca2+]i) in strips exposed briefly to prostaglandin F2α (PGF).
PGF-induced increases in tension were underpinned by action potentials followed by sustained depolarization and biphasic increases in [Ca2+]i at mid- (peak, 879 ± 199 nM; sustained, 298 ± 35 nM, n = 11) and late pregnancy (peak, 989 ± 302 nM; sustained 178 ± 33 nM, n = 8).
At mid- and late pregnancy, nifedipine (10−6 M) reduced (a) the PGF-induced increase in tension to 84 and 35 %, (b) the level attained during the depolarization by 2 and 12 mV and (c) the peak rise in [Ca2+]i to 42 and 17 %. The sustained rises in [Ca2+]i were resistant to nifedipine.
In Ca2+-free solution (containing 1 mM EGTA), PGF elicited an increase in tension that was 26 % of that in 2·5 mM Ca2+ and an increase in [Ca2+]i (24 % of the sustained level) at mid-pregnancy but no increase in tension or [Ca2+]i at term.
At both stages of pregnancy, PGF decreased the level of [Ca2+]i required to elicit increases in tension comparable to those evoked by high . The slope of the tension-[Ca2+]i curves were steeper in mid- than in late pregnancy.
In conclusion, at mid-pregnancy, the contractile response of the guinea-pig myometrium to PGF involves Ca2+ influx through L-type voltage-operated Ca2+ channels (VOCCs) and by receptor-operated mechanisms, release of Ca2+ from intracellular stores, and an increase in the sensitivity of the contractile apparatus to Ca2+. At term the situation is different: a modest increase in the sensitivity of the contractile apparatus to Ca2+ persists and there is a major reliance on Ca2+ influx through VOCCs.
The contractile activity of the uterus changes markedly from relative quiescence during pregnancy to the generation of strong, co-ordinated contractions during labour. This transformation has been referred to as ‘activation’ (Challis & Lye, 1994) and is envisaged to involve changes in the identity and number of ion channels, the proteins of the contractile apparatus, second messenger systems and gap junction formation. Several classes of the K+ channels in myometrium that are influenced by the sex steroids (in rats, Boyle et al. 1987; Toro et al. 1990) or by pregnancy (women, Khan et al. 1993; rats, Wang et al. 1998) could contribute to the changes in excitability that occur during labour. The inward Ca2+ current in isolated uterine smooth muscle cells in rats appears to be enhanced by progesterone (Rendt et al. 1992), while a Na+ current increases during pregnancy (Inoue & Sperelakis, 1991). Coupling of G proteins to second messengers in guinea-pig myometrium (Arkinstall & Jones, 1990) and the levels of mRNA for various isoforms of phospholipase C change during pregnancy in rats (Bieber et al. 1998) but the results are not always easily extrapolated to tissue contraction.
The contractile state of smooth muscle is determined predominantly by the level of phosphorylated myosin, achieved largely via myosin light chain kinase (MLCK) whose activity is regulated by Ca2+-calmodulin (Somlyo & Somlyo, 1994; Walsh et al. 1996). The level of cytoplasmic free Ca2+ ([Ca2+]i) is influenced by the complement of ion channels, pumps and exchangers in the plasma membrane and as a result of Ca2+ release from the endoplasmic reticulum (see Ashida et al. 1988; Nelson et al. 1990). Dephosphorylation of myosin by myosin light chain phosphatase (MLCP) facilitates relaxation. Inactivation of MLCP, as a result of its phosphorylation by second messengers such as rho kinase or protein kinase C, can give rise to an increase in the level of phosphorylated myosin in the face of constant levels of [Ca2+]i and activated MLCK. The resulting increase in the sensitivity of the contractile apparatus to Ca2+ accounts for the large contractile response of many smooth muscles to excitatory agonists (Somlyo & Somlyo, 1994; Walsh et al. 1996), including the uterus (Izumi et al. 1996). The levels of tension and phosphorylated MLC are well correlated in myometrium, and the tension per phosphorylated MLC is increased during pregnancy in women (Word et al. 1993).
The aims of this study were to investigate (1) Ca2+ influx, (2) release of Ca2+ from intracellular stores and (3) the sensitivity of the contractile apparatus to Ca2+, in order to determine the relative significance of these mechanisms in contraction of the myometrium. We also investigated whether the relative contribution of the various mechanisms to contraction might change during pregnancy. Tissues from guinea-pigs were studied because their oestrogen and progesterone profiles during pregnancy and labour resemble those of humans most closely (Bedford et al. 1972).
METHODS
Tissues
Guinea-pigs were killed by decapitation (Monash University Animal Experimentation Ethics Committee permit number 95077) at mid-pregnancy, days 31–38, and at term, days 62–66 of pregnancy. The day on which a plug of semen was found in the vagina was designated day 1 of pregnancy and the length of pregnancy in our colony was 67 ± 1 days (n = 121). Strips of outer longitudinal uterine smooth muscle, approximately 200 μm wide (one bundle), 3 mm long and full thickness, were prepared and transferred to a recording chamber (about 0.5 ml capacity), and one end was attached to an isometric force transducer (AE801, SensoNor, Horton, Norway). For simultaneous recording of membrane potential and tension, the other end was pinned to the silicone rubber base of the chamber. For simultaneous determination of cytoplasmic calcium ([Ca2+]i) and tension, the free end was attached to the glass bottom of the recording chamber with SupaGlue. The tissues were continuously superfused at 3 ml min−1 with physiological saline solution (PSS) containing (mM): NaCl, 120; KCl, 5; NaHCO3, 25; KH2PO4, 1; MgSO4, 1.2; glucose, 11; CaCl2, 2.5, bubbled with 95 % O2 and 5 % CO2 and maintained at 35°C. Membrane potential was recorded using intracellular glass microelectrodes filled with 1 M KCl and having resistances of 60–100 MΩ, as described previously (Parkington et al. 1999b).
Determination of [Ca2+]i
The method used to determine [Ca2+]i simultaneously with tension has been described previously (Parkington et al. 1999b). Briefly, the tissues were alternately illuminated at 340 and 380 nm (100 Hz) and the emission at 505 nm was collected by a photomultiplier tube. Autofluorescence and background fluorescence were determined. The tissues were then incubated with solution containing (mM): NaCl, 135; KCl, 5; Hepes, 10; glucose, 11; CaCl2, 2.5; 5 μM fura-2 acetoxymethyl ester (fura-2 AM) and 0.01 % pluronic F127 for 50–60 min at room temperature (21°C). Hepes was used as the buffer in the loading solution because flow was suspended during this time. Following loading, solution flow was resumed using normal PSS at 35°C. Prior to the commencement of experiments, fura-2 was removed from the extracellular environment during a 30 min wash period. During the experiments, the ratio of the fluorescence emitted upon illumination at 340 and 380 nm, R340/380, was recorded at rest, during spontaneous contractions and during application of a maximally effective concentration of PGF (10−5 M) (established in preliminary experiments). Responses were also examined in the absence and presence of various pharmacological tools (see Results). At the end of each experiment, an attempt was made to calibrate the fluorescence signal. Rmax, the maximal R340/380 signal, was determined using Hepes-buffered solution (see above) containing the Ca2+ ionophore ionomycin (2 × 10−5 M) and 5 mM [Ca2+]o, and Rmin, the minimal R340/380 signal, was determined by switching to Ca2+-free solution containing EGTA (3 × 10−3 M). R340/380, Rmax, Rmin and a Kd of 224 nM (Grynkiewicz et al. 1985) were then used to express the fluorescence signal in terms of [Ca2+]i. In the experiment designed to test the sensitivity of the contractile apparatus to Ca2+, and in those in which the [Ca2+]i profile of the response to PGF was quantified, data from some experiments were excluded from consideration in the results because of failure of the calibration. In other experiments, e.g. testing thapsigargin, cyclopiazonic acid (CPA) and caffeine, the changes in R340/380 (e.g. in the presence of nifedipine) were small, that is, R340/380 lay within the essentially linear range of the Ca2+-ratio curve (see Hayes et al. 1996; Haworth & Redon, 1998), hence it was possible to use the values of R340/380 as indicators of [Ca2+]i. Possible loading of intracellular organelles was tested using 5 × 10−5 M saponin for 20 min to disrupt the plasma membrane. This caused a precipitous drop in R340/380 to near background levels. Tissues were then exposed to 1 % Triton X-100 for a further 20 min to disrupt the membranes of organelles. There was no additional change in R340/380. These results provided compelling evidence against significant loading of organelles with fura-2 in this study.
Responses in Ca2+-free solution
The response to PGF in the absence of extracellular Ca2+ was also tested. Ca2+ was omitted from normal PSS and 10−3 M EGTA was added, to give ‘Ca2+-free’ PSS. The flow rate of the solution was increased to 7 ml min−1 for these experiments in order to facilitate more complete removal of . Tissues were superfused with Ca2+-free PSS for 2 min prior to and during testing the response to PGF.
