Abstract
Cytochemical and in vitro whole-cell patch clamp techniques were used to investigate granule cell hyperexcitability in the dentate gyrus 1 week after fluid percussion head trauma.
The percentage decrease in the number of hilar interneurones labelled with either GAD67 or parvalbumin mRNA probes following trauma was not different from the decrease in the total population of hilar cells, indicating no preferential survival of interneurones with respect to the non-GABAergic hilar cells, i.e. the mossy cells.
Dentate granule cells following trauma showed enhanced action potential discharges, and longer-lasting depolarizations, in response to perforant path stimulation, in the presence of the GABAA receptor antagonist bicuculline.
There was no post-traumatic alteration in the perforant path-evoked monosynaptic excitatory postsynaptic currents (EPSCs), or in the intrinsic properties of granule cells. However, after trauma, the monosynaptic EPSC was followed by late, polysynaptic EPSCs, which were not present in controls.
The late EPSCs in granule cells from fluid percussion-injured rats were not blocked by the NMDA receptor antagonist 2-amino-5-phosphonovaleric acid (APV), but were eliminated by both the non-NMDA glutamate receptor antagonist 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) and the AMPA receptor antagonist GYKI 53655.
In addition, the late EPSCs were not present in low (0·5 mM) extracellular calcium, and they were also eliminated by the removal of the dentate hilus from the slice.
Mossy hilar cells in the traumatic dentate gyrus responded with significantly enhanced, prolonged trains of action potential discharges to perforant path stimulation.
These data indicate that surviving mossy cells play a crucial role in the hyperexcitable responses of the post-traumatic dentate gyrus.
The dentate gyrus is known to be preferentially sensitive to traumatic head injury both in humans and in experimental animals (Margerison & Corsellis, 1966; Bruton, 1988; Lowenstein et al. 1992; Toth et al. 1997). The dentate gyrus plays a central role in regulating the entorhino-hippocampal interplay (Amaral, 1978; Buzsáki et al. 1983); therefore, it is likely that alterations in the dentate neuronal circuitry contribute to the post-traumatic neurological disturbances affecting a large number of head-injured patients (Annegers et al. 1980; Salazar, 1992). However, the exact consequences of the loss, or the survival with modified functional properties, of the various GABAergic and glutamatergic components of the neuronal network of the dentate gyrus are not understood.
The glutamatergic mossy cells of the dentate hilus provide an important recurrent excitatory feedback circuit to granule cells, and mossy cells also excite GABAergic interneurones (Amaral, 1978; Frotscher et al. 1991; Buckmaster et al. 1992; Soltesz et al. 1993; Buckmaster & Schwartzkroin, 1994; Soriano & Frotscher, 1994; Scharfman, 1995; Jackson & Scharfman, 1996). Mossy cells are believed to be one of the most vulnerable types of neurone in the mammalian brain to various forms of injury, including epilepsy, ischaemia and traumatic head injury (for reviews, see Buckmaster & Schwartzkroin, 1994; Sloviter, 1994). The alleged selective vulnerability of mossy hilar cells led to the suggestion that the selective loss of these excitatory hilar cells is one of the key factors in hyperexcitability (Sloviter, 1991). However, surprisingly little direct evidence is available regarding the preferential loss of mossy cells (with respect to GABAergic hilar cells) following various insults, mostly because, until recently (Leránth et al. 1996), there has been no specific immunocytochemical marker for mossy cells. In addition to the paucity of information regarding the relative degree of the post-insult loss of mossy cells, the role of the surviving mossy cells in the excitatory circuitry of the dentate gyrus following injury is not known.
This study was carried out to determine key synaptic, cellular factors within the dentate gyrus that contribute to altering the way granule cells respond to perforant path activation following traumatic brain injury in rats. Brain trauma was modelled by the controlled, rapid delivery of a single, moderate (2.0–2.2 atm) pressure wave transient via a jet of fluid onto the neocortex of anaesthetized rats (fluid percussion injury; FPI) (Dixon et al. 1989; McIntosh et al. 1989). The data show that surviving mossy hilar cells following FPI play important roles in producing abnormal discharge patterns in granule cells. Therefore, the results suggest that not only the loss, but also the survival of mossy hilar cells may contribute to the major consequences of head trauma, including the development of post-traumatic seizures and memory deficits.
METHODS
All procedures described in this study were approved by the University of California, Irvine Institutional Animal Care and Use Committee.
Lateral fluid percussion injury
The lateral fluid percussion technique was carried out as described previously (Dixon et al. 1989; McIntosh et al. 1989; Lowenstein et al. 1992; Toth et al. 1997). Briefly, adult (200 g) male Wistar rats were anaesthetized with Nembutal (65 mg kg−1i.p.; adequate anaesthesia was repeatedly ascertained by the lack of the ocular reflex and by the absence of a withdrawal response to a pinch of the hindlimb), placed in a stereotaxic frame, and the scalp sagittally incised. A 2 mm hole was trephined to the skull at −3 mm (i.e. caudal) from bregma, 3.5 mm lateral from the sagittal suture. Two steel screws were placed 1 mm rostral to bregma and 1 mm caudal to lambda. A Luer-Loc syringe hub with a 2.6 mm inside diameter was placed over the exposed dura and bonded to the skull with cyanoacrylate adhesive. Dental acrylic was poured around the injury tube and skull screws, and allowed to harden, and the scalp was sutured. Bacitracin was applied to the wound, and the animal was returned to its home cage. A day later, the rats were anaesthetized with halothane in a 2 l chamber (surgical level of anaesthesia was ascertained as described above), and subsequently removed from the anaesthetizing chamber and immediately connected to the injury device (see below). The establishment of the connection to the device took 5 s, and the actual injury (release of the pendulum) took another 5 s; therefore, the animal was fully anaesthetized at the time of injury, even though the halothane anaesthesia was not actively administered at the time of injury. All animals were immediately ventilated with room air. The animals were injured by a moderate (2.0–2.2 atm (∼202–222.2 kPa)) impact. The moderate level of injury was selected to produce neuronal degeneration in the dentate gyrus similar to that reported previously (Lowenstein et al. 1992; Toth et al. 1997). Age-matched, sham-operated control animals were treated in the same way, including the connection to the FPI device, but the pendulum (see below) was not released. The animals recovered fully from anaesthesia within 10–15 min, and their subsequent behaviour, such as feeding and grooming, was normal. After survival periods of 1 week or 5 months, the animals were deeply anaesthetized with Nembutal (65 mg kg−1i.p.) and killed either by decapitation for slice physiology, or by transcardial perfusion of fixative for morphological studies (see below). For the experiments involving recordings from mossy cells in the hilus with infrared video microscopy-aided patch clamp techniques (see below), younger animals (postnatal day (P)20-P25) were used to allow visual identification of the neurones. Experiments carried out with the ‘dark neurone’ histological silver staining method of Gallyas (e.g. Toth et al. 1997) unequivocally determined that the pattern of neuronal damage in these younger animals (i.e. the hilar cells, and interneurones in the granule cell layer, were darkly stained, indicating injury, whereas the granule cells were unstained; data not shown) was identical to that seen in more mature animals (Toth et al. 1997).
The fluid percussion device (Department of Biomedical Engineering, Virgina Commonwealth University, Richmond, VA, USA) was identical to that used by several other laboratories (Dixon et al. 1989; McIntosh et al. 1989; Lowenstein et al. 1992; Povlishok et al. 1994; Coulter et al. 1996) and previously by us (Toth et al. 1997). Briefly, the device consisted of a Plexiglass cylinder reservoir, 60 cm long and 4.5 cm in diameter. At one end of the cylinder a rubber-covered Plexiglass piston was mounted on O rings. The opposite end of the cylinder had an 8 cm long metal housing that contained a transducer. Fitted at the end of the metal housing was a 5 mm tube with a 2 mm inner diameter, which terminated in a male Luer-Loc fitting. This fitting was then connected to the female fitting, which had been chronically implanted. The entire system was filled with saline. The injury was produced by a metal pendulum striking the piston of the injury device. The resulting pressure pulse was recorded extracranially by a transducer and expressed in atmospheres. This injury device injected a small volume of saline into the closed cranial cavity and produced a brief (20 ms) displacement and deformation of brain tissue. The magnitude of injury was controlled by varying the height from which the pendulum was released (in these experiments, it was 12–13.5 deg, which produced a 2.0–2.2 atm pressure wave). The delivery of the pressure pulse was associated with brief (less than 120–200 s), transient traumatic unconsciousness (as assessed by the duration of post-impact immobility and the suppression of the withdrawal reflex; in contrast, control animals started to spontaneously move around in the cage, and showed vigorous withdrawal responses, within 30 s following sham injury). Although the injured animals in this study, subjected only to moderate impacts, did not exhibit any obvious lasting behavioural deficit or seizure activity, fluid percussion head injury, especially at higher impact forces, can lead to detectable motor and memory deficits lasting for days (McIntosh et al. 1989; Povlishock et al. 1994). Before and during each experiment, great care was taken to ensure that no air bubble was trapped or formed in the device. Furthermore, in each experiment the pressure wave was closely examined on the oscilloscope for any sign of a jagged rising edge (which would indicate the presence of air bubbles in the system), and the amplitude of the oscilloscope reading of the pressure wave was recorded.
Slice preparation
Brain slices were prepared as previously described (Soltesz & Mody, 1994; Chen et al. 1999). The fluid percussion-injured and the sham-operated, age-matched rats were anaesthetized with sodium pentobarbital (75 mg kg−1i.p.). Anaesthetized rats were decapitated, and the brains were removed and cooled in 4°C oxygenated (95 % O2−5 % CO2) artificial cerebrospinal fluid (ACSF) composed of (mM): 126 NaCl, 2.5 KCl, 26 NaHCO3, 2 CaCl2, 2 MgCl2, 1.25 NaH2PO4 and 10 glucose. Horizontal brain slices (400 μm for blind patch clamp; 300 μm for visualized patch clamp) were prepared with a vibratome tissue sectioner (Lancer Series 1000) from the ventral-to-mid section of the hippocampus (see Toth et al. 1997, for rationale and details). This procedure yielded about six slices. The brain slices were sagittally bisected into two hemispheric components and the slices ipsilateral to the injury were incubated submerged in 32°C ACSF for 1 h in a holding chamber (the contralateral slices were not examined in these experiments).
For the experiments involving the removal of the hilus, immediately after preparation of the slices with the vibrotome, sections were placed in a Petri dish in ice-cold ACSF, and in randomly chosen sections the hilus was cut away under visual control using a razor blade held with a haemostat (Soltesz & Mody, 1995; Hollrigel et al. 1996). This procedure was carried out on sections from both control and FPI animals. Cold ACSF offers neuroprotection in this system (Soltesz & Mody, 1995). The knife-cut through the slice was done along the line connecting the tip of the dorsal blade of the granule cell layer to the middle of the crest of the granule cell layer. A second cut was made perpendicular to this at the middle of the crest as in Fig. 7A inset. Therefore, this procedure resulted in the disconnection of a large portion of the hilus from the granule cell layer. The cut part of the dentate gyrus (containing a large part of the hilus) was physically removed from the rest of the slice (in order to ensure that the disconnection was complete). A similar procedure was carried out in experiments involving the removal of the CA3c region. In the latter experiments, however, the two cuts were done along the lines which connect the tip of the CA3c layer with the tips of the granule cell layer.
