Abstract
Whole-cell patch-clamp recordings were made from the neurons in the superficial trigeminal caudal nucleus (substantia gelatinosa) visually identified in a parasagittal brainstem slice of neonatal rat with the mandibular nerve attached.
Stimulation of the mandibular nerve at 0·03 Hz evoked compound excitatory postsynaptic potentials (EPSPs) or currents (EPSCs) in trigeminal caudal neurons. When stimulated at higher frequency (> 0·5 Hz), compound synaptic responses were largely attenuated and a small component remained. This component had a monosynaptic nature, following high-frequency stimulation (33–50 Hz) with a stable synaptic latency.
The N-methyl-D-aspartate (NMDA) receptor antagonist D(-)-2-amino-5-phosphonopentanoic acid (D-AP5, 50 μM) largely attenuated the slow polysynaptic EPSCs. The AMPA/kainate receptor antagonist 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX, 10 μM) largely attenuated monosynaptic EPSCs, but only weakly attenuated slow polysynaptic EPSCs. Simultaneous application of CNQX and D-AP5 completely abolished EPSCs. The monosynaptic EPSCs isolated by repetitive stimulation had both NMDA and non-NMDA components.
Monosynaptic EPSCs having high threshold had a relatively long latency. During repetitive stimulation (0·5–5·0 Hz), EPSCs having high threshold and long latency underwent a stepwise potentiation in an activity-dependent manner. The conduction velocity estimated for these EPSCs fell into the range of C-fibres. The activity-dependent potentiation was observed for both non-NMDA and NMDA EPSCs and was accompanied by a significant decrease in the coefficient of variation of EPSC amplitude.
We suggest that the activity-dependent potentiation of EPSCs is induced presynaptically and that it may underlie the wind-up phenomenon, an activity-dependent hyperexcitability of the primary afferent C-fibres.
The primary afferent synaptic response conveying nociceptive information has been characterized in the dorsal horn of spinal cord, where the fundamental role of glutamate receptors has been established (Schneider & Perl, 1988; Yoshimura & Jessell, 1990; King & Lopez-Garcia, 1993; Miller & Woolf, 1996). In the spinal cord, the activity-dependent neuronal hyperexcitability, the so called wind-up phenomenon, has been established for the C-fibre input (Mendell & Wall, 1965) and is thought to underlie the development and maintenance of hyperalgesia (Woolf & Thompson, 1991; for review, see Urban et al. 1994). In the brainstem, the main nociceptive relay station is the superficial layer of trigeminal caudal subnucleus (substantia gelatinosa, SG), which receives primary afferent inputs of myelinated Aβ-, Aδ- and unmyelinated C-fibres from oro-facial regions such as tooth pulp, oral mucous and facial skin (Hu et al. 1981; for reviews, see Dubner & Bennett, 1983; Sessle, 1987). The wind-up phenomenon and post-stimulus potentiation of EPSPs similar to those in the spinal cord have been observed exclusively for C-fibre inputs in the trigeminal caudal subnucleus (Hamba et al. 1992; Hamba, 1998). Whilst synaptic responses in the spinal cord have been well characterized, relatively little is known about those in the brainstem trigeminal neurons. In the present study, we have characterized the excitatory postsynaptic responses evoked in the trigeminal caudal neurons by the mandibular nerve stimulation by applying the whole-cell patch-clamp techniques to the brainstem slice preparation of young rats. Our results indicate that NMDA receptors substantially contribute to polysynaptic transmission and that the EPSCs having high threshold and long latency preferentially undergo a stepwise amplitude potentiation by repetitive stimulation. Some of these findings have been reported in a preliminary form (Onodera et al. 1998).
METHODS
Slice preparation
All experiments were performed in accordance with the guidelines of the Physiological Society of Japan. A longitudinal sagittal slice including the trigeminal caudal nucleus with mandibular nerve trunks attached was prepared from Wistar rats, 7–10 days old, after decapitation under halothane anaesthesia. All dissecting procedures were performed as reported previously (Hamba, 1998; Hamba & Onimaru, 1998) in an oxygenated (95 % O2-5 % CO2) artificial cerebrospinal fluid (aCSF) of the following composition (mM): NaCl, 124; KCl, 2.5; CaCl2, 2; MgCl2, 0.5 (unless otherwise noted); NaHCO3, 26; NaH2PO4, 2.5; glucose, 30 (pH 7.4 when equilibrated with 95 % O2 and 5 % CO2). Briefly, after the brainstem was isolated together with a mandibular nerve trunk, dye markings were made (with Pontamin Sky-Blue) at two points on the dorsal surface of the brainstem along the medial border of the trigeminal spinal tract nucleus; one at the height of obex and the other 2 mm caudal to the obex at 0.8 mm and 0.4 mm from the lateral margin of brainstem, respectively. The brainstem was then parasagittally cut with a surgical knife along the line passing through the points and then sectioned transversely at 2 mm caudal to the obex (Fig. 1A). Since the trigeminals caudalis contains thick laminated structures of SG in the medial border area (Sugimoto et al. 1997), the cutting line was made perpendicularly to line up SG neurons in a wide range of cut surface. The slice attached to a mandibular nerve trunk was submerged in a recording chamber (1.5 ml) and immobilized with a platinum grid (Edwards et al. 1989), with the cut surface up (Fig. 1B). The thickness of the slice was about 200 μm at the substantia gelatinosa region. The recording chamber was continuously superfused at 2.0-2.5 ml min−1 with the aCSF equilibrated with 95 % O2 and 5 % CO2. To block inhibitory transmission, strychnine sulfate (0.5 μM) and bicuculline methiodide (10 μM) were included in the superfusate. All experiments were carried out at room temperature (24-26°C).
