Abstract
N-Methyl-D-aspartate (NMDA) receptor function can be modified by the action of several endogenous and exogenous modulatory processes. In the present study, we report that brief pulses of light potentiate NMDA, but not non-NMDA glutamatergic receptor-mediated whole-cell and single channel currents in rat cortical neurones in vitro. In addition, light also potentiated NMDA receptor-mediated whole-cell responses in isolated rat retinal neurones.
Potentiation of NMDA whole-cell currents in cortical neurones was readily observed during and following a brief (< 2 s) exposure of neurones to wavelengths of less than 324 nm of relatively bright light (0·09 μW μm−2). In addition, prolonged exposures (> 30 s) to visible wavelengths (> 380 nm) or to attenuated light (1-3 % transmittance of non-attenuated light) were also sufficient to enhance NMDA receptor-mediated responses.
The light-induced potentiation of NMDA receptor-mediated currents persisted for several minutes, slowly reversing to control levels with a time constant of approximately 5 min. A subsequent exposure to light could potentiate NMDA receptor-mediated currents for a second time.
Light did not alter the apparent affinity of the NMDA receptor for the co-agonists NMDA and glycine. Additionally, potentiation of the NMDA-induced currents was not mediated by a change in the pH sensitivity of the receptor. In excised outside-out membrane patches, the effects of light on NMDA-activated unitary currents were manifested as a twofold increase in channel open frequency without alterations in single channel amplitude or open time.
Our results suggest the presence of a light-sensitive moiety within the NMDA receptor, or in a closely associated structure, which affects channel properties. This previously unrecognized form of NMDA receptor modulation may provide a tool for understanding the conformational changes associated with its gating. In addition, it is possible that light may affect NMDA receptor-mediated function or dysfunction in the retina.
Activation of N-methyl-D-aspartate (NMDA) receptors and subsequent Ca2+ entry into neurones has been associated with critical physiological processes in the brain, including synaptic transmission, long-term changes in synaptic efficacy, and neuronal differentiation (Ozawa et al. 1998). Additionally, excessive NMDA receptor stimulation, leading to altered intracellular Ca2+ homeostasis and neuronal cell death, can be caused by abnormally high levels of glutamate released during stroke, trauma and seizures (Doble, 1999). Because of the multitude of roles that the NMDA receptor plays in neuronal function and survival, its activity is tightly regulated. A number of different intracellular and extracellular factors have been shown to modulate NMDA receptor function (McBain & Mayer, 1994; Dingledine et al. 1999). For example, the receptor is sensitive to extracellular and intracellular Mg2+ block which is relieved by membrane depolarization or hyperpolarization, respectively (Nowak et al. 1984; Mayer et al. 1984; Johnson & Ascher, 1990). In addition, extracellular zinc has also been shown to block NMDA-activated currents in a subunit-dependent fashion (Legendre & Westbrook, 1990; Williams, 1996; Chen et al. 1997; Paoletti et al. 1997). Extracellular pH (Traynelis & Cull-Candy, 1990; Tang et al. 1990), redox-active substances (Aizenman et al. 1989; Tang & Aizenman, 1993), polyamines (Ransom & Stec, 1988; Williams, 1995), intracellular Ca2+ (Clark, 1990; Krupp et al. 1998; Villarroel et al. 1998), phosphorylation events (Leonard & Hell, 1997; Tingley et al. 1997; Zheng et al. 1998), and even mechanical stretch (Paoletti & Ascher, 1994; Casado & Ascher, 1999), have all been shown to modify NMDA receptor activity. Studies investigating the mode of action of these various modulators have provided important information about NMDA receptor structure and function and, significantly, enhanced our understanding of brain pathology and generated new therapeutic strategies for limiting excitotoxic neuronal injury (Dingledine et al. 1999).
We report here that the NMDA receptor can be modulated by a novel stimulus. We observed that short duration pulses of light, directed onto the soma and proximal dendrites of cultured neurones by an optical fibre, strongly potentiate NMDA receptor currents. We have investigated the mode of action of this novel modulator of the NMDA receptor using electrophysiological techniques in cultured neurones. Based on our findings, we propose that light-induced potentiation is a reversible modification of the NMDA receptor or a closely associated structure, manifested as an increase in channel open frequency. Further characterization of the actions of this novel modulator is likely to help elucidate additional structural features involved in NMDA receptor channel function. Some of the results presented here have been published in abstract form (Leszkiewicz et al. 1999).
METHODS
Tissue culture
All drugs and chemicals were obtained from Sigma unless noted otherwise. Primary cultures of neurones were obtained from the cerebral cortices of E16 Sprague-Dawley C-D rats and dissociated according to previously described methods (Hartnett et al. 1997). Pregnant rats were killed by CO2 inhalation immediately prior to removal of embryos, in accordance with national guidelines. Embryonic cortical cells were dissociated by incubation with trypsin and plated onto 35 mm tissue culture dishes containing five poly-L-lysine-coated glass coverslips. After 15 days in culture, non-neuronal cell growth was arrested using 2 μM cytosine arabinoside. Cells were maintained in culture for 20–30 days before electrophysiological recordings. The eyes from the rat embryos from which the cortical tissue was obtained were sometimes harvested to obtain acute dissociated cultures of retinal cells. Isolated retinae were treated with papain, dissociated by gentle trituration, and cultured essentially as described previously (Aizenman et al. 1988; Barres et al. 1988). Electrophysiological experiments were started 6–8 h after plating. Recordings were obtained from putative Y or α-like retinal ganglion cells, which were identified by their large size and morphology (Fukada, 1977; Perry, 1979). Acutely dissociated retinal ganglion cells have previously been shown to express functional NMDA receptors (Aizenman et al. 1988).