Sensitivity of the contractile apparatus to [Ca2+]
Tissues were superfused with PSS containing 100 mM K+ (isosmotic replacement of Na+) to activate voltage-operated Ca2+ channels, and 10−6 M thapsigargin to prevent Ca2+ uptake into intracellular stores (HiK PSS). In HiK PSS, [Ca2+]o was increased stepwise from 0 mM to 0.5, 1, 2.5, 5, 10 and 20 mM at 3 min intervals, thus allowing sufficient time for the [Ca2+]i and tension to stabilize at each new level. The tissue was then rested in normal PSS (containing 2.5 mM Ca2+) for 20 min. The procedure was repeated, this time including PGF (10−5 M) in the HiK PSS as [Ca2+]o was increased, which was added from the final 3 min of exposure to 0 mM [Ca2+]o onwards. The fluorescence signal was calibrated immediately following 10 min washout of the HiK PSS. Calibration was successful in 74 % of tissues and only these data are presented in the Results (see p. 794). This method was used to investigate the effects of PGF on the sensitivity of the contractile apparatus to Ca2+ because high concentrations of the pore-forming agent staphylococcal α-toxin are required for guinea-pig tissues. Thus, it is possible that the toxin might fail to reach all of the smooth muscle cells within the tissue.
Drugs used
The stable form of PGF, dinoprost, stock 10−2 M, was used exclusively and was a gift from Upjohn (Kalamazoo, USA). Stock solutions of the following drugs were made: nifedipine (10−2 M in DMSO), thapsigargin (10−2 M in DMSO), cyclopiazonic acid (10−1 M in DMSO) and caffeine (dissolved directly in PSS) (all from Sigma). Dimethyl sulfoxide (DMSO) was without a detectable effect on uterine smooth muscle activity when applied alone at 10−4 M.
Analysis of data
Membrane potential and tension were recorded and stored on videocassette and [Ca2+]i and tension were stored on an AmLab data acquisition system (AmLab Technology, Sydney) (see Parkington et al. 1999b). The data were analysed later using the statistical software Prism (GraphPad, USA) and Origin (Microcal, USA). The normality of the data was tested using the method of Kolmogorov & Smirnov and equivalence between standard deviations was tested using Bartlett's method (both tests achieved using GraphPad Instat). The data passed these tests and subsequent analysis of variance was used to test between the two stages of pregnancy and between treatments, and Student's t test was used to compare means. Means ±s.e.m. are quoted throughout. In all instances n designates the number of animals studied. A level of P < 0.05 was accepted as indicating significant difference.
To assess the sensitivity of the contractile apparatus to Ca2+, tension was plotted against [Ca2+]i. In permeabilized blood vessels stimulated with noradrenaline, the tension-Ca2+ relationship is sigmoid (Nishimura & van Breemen, 1989), and thus the lower part of the sigmoid curve is essentially exponential. Hence, an exponential function was fitted to the force-[Ca2+]i data obtained in control solution and in the presence of PGF for each tissue. The curves fitted the data with r2 > 0.9 for all tissues (an example is illustrated in Fig. 10B). From each curve, the concentrations of Ca2+ required to evoke contractions that were 50 and 100 % of that elicited by high K+ were calculated within each individual tissue. The values obtained for tissues from sevenguinea-pigs were averaged to provide the data shown in Table 2.
Figure 10. Effect of increasing extracellular Ca2+ on [Ca2+]i and tension and the influence of PGF on the relationship between [Ca2+]i and tension in guinea-pig myometrium during pregnancy.
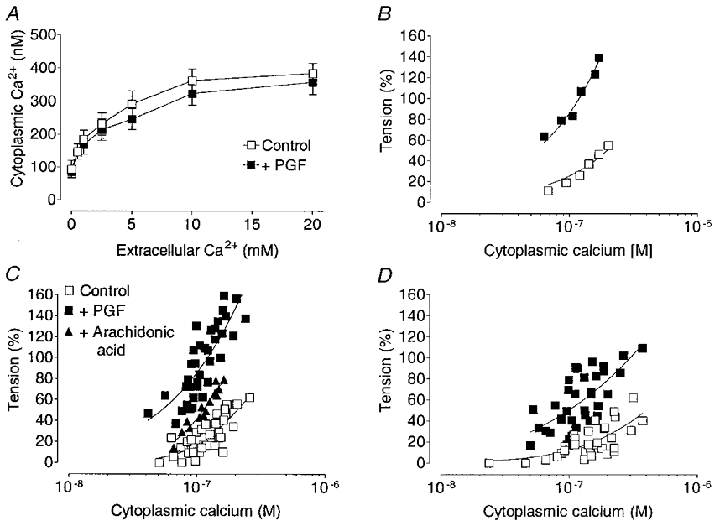
A, increasing induced similar increases in [Ca2+]i in PSS containing high K+ (100 mM, control) and in PSS containing both high K+ and PGF (10−5 M). B, an example of the effect of increasing levels of [Ca2+]i on tension (expressed as a percentage of the initial peak contraction evoked by high K+ in normal 2.5 mM Ca2+) in an individual strip illustrates that the relationship was well fitted by an exponential function (continuous lines). The relationship between [Ca2+]i and tension in control solution, was not different in mid- (C) and late (D) pregnancy. PGF (10−5 M) increased the tension response for any given value of [Ca2+]i at both mid- (C, n = 7) and late (D, n = 7) pregnancy but was more effective at mid-pregnancy. At mid-pregnancy, arachidonic acid (10−5 M) (n = 4) also increased the sensitivity of the contractile apparatus to [Ca2+]i but to a lesser extent than PGF (C).
Table 2.
Effect of PGF on the [Ca2+]i at which tissues produced tension that was 50% and 100% of the tension evoked by 100 mm
| [Ca2+]i producing 50% tension (nm) | [Ca2+]i producing 100% tension (nm) | |||
|---|---|---|---|---|
| Mid | Late | Mid | Late | |
| Control | 191 ± 15 | 236 ± 30 | 550 ± 21* | 999 ± 26*† |
| +PGF | 73 ± 7‡ | 117 ± 21‡ | 114 ± 8‡ | 203 ± 24†‡ |
| +Arachidonic acid | 116 ± 12‡ | — | 249 ± 9*‡ | — |
In strips from 7 mid-pregnant guinea-pigs, and from 7 guinea-pigs at late pregnancy, PGF (10−5m) decreased by approximately half, the concentration of [Ca2+]i required to evoke contraction that was 50% of that elicited by 100 mm in the same strip. In 4 of the mid-pregnant tissues the effects of arachidonic acid (10−5m) were also tested and it was found to decrease the [Ca2+]i requirement for contraction. In order to elicit tension that was equal to 100% of that evoked by 100 mm only one-fifth the concentration of [Ca2+]i was required in the presence of PGF. Data from individual animals were used to obtain these means and s.e.m.
Data were obtained by extrapolation.
Significantly different from mid-pregnant.
Significantly different from control.
RESULTS
Spontaneous contractions
Within approximately 30 min of being mounted in the chamber, spontaneous contractions occurred in all tissues obtained at mid-pregnancy (Fig. 1). The amplitudes of these contractions were 96 ± 4 % (n = 33) of the contraction elicited by 100 mM (Fig. 2A). Each contraction was preceded by a complex action potential that consisted of an initial spike followed by a plateau of depolarization to −25 ± 1 mV (n = 37) (Fig. 3A). The most negative level of the membrane potential, between complex action potentials, was −56 ± 1 mV (n = 37).
Figure 1. Spontaneous and PGF-induced electrical and contractile activity in control solution and in the presence of nifedipine in myometrium obtained from guinea-pigs at mid- and late pregnancy.
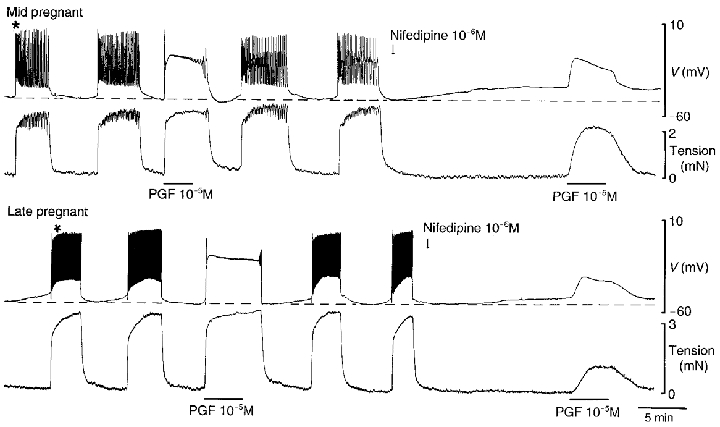
Spontaneous action potentials (upper traces) were accompanied by large increases in tension (lower traces). PGF induced an initial spike followed by sustained depolarization at both stages of pregnancy. In nifedipine, commenced at the time indicated by the arrow, spontaneous activity was abolished and the membrane depolarized to a greater extent at mid- compared with at late pregnancy. The sustained membrane potential and tension responses to PGF were largely resistant to nifedipine in mid-pregnancy, while they were considerably reduced at term. Portions of the spontaneous action potentials marked * have been shown on an expanded time scale in Fig. 3.