Figure 1. Schematic illustrations for the parasagittal brainstem slice and the experimental arrangement.

A, dorsal and ventral views of the brainstem attached with mandibular nerve trunks. A parasagittal slice including the trigeminal spinal tract nucleus was trimmed out of the brainstem along the thick line indicated. B, the slice attached to a mandibular nerve trunk was immobilized with its cut surface upward with nylon threads glued onto a U-shaped platinum frame (Edwards et al. 1989). Whole-cell recordings were made from neurons visually identified in substantia gelatinosa in subnucleus caudalis. D, dorsal side; V, ventral side. The middle thread is approximately at the height of the obex in this scheme.
Recordings
Trigeminal neurons were visually identified under a × 40 water-immersion objective (Karl Zeiss) at the superficial layer of brainstem (50-200 μm from the dorsal margin). Whole-cell recordings were made (Edwards et al. 1989) with a patch pipette pulled from borosilicate glass (Clark Electrochemical, 1.5 mm in outer diameter, standard wall with inner filaments) and filled with an internal solution of the following composition (mM): potassium gluconate, 110; KCl, 30; Hepes, 10; MgCl2, 1; EGTA, 5; MgATP, 2; Na3GTP, 0.3. The electrode resistance was typically 5–7 MΩ. For improved voltage-clamp conditions, in experiments of the current-voltage relationship of EPSCs, potassium conductance was blocked by substitution of potassium gluconate with caesium gluconate. Access resistance of recordings (20-40 MΩ) was continuously monitored and when the value changed by > 20 %, data were discarded. The mean capacitance and resting potential of trigeminal neurons was 17 ± 1 pF (mean ±s.e.m., n = 40 cells) and -63 ± 6 mV (n = 18), respectively. In voltage-clamp recordings, the holding potential was -67 mV unless otherwise noted. The liquid junction potential between the external and internal solutions was +5.7 mV and was corrected for. To evoke EPSCs, the mandibular nerve was stimulated at 1.0 mm distal to the ganglion with a square voltage pulse (1-9 V, 0.3 ms) delivered through a bipolar tungsten electrode (Parylene coated, A-M Systems, Carlsborg, WA, USA). In some early experiments, longer pulse duration (up to 1.3 ms) was used for supramaximal stimulation. The conduction distance (7.0 mm) was estimated by measuring the length of the peripheral (1.0 mm) and central (1.5 mm) processes of the mandibular nerve and afferents descending in the trigeminal spinal tract to the recording site (4.5 mm). Data were sampled at 1–10 kHz with an LM-12 interface (Dagan). Significant difference was evaluated by Student's unpaired t test, with P < 0.05 taken as being significant.
Drugs
D-AP5 and CNQX were purchased from Tocris Cookson (Bristol, UK), (-)-bicuculline methiodide, strychnine sulfate and Pontamin Sky-Blue from Sigma (St Louis, MO, USA).
RESULTS
The primary afferent excitatory postsynaptic responses
After a whole-cell recording was established in the trigeminal caudal neurons, EPSPs or EPSCs were evoked by stimulating the mandibular nerve at low frequency (0.03 Hz) under current-clamp or voltage-clamp mode. When the stimulus intensity was gradually increased, a small and brief EPSP component first appeared, in a subset of neurons, followed by larger and slower components often accompanied by action potentials at stronger stimuli (Fig. 2Aa). However, in another subset, slow components first appeared and stronger stimuli elicited a fast component (Fig. 2Ab). These results suggest that the afferent fibres having different conduction velocities innervate trigeminal caudal neurons through monosynaptic and polysynaptic pathways. The long-lasting slow response was observed at suprathreshold stimulation in most neurons studied provided that the stimulus frequency was kept as low as 0.03 Hz.
Figure 2. Excitatory postsynaptic responses recorded from trigeminal caudal neurons in response to mandibular nerve stimulation.
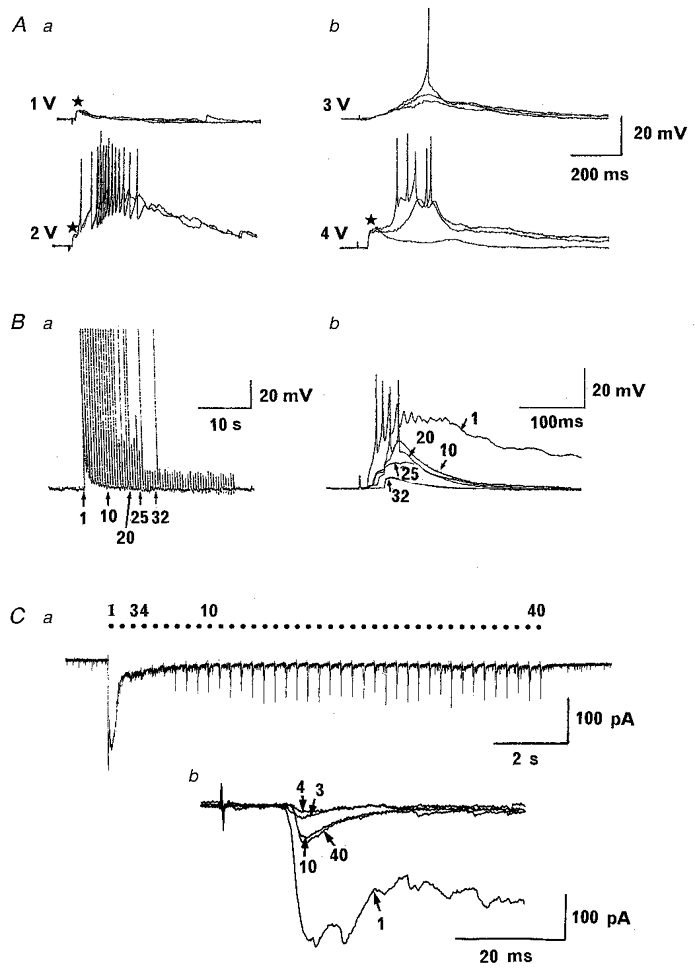
Excitatory postsynaptic potentials (EPSPs, A and B) and currents (EPSCs, C) evoked in trigeminal caudal neurons. A, mandibular nerves were stimulated at 0.03 Hz with a square pulse (0.3 ms) of different intensities as indicated in two different neurons. A short-latency fast EPSP component (asterisks) had lower threshold than slow components in one cell (a), but vice versa in another cell (b). Resting membrane potential was -64 mV in a and -71 mV in b. B and C, effects of repetitive stimulation on excitatory postsynaptic responses. B, gradual attenuation of EPSPs during 2 Hz stimulation (resting potential, -64 mV). The 1st, 10th, 20th, 25th and 32nd EPSPs are superimposed at a fast time scale (b). Note that an EPSP component with a relatively long latency remains unblocked after the 32nd stimulation. C, suppression of EPSCs during a 3.3 Hz train of stimulation followed by a potentiation in another cell under voltage clamp. The 2nd-6th EPSC was largely attenuated, but potentiated thereafter till the end of stimulation (a). Note that the 10th and 40th EPSCs had a slightly longer latency (13.8 ms) than the 1st, 3rd and 4th EPSCs (10.2 ms) (b). Synaptic responses were evoked with the supra-maximal stimulus intensity.