Electrophysiological recordings
Electrophysiological recordings were conducted using both whole-cell and outside-out patch configurations of the patch clamp technique as described earlier (Tang & Aizenman, 1993; Brimecombe et al. 1997). The extracellular recording solution contained (mM): NaCl, 150; KCl, 2.8; CaCl2, 1.0; Hepes, 10; pH was adjusted to 7.2 with 0.3 M NaOH; 0.3 μM tetrodotoxin (Calbiochem) was added when performing whole-cell recordings. In addition, 10 μM glycine was also added to this solution, except where indicated. The intracellular (pipette) solution contained (mM): CsF, 140; EGTA, 10; CaCl2, 1.0; pH was adjusted to 7.2 with CsOH. Whole-cell recordings were performed under voltage clamp with 2–3 MΩ electrodes. Partial compensation for series resistance (80 %) was performed in some experiments. Whole-cell currents were filtered at 0.5-1 kHz and digitized at 1–2 kHz. Drugs were dissolved in external solution and applied to cells using a multi-barrel fast perfusion system (Warner Instruments, Hamden, CT, USA). NMDA-induced whole-cell currents were elicited repeatedly at the beginning of each experiment and allowed to stabilize prior to light stimulation. Unitary currents were measured using 10–15 MΩ pipettes in patches exposed to 3 μM NMDA and 3 μM glycine. Single channel records were filtered at 2 kHz and digitized at 10 kHz. Open time, channel open frequency and channel amplitudes were analysed using pCLAMP 6 (Axon Instruments, Foster City, CA, USA) at a 50 % threshold detection criterion, as described earlier (Brimecombe et al. 1997). Events briefer than 180 μs were ignored. All results are expressed as means ±s.e.m.
Light stimulation
Light was directed onto cells as previously described (Kandler et al. 1998, 1999). Briefly, light from a 100 W mercury lamp (Oriel Corporation, Stratford, CT, USA) was guided onto the preparation using a 50 μm (i.d.) fused silica fibre. A 280 nm long-pass filter (Oriel) was normally placed in the path of the light beam to minimize UV damage to the cell (except where indicated). A computer-controlled shutter (Vincent Associates, Rochester, NY, USA) was used to determine the duration of the light stimulus. The fibre produced an ∼80 μm diameter light spot covering the soma and proximal dendrites of a cultured cortical neurone. For stimulating excised patches, the centre of the fibre was aligned with the electrode tip. Light emitted by the fibre was measured with a light meter (TQ8210, Advantest, Japan) in microwatts per square micrometre. This value was converted to photons per second per square micrometre, as 1 W = 1 erg s−1, and the energy of a single photon, in ergs, being E =hν. The term h is Plank's constant (6.6 × 10−27 erg s−1), and ν, frequency (1 s−1), equals C/λ, where C is the speed of light (3 × 1010 cm s−1) and λ is the wavelength of the light. Measurements were performed at 404 nm as this is the shortest wavelength that can be accurately detected with our light meter. In addition, the power spectrum of the mercury lamp used in our experiments has a substantial peak very near this wavelength (404.7 nm, Oriel). Therefore, our intensity measurements are higher than the average power spectrum for the light illuminating the cell being investigated. Finally, we evaluated whether the light stimuli used could affect the temperature of the cell under study by placing a micro-thermocoupler probe (Omega Engineering, Stamford, CT, USA) in close apposition to the fibre. No changes in temperature were recorded by the probe even after 3 min of continuous exposure to unfiltered light.
RESULTS
NMDA receptor-mediated currents are potentiated by a light stimulus
Photolysis of caged glutamate (p-hydroxyphenacyl-glutamate, 30 μM) induced by 50 ms flashes of light (>280 nm) elicited whole-cell inward currents in rat cortical neurones in culture. Non-NMDA glutamatergic receptor-mediated currents were isolated by bathing neurones in a solution containing the competitive NMDA receptor antagonist CGS-19755 (30 μM). The amplitude of these currents did not change between stimuli (Fig. 1A). Surprisingly, the amplitude of the responses in the absence of CGS-19755 (i.e. inclusion of the NMDA component of the response) increased during sequential stimuli (Fig. 1A). In order to determine the mechanism underlying the potentiation of the NMDA component of the glutamate response, we evaluated whether light could alter glutamatergic receptor function independently of its uncaging actions. In the absence of any caged compound, whole-cell currents were measured in response to NMDA (10 μM) or kainate (30 μM) both before and shortly after (< 5 s) a 600 ms light flash (>280 nm). These light stimuli induced a substantial potentiation of NMDA-activated responses (40 %± 10, n = 4). This potentiation was specific for NMDA receptor-mediated responses as the currents elicited by kainate remained unchanged (3 %± 3, n = 4; Fig. 1B). These results suggest that the aforementioned increases in whole-cell current amplitude after photolysis of caged glutamate are likely due to a potentiation of NMDA currents caused by light itself. In addition, the fact that the kainate-induced currents remained unaltered suggests that the effects of light on the NMDA receptor are most likely the result of a modification of the NMDA receptor rather than the result of non-specific cell damage. Furthermore, as all recordings were performed in the absence of extracellular Mg2+ (see Methods), the effect of light on the NMDA receptor is unlikely to reflect an alteration in the channel-blocking properties of this cation.
Figure 1. NMDA-activated responses are potentiated by light.
