Abstract
Changes in muscle strength, vastus lateralis fibre characteristics and myosin heavy-chain (MyoHC) gene expression were examined in 48 men and women following 3 weeks of knee immobilization and after 12 weeks of retraining with 1866 eccentric, concentric or mixed contractions.
Immobilization reduced eccentric, concentric and isometric strength by 47 %. After 2 weeks of spontaneous recovery there still was an average strength deficit of 11 %. With eccentric and mixed compared with concentric retraining the rate of strength recovery was faster and the eccentric and isometric strength gains greater.
Immobilization reduced type I, IIa and IIx muscle fibre areas by 13, 10 and 10 %, respectively and after 2 weeks of spontaneous recovery from immobilization these fibres were 5 % smaller than at baseline. Hypertrophy of type I, IIa and IIx fibres relative to baseline was 10, 16 and 16 % after eccentric and 11, 9 and 10 % after mixed training (all P < 0.05), exceeding the 4, 5 and 5 % gains after concentric training. Type IIa and IIx fibre enlargements were greatest after eccentric training.
Total RNA/wet muscle weight and type I, IIa and IIx MyoHC mRNA levels did not change differently after immobilization and retraining. Immobilization downregulated the expression of type I MyoHC mRNA to 0.72-fold of baseline and exercise training upregulated it to 0.95 of baseline. No changes occurred in type IIa MyoHC mRNA. Immobilization and exercise training upregulated type IIx MyoHC mRNA 2.9-fold and 1.2-fold, respectively. For the immobilization segment, type I, IIa and IIx fibre area and type I, IIa and IIx MyoHC mRNA correlated (r = 0.66, r = 0.07 and r = −0.71, respectively).
The present data underscore the role muscle lengthening plays in human neuromuscular function and adaptation.
The debilitating effects of disuse on muscle strength and size are well documented. A substantial amount of skeletal muscle mass and strength is lost during joint surgery-related immobilization (MacDougall et al. 1977), unloading (Berg et al. 1991), space flight (Edgerton et al. 1995), bed rest (LeBlanc et al. 1988), myopathies (LeBlanc et al. 1988) and aging (Larsson et al. 1979). Atrophy and strength loss appear to be the greatest in the lower limb extensors (Booth, 1982) and exceed 40 % following immobilization (Sargeant et al. 1977). Reduced function may persist for months as athletes who had undergone immobilization in conjunction with knee surgery showed only 80–90 % of recovery of the operated leg 14 months after full resumption of their athletic activities (Grimby et al. 1980).
Immobilization places the limb in a passive state (Yue et al. 1997) and reduces reflex potentiation (Sale et al. 1983), EMG activity, motor unit firing rate (Duchateau & Hainaut, 1990), resting membrane potential, neurotrophic factors (Booth, 1982), and affects the mechanical properties of the muscle (Davies et al. 1987; Duchateau & Hainaut, 1987). It is still unclear whether a reduction in muscle size is due to the preferential atrophy of the fast or slow twitch muscle fibres (Appell, 1986). In vivo studies suggest that a fall in the rate of protein synthesis rather than an increase in protein degradation is the predominant mechanism of atrophy at least during the first few weeks of immobilization in rats (Booth & Seider, 1979) as well as in humans (Gibson et al. 1987).
There is a paucity of information on the effects of knee immobilization in injury-free humans and the correlated changes in biochemical and mechanical properties of skeletal muscle (Veldhuizen et al. 1993). None of the prior studies have examined the time course of recovery and the nature of the mechanical stimulus that is most effective in restoring muscle function after knee joint immobilization. In his pioneering work D. F. Goldspink (1977) demonstrated that when the extensor digitorum longus of young rats was chronically stretched while immobilized, the muscle actually underwent hypertrophy. Although this chronic stretch may not be qualitatively equivalent to dynamic muscle lengthening, there is now cumulative evidence to suggest that strength gains (Dudley et al. 1991), muscle hypertrophy (Hather et al. 1991) and myosin heavy-chain (MyoHC) gene expression are specific to the type of mechanical loading (Booth & Thomason, 1991), with greater adaptations occurring if the mechanical stimulus contains muscle lengthening or eccentric contractions compared with concentric contractions. Based on these data we hypothesized that the rate of muscle strength recovery from immobilization would be faster and that the strength gains and hypertrophy after retraining from knee immobilization would be greater when the mechanical stimulus involves eccentric contractions compared with concentric contractions. Directional changes in MyoHC gene expression specific to inactivity and contractile activity, respectively, have also been reported in animal models (Caiozzo et al. 1996a) but such data are non-existent in humans. Therefore the purpose of the present work was to examine changes in muscle strength, muscle fibre size and MyoHC gene expression after 3 weeks of knee immobilization and 12 weeks of retraining in previously sedentary young men and women.
METHODS
Subjects and design
Twenty-four men and twenty-four women (mean ±s.d. for age, height and weight: 22.0 ± 3.0 years, 1.68 ± 0.09 m and 73.0 ± 15.3 kg, respectively) volunteered for the study. Subjects were recreationally active college students who walked or bicycled to school but had not been involved in systematic physical exercise for 1 year before the study. Subjects were excluded who had had knee surgery or had had any other orthopaedic abnormalities of the lower extremities, who had hypertension (systolic and diastolic blood pressure > 140 and 90 mmHg) or smoked. Before testing, subjects read and signed a written informed consent approved by the University's Policy and Review Committee on Human Research.
Figure 1 shows the study design. The study involved two sessions of familiarization with the procedures, 3 weeks of unilateral lower extremity immobilization and 12 weeks of retraining with resistive exercise. Subjects were randomly assigned to a control group or one of three exercise groups after 3 weeks of left leg immobilization. Within the control group, three men and three women were randomly assigned to a non-immobilized and non-exercising group and another three men and three women to an immobilized and non-exercising group. Test 1 (baseline), Test 2a (after immobilization) and Test 3 (after exercise training) included unilateral strength and surface electromyography (EMG) assessment of both legs and a muscle biopsy of the left vastus lateralis. The six immobilized control subjects were allowed to spontaneously recover after the cast was removed and were tested 2 weeks (Test 2b) as well as 12 weeks (Test 3) after normal daily activity.
Figure 1. Study design.
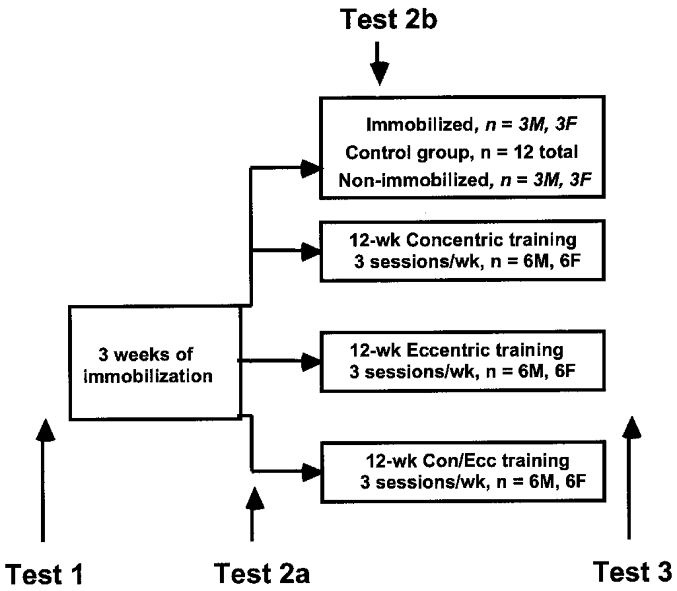
Twenty-four young male (M) and female (F) subjects were randomly assigned to a control group and three exercise groups following 3 weeks of left leg immobilization of 42 subjects. Tests 1, 2a and 3 included unilateral strength and surface electromyography assessment of both legs and muscle biopsy of the left vastus lateralis. Test 2b involved the same measurements as Test 2a but were administered in 6 subjects after 2 weeks of spontaneous recovery from immobilization. Exercising subjects performed a total of 1866 maximal quadriceps contractions over 12 weeks using 4–6 bouts of 6–12 repetitions per session.
