Abstract
Post-weaning Escherichia coli diarrhea (PWECD) in Ontario was investigated using a case-control study involving 50 Ontario nurseries. The clinical signs and the impact on productive parameters were determined by means of a producer survey. The hemolytic E. coli serogroups involved in PWECD (O149:K91:K88) were examined in this study. Based on a polymerase chain reaction test, the hemolytic E. coli from 82% of the case herds were positive for 3 enterotoxins (STa, STb, and LT), those from 12% of the case herds were positive for STb and LT only, and those from one herd (6%) were positive for 3 enterotoxins, as well as for verotoxin and F18 pili. The E. coli involved in disease were resistant to multiple antibiotics. Case farms commonly used a wide variety of antibiotics either in the feed or water, or as injectable drugs. The most common antibiotic used to treat PWECD on the study farms was apramycin, but evidence of resistance to this antibiotic was noted. The PWECD problem was commonly seen within a week of weaning but onset of diarrhea was reported as late as the grower-finisher stage. Growth rate was poorer in case herds and mortality was higher than in control herds, demonstrating that PWECD is an economically important disease in Ontario.
Introduction
Enterotoxigenic Escherichia coli (ETEC) strains that express K88 (F4) fimbriae are a major cause of diarrhea and death in neonatal and newly weaned pigs. Enterotoxigenic E. coli adhere to the small intestinal microvilli and produce enterotoxins that act locally on enterocytes. This action results in hypersecretion of water and electrolytes, and reduced absorption (7,18,19).
Enterotoxigenic E. coli implicated in post-weaning diarrhea in pigs most frequently produce either the K88 or F18 fimbrial adhesin (19). The most frequently observed enterotoxin combinations are LT and STb, or LT, STa, and STb (2). Isolates that are nonenterotoxigenic and induce attaching and effacing (AE) lesions are detected in about 6% of pigs with diarrhea in the post-weaning period (7).
In the fall and winter of 1997, reports of post-weaning diarrhea and mortality caused by K88-positive E. coli emerged as a concern in Ontario. Information accompanying diagnostic laboratory submissions indicated that the infection sometimes progressed so rapidly that pigs 2 to 8 wk of age were found dead before clinical signs were observed. Compared with isolations from the previous year, there was a 3-fold increase in the isolation of ETEC, specifically of O149:K88 serogroup (14,15,16). Pure cultures of the ETEC organisms were grown from small intestine swabs of pigs with post-weaning diarrhea. There have been speculations that newly emerging problems with PWECD are sometimes caused by E. coli strains that are more virulent in individual animals and more persistent within a herd than previous E. coli strains (22).
The objectives of this study were to determine the common K88-positive E. coli serogroups and pathotypes involved in PWECD problems in nursery units in Ontario, to evaluate the impact of the disease on certain productivity parameters, and to characterize the presentation of the disease on these pig farms.
Materials and methods
Selection of the farms
A total of 50 farms were visited in the summer of 1999 as part of a case-control study. Case and control herds were selected from the records of the Animal Health Laboratory, Guelph, Ontario, and with the assistance of several swine practitioners. Farms were selected based on the presence or absence of K88 E. coli and a history of diarrhea and/or sudden death in the previous year (March–December, 1998). Twenty-five farms in each category were sought in March of 1999.
A case farm met the following criteria: post-weaning pigs that had clinical signs of E. coli diarrhea and mortality as well as positive cultures of K88-positive E. coli. A farm was classified as a control if the herd did not have a history of PWECD and the disease was not diagnosed at the time of the visit. Control farms were selected from the list of herds submitting samples to the Animal Health Laboratories during the same time period as the case farms.
Survey information
Information on average daily gain (ADG), mortality, presentation and management of diarrhea problems, and antimicrobial drug usage was collected on each farm visit. Growth rate was obtained either from farm records or by subtracting the weaning weight from the weight of the pigs when they were moved out of the nursery, divided by the number of days in the nursery. Preweaning ADG was calculated by subtracting an average birth weight of 1.5 kg from the average weight at weaning, divided by the average weaning days. Data on clinical problems that have been associated with a diarrhea problem were collected in the case and control farms. A standard protocol was followed on each farm.