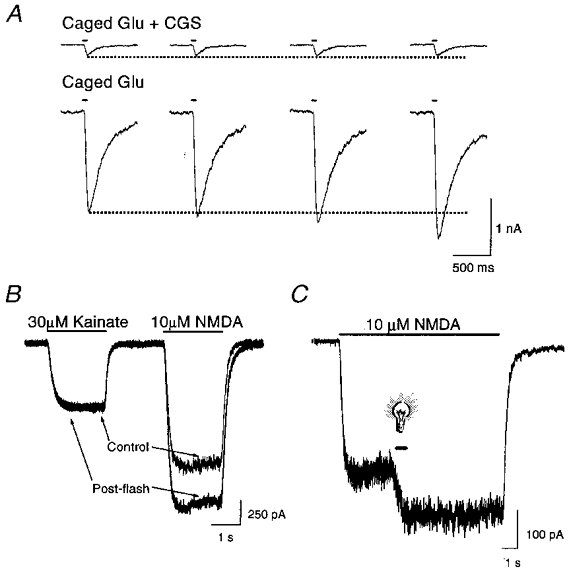
A, whole-cell currents (membrane potential: -60 mV in this and all subsequent figures) in a rat cortical neurone in vitro in response to photolysis (50 ms, > 280 nm) of caged glutamate (Glu, 30 μM) in the presence and absence of 30 μM CGS-19755 (CGS). Sample traces represent consecutive stimuli, 60 s apart, in a single neurone. Similar effects were seen in at least 4 additional cells. B, whole-cell currents elicited by 30 μM kainate and 10 μM NMDA in a cortical neurone before and shortly after (≈5 s) a brief light stimulus to the cell in the absence of caged compounds. Traces are representative of a total of 4 cells. C, whole-cell response of a neurone exposed to light (600 ms, > 280 nm; light bulb symbol) during the application of 10 μM NMDA. Similar effects were seen in 4 additional cells.
In order to observe better the rate at which NMDA-mediated responses were enhanced by light, we stimulated neurones with light during agonist exposure. A 600 ms light pulse (>280 nm) applied during a long agonist application led to a rapid potentiation of the NMDA current. The magnitude of this effect continued to increase during the light stimulus. The current remained potentiated at a steady level for the remainder of the NMDA perfusion, even after the end of the light flash (Fig. 1C). Under these conditions, it was difficult to establish the maximal effect light had on the NMDA response and to accurately determine the kinetics of the potentiation, since prolonged exposure to light which includes short wavelengths invariably damaged the cells (Mendez & Penner, 1998). However, the nearly immediate onset of the light-induced potentiation argues against the possibility that the observed current enhancement is mediated by the modification of a signal transduction pathway which would indirectly alter NMDA receptor function. Instead, potentiation of the NMDA receptor currents by light is probably due to a fast-acting direct modification of the receptor protein itself, or a closely associated structure.
We next evaluated whether light-induced potentiation of NMDA receptor-mediated responses was a reversible process. In these experiments, NMDA-activated currents were measured at several intervals following light stimulation (600 ms, >280 nm; Fig. 2A). The current amplitudes obtained at various time points following the light stimulus were normalized to the last pre-flash response and the resulting distribution was fitted with an exponential function. The resulting curve shows that the light-mediated potentiation of the responses decays with a time constant of nearly 5 min (n = 8; Fig. 2B). In these experiments, we also tested whether an additional light stimulus, presented approximately 5 min after the initial flash, could potentiate the currents a second time, which was indeed the case (Fig. 2A). The recovery of the NMDA receptor-mediated currents following the first flash, and the enhancement by the second flash, indicates that the effects of light on the receptor represent a reversible process.
Figure 2. Light-induced potentiation of NMDA-induced currents is reversible.
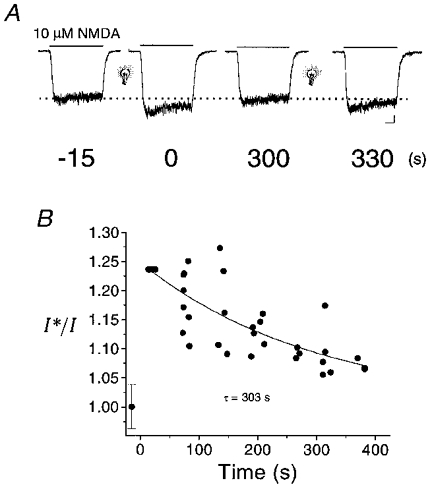
A, NMDA (10 μM)-elicited responses in a neurone before and several times after exposure to light. Numbers under the traces represent the elapsed interval (in seconds) before and following the initial flash (600 ms, > 280 nm; light bulb). A second light stimulus was given after 300 s. B, peak response amplitudes for traces such as those shown above were measured in a total of 8 cells and plotted relative to the pre-flash response amplitude (I*/I) with respect to the post-flash interval. A single exponential function was fitted to the data to estimate the time constant (τ) of the reversal of the effect of light on the NMDA response. Scale bars below right-hand trace in A represent 100 pA and 1 s.
Light-induced potentiation might be due to a direct modification of the NMDA receptor
In a first step to study the mechanism by which light potentiates NMDA receptor-mediated currents, we used single channel recordings to investigate if this type of modulation could be evoked in a cell-free environment. Unitary events were recorded from outside-out patches obtained from cortical neurones. Patches were exposed to NMDA (3 μM) both before and after light stimulation (2 s, >280 nm). Immediately after the light stimulus single channel activity was strongly increased compared to the pre-flash recording (Fig. 3A). In fact, the frequency of channel opening (F, events s−1) nearly doubled following the light stimulation (Fflash/Fcontrol= 1.8 ± 0.2; n = 4; P < 0.05, Student's two-tailed one-sample t test; Fig. 3B). In contrast, unitary amplitude (control, 3.1 ± 0.1 pA; post-flash, 3.0 ± 0.2 pA; -60 mV) and open time (control, 6.0 ± 0.5 ms; post-flash, 6.2 ± 0.2 ms) were not affected by the light exposure. These data indicate that light exerts its effects on the NMDA receptor by increasing channel opening frequency without affecting channel open time or conductance. The single channel results suggest that either the NMDA receptor itself, or a very tightly associated structure, contain the light-sensitive moiety.
Figure 3. Light increases the open channel frequency of the NMDA receptor.