Immobilization
The left leg was immobilized at 3 deg of knee flexion for 3 weeks with a tack-free fibreglass cast weighing a maximum of 2.2 % or 1–2 kg of body mass (Kirschner, Inc., Timonium, MD, USA). The cast was applied from just above the malleolii to just below the groin over a powdered stump sock. To relieve pressure, the cast over the patella was cut out and a felt relief pad was glued on the stump sock over the fibula head. Subjects ambulated on crutches and were required to minimize the use of the left leg. Each subject reported to the laboratory twice a week for cast inspection. To assess the magnitude of muscle activity in the cast, the EMG activity of the vastus lateralis, medialis and medial hamstring muscles of the immobilized leg was recorded in six subjects while ambulating 5 m in the laboratory. This testing was done once, on the 4th day of the week, for 3 weeks of immobilization. EMG electrodes were placed on the skin through openings cut in the cast. Data were collected for five walking trials of five steps.
Strength testing and electromyography (EMG)
Unilateral maximal voluntary isometric and isokinetic eccentric and concentric strength of the left and right quadriceps and medial hamstring were measured on a dynamometer (Kin-Com, 500H, Chattecx, Inc., Chattanooga, TN, USA). Subjects sat on the dynamometer seat with a knee and hip joint angle of ∼1.57 rad and arms folded in front of the chest. The anatomical zero was set at a knee angle of 3.14 rad. Extraneous movement of the upper body and the involved leg was limited by two crossover shoulder harnesses, a lap belt, a thigh strap and an ankle cuff. The transverse axis of the knee joint was aligned with the transverse axis of the dynamometer's power shaft. The length of the lever arm was individually determined. Force was measured by a strain gauge embedded in the ankle cuff. The force values were corrected by the software for leg mass that was measured in the horizontal position.
Familiarization with strength testing included three submaximal (25, 50 and 90 %) practice trials for each contraction mode. Maximal isometric force was measured at a knee angle of 2.36 rad. Two, maximal effort, 5 s trials were performed with 1 min of rest between trials. Maximal concentric and eccentric force of the knee extensors was measured at 1.05 rad s−1. Subjects performed two repetitions with a 1 s pause at either end of the range of motion to avoid the facilitating effects of the preceding contraction. The order of isometric versus dynamic actions and eccentric versus concentric actions was counterbalanced across subjects. The higher value of two trials was used in the analysis.
Surface EMG activity was recorded from the vastus lateralis, vastus medialis and the medial hamstring. We recorded from these synergistic vastii muscles to represent a larger portion of the quadriceps muscle. The skin surface was cleaned with alcohol. One box electrode with a built-in preamplifier (Motion Control, Inc., Salt Lake City, UT, USA), powered by 9 V batteries, was placed axially, taped and ace-bandaged on each muscle belly. The three electrodes had similar electronic characteristics: a common mode rejection ratio of 370 dB, a bandwidth of 8 Hz to 28 kHz, quiescent current of 0.12 mA and a DC input impedance of 1 MΩ.
The force and the goniometer signals from the dynamometer's A/D board and the three EMG signals were input to a digital adapter (Model 4000A, Vetter Co., Rebersburg, PA, USA) that sampled the signals at 80 MHz. The adapter was connected to a modified video recorder (JVC, HR-D86OU, Model 500C, Vetter Co.). Data from the video tape were transferred through a 12-bit A/D board (Data Translation, Model 2801A, Marlboro, MA, USA). The Myosoft software (Noraxon, Inc., Scottsdale, AZ, USA) package was used to store and digitize the data. Prior to digitization, each data file was inspected and if needed, adjusted for baseline DC shift. The root mean square (RMS) of the direct EMG data was obtained using a 20 ms smoothing window. Across all channels, the first marker was placed at peak force and a second marker was placed 250 ms before the first marker. Within this 250 ms window the highest RMS value was taken as peak EMG (μV) activity.
EMG data during gait of the immobilized leg were RMS processed and the RMS peak identified for each of the five steps. These peak values were averaged for the five steps within each trial then averaged across the five trials and expressed as a percentage of maximal EMG activity measured during isometric knee extension and flexion.
Muscle biopsy and histochemistry
Vastus lateralis samples were taken before immobilization and any strength testing, 3 h after the cast was removed, and 3 h after the last exercise bout. Immobilized and non-exercising control subjects were biopsied twice, at baseline and after the cast was removed. Non-immobilized and non-exercising controls were biopsied also twice, at baseline and 15 weeks later. Under local anaesthesia (5 ml of 1 % lidocaine (lignocaine)), a 20–120 mg sample was removed from the belly of the vastus lateralis by applying suction to a 5 mm Bergström needle. The repeat biopsy was taken 2–3 cm proximal to the previous sample at the same depth of 4–5 cm. The specimens were dissected of visible fat and connective tissue and a small section was mounted in an O.C.T. trigacanth gum mixture (Miles Inc., Elkhart, IN, USA), frozen in pre-cooled isopentane and stored in liquid nitrogen. Type I, IIa and IIx muscle fibres were determined from 10 μm sections using myosin ATPase staining at pre-incubations of pH 10.3 and 4.54 (Brooke & Kaiser, 1970). In line with the most current interpretation, what have previously been referred to as IIb muscle fibres in humans, we refer to these fibres as type IIx (Smerdu et al. 1994; Ennion et al. 1995). Fibre cross-sectional area was calculated from about 25 type I, IIa and IIx fibres by computerized digitometry (Autosketch 2.0, Sausalito, CA, USA). The other portion of the sample was immediately frozen in liquid nitrogen for RNA analysis.
RNA isolation
Total RNA was isolated with TRIzol reagent (Gibco-BRL, Gaithersburg, MD, USA) according to the manufacturer's instructions and as described previously (O'Neill et al. 1999). The frozen muscle samples were homogenized in 1 ml of TRIzol. To each sample 200 μl of chloroform were added, vortexed vigorously for 30 s and incubated at room temperature for 5 min. Samples were microfuged for 15 min (12 000 g) at 4°C and 400 μl of the top aqueous layer was transferred to a fresh microfuge tube. RNA was precipitated by the addition of an equal volume of isopropanol and incubated at room temperature for 10 min. Samples were microfuged for 10 min (12 000 g) at 4°C. RNA pellets were washed with 1 ml of 70 % ethanol. The air-dried pellets were resuspended in 50 μl of diethyl pyrocarbonate-treated water. Total RNA was expressed as μg (mg wet weight)−1 of the muscle sample.
RNase protection assay (RPA)
Type I, IIa and IIx MyoHC mRNA levels were determined by a method described previously (O'Neill et al. 1999). All samples for a subject were analysed at the same time on adjacent lanes. The cDNA probe for the DNA complementary to type I MyoHC mRNA was obtained from Kirti Bhatt (University of Rochester, Rochester; see Welle et al. 1996). The cDNA probes for type IIa and IIx MyoHC mRNA were obtained from Leslie Leiwand (University of Colorado, Boulder; see Smerdu et al. 1994). The identity and specificity of the probes were described in these papers (Smerdu et al. 1994; Welle et al. 1996). In independent experiments we also verified the RPA assay by comparing single probe hybridization with multiprobe hybridization. In these experiments the linear range of the assay for each isoform was verified by using 20 μg yeast RNA, 1, 2 and 5 μg total RNA from human muscle as well as by hybridization using double amount of full-length probes. The 20 μg yeast RNA also served as a negative control of RNase digestion in which a 1:1000 dilution of RNase A/RNase T1 mix was used (Ambion, Austin, TX, USA). The molecular size of the protected fragments and type I, IIa and IIx MyoHC full-length probes were verified by comparing them with an RNA molecular ladder (Century Size Markers, Ambion). To minimize overexposure of the autoradiograph, the amount of full-length probes loaded on the gel was equivalent to only one-twelfth of the amount used for the hybridization with the sample RNA and the radioactivity of the full-length probes on the gel was still higher than the protected RNA fragments. A total of 2 μg of RNA per sample was used in the assays.
Type I human MyoHC I plasmid was linearized with Xho I. Type IIa and IIx MyoHC plasmids were linearized with Xba I and Xho I, respectively. Labelled antisense RNA probes for type I, IIa and IIx MyoHC mRNA were synthesized using [32P]UTP and the RNA polymerase T3/T7 MAXscript in vitro transcription kit (Ambion). The T7 RNA polymerase was used to generate a human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antisense RNA probe from linearized plasmid (Ambion). The RNase protection assay was performed with a commercially available kit (RPA II, Ambion). A 1:1000 dilution of RNase A/T1 mixture (Ambion) was used for digestion. The protected RNA samples were size-fractioned on a 6 % polyacrylamide gel containing 7 M urea, visualized by autoradiography and quantified by phosphorimager analysis (Molecular Dynamics, Sunnyvale, CA, USA). Figure 2 shows a radiograph of polyacrylamide gels from a ribonuclease protection assay for type I, IIa and IIx MyoHC mRNA isoforms before and after immobilization and after exercise training.