Bacteriology and antibiotic sensitivity
Rectal swabs of 10 weaned pigs were collected from each of the farms visited to ensure a consistency in culturing for the presence of K88-positive E. coli at the time of the visit. The samples were taken from pigs within 1 or 2 wk of weaning that showed clinical signs of diarrhea; in herds where no diarrhea was present, pigs were sampled in a random manner from the same age group. Depending on the size of the farm, 1 or 2 samples were taken from each pen. These samples were sent to Gallant Custom Laboratories in Cambridge, Ontario, where E. coli were isolated and slide agglutination tests for K88 antigen and for O and K serogroups implicated in PWECD were performed on hemolytic E. coli isolates by using standard techniques (6). Only O149:K91:K88 isolates were tested for antimicrobial sensitivity.
Diffusion sensitivity testing (Kirby-Bauer) was conducted for the following antibiotics: ampicillin, carbenicillin, cefadroxil, gentamicin, amikacin, tobramycin, enrofloxacin, neomycin, ciprofloxacin, polymyxin B, spectinomycin, sulfamethoxazole, tetracycline, ceftiofur, and apramycin. The criteria used to indicate resistance versus susceptibility were based on the proposed standards of the National Committee for Clinical Laboratory Standards.
Subsequently, blood agar plates with a single representative colony type from each of the positive farms were submitted to the Faculté de médecine vétérinaire, Université de Montréal. Isolates were tested for the presence of genes for K88 (F4) and F18 fimbriae and for toxins associated with PWECD (STa, STb, LT, and VT) by a polymerase chain reaction (PCR) technique as described previously (3) using primers for the detection of the appropriate genes (8,12,17,20,21).
Statistical analysis
The simple association between case and control herd status and putative disease factors was determined using the chi-squared test for qualitative variables and Student's t-test for quantitative variables. Numerical variables that were not normally distributed according to the Wilk-Shapiro test were tested with the Mann-Whitney test. Variables different at P ≤ 0.05 were considered significant. Variables with a P-value between 0.06 and 0.1 were considered numerically reportable as potential trends. Statistical analyses were completed by using a statistical software program (Statistix v. 1.0 for Windows; Analytical Software, Tallahassee, Florida, USA).
Results
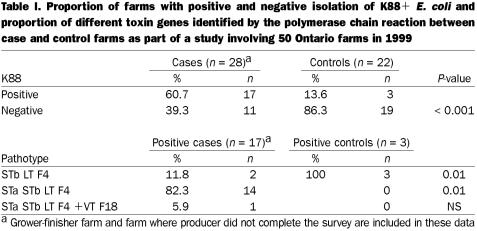
Three control herds developed diarrhea problems and were diagnosed with K88-positive E. coli prior to the farm visit. These 3 farms were considered cases. One survey from a case farm was not completed at the time of the visit. The farmer was asked to complete and return the survey but failed to do so. Another case farm was a grower-finisher operation. Both these farms were dropped from the survey analysis, although rectal swabs were taken from each farm; these were cultured, and the hemolytic E. coli isolates were serogrouped and tested for sensitivity (Table I).
Table I.
Nursery inventory ranged from 20 to 4400 (1099.2 ± 1086) pigs on case farms and from 60 to 3000 (1194 ± 926.2) pigs on control farms. The type of management system used by case and control farms included farrow-to-finish, farrow-to-partly finish, farrow-to-feeder, and off-site nurseries.
There were 28 case farms and 22 control farms in the analysis for the serogroup and antibiotic resistance study. Seventeen case farms and 3 control farms had pigs whose hemolytic E. coli were identified as O149:K91:K88. The 3 control farms that had pigs with K88- positive E. coli were left in the control group because no diarrhea was observed at the time of the study and there was no history of an E. coli-related diarrhea problem prior to the study. The PCR results are summarized in Table I. Three case farms with K88 E. coli also had isolates of serogroup O139:K82, and E. coli of serogroup O138:K81 were isolated on a case farm where K88 E. coli were not isolated at the time of the visit. Two case farms whose pigs lacked K88-positive E. coli had pigs from which VTF18-positive E. coli of serogroups O138:K81 and O139:K82 were isolated.