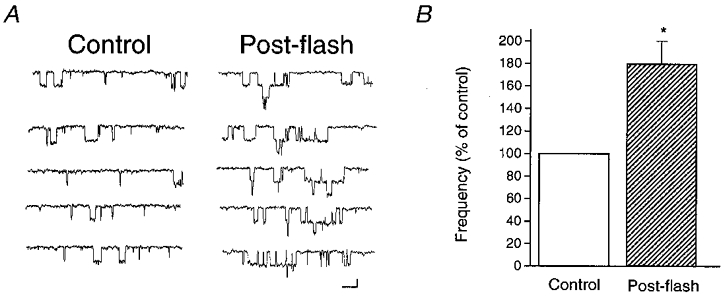
A, sample traces from an outside-out patch excised from a cortical neurone. Single channel events were activated by 3 μM NMDA. In this patch, the open channel frequency increased from 35.9 events s−1 to 62.9 events s−1 following a light pulse (2 s, > 280 nm). Total amplitude histograms and open time measurements in this and the other three patches revealed that light did not affect these two parameters (see Results). In this patch, the single channel conductance and open time values were 52.5 pS and 6.1 ms before the flash, and 49.7 pS and 6.4 ms following photostimulation. Scale bars: 2 pA, 20 ms. B, similar effects of light on open channel frequency were seen in a total of 4 patches which remained stable following the light stimulus (*P < 0.05, Student's two-tailed one sample t test, the null hypothesis being Fflash/Fcontrol= 1, where F is the frequency of channel opening).
Short wavelengths are more effective than long wavelengths in potentiating NMDA-activated currents
In all of the aforementioned experiments, we had placed a filter in the path of the light reaching the preparation that permitted only wavelengths longer that 280 nm to pass. This was done to minimize UVC light (< 290 nm) damage to the cell under study. In an additional set of studies, we employed different filters in order to determine the wavelengths at which the NMDA receptor could be modified by light. We observed that for relatively brief (600 ms) light stimuli, wavelengths longer than 324 nm did not potentiate NMDA receptor-mediated currents (Fig. 4A). This result argues that the putative protein moiety altering NMDA receptor function following photostimulation best absorbs light at short wavelengths (< 324 nm; UVB), suggesting that alterations of tryptophan, tyrosine or other ring-containing residues may be critical for this effect. Temporary disruption of these aromatic residues could affect important π orbital-dependent interactions within the protein structure (Dougherty, 1996). Since the filters used would allow the passage of equivalent levels of heat, these results also suggest that thermal energy from the light stimulus is not the causative agent in inducing potentiation of the NMDA receptor-mediated currents. Although less effective, light at wavelengths longer than 324 nm could potentiate NMDA receptor-mediated currents, provided the duration of the light pulse was increased. For instance, a 60 s stimulus utilizing a 385 nm filter was sufficient to induce an enhancement of the NMDA receptor-mediated currents (Fig. 4B). This observation raises the intriguing possibility that light may modulate retinal function via a modification of the NMDA receptor.
Figure 4. Light potentiates NMDA-activated currents at various wavelengths.
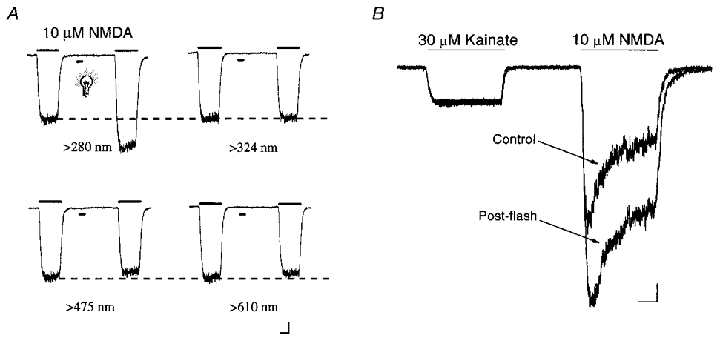
A, whole-cell currents elicited by 10 μM NMDA before and shortly after a 600 ms light pulse. Filters allowing wavelengths longer than 280 nm (n = 9), 324 nm (n = 9), 475 nm (n = 5) and 610 nm (n = 5) were placed in the path of the light reaching the cells. Scale bars: 250 pA, 1 s. B, whole-cell currents measured in a neurone in response to 10 μM NMDA before and shortly after a 60 s exposure to light using a filter that allows wavelengths longer than 385 nm to reach the cell. Scale bars: 250 pA, 0.5 s.
Proton, NMDA and glycine sites are largely unaffected by light
Changes in extracellular pH are known to modulate NMDA receptor function, as protons can non-competitively block the receptor (Traynelis & Cull-Candy, 1990; Tang et al. 1990). The pKa (negative log of the acid dissociation constant) for proton block of the NMDA receptor has been reported to be slightly more basic, but within the range for that of the amino acid histidine (Traynelis & Cull-Candy, 1990; Tang et al. 1990; Wu & Christensen, 1996). Interestingly, protonated histidine residues can be preferentially stabilized by nearby aromatic amino acids as a result of cation-π interactions (i.e. shifting the pK to the right: Loewenthal et al. 1992; Dougherty et al. 1996). We evaluated the possibility that disruption of aromatic π orbitals by the light stimulus might shift the pKa of the proton block to the left. This effect would be manifested as a response potentiation, as the NMDA receptor is normally 50 % inhibited at neutral pH (Traynelis & Cull-Candy, 1990; Tang et al. 1990). We tested the effects of a 2 s light stimulus on NMDA (30 μM)-induced whole-cell currents at a range of different extracellular pH values (5.3-9.3). Any proton-activated transient inward currents elicited by a solution change to a very acidic pH (Grantyn & Lux, 1988) were allowed to decay prior to NMDA application. Data from 22 cells were normalized to the responses obtained at pH 7.3 prior to the flash, and fitted with a logistic function (DeLean et al. 1978; Tang & Aizenman, 1993; Fig. 5A and B). We observed no significant change in the pKa of proton block after a light stimulus (6.63 ± 0.13) when compared to control (6.77 ± 0.07). This result argues against a scenario by which light indirectly affects the histidine residues responsible for proton inhibition by disrupting nearby aromatic π orbitals.
Figure 5. Proton, NMDA and glycine sites are not affected by light stimulation.