Figure 2. Contraction, membrane potential response (A) and [Ca2+]i (B) evoked by 100 mM in myometrium from a mid-pregnant guinea-pig.
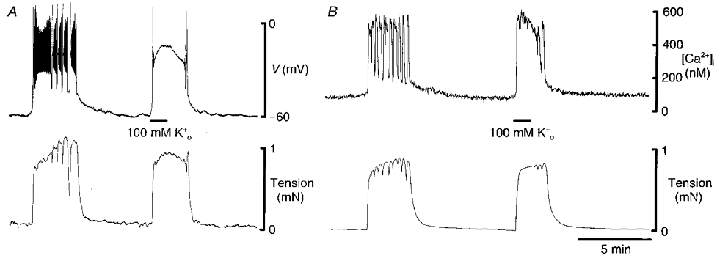
A, spontaneous contraction (lower trace) was underpinned by a complex action potential (upper trace). Replacement of Na+ with 100 mM K+ led to prompt depolarization, the initiation of spikes and contraction. B, spontaneous contraction (lower trace) was preceded by an increase in [Ca2+]i (upper trace). Replacement of Na+ with 100 mM also evoked a rapid increase in [Ca2+]i and contraction. Both tissues from the same mid-pregnant guinea-pig.
Figure 3. Spontaneous electrical activity occurring in pregnant guinea-pig myometrium.
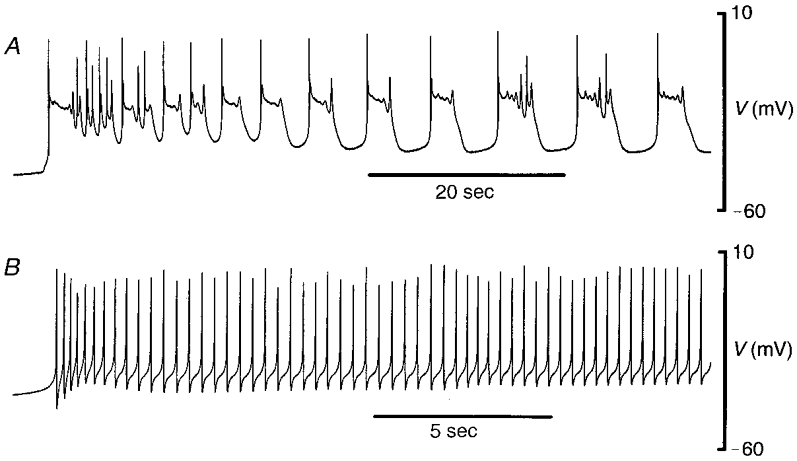
A, in a tissue obtained at mid-pregnancy, action potentials were complex in form, with spikes and plateaux of depolarization. B, at term, only simple spikes were recorded. Each trace is a part of the first burst of spontaneous activity from Fig. 1 (marked *), reproduced on an expanded time scale.
In preparations obtained from term pregnant guinea-pigs the resting membrane potential was −58 ± 3 mV (n = 51). At this stage of pregnancy, spontaneous contractions occurred in only 31 % of tissues and these contractions were 106 ± 5 % of a high K+-induced contraction (n = 12, not different compared with mid-pregnancy, P = 0.1782). The contractions were underpinned by a burst of spikes upon a depolarization to −42 ± 5 mV (n = 11) in most instances (Figs 1 and 3B). The first spike was terminated by an undershooting hyperpolarization. Complex action potentials similar to those that occurred in mid-pregnancy were rare (plateau to −36 ± 3 mV, n = 5).
Resting [Ca2+]i was not different in tissues obtained at mid- (88 ± 17 nM, n = 11) and late (91 ± 15 nM, n = 11) pregnancy (P = 0.8960). Spontaneous contractions were preceded by similar (P = 0.4601) increases in [Ca2+]i at mid- (peak at 630 ± 28 nM, n = 11) and late pregnancy (peak at 669 ± 34 nM, n = 4) (see Fig. 4). High K+ solution also evoked similar peak increases in [Ca2+]i, 623 ± 21 nM (n = 5) (see Fig. 2B) and 654 ± 23 nM (n = 4) at mid- and late pregnancy, respectively.
Figure 4. Spontaneous and PGF-induced increases in [Ca2+]i and contractile activity in control solution and in the presence of nifedipine in myometrium obtained from guinea-pigs at mid- and late pregnancy.
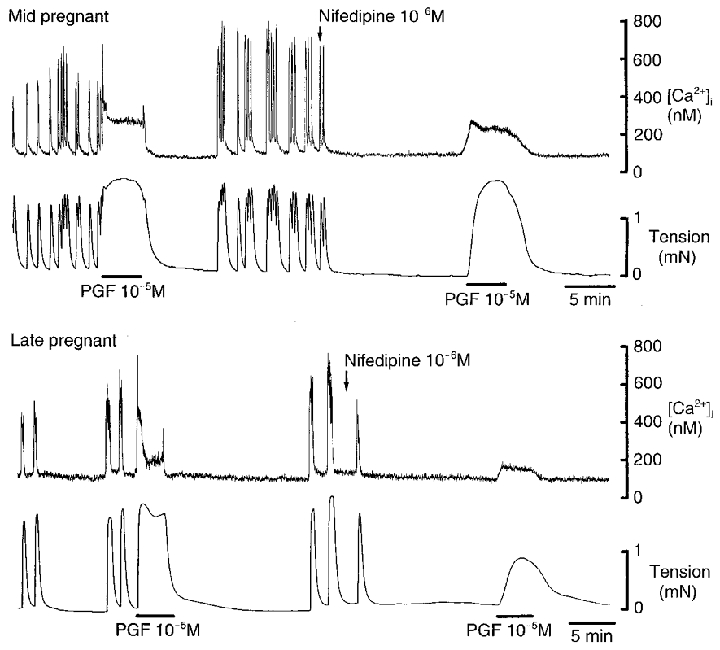
Spontaneous increases in tension (lower traces) were preceded by increases in [Ca2+]i (upper traces). PGF induced a biphasic increase in [Ca2+]i, with a transient peak followed by a lower sustained increase. Nifedipine, introduced as indicated by the arrow, markedly reduced the PGF-induced peak in [Ca2+]i and the tension response at term. The sustained rise in [Ca2+]i persisted to a greater extent, as did the tension response at mid-pregnancy.
Prostaglandin-induced responses
The membrane potential response to a maximally effective concentration of PGF (10−5 M for 2–5 min) in normal PSS consisted of the initiation of a spike action potential which was followed by depolarization that peaked at −17 ± 1 mV at mid-pregnancy (n = 21) and −20 ± 2 mV at late pregnancy (n = 18) (P = 0.1695) (Fig. 1). The depolarization then declined to a sustained level of −20 ± 1 mV (n = 21) in mid-pregnancy and −22 ± 1 mV at term (n = 18) (P = 0.1682). These responses were accompanied by contraction that peaked at 157 ± 7 % (n = 21) and 147 ± 6 % (n = 18, P = 0.2936) of the peak of the contraction elicited by 100 mM in tissue obtained at mid- and late pregnancy, respectively.
The contraction evoked by PGF (10−5 M) was accompanied by a biphasic increase in [Ca2+]i (Fig. 4). The initial peak, similar in amplitude at mid- and late pregnancy, decreased to a significantly lower sustained level which was larger in mid- than in late pregnancy (Table 1).
Table 1.
The membrane potential, [Ca2+]i and tension responses to PGF (10−5 M) and the effect of nifedipine (10−6 M) on these responses in myometrium from guinea-pigs at mid- and late pregnancy
| Initial component of response | Sustained component of response | |||
|---|---|---|---|---|
| Mid | Late | Mid | Late | |
| Depolarization level (mV) | ||||
| Control | −17 ± 1(21) | −20 ± 2(18) | −20 ± 1(21) | −22 ± 1(18) |
| Nifedipine | −20 ± 2(21) | −31 ± 2*†(18) | −22 ± 1(21) | −30 ± 2*†(18) |
| Cytoplasmic calcium (nM) | ||||
| Control | 879 ± 199(11) | 989 ± 302(8) | 298 ± 35(11) | 178 ± 33*(8) |
| Nifedipine | 367 ± 141†(11) | 173 ± 28 †(8) | 230 ± 32(11) | 163 ± 36(8) |
| Tension (%) | ||||
| Control | — | — | 100 | 100 |
| Nifedipine | — | — | 84 ± 3(19) | 35 ± 2*(9) |
PGF evoked membrane depolarization, an increase in [Ca2+]i and an increase in tension. Nifedipine decreased the initial transient increase in [Ca2+]i at both stages of pregnancy. The blocker had little effect on the depolarization, the sustained rise in [Ca2+]i or on tension at mid-pregnancy but markedly suppressed these parameters at late pregnancy. The number of tissues examined is given in parentheses.