Case herds were more likely than control farms to have E. coli of pathotype STa STb LT (P = 0.01). The K88-positive E. coli from pigs on 3 control farms were of pathotype STb LT, and among the K88- negative control farms, 2 were eae-positive (E. coli attaching and effacing).
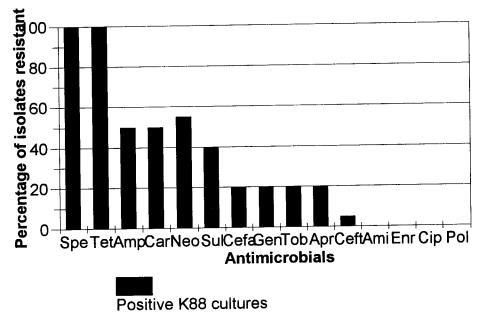
A total of 75 isolates from 20 K88-positive case farms were assayed for antibiotic sensitivity. Multiple antimicrobial resistance, as typified by resistance to at least 2 distinct antimicrobial classes, was observed in 100% of the isolates that were assayed (Figure 1).
Figure 1. Resistance patterns of 75 K88-positive isolates from 20 K88-positive farms. Results were expressed as percentage. The black bar represents the percentage of antimicrobial resistance to an specific antibiotic. The antimicrobials tested were: Spe (spectinomycin), Tet (tetracycline), Amp (ampicillin), Car (carbenicillin), Neo (neomycin), Sul (sulfamethoxazole), Cefa (cefadroxil), Gen (gentamicin), Tob (tobramycin), Apr (apramycin), Ceft (ceftiofur), Ami (amikacin), Enr (enrofloxacin), Cip (ciprofloxacin), Pol (polymyxin B).
For the survey analysis, 26 farms were designated as cases and 22 farms were designated as controls. K88 E. coli were isolated from 15 of the 26 case farms during this study.
In 23 case farms (88.5%), the diarrhea and mortality began within a week of weaning, whereas only one control farm reported severe diarrhea problems within this period (P 0.001). Three case farms (11.5%) had the worst problem of diarrhea at 2 to 3 wk after weaning, and 4 case farms (15.38%) also reported diarrhea problems in the preweaning period.
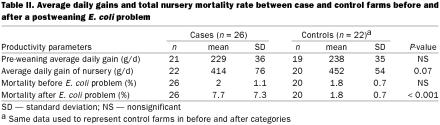
There were no significant differences in growth rate of pigs in the preweaning period between case and control farms. After weaning, growth rate in the nursery tended to be better on control farms than on case farms (452 g/d vs 414 g/d, respectively) (P = 0.07). There was no difference in mortality rate between the 2 groups before the E. coli outbreak occurred. After the disease outbreak, the mortality in case farms (7.7%) was higher than on control farms (1.8%) (P 0.001) (this P value was obtained from the Mann-Whitney test). The mean mortality in case herds ranged from 0.5% to 10–30% (Table II).
Table II.
The prevalence of clinical signs associated with an outbreak of PWECD in a case farm were: sudden death (84.6%), watery diarrhea (73%), a thin or unthrifty appearance (50%), vomition (19.2%), and dehydration (77%). Other observations reported by the farmers were: pigs with purple coloration of the body before death, and the impression that certain pens were more frequently affected than other pens.
Management and feed changes to control diarrhea problems were more commonly reported in case farms than in control farms (P 0.001). The most common management changes were: increased age at weaning, better control of temperature and ventilation, creation of sick pens, decreased density in pens, and reduced mixing of pigs. The most common feed changes reported were: purchasing feed from a different supplier, changing the feed medication, offering a limited amount of feed 4 or 5 times per day, blending of feed between phases, adding high levels of zinc oxide, decreasing the level of protein, and increasing the level of fibre.