Figure 2. Myosin heavy chain mRNA analysis.
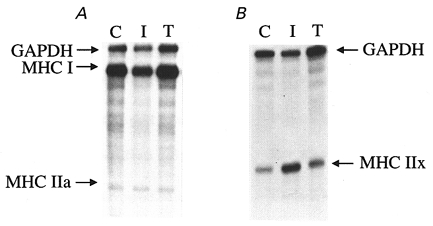
Radiograph of polyacrylamide gels from ribonuclease protection assay for type I and IIa (A) and IIx (B) MyoHC mRNAs before (C, control), after 3 weeks of leg immobilization (I) and after 12 weeks of eccentric exercise training (T). In each panel the data from one subject are shown. Vastus lateralis samples were taken 3 h after the cast was removed and 3 h after last contraction.
We expressed the MyoHC mRNA data in three independent ways. First, data were normalized relative to GAPDH mRNA. By assuming uniform loading, the changes in the GAPDH-corrected levels were computed by assigning a value of 1.0 to the before immobilization data. Second, each MyoHC mRNA isoform was also expressed relative to its baseline, uncorrected for GAPDH mRNA, and relative changes were computed. Third, type I and IIx MyoHC mRNA isoforms were normalized relative to type IIa MyoHC mRNA isoform that remained unaltered after immobilization and exercise. With either method, the MyoHC mRNA levels were expressed as dimensionless ratios.
Exercise training
Exercise training was conducted on the same dynamometer as testing was done. The training regimen consisted of 12 weeks of isokinetic concentric (CON), eccentric (ECC) or mixed (MIX) quadriceps strengthening of the left leg at 1.05 rad s−1. The subjects performed 1866 maximum effort repetitions distributed over 36 training sessions in fluctuating four to six bouts of 8–12 repetitions from week to week according to the periodization principle (Hortobágyi et al. 1996b). Maximum effort contractions were used to maximize the treatment effect. There was a 1 min rest period between bouts. Every third session the force production of each repetition was recorded and averaged to determine the rate of recovery from immobilization relative to the baseline before immobilization.
Statistical analysis
The BMDP PC-90 statistical package was used to perform all statistical data analyses. Percentage strength loss and percentage changes in the associated EMG activity were compared with a one-way analysis of variance between immobilized leg, non-immobilized leg, 2 week recovery of immobilized leg, 2 week recovery of non-immobilized leg and both legs combined of non-immobilized controls. Changes in EMG activity while immobilized, were analysed with a one-way repeated measures ANOVA for the three observations. Rate of muscle strength recovery from immobilization was compared within a group (eccentric, concentric, mixed) by time (12 weeks) ANOVA with orthogonal polynomial decomposition for linear and quadratic components. A group by contraction-mode ANOVA with repeated measures for the latter factor was used for the comparison between the three groups in percentage changes in muscle strength between pre-immobilization and after training. In the case of a significant F ratio, a Tukey's post-hoc contrast was performed to determine the means that were different at the significance level of P < 0.05.
RESULTS
Changes in strength and EMG
Figure 3 summarizes the immobilization-induced changes in muscle strength. Immobilization significantly but uniformly reduced eccentric, concentric and isometric force by −48 ± 17, −45 ± 14 and −48 ± 15 % (P < 0.05; average reduction of 47 ± 15 %). Changes in eccentric, concentric and isometric force of the non-immobilized leg in the immobilized subjects were −5 ± 9, 8 ± 14 and 3 ± 10 % (P > 0.05; average change of 2 ± 11 %). After 2 weeks of spontaneous recovery, eccentric, concentric and isometric forces of the immobilized leg were −12 ± 3, −11 ± 2 and −10 ± 2 % depressed, compared with control (P < 0.05; average change −11 ± 4 %). Over the 3 weeks, no changes occurred in eccentric, concentric or isometric forces in either leg of the non-immobilized controls and the changes for both legs and three contraction modes averaged 4 ± 11 % (P > 0.05).
Figure 3. Immobilization and muscle strength.
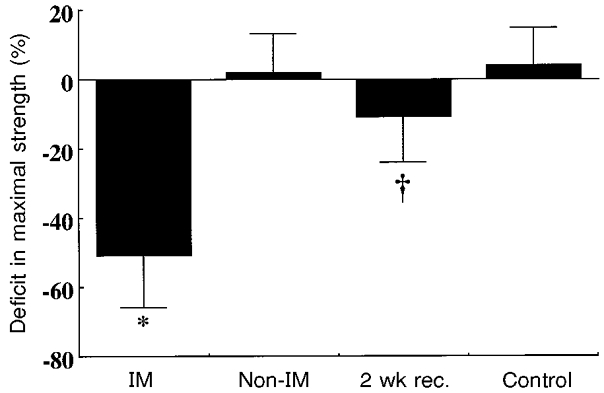
Changes in quadriceps muscle strength following 3 weeks of leg immobilization. For IM, non-IM and 2 wk rec. data are the averages of eccentric, concentric and isometric quadriceps strength, while the control data are the averages of eccentric, concentric and isometric quadriceps strength of both legs. IM, immobilized leg; Non-IM, non-immobilized leg of subjects whose other leg was immobilized; 2wk rec., muscle strength after 2 weeks of spontaneous recovery from 3 weeks of immobilization; and Control, non-immobilized and non-exercised subjects' strength data 15 weeks after an initial test. *P < 0.05 compared with all other groups and †P < 0.05 compared with non-IM and control. Vertical bars denote + or – 1 s.d.
Immobilization altered surface EMG activity of the vastus lateralis and medialis similarly and in parallel with the force data. There were no differences in the percentage changes in EMG activity associated with eccentric, concentric and isometric contractions in either muscle. The average reduction in vastus lateralis and medialis EMG activity was −38 ± 20 and −43 ± 24 % (both P < 0.05). EMG activity also recovered in parallel with strength after 2 weeks of spontaneous recovery and did not change in the non-immobilized leg or in the control subjects.
Figure 4 shows that the rate of muscle strength recovery was significantly faster and the linear polynomial component significantly greater after eccentric training and mixed training compared with training with concentric contractions alone (group by time interaction, F = 12.4, P = 0.0001; group by linear polynomial time component interaction, F = 8.7, P = 0.0001). Whereas recovery was complete by week 4 with concentric training, recovery was complete by about week 2 with eccentric and mixed training.
Figure 4. Immobilization and strength recovery.
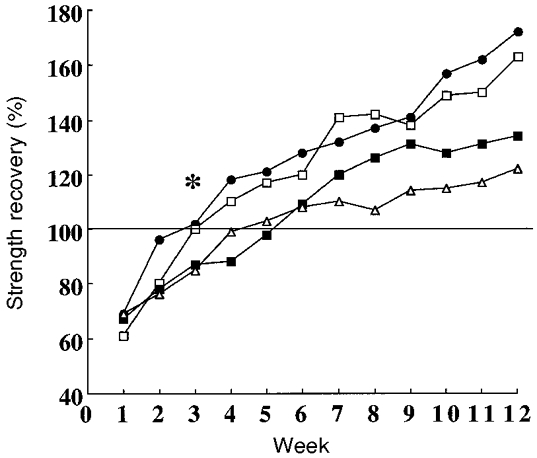
Rate of muscle strength recovery from immobilization. * Recovery occurred at a significantly faster rate at about week 2 in eccentric strength with eccentric (•) or mixed (□) training compared with the recovery of concentric strength with mixed (▪) or concentric training (▵). In this latter group recovery occurred at about week 4. The horizontal line at 100 % indicates recovery to strength levels before immobilization. Standard deviations are omitted for clarity.
Figure 5 displays the group by contraction mode ANOVA for comparison between the three training groups in percentage changes in muscle strength between pre-immobilization and after training. Eccentric training improved eccentric, isometric and concentric strength by 86, 42 and 25 % (all P < 0.05). Concentric training improved eccentric, isometric and concentric strength by 20, 31 and 44 % (the latter two P < 0.05). Mixed training improved eccentric, isometric and concentric strength by 70, 38 and 40 % (all P < 0.05). Eccentric training improved eccentric strength significantly more than concentric training increased concentric strength. The greatest percentage change in the control group was 6 % (P > 0.05). Eccentric and mixed training improved isometric strength by 52 and 46 %, significantly more than the 31 % by concentric training (P < 0.05).
Figure 5. Strength training effects after immobilization.