One of the widely used criteria for identifying monosynaptic responses is the relative resistance to repetitive stimulation (e.g. Gerber et al. 1991; Baba et al. 1994). When the stimulus frequency was raised from 0.03 Hz to 2–3 Hz, primary afferent synaptic responses became smaller and shorter, presumably due to the low safety factor for polysynaptic transmission (Fig. 2B and C). The long-lasting slow component was particularly sensitive to repetitive stimulation, disappearing in the 2nd or 3rd stimulation. In 83 out of 120 trigeminal caudal neurons studied, synaptic responses were attenuated in amplitude and duration during repetitive stimulation (1-5 Hz) and a small and short-lasting component persisted (Fig. 2B and C). In another 37 neurons, the synaptic response became undetectable during repetitive stimulation suggesting the lack of monosynaptic connection (data not shown). In 26 out of 83 neurons, synaptic responses were diminished to very small or disappeared but potentiated or reappeared during repetitive stimulation (Fig. 2C), presumably due to the activity-dependent potentiation as described below (Fig. 7).
Figure 7. Activity-dependent potentiation of EPSCs.

A, monosynaptic non-NMDA EPSCs recorded in the presence of D-AP5 (50 μM) underwent an abrupt potentiation during repetitive stimulations (0.6-5 Hz). At a stimulus frequency below 0.5 Hz, no potentiation was observed (data not shown). The potentiation occurred earlier at higher stimulus frequency (abscissa, sequence number of stimulation). Sample traces are EPSCs (3 events superimposed) at 0.8 Hz before (a) and after (b) potentiation. B, NMDA EPSCs in the presence of CNQX (10 μM) evoked at 1 and 2 Hz. Only the first NMDA EPSC had a clear polysynaptic component. Monosynaptic NMDA EPSCs showed a potentiation after the 15th (2 Hz) or 16th (1 Hz) event (b). Sample traces are whole time course at 1 Hz stimulation (left) and NMDA EPSCs before (a) and after (b) potentiation (1 Hz) superimposed (right). EPSCs were evoked by the suprathreshold stimulus.
The primary afferent monosynaptic EPSCs
The EPSCs remaining during stimulation at 3.3 Hz had synaptic latencies falling into a narrow Gaussian distribution. Figure 3 illustrates two examples of EPSCs, one having a short latency (A) and the other having a relatively long latency (B). The short latency EPSCs had a relatively low threshold (Fig. 6) and persisted up to 50 Hz stimulation with a stable latency and kinetics, although diminished in amplitude (Fig. 3A). The long latency EPSCs had high threshold and persisted up to 33 Hz also with a stable latency and kinetics (Fig. 3B). Thus, EPSCs recorded at 1–5 Hz were largely monosynaptic in nature, although minor polysynaptic components sensitive to higher frequency stimulation (> 10 Hz) were sometimes discerned.
Figure 3. Monosynaptic nature of EPSCs remaining after repetitive stimulation.
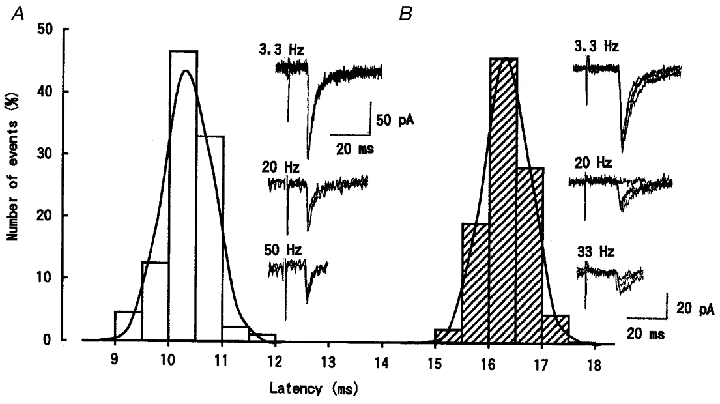
The latency histograms of EPSCs evoked at 3.3 Hz in two different trigeminal caudal neurons. EPSCs in one neuron (A) had a threshold of 1.8 V and mean latency of 10.1 ms (88 events fitted with a Gaussian distribution, □). These EPSCs persisted up to 50 Hz with a stable latency (3-4 traces superimposed in sample records). EPSCs recorded from another neuron (B) had a threshold of 5.0 V and mean latency of 16.1 ms (105 events fitted as above, ) and persisted up to 33 Hz with a stable latency (4 traces superimposed).