Significantly different from mid-pregnancy.
Significantly different from control.
Voltage-operated Ca2+ channels: effect of nifedipine
Nifedipine (10−6 M) was used to block Ca2+ influx through voltage-operated Ca2+ channels (VOCCs). That this occurred successfully was verified by the abolition of all spikes, increases in [Ca2+]i, and contraction in response to 100 mM (not shown). Following the addition of nifedipine, spontaneous contractions did not occur. The cells no longer generated action potentials and the membrane potential depolarized to a stable level of −43 ± 1 mV (n = 33) at mid-pregnancy (Fig. 1). The depolarization observed in the presence of nifedipine was 13 ± 1 mV (P < 0.0001, paired t test).
At term, the membrane depolarized to a stable level of −52 ± 2 mV (n = 22) in nifedipine (Fig. 1). This was 6 ± 1 mV more depolarized than the value of the membrane potential prior to nifedipine in the same tissues. Although this depolarization was significant (P = 0.0107, paired t test), it was not as large as that which occurred at mid-pregnancy (P < 0.0001).
Nifedipine had no detectable effect on resting [Ca2+]i at either stage of pregnancy (90 ± 18 nM, n = 11 at mid-pregnancy and 89 ± 14 nM, n = 11 at term) (Fig. 4).
Effect of nifedipine on the responses to PGF
It has been suggested that the dihydropyridine VOCC blocking drugs, especially at higher concentrations, may be capable of interfering with agonist-induced contractile mechanisms in smooth muscle (Kobayashi et al. 1991). In tissues obtained at mid-pregnancy, increasing the concentration of nifedipine decreased the maximum rate of rise of the initial spike on the upstroke of the membrane potential response to PGF, from 11 ± 2 V s−1 at 10−9 M nifedipine to 1 ± 1 V s−1 (n = 6) at 10−7 M. Spikes were abolished at higher concentrations of the blocker (Fig. 5). However, the sustained depolarization phase of the response persisted (only a 2 mV reduction) and was not affected when the concentration of nifedipine was increased to 10−5 M. In nifedipine, the PGF-induced increase in tension was 84 ± 3 % (n = 19) of the control response to PGF (Fig. 1 and Table 1).
Figure 5. Effect of nifedipine and Cd2+ on the membrane potential and tension responses to PGF in myometrium of guinea-pig at mid-pregnancy.
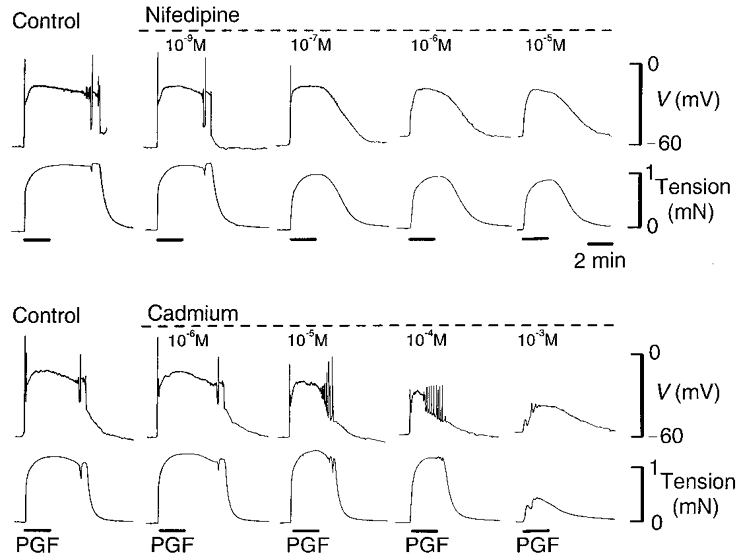
Increasing concentrations of nifedipine reduced and then abolished the spike component of the membrane potential responses (upper traces) evoked by PGF (10−5 M) while leaving intact the sustained depolarization and the increase in tension (lower traces). Increasing concentrations of Cd2+ progressively suppressed both the spikes and the depolarization but only decreased the amplitude of the tension response at the highest concentration.
Treatment with nifedipine had a dramatic effect on the responses evoked by PGF at term. Again spikes were abolished but, in addition, the depolarization phase of the membrane potential response reached only −30 ± 2 mV (n = 9) (a 12 mV reduction) and tension was reduced to 35 ± 2 % (n = 9) of control (Fig. 1 and Table 1).
At both stages of pregnancy the initial peak in [Ca2+]i evoked by PGF was significantly reduced in the presence of nifedipine (Fig. 4 and Table 1). The reduction was to 42 % at mid- and to 17 % at late pregnancy. In contrast, the sustained increases in [Ca2+]i were not significantly different in nifedipine compared with control (paired t tests) at either stage of pregnancy (Fig. 4 and Table 1).
Ca2+ influx during depolarization and contraction
Ca2+ can enter smooth muscle cells via pathways other than through VOCCs, e.g. Ca2+‘leak’ or store-depleted Ca2+ influx, and the involvement of such mechanisms can be tested by varying [Ca2+]o and by using other divalent cations, such as Cd2+ (Somlyo & Somlyo, 1994; Pacaud et al. 1996). In the presence of nifedipine, [Ca2+]o was decreased for 2 or 5 min prior to and during exposure to PGF and tension was expressed as a percentage of the response to PGF in PSS containing normal 2.5 mM , also in the presence of nifedipine. The level of depolarization and the amplitude of the tension response evoked by 10−5 M PGF in tissues obtained at both stages of pregnancy progressively decreased as [Ca2+]o was lowered from 2.5 mM to 1, 0.3 and 0 mM (Fig. 6A). The level of membrane potential attained during the sustained depolarization decreased by 12.1 ± 0.7 mV (n = 6) and tension declined by 43 ± 3 % per 10-fold decrease in [Ca2+]o (Fig. 6A). The rate of the decrease was less steep at term (4.4 ± 0.7 mV and 31 ± 4 % per 10-fold decrease in [Ca2+]o, respectively, n = 6, Fig. 6A).
Figure 6. Effect of low , nifedipine, Cd2+ and CPA on the depolarization and tension responses evoked by PGF in myometrium of pregnant guinea-pigs.
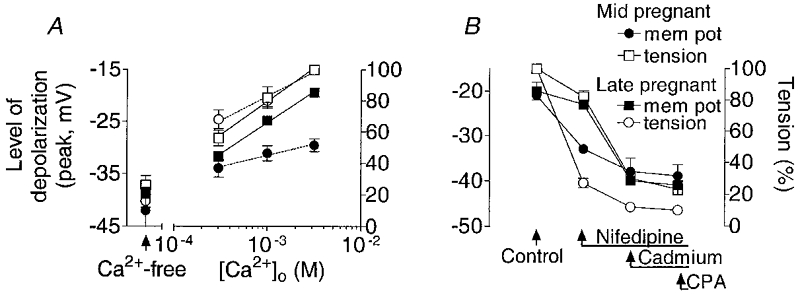
A, the level of membrane depolarization evoked by PGF (10−5 M), applied following 2–5 min in low Ca2+ and nifedipine (10−6 M), was plotted against [Ca2+]o. The increase in tension was expressed as a percentage of the responses in normal 2.5 mM at mid- (n = 6) and late (n = 6) pregnancy. The responses in nominally Ca2+-free solution (no EGTA) are shown on the left. B, Cd2+ (10−3 M) caused the greatest reduction in the depolarization and tension responses to PGF (10−5 M) at mid-pregnancy (n = 5) while nifedipine (10−6 M) had the greatest effect at term (n = 5). Cyclopiazonic acid (CPA, 2 × 10−5 M) had no further effect on the responses at either stage of pregnancy.
Increasing the concentration of Cd2+ in the perfusate between 10−5 and 10−3 M progressively reduced the level of the depolarization and the contraction in response to PGF at both stages of pregnancy in the absence of nifedipine (illustrated for mid-pregnancy in Fig. 5). In the presence of nifedipine, Cd2+ (10−3 M) reduced the level of the PGF-induced depolarization to −40 ± 1 mV and the contraction to 35 ± 3 % (n = 5) of control at mid-pregnancy. At term, these parameters were reduced to −38 ± 3 mV and 12 ± 2 % (n = 5), respectively (Fig. 6B). Ni2+ was without effect on PGF-evoked depolarization or increase in tension, in the presence of nifedipine, until the concentration was increased above 2 × 10−3 M (n = 5, data not shown).