Figure 7. The hilus is necessary for the post-FPI enhancement of granule cell responses.
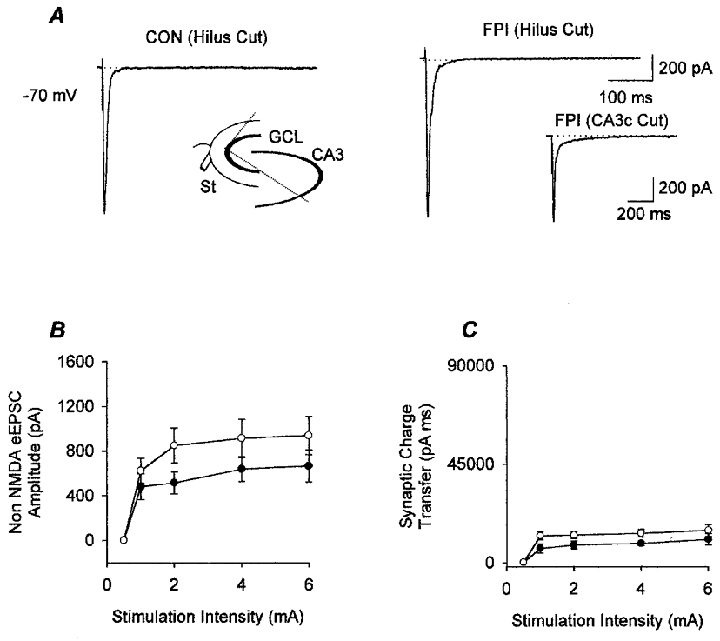
A, means of representative traces from granule cells in slices from control (CON) and FPI animals with the hilus largely removed (as illustrated in the schematic drawing in the inset in the left panel; GCL, granule cell layer; St, stimulating electrode; see Methods), illustrate that, after the removal of the hilus, the late inward current shown in Fig. 4 from FPI animals could not be observed. Recordings were obtained in the presence of bicuculline (20 μM) and APV (20 μM). Note also that the second peak seen in granule cells from ‘uncut’ (i.e. hilus intact) slices from control animals (Fig. 4A) is reduced in the ‘hilus cut’ slices from control animals. The inset in the right panel shows that removal of the CA3c alone (i.e. cutting along the two lines which connect the tip of the CA3c with the tips of the granule cell layer, without removal of the hilus) did not eliminate the late inward current in slices from FPI animals (compare with Figs 4A and 7A); however, there appeared to be a reduction, albeit a non-significant one, in the late EPSC at all stimulation intensities (e.g. at 4 mA stimulation, the charge transfer in granule cells from intact slices from FPI animals was 69 395 ± 13 780 pA ms, as shown in Fig. 4C, versus 39 179 ± 16 183 pA ms in granule cells from ‘CA3c cut’ FPI slices; by comparison, the charge transfer in granule cells from hilus cut FPI slices at the same stimulation intensity was 13 522 ± 1765 pA ms, as shown in Fig. 7C). B, summary data showing the lack of a difference between the amplitudes of the monosynaptic EPSCs in granule cells from slices with the hilus removed from control (•, n = 9) and FPI (○, n = 6) animals. C, the post-FPI enhancement of the synaptic charge transfer shown in Fig. 4E was also eliminated in slices with the hilus largely removed.
Electrophysiology
Individual slices were transferred to a recording chamber (Soltesz et al. 1995; Hollrigel et al. 1996) perfused with ACSF, in most cases together with 20 μM bicuculline methiodide, and 20 μM 2-amino-5-phosphonovaleric acid (APV), or 10 μM 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) or 20 μM GYKI 53655. In some experiments (see Results; Fig. 5B) the extracellular calcium concentration of ACSF was lowered to 0.5 mM (and the Mg2+ increased to 3.5 mM). The brain slices rested on filter paper and were stabilized with platinum wire weights. The tissue was continuously superfused with humidified 95 % O2−5 % CO2 and the temperature of the perfusion solution was maintained at 36°C. All salts were obtained from Fluka; APV and CNQX were purchased from Tocris; bicuculline methiodide was from Research Biochemicals International; and GYKI 53655 was a generous gift from Dr I. Tarnawa (Gedeon Richter Ltd, Budapest).
Figure 5. Post-traumatic enhancement of granule cell responses.
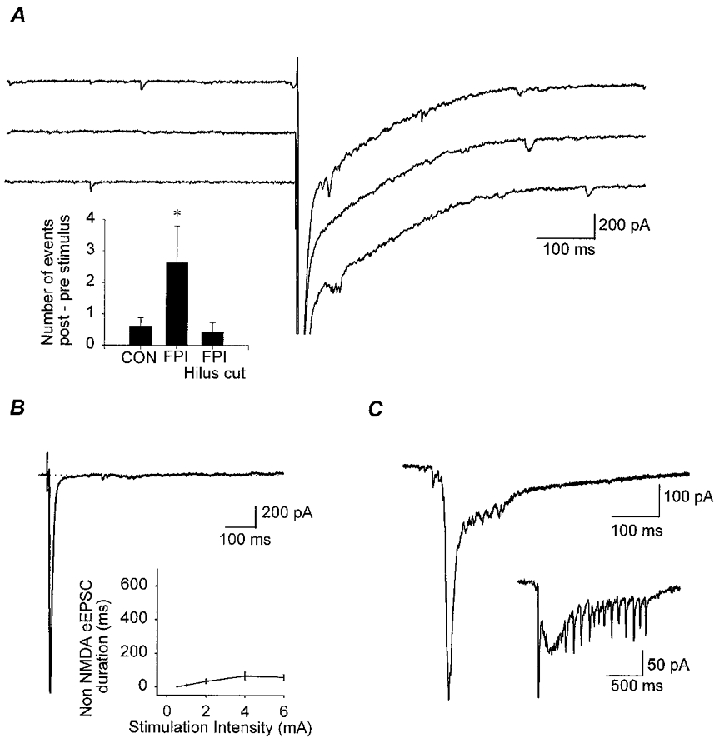
A, consecutive traces from the same granule cell illustrating the increased number of late EPSCs following the stimulus to the perforant path, compared to the immediate pre-stimulus period. Note the bursts of EPSCs during the first 100 ms following the stimulus, which frequently show up as a second peak on the averaged EPSC responses (e.g. Fig. 4A). Inset shows a summary graph of the number of EPSCs during the 500 ms following the stimulus minus the number of events during the 500 ms before the stimulus. Note the increase in the number of EPSCs in the granule cells (n = 3) following FPI, compared to both control cells (n = 8) and the cells from the FPI slices with the hilus cut (n = 3) (* P≤ 0.5). B, in low-Ca2+, high-Mg2+ ACSF (also containing bicuculline and APV), the late EPSCs could not be observed in granule cells (n = 4) following FPI, as indicated by the short duration of the EPSC (inset; the y-axis was intentionally set to facilitate comparison with Fig. 4D). C, spontaneous bursts of EPSCs, resembling the evoked EPSCs (e.g. compare with the traces in A), could be observed in 5/23 granule cells (21.7 %) in FPI animals, but not in control granule cells (n = 15 examined), or in FPI granule cells with the hilus cut. In some of the bursts (inset) the individual EPSCs during the late phase of the burst could be especially clearly resolved.
Patch pipettes were pulled from borosilicate (KG-33) glass capillary tubing (1.5 mm o.d.; Garner Glass) with a Narishige PP-83 two-stage electrode puller. Pipette solutions consisted of (mM): 140 potassium or caesium gluconate, 2 MgCl2 and 10 Hepes; in some experiments, the intracellular solution also included biocytin (0.3 %); for the voltage clamp experiments described in Fig. 5A and B and Fig. 7, the sodium channel blocker QX-314 (3 mM) was also included. ‘Blind’ whole-cell recordings were obtained as previously described (e.g. Soltesz & Mody, 1994); for mossy cells, infrared video microscopy-aided visualized techniques were used (Axioscope FS, Zeiss; Stuart et al. 1993). Recordings were obtained with an Axopatch 200A or 200B amplifier (Axon Instruments) and digitized at 88 kHz (Neurocorder, NeuroData) before being stored in PCM form on videotape. The series resistance was monitored throughout the recordings, and the data were rejected if it significantly increased. Field recordings of orthodromic population spikes in the granule cell layer of the dentate gyrus were conducted using patch pipettes filled with ACSF. To evoke the field or whole-cell responses, constant-current stimuli (0.2–8 mA, 50–200 μs) were applied at 0.1 Hz through a bipolar 90 μm tungsten stimulating electrode placed in the perforant path at the junction of the dorsal blade and the crest. The field responses in the granule cell layer were measured at five pre-determined points in each slice (Toth et al. 1997), including the tips of the dorsal and the ventral blades, the middle of the dorsal and ventral blades and the middle of the crest, and the largest response was studied further.
To visualize recorded cells filled with biocytin, slices were processed as whole mounts (e.g. Hollrigel et al. 1997). Briefly, after the tracer had been allowed to diffuse for 20–60 min in the recording chamber, the slice was fixed in a solution of 4 % paraformaldehyde and 0.5 % glutaraldehyde overnight at 4°C, then washed thoroughly before being reacted with 0.015 % 3,3′-diaminobenzidine (DAB) and 0.006 % H2O2. The reacted slices were cleared in an ascending series of glycerol and coverslipped before the cells were reconstructed with a camera lucida.
Analysis
Recordings of EPSCs were filtered at 3 kHz before digitization at 20 kHz by a personal computer for analysis using Strathclyde Electrophysiology Software (courtesy of Dr J. Dempster, University of Strathclyde, UK) and Synapse software (courtesy of Dr Y. De Koninck, McGill University, Montreal, Canada). Statistical analyses (Student's t test or ANOVA) were performed with SPSS for Windows or SigmaPlot, with a level of significance of P≤ 0.05. Data are presented as means ±s.e.m.