) and persisted up to 33 Hz with a stable latency (4 traces superimposed).
Figure 6. Threshold and latency of monosynaptic EPSCs.
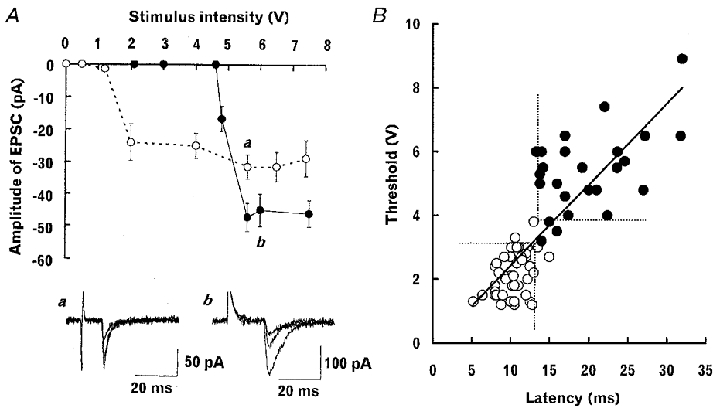
A, the relationship between the stimulus intensity and amplitude of low threshold (^) and high threshold (•) EPSCs each evoked at 2 Hz in two different trigeminal caudal neurons (superimposed sample traces in the bottom panel are 3 consecutive EPSCs at a and b, respectively). Mean amplitudes and s.e.m.s of EPSCs (10-17 events including failures) are plotted against stimulus intensity (V). B, the relationship between threshold (V) and latency (ms) for EPSCs recorded from 75 trigeminal caudal neurons. Threshold and latency are positively correlated (r = 0.81). A regression line was drawn with the least square method. Filled symbols (•) indicate EPSCs which underwent activity-dependent potentiation during 0.6-5 Hz stimulation (see Fig. 7). The dashed lines separate the high-threshold long-latency EPSCs with activity-dependent potentiation (•) from the low-threshold short-latency EPSCs with no activity-dependent potentiation (^). Most EPSCs (89 %) could be classified into one of these categories.
The voltage dependence of the monosynaptic EPSCs remaining at 1–5 Hz stimulation was studied (Fig. 4). When the trigeminal caudal neurons were depolarized under voltage clamp, the peak amplitudes of EPSCs having short latency (Fig. 4A, ^) and long latency (Fig. 4B, ▵) were both diminished and reversed their current polarities at around 0 mV. The reversed EPSCs at positive membrane potentials had clear slow components, which were blocked by D-AP5 (50 μM, Fig. 4Ab), whereas fast components were largely blocked by CNQX (10 μM) (Fig. 4Ab and Bb). Thus monosynaptic EPSCs are mediated by the AMPA/kainate and NMDA receptors. The current-voltage relationships of non-NMDA EPSCs were linear between -85 and +45 mV (Fig. 4C), whereas the NMDA EPSCs showed a typical outward rectification at the negative membrane potential (Fig. 4C, ▪) presumably due to the voltage-dependent Mg2+ block of NMDA receptor channels (Nowak et al. 1984).
Figure 4. Voltage dependence of monosynaptic EPSCs.

Voltage dependence of EPSCs recorded from two different trigeminal caudal neurons. Aa, EPSCs evoked at 3.3 Hz having a low threshold (1.8 V) and short latency (10.2 ms). Averaged EPSC (from 5 consecutive events in this and following figures) at each holding potential is superimposed. Ab, non-NMDA component isolated by D-AP5 (50 μM, left) and NMDA component isolated by CNQX (10 μM, right) at holding potentials of +45 and -65 mV (4 traces superimposed). An asterisk indicates a spontaneous EPSC. Ba, EPSCs evoked at 5 Hz having a relatively high threshold (6.0 V) and long latency (19.7 ms) superimposed as above. Bb, NMDA component isolated by CNQX (10 μM) at each holding potential superimposed. C, current-voltage relationships for the peaks of non-NMDA EPSCs (Aa, ^; Ba, ▵) and NMDA EPSCs (Bb, ▪).
Pharmacological properties of compound EPSCs
We next examined pharmacological properties of the compound EPSCs evoked at 0.03 Hz stimulation. The NMDA receptor antagonist D-AP5 largely suppressed the compound EPSCs, sparing only the small and short-lasting component (Fig. 5Aa and Ba). This non-NMDA component followed with a stable latency during 10 Hz stimulation, but the decay time of EPSCs became shorter at higher frequency (cf. Fig. 3), suggesting that the non-NMDA EPSCs contained both monosynaptic and polysynaptic components (Fig. 5Ab). In contrast to D-AP5, the AMPA/kainate receptor antagonist CNQX (10 μM) attenuated the fast EPSC components with less effect on the slow components (Fig. 5Ac and Bb). In the presence of CNQX, mandibular nerve stimulation could still elicit a large and slow EPSP accompanied by a burst of action potentials (Fig. 5Ad). Simultaneous application of D-AP5 (50 μM) and CNQX (10 μM) completely abolished EPSCs (Fig. 5Aa and Ba) indicating that the primary afferent EPSCs are predominantly mediated by ionotropic glutamate receptors. These pharmacological features of EPSCs were consistent in all trigeminal caudal neurons examined (n = 7) irrespective of whether the inhibitory synaptic transmission was blocked by bicuculline and strychnine (Fig. 5B) or not.
Figure 5. Pharmacological properties of EPSCs.