The endoplasmic reticulum
Release of Ca2+ from the endoplasmic reticulum (ER) contributes to the initial rise in [Ca2+]i and to the contraction evoked by many spasmogens of smooth muscle and a role for this system in the response of the guinea-pig myometrium to PGF was investigated. Cyclopiazonic acid (CPA, 1 or 2 × 10−5 M), which prevents refilling of the ER with Ca2+, evoked action potential discharge and contraction in three quiescent tissues obtained at late pregnancy and these were abolished by nifedipine (data not shown). At mid-pregnancy, CPA increased activity by either increasing the frequency (with a decrease in the duration) or an increase in the duration (with a decrease in frequency) (Fig. 7) of bursts of action potentials/contractions, with no effect on the amplitude of contraction (89 ± 5 % of prior values, n = 5, P = 0.0858). The variability in the response was reflected in large standard errors and consequent lack of statistical significance in the changes in frequency (104 ± 12 %, n = 5, P = 0.7174) and duration (111 ± 20 %, n = 5, P = 0.617). The underlying depolarization evoked by CPA was clearly revealed in nifedipine (effect at mid-pregnancy shown in Fig. 8). The amplitude of this depolarization was greater at mid-pregnancy (12 ± 2 mV, n = 6) than at late pregnancy (7 ± 1 mV, n = 5, P = 0.0457). In the absence of nifedipine, CPA was without effect on the amplitude of the contractions evoked by 100 mM (98 ± 2 %, n = 10) or PGF (98 ± 3 %, n = 10) at mid-pregnancy. In the presence of nifedipine, the level of depolarization and tension attained by PGF were unaltered by CPA (Fig. 8).
Figure 7. Effect of CPA on membrane potential and tension in normal solution.
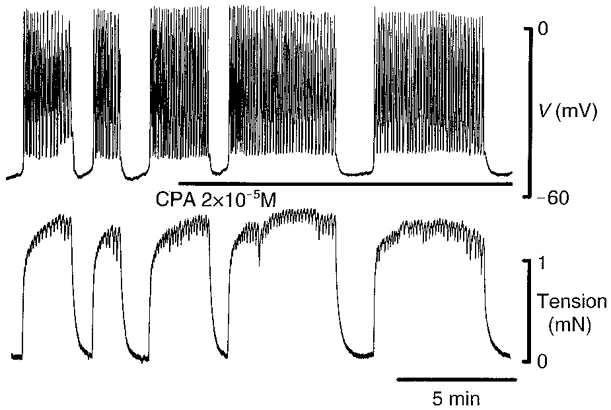
Cyclopiazonic acid (CPA, 2 × 10−5 M) increased the period of firing of spikes (upper trace) and prolonged the duration of contraction (lower trace).
Figure 8. Effect of thapsigargin and CPA on the membrane potential and tension responses in the presence of nifedipine in myometrium of guinea-pig at mid-pregnancy.
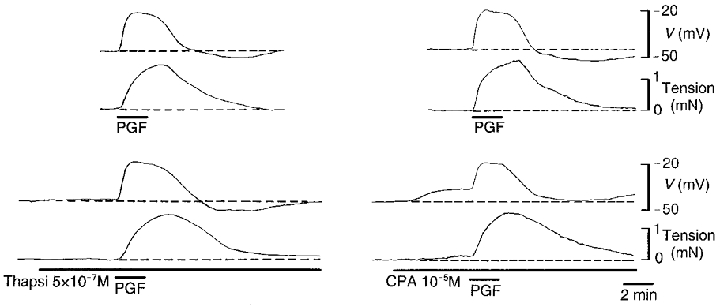
In the presence of nifedipine (10−6 M), thapsigargin (Thapsi) had little effect on resting membrane potential or tension or on the responses to PGF (10−5 M). Cyclopiazonic acid (CPA) alone evoked depolarization and an increase in tension but had no effect on the responses to PGF. Upper traces, membrane potential; lower traces, tension.
Thapsigargin (10−6 M), a structurally unrelated inhibitor of the ER Ca2+ pump, was without effect on spontaneous action potentials or on the frequency (97 ± 2 % of prior values, n = 5), duration (103 ± 5 %, n = 5) or amplitude (100 ± 3 %, n = 5) of the attendant contractions at mid-pregnancy. Thapsigargin was also without effect on membrane potential in nifedipine (Fig. 8).
The effects of thapsigargin (5 × 10−7 or 10−6 M) and CPA (1 or 2 × 10−5 M) (for 10–20 min) on R340/380, an indication of [Ca2+]i, and on tension were also examined in the presence of nifedipine. Thapsigargin was without effect on resting R340/380 or on tension at mid- (n = 5) or late (n = 5) pregnancy. In the same tissues and in the presence or absence of thapsigargin, CPA increased R340/380 by 20 ± 3 % of that induced by high (obtained prior to application of nifedipine) at mid-pregnancy and by 9 ± 3 % at term. The associated increases in tension were 11 ± 4 and 1 ± 0.3 %, respectively, of the peak tension induced by 100 mM . Neither of the pump inhibitors had a measurable effect on the increase in R340/380 or on tension in response to a first or a second application of PGF (n = 4) in the presence of nifedipine (data not shown).
We examined the possibility that Ca2+ influx through VOCCs might induce Ca2+ release from the ER (Ca2+-induced Ca2+ release, CICR). Ryanodine (2 × 10−5 M), which releases this store (Taggart & Wray, 1998), had no effect on spontaneous activity or on the responses to PGF (not shown). Caffeine (10−2 M), which also releases Ca2+ from the CICR store (Somlyo & Somlyo, 1994), did not increase R340/380 in tissues obtained at mid-pregnancy (n = 7; see example in Ca2+-free PSS, Fig. 9C) or late pregnancy (n = 5). In tissues in which calibration was successful, caffeine reduced [Ca2+]i to 17 ± 9 nM (n = 4).
Figure 9. Effect of thapsigargin and caffeine on the increase in [Ca2+]i and tension evoked by PGF in Ca2+-free solution, containing 1 mM EGTA, in myometrium obtained from a guinea-pig at mid-pregnancy.
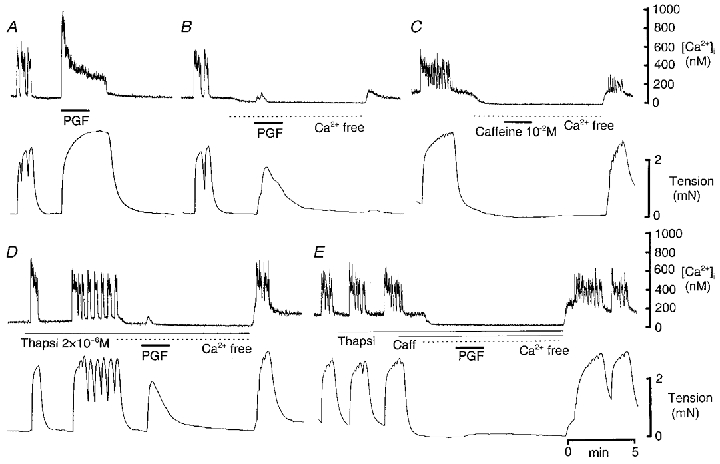
A, PGF (10−5 M) evoked the usual biphasic increase in [Ca2+]i and a large increase in tension in control solution. B, these responses were markedly reduced in Ca2+-free solution containing 1 mM EGTA. C, caffeine failed to evoke a response in Ca2+-free solution. D, thapsigargin (Thapsi) abolished the earlier component of the rise in [Ca2+]i evoked by PGF in Ca2+-free solution. E, thapsigargin plus caffeine (Caff) abolished all responses to PGF in Ca2+-free solution. Upper traces, [Ca2+]i; lower traces, tension.
In view of the fact that an initial peak in [Ca2+]i in response to PGF persisted in the presence of nifedipine and/or thapsigargin, we tested the effect of PGF on [Ca2+]i in PSS from which Ca2+ had been omitted and which contained 1 mM EGTA. Superfusion with this solution caused a prompt decline in [Ca2+]i (Fig. 9). After 2 min, and still in Ca2+-free solution, PGF elicited a brief increase in [Ca2+]i that was 24 ± 7 % of the sustained, nifedipine-resistant component of the PGF-induced increase in [Ca2+]i in the seven tissues tested at mid-pregnancy (Fig. 9B). This increase in [Ca2+]i was accompanied by an increase in tension that was 26 ± 9 % (n = 7) of that elicited in normal PSS. In four of these tissues the increase in [Ca2+]i consisted of two distinct peaks (the response in one of these tissues is illustrated in Fig. 9), and in one of the remaining three an inflection on the rise in [Ca2+]i suggested the possibility of more than one component. Inflections were apparent on the associated increases in tension (Fig. 9B). The earlier of the two peaks in [Ca2+]i was abolished when thapsigargin was included in the Ca2+-free PSS, leaving the second peak intact (Fig. 9D). All increases in [Ca2+]i were abolished in the combined presence of thapsigargin and caffeine (Fig. 9E). In three tissues examined at term, PGF did not evoke an increase in [Ca2+]i or contraction in Ca2+-free PSS (data not shown).