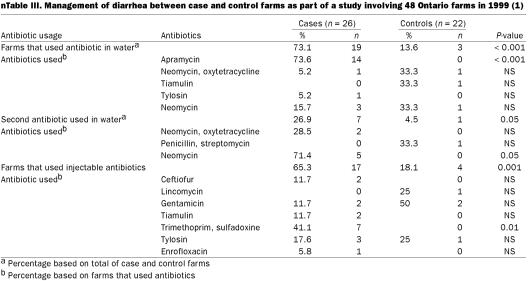
Case farms commonly switched feed antibiotics when diarrhea problems were present (46.2%). The most common antibiotics used on farms that had switched in-feed antibiotics were: carbadox (30.8%), chlortetracycline (23%), and a combination of chlortetracycline, sulfamethazine, and procaine penicillin (15.4%). However, significant differences were not found between the use of these antibiotics and the ones used on control farms. Case farms were more likely to use antibiotics in water (73.1%) than control farms (13.6%) (P 0.001), with apramycin being the first water medication of choice in case farms. Case farms were more likely to change to a second choice of antibiotic in water when no results were observed with the first choice of antibiotic. Neomycin was the antibiotic of choice in these cases (P = 0.05) (Table III).
Table III.
Case farms were more likely to use injectable antibiotics on individual pigs than were control farms (63.4 and 18.2%, respectively) (P = 0.001). The injectable antibiotic most commonly used to treat PWECD was trimethoprim with sulfadoxine (41.2%) (P = 0.01) (Table III).
The use of E. coli vaccines in nursery pigs, probiotics, and acidifiers in water or feed were other forms of prevention employed by a few of the case farms. Sanitizers in water were used on 19.2% of the case farms and just in one (4.5%) of the control farms. More case farms (61.5%) used high levels of zinc oxide (≥ 2.5 kg/T) than control farms (36.4%) (P = 0.08). Case farms (42.3%) tended to be more likely to use electrolytes for nursery pigs than control farms (18.2%) (P = 0.07) (Table IV).
Table IV.
Discussion
This study demonstrated that PWECD is an economically important disease in pigs. Farmers reported that, on average, mortality increased from 2% to 7% following an outbreak of PWECD, and some farms reported having had the problem for more than one year. If it is assumed that a newly weaned pig is worth approximately $40, then this level of mortality in a 500-sow herd would equal a loss of about $20 000 annually. However, some farms experienced mortalities as high as 20–30%, over a 1- to 2-month time span. Average daily gain (ADG) tended to be lower on farms with the problem compared with the farms that did not have the disease (452 g/d and 414 g/d, respectively). Similar decreases in growth rates and mortality were reported by Josephson et al (14). In addition, case farms used more antibiotics and other treatments such as vaccines, acidifiers, probiotics, high levels of zinc oxide, and injection of individual pigs, which reflect an increased production cost and labor cost.
In this study, it was noted that PWECD occurred most commonly in the first week after weaning, but in agreement with Fairbrother (7), the disease was also observed to affect pigs after 2 to 3 wk following weaning, or in at least one case diarrhea occurred following the transfer of pigs to the grower-finisher unit.
It has been reported that problems of PWECD occur sporadically. The findings in this study are consistent with this observation as case farms with a recent history of PWECD were sometimes free of clinical signs at the time of the survey and K88-positive E. coli were not isolated. Producers have often experienced short periods of success in combating the disease, only to have the disease reappear a few weeks later. The clinical signs observed by the farmers in this study are similar to those described elsewhere (1,7,10).
The O149:K91:K88 ETEC E. coli strains were the most commonly isolated in pure cultures from pigs with PWECD problems. This agrees with a previous report (13). The O139:K82 serogroup was isolated from some farms, and this is the serogroup most commonly isolated from cases of edema disease (9). The most frequently observed enterotoxin combination was STa, STb, LT, which agrees with reports made by Celemin et al (2). The emergence of this E. coli with 3 enterotoxins has prompted Fairbrother (7) to suggest this may indicate the emergence of a new and more virulent pathotype. The combination of just 2 toxins (STb, LT) was observed less commonly in case farms, but was present on the 3 positive control farms, where clinical problems were not apparent. The presence of multiple adhesins and toxins was observed in at least one farm which had a serious diarrhea problem (STa, STb, LT F4 and VT+F18). Nagy and Fekete (19) reported that some ETEC strains may produce more than one adhesin factor. Other authors have suggested that the presence of verotoxin (VT), specifically VT2e, is associated with edema disease, but can play a role in PWECD (7,10,18). Attaching-effacing isolates were found in pigs from 2 control farms, but were not associated with a diarrhea problem.