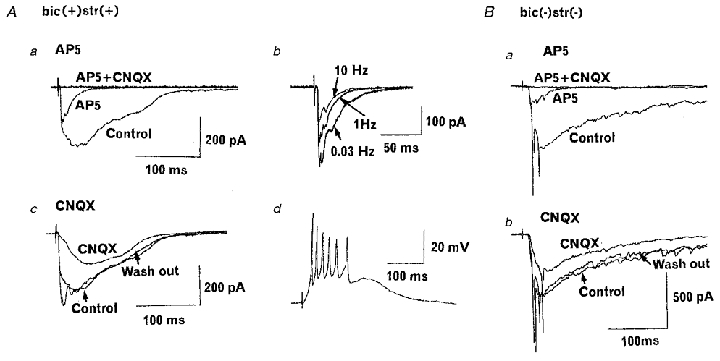
A, EPSCs evoked at 0.03 Hz in a caudal neuron. The aCSF contained bicuculline (10 μM), strychnine (0.5 μM) and 1 mM Mg2+. Aa, D-AP5 (50 μM) largely attenuated slow EPSC components (AP5) and further addition of CNQX (10 μM) completely abolished the synaptic current (AP5 + CNQX). Averaged records from 5 events each are superimposed. Ab, EPSCs remaining in the presence of D-AP5 evoked at 0.03, 1 and 10 Hz (averaged EPSCs superimposed). Ac, CNQX (10 μM) attenuated EPSCs in a reversible manner. Ad, EPSPs remaining in the presence of CNQX trigger action potentials. Resting potential was -67 mV. B, essentially the same results as above obtained in the absence of bicuculline and strychnine and in aCSF containing 0.5 mM Mg2+.
Threshold and latency of the monosynaptic EPSCs
The monosynaptic EPSC evoked at 1–5 Hz showed a discrete threshold for the graded stimulus intensity. Figure 6A illustrates typical examples for low-threshold EPSCs (a) and high-threshold EPSCs (b) recorded from two different trigeminal caudal neurons. The threshold and latency largely varied among inputs but the EPSCs having higher threshold tended to have a longer latency and vice versa with a clear positive correlation between these parameters (Fig. 6B, correlation coefficient r = 0.81). Among 83 trigeminal caudal neurons receiving monosynaptic inputs, 61 neurons (73 %) showed either high-threshold long-latency or low-threshold short-latency EPSCs, whereas in 22 neurons (27 %) both types of EPSCs could be evoked with different stimulus strength.
Activity-dependent potentiation of monosynaptic EPSC
A peculiar phenomenon was observed for the high-threshold long-latency EPSCs. During repetitive stimulation (0.6-5.0 Hz) with suprathreshold intensity, the monosynaptic non-NMDA EPSC recorded in the presence of D-AP5 abruptly underwent a potentiation, reaching a new greater amplitude (Fig. 7A). This stepwise potentiation was apparently activity dependent, since it occurred earlier at higher frequencies of stimulation. After potentiation, EPSCs sometimes went back and forth between the original and new levels (see 0.8 Hz), or further increased to the third level (observed in 8 neurons, not shown) during the repetitive stimulation. When the stimulus frequency was returned to 0.03 Hz, EPSCs returned to the original level within a few minutes (data not shown). A similar activity-dependent potentiation was observed also for the monosynaptic NMDA EPSCs at 1–2 Hz in the presence of CNQX (observed in 4 neurons, Fig. 7B). In the case illustrated in Fig. 7B, an NMDA EPSC was not detectable up to the 14th (at 2 Hz, ^) or the 15th (at 1 Hz, •, a) response, but abruptly potentiated thereafter (b). A similar phenomenon observed for both non-NMDA and NMDA EPSCs suggests that a presynaptic mechanism may underlie this phenomenon. To further examine whether the induction site is presynaptic or postsynaptic, we have measured the coefficient of variation (c.v. =s.d./mean) in the amplitude of non-NMDA EPSCs before and after the potentiation. This parameter decreases when the transmitter release is increased and vice versa (del Castillo & Katz, 1954), and is therefore widely used for identifying the site of drug actions (e.g. Forsythe & Clements, 1990). In an example illustrated in Fig. 7A, the c.v. of EPSCs (0.8 Hz) was 0.15 before potentiaton but decreased to 0.11 after potentiation. In eight neurons, the c.v. of non-NMDA EPSCs after potentiation was significantly less than control (52 ± 6.6 %, P < 0.01). These results strongly suggest that the site for the induction of the activity-dependent potentiation is presynaptic.
This activity-dependent potentiation was observed in 35 % (26 out of 75) of EPSCs tested, and predominantly for the high-threshold long-latency EPSCs (Fig. 6B, •). Thus, based upon three criteria, (i) threshold, (ii) latency and (iii) whether or not the activity-dependent potentiation is induced, monosynaptic EPSCs recorded from trigeminal caudal neurons can be classified into two main categories. One category constitutes 29 % of EPSCs and had high threshold (mean, 5.7 ± 0.2 V), long latency (20 ± 1.2 ms) and activity-dependent potentiation (n = 22). Another category (60 %, n = 45) had relatively low threshold (2.0 ± 0.1 V), short latency (10 ± 0.3 ms) and did not show activity-dependent potentiation. Eight EPSCs (11 %) fell outside of these categories.