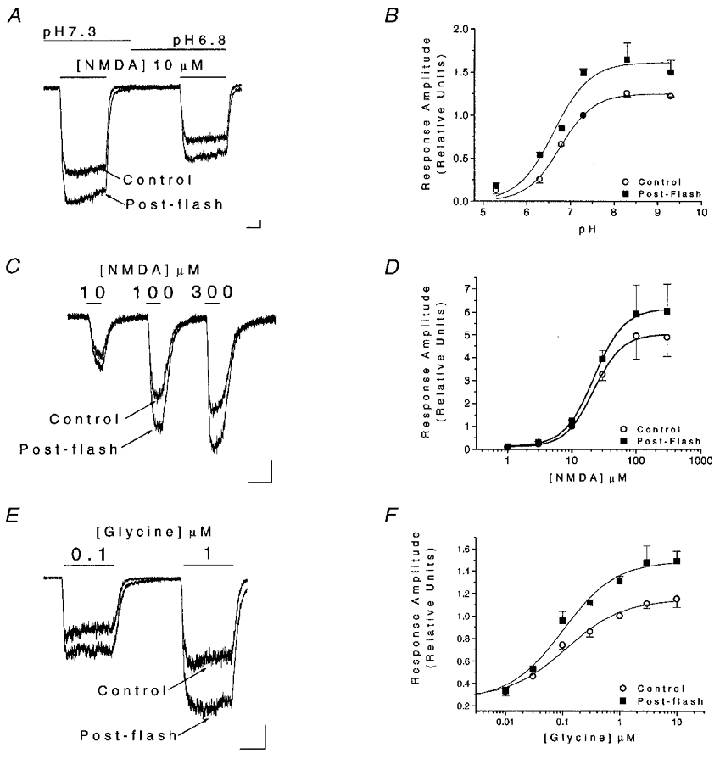
A, whole-cell currents in cultured cortical neurones elicited by 10 μM NMDA at two different extracellular pH values (7.3 and 6.8) obtained before and shortly after a light stimulus (2 s, > 280 nm). B, data such as those shown in A were obtained at extracellular pH values ranging from 5.3 to 9.3 and normalized to the pre-flash response recorded at pH 7.3. Data from a total of 22 cells were fitted with logistic functions to estimate the pK of proton inhibition before (6.8 ± 0.1) and after (6.8 ± 0.1) light stimulation. The logistic function used has the form Y = {[a -d]/[1 + (X/c)b]} +d, where Y is the response, X is the pH (or drug concentration), a is the response when X = 0, d is the response for an infinite dose, c is the pK (or EC50) and b is the ‘slope factor’, which determines the steepness of the curve and is analogous to the Hill coefficient (DeLean et al. 1978). No parameters were fixed in these fits. C, whole-cell currents elicited by increasing concentrations of NMDA (10, 100, 300 μM) in the presence of 10 μM glycine in neurones before and shortly after light stimulation (2 s, > 280 nm). D, data such as those shown in C were obtained at a range of NMDA concentrations between 1 and 300 μM in a total of 18 cells, and normalized to the pre-flash 10 μM NMDA-induced response. The resulting concentration-response relationships were fitted with logistic functions (no fixed parameters) to estimate the agonist EC50 before (21.9 ± 1.5 μM) and after (22.8 ± 0.3 μM) the flash. The ‘slope factors’ were also similar before and after the flash (1.9 and 1.8). E, whole-cell currents elicited at two concentrations of glycine (0.1 and 1 μM) in the presence of 100 μM NMDA in neurones before and shortly after photostimulation (2 s, > 280 nm). F, data such as those shown in D were obtained at a range of glycine concentrations between 0.01 and 3 μM in a total of 18 cells, and normalized to the pre-flash 1 μM glycine-induced response. The resulting concentration-response relationships were fitted with logistic functions to estimate the agonist EC50 before (115.1 ± 27.6 nM) and after (101.4 ± 24.6 nM) the flash. The ‘slope factors’ were the same for both fits (0.9). For these logistic functions a was fixed to Y when X = 0.01 μM as the background glycine concentration has been estimated to be within this range. All scale bars represent 150 pA and 1 s.
Another possible way in which light could change NMDA receptor function is by alterations in the apparent affinity of the receptor for either of its two co-agonists, NMDA and glycine. To test this, we measured the peak amplitudes of whole-cell currents in response to various concentrations of NMDA (1-300 μM, in the presence of 10 μM glycine; n = 18) before and after a light stimulus (2 s, > 280 nm). A glycine concentration-response curve was generated by measuring current in response to varying concentrations of glycine (0.01-3 μM) in the presence of 100 μM NMDA (n = 18). The mean amplitude data were normalized to the 10 μM NMDA or the 1 μM glycine mean responses, respectively, and then fitted with logistic functions (DeLean et al. 1978; Tang & Aizenman, 1993; Fig. 5C–F). The EC50 values for both NMDA (control, 21.9 ± 1.5 μM; post-flash, 22.8 ± 0.3 μM), and glycine (control, 105.5 ± 24.0 nM; post-flash, 100.0 ± 24.4 nM) were not altered by the light stimulus, suggesting that changes in apparent affinity for these agonists do not account for light-induced NMDA response potentiation.
Light at physiological intensities can modify NMDA receptor function
Using a light meter, we have measured the amount of light transmitted by the 50 μm (i.d.) optical fibre used in our studies. The measured intensity in air was 0.09 μW μm−2 at 404 nm. This corresponds to approximately 2 × 1011 photons s−1μm−2 at this wavelength (see Methods), which is the shortest wavelength we can accurately measure with our meter. We also measured sunlight (at 10.00 h) in air as 0.001 μW μm−2 (2 × 109 photons s−1μm−2) at 404 nm. This means that, at least at 404 nm, we have utilized nearly 90 times more light in our aforementioned experiments than relatively bright sunlight. We therefore determined whether light intensities closer to more physiological levels could affect the function of the NMDA receptor. These experiments were performed to begin to address the question of whether light modulation of the NMDA receptor may possibly have functional implications in the retina. For these experiments, we used light which included non-visible wavelengths (> 280 nm), as (i) the wavelength transmittance threshold for the corneas of most vertebrate species is approximately 280 nm (Fatt & Weissman, 1992), and (ii) UV light can reach the retina (Lerman, 1984). In fact, certain mammalian species possess retinal photoreceptors that are maximally sensitive in the UVA range (∼350 nm; Jacobs et al. 1991).