In situ hybridization
Rats deeply anaesthetized with Nembutal (see above) were transcardially perfused with 4 % paraformaldehyde in 0.1 M phosphate buffer. The brain was removed from the skull, postfixed for 2 h, cryoprotected in RNase-free 25 % sucrose-phosphate-buffered saline (24 h), frozen in isopentane (at −40°C) and stored at −70°C. Horizontal sections (30 μm) of the hippocampus from the impact side of the brain (left) were cut on a cryotome. In a manner similar to that described in Toth et al. (1997), sections taken at regular intervals (every 600 μm) were processed for parvalbumin in situ hybridization, parvalbumin immunocytochemistry, GAD67 in situ hybridization or Nissl staining. The six most ventral sections were evaluated. Parvalbumin riboprobes were synthesized using the PCR technique. A pair of primers was chosen to amplify a 340 bp fragment of rat parvalbumin cDNA (Epstein et al. 1986). Primer sequences were complementary to bases 81–103 (downstream) and 420–397 (upstream). A second primer pair was synthesized containing the same sequences, but including the T7 promoter sequence. PCR using a combination of promoter-containing and promoter-less primers resulted in the synthesis of DNA fragments that could be used as templates to synthesize riboprobes in either the sense or the antisense direction. Riboprobes were transcribed from 0.3 μg purified template DNA in the presence of digoxigenin (DIG)-UTP according to the manufacturer's recommendation (Boehringer, Mannheim, Germany). The labelled RNA (about 5–10 μg) was dissolved in 50 μl of diethylpyrocarbonate (DEPC)-treated H2O. The probes were tested and stored at −20°C for more than 6 months without noticeable loss of activity. To determine the fate of hilar interneurones, GAD67 mRNA was also used as a marker. As shown by Houser & Esclapez (1994), GAD67 mRNA is expressed by virtually all GABAergic neurones in the hippocampus. Riboprobes (sense and antisense) were transcribed from 1 μg linearized pBluescript vector containing rat GAD67 cDNA (Erlander et al. 1991) as described above. For in situ hybridization, sections were washed in 2 × SSC (0.3 M NaCl, 0.03 M sodium citrate), twice for 15 min, to remove the sucrose. Sections were then transferred to a mixture (1:1) of 2 × SSC and hybridization solution (15 min) before being prehybridized with hybridization solution (50 % formamide, 4 × SSC, 250 μg ml−1 denatured salmon sperm DNA, 100 μg ml−1 yeast tRNA, 5 % dextrane sulfate, 5 × Denhardt's solution) at 50°C for 1 h. For hybridization, DIG-labelled probes were added (parvalbumin, sense and antisense, 1:600 dilution; GAD67, sense and antisense, 1:1000 dilution) and the sections were incubated overnight at 50°C. For all steps RNase-free (DEPC-treated) solutions and sterile six-well plates were used. After washing (at room temperature: 2 × SSC, twice for 20 min; at 60°C: 2 × SSC-50 % formamide, once for 20 min, followed by 0.1 × SSC-50 % formamide, once for 40 min, and 0.1 × SSC once for 20 min), hybrid molecules were detected with an anti-DIG antibody conjugated to alkaline phosphatase according to the manufacturer's protocol (Boehringer). Staining was carried out using NBT/BCIP-substrates (Boehringer; NBT, nitroblue tetrazolium salt; BCIP, 5-bromo-4-chloro-3-indolyl-phosphate). Finally, sections were mounted on glass slides, embedded with gelatin and coverslipped. When antisense and sense probes were tested, antisense probes (GAD67 after 1–2 h; parvalbumin after 4–6 h), but not the corresponding sense probes, revealed clear and distinct signals. For immunocytochemistry, sections were incubated with a monoclonal antibody against rat parvalbumin (Celio et al. 1988) for 48 h at 4°C. The antibody was diluted 1:10 000 in 0.1 M phosphate buffer and 2 % normal horse serum. After incubation with the primary antibody, sections were washed (3 × 10 min in 0.1 M phosphate buffer) before being incubated with the secondary antibody (biotinylated anti-mouse IgG; Vectastain ABC Kit, Vector Laboratories, Burlingame, CA, USA; diluted 1:200 in 0.1 M phosphate buffer) for 24 h at 4°C. The immunoreaction was visualized by an ABC reaction (Vectastain ABC Kit, Standard) with DAB (0.07 % DAB and 0.002 % hydrogen peroxide in 0.1 M phosphate buffer for 15 min). Finally, sections were mounted on glass slides, dehydrated in graded ethanol, and coverslipped using Histokit (Shandon, Pittsburgh, PA, USA). The immunoreactive cell bodies in the hilus, or the Nissl-stained hilar cells, from each section were drawn using a camera lucida and counted as described previously in detail (Toth et al. 1997). The hilus was defined as the area between the granule cell layer and the two lines connecting the two tips of the granule cell layer to the tip of the CA3c region. As in a previous study (Toth et al. 1997), in the case of those cells that were situated at the border of the hilus and the granule cell layer, the cells were classified as hilar cells if more than half of the cell body was in the hilus.
RESULTS
Lack of preferential survival of interneurones
It has been shown that, following FPI at moderate impact strength under our conditions, there is a 50–60 % decrease in the number of hilar cells labelled with glutamate receptor (GluR)2/3 antibodies (Toth et al. 1997). Antibodies against GluR2/3 specifically label mossy cells within the hilus (Leránth et al. 1996). The observed decrease in the number of GluR2/3 antibody-labelled mossy cells was also demonstrated to be similar to the relative decrease in the number of parvalbumin (or cholecystokinin) antibody-labelled hilar interneurones, as well as to the percentage loss in the total population of hilar cells (for details, see Toth et al. 1997). The decrease in the number of the various hilar cell populations was previously shown to be complete by 1 week, i.e. the cell counts were essentially identical at time points 1 week and several weeks and months post-injury (Toth et al. 1997). Based on these data, it has been suggested that mossy cells may not show a preferential loss following head trauma (Toth et al. 1997), contrary to widely held assumptions regarding their hypersensitivity to various insults. However, a potential caveat could be that the interneurones may only lose their protein marker (e.g. parvalbumin) following FPI, and still preferentially survive. In this scenario, the preferential survival of interneurones would indicate the preferential loss of mossy cells, since the GABAergic interneurones and the glutamatergic mossy cells together make up the total population of hilar cells.
In order to determine whether it is only the parvalbumin protein content of the basket- and axo-axonic cells (for a review, see Freund & Buzsáki, 1996) that is decreased after head injury, non-radioactive in situ hybridization experiments were carried out on the dentate gyrus of animals that had experienced FPI, and on age-matched, sham-operated control rats. The data showed that the percentage decrease in the number of cells labelled by the parvalbumin mRNA probe was not detectably lower (or higher) than the percentage decrease in the total hilar cell population (i.e. the percentage decrease in the number of Nissl-stained cells) 5 months following FPI, and it was also similar to the percentage decrease in the number of hilar cells positive for parvalbumin protein at both 5 months and 1 week after impact (Fig. 1). These data suggest that not only is the parvalbumin protein content downregulated following trauma, but also the number of cells positive for parvalbumin mRNA is decreased.
Figure 1. Lack of preferential survival of interneurones after trauma.
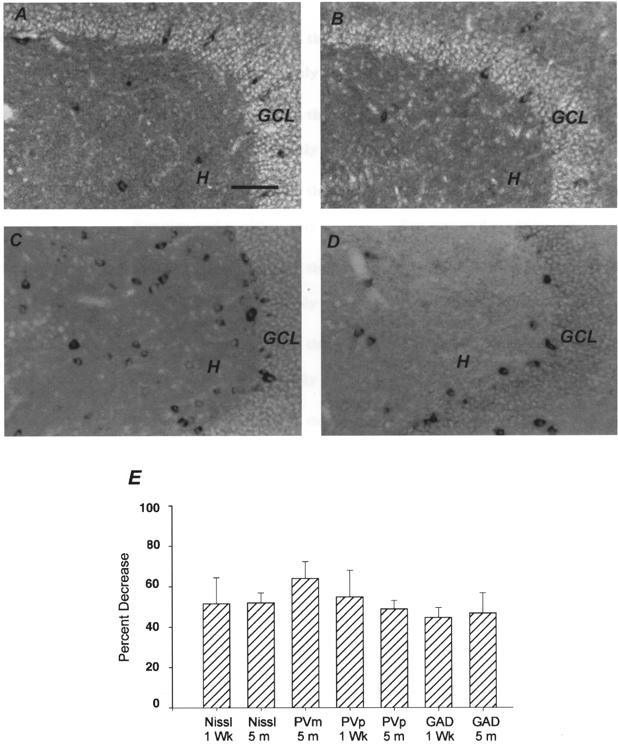
A, parvalbumin mRNA labelling shows interneurones in an age-matched, sham-operated control animal. B, 5 months following FPI, there is a decrease in the number of parvalbumin mRNA-positive cells in the hilus (as shown previously, the number of parvalbumin-immunopositive cells in the granule cell layer does not change following FPI; Toth et al. 1997). C, GAD67 mRNA labelling illustrates GABAergic cells in the hilus in an age-matched, sham-operated control animal. D, note the decrease in the number of GAD67 mRNA-positive cells 1 week after FPI. GCL, granule cell layer; H, hilus. Scale bar in A (also applies to B–D), 60 μm. E, summary data showing that the percentage decrease in the total number of hilar cells (Nissl) is statistically similar (ANOVA) to the percentage decrease in the number of parvalbumin (mRNA or protein)-positive or GAD67 mRNA-positive interneurones in the hilus. 5 m, 5 months post-FPI; 1 Wk, 1 week after FPI; PVm, parvalbumin mRNA (i.e. interneurones labelled by in situ hybridization); PVp, parvalbumin protein; GAD, GAD67 mRNA. Number of animals: 1 week, three control and three FPI for each group; 5 months, four control and four FPI for each group.
It is possible, however, that both the parvalbumin protein and the mRNA for parvalbumin are decreased following FPI, and the cells still survive the trauma. Clearly, this possibility cannot be fully excluded. A further limitation in this regard is that no second marker is available for the parvalbumin-positive hilar neurones (and even if it were, it could be argued that the second marker may also be only downregulated following FPI, without a loss of the cells). However, the parvalbumin-positive cells constitute only a small, albeit functionally significant, proportion of the total interneuronal population in the hilus (Freund & Buzsáki, 1996). Importantly, results obtained with a GAD67 mRNA probe (the enzyme for the synthesis of GABA) proved to be similar to those described above for parvalbumin, i.e. the percentage decrease in the number of GAD67-positive cells was also statistically similar to the percentage decrease in the total number of hilar cells (Fig. 1), both at 5 months and at 1 week post-FPI. Therefore, there is no evidence of a preferential survival of interneurones. Consequently, these data are consistent with the suggestion that interneurones and mossy cells appear to be decreased in number in approximately the same relative proportions following impact (Toth et al. 1997).
Post-traumatic hyperexcitability in dentate excitatory circuits
It has been shown previously that the population spikes evoked by perforant path stimulation in vivo, or in vitro in control ACSF, are significantly enhanced 1 week following FPI (Lowenstein et al. 1992; Coulter et al. 1996; Toth et al. 1997); the electrophysiological data on the functional role of mossy cells following FPI in the rest of the paper are focused on the 1 week post-injury time point, and the question of hyperexcitability months after trauma will not be addressed (see Discussion). The post-impact enhancement of the population spikes has been suggested to be at least partly due to a decreased inhibitory control of granule cell discharges related to the post-traumatic perturbation of interneuronal networks within the dentate gyrus (Toth et al. 1997). In addition to this ‘GABAergic component’, however, the glutamatergic cells of the dentate gyrus may be a source of hyperexcitability. To determine whether there was a significant difference in excitability between slices obtained from FPI and age-matched, sham-operated control animals even after fast GABAergic inhibition had been blocked pharmacologically, field recording experiments were carried out in the presence of the GABAA receptor antagonist bicuculline (20 μM). The results clearly showed that significantly enhanced population spike discharges in slices obtained from animals that had experienced FPI (with respect to slices from control animals) were readily observed even in the presence of bicuculline (Fig. 2). Therefore, these data suggest that the glutamatergic networks of the dentate gyrus may be hyperexcitable following trauma (see below).
Figure 2. Post-traumatic hyperexcitability in the dentate gyrus.