DISCUSSION
The superficial trigeminal caudal nucleus is a nociceptive relay station, where myelinated Aβ-, Aδ- and unmyelinated C-fibres converge (Hu et al. 1981; Dubner & Bennett, 1983; Hayashi, 1985; Sessle 1987; Sugimoto et al. 1997). Excitatory postsynaptic responses evoked in the trigeminal caudal neurons by suprathreshold mandibular nerve stimulation had multiple components with different time course and synaptic latency. The long-lasting slow component of compound EPSCs was highly sensitive to repetitive stimulation and could remain stable only at a low frequency such as 0.03 Hz. According to the conventionally used criterion for the monosynaptic responses (Gerber et al. 1991; Baba et al. 1994), those EPSCs disappearing during 1–5 Hz stimulation are likely to be mediated polysynaptically. It might be argued that the slow EPSCs are monosynaptically mediated but sensitive to repetitive stimulation because of postsynaptic receptor desensitization. However, this is unlikely because the slow EPSCs are sensitive to D-AP5 (Fig. 5), whereas the D-AP5-sensitive EPSCs remained throughout 1–5 Hz stimulation (Figs 4 and 7). The monosynaptic EPSCs isolated by repetitive stimulation had both non-NMDA and NMDA receptor components. These features of primary afferent EPSCs were common for both the long-latency and short-latency responses.
The monosynaptic EPSCs recorded from trigeminal caudal neurons by mandibular nerve stimulation can be classified into two categories on the criteria of threshold, synaptic latency and whether or not an activity-dependent potentiation occurs during repetitive stimulation. The mean synaptic latency of the EPSCs having high threshold and activity-dependent potentiation was 20 ms. From the approximate distance between the stimulating and recording electrodes (7 mm) and allowing 1 ms for the onset of action potential and a synaptic delay, the conduction velocity is estimated to be 0.4 m s−1. This value falls into the range of the conduction velocity of C-fibres (Fulton, 1987), which remains similar during postnatal development (Fulton, 1987; Fitzgerald, 1987; Hamba, 1998). In contrast, conduction velocity estimated for the low-threshold and short-latency EPSCs expressing no stepwise potentiation was 0.8 m s−1. This is slower than the conduction velocity of mature A-fibres (Villière & McLachlan, 1996) but may represent the conduction velocity of a promyelinated form of Aβ- or Aδ-fibres found in neonatal animals (Fulton, 1987). EPSCs evoked by suprathreshold stimulation contained both mono- and polysynaptic components arising from multiple inputs having different conduction velocity. This may explain the apparent shift in the latency of the earliest EPSC component during repetitive stimulation (Fig. 2B and C).
In adult rats, the conduction velocity of C-fibre has been reported to slow down substantially during high-frequency stimulation, possibly due to a prolonged after-hyperpolarization caused by Ca2+-dependent K+ conductances (Gee et al. 1996). However, the high-threshold long-latency EPSCs recorded from trigeminal caudal neurons did not show slowing of synaptic latency during high-frequency stimulation. This discrepancy may be due to the fewer stimuli used (< 20) and shorter conduction distance in this present study. However, it is also possible that the mechanism required for slowing conduction velocity has not been fully established in C-fibres of neonatal rats.
Our results indicate a substantial contribution of NMDA receptors to polysynaptic transmission. This contrasts with reports for the adult rat spinal cord, where non-NMDA receptors play a major role in both the monosynaptic and polysynaptic transmission (Yoshimura & Jessell, 1990; King & Lopez-Garcia, 1993; Yoshimura & Nishi, 1993). However, a significant role for NMDA receptors in synaptic transmission has been reported recently in the neonatal rat spinal cord (Bardoni et al. 1998; Bellingham et al. 1998). Thus, the major contribution of NMDA receptors to polysynaptic transmission observed in the present study may be due, at least in part, to the neonatal stage of the animals used. Since polymodal nociceptors are totally mature at birth (Fitzgerald, 1987), through polysynaptic transmission, NMDA receptors may play an important role in the amplification of nociceptive signals in neonatal animals.
In the present study, even after repetitive stimulation, we were unable to evoke non-glutamatergic responses such as those mediated by tachykinins (Urban & Randic, 1984; for review see Otsuka & Yoshioka, 1993). This may not be surprising since neurokinin receptors are sparse in substantia gelatinosa (Bleazard et al. 1994). However, it is also possible that the non-glutamatergic responses might be masked by the opiate receptor-mediated presynaptic inhibition (Hori et al. 1992).
The activity-dependent stepwise potentiation was observed specifically for the high-threshold long-latency EPSCs possibly mediated by C-fibres. Occurrence of this phenomenon for both the non-NMDA and NMDA EPSCs, together with a decrease in the coefficient of variation of EPSC amplitude after potentiation, suggests a presynaptic mechanism. Upon repetitive activation of peripheral nerves, facilitation of transmitter release may occur simultaneously at many C-fibre terminals. The activity-dependent increase in neuronal excitability during stimulation, wind-up (Mendell & Wall, 1965), and short-term and long-term potentiations after conditioning stimulation are suggested to underlie some forms of hyperalgesia (Urban et al. 1994). The mechanisms underlying wind-up, short-term and long-term potentiations have been attributed to postsynaptic changes involving NMDA receptors (Dickenson & Sullivan, 1987; Woolf & Thompson, 1991; Randic et al. 1993; Hamba, 1998) and neurokinin receptors (Urban et al. 1994). However, our present results suggest a possible involvement of presynaptic mechanisms in the wind-up phenomenon, at least in these neonatal animals. The NMDA receptor antagonists blocked wind-up (Dickenson & Sullivan, 1987; Woolf & Thompson, 1991) in adult rat spinal cord and short-term potentiation of trigeminal EPSPs in neonatal animals (Hamba, 1998). However, if polysynaptic EPSCs are attenuated by the NMDA receptor antagonists as observed here, polysynaptic amplification of the central sensitization such as wind-up or post-stimulus potentiation would be reduced. It remains to be determined whether the stepwise potentiation persists in trigeminal C-fibre afferent synaptic responses in adult animals.