Sensitivity of the contractile apparatus to Ca2+
When Ca2+ in the PSS superfusing strips of myometrium was increased from 0 to 20 mM, in the presence of 100 mm and 10−6 M thapsigargin, there was a concentration-dependent increase in [Ca2+]i (Fig. 10A) and an increase in tension (Fig. 10B). The relationship between tension and [Ca2+]i in control solution, that is, in the absence of PGF, was not different at mid- (Fig. 10C) and late (Fig. 10D) pregnancy. When [Ca2+]o was raised in the presence of PGF, [Ca2+]i increased as in control solution (Fig. 10A) but the tension was dramatically increased (Fig. 10B) at both stages of pregnancy. This increase in the sensitivity of the contractile apparatus to Ca2+ in mid-pregnant tissues was unaffected by staurosporine (10−7 M) (n = 4, data not shown).
At both stages of pregnancy, equivalent tension development required a lower level of [Ca2+]i in the presence of PGF than in its absence (Fig. 10C and D). At lower [Ca2+]i, the increase in the sensitivity of the contractile apparatus to Ca2+ brought about by PGF appeared to be similar at mid- (73 ± 7 nM, n = 7) and late (117 ± 21 nM, n = 7) pregnancy (increases in tension that were 50 % of those evoked by high K+, Table 2). However, as [Ca2+]i increased, the sensitivity to Ca2+ was significantly greater in tissues obtained at mid-pregnancy (only 114 ± 8 nM Ca2+ required to achieve a level of tension that was 100 % of that in response to high ) compared with late pregnancy (203 ± 24 nM Ca2+ required to achieve a 100 % increase in tension, Table 2.
It has been suggested that arachidonic acid may contribute to the increase in the sensitivity of the contractile apparatus to Ca2+ evoked by spasmogens in some smooth muscles (Somlyo & Somlyo, 1994). Since PGF induced a greater sensitivity to Ca2+ at mid-pregnancy, arachidonic acid was tested in these tissues. The level of [Ca2+]i required to achieve tensions that were 50 % and 100 % of that evoked by high was decreased in the presence of 10−5 M arachidonic acid, but the effect was not as great as that which was achieved by PGF (Fig. 10C and Table 2).
DISCUSSION
The results of this study demonstrate that the relative importance of the mechanisms underpinning PGF-induced contraction of uterine smooth muscle changes dramatically between mid- and late pregnancy in guinea-pigs. At mid-pregnancy, PGF stimulated Ca2+ entry into the smooth muscle cells through VOCCs and through a receptor-operated mechanism, Ca2+ was released from intracellular stores, and there was an increase in the sensitivity of the contractile apparatus to Ca2+. At term, Ca2+ influx was predominantly through VOCCs, with direct receptor-mediated Ca2+ influx markedly reduced, little if any release of Ca2+ from stores, and a blunted ability of PGF to increase the sensitivity of the contractile apparatus to Ca2+.
Effects of pregnancy
It is likely that the ion channel profile in the myometrium of guinea-pigs changes between mid- and late pregnancy as indicated by the following observations. (1) The action potential in mid-pregnant tissues was complex in form, consisting of simple spikes followed by a sustained depolarization to around −25 mV, while only simple spikes occurred at term. It has been suggested previously that the plateau potential in the circular myometrium of pregnant rats reflects a weaker K+ and a stronger Ca2+ conductance (Osa & Kawarabayashi, 1977), and in that tissue there is also a switch from complex action potential to simple spikes at term (Bengtsson et al. 1984; Osa & Ogasawara, 1984). (2) A pronounced after-hyperpolarization terminated the first spike action potential at term but was never recorded in tissues obtained at mid-pregnancy. The outward K+ current density in rat myometrial cells is almost doubled in late pregnancy (Wang et al. 1998), an additional persistent K+ current is recorded during labour in human myometrial cells (Khan et al. 1993), and oestrogen, elevated in late pregnancy and labour, stimulates the appearance of a K+ current in rat (Mironneau & Savineau, 1980; Boyle et al. 1987; Toro et al. 1990) and guinea-pig (Vassort, 1975) myometrium. These currents resulted from voltage-dependent K+ (KV) channels, rather than Ca2+-activated K+ (KCa) channels. (3) Nifedipine was associated with a significantly greater membrane depolarization, by 7 mV, at mid- versus late pregnancy. Suppression of activity of KV and KCa channels has been reported for this drug, including one study involving KCa channels in rat myometrium (Miyoshi et al. 1991). (4) Significantly fewer tissues displayed spontaneous contractile activity at term, which could reflect a decline in pacemaker activity. However, it could be that the membrane potential recorded in the presence of nifedipine reflects the ‘true’ resting level and that the value recorded between action potentials at mid-pregnancy reflects the activation of KCa channels resulting from Ca2+ influx through VOCCs. In that case, a decrease in the importance of KCa with a greater reliance on KV at term (rat, Wang et al. 1998; human, Khan et al. 1993) could explain the reduced incidence of spontaneous activity, the after-hyperpolarization which terminates the action potential, the more negative membrane potential in nifedipine, and perhaps the absence of the plateau component that determines the shape of the complex action potential at this time.
Frequent spontaneous contractions have been observed universally in myometrium isolated from rats (Osa & Ogasawara, 1984), mice (Osa, 1974), rabbits (Kleinhaus & Kao, 1969), cats (Bülbring et al. 1968), sheep (Parkington, 1985), women (Word et al. 1992; Parkington et al. 1999a,b), and now guinea-pigs, during mid-pregnancy, a time when uterine smooth muscle is relatively quiescent in vivo. This suggests that endogenous inhibitory influences act to maintain a normal pregnancy but the myometrium is capable of contracting if necessary, for instance, in the event of fetal death. Myometrium isolated from guinea-pigs, sheep and women at term is more quiescent. These observations suggest that a large proportion of the VOCCs are in the closed, available state and hence can facilitate maximal Ca2+ influx upon depolarization by stimulatory hormones during labour, thus allowing greater control by these hormones.
Voltage-operated Ca2+ channels and contraction
During mid-pregnancy, PGF is capable of eliciting a substantial increase in tension, underpinned by depolarization and a sustained increase in [Ca2+]i that is independent of Ca2+ influx through VOCCs. A similar depolarization is evoked by oxytocin in rat myometrium as a result of activation of a cation channel with significant Ca2+ permeation. Ca2+ released from intracellular stores has been implicated in the activation of these channels (Arnaudeau et al. 1994). A biphasic increase in [Ca2+]i, also with a plateau to around 270 nM, is evoked by bradykinin and histamine in human airway smooth muscle (Murray & Kotlikoff, 1991). The PGF-induced depolarization and the sustained component of [Ca2+]i reported here in myometrium decreased significantly at term (Table 1). Of note is the greater effectiveness of Cd2+ in reducing the nifedipine-resistant depolarization in mid-pregnant tissues than at term (Fig. 6B), and this strengthens the notion of greater Ca2+ permeation in response to PGF in mid-pregnancy. The increased effectiveness of nifedipine at term revealed the importance of Ca2+ influx through VOCCs at this time. Again, this is very similar to observations in term human myometrium (Parkington et al. 1999a).
The endoplasmic reticulum
In the present study, the existence of a Ca2+ store that is released by PGF has been demonstrated in mid-pregnant tissues, confirming previous observations in our laboratory in which contractility alone was recorded (Coleman et al. 1988). However, thapsigargin or CPA, which prevent filling of intracellular stores, were without effect on the response to PGF, despite the ability of these agents to suppress the transient increase in [Ca2+]i by PGF in Ca2+-free PSS. CPA caused depolarization and an increase in [Ca2+]i. Thapsigargin did not induce depolarization, but observations in vascular smooth muscle raise the possibility that thapsigargin may inhibit the Ca2+ pump in only a component of the endoplasmic reticulum, unlike CPA which appears to deplete the entire store (Shima & Blaustein, 1992). Another possibility is that thapsigargin may be less effective in blocking the pump than CPA (see Taylor & Broad, 1998). It has been suggested that when the endoplasmic reticulum is replete with Ca2+, some of that Ca2+ is released into the space between the endoplasmic reticulum and the plasma membrane, where it activates KCa channels before being extruded from the cell by pumps or exchangers. Depolarization by CPA has been explained in terms of removal of this mechanism (Suzuki et al. 1992). Similar activation of Ca2+-dependent Cl− channels is also a possibility.