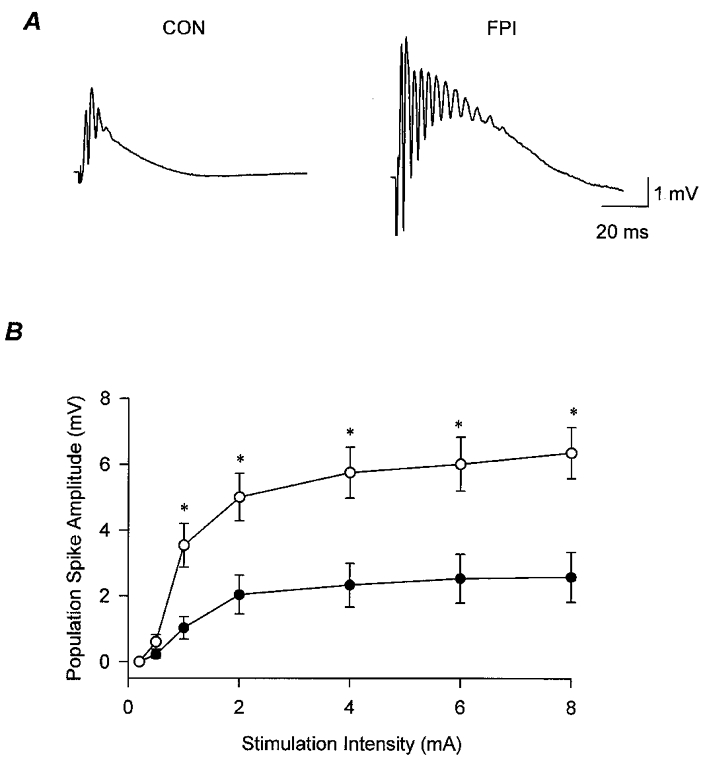
A, representative population responses obtained from the granule cell layer of slices from an age-matched, sham-operated control (CON) and a head-injured animal (FPI), illustrating the enhanced excitability of granule cells in response to stimulation of the perforant path (stimulation intensity, 6 mA). B, summary data similar to those shown in A, from slices from control (•, n = 9) and FPI (○, n = 12) animals. Note that these results were obtained in the presence of the GABAA receptor antagonist bicuculline (20 μM), indicating that the excitatory network of the dentate gyrus is hyperexcitable following FPI. Asterisks indicate significant difference from control (P≤ 0.05).
Next, whole-cell current clamp recordings were performed on granule cells to determine the single-cell responses underlying the field recording data. As illustrated in Fig. 3, in the presence of bicuculline (20 μM), granule cells responded with a higher number of action potentials and longer-lasting postsynaptic depolarizations to stimulation of the perforant path in FPI animals, compared to controls. Therefore, these field and patch clamp data indicate the existence of hyperexcitable glutamatergic networks in the dentate gyrus following traumatic head injury.
Figure 3. Enhanced excitability of granule cells following impact.
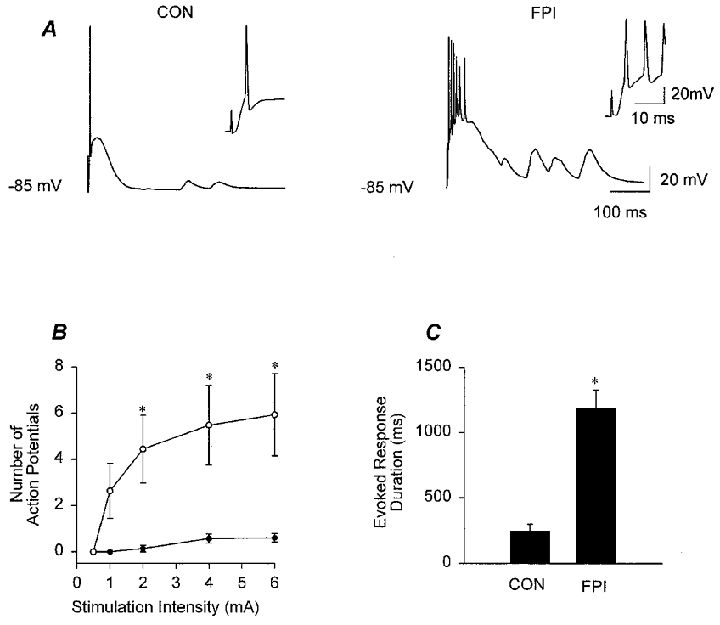
A, representative voltage traces from granule cells of slices from control (CON) and FPI animals illustrating enhanced discharges in response to stimulation of the perforant path following head trauma (stimulation intensity, 6 mA). Whole-cell patch clamp recording with potassium gluconate-containing pipettes, at −85 mV (i.e. close to the mean resting membrane potential of granule cells), in the presence of bicuculline (20 μM). The insets in A show the responses on an expanded time scale. B, summary data showing an enhanced number of action potentials in the granule cells from FPI animals (○, n = 9), compared to controls (•, n = 7). C, the duration of the evoked depolarization was also longer in granule cells following impact (the end of the response was defined as the return of the voltage response to < 2 mV of baseline, for at least 12 ms). Asterisks in B and C indicate significant difference from control (P≤ 0.05).
Abnormal perforant path responses in granule cells after trauma
Granule cells did not show any post-FPI alterations in resting membrane potential (control, −78.0 ± 1.8 mV, n = 5 cells; FPI, −77.0 ± 1.3 mV, n = 7), input resistance (control, 141.7 ± 16.7 MΩ, n = 5; FPI, 161.5 ± 18.1 MΩ, n = 7), or action potential characteristics, including action potential threshold (control, −43.5 ± 1.8 mV, n = 5; FPI, −42.7 ± 1.6 mV, n = 7), amplitude (control, 86.6 ± 0.7 mV, n = 5; FPI, 84.5 ± 1.8 mV, n = 7) and half-width (control, 0.51 ± 0.01 ms, n = 5; FPI, 0.55 ± 0.06 ms, n = 7). Furthermore, the amplitude of the monosynaptic, non-NMDA receptor-mediated EPSC (recorded in the presence of 20 μM APV and 20 μM bicuculline) was also unchanged in granule cells from FPI animals (Fig. 4A and B). However, in agreement with the current clamp data presented above, the monosynaptic EPSC from FPI animals was followed by a significantly enhanced long-lasting, most probably polysynaptic component (Fig. 4). The late component was smaller in amplitude than the monosynaptic EPSC (Fig. 4C; compare with Fig. 4B), but it lasted for about half a second on average (Fig. 4D), effectively increasing the post-FPI excitatory synaptic charge transfer following activation of the perforant path (Fig. 4E). Based on its post-FPI appearance and time course, the late, long-lasting, slow EPSC probably underlies the enhanced, long-lasting excitatory response of granule cells observed in current clamp (Fig. 3).
Figure 4. Post-FPI increase in the late component of the perforant path-evoked EPSC in granule cells.
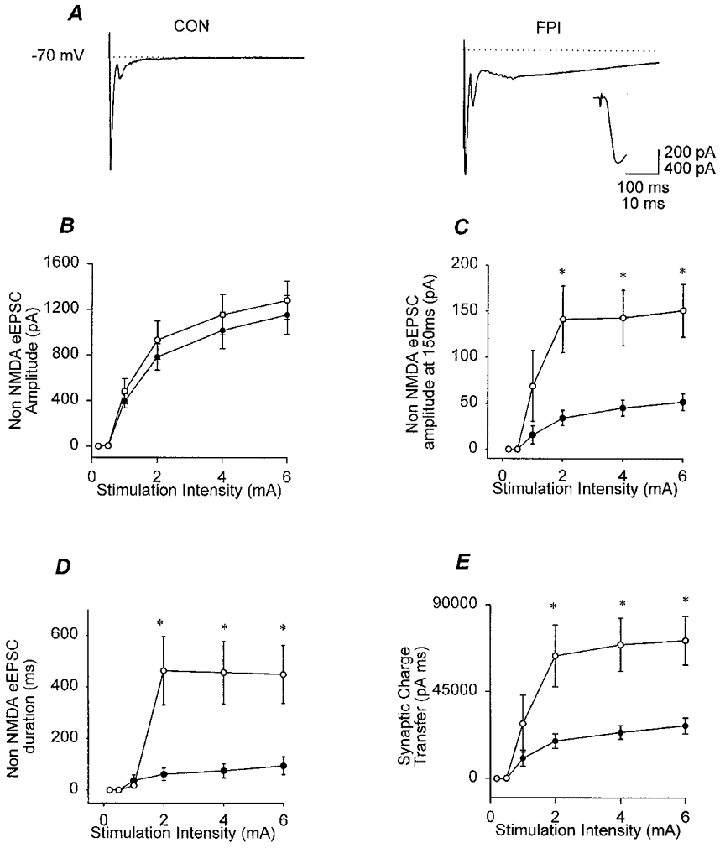
A, averaged representative traces (n = 3–5) obtained in voltage clamp from granule cells from control (CON) and FPI rats, illustrating that the monosynaptic EPSC is not altered by the trauma; however, FPI results in the appearance of late, slow components following the monosynaptic EPSC. Recordings were obtained in the presence of bicuculline (20 μM) and the NMDA receptor antagonist APV (20 μM). Note that a small second peak following the monosynaptic EPSC is visible even in the control trace, presumably due to a reverberation of the activity in response to a stimulus to the perforant path in the presence of bicuculline. The inset shows the monosynaptic response from the FPI animal on an expanded scale (x and y scales for inset: 10 ms, 400 pA). B, summary data illustrating the lack of a post-traumatic alteration in the peak amplitude of the fast, monosynaptic evoked EPSC (eEPSC; control, •, n = 9; FPI, ○, n = 10). C, following FPI, the amplitude of the late component of the EPSC increased (amplitude measured at 150 ms following the stimulus). D, the late component lasted significantly longer in FPI animals compared to controls. E, the post-impact appearance of the late, slow component of the inward current led to the effective enhancement of the synaptic charge transfer in granule cells from FPI animals. Asterisks in C–E indicate significant difference from control (P≤ 0.05).
The polysynaptic nature of the late, long-lasting, slow EPSC was indicated by the increase in the number of resolvable individual, smaller EPSCs with variable latencies (see consecutive traces in Fig. 5A) following a stimulus, compared to the immediate pre-stimulus period in the same cells (note also that small ‘ripples’ can be seen even on the averages of late, slow EPSC traces, e.g. Fig. 4A, right panel), as well as by the fact that separating the granule cell layer from the hilus resulted in the loss of the late component (see below). To further examine the polysynaptic nature of the late EPSCs, granule cell responses were recorded in a low-Ca2+, high-Mg2+ medium (0.5 mM Ca2+, 3.5 mM Mg2+). As expected from the above data, the late EPSC could not be observed in the low-Ca2+, high-Mg2+ medium in granule cells (n = 4) following FPI (Fig. 5B).
In addition, in 5/23 granule cells (21.7 %) from FPI animals, large, spontaneous bursts of EPSCs could be observed, which appeared similar to the stimulus-evoked EPSC responses from FPI animals (Fig. 5C; compare with Figs 5A and 4A, left panel). The similarity between the evoked EPSCs and the spontaneous bursts of EPSCs included the large-amplitude leading EPSC, and the presence of individual smaller EPSCs which could be resolved during the late phase of the events (Fig. 5C; these experiments were done in ACSF containing bicuculline, APV and normal extracellular [Ca2+]). The spontaneous bursts of EPSCs (a ‘burst’ was arbitrarily defined as an event which began with an EPSC > 100 pA in peak amplitude, and lasted for > 200 ms, with the end of the burst marked by the return of the current value to 10 % of its peak; burst characteristics after FPI: peak amplitude, 796.4 ± 52.1 pA; frequency, 0.21 ± 0.06 Hz; duration, 525.5 ± 154.4 ms) were not present in control granule cells (n = 15 examined), or in granule cells from FPI slices with the hilus removed (see below).