The activity-dependent stepwise potentiation observed for the high-threshold input in trigeminal neurons may be compared to the presynaptically mediated frequency facilitation at the hippocampal mossy fibre-CA3 pyramidal synapse (Salin et al. 1996). Discrete steps of potentiation observed for trigeminal synapses may be due to a limited number of release sites simultaneously recruited at the trigeminal synapse. One of the possible mechanisms underlying the stepwise potentiation at the trigeminal synapse would be the presynaptic residual Ca2+ by the preceding synaptic activation, which may recruit otherwise ‘silent’ presynaptic release sites for transmitter release. Another possible mechanism would be the summed effect of residual activity from a preceding action potential, which may overcome branch point conduction failure (Debanne et al. 1997). Whatever the mechanism, this short-term synaptic plasticity together with the long-term plasticity (Hamba et al. 2000) seems a significant property for the C-fibre inputs and therefore may be important in the modulation of nociceptive information.
Acknowledgments
We are grateful to Drs Motoy Kuno, Toshiya Manabe, Akiko Momiyama, Brian Robertson, Akira Takeuchi and Megumu Yoshimura for critical readings of this manuscript. This study was supported by the ‘Research for the Future’ Program by The Japan Society for the Promotion of Sciences to T.T.
References
- Baba H, Yoshimura M, Nishi S, Shimoji K. Synaptic responses of substantia gelatinosa neurones to dorsal column stimulation in rat spinal cord in vitro. The Journal of Physiology. 1994;478:87–99. doi: 10.1113/jphysiol.1994.sp020232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardoni R, Magherini PC, MacDermott AB. NMDA EPSCs at glutamatergic synapses in the spinal cord dorsal horn of the postnatal rat. Journal of Neuroscience. 1998;18:6558–6567. doi: 10.1523/JNEUROSCI.18-16-06558.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellingham MC, Lim R, Walmsley B. Developmental changes in EPSC quantal size and quantal content at a central glutamatergic synapse in rat. The Journal of Physiology. 1998;511:861–869. doi: 10.1111/j.1469-7793.1998.861bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleazard L, Hill RG, Morris R. The correlation between the distribution of the NK1 receptor and the actions of tachykinin agonists in the dorsal horn of the rat indicates that substance P does not have a functional role on substantia gelatinosa (lamina II) neurons. Journal of Neuroscience. 1994;14:7655–7664. doi: 10.1523/JNEUROSCI.14-12-07655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debanne D, Guerineau NC, Gahwiler BH, Thompson SM. Action-potential propagation gated by an axonal IA- like K+ conductance in hippocampus. Nature. 1997;389:286–289. doi: 10.1038/38502. [DOI] [PubMed] [Google Scholar]
- Del Castillo J, Katz B. Quantal components of the end-plate potential. The Journal of Physiology. 1954;124:560–573. doi: 10.1113/jphysiol.1954.sp005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickenson AH, Sullivan AF. Evidence for a role of the NMDA receptor in the frequency dependent potentiation of deep rat dorsal horn nociceptive neurones following C fibre stimulation. Neuropharmacology. 1987;26:1235–1238. doi: 10.1016/0028-3908(87)90275-9. [DOI] [PubMed] [Google Scholar]
- Dubner R, Bennett GJ. Spinal and trigeminal mechanisms of nociception. Annual Review of Neuroscience. 1983;6:381–418. doi: 10.1146/annurev.ne.06.030183.002121. [DOI] [PubMed] [Google Scholar]
- Edwards FA, Konnerth A, Sakmann B, Takahashi T. A thin slice preparation for patch clamp recordings from neurones of the mammalian central nervous system. Pflügers Archiv. 1989;414:600–612. doi: 10.1007/BF00580998. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M. Cutaneous primary afferent properties in the hind limb of the neonatal rat. The Journal of Physiology. 1987;383:79–92. doi: 10.1113/jphysiol.1987.sp016397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsythe ID, Clements JD. Presynaptic glutamate receptors depress excitatory monosynaptic transmission between mouse hippocampal neurones. The Journal of Physiology. 1990;429:1–16. doi: 10.1113/jphysiol.1990.sp018240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton BP. Postnatal changes in conduction velocity and soma action potential parameters of rat dorsal root ganglion neurones. Neuroscience Letters. 1987;73:125–130. doi: 10.1016/0304-3940(87)90005-x. [DOI] [PubMed] [Google Scholar]
- Gee MD, Lynn B, Cotsell B. Activity-dependent slowing of conduction velocity provides a method for identifying different functional classes of C-fiber in the rat saphenous nerve. Neuroscience. 1996;73:667–675. doi: 10.1016/0306-4522(96)00070-x. [DOI] [PubMed] [Google Scholar]
- Gerber G, Cerne R, Randic M. Participation of excitatory amino acid receptors in the slow excitatory synaptic transmission in rat spinal dorsal horn. Brain Research. 1991;561:236–251. doi: 10.1016/0006-8993(91)91600-6. [DOI] [PubMed] [Google Scholar]
- Hamba M. Stimulation-induced responses of the trigeminal caudal neurons in the brainstem preparation isolated from newborn rats. Brain Research. 1998;785:66–74. doi: 10.1016/s0006-8993(97)01382-6. [DOI] [PubMed] [Google Scholar]
- Hamba M, Hisamitsu H, Muro M. Wind-up of tooth pulp-evoked responses and its suppression in rat trigeminal caudal neurons. Brain Research Bulletin. 1992;29:883–889. doi: 10.1016/0361-9230(92)90160-y. [DOI] [PubMed] [Google Scholar]