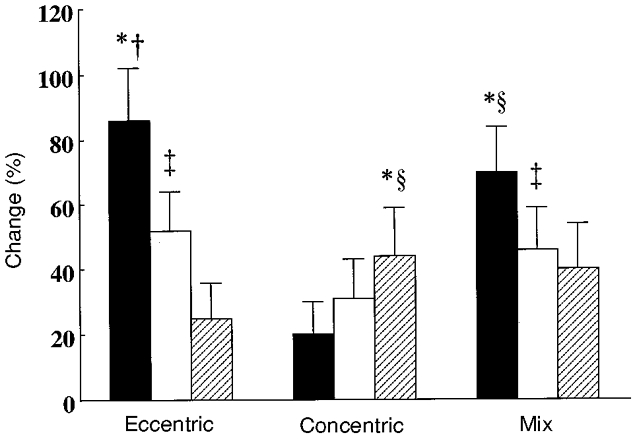
Percentage changes in eccentric (▪), isometric (□) and concentric ( ) quadriceps muscle strength following 1866 eccentric, concentric or mixed contractions for the comparisons between Tests 1 and 3 (cf. Fig. 1). * Significantly more change than other changes within the same group (P < 0.05); † significantly more change than the changes in eccentric, isometric and concentric strength in the other two groups; ² significantly more change than isometric and eccentric strength in the concentric group; ³ significantly more change than concentric strength in eccentric group. Data for the control group are not shown for clarity. Vertical bars denote +1 s.d.
) quadriceps muscle strength following 1866 eccentric, concentric or mixed contractions for the comparisons between Tests 1 and 3 (cf. Fig. 1). * Significantly more change than other changes within the same group (P < 0.05); † significantly more change than the changes in eccentric, isometric and concentric strength in the other two groups; ² significantly more change than isometric and eccentric strength in the concentric group; ³ significantly more change than concentric strength in eccentric group. Data for the control group are not shown for clarity. Vertical bars denote +1 s.d.
Changes in vastus lateralis and medialis EMG activity paralleled the changes in force. The greatest change of 67 % occurred in EMG measured during eccentric test contraction after eccentric training and the smallest of 20 % during the eccentric test contraction after concentric training.
EMG activity during locomotion while immobilized
In six subjects, vastus lateralis EMG activity of the immobilized limb while walking on crutches was 8 ± 7 %, (week 1), 6 ± 9 % (week 2) and 11 ± 15 % (week 3) of the EMG activity measured during a maximal isometric knee extension. The corresponding values for vastus medialis were 9 ± 10, 8 ± 7 and 8 ± 9 % and for the medial hamstring were 10 ± 14, 12 ± 9 and 8 ± 10 %, respectively.
Histochemistry, RNA and MyoHC mRNA levels
The total number of muscle fibres counted averaged 523 ± 67, 589 ± 88, 566 ± 58 and 612 ± 71 at Tests 1, 2a, 2b and 3, respectively. Immobilization or exercise training did not change fibre type composition differently between the groups. Figure 6 shows that, compared with baseline, immobilization significantly reduced (by 9 %) and the ensuing exercise training significantly increased (by 13 %) the percentage of type I fibres (P < 0.05). Two weeks of spontaneous activity after immobilization resulted in type I fibre percentages comparable to those observed before immobilization or after 12 weeks in control subjects. Immobilization did not affect but exercise training significantly increased by 7 % the proportion of type IIa fibres (P < 0.05). Immobilization significantly increased (by 7 %) and exercise training reduced (by 11 %) the proportion of type IIx fibres (P < 0.05). Two weeks of spontaneous activity after immobilization resulted in type IIx fibre proportions similar to those observed before immobilization or after 12 weeks in control subjects.
Figure 6. Changes in fibre type composition.
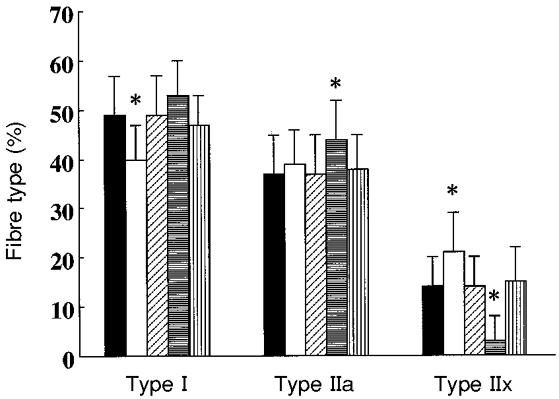
Fibre type distribution in the vastus lateralis muscle before immobilization (▪, n = 36), after 3 weeks of immobilization (□, n = 36), after 2 weeks of spontaneous recovery from immobilization ( , n = 6), after 12 weeks of knee extension exercise training (
, n = 6), after 12 weeks of knee extension exercise training ( , n = 36) and after 15 weeks of spontaneous activity (
, n = 36) and after 15 weeks of spontaneous activity ( , n = 6). * Significantly different compared with all other conditions (P < 0.05). Vertical bars denote + 1 s.d.
, n = 6). * Significantly different compared with all other conditions (P < 0.05). Vertical bars denote + 1 s.d.
Table 1 shows that 3 weeks of immobilization significantly and uniformly reduced type I, IIa and IIx muscle fibre areas by 13, 10 and 10 %, respectively. After 2 weeks of spontaneous recovery from immobilization the size of type I, IIa and IIx muscle fibres were, on average, still reduced by 5 %. Hypertrophy of type I, IIa and IIx fibres relative to baseline was 10, 16 and 16 % after eccentric and 11, 9 and 10 % after mixed training (all P < 0.05) and these gains were significantly (P < 0.05) greater than the hypertrophy after concentric training (4, 5 and 5 %). In addition, the type IIa and IIx fibres were significantly larger after eccentric than after mixed training (P < 0.05).
Table 1.
Changes in muscle fibre area
| Type I | Type IIa | Type IIx | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group | n | T1 | T2 | T3 | T1 | T2 | T3 | T1 | T2 | T3 |
| Eccentric | 12 | 42.6 | 36.0* | 46.6† | 62.4 | 56.6* | 72.2† | 54.4 | 48.7* | 63.3† |
| Mixed | 12 | 42.7 | 37.4* | 47.2† | 63.4 | 55.1* | 68.9†‡ | 55.5 | 50.6* | 60.8†‡ |
| Concentric | 12 | 42.5 | 37.3* | 44.3‡ | 61.8 | 56.2* | 65.0§ | 53.9 | 48.7* | 56.7§ |
| Control1 | 6 | 42.1 | 40.0* | — | 62.6 | 59.5* | — | 54.7 | 51.9* | — |
| Control2 | 6 | 42.9 | — | 43.9 | 62.7 | — | 62.1 | 55.5 | — | 54.3 |
Values are expressed in μm2 × 10−2. T1, baseline test; T2, test after 3 weeks of immobilization; T3, test after 12 weeks of exercise training following 3 weeks of immobilization. Control1, immobilized and non-exercising controls whose T2 occurred 2 weeks after spontaneous recovery from immobilization. Control2, non-immobilized and non-exercising controls.
Less than at T1 (P < 0.05)
greater than at T1 and T2 (P < 0.05)
greater than at T1 and T2 but less than eccentric training group (P < 0.05)
greater than at T1 and T2 but less than eccentric and mixed training groups (P < 0.05).
A sufficient amount of muscle sample was obtained to perform total RNA and MyoHC mRNA analyses in 7, 7 and 8 subjects in the eccentric, concentric and mixed training groups, respectively. No samples were obtained for RNA analyses in the control subjects. In the three training groups combined (n = 22), total RNA levels normalized for wet muscle weight were unaltered by immobilization (0.43 ± 0.15 μg (mg wet weight)−1) or subsequent exercise training (0.41 ± 0.008 μg (mg wet weight)−1) compared with baseline (0.47 ± 0.21 μg (mg wet weight)−1).
We used three different techniques to normalize MyoHC mRNA data and the interpretation of the data was identical using either normalization approach. Figure 7 shows the changes in type I, IIa and IIx MyoHC mRNA levels normalized for GAPDH mRNA levels after immobilization and subsequent exercise training. Immobilization significantly downregulated the expression of type I MyoHC mRNA to 0.72-fold of baseline and exercise training upregulated it to 0.95-fold of baseline (both changes P < 0.05). Neither immobilization nor exercise training altered the expression of type IIa MyoHC mRNA. The levels of type IIx MyoHC mRNA were 2.9-fold (±1.7) upregulated after immobilization (P < 0.05) but remained unchanged after exercise training. In agreement with previous exercise data (O'Neill et al. 1999), GAPDH mRNA levels did not change with immobilization or exercise. The percentage changes in GAPDH mRNA relative to baseline were 9 ± 6 and 12 ± 9 % after immobilization and retraining, respectively (both P > 0.05). The percentage changes in type I MyoHC mRNA relative to type IIa MyoHC mRNA were -36 ± 8 % (P < 0.05) and 2 ± 9 % (P > 0.05) and the changes in type IIx MyoHC mRNA relative to the IIa isoform were 266 ± 136 % (P < 0.05) and 25 ± 18 % (P > 0.05) after immobilization and retraining, respectively.
