In the CA1 region of the hippocampus pyramidal neuron basilar dendrites extend into the stratum oriens-alveus while the apical dendrites project deep into the stratum lacunosum-moleculare, a distance several hundred micrometres in extent. This extended dendritic arbor provides a large surface area for afferent input. For example, the axons of CA3 pyramidal neurons synapse onto pyramidal cell dendritic spines across ∼2/3 of the apical dendritic tree. In contrast a diverse population of local-circuit GABAergic inhibitory interneurons selectively innervate specific postsynaptic domains of principal cells (for review see Freund & Buzsaki, 1996). These cells target their axons either to the axon initial segment, somata, or proximal and distal dendrites, with each cell type implicated in a particular operational role. However, rigid classification of the numerous subpopulations has been problematic (see Parra et al. 1998 for further discussion).
One subpopulation of stratum oriens-alveus inhibitory interneurons has a somato-dendritic axis parallel to the stratum pyramidale. These cells primarily receive excitatory input from CA1 pyramidal cells (Blasco-Ibanez & Freund, 1995), i.e. they are activated in a ‘feedback’ manner. A functional demonstration of this has been provided for only one horizontally oriented cell type, the O-LM cell (Maccaferri & McBain, 1995), whose axon primarily terminates in the stratum lacunosum-moleculare on the distal dendrites of pyramidal neurons. An article by Maccaferri et al. (2000) in this issue of TheJournal of Physiology provides a detailed examination of the nature of transmission between several types of str. oriens-alveus interneurons and their pyramidal cell targets, combining paired whole cell recordings with immunolabelling and light and electron microscopy. In a technical tour de force they demonstrate that this class of interneurons with horizontal dendrites not only includes the previously described O-LM cell, but bistratified cells (O-BiCs) whose axons target pyramidal dendrites in str. oriens and radiatum and basket cells that target the soma and proximal dendrites (BCs). Both O-LM and O-BiCs are somatostatin positive while BCs are parvalbumin or CCK positive. These data strongly indicate that recurrent inhibitory input may be channelled to distinct ‘zones’ across the pyramidal cell axis depending on the inhibitory cell type activated (Fig. 1).
Figure 1. A diverse population of stratum oriens-alveus interneurons with horizontally oriented dendrites make domain specific afferent input onto CA1 pyramidal cells.
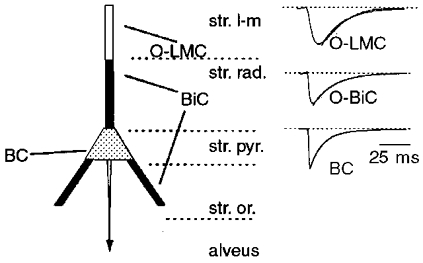
O-LMCs, BiCs and BCs target the most distal dendrites (white) the proximal apical and basilar dendrites (black) and the soma (grey) respectively. Right panels, averaged unitary IPSCs from recordings of connected pairs reveal that currents generated by O-LM cells possess slower rise times and decay time constants than IPSCs generated by O-BiCs or BCs, consistent with dendritic filtering being a major determinant of the kinetics recorded in whole-cell mode at the soma.
The kinetic properties of the somatically recorded unitary IPSCs are strongly correlated with the innervated cell surface domain suggesting that electrotonic filtering is the major determinant of the observed kinetics (Fig. 1). Consequently these data provide little information about the kinetics of transmission at the actual dendritic site; however, they do unequivocally demonstrate that inhibitory inputs arriving at the most distal dendrites could influence not only dendritic, but also somatic and axonal excitability. This is an important observation given the strict division of labour suggested by Miles et al. (1996), that interneuron axons targeted to dendrites control dendritic calcium electrogenesis and axons targeted to axons and somata control sodium-spike generation. Pearce (1993) described pharmacologically and kinetically distinct GABAA receptor-mediated IPSCs on CA1 pyramidal cells, suggesting that the molecular identity of GABAA receptors may vary between synapses made by specific interneuron subtypes. None of the IPSC kinetics generated from any interneuron subtype in this study approximated the slow IPSCs described by Pearce (1993) suggesting that the neurons responsible for these events were not among the cells tested here and remain elusive. In contrast either BCs or axo-axonic cells may generate the IPSCs with rapid kinetics described by Pearce (1993).
The degree of paired pulse depression and the postsynaptic response to a train of presynaptic action potentials differ markedly for each cell type. IPSCs generated by O-LM and O-Bi cells show no paired pulse depression and IPSCs evoked by a sustained train of action potentials show little amplitude decrement. In contrast IPSCs generated by BCs possess marked paired pulse depression and strong depression of transmission generated by a train of presynaptic action potentials. How then might this diverse class of interneurons influence their target pyramidal cells? The differential response to sustained presynaptic activity suggests that recurrent inhibition onto the soma and axon initial segment will be most effective at lower firing rates with transmission falling off at higher frequencies. In contrast rapid recurrent activation of O-LM and O-Bi cells will result in a sustained charge transfer influencing large portions of pyramidal cell dendrites.
However, in addition to IPSC properties a large array of both ligand- and voltage-gated ion channels and receptors are differentially expressed across the somato-dendritic axis. Each is capable of influencing different components of cell excitability, integration of converging signals and ultimately the efferent output of the neuron. How interneuron afferent activity interacts with this mosaic of properties remains to be tested. Nevertheless this work clearly demonstrates that recurrent inhibition may be much more complex than originally assumed.
However, in addition to IPSC properties a large array of both ligand- and voltage-gated ion channels and receptors are differentially expressed across the somato-dendritic axis. Each is capable of influencing different components of cell excitability, integration of converging signals and ultimately the efferent output of the neuron. How interneuron afferent activity interacts with this mosaic of properties remains to be tested. Nevertheless this work clearly demonstrates that recurrent inhibition may be much more complex than originally assumed.
References
- Blasco-Ibanez JM, Freund TF. European Journal of Neuroscience. 1995;7:2170–2180. doi: 10.1111/j.1460-9568.1995.tb00638.x. [DOI] [PubMed] [Google Scholar]
- Freund TF, Buzsaki G. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Maccaferri G, McBain CJ. Neuron. 1995;15:137–145. doi: 10.1016/0896-6273(95)90071-3. [DOI] [PubMed] [Google Scholar]
- Maccaferri G, Roberts JDB, Szucs P, Cottingham CA, Somogyi P. Journal of Physiology. 2000;524:91–116. doi: 10.1111/j.1469-7793.2000.t01-3-00091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles R, Toth K, Gulyas AI, Hajos N, Freund TF. Neuron. 1996;16:815–823. doi: 10.1016/s0896-6273(00)80101-4. [DOI] [PubMed] [Google Scholar]
- Parra P, Gulyas AI, Miles R. Neuron. 1998;20:983–993. doi: 10.1016/s0896-6273(00)80479-1. [DOI] [PubMed] [Google Scholar]
- Pearce RA. Neuron. 1993;10:189–200. doi: 10.1016/0896-6273(93)90310-n. [DOI] [PubMed] [Google Scholar]