- Hamba M, Onimaru H. Newborn rat brainstem preparation with the trigeminal nerve attached for pain study. Brain Research Protocols. 1998;3:7–13. doi: 10.1016/s1385-299x(98)00015-4. [DOI] [PubMed] [Google Scholar]
- Hamba M, Onodera K, Takahashi T. Long-term potentiation of primary afferent neurotransmission at trigeminal synapses of juvenile rats. European Journal of Neuroscience. 2000 doi: 10.1046/j.1460-9568.2000.01028.x. in the Press. [DOI] [PubMed] [Google Scholar]
- Hayashi H. Morphology of terminations of small and large myelinated trigeminal primary afferent fibers in the cat. Journal of Comparative Neurology. 1985;240:71–89. doi: 10.1002/cne.902400106. [DOI] [PubMed] [Google Scholar]
- Hori Y, Endo K, Takahashi T. Presynaptic inhibitory action of enkephalin on excitatory transmission in superficial dorsal horn of rat spinal cord. The Journal of Physiology. 1992;450:673–685. doi: 10.1113/jphysiol.1992.sp019149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu JW, Dostrovsky JO, Sessle BJ. Functional properties of neurons in cat trigeminal subnucleus caudalis (medullary dorsal horn): I. Responses to oral-facial noxious and nonnoxious stimuli and projections to thalamus and subnucleus oralis. Journal of Neurophysiology. 1981;45:173–192. doi: 10.1152/jn.1981.45.2.173. [DOI] [PubMed] [Google Scholar]
- King AE, Lopez-Garcia JA. Excitatory amino acid receptor-mediated neurotransmission from cutaneous afferents in rat dorsal horn in vitro. The Journal of Physiology. 1993;472:443–457. doi: 10.1113/jphysiol.1993.sp019955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendell LM, Wall PD. Responses of single dorsal cord cells to peripheral cutaneous unmyelinated fibres. Nature. 1965;206:97–99. doi: 10.1038/206097a0. [DOI] [PubMed] [Google Scholar]
- Miller BA, Woolf CJ. Glutamate-mediated slow synaptic currents in neonatal rat deep dorsal horn neurons in vitro. Journal of Neurophysiology. 1996;76:1465–1476. doi: 10.1152/jn.1996.76.3.1465. [DOI] [PubMed] [Google Scholar]
- Nowak L, Bregestovski P, Ascher P, Herbet A, Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurons. Nature. 1984;307:462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Onodera K, Hamba M, Takahashi T. Activity-dependent potentiation of EPSCs induced by the stimulation of nociceptive afferent in rat trigeminal caudalis neurons. Proceedings of the Australian Physiological and Pharmacological Society. 1998;29:168P. [Google Scholar]
- Otsuka M, Yoshioka K. Neurotransmitter functions of mammalian tachykinins. Physiological Reviews. 1993;73:229–308. doi: 10.1152/physrev.1993.73.2.229. [DOI] [PubMed] [Google Scholar]
- Randic M, Jiang MC, Cerne R. Long-term potentiation and long-term depression of primary afferent neurotransmission in the rat spinal cord. Journal of Neuroscience. 1993;13:5228–5241. doi: 10.1523/JNEUROSCI.13-12-05228.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salin PA, Scanziani M, Malenka RC, Nicoll RA. Distinct short-term plasticity at two excitatory synapses in the hippocampus. Proceedings of the National Academy of Sciences of the USA. 1996;93:13304–13309. doi: 10.1073/pnas.93.23.13304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider SP, Perl ER. Comparison of primary afferent and glutamate excitation of neurons in the mammalian spinal dorsal horn. Journal of Neuroscience. 1988;8:2062–2073. doi: 10.1523/JNEUROSCI.08-06-02062.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessle BJ. The neurobiology of facial and dental pain: present knowledge, future directions. Journal of Dental Research. 1987;66:962–981. doi: 10.1177/00220345870660052201. [DOI] [PubMed] [Google Scholar]
- Sugimoto T, Fujiyoshi Y, He Y-F, Xiao C, Ichikawa H. Trigeminal primary projection to the rat brain stem sensory trigeminal nuclear complex and surrounding structures revealed by anterograde transport of cholera toxin B subunit-conjugated and Bandeiraea simplicifolia isolectin B4-conjugated horseradish peroxidase. Neuroscience Research. 1997;28:361–371. doi: 10.1016/s0168-0102(97)00064-3. [DOI] [PubMed] [Google Scholar]
- Urban L, Randic M. Slow excitatory transmission in rat dorsal horn: possible mediation by peptides. Brain Research. 1984;290:336–341. doi: 10.1016/0006-8993(84)90952-1. [DOI] [PubMed] [Google Scholar]
- Urban L, Thompson SWN, Dray A. Modulation of spinal excitability: co-operation between neurokinin and excitatory amino acid neurotransmitters. Trends in Neurosciences. 1994;17:432–438. doi: 10.1016/0166-2236(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Villière V, McLachlan EM. Electrophysiological properties of neurons in intact rat dorsal root ganglia classified by conduction velocity and action potential duration. Journal of Neurophysiology. 1996;76:1924–1941. doi: 10.1152/jn.1996.76.3.1924. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Thompson SWN. The induction and maintenance of central sensitization is dependent on N-methyl-D-aspartic acid receptor activation; implications for the treatment of post-injury pain hypersensitivity states. Pain. 1991;44:293–299. doi: 10.1016/0304-3959(91)90100-C. [DOI] [PubMed] [Google Scholar]
- Yoshimura M, Jessell T. Amino acid-mediated EPSPs at primary afferent synapses with substantia gelatinosa neurones in the rat spinal cord. The Journal of Physiology. 1990;430:315–335. doi: 10.1113/jphysiol.1990.sp018293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura M, Nishi S. Blind patch-clamp recordings from substantia gelatinosa neurons in adult rat spinal cord slices: pharmacological properties of synaptic currents. Neuroscience. 1993;53:519–526. doi: 10.1016/0306-4522(93)90216-3. [DOI] [PubMed] [Google Scholar]