Neutral density filters (fused silica metallic filters; Oriel) which attenuate an incident light beam by 97 %, 99 % and 99.97 % (3 %, 1 % and 0.03 % transmittance) were used to decrease light intensity. According to the manufacturer, these filters show a substantial decrease in transmittance beginning at about 350 nm, maximally dipping near 250 nm. Hence, the attenuation at UVC and at lower UVB wavelengths is much more pronounced than at longer wavelengths. By comparison, light transmission through the human cornea is uniformly greater than 80–96 % above 400 nm, but transmittance decreases substantially at wavelengths less than 350 nm (Beems & Van Best, 1990). Similar transmission properties are also observed in the aqueous humour and in the healthy ocular lens (Lerman, 1984). Therefore, the spectral transmittance profile of the neutral density filters used in these experiments is similar to that of the human eye.
Following the recording of control NMDA-induced whole-cell responses, the attenuated light stimulus was continuously applied to the cells while NMDA responses were obtained every 30 s. We observed that the NMDA receptor-mediated currents could, in fact, be potentiated by light attenuated by 97 % and 99 %, but not by 99.97 % (Fig. 6). Thus light intensities of 0.0027 μW μm−2 and 0.0009 μW μm−2 (at 404 nm) were sufficient to induce potentiation of NMDA receptor-mediated currents, albeit requiring prolonged applications. In the future, it may be interesting to determine whether long exposure to ambient light, or perhaps even to light from the recording microscope itself, is sufficient to induce a change in NMDA receptor properties.
Figure 6. Potentiation of NMDA receptor-mediated currents by light at physiological intensities.
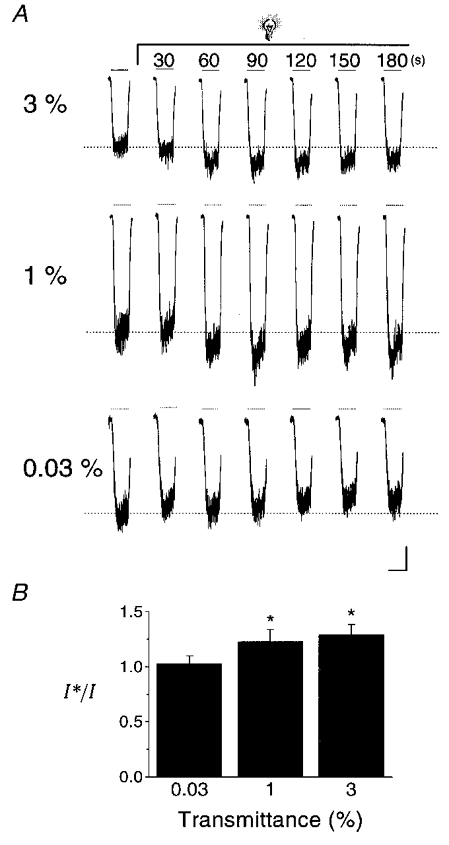
A, NMDA (10 μM)-elicited responses in cortical neurones before and during continuous light stimulation with light attenuated by neutral density filters. NMDA applications are represented by the lines above the traces. The transmittance (%) of non-attenuated light (0.09 μW μm−2 at 404 nm; see Methods) is indicated to the left of each set of traces. Time records above the traces represent the time elapsed since the initiation of the flash. B, peak response amplitudes for traces such as those shown above were measured in a total of 3–4 cells per filter and plotted relative to the pre-flash response amplitude (I*/I). A one-way analysis of variance followed by Tukey's multiple comparison tests revealed a significant difference between 0.03 % transmittance and the 1 % and 3 % transmittance groups (*P < 0.05). Scale bars: 100 pA, 2 s.
It became important at this point to examine whether NMDA receptors expressed by retinal cells could be affected by light in a manner similar to cortical receptors. Indeed, NMDA-mediated whole-cell currents obtained from acutely dissociated retinal neurones, putatively identified as ganglion cells, were effectively enhanced 2.1 (± 0.7)-fold by a 2 s light stimulus (n = 4; Fig. 7). These data suggest that NMDA receptors which are present in retinal neurones are similar to those expressed by cortical neurones, in terms of their sensitivity to light.
Figure 7. NMDA receptor-mediated responses in retinal neurones are potentiated by light exposure.
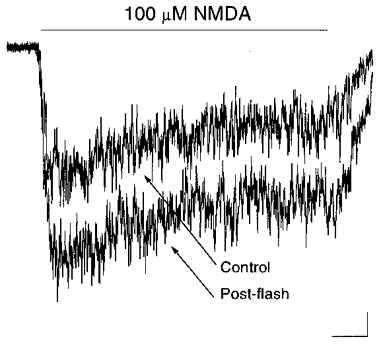
Whole-cell responses induced by 100 μM NMDA in an acutely dissociated retinal neurone before and shortly after a 2 s light stimulus (> 280 nm). Similar effects were seen in a total of 4 retinal neurones putatively identified morphologically as ganglion cells. Scale bars: 25 pA, 0.25 s.
DISCUSSION
The results presented here demonstrate that short pulses of light can modulate NMDA receptor function by potentiating agonist-activated currents. This potentiation occurred very rapidly following a high intensity light stimulus and was slowly reversible. The effects of light appeared to be selective for glutamatergic NMDA receptors as kainate-activated non-NMDA receptor-mediated currents were not affected by exposures to light. Whether other types of ligand-gated channels are affected by light is not known at this time.
The selectivity of the light effect for the NMDA receptor implies that a previously described UV light-mediated phototoxic membrane current (Mendez & Penner, 1998) does not underlie the phenomenon we describe in this study. It is noteworthy that membrane conductances induced as a consequence of the phototoxic actions of light are only observed following more than 100 s of continuous exposure to 340 nm light (Mendez & Penner, 1998). In addition to the specificity of the effect for NMDA-induced currents, the fact that the potentiation was reversible and could be induced again by a second light stimulus argues against non-specific membrane or protein damage accounting for our observations.