In agreement with other studies (1,5,7,18) ETEC isolates from pigs with post-weaning diarrhea showed a high frequency of resistance to multiple antibiotics. Increased risk of resistance among E. coli has been associated with the use of various antimicrobials (1,5). The use of various antibiotic drugs via feed, water, or injection was a common finding on farms that had PWECD problems.
More antibiotics were used on case farms, so it is not surprising that the E. coli on case farms showed more resistance in general. Antibiotics appear to be a short term solution and as farmers move from one antibiotic to the next, as illustrated in this study, antibiotic resistance is likely to develop.
Dunlop et al (4) reported that, in Canada, the medications most commonly added to creep and starter rations were a combination of chlortetracycline-sulfamethazine-penicillin, carbadox, and tylosin plus furazolidone. In agreement with Dunlop's findings, except for furazolidone, which has been withdrawn as an approved in-feed medication for swine rations, farms in this study tended to use these antimicrobial drugs as growth promoting agents and as therapeutic drugs to treat diarrhea.
According to Fairbrother (7), apramycin remains the antimicrobial of choice for use in water. In the Fairbrother study, most isolates showed sensitivity to apramycin and amikacin. In this present study, apramycin was a commonly used treatment and the development of resistance was evident (23.5% of the E. coli isolates). In contrast, no resistance was observed for amikacin (a drug not used in swine production) in any of the isolates from this study. In a survey study developed in the United Kingdom (11), a high level of resistance was also found to apramycin in pigs of all ages and in humans, including hospital patients and one pig worker. Also, apramycin-resistant E. coli were found to persist in a dry environment in a pig pen that had been empty for 10 mo (11). Resistance to apramycin provides cross-resistance with other aminoglycosides, such as gentamicin and tobramycin (11), as well as neomycin, which makes this drug a curious second choice on farms that have switched from apramycin. There were some other antibiotics included in the study which are not commonly used in the swine industry; however, it is interesting to note the resistance patterns because of their relevance in human medicine.
This was the first reported study of PWECD in Ontario. The disease appears to be of economic significance and poses a challenge as far as control. The common approach of treating PWECD with mass antibiotic medication will likely lead to a build up of antibiotic resistance. There is a need to conduct more carefully designed and evaluated field trials with controls to examine the true efficacy of the many treatments and control measures instituted on case farms as revealed by this study. The E. coli involved in PWECD tend to persist in spite of attention to the usual management, environmental and hygiene factors.
Footnotes
Acknowledgments
We would like to thank Ontario Pork and the Ontario Ministry of Agriculture, Food and Rural Affairs for funding this project, and all the producers and practitioners who participated in this project. We would also like to acknowledge the assistance of Jackie Gallant, Dr. Gaylan Josephson, Dr. Beverly McEwen, and Dr. Steve Wolfgram.
Address correspondence and reprint requests to Dr. Robert Friendship, telephone: 519-824-4120 ext. 4022; fax: 519-763-8621; e-mail: rfriends@ovc.uoguelph.ca
Received August 9, 2001. Accepted January 7, 2002.
References
- 1.Bertshinger HU. Post-weaning Escherichia coli diarrhea and edema disease. In: Straw B, D'Allaire S, Mengeling W, Taylor D. Disease of Swine. 8th ed. Ames, Iowa: Iowa State University Press, 1999:441–54.
- 2.Celemin C, Rubio P, Echeverria P, Suarez S. Gene toxin patterns of Escherichia coli isolated from diseased and healthy piglets. Vet Microbiol 1995;45:121–127. [DOI] [PubMed]
- 3.Desrosiers A, Fairbrother JM, Johnson RP, Desautels C, Letellier A, Quessy S. Phenotypic and genotypic characterization of Escherichia coli verotoxin-producing isolates from humans and pigs. J Food Prot 2002. In press. [DOI] [PubMed]
- 4.Dunlop RH, McEwen SA, Meek AH, Black WD, Clarke RC, Friendship RM. Individual and group antimicrobial usage rates on 34 farrow-to-finish swine farms in Ontario, Canada. Prev Vet Med 1998;34:247–264. [DOI] [PubMed]
- 5.Dunlop RH. Antimicrobial treatments and antimicrobial resistance of fecal Escherichia coli of swine in Ontario, Canada [PhD thesis]. Guelph, Ontario. Univ of Guelph, 1996:275–285.