Figure 7. Myosin heavy chain gene expression.
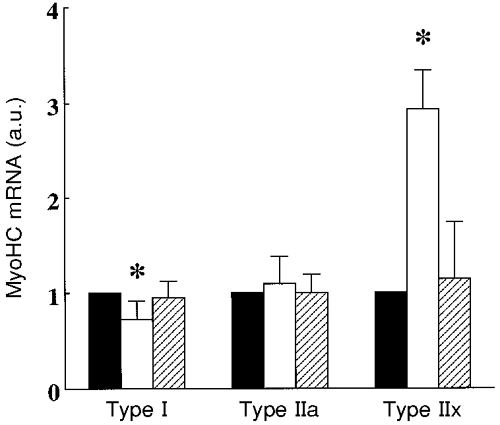
Changes in type I, IIa and IIx myosin heavy chain mRNA levels (in arbitrary units, a.u.) at baseline (▪), after 3 weeks of immobilization (□) and after 12 weeks of resistive exercise training of the human quadriceps muscle ( ). * Significantly different compared with baseline and exercise training (P < 0.05, n = 22). Vertical bars denote + 1 s.d.
). * Significantly different compared with baseline and exercise training (P < 0.05, n = 22). Vertical bars denote + 1 s.d.
Table 2 shows that the average correlation between percentage strength loss and percentage muscle fibre atrophy was r = 0.69 (P < 0.05) and it was r = 0.62 between percentage strength gain and percentage muscle fibre hypertrophy.
Table 2.
Correlations between percentage strength loss and fibre atrophy due to immobilization and between strength gains and muscle fibre hypertrophy due to exercise retraining after immobilization
| Immobilization | Exercise | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Exercise group | n | I | IIa | IIx | Average | n | I | IIa | IIx | Average |
| Eccentric | 12 | 0.66 | 0.79 | 0.77 | 0.74 | 12 | 0.65 | 0.70 | 0.77 | 0.71 |
| Mixed | 12 | 0.75 | 0.60 | 0.60 | 0.66 | 12 | 0.71 | 0.68 | 0.70 | 0.69 |
| Concentric | 12 | 0.64 | 0.63 | 0.74 | 0.68 | 12 | 0.49 | 0.21 | 0.53 | 0.42 |
| Average | 0.69 | 0.68 | 0.72 | 0.69 | 0.63 | 0.56 | 0.68 | 0.62 | ||
Strength loss is computed as the mean percentage loss in isometric, concentric and eccentric strength. Strength gain is computed as the percentage change in eccentric strength for the eccentric group, percentage change in concentric strength for the concentric group and the average percentage change in concentric and eccentric strength for the mixed group. Average correlation is computed by converting each r value to a z score, averaging these z scores and converting this average z score back to an r value. P < 0.05 for r = 0.58 (n = 12).
Percentage changes in type I, IIa and IIx fibre area were also correlated with percentage changes in type I, IIa and IIx MyoHC mRNA, respectively, by combining the data from the three training groups for the changes caused by immobilization (n = 22) and exercise (n = 22), yielding six coefficients each comprised of 22 data points. For the immobilization segment, the correlations were r = 0.66 (P < 0.05), r = 0.07 (P > 0.05) and r = −0.71 (P < 0.05) and for the exercise segment the correlations were r = 0.22, r = 0.01 and r = 0.33 (P > 0.05) between changes in type I, IIa and IIx fibre area and type I, IIa and IIx MyoHC mRNA, respectively.
DISCUSSION
The effects of knee immobilization
The present work provides new data on human muscle plasticity because in previous knee immobilization studies the magnitude of strength loss and muscle atrophy were confounded by various injuries (Sargeant et al. 1977; Grimby et al. 1980; Häggmark et al. 1981; Gibson et al. 1987). In contrast to the 10–20 % strength deficit observed in the involved leg of athletes 14 months after full resumption of activities following knee surgery and immobilization (Grimby et al. 1980), when injury-free subjects are immobilized, spontaneous activity after cast removal results in almost full recovery of leg muscles in a few weeks (current study) or even days in the arm (Yue et al. 1997). In the present work, 3 weeks of knee immobilization resulted in 47 % strength loss – similar to the 52 % reduction after 4 weeks of knee immobilization reported in the only previous work on injury-free subjects' legs (Veldhuizen et al. 1993). Since in injury-free subjects the greatest strength loss of 50 % seems to occur after knee immobilization in the quadriceps muscle and in hand muscles (Sale et al. 1983; Duchateau & Hainaut, 1987) followed by a 35 % value for arm extensors and flexors (MacDougall et al. 1977, 1980; Yue et al. 1997) and a 20 % reduction in plantarflexors (White et al. 1984), there seems to be no clear relationship between strength loss, the size of the muscle or its location.
Interestingly, 4–6 weeks of lower limb unloading reduced quadriceps strength by only 20 % (Berg et al. 1991), suggesting that, even though non-weight bearing is a common component in both models, the magnitude of strength loss is different after limb immobilization and unloading. The cast is applied to the straight knee and the quadriceps is in a shortened position that is probably a key factor in causing the 50 % strength reduction measured immediately after immobilization. In contrast, the lengthened position of the quadriceps and the allowance for joint mobility in the unloading model may alleviate many of the disuse-related effects (G. Goldspink, 1974). It is also worth noting the uniform reduction in eccentric, concentric and isometric quadriceps forces after immobilization. Because resistance of passive elements augments force production during muscle stretch and as short as 9 days of immobilization increases the quantity and stiffness of these passive elements in animal models (Williams et al. 1988), eccentric strength loss is expected to be less after immobilization compared with concentric or isometric strength losses. This clearly was not the case, suggesting possible differences in the adaptations to disuse between animal and human models related to joint position, duration of immobilization, weight bearing, tissue properties and age (Booth, 1987).
The obvious but rarely assessed cause of functional decline following immobilization is inactivity. Chronic EMG recordings of the human biceps brachii was less than 10 % of the non-immobilized biceps brachii (Yue et al. 1997). We did not measure EMG activity of the leg muscles for 12–18 h periods but EMG activity recorded once a week for 3 weeks from the immobilized thigh and hamstring muscles revealed about 10 % of maximal EMG activity. These data suggest that knee immobilization severely but not fully restricts muscle activity during locomotion. While in the cast, unintended isometric muscle tensing or reflexive postural adjustments must occur associated with balance-seeking efforts.
Even though strength loss correlated with muscle fibre atrophy (r = 0.75), the magnitude of strength loss (47 %) was almost 4-fold greater than the magnitude of fibre atrophy (11 %), suggesting that disuse must affect neural activation. Indeed, immobilization appears to make neural drive less effective to voluntarily activated skeletal muscles because in a prior study electrically evoked maximal force declined 35 % compared with a 55 % reduction in maximal voluntary force (Duchateau & Hainout, 1987). That neural factors are important in immobilization-induced strength loss is also illustrated by the observation that maximal isometric elbow extension force was reduced by 30 % without change in muscle size and that muscle proton relaxation time (T2) increased less with exercise after disuse (Yue et al. 1997). Thus, our finding of a 40 % reduction in vastus lateralis and vastus medialis EMG activity after knee immobilization is consistent with the notion that reduced muscle activation contributes to strength loss. The mechanism mediating reduced muscle activation with immobilization is unknown but a 5 mV reduction in resting membrane potential, a 60 % decline in the frequency of miniature end-plate potentials and a 25 % decrease in [3H]ouabain binding collectively indicate an impairment of sarcolemmal excitability (Zemkováet al. 1990). Central adaptations may also occur during immobilization. Transcranial magnetic stimulation in 22 patients whose ankle joints were immobilized for up to 60 weeks revealed that after only 4 weeks the cortical motor area of the tibialis anterior was reduced to 13.5 cm2 compared with 22.6 cm2 of control without altering spinal excitability or motor threshold, contrasting with the animal data of Zemkováet al. (Liepert et al. 1995).