The late, slow EPSC did not require NMDA receptor activation, since it could be observed in the presence of APV (Fig. 4). However, it could be blocked by the non-NMDA ionotropic glutamatergic receptor antagonist CNQX (Fig. 6A, trace labelled −60 mV, recorded from the FPI animal), as well as by the selective AMPA receptor antagonist GYKI 53655 (n = 4; data not shown). In addition, there was no post-traumatic change in the amplitude of the NMDA receptor-dependent EPSCs (recorded in the presence of CNQX and bicuculline; Fig. 6). These data indicate that there is no post-traumatic change in the monosynaptic NMDA or non-NMDA receptor-dependent EPSCs evoked by stimulation of the perforant path, or in the intrinsic properties of granule cells. Therefore, a major source of the hyperexcitability observed in granule cells following FPI is related to the late, slow EPSC, the origin of which is likely to be located in the hilus (see below).
Figure 6. Lack of a post-traumatic alteration in the NMDA receptor-mediated EPSC in granule cells.
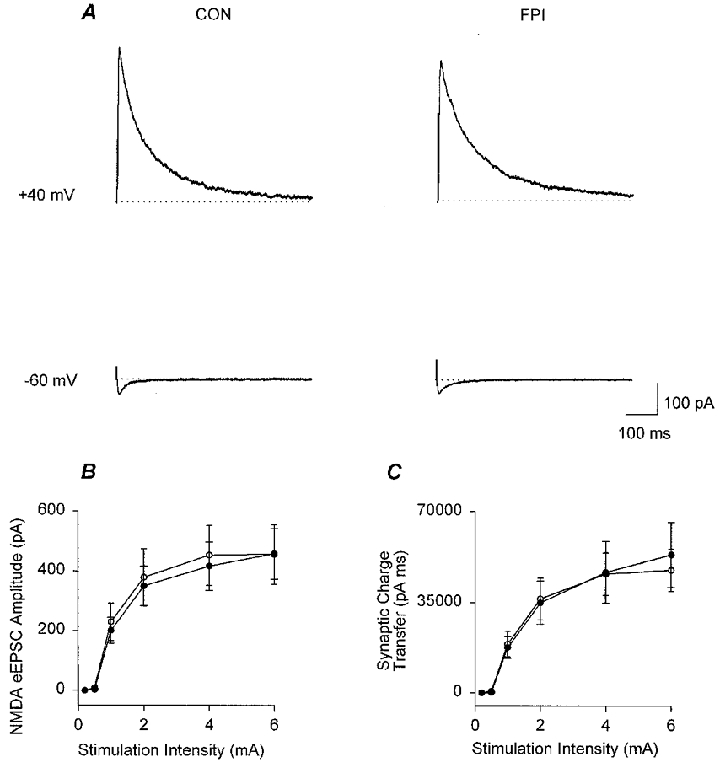
A, means of representative traces are shown for granule cells from control (CON) and FPI rats, obtained in voltage clamp following stimulation of the perforant path in the presence of bicuculline (20 μM) and CNQX (10 μM), at +40 mV (upper traces), and at −60 mV (lower traces). Note the absence of the late component of the EPSC (compare lower traces with Fig. 4) in the presence of CNQX at −60 mV (the late component was also abolished by the selective AMPA receptor antagonist GYKI 53655; not shown). B and C, summary data showing the lack of a post-FPI alteration in the amplitude (B) and synaptic charge transfer (C) of the NMDA receptor-dependent EPSCs (measured at +40 mV; control, •, n = 7; FPI, ○, n = 10).
The hilus is necessary for granule cell hyperexcitability
In the next series of experiments a large portion of the dentate hilus was removed with a knife-cut from slices from both FPI and control animals (the cut is indicated by the schematic drawing in the inset of Fig. 7A, left panel; see Methods). The characteristic post-impact late component of the perforant path-evoked EPSC in granule cells (Fig. 4) was no longer observed in slices with the hilus removed (Fig. 7; compare with Fig. 4). In these ‘cut’ slices from control and FPI animals, there was still no difference between the amplitudes of the monosynaptic EPSCs (Fig. 7B). Furthermore, the enhanced synaptic charge transfer observed in granule cells from FPI rats (Fig. 4E) was not observed in slices with the hilus removed (Fig. 7C). Therefore, the hyperexcitable responses of granule cells seen in the presence of bicuculline following perforant path stimulation in FPI animals requires the excitatory circuitry of the hilus. Note that the monosynaptic EPSC in Fig. 4A is followed by a small second peak even in slices from control animals, presumably indicating reverberation of activity in the presence of bicuculline in response to stimulation of the perforant path; following the removal of a large part of the hilus, this small second peak was also reduced in controls (as well as in granule cells from FPI rats; Fig. 7).
Since removal of the hilus (Fig. 7A, inset of left panel) disconnected the CA3c region as well, further experiments were carried out involving the removal of the CA3c region alone (i.e. without the hilus). When the CA3c region (together with the rest of CA3) was removed from the FPI slices (by two cuts connecting the tip of the CA3c layer with the tips of the granule cell layer), the late EPSC, observed in granule cells in intact slices from FPI animals (Fig. 4A), was not eliminated, although it appeared to be reduced in amplitude (Fig. 7A, inset of right panel; the reduction in charge transfer did not reach statistical significance, see legend). These data further indicate that the excitatory cells in the hilar circuitry (i.e. the mossy cells, see below) are important in generating the post-traumatic hyperexcitable responses in granule cells, and these results also suggest that the CA3c region may contribute to the late EPSCs seen in granule cells from FPI animals.
Hyperexcitability of surviving mossy cells
A final series of experiments was conducted to directly determine whether mossy cells show post-traumatic hyperexcitability in response to stimulation of the perforant path. (Mossy cells may receive monosynaptic or disynaptic inputs from the perforant path (Scharfman & Schwartzkroin, 1989; Soltesz & Deschênes, 1993; Buckmaster & Schwartzkroin, 1994; Scharfman, 1995), e.g. via entorhinal fibres in the molecular layer and the hilus (Deller et al. 1996) as well as through granule cell activation.) Since the mossy cell recordings were carried out using visualized patch clamp techniques in younger animals (FPI at P20-P25, recordings 1 week later), whereas the granule cell hyperexcitability data were obtained from fully mature animals, experiments were performed which determined that granule cells in these younger animals also showed prolonged responses to perforant path stimulation, similar to their adult counterparts (data not shown); the duration of the evoked EPSCs in these young animals was almost ten times longer after FPI even at low stimulation intensity (2 mA; 234.0 ± 19.4 ms, n = 3) compared to age-matched, sham-operated controls (25.3 ± 4.5 ms, n = 3). The biocytin-labelled mossy hilar cells had large somata, their dendrites were confined to the hilus and they displayed complex spines on their proximal dendrites. Furthermore, their intrinsic electrophysiological properties were similar to those published previously; there was no significant difference between control and FPI intrinsic electrophysiological properties, including the characteristic small amplitude of the slow after-hyperpolarization following a burst of action potentials (control, 3.1 ± 1.9 mV, n = 3; FPI, 2.7 ± 0.9 mV, n = 3), the action potential amplitude (control, 89.8 ± 1.1 mV; FPI, 91.9 ± 3.8 mV), action potential threshold (control, 40.5 ± 2.8 mV; FPI, 40.2 ± 2.9 mV) and resting membrane potential (control, −59.7 ± 4.9 mV; FPI, −55.0 ± 2.8 mV; Buckmaster et al. 1992; Soltesz et al. 1993; Scharfman, 1995; Lübke et al. 1998). However, mossy cells from the FPI animals responded with significantly more action potentials to perforant path stimulation, compared to controls (Fig. 8C and D). In addition, the return of the voltage trace to baseline following perforant path stimulation was significantly prolonged in cells following trauma (Fig. 8C and E). These data, indicating the post-traumatic hyperexcitability of mossy hilar cells, are in agreement with the experiments described above showing that removal of the hilus eliminates the late component of the evoked EPSCs in dentate granule cells.
Figure 8. Mossy hilar cells in control and head-injured rats.
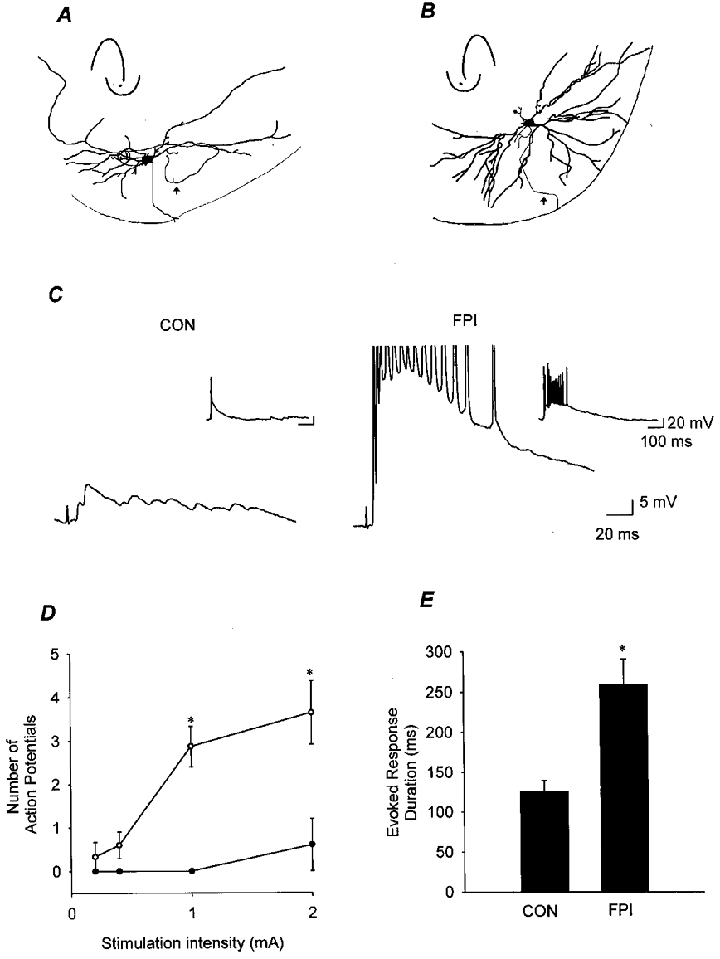
A and B, camera lucida drawings of biocytin-filled mossy cells from control (A) and FPI animals (B). Insets illustrate the position of the cells in the dentate hilus. The arrows point to the axons. C, representative traces from identified mossy hilar cells from control (CON) and FPI rats illustrating that mossy cells following FPI discharge a higher number of action potentials and show a longer-duration depolarization in response to stimulation of the perforant path (intensity, 2 mA; −70 mV) with bicuculline (20 μM) present in the perfusing medium. The inset in the left panel illustrates the response of a control mossy cell which displayed action potential discharge following perforant path stimulation. The inset in the right panel shows the response of the FPI mossy cell on an expanded time scale and a larger y-axis. Scale bars for the two main traces (5 mV, 20 ms) and the two inset traces (20 mV, 100 ms) are indicated on the right. D, summary data showing the increased number of perforant path stimulation-evoked action potentials in mossy cells following FPI (○, n = 3), compared to control (•, n = 3). E, the duration of the depolarization following perforant path stimulation was longer in mossy cells from FPI animals (the end of the response was defined as the return of the voltage response to < 2 mV of baseline, for at least 12 ms), measured at 2 mA stimulation intensity. Asterisks in D and E indicate significant difference from control (P≤ 0.05).