- 6.Edwards PR, Ewing WH: The genus Escherichia. In: Identification of Enterobacteriaceae. 3rd ed. Minneapolis, Minnesota: Burgess Publishing, 1972:67–107.
- 7.Fairbrother JM. Identification, nomenclature, and diagnosis of pathogenic Escherichia coli. Proc Ann Meet West Can Assoc Swine Pract. Saskatoon, Saskatchewan, 1999:21–31.
- 8.Furrer B, Candrian U, Lüthy J. Detection and identification of E. coli producing heat-labile enterotoxin type I by enzymatic amplification of a specific DNA fragment. Lett Appl Microbiol 1990;10:31–34. [DOI] [PubMed]
- 9.Gyles C. Escherichia coli in diseases of weaned pigs: Biological aspect. In: Enteric Diseases of Nursery Pigs. Proc Ann Meet Am Assoc Swine Pract, 2001:29–41.
- 10.Hampson DJ. Post-weaning Escherichia coli diarrhea in pigs. In: Gyles CL, ed. Escherichia coli in domestic animals and humans. London: CABI, 1994:171–191.
- 11.Hunter J. Some studies on multiple resistant E. coli and the use of antibiotic in the treatment of diarrhea in pigs. Pig Vet J 1993;31:143–151.
- 12.Imberechts H, De Greve H, Bouchet H, et al. Characterization of F107 fimbriae of Escherichia coli 107/86, which causes edema disease in pigs, and nucleotide sequence of the F107 major fimbrial subunit gene, fedA. Infect Immun 1992;60:1963–1971. [DOI] [PMC free article] [PubMed]
- 13.Josephson G, Archambault M. Colibacillosis in pigs in 1999. Animal Health Laboratory Newsletter. Guelph: University of Guelph, 2000;4:8.
- 14.Josephson G, Smart N, McEwen B., Gough J. K88+ E. coli diarrhea in post weaning pigs. Guelph: Animal Health Laboratory, University of Guelph. 18th Annu Centralia Swine Res Update, 1999:38–39.
- 15.Josephson G, Smart N. K88 strains of E. coli. Animal Health Laboratory Newsletter. Guelph: University of Guelph, 1998a;2:4.
- 16.Josephson G, Smart N. Update on K88 positive E. coli. Animal Health Laboratory Newsletter. Guelph: University of Guelph, 1998b;2:2.
- 17.Lortie LA, Dubreuil JD, Harel J. Characterization of Escherichia coli strains producing heat-stable enterotoxin b (STb) isolated from humans with diarrhea. J Clin Microbiol 1991;29:656–659. [DOI] [PMC free article] [PubMed]
- 18.Mackinnon JD. Enteritis in the young pig caused by Escherichia coli. Pig Vet J 1999;41:227–255.
- 19.Nagy B, Fekete PZ. Enterotoxigenic Escherichia coli (ETEC) in farm animals. Vet Res 1999;30:259–284. [PubMed]
- 20.Ojeniyi B, Ahrens P, Meyling A. Detection of fimbrial and toxin genes in Escherichia coli and their prevalence in piglets with diarrhea. The application of colony hybridization assay, polymerase chain reaction and phenotypic assays. Zentralbl Veterinarmed (J Vet Med) B 1994;41:49–59. [DOI] [PubMed]
- 21.So M, McCarthy BJ. Nucleotide sequence of the bacterial transposon Tn1681 encoding a heat-stable (ST) toxin and its identification in enterotoxigenic Escherichia coli strains. Proc Natl Acad Sci USA 1980;77:4011–4015. [DOI] [PMC free article] [PubMed]
- 22.Wood EN. Fashionable and future diseases. Vet J 1991;27: 193–197.