Ca2+ influx through VOCCs can release Ca2+ from stores in some smooth muscles, presumably via CICR (Imaizumi et al. 1998). On the one hand, caffeine, which releases this store in many tissues, abolished the transient rise in [Ca2+]i evoked by PGF in Ca2+-free solution. On the other hand, even prolonged exposure to ryanodine, which putatively releases the store involved in CICR, was entirely without effect on spontaneous or PGF-induced contraction or [Ca2+]i, and caffeine itself was ineffective in releasing Ca2+. A similar lack of effect of caffeine in rat myometrium has been interpreted in terms of the absence of CICR in this tissue (Savineau & Mironneau, 1990; Taggart & Wray, 1998). Other putative actions of caffeine, e.g. inhibition of phosphodiesterases, or inhibition of the Ins(1,4,5)P3 receptor, could account for these anomalies (Ehrlich et al. 1994). The decrease in basal tone by caffeine was preceded by a lowering of [Ca2+]i and this may reflect inhibition of phosphodiesterases.
Sensitivity of the contractile apparatus to Ca2+
A tetanic contractile response was maintained for the entire 2–5 min exposure to PGF, despite a significant decline in [Ca2+]i within approximately 1 min. An increase in the sensitivity of the contractile apparatus to Ca2+ could account for this, and a similar increase in sensitivity by stimulatory agonists has been described previously for rat uterine smooth muscle (Izumi et al. 1996; Taggart & Wray, 1998). The present work probed these effects further and extended observations to encompass a wider range of [Ca2+]i and to investigate changes in the extent of sensitization with pregnancy. The fura-2 method of estimating [Ca2+]i used in the present study is not without its pitfalls (see Hayes et al. 1996; Haworth & Redon, 1998). However, it has the advantage that (1) the tissue is studied under conditions identical to those used for electrophysiology, (2) it circumvents the possibility that permeabilizing agents might fail to reach all of the smooth muscle cells within the tissue, and (3) a not dissimilar approach has previously been used to study myometrium (Taggart & Wray, 1998). Special care was taken with these experiments, and only tissues with successful calibrations were included in this aspect of the study. Thus, while errors will be consistent within tissues and are likely to be consistent between tissues, the values of [Ca2+]i given may not represent absolute levels. Nonetheless, upon inclusion of PGF in Ca2+-free, high K+ solution there was an immediate increase in tension without any change in the 340/380 nm ratio, clearly demonstrating an increase in the sensitivity of the contractile apparatus to Ca2+ induced by PGF. A G protein appears to be involved in the sensitization of the contractile apparatus to Ca2+ in smooth muscle (Nishimura & van Breemen, 1989; Somlyo & Somlyo, 1994), including the myometrium (Izumi et al. 1996). Resistance to staurosporine in the present study suggests the involvement of a small monomeric rather than a large trimeric G protein in guinea-pig myometrium, analogous to the implication of Rho in sensitization in vascular (Hirata et al. 1992) and visceral (ileum, Itagaki et al. 1995) smooth muscles. A small monomeric G protein Rnd1, related to Rho but not possessing GTPase activity, has recently been found to suppress the sensitization evoked by carbachol in rat ileum (Loirand et al. 1999). Of interest to the present argument is the finding that the expression of mRNA for Rnd1 was increased by oestrogen and progesterone (Loirand et al. 1999). In guinea-pigs, as in women, the circulating levels of both of these steroids increase up to and during labour (Bedford et al. 1972) and hence this could account for the depressed ability of PGF to increase the sensitivity of the contractile apparatus to Ca2+ at term.
In conclusion, the results described here demonstrate that uterine contractions in response to PGF during mid-pregnancy in guinea-pigs are underpinned by a broad suite of mechanisms including Ca2+ influx through VOCCs and via receptor-operated mechanisms, release of Ca2+ from intracellular stores, and an increase in the sensitivity of the contractile apparatus to Ca2+. The receptor-operated Ca2+ mechanisms and store release decline to insignificance at term, leaving a marked reliance on Ca2+ influx through VOCCs, supported by a persistent, though blunted sensitization to Ca2+. It is suggested that this reliance on VOCCs, together with the absence of spontaneous action potential activity at term in guinea-pigs (present study) and in women (Parkington et al. 1999a) would be conducive to greater control by local or circulating hormones. Mechanisms similar to those described here in guinea-pigs are also observed in the uterus of women at term.
Acknowledgments
The authors thank Drs M. Tare and I. Wendt for discussion of the manuscript. This work was supported by the National Health and Medical Research Council of Australia.
References
- Arkinstall SJ, Jones CT. Pregnancy suppresses G protein coupling to phosphoinositide hydrolysis in guinea pig myometrium. American Journal of Physiology. 1990;259:E57–65. doi: 10.1152/ajpendo.1990.259.1.E57. [DOI] [PubMed] [Google Scholar]
- Arnaudeau S, Lepretre N, Mironneau J. Chloride and monovalent ion-selective cation currents activated by oxytocin in pregnant rat myometrial cells. American Journal of Obstetrics and Gynecology. 1994;171:491–501. doi: 10.1016/0002-9378(94)90288-7. [DOI] [PubMed] [Google Scholar]
- Ashida T, Schaeffer J, Goldman WF, Wade JB, Blaustein MP. Role of sarcoplasmic reticulum in arterial contraction: comparison of ryanodine's effect in a conduit and a muscular artery. Circulation Research. 1988;62:854–863. doi: 10.1161/01.res.62.4.854. [DOI] [PubMed] [Google Scholar]
- Bedford CA, Challis JRG, Harrison FA, Heap RB. The role of oestrogens and progesterone in the onset of parturition in various species. Journal of Reproduction and Fertility. 1972;16:1–23. [PubMed] [Google Scholar]
- Bengtsson B, Chow EMH, Marshall JM. Activity of circular muscle of rat uterus at different times in pregnancy. American Journal of Physiology. 1984;246:C216–223. doi: 10.1152/ajpcell.1984.246.3.C216. [DOI] [PubMed] [Google Scholar]
- Bieber E, Stratman T, Sanseverino M, Sangueza J, Phillippe M. Phosphatidylinositol-specific phospholipase C isoform expression in pregnant and nonpregnant rat myometrial tissue. American Journal of Obstetrics and Gynecology. 1998;178:848–854. doi: 10.1016/s0002-9378(98)70502-2. [DOI] [PubMed] [Google Scholar]
- Boyle MB, MacLusky NJ, Naftolin F, Kaczmarek LK. Hormonal regulation of K+-channel messenger RNA in rat myometrium during oestrus cycle and in pregnancy. Nature. 1987;330:373–375. doi: 10.1038/330373a0. [DOI] [PubMed] [Google Scholar]
- Bülbring E, Casteels R, Kuriyama H. Membrane potential and ion content in cat and guinea-pig myometrium and the response to adrenaline and noradrenaline. British Journal of Pharmacology. 1968;34:388–407. doi: 10.1111/j.1476-5381.1968.tb07060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challis JRG, Lye SJ. Parturition. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. 2. New York: Raven Press; 1994. pp. 985–1031. [Google Scholar]
- Coleman HA, McShane PG, Parkington HC. Gestational changes in the utilization of intracellularly stored calcium in the myometrium of guinea-pigs. The Journal of Physiology. 1988;399:13–32. doi: 10.1113/jphysiol.1988.sp017065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich BE, Kaftan E, Bezprozvannaya S, Bezprozvanny I. The pharmacology of intracellular Ca2+-release channels. Trends in Pharmacological Sciences. 1994;15:145–149. doi: 10.1016/0165-6147(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. Journal of Biological Chemistry. 1985;260:3440–3449. [PubMed] [Google Scholar]
- Haworth RA, Redon D. Calibration of intracellular Ca transients of isolated adult heart cells labelled with fura-2 by acetoxymethyl ester loading. Cell Calcium. 1998;24:263–273. doi: 10.1016/s0143-4160(98)90050-1. [DOI] [PubMed] [Google Scholar]
- Hayes A, Cody SH, Williams DA. Measuring cytosolic Ca2+ in cells with fluorescent probes: an aid to understanding cell pathophysiology. International Review of Experimental Pathology. 1996;36:197–212. [PubMed] [Google Scholar]
- Hirata K, Kikuchi A, Sasaki T, Kuroda S, Kaibuchi K, Matsuura Y, Seki H, Saida K, Takai Y. Involvement of rho p21 in the GTP-enhanced calcium ion sensitivity of smooth muscle contraction. Journal of Biological Chemistry. 1992;267:8719–8722. [PubMed] [Google Scholar]