As is the case for strength loss, limited information is available on disuse-induced muscle atrophy in injury-free human lower extremity muscles. Greater type II than type I muscle fibre atrophy occurred after elbow immobilization in the triceps brachii (30 vs. 25 %, MacDougall et al. 1980), in the vastus lateralis after an 11 day space flight (36 vs. 16 %, Edgerton et al. 1995), lower leg suspension (12 vs. 6 %, Berg et al. 1993) and knee immobilization (19 vs. 15 %, Veldhuizen et al. 1993). In contrast, others reported that atrophy was greater in type I than type II fibres (46 vs. 37 %, Sargeant et al. 1977; 26 vs. 1 %, Häggmark et al. 1981). In the present work type I and type II fibres atrophied to about the same extent, 13 and 10 %. It is interesting to note that, in general, there was a somewhat larger type II than type I fibre atrophy in injury-free subjects. The mechanism dictating the difference in fibre atrophy could be related to pain experienced by the injured but not by the injury-free subjects as moderate pain seems to inhibit the firing of low threshold motors units innervating type I muscle fibres (Häggmark et al. 1981).
Unlike the fibre size data, there is a remarkable consistency in the literature on the decrease in the percentage of type I fibres and increase in the percentage type II fibres after immobilization regardless of whether the subjects were injured (Häggmark et al. 1986) or injury-free (Veldhuizen et al. 1993). The present work is in line with previous data as the percentage of type I fibres increased by 9 % and the percentage of type IIx fibres decreased by 7 % (Fig. 6). These observations are in agreement with the hypothesis that reduced use makes muscles faster (Caiozzo et al. 1996a,b) as electrical stimulation, cross-innervation and denervation studies have consistently demonstrated that neural impulse properties determine muscle phenotype (Kraus et al. 1994).
In humans, only a few reports have been published on gene expression of contractile proteins. Growth hormone administration elevated MyoHC mRNA levels (Fong et al. 1989). MyoHC mRNA abundance was similar in young and old adults (Welle et al. 1996) whereas a mismatch between MyoHC mRNA and protein distribution was found in human skeletal muscle fibres (Andersen & Schiaffino, 1997). O'Neill et al. (1999) examined the acute effects of endurance exercise and reported 30 % downregulation of the type IIx MyoHC mRNA isoform 3 h after the 7th exercise bout without changes in the other isoforms.
The present report is the first to address changes in gene expression by systematically manipulating contractile activity through immobilization and retraining in humans. After immobilization type I MyoHC mRNA isoform was about 30 % downregulated, type IIx MyoHC mRNA was almost 300 % upregulated and type IIa MyoHC mRNA isoform was not altered. These reciprocal changes were correlated (r = 0.66, type I; r = −0.71, type IIx) with changes in fibre size, suggesting a reasonable synchronization between gene expression and MyoHC phenotype. That the relationship between gene expression and ATPase-based fibre atrophy is only moderate is most likely due to mismatch between the MyoHC mRNA and the protein distribution being augmented by the ATPase method (Andersen et al. 1997), although fibre type area and myosin heavy chain content in human skeletal muscle are highly correlated (Fry et al. 1994).
Animal data suggested marked differences in the cellular changes induced by immobilization when the muscle was in the shortened position compared with those when the muscle was in the lengthened position. Slow myosin RNA expression increased in the shortened position and decreased in the lengthened position (G. Goldspink et al. 1992). Booth & Kirby (1992) reported small changes in message levels during the early stages of atrophy after immobilization in the shortened position. They proposed that the initial changes occur at the level of translation and proteolysis and not at the level of transcription. In the present work, the quadriceps muscle was in the shortened position and the type I MyoHC mRNA expression decreased. However, the current human data are in qualitative agreement with the directional changes observed in rats exposed to microgravity where type I MyoHC mRNA levels decreased and type IIb and IIx isoforms increased (Caiozzo et al. 1996b). Unfortunately from these data we cannot determine how soon into immobilization such reciprocal changes occur, or whether such changes are long term or only transient. Although MyoHC gene expression is altered by one of two mechanisms, i.e. replacement of a downregulated isoform by another isoform and isoform co-expression, the interpretation of the present data is further complicated by the disproportionate isoform switching. In addition, MyoHC gene expression was not reciprocally altered by the ensuing exercise programme beyond control levels (see below). Clearly, additional research is needed to elucidate the time course of adaptation and the dose-response relationship of MyoHC gene expression in humans (Caiozzo et al. 1996a).
Recovery from immobilization
We are not aware of studies that examined the time course of strength recovery of lower extremity muscles from immobilization using retraining. Information on spontaneous recovery from immobilization is also scant. In the current work, resumption of spontaneous activity for 2 weeks after the cast was removed resulted in about 90 % recovery of muscle strength and 95 % recovery of muscle fibre size. In contrast, a complete recovery of elbow extensor isometric force occurred in 2 weeks after 4 weeks of elbow joint immobilization (Yue et al. 1997). Four days of uncontrolled activity after 4 weeks of lower limb unloading resulted in 90 % recovery of quadriceps strength and after 7 weeks of spontaneous activity muscle strength returned to normal (Berg et al. 1991). The strength loss in the studies of Yue et al. (1997) and Berg et al. (1991) was about half of the strength loss that occurred in the present work, so the rate of recovery appears to be related to the magnitude of strength loss but independent of whether the immobilized muscle is postural (present work and Berg et al. 1991) or non-postural (MacDougall et al. 1980; Yue et al. 1997).
There is only one study in which physical activity was used to facilitate strength recovery from immobilization (MacDougall et al. 1980). These authors reported about 100 % increase in elbow extension strength after 5–6 months of strength training subsequent to 5–6 weeks of elbow joint immobilization in three subjects. MacDougall et al. (1980) observed a few type I and IIa fibres that somehow remained atrophied after 5–6 months of retraining from immobilization. In the present work immobilization did not affect the sensitivity of the muscle to the exercise stimulus and we noticed no atrophied fibres after retraining or spontaneous recovery from immobilization. We observed a significantly faster rate of strength recovery when the exercise programme contained eccentric contractions. Muscle strength recovery after 4 weeks was complete when subjects exercised with concentric contractions whereas recovery to initial levels occurred about 2 weeks faster when pure eccentric contractions were used or added to concentric contractions (Fig. 4). Whereas athletes still show up to 20 % strength deficit after months of spontaneous recovery and weeks of exercise rehabilitation from injury-related immobilization (Grimby et al. 1980), our non-exercising controls were fully recovered after 12 weeks. Perhaps the nature and rate of recovery is different in subjects who were immobilized after an injury compared with the recovery patterns from short-term limb immobilization of initially injury-free subjects (present study and MacDougall et al. 1980).
Changes due to retraining
Not only was the rate of strength recovery faster with pure eccentric or mixed eccentric and concentric contractions but the strength gains and muscle fibre hypertrophy were also substantially greater compared with pure concentric contractions, confirming most (Komi & Buskirk, 1972; Dudley et al. 1991; Hather et al. 1991; Hortobágyi et al. 1996a, b) but not all (Jones & Rutherford, 1987; Smith & Rutherford, 1995) prior reports. The exercise training programmes were designed to maximize the treatment effect and the groups were not matched for absolute force produced during shortening and lengthening contractions. The possibility exists that the faster recovery and the greater strength gains were caused by the larger amount of work done during eccentric contractions. However, it was demonstrated that exercising at the same absolute eccentric and concentric force levels, the adaptations after eccentric training still significantly exceeded those of concentric training (Hortobágyi et al. 1996a). Smith & Rutherford (1995) also reported that perhaps metabolic cost and not high forces are involved in the stimuli for muscle hypertrophy and strength gains following high-resistance training. Finally, most recently it was shown that eccentric compared with concentric bicycle exercise training, when matched for work rate in the final phase of the programme, still resulted in substantially greater isometric strength gains (Lastayo et al. 1999). These findings suggest that faster rate of strength recovery and the greater strength gains and type II fibre hypertrophy after eccentric retraining were probably related to the unique aspects of muscle lengthening and not to 10–20 % greater eccentric compared with concentric forces during training (Hortobágyi et al. 1996b). It remains to be seen whether this unique aspect of muscle lengthening is associated with eccentric contractions, involves fatigue resistance, greater efficiency, the dissipation rather than generation of potential energy, modified actinomyosin-ATP stoichiometry, the reinforcement of the passive element scaffolding or specific motor unit recruitment.