Light affected NMDA-induced single channel responses obtained in patches excised from neuronal membranes. These single-channel results strongly suggest that the effects of light on NMDA receptor function are due to changes in the receptor structure and not to alterations in other sub-cellular processes which can affect channel behaviour. Our experiments, however, cannot exclude the possibility that proteins that remain closely associated with the receptor even in excised patches, or perhaps even specific lipid residues, may contribute to the effects of light. Indeed, UV light near the visible range can act in similar ways to ionizing radiation or oxidative stress, leading to alterations of membrane structures, including lipid peroxidation (Morliere et al. 1991). Hence, it is possible that light-induced alteration in NMDA receptor function may be mediated by altering the interaction of the protein structure with the lipid membrane (Casado & Ascher, 1999).
Although light might alter different modulatory sites on the NMDA receptor, our studies have ruled out the agonist recognition sites as well as the proton inhibitory site as likely targets for light-induced potentiation. As the proton site shares many structural features with the zinc and redox modulatory sites (Traynelis et al. 1998), it is also not likely, albeit not yet determined, that these two latter sites are altered by light. Because the optimal wavelengths at which the effects of light were seen are in the UV range, we currently favour the hypothesis that light disrupts aromatic structures that may be critical in processes such as channel gating, but perhaps not in agonist binding (Zhong et al. 1998). As such, more detailed kinetic analyses investigating the effects of light on NMDA receptor function may be useful for determining the structural features of the protein that are involved in gating, which thus far have alluded investigators.
The observations that prolonged exposures to light above 385 nm, and to attenuated light, also altered NMDA-induced currents are intriguing because they raise the possibility that light at wavelengths and intensities encountered by cells in the retina can have non-photoreceptor-mediated physiological or pathophysiological effects. The light-induced potentiation that we observed with the 385 nm long-pass filter is not due to residual UV light transmitted by the filter. According to the manufacturer (Oriel), with a 385 nm filter there is at least a 10−7 attenuation of wavelengths below 340 nm, which, with a 60 s exposure (Fig. 4B), would be equivalent to a 6 μs stimulus at these shorter wavelengths. Such a short equivalent exposure to UV wavelengths would be insufficient to account for the potentiation (see Fig. 1C). However, we do not know if the potentiation observed at the longer wavelengths is mediated by the same mechanism as that induced by brief exposures to short wavelengths. It also remains to be shown whether NMDA receptor potentiation with long exposures at near-visible wavelengths or by attenuated light represents a physiologically relevant phenomenon, for example in retinal function or dysfunction. The fact that NMDA receptors expressed by retinal cells are also affected by light further suggests that the phenomenon described in this study may have physiological or pathophysiological implications, although this possibility clearly remains to be demonstrated in a more intact retinal preparation.
Our results also emphasize the importance of controlling for the effects of light when using photolabile ‘caged’ precursors of biologically active molecules, including NMDA (Gee et al. 1999), as has been done in some investigations (see, for example, Yang et al. 1999). However, using uncaging wavelengths longer than 324 nm (Schiller et al. 1998), and/or limiting the light exposure to a few milliseconds (Kandler et al. 1998) will help to minimize the effects of light stimulation on NMDA receptor-mediated physiological processes, unless a large number of brief stimuli are applied during a short time. Naturally, the interpretation of studies examining non-NMDA receptor-mediated responses should not be affected by our present findings.
In summary, we have found that the NMDA receptor can be modulated by light. We observed that short duration pulses of light focused onto the soma and proximal dendrites of cultured neurones strongly potentiate NMDA receptor whole-cell currents. Based on our findings, we propose that light-induced potentiation is a reversible modification of the NMDA receptor or a closely associated structure, and is manifested microscopically as an increase in channel open frequency. Further characterization of the mechanism and physiological relevance of this novel modulator is likely to help elucidate additional structural features involved in NMDA receptor channel function.
Acknowledgments
We thank W. Potthoff for aiding with the initial experiments, K. Hartnett for technical assistance, Dr Z.-P. Mi for help in establishing the retinal cultures, Dr R. Givens for p-hydroxyphenacyl-glutamate, G. A. Herin and Drs M. Cascio, E. Frank and J. Horn for helpful discussions throughout the course of these investigations. This work was supported in part by NIH grants NS29365 (E.A.) and DC04199 (K.K.), by an American Heart Association grant (D.N.L.), and by a grant from the National Alliance for Research in Schizophrenia and Depression (E.A.).