DISCUSSION
Limitations and constraints
The following limitations and constraints apply to this study: (i) the main focus of this investigation was on the early post-traumatic time period (see below); (ii) the exact pre- and/or postsynaptic mechanisms underlying mossy cell hyperexcitability are not yet known; (iii) in the experiments involving a knife-cut through the hilus, not all hilar cells were removed; and (iv) the experiments did not directly investigate the hyperexcitability of CA3c cells. However, these limitations do not alter the main conclusions of this study.
The importance of the early post-traumatic period
The electrophysiological, and part of the cytochemical, data were obtained at 1 week post-injury. We chose this time point because by 1 week, the loss of mossy cells and other hilar neurones is complete in our experimental model (Toth et al. 1997; present study, see Fig. 1). Furthermore, the early (≤ 1 week) post-traumatic period is known to be crucially important in determining the long-term outcome of human head injuries (e.g. Grady & Lam, 1995). For example, the presence of early (≤ 1 week) post-traumatic seizures has been identified to be among the major risk factors for late (> weeks and months) seizures (Jennett & Lewin, 1960; Jennett, 1975; Willmore, 1997; Asikainen et al. 1999). The early post-injury period is also frequently characterized by short-term amnesia and memory problems, as part of the ‘post-concussion syndrome’ (Binder, 1986; Lowenstein et al. 1992). Therefore, understanding the pathophysiological events taking place during the early post-traumatic period (the ‘therapeutical time window’) is an important goal of research into the neuronal mechanisms underlying the neurological problems following traumatic brain injuries. Indeed, recent results showed that pharmacological interventions, such as the application of tetrodotoxin to the mechanically injured cortex, are effective in preventing the long-term development of post-traumatic hyperexcitability when applied shortly after injury, but not when applied 11 days following trauma (Graber & Prince, 1999). These data further emphasize that events taking place during the first week following trauma are crucially important in shaping the long-term consequences of traumatic brain injury. Finally, since mossy fibre sprouting typically takes weeks or months to fully develop (e.g. Cavazos et al. 1992; Buckmaster & Dudek, 1997; granule cells in control animals do not significantly innervate other granule cells), the 1 week post-injury time point also allowed us to study a post-traumatic, hyperexcitable system without the confounding factor related to the appearance of new recurrent excitatory circuits, which would make interpretation of the data difficult.
Non-interneurone-related mechanisms of post-traumatic dentate hyperexcitability
The results show that hyperexcitability following FPI exists in the dentate gyrus even after GABAA receptors have been blocked, indicating that modifications involving interneurones (e.g. Toth et al. 1997) cannot fully explain post-traumatic hyperexcitability in the dentate, and that the dentate glutamatergic network itself is a major source of hyperexcitability after trauma. Furthermore, the immunocytochemical data presented in this paper, supporting the results of our previous study (Toth et al. 1997), indicate that mossy hilar cells do not seem to be preferentially lost following FPI, compared to non-mossy, GABAergic hilar cells. In addition, the electrophysiological data strongly suggest that the hilar excitatory feedback loop (Buckmaster & Schwartzkroin, 1994; Jackson & Scharfman, 1996) is necessary for the post-traumatic hyperexcitability of granule cells, since the removal of a large section of the hilus resulted in the near-complete elimination of the FPI-related late-component EPSCs which underlie the hyperexcitable responses of granule cells. Furthermore, the fact that in low-Ca2+, high-Mg2+ medium the late EPSCs could not be observed in granule cells following FPI also indicates the importance of polysynaptic pathways. Also, direct evidence was presented to show that the surviving mossy cells are indeed hyperexcitable following a single episode of head trauma. Therefore, based on these results, we suggest that the survival of the excitatory mossy hilar cells with altered synaptic response properties and roles in the circuit may be an important contributor to post-traumatic hyperexcitability, which is likely to add to the hyperexcitable effects resulting from the post-traumatic perturbation of interneuronal networks (Toth et al. 1997). The hypothesis that mossy cells play an important role in early post-traumatic hyperexcitability will be referred to below as the ‘irritable mossy cell’ hypothesis (IMCH; Fig. 9). Although the removal of the CA3c region did not eliminate the late EPSCs, the post-FPI mossy cells may act in concert with the CA3c cells, which are similar to mossy cells in many (Lorente de No, 1934; Scharfman, 1992), albeit not all (Buckmaster et al. 1993), aspects of their basic electrophysiological properties and connectivity (especially in the ventral hippocampus, where CA3c cells are known to innervate granule cells; Hsu & Buzsáki, 1994).
Figure 9. The ‘irritable mossy cell’ hypothesis.
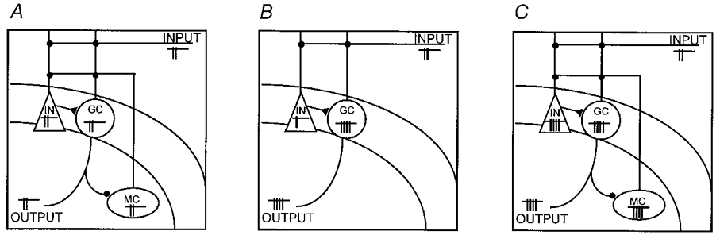
A, schematic diagram of the connectivity of the control dentate gyrus. Stimulation of the perforant path (INPUT) discharges both interneurones (IN) and granule cells (GC), and granule cells excite the mossy cells (MC) (perforant path inputs may also directly activate mossy cells; not indicated). The granule cell discharge is the main signal that leaves the dentate gyrus (OUTPUT). Neuronal discharge is schematically indicated next to the INPUT, the OUTPUT and the three cell types. B, the ‘dormant basket cell’ hypothesis (DBCH; Sloviter, 1991) proposes that the loss of mossy cells is the central event leading to the development of hyperexcitability (hyperexcitability is indicated by the increased number of action potentials in granule cells). C, the ‘irritable mossy cell’ hypothesis (IMCH) suggests that the key event is the survival of mossy cells, which form a hyperexcitable excitatory feedback pathway to granule cells. Note that the loss of some mossy cells is not indicated for clarity. Also note that although enhanced firing of interneurones is indicated in C (since this is a likely prediction of the IMCH), in this paper the issue of the excitatory drive to GABAergic cells after FPI is not directly addressed (however, in other models of hyperexcitability, enhanced excitatory drive to interneurones has been observed, e.g. Buhl et al. 1996).
The ‘irritable mossy cell’ hypothesis: comparisons
A comparison (Fig. 9) can be made between the IMCH and the ‘dormant basket cell’ hypothesis (DBCH; Sloviter, 1991) regarding their relevance to early post-traumatic hyperexcitability in the FPI model of experimental head injury. The DBCH was originally proposed based on data from one experimental model of hyperexcitability involving prolonged stimulation of the perforant path. Therefore, it was not put forward directly for the purpose of explaining, for example, post-traumatic or other forms of hyperexcitability. Nonetheless, the DBCH has been quite contributive in providing a potentially falsifiable hypothesis whose generality across models could be determined, as evidenced by the debate which followed its original publication (e.g. Buckmaster & Schwartzkroin, 1994; Buhl et al. 1996; Bernard et al. 1998; Jefferys & Traub, 1998; Prince & Jacobs, 1998). A central idea of the DBCH is that the loss of mossy hilar neurones is the key reason for the development of hyperexcitability (Sloviter, 1991) (Fig. 9A and B). Specifically, the DBCH proposed that the degeneration of the hyper-vulnerable mossy cells following a prolonged period of increased glutamate release from the perforant path leads to a decrease in the excitatory drive to interneurones, rendering them ‘dormant’, which, in turn, leads to granule cell hyperexcitability (Sloviter, 1991). Taken together, the data in this paper suggest that, after head injury, an alternative scenario seems to be more likely, namely, that the survival (as opposed to the loss) of mossy cells is important in hyperexcitability. In addition, the involvement of interneurones is central to the DBCH, whereas our data indicate increased excitability of post-FPI granule cells even in the presence of a GABAA receptor antagonist. Finally, removal of the hilus would not necessarily be expected to eliminate hyperexcitability if the DBCH model applied to the early post-traumatic dentate gyrus (since, according to the DBCH, removal of the hilus would be expected to remove even more excitatory inputs to interneurones in the granule cell layer, rendering the interneurones more dormant, leading to increased hyperexcitability). Therefore, it appears that the IMCH model may be especially well-suited to explain the post-FPI hyperexcitable phenomena observed in this paper.
It should be noted, however, that these data alone do not necessarily exclude the DBCH in FPI. For example, it is conceivable that mossy cells exhibit hyperexcitable responses at the same time as the efficacy of EPSPs onto interneurones is reduced. However, recent experiments showed that, at least during the immediate post-traumatic period (< 4 days), interneurones in the granule cell layer respond to perforant path stimulation with a significantly lower (and not higher) threshold, due to a selective, interneurone-specific, post-traumatic depolarization of their membrane potential (Ross et al. 1999). At later time points, the efficacy of the excitatory drive to interneurones also seemed to be increased, and not decreased, as indicated by the effectiveness of glutamate receptor antagonists in reducing the frequency of spontaneous IPSCs in granule cells in FPI, but not in control granule cells (Jeng et al. 1998). In other models of hyperexcitability, similar enhancement of the excitatory drive to interneurones has also been reported (e.g. Buhl et al. 1996).
Experimental considerations
A cautionary note is due here with regard to the experiments involving the removal of the hilus. While severance of the granule cell axons is an inevitable outcome of these experiments, the cold medium (known to be an effective, albeit not perfect, neuroprotectant in mechanical trauma to granule cells; Soltesz & Mody, 1995) used during the knife-cuts would be expected to limit cellular damage. The amplitude of the monosynaptic EPSCs in granule cells from the control and FPI slices with the hilus removed appeared similar (Fig. 7A and B), indicating that it is unlikely that the mossy fibre axotomy resulted in a preferential damage to FPI versus control granule cells. Consequently, the elimination of the late EPSCs in FPI slices following the removal of the hilus would be difficult to explain by preferential damage to granule cells in slices from FPI animals. When comparisons are made between the amplitude of the monosynaptic responses obtained in intact slices versus slices with the hilus removed, it is apparent that the perforant path-evoked monosynaptic EPSCs are quite similar at lower stimulation intensities (e.g. at 1 mA, see Fig. 4B versus Fig. 7B) for both control and FPI animals, but the intact slices tend to produce larger responses at the highest stimulation intensities, probably indicating some damage to granule cells as a result of the axotomized mossy fibre pathway.