In rodents, resistive exercise resulted in contraction-type specific effects in terms of protein synthesis and mRNA (Booth & Thomason, 1991). Even though there was some chronic effect of the 1866 eccentric, concentric and mixed contractions on MyoHC gene expression in our subjects, this effect was independent of contraction type. We are aware of only one study that examined not the chronic but the acute effects of exercise on MyoHC gene expression. O'Neill et al. reported no changes 3 h after a single 1 h bout of cycling in type I and IIa MyoHC mRNA isoforms but found a 30 % significant downregulation of the type IIx MyoHC mRNA isoform 3 h after the 7th bout of cycling without changes in the other isoforms (O'Neill et al. 1999). These data suggest that the time course of MyoHC gene expression may shift with exercise. Further, in contrast to the effects of immobilization where the changes in ATPase-based measured fibre atrophy and MyoHC mRNA levels were correlated, the changes in these two variables became uncoupled after exercise training. Such uncoupling has also been reported in a few cases in rat muscles examined under microgravity (Caiozzo et al. 1996b). The uncoupling is most likely related to temporal differences in the time course as well as in the magnitude of protein synthesis and MyoHC gene expression.
When muscle is subjected to force generation, its slow myosin genes are expressed and fast MyoHC genes are repressed (G. Goldspink et al. 1992; Caiozzo et al. 1996a,b). In contrast, when the muscle is inactive, fast MyoHC genes are expressed by ‘default’ (G. Goldspink et al. 1992), as was the case for the immobilization segment of the present work (Figs 2 and 7). The discrepancy between the current data, i.e. no change in MyoHC gene expression after exercise, and observations in different animal models is unclear. Possible explanations include a difference between the animal models and the current human data in the quantity of exercise (less work done by human subjects), the nature of muscle activation (voluntary vs. artificial), time course of gene expression and assay sensitivity due to muscle sample size. The most important of these possibilities could be the time course as the time course of adaptation in response to high-resistance exercise training in rats revealed no further alterations in any of the four isoforms examined after about 8 days of exercise (Caiozzo et al. 1996a), suggesting that steps other than transcription are more important in protein assembly under chronic exercise conditions in both human and animal models.
Acknowledgments
This work was supported in part by NICHD-30422 and Research/Creative Activity grants from East Carolina University's Faculty Senate (to T.H.) and by a DK38416 grant (L.D). We thank: Karl Tyndell of the Carolina Ortho-Prosthetics, Greenville, NC, for his initial assistance with cast preparation and mounting, and David Johnson, Kevin Jones and Scott Oaks, physical therapy students, for their technical assistance. MyoHC IIa and IIx plasmids were provided by Dr L. Leinwand (University of Colorado, Boulder) and the MyoHC I by Dr K. Bhatt (University of Rochester, Rochester).
References
- Andersen JL, Schiaffino S. Mismatch between myosin heavy chain mRNA and protein distribution in human skeletal muscle fibres. American Journal of Physiology. 1997;272:C1881–1889. doi: 10.1152/ajpcell.1997.272.6.C1881. [DOI] [PubMed] [Google Scholar]
- Appell HJ. Skeletal muscle atrophy during immobilization. International Journal of Sports Medicine. 1986;7:1–5. doi: 10.1055/s-2008-1025725. [DOI] [PubMed] [Google Scholar]
- Berg HE, Dudley GA, Häggmark T, Ohlsén H, Tesch PA. Effects of lower limb unloading on skeletal muscle mass and function in humans. Journal of Applied Physiology. 1991;70:1882–1885. doi: 10.1152/jappl.1991.70.4.1882. [DOI] [PubMed] [Google Scholar]
- Berg HE, Dudley GA, Hather B, Tesch PA. Work capacity and metabolic and morphologic characteristics of the human quadriceps muscle in response to unloading. Clinical Physiology. 1993;13:337–347. doi: 10.1111/j.1475-097x.1993.tb00334.x. [DOI] [PubMed] [Google Scholar]
- Booth FW. Effect of limb immobilization on skeletal muscle. Journal of Applied Physiology. 1982;52:1113–1118. doi: 10.1152/jappl.1982.52.5.1113. [DOI] [PubMed] [Google Scholar]
- Booth FW. Physiologic and biochemical effects of immobilization on muscle. Clinical Orthopaedics and Related Research. 1987;219:15–20. [PubMed] [Google Scholar]
- Booth FW, Kirby CR. Changes in skeletal muscle gene expression consequent to altered weight bearing. American Journal of Physiology. 1992;262:R329–332. doi: 10.1152/ajpregu.1992.262.3.R329. [DOI] [PubMed] [Google Scholar]
- Booth FW, Seider MJ. Early changes in skeletal muscle protein synthesis after limb immobilization of rats. Journal of Applied Physiology. 1979;47:974–977. doi: 10.1152/jappl.1979.47.5.974. [DOI] [PubMed] [Google Scholar]
- Booth FW, Thomason DB. Molecular and cellular adaptation of muscle in response to exercise: Perspectives of various models. Physiological Reviews. 1991;71:541–585. doi: 10.1152/physrev.1991.71.2.541. [DOI] [PubMed] [Google Scholar]
- Brooke MH, Kaiser KK. The myosin adenosine triphosphatase systems: the nature of their pH lability and sulfhydryl dependence. Journal of Histochemistry and Cytochemistry. 1970;18:670–672. doi: 10.1177/18.9.670. [DOI] [PubMed] [Google Scholar]
- Caiozzo VJ, Haddad F, Baker MJ, Baldwin KM. Influence of mechanical loading on myosin heavy-chain protein and mRNA isoform expression. Journal of Applied Physiology. 1996a;80:1503–1512. doi: 10.1152/jappl.1996.80.5.1503. [DOI] [PubMed] [Google Scholar]
- Caiozzo VJ, Haddad F, Baker MJ, Herrick RE, Prietto N, Baldwin KM. Microgravity-induced transformation of myosin isoforms and contractile properties of skeletal muscle. Journal of Applied Physiology. 1996b;81:123–132. doi: 10.1152/jappl.1996.81.1.123. [DOI] [PubMed] [Google Scholar]
- Davies CTM, Rutherford IC, Thomas DO. Electrically evoked contractions of the triceps surae during and following 21 days of voluntary leg immobilization. European Journal of Applied Physiology and Occupational Physiology. 1987;56:306–312. doi: 10.1007/BF00690897. [DOI] [PubMed] [Google Scholar]
- Duchateau J, Hainaut K. Electrical and mechanical changes in immobilized human muscle. Journal of Applied Physiology. 1987;62:2168–2173. doi: 10.1152/jappl.1987.62.6.2168. [DOI] [PubMed] [Google Scholar]
- Duchateau J, Hainaut K. Effects of immobilization on contractile properties, recruitment and firing rates of human motor units. The Journal of Physiology. 1990;422:55–65. doi: 10.1113/jphysiol.1990.sp017972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley GA, Tesch PA, Miller BJ, Buchanan P. Importance of eccentric actions in performance adaptations to resistance training. Aviation, Space and Environmental Medicine. 1991;62:543–550. [PubMed] [Google Scholar]
- Edgerton VR, Zhou MY, Ohira Y, Klitgaard H, Jiang B, Bell G, Harris B, Saltin B, Gollnick PD, Roy RR, Day MK, Greenisen M. Human fibre size and enzymatic properties after 5 and 11 days of spaceflight. Journal of Applied Physiology. 1995;78:1733–1739. doi: 10.1152/jappl.1995.78.5.1733. [DOI] [PubMed] [Google Scholar]
- Ennion S, Sant'Ana Pereira J, Sargeant AJ, Young A, Goldspink G. Characterization of human skeletal muscle fibres according to the myosin heavy chains they express. Journal of Muscle Research and Cell Motility. 1995;16:35–43. doi: 10.1007/BF00125308. [DOI] [PubMed] [Google Scholar]