- Imaizumi Y, Torii Y, Ohi Y, Nagano N, Atsuki K, Yamamura H, Muraki K, Watanabe M, Bolton TB. Ca2+ images and K+ current during depolarization in smooth muscle cells of the guinea-pig vas deferens and urinary bladder. The Journal of Physiology. 1998;510:705–719. doi: 10.1111/j.1469-7793.1998.705bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue Y, Sperelakis N. Gestational change in Na+ and Ca2+ channel current densities in rat myometrial smooth muscle cells. American Journal of Physiology. 1991;260:C658–662. doi: 10.1152/ajpcell.1991.260.3.C658. [DOI] [PubMed] [Google Scholar]
- Itagaki M, Komori S, Unno T, Syuto B, Ohashi H. Possible involvement of a small G-protein sensitive to exoenzyme C3 of Clostridium botulinum in the regulation of myofilament Ca2+ sensitivity in β-escin skinned smooth muscle of guinea-pig ileum. Japanese Journal of Pharmacology. 1995;67:1–7. doi: 10.1254/jjp.67.1. [DOI] [PubMed] [Google Scholar]
- Izumi H, Bian K, Bukoski RD, Garfield RE. Agonists increase the sensitivity of contractile elements for Ca2+ in pregnant rat myometrium. American Journal of Obstetrics and Gynecology. 1996;175:199–206. doi: 10.1016/s0002-9378(96)70275-2. [DOI] [PubMed] [Google Scholar]
- Khan RN, Smith SK, Morrison JJ, Ashford MLJ. Properties of large-conductance K+-channels in human myometrium during pregnancy and labour. Proceedings of the Royal Society. 1993;B 251:9–15. doi: 10.1098/rspb.1993.0002. [DOI] [PubMed] [Google Scholar]
- Kleinhaus AL, Kao CY. Electrophysiological actions of oxytocin on the rabbit myometrium. Journal of General Physiology. 1969;53:758–780. doi: 10.1085/jgp.53.6.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S, Gong ML, Somlyo AV, Somylo AP. Ca2+ channel blockers distinguish between G protein-coupled pharmacomechanical Ca2+ release and Ca2+ sensitization. American Journal of Physiology. 1991;260:C364–370. doi: 10.1152/ajpcell.1991.260.2.C364. [DOI] [PubMed] [Google Scholar]
- Loirand G, Cario-Toumaniantz C, Chardin P, Pacaud P. The Rho-related protein Rnd1 inhibits Ca2+ sensitization of rat smooth muscle. The Journal of Physiology. 1999;516:825–834. doi: 10.1111/j.1469-7793.1999.0825u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mironneau J, Savineau JP. Effects of calcium ions on outward membrane currents in rat uterine smooth muscle. The Journal of Physiology. 1980;302:411–425. doi: 10.1113/jphysiol.1980.sp013253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi H, Urabe T, Fujiwara A. Electrophysiological properties of membrane currents in single myometrial cells isolated from pregnant rats. Pflügers Archiv. 1991;419:386–393. doi: 10.1007/BF00371121. [DOI] [PubMed] [Google Scholar]
- Murray RK, Kotlikoff MI. Receptor-activated calcium influx in human airway smooth muscle cells. The Journal of Physiology. 1991;435:123–144. doi: 10.1113/jphysiol.1991.sp018501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson MT, Patlak JB, Worley JF, Standen NB. Calcium channels, potassium channels and voltage dependence of arterial smooth muscle tone. American Journal of Physiology. 1990;259:C3–18. doi: 10.1152/ajpcell.1990.259.1.C3. [DOI] [PubMed] [Google Scholar]
- Nishimura J van, Breemen C. Direct regulation of smooth muscle contractile elements by second messengers. Biochemical and Biophysical Research Communications. 1989;163:929–935. doi: 10.1016/0006-291x(89)92311-5. [DOI] [PubMed] [Google Scholar]
- Osa T. An interaction between the electrical activities of longitudinal and circular smooth muscles of pregnant mouse uterus. Japanese The Journal of Physiology. 1974;24:189–203. doi: 10.2170/jjphysiol.24.189. [DOI] [PubMed] [Google Scholar]
- Osa T, Kawarabayashi T. Effects of ions and drugs on the plateau potential in the circular muscle of pregnant rat myometrium. Japanese The Journal of Physiology. 1977;27:111–121. doi: 10.2170/jjphysiol.27.111. [DOI] [PubMed] [Google Scholar]
- Osa T, Ogasawara T. Changes in the adrenergic effects and membrane activity of the circular muscle of the rat uterus during late postpartum. Japanese The Journal of Physiology. 1984;34:113–126. doi: 10.2170/jjphysiol.34.113. [DOI] [PubMed] [Google Scholar]
- Pacaud P, Grégoire G, Loirand G. Mechanisms of Ca2+ entry into smooth muscle after Ca2+ store depletion. In: Bolton TB, Tomita T, editors. Smooth Muscle Excitation. London: Academic Press; 1996. pp. 55–62. [Google Scholar]
- Parkington HC. Some properties of the circular myometrium of the sheep throughout pregnancy and during labour. The Journal of Physiology. 1985;359:1–15. doi: 10.1113/jphysiol.1985.sp015571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkington HC, Tonta MA, Brennecke SP, Coleman HA. Contractile activity, membrane potential, and cytoplasmic calcium in human uterine smooth muscle in the third trimester of pregnancy and during labor. American Journal of Obstetrics and Gynecology. 1999a;181:1445–1451. doi: 10.1016/s0002-9378(99)70390-x. [DOI] [PubMed] [Google Scholar]
- Parkington HC, Tonta MA, Davies NK, Brennecke SP, Coleman HA. Hyperpolarization and slowing of the rate of contraction in human uterus in pregnancy by prostaglandins E2 and F2α: involvement of the Na+ pump. The Journal of Physiology. 1999b;514:229–243. doi: 10.1111/j.1469-7793.1999.229af.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendt JM, Toro L, Stefani E, Erulkar SD. Progesterone increases Ca2+ currents in myometrial cells from immature and nonpregnant adult rats. American Journal of Physiology. 1992;262:C293–301. doi: 10.1152/ajpcell.1992.262.2.C293. [DOI] [PubMed] [Google Scholar]
- Savineau JP, Mironneau J. Caffeine acting on pregnant rat myometrium: analysis of its relaxant action and its failure to release Ca2+ from intracellular stores. British Journal of Pharmacology. 1990;99:261–266. doi: 10.1111/j.1476-5381.1990.tb14691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shima H, Blaustein MP. Modulation of evoked contractions in rat arteries by ryanodine, thapsigargin and cyclopiazonic acid. Circulation Research. 1992;70:968–977. doi: 10.1161/01.res.70.5.968. [DOI] [PubMed] [Google Scholar]
- Somlyo AP, Somlyo AV. Signal transduction and regulation in smooth muscle. Nature. 1994;372:231–236. doi: 10.1038/372231a0. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Muraki K, Imaizumi Y, Watanabe M. Cyclopiazonic acid, an inhibitor of sarcoplasmic reticulum Ca2+-pump, reduces Ca2+-dependent K+ current in smooth muscle cells. British Journal of Pharmacology. 1992;107:134–140. doi: 10.1111/j.1476-5381.1992.tb14475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taggart MJ, Wray S. Contribution of sarcoplasmic reticular calcium to smooth muscle contractile activity: gestational dependence in isolated rat uterus. The Journal of Physiology. 1998;511:133–144. doi: 10.1111/j.1469-7793.1998.133bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor CW, Broad LM. Pharmacological analysis of intracellular Ca2+ signalling: problems and pitfalls. Trends in Pharmacological Sciences. 1998;19:370–375. doi: 10.1016/s0165-6147(98)01243-7. [DOI] [PubMed] [Google Scholar]
- Toro L, Stefani E, Erulkar S. Hormonal regulation of potassium currents in single myometrial cells. Proceedings of the National Academy of Sciences of the USA. 1990;87:2892–2895. doi: 10.1073/pnas.87.8.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassort G. Voltage-clamp analysis of transmembrane ionic currents in guinea-pig myometrium: Evidence for an initial potassium activation triggered by calcium influx. The Journal of Physiology. 1975;252:713–734. doi: 10.1113/jphysiol.1975.sp011167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh MP, Horowitz A, Clément-Chomienne O, Andrea JE, Allen BG, Morgan KG. Protein kinase C mediation of Ca2+-independent contractions of vascular smooth muscle. Biochemistry and Cell Biology. 1996;74:485–502. doi: 10.1139/o96-053. [DOI] [PubMed] [Google Scholar]
- Wang SY, Yoshino M, Sui JL, Wakui M, Kao PN, Kao CY. Potassium currents in freshly dissociated uterine myocytes from nonpregnant and late-pregnant rats. Journal of General Physiology. 1998;112:737–756. doi: 10.1085/jgp.112.6.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Word RA, Kamm KE, Casey ML. Contractile effects of prostaglandins, oxytocin, and endothelin-1 in human myometrium in vitro: Refractoriness of myometrial tissue of pregnant women to prostaglandins E2 and F2α. Journal of Clinical Endocrinology and Metabolism. 1992;75:1027–1032. doi: 10.1210/jcem.75.4.1400867. [DOI] [PubMed] [Google Scholar]
- Word RA, Stull JT, Casey ML, Kamm KE. Contractile elements and myosin light chain phosphorylation in myometrial tissue from nonpregnant and pregnant women. Journal of Clinical Investigation. 1993;92:29–37. doi: 10.1172/JCI116564. [DOI] [PMC free article] [PubMed] [Google Scholar]