Hyperexcitable responses following FPI were shown in previous papers in vivo (Lowenstein et al. 1992), as well as in control medium (i.e. without bicuculline; Toth et al. 1997). The experiments included in this paper indicate that the hyperexcitable responses in field recordings in the granule cell layer in response to perforant path stimulation following FPI persist in the presence of bicuculline, and are reflected in the increased action potential discharges of granule cells. The enhanced granule cell discharges, in turn, were linked to the late EPSCs underlying the granule cell depolarization in the FPI animals. Therefore, the late EPSC is likely to be a key component towards understanding the post-traumatic hyperexcitable state within the limbic system. Although the experiments in this paper provide evidence that the hilar excitatory feedback to granule cells contributes to the late EPSCs (and, therefore, to the hyperexcitable dentate gyrus), it is important to note that several other synaptic, cellular and non-synaptic factors (e.g. post-traumatic deficiencies in electrogenic ion pumps and K+ buffering), in neurones and/or glial cells, may also contribute to the late inward current seen following stimulation of the perforant path in FPI animals. These additional components of granule cell hyperexcitability will need to be investigated in subsequent studies. The fact that in the FPI granule cells spontaneous bursts of EPSCs, closely resembling the perforant path-evoked EPSCs, could be observed, indicates that the late component of the post-traumatic evoked response may be generated by the circuitry spontaneously, in the absence of externally applied stimuli. Where these spontaneous bursts originate is not known; however, since they could not be observed in the slices with the hilus removed, it is likely that the hilar circuitry is important in the generation of these events as well.
A final point on the experimental model concerns the fact that the bulk of the results were obtained using ‘blind’ patch clamp techniques in fully adult animals, whereas the mossy cell recordings were done with infrared video microscopy-aided visualized patch clamp techniques in younger animals. Because of the differences in the two setups (the infrared setup uses fully submerged slices, whereas the ‘blind’ setup works on semi-submerged slices which have only a thin layer of ACSF perfusate on the top), as well as the age difference, direct comparisons of the absolute values of stimulation intensities used to elicit responses in granule cells (e.g. Fig. 3B) and in mossy cells (Fig. 8D) cannot be made. However, this limitation does not affect the comparisons between control and FPI granule cells, and between control and FPI mossy cells. In addition, granule cells from younger animals showed enhanced late EPSCs, similar to their adult counterparts, indicating that the difference in the age of the animals used for the two sets of experiments does not alter the hyperexcitability of the dentate gyrus following trauma.
Possible significance for post-traumatic seizures and memory disturbances
Traumatic brain injury affects millions of people world-wide (Salazar, 1992; LeRoux & Grady, 1995; see also Toth et al. 1997, for additional references). Two major consequences of head injury are post-traumatic seizures and memory dysfunction, both of which are likely to involve the hippocampal formation (Jennet, 1975; Binder, 1986; Rempel-Clower, 1996; Asikainen et al. 1999).
The present data indicate that future efforts to reduce early post-traumatic hyperexcitability may target the selective dampening of hyperexcitability in the surviving mossy hilar cells. To achieve this goal, the mechanisms underlying the post-traumatic hyperexcitability of mossy cells will need to be investigated and understood in detail. Several properties of mossy cells already provide important clues towards understanding mossy cell hyperexcitability, including the post-depolarization enhancement of mossy cell excitability (Strowbridge & Schwartzkroin, 1996), and the ability of chelators of excess intracellular Ca2+ to reduce mossy cell damage (Scharfman & Schwartzkroin, 1989). Mossy cells respond to perforant path stimulation with a series of EPSPs/EPSCs even in control animals (e.g. Fig. 8C), presumably reflecting the reverberation of excitatory activity in the disinhibited network through polysynaptic pathways and/or the non-synchronous release of glutamate from mossy fibres. Therefore, it will be important to determine whether the enhancement of the mossy cell response following FPI is due to the amplification of mechanisms that already play a role even in control animals, or the post-traumatic recruitment of new synaptic, cellular processes following the impact. It is also interesting to speculate that the issue of the loss versus survival (with altered properties) of mossy cells will need to be considered in experiments attempting to replace lost hilar cells after trauma with injections of hilar cell implants, since such interventions may only be beneficial if the implanted mossy cells are not hyperexcitable. In other words, according to the IMCH, even successful re-wiring of the dentate circuitry following cell loss is unlikely to be enough to eliminate hyperexcitability without a concurrent decrease in mossy cell hyperexcitability.
In addition to post-traumatic hyperexcitability, the findings presented in this paper may be relevant to post-traumatic memory disturbances (FPI has been shown to lead to cognitive dysfunction involving the hippocampus, e.g. Bramlett et al. 1997). However, since the precise roles of the dentate gyrus in general, and of the mossy cells in particular, in memory functions are not understood, it is difficult at the present time to assess exactly how the disturbed mossy cell excitability might influence memory processes. Nonetheless, an insight may be provided by a recent hypothesis (Lisman, 1999), which proposed that the recall of memory sequences is critically dependent on the integrity of the dentate recurrent excitatory network, and that the accuracy of sequence recall should be reduced by modification of the timing of the synaptic events within the recurrent network. Our findings show that, following head injury, granule cells alter their response to perforant path activation, and that this post-traumatic change in the timing of granule cell spikes is dependent on the recurrent feedback excitation from the hilar cells. Therefore, it seems reasonable to speculate that the observed long-duration response of granule cells to incoming synaptic signals from the perforant path following trauma may play a role in reducing the accuracy of recall of memory traces.
Conclusions and outlook
In summary, the hilar recurrent excitatory circuitry (and, specifically, the mossy cells) were shown to be crucial in sustaining post-traumatic hyperexcitable responses of granule cells to perforant path stimulation. The IMCH was proposed to provide a conceptual framework for future studies, both for those focusing on head trauma (e.g. what are the pre- and/or postsynaptic mechanisms underlying mossy cell hyperexcitability following FPI?), as well as for investigations involving other models of hyperexcitability (to test the generality of the IMCH).
Acknowledgments
The authors wish to thank Drs Niranjala Tillakaratne and Allen Tobin for kindly providing the transcription vector containing rat GAD67 cDNA, Dr Istvan Tarnawa for GYKI 53655, and Ms R. Zhu for expert technical assistance. The work was financially supported by the NIH (NS35916 to I.S.), and by the Deutsche Forschungsgemeinschaft (SFB 505 to M.F.). We also thank Dr O. Steward for his support.
References
- Amaral DG. A Golgi study of cell types in the hilar region of the hippocampus in the rat. Journal of Comparative Neurology. 1978;182:851–914. doi: 10.1002/cne.901820508. [DOI] [PubMed] [Google Scholar]
- Annegers JF, Grabow JD, Groover RV, Laws ER, Jr, Eiveback LR, Kurkland LT. Seizures after head trauma: A population study. Neurology. 1980;30:683–689. doi: 10.1212/wnl.30.7.683. [DOI] [PubMed] [Google Scholar]
- Asikainen I, Kaste M, Sarna S. Early and late posttraumatic seizures in traumatic brain injury rehabilitation patients: brain injury factors causing late seizures and influence of seizures on long-term outcome. Epilepsia. 1999;40:584–589. doi: 10.1111/j.1528-1157.1999.tb05560.x. [DOI] [PubMed] [Google Scholar]
- Bernard C, Esclapez M, Hirsch JC, Ben-Ari Y. Interneurones are not so dormant in temporal lobe epilepsy: a critical reappraisal of the dormant basket cell hypothesis. Epilepsy Research. 1998;32:93–103. doi: 10.1016/s0920-1211(98)00043-6. [DOI] [PubMed] [Google Scholar]
- Binder LM. Persisting symptoms after mild head injury: a review of the postconcussive syndrome. Journal of Clinical and Experimental Neuropsychology. 1986;8:323–346. doi: 10.1080/01688638608401325. [DOI] [PubMed] [Google Scholar]
- Bramlett HM, Green EJ, Dietrich WD. Hippocampally dependent and independent chronic spatial navigational deficits following parasagittal fluid percussion brain injury in the rat. Brain Research. 1997;762:195–202. doi: 10.1016/s0006-8993(97)00387-9. [DOI] [PubMed] [Google Scholar]
- Bruton C. The Neuropathology of Temporal Lobe Epilepsy. New York: Oxford University Press; 1988. [Google Scholar]
- Buckmaster PS, Dudek FE. Network properties of the dentate gyrus in epileptic rats with hilar neuron loss and granule cell axon reorganization. Journal of Neurophysiology. 1997;77:2685–2696. doi: 10.1152/jn.1997.77.5.2685. [DOI] [PubMed] [Google Scholar]
- Buckmaster PS, Schwartzkroin PA. Hippocampal mossy cell function: a speculative view. Hippocampus. 1994;4:393–402. doi: 10.1002/hipo.450040402. [DOI] [PubMed] [Google Scholar]
- Buckmaster PS, Strowbridge BW, Kunkel DD, Schmiege DL, Schwartzkroin PA. Mossy cell axonal projections to the dentate gyrus molecular layer in the rat hippocampal slice. Hippocampus. 1992;2:349–362. doi: 10.1002/hipo.450020403. [DOI] [PubMed] [Google Scholar]
- Buckmaster PS, Strowbridge BW, Schwartzkroin PA. A comparison of rat hippocampal mossy cells and CA3c pyramidal cells. Journal of Neurophysiology. 1993;70:1281–1299. doi: 10.1152/jn.1993.70.4.1281. [DOI] [PubMed] [Google Scholar]
- Buhl EH, Otis TS, Mody I. Zinc-induced collapse of augmented inhibition by GABA in a temporal lobe epilepsy model. Science. 1996;271:369–373. doi: 10.1126/science.271.5247.369. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Leung LW, Vanderwolf CH. Cellular bases of hippocampal EEG in the behaving rat. Brain Research. 1983;287:139–171. doi: 10.1016/0165-0173(83)90037-1. [DOI] [PubMed] [Google Scholar]
- Cavazos JE, Golarai G, Sutula TP. Septotemporal variation of the supragranular projection of the mossy fiber pathway in the dentate gyrus of normal and kindled rats. Hippocampus. 1992;2:363–372. doi: 10.1002/hipo.450020404. [DOI] [PubMed] [Google Scholar]