- Fong Y, Rosenbaum M, Tracey K, Raman G, Hesse D. Recombinant growth hormone enhances muscle myosin heavy-chain mRNA accumulation and amino acid accrual in humans. Proceedings of the National Academy of Sciences of the USA. 1989;86:3371–3374. doi: 10.1073/pnas.86.9.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry AC, Allemeier CA, Staron RS. Correlation between percentage fibre type area and myosin heavy chain content in human skeletal muscle. European Journal of Applied Physiology and Occupational Physiology. 1994;68:246–251. doi: 10.1007/BF00376773. [DOI] [PubMed] [Google Scholar]
- Gibson JNA, Halliday D, Morrison WL, Stoward PJ, Hornsby GA, Watt PW, Murdoch G, Rennie MJ. Decrease in human quadriceps muscle protein turnover consequent upon leg immobilization. Clinical Science. 1987;72:503–509. doi: 10.1042/cs0720503. [DOI] [PubMed] [Google Scholar]
- Goldspink DF. The influence of immobilization and stretch on protein turnover of rat skeletal muscle. The Journal of Physiology. 1977;264:267–282. doi: 10.1113/jphysiol.1977.sp011667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldspink G. The influence of activity on muscle size and protein turnover. The Journal of Physiology. 1974;264:283–296. doi: 10.1113/jphysiol.1977.sp011668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldspink G, Scutt A, Loughna P, Wells DJ, Gerlach G, Jaenicke T. Gene expression in skeletal muscle in response to stretch and force generation. American Journal of Physiology. 1992;262:R356–363. doi: 10.1152/ajpregu.1992.262.3.R356. [DOI] [PubMed] [Google Scholar]
- Grimby G, Gustafsson E, Peterson L, Renström P. Quadriceps function and training after knee ligament surgery. Medicine and Science in Sports Exercise. 1980;12:70–75. [PubMed] [Google Scholar]
- Häggmark T, Eriksson E, Jansson E. Muscle fibre type changes in human skeletal muscle after injuries and immobilization. Orthopedics. 1986;9:181–185. doi: 10.3928/0147-7447-19860201-08. [DOI] [PubMed] [Google Scholar]
- Häggmark T, Jansson E, Eriksson E. Fibre type and metabolic potential of the thigh muscle in man after knee surgery and immobilization. International Journal of Sports Medicine. 1981;2:12–17. doi: 10.1055/s-2008-1034577. [DOI] [PubMed] [Google Scholar]
- Hather BM, Tesch PA, Buchanan P, Dudley GA. Influence of eccentric actions on skeletal muscle adaptations to resistance training. Acta Physiologica Scandinavica. 1991;143:177–185. doi: 10.1111/j.1748-1716.1991.tb09219.x. [DOI] [PubMed] [Google Scholar]
- Hortobágyi T, Barrier J, Beard D, Braspennincx J, Koens P, DeVita P, Dempsey L, Lambert J. Greater initial adaptations to submaximal lengthening than maximal shortening. Journal of Applied Physiology. 1996b;81:1677–1682. doi: 10.1152/jappl.1996.81.4.1677. [DOI] [PubMed] [Google Scholar]
- Hortobágyi T, Hill JP, Houmard JA, Fraser DD, Lambert NJ, Israel RG. Adaptive responses to muscle lengthening and shortening in humans. Journal of Applied Physiology. 1996a;80:765–772. doi: 10.1152/jappl.1996.80.3.765. [DOI] [PubMed] [Google Scholar]
- Jones DA, Rutherford OM. Human muscle strength training: the effects of three different regimes and the nature of the resultant changes. The Journal of Physiology. 1987;391:1–11. doi: 10.1113/jphysiol.1987.sp016721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komi PV, Buskirk ER. Effect of eccentric and concentric muscle conditioning on tension and electrical activity of human muscle. Ergonomics. 1972;15:417–434. doi: 10.1080/00140137208924444. [DOI] [PubMed] [Google Scholar]
- Kraus WE, Torgan CE, Taylor DA. Skeletal muscle adaptation to chronic low-frequency motor nerve stimulation. Exercise and Sport Sciences Reviews. 1994;22:313–360. [PubMed] [Google Scholar]
- Larsson L, Grimby G, Karlsson J. Muscle strength and speed of movement in relation to age and muscle morphology. Journal of Applied Physiology. 1979;46:451–456. doi: 10.1152/jappl.1979.46.3.451. [DOI] [PubMed] [Google Scholar]
- Lastayo PC, Reich TE, Urquhart M, Hoppeler H, Lindstedt SL. Chronic eccentric exercise: improvements in muscle strength can occur with little demand for oxygen. American Journal of Physiology. 1999;276:R611–615. doi: 10.1152/ajpregu.1999.276.2.R611. [DOI] [PubMed] [Google Scholar]
- LeBlanc A, Gogia P, Schneider V, Krebbs J, Schonfeld E, Evans H. Calf muscle area and strength changes after five weeks of horizontal bed rest. American Journal of Sports Medicine. 1988;16:624–629. doi: 10.1177/036354658801600612. [DOI] [PubMed] [Google Scholar]
- Liepert J, Tegenthoff M, Malin JP. Changes in cortical motor area size during immobilization. Electroencephalography and Clinical Neurophysiology. 1995;97:382–386. doi: 10.1016/0924-980x(95)00194-p. [DOI] [PubMed] [Google Scholar]
- MacDougall JD, Elder GCB, Sale DG, Moroz JR, Sutton JR. Effects of strength training and immobilization on human muscle fibres. European Journal of Applied Physiology and Occupational Physiology. 1980;43:25–34. doi: 10.1007/BF00421352. [DOI] [PubMed] [Google Scholar]
- MacDougall JD, Ward GR, Sale DG, Sutton JR. Biochemical adaptations of human skeletal muscle to heavy resistance training and immobilization. Journal of Applied Physiology. 1977;43:700–703. doi: 10.1152/jappl.1977.43.4.700. [DOI] [PubMed] [Google Scholar]
- O'Neill DS, Zheng D, Anderson WK, Dohm GL, Houmard JA. Effect of endurance exercise on myosin heavy chain gene regulation in human skeletal muscle. American Journal of Physiology. 1999;276:R414–419. doi: 10.1152/ajpregu.1999.276.2.R414. [DOI] [PubMed] [Google Scholar]
- Sale DG, MacDougall JD, Upton ARM, McComas AJ. Effect of strength training upon motoneuron excitability in man. Medicine and Science in Sports and Exercise. 1983;15:57–62. [PubMed] [Google Scholar]
- Sargeant AJ, Davies CTM, Edwards RHT, Maunder C, Young A. Functional and structural changes after disuse of human muscle. Clinical Science and Molecular Medicine. 1977;52:337–342. doi: 10.1042/cs0520337. [DOI] [PubMed] [Google Scholar]
- Smerdu V, Karch-Mizrachi I, Campione M, Leinwand L, Schiaffino S. Type IIx myosin heavy chain transcripts are expressed in type IIb fibres of human skeletal muscle. American Journal of Physiology. 1994;267:C1723–1728. doi: 10.1152/ajpcell.1994.267.6.C1723. [DOI] [PubMed] [Google Scholar]
- Smith RC, Rutherford OM. The role of metabolites in strength training. A comparison of eccentric and concentric contractions. European Journal of Applied Physiology and Occupational Physiology. 1995;71:332–336. doi: 10.1007/BF00240413. [DOI] [PubMed] [Google Scholar]
- Veldhuizen JW, Verstappen FTJ, Vroemen J, Kuipers H, Greep JM. Functional and morphological adaptations following four weeks of knee immobilization. International Journal of Sports Medicine. 1993;14:283–287. doi: 10.1055/s-2007-1021178. [DOI] [PubMed] [Google Scholar]
- Welle S, Bhatt K, Thorton C. Polyadenylated RNA, actin mRNA, and myosin heavy chain mRNA in young and old human skeletal muscle. American Journal of Physiology. 1996;270:E224–229. doi: 10.1152/ajpendo.1996.270.2.E224. [DOI] [PubMed] [Google Scholar]
- White MJ, Davies CT, Brooksby P. The effects of short-term immobilization on the contractile properties of human triceps surae. Quarterly Journal of Experimental Physiology. 1984;69:685–691. doi: 10.1113/expphysiol.1984.sp002860. [DOI] [PubMed] [Google Scholar]
- Williams PE, Catanese T, Lucey EG, Goldspink G. The importance of stretch and contractile activity in the prevention of connective tissue accumulation in muscle. Journal of Anatomy. 1988;158:109–114. [PMC free article] [PubMed] [Google Scholar]
- Yue GH, Bilodeau M, Hardy PA, Enoka RM. Task-dependent effect of limb immobilization on the fatigability of the elbow flexor muscles in humans. Experimental Physiology. 1997;82:567–592. doi: 10.1113/expphysiol.1997.sp004048. [DOI] [PubMed] [Google Scholar]
- Zemková H, Teisinger J, Almon RR, Vejsada R, Hník P, Vyskocyl F. Immobilization atrophy and membrane properties in rat skeletal muscle. Pflügers Archiv. 1990;416:126–129. doi: 10.1007/BF00370233. [DOI] [PubMed] [Google Scholar]