- Celio MR, Baier W, Schärer L, de Viragh PA, Gerday C. Monoclonal antibodies directed against the calcium binding protein parvalbumin. Cell Calcium. 1988;9:81–86. doi: 10.1016/0143-4160(88)90027-9. [DOI] [PubMed] [Google Scholar]
- Chen K, Baram TZ, Soltesz I. Febrile seizures in the developing brain result in persistent modification of neuronal excitability in limbic circuits. Nature Medicine. 1999;5:888–894. doi: 10.1038/11330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulter DA, Rafiq A, Shumate M, Gong QZ, DeLorenzo RJ, Lyeth BG. Brain injury-induced enhanced limbic epileptogenesis: anatomical and physiological parallels to an animal model of temporal lobe epilepsy. Epilepsy Research. 1996;6:81–91. doi: 10.1016/s0920-1211(96)00044-7. [DOI] [PubMed] [Google Scholar]
- Deller T, Martinez A, Nitsch R, Frotscher M. A novel entorhinal projection to the rat dentate gyrus: direct innervation of proximal dendrites and cell bodies of granule cells and GABAergic neurons. Journal of Neuroscience. 1996;16:3322–3333. doi: 10.1523/JNEUROSCI.16-10-03322.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon CE, Lyeth BG, Povlishock JT, Findling RL, Hamm RJ, Marmarou A, Young HF, Hayes RL. A fluid percussion model of experimental brain injury in the rat. Journal of Neurosurgery. 1989;67:110–119. doi: 10.3171/jns.1987.67.1.0110. [DOI] [PubMed] [Google Scholar]
- Epstein P, Means AR, Berchtold MW. Isolation of a rat parvalbumin gene and full length cDNA. Journal of Biological Chemistry. 1986;261:5886–5891. [PubMed] [Google Scholar]
- Erlander MG, Tillakaratne NJK, Feldblum S, Patel N, Tobin AJ. Two genes encode distinct glutamate decarboxylases. Neuron. 1991;7:91–100. doi: 10.1016/0896-6273(91)90077-d. [DOI] [PubMed] [Google Scholar]
- Freund TF, Buzsáki G. Interneurons of the hippocampus. Hippocampus. 1996;6:345–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Frotscher M, Seress L, Schwerdtfeger WK, Buhl E. The mossy cells of the fascia dentata: a comparative study of their fine structure and synaptic connections in rodents and primates. Journal of Comparative Neurology. 1991;312:145–163. doi: 10.1002/cne.903120111. [DOI] [PubMed] [Google Scholar]
- Graber KD, Prince DA. Tetrodotoxin prevents post-traumatic epileptogenesis in rats. Annals of Neurology. 1999;46:234–242. doi: 10.1002/1531-8249(199908)46:2<234::aid-ana13>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Grady MS, Lam AM. Management of acute head injury. In: Lam AM, editor. Anesthetic Management of Head Injury. NY, USA: McGraw-Hill, Inc.; 1995. pp. 87–100. [Google Scholar]
- Hollrigel GS, Soltesz I. Slow kinetics of miniature IPSCs during early postnatal development in granule cells of the dentate gyrus. Journal of Neuroscience. 1997;17:5119–5128. doi: 10.1523/JNEUROSCI.17-13-05119.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollrigel GS, Toth K, Soltesz I. Neuroprotection by propofol in acute mechanical injury: role of GABAergic inhibition. Journal of Neurophysiology. 1996;76:2412–2422. doi: 10.1152/jn.1996.76.4.2412. [DOI] [PubMed] [Google Scholar]
- Houser CR, Esclapez M. Localization of mRNAs encoding two forms of glutamic acid decarboxylase in the rat hippocampal formation. Hippocampus. 1994;4:530–545. doi: 10.1002/hipo.450040503. [DOI] [PubMed] [Google Scholar]
- Hsu M, Buzsáki G. Vulnerability of mossy fiber targets in the rat hippocampus to forebrain ischemia. Journal of Neuroscience. 1993;13:3964–3979. doi: 10.1523/JNEUROSCI.13-09-03964.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson MB, Scharfman HE. Positive feedback from hilar mossy cells to granule cells in the dentate gyrus revealed by voltage-sensitive dye and microelectrode recording. Journal of Neurophysiology. 1996;76:601–616. doi: 10.1152/jn.1996.76.1.601. [DOI] [PubMed] [Google Scholar]
- Jefferys JGR, Traub RD. lsquo;Dormant’ inhibitory neurons: do they exist and what is their functional impact? Epilepsy Research. 1998;32:104–113. doi: 10.1016/s0920-1211(98)00044-8. [DOI] [PubMed] [Google Scholar]
- Jeng J-M, Toth Z, Soltesz I. Rapid increase in excitatory glutamatergic drive to dentate interneurons following head trauma. Society for Neuroscience Abstracts. 1998:129.9. [Google Scholar]
- Jennet B. Epilepsy After Non-missile Head Injuries. UK: W. Heinemann Medical Books; 1975. [Google Scholar]
- Jennett B, Lewin W. Traumatic epilepsy after closed head injuries. Journal of Neurology, Neurosurgery and Psychiatry. 1960;23:295–301. doi: 10.1136/jnnp.23.4.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leránth C, Szeidemann Z, Hsu M, Buzsáki G. AMPA receptors in the rat and primate hippocampus: a possible absence of GluR2/3 subunits in most interneurons. Neuroscience. 1996;70:631–652. doi: 10.1016/s0306-4522(96)83003-x. [DOI] [PubMed] [Google Scholar]
- LeRoux PD, Grady MS. Epidemology of head injury. In: Lam AM, editor. Anesthetic Management of Head Injury. NY, USA: McGraw-Hill, Inc.; 1995. pp. 1–11. [Google Scholar]
- Lisman JE. Relating hippocampal circuitry to function: recall of memory sequences by reciprocal dentate-CA3 interactions. Neuron. 1999;22:233–242. doi: 10.1016/s0896-6273(00)81085-5. [DOI] [PubMed] [Google Scholar]
- Lorente de No R. Studies on the structure of the cerebral cortex. II. Continuation of the study of the ammonic system. Journal of Psychology and Neurology Leipzig. 1934;46:113–177. [Google Scholar]
- Lowenstein DH, Thomas MJ, Smith DH, McIntosh TK. Selective vulnerability of dentate hilar neurons following traumatic brain injury: a potential mechanistic link between head trauma and disorders of the hippocampus. Journal of Neuroscience. 1992;12:4846–4853. doi: 10.1523/JNEUROSCI.12-12-04846.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lübke J, Frotscher M, Spruston N. Specialized electrophysiological properties of anatomically identified neurons in the hilar region of the rat fascia dentata. Journal of Neurophysiology. 1998;79:1518–1534. doi: 10.1152/jn.1998.79.3.1518. [DOI] [PubMed] [Google Scholar]
- McIntosh TK, Vink R, Noble L, Yamakami I, Fernyak S, Soares H, Faden AL. Traumatic brain injury in the rat: characterization of a lateral fluid-percussion model. Neuroscience. 1989;28:233–244. doi: 10.1016/0306-4522(89)90247-9. [DOI] [PubMed] [Google Scholar]
- Margerison JH, Corsellis JA. Epilepsy and the temporal lobes. A clinical, electroencephalographic and neuropathological study of the brain in epilepsy, with particular reference to the temporal lobes. Brain. 1966;89:499–530. doi: 10.1093/brain/89.3.499. [DOI] [PubMed] [Google Scholar]
- Povlishock JT, Hayes RL, Michel ME, McIntosh TK. Workshop on animal models of traumatic brain injury. Journal of Neurotrauma. 1994;11:723–732. doi: 10.1089/neu.1994.11.723. [DOI] [PubMed] [Google Scholar]
- Prince DA, Jacobs K. Inhibitory function in two models of chronic epileptogenesis. Epilepsy Research. 1998;32:83–92. doi: 10.1016/s0920-1211(98)00042-4. [DOI] [PubMed] [Google Scholar]
- Rempel-Clower NL, Zola SM, Squire LR, Amaral DG. Three cases of enduring memory impairment after bilateral damage limited to the hippocampal formation. Journal of Neuroscience. 1996;16:5233–5255. doi: 10.1523/JNEUROSCI.16-16-05233.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross ST, Toth Z, Jeng J-M, Soltesz I. Downregulation of interneuronal Na+/K+-ATPase function and long-lasting amplification of inhibition in the dentate gyrus. Society for Neuroscience Abstracts. 1999:398.8. [Google Scholar]
- Salazar AM. Traumatic brain injury: the continuing epidemic. In: Hachinski VC, editor. Challenges in Neurology. Philadelphia, PA, USA: Davis; 1992. pp. 55–67. [Google Scholar]
- Scharfman HE. Differentiation of rat dentate neurons by morphology and electrophysiology in hippocampal slices: granule cells, spiny hilar neurons, and ‘fast spiking’ cells. Epilepsy Research. 1992;(suppl. 7):93–109. [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE. Electrophysiological evidence that dentate hilar mossy cells are excitatory and innervate both granule cells and interneurons. Journal of Neurophysiology. 1995;74:179–194. doi: 10.1152/jn.1995.74.1.179. [DOI] [PubMed] [Google Scholar]
- Scharfman HE, Schwartzkroin PA. Protection of dentate hilar cells from prolonged stimulation by intracellular calcium chelation. Science. 1989;246:257–260. doi: 10.1126/science.2508225. [DOI] [PubMed] [Google Scholar]
- Sloviter RS. Permanently altered hippocampal structure, excitability, and inhibition after experimental status epilepticus in the rat: the ‘dormant basket cell’ hypothesis and its possible relevance to temporal lobe epilepsy. Hippocampus. 1991;1:41–66. doi: 10.1002/hipo.450010106. [DOI] [PubMed] [Google Scholar]
- Sloviter RS. On the relationship between neuropathology and pathophysiology in the epileptic hippocampus of humans and experimental animals. Hippocampus. 1994;4:250–253. doi: 10.1002/hipo.450040304. [DOI] [PubMed] [Google Scholar]
- Soltesz I, Bourassa J, Deschenes M. The behavior of mossy cells of rat dentate gyrus during theta oscllations in vivo. Neuroscience. 1993;57:555–564. doi: 10.1016/0306-4522(93)90005-z. [DOI] [PubMed] [Google Scholar]
- Soltesz I, Mody I. Patch-clamp recordings reveal powerful GABAergic inhibition in dentate hilar neurons. Journal of Neuroscience. 1994;14:2365–2376. doi: 10.1523/JNEUROSCI.14-04-02365.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltesz I, Mody I. Ca2+-dependent plasticity of miniature inhibitory postsynaptic currents after amputation of dendrites in central neurons. Journal of Neurophysiology. 1995;73:1763–1773. doi: 10.1152/jn.1995.73.5.1763. [DOI] [PubMed] [Google Scholar]
- Soltesz I, Smetters DK, Mody I. Tonic inhibition originates from synapses close to the soma. Neuron. 1995;14:1273–1283. doi: 10.1016/0896-6273(95)90274-0. [DOI] [PubMed] [Google Scholar]
- Soriano E, Frotscher M. Mossy cells of the rat fascia dentata are glutamate-immunoreactive. Hippocampus. 1994;4:65–69. doi: 10.1002/hipo.450040108. [DOI] [PubMed] [Google Scholar]
- Strowbridge BW, Schwartzkroin PA. Transient potentiation of spontaneous EPSPs in rat mossy cells induced by depolarization of a single neurone. The Journal of Physiology. 1996;494:493–510. doi: 10.1113/jphysiol.1996.sp021508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart GJ, Dodt HU, Sakmann B. Patch-clamp recordings from the soma and dendrites of neurons in brain slices using infrared video microscopy. Pflügers Archiv. 1993;423:511–518. doi: 10.1007/BF00374949. [DOI] [PubMed] [Google Scholar]
- Toth Z, Hollrigel GS, Gorcs T, Soltesz I. Instantaneous perturbation of interneuronal networks by a pressure wave-transient delivered to the neocortex. Journal of Neuroscience. 1997;17:8106–8117. doi: 10.1523/JNEUROSCI.17-21-08106.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willmore LJ. Prophylactic treatment. In: Engel J, Pedley TA, editors. Epilepsy: A Comprehensive Textbook. Philadelphia, PA, USA: Lippincott-Raven Publishers; 1997. pp. 1333–1337. [Google Scholar]