References
- Aizenman E, Frosch MP, Lipton SA. Responses mediated by excitatory amino acid receptors in solitary retinal ganglion cells from rat. The Journal of Physiology. 1988;396:75–91. doi: 10.1113/jphysiol.1988.sp016951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizenman E, Lipton SA, Loring RH. Selective modulation of NMDA responses by reduction and oxidation. Neuron. 1989;2:1257–1263. doi: 10.1016/0896-6273(89)90310-3. [DOI] [PubMed] [Google Scholar]
- Barres BA, Silverstein BE, Corey DP, Chun LLY. Immunological, morphological, and electrophysiological variation among retinal ganglion cells purified by panning. Neuron. 1988;1:791–803. doi: 10.1016/0896-6273(88)90127-4. [DOI] [PubMed] [Google Scholar]
- Beems EM, Van Best JA. Light transmission of the cornea in whole human eyes. Experimental Eye Research. 1990;50:393–395. doi: 10.1016/0014-4835(90)90140-p. [DOI] [PubMed] [Google Scholar]
- Brimecombe JC, Boeckman FA, Aizenman E. Functional consequences of NR2 subunit composition in single recombinant N-methyl-D-aspartate receptors. Proceedings of the National Academy of Sciences of the USA. 1997;94:11019–11024. doi: 10.1073/pnas.94.20.11019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casado M, Ascher P. Opposite modulation of NMDA receptors by lysophopholipids and arachidonic acid: common features with mechanosensitivity. The Journal of Physiology. 1999;513:317–330. doi: 10.1111/j.1469-7793.1998.317bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N, Moshaver A, Raymond LA. Differential sensitivity of recombinant N-methyl-D-aspartate receptor subtypes to zinc inhibition. Molecular Pharmacology. 1997;51:1015–1023. doi: 10.1124/mol.51.6.1015. [DOI] [PubMed] [Google Scholar]
- Clark GD. The effect of agonist concentration, membrane voltage and calcium on N-methyl-D-aspartate receptor desensitization. Neuroscience. 1990;39:787–797. doi: 10.1016/0306-4522(90)90261-2. [DOI] [PubMed] [Google Scholar]
- DeLean A, Munson PJ, Rodbard D. Simultaneous analysis of families of sigmoidal curves: application to bioassay, radioligand assay, and physiological dose-response curves. American Journal of Physiology. 1978;235:E97–102. doi: 10.1152/ajpendo.1978.235.2.E97. [DOI] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacological Reviews. 1999;51:7–61. [PubMed] [Google Scholar]
- Doble A. The role of excitotoxicity in neurodegenerative diseases: implications for therapy. Pharmacology and Therapeutics. 1999;81:163–221. doi: 10.1016/s0163-7258(98)00042-4. [DOI] [PubMed] [Google Scholar]
- Dougherty DA. Cation-pi interactions in chemistry and biology: a new view of benzene. Science. 1996;271:163–168. doi: 10.1126/science.271.5246.163. [DOI] [PubMed] [Google Scholar]
- Fatt I, Weissman BA. Physiology of the Eye. An Introduction to the Vegetative Functions. Boston: Butterworth-Heinemann; 1992. [Google Scholar]
- Fukada Y. A three-group classification of rat retinal ganglion cells: histological and physiological studies. Brain Research. 1977;119:327–344. doi: 10.1016/0006-8993(77)90314-6. [DOI] [PubMed] [Google Scholar]
- Gee KR, Niu L, Schaper K, Jayaraman V, Hess GP. Synthesis and photochemistry of a photolabile precursor of N-methyl-D-aspartate (NMDA) that is photolyzed in the microsecond time region and is suitable for chemical kinetic investigations of the NMDA receptor. Biochemistry. 1999;38:3140–3147. doi: 10.1021/bi9826557. [DOI] [PubMed] [Google Scholar]
- Grantyn R, Lux HD. Similarity and mutual exclusion of NMDA- and proton-activated transient Na+-currents in rat tectal neurons. Neuroscience Letters. 1988;89:198–203. doi: 10.1016/0304-3940(88)90381-3. [DOI] [PubMed] [Google Scholar]
- Hartnett KA, Stout AK, Rajdev S, Rosenberg PA, Reynolds IJ, Aizenman E. NMDA receptor-mediated neurotoxicity: a paradoxical requirement for extracellular Mg2+ in Na+/Ca2+-free solutions in rat cortical neurons in vitro. Journal of Neurochemistry. 1997;68:1836–1845. doi: 10.1046/j.1471-4159.1997.68051836.x. [DOI] [PubMed] [Google Scholar]
- Jacobs G, Neitz J, Deegan JF., II Retinal receptors in rodents maximally sensitive to ultraviolet light. Science. 1991;353:655–656. doi: 10.1038/353655a0. [DOI] [PubMed] [Google Scholar]
- Johnson JW, Ascher P. Voltage-dependent block by intracellular Mg2+ of N-methyl-D-aspartate activated channels. Biophysical Journal. 1990;57:1085–1090. doi: 10.1016/S0006-3495(90)82626-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandler K, Givens R, Katz LC. Photostimulation with caged glutamate. In: Yuste R, Lanni F, Konnerth A, editors. Imaging Neurons: a Laboratory Manual. NY, USA: Cold Spring Harbor Laboratory Press; 1999. pp. 27.1–27.9.. [Google Scholar]
- Kandler K, Katz LC, Kauer JA. Focal photolysis of caged glutamate produces long-term depression of hippocampal glutamate receptors. Nature Neuroscience. 1998;1:119–123. doi: 10.1038/368. [DOI] [PubMed] [Google Scholar]
- Krupp JJ, Vissel B, Heinemann SF, Westbrook GL. N-terminal domains in the NR2 subunit control desensitization of NMDA receptors. Neuron. 1998;20:317–327. doi: 10.1016/s0896-6273(00)80459-6. [DOI] [PubMed] [Google Scholar]
- Legendre P, Westbrook GL. The inhibition of single N-methyl-D-aspartate-activated channels by zinc ions on cultured rat neurones. The Journal of Physiology. 1990;429:429–449. doi: 10.1113/jphysiol.1990.sp018266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard AS, Hell JW. Cyclic AMP-dependent protein kinase and protein kinase C phosphorylate N-methyl-D-aspartate receptors at different sites. Journal of Biological Chemistry. 1997;272:12107–12115. doi: 10.1074/jbc.272.18.12107. [DOI] [PubMed] [Google Scholar]
- Lerman S. Biophysical aspects of corneal and lenticular transparency. Current Eye Research. 1984;3:3–14. doi: 10.3109/02713688408997182. [DOI] [PubMed] [Google Scholar]
- Leszkiewicz DN, Potthoff WK, Kandler K, Aizenman E. Selective enhancement of NMDA receptor-mediated currents by light. Society for Neuroscience Abstracts. 1999;25:1978. [Google Scholar]
- Loewenthal R, Sancho J, Fersht AR. Histidine-aromatic interactions in barnase. Elevation of histidine pKa and contribution to protein stability. Journal of Molecular Biology. 1992;224:759–770. doi: 10.1016/0022-2836(92)90560-7. [DOI] [PubMed] [Google Scholar]
- McBain CJ, Mayer ML. N-Methyl-D-aspartic acid receptor structure and function. Physiological Reviews. 1994;74:723–760. doi: 10.1152/physrev.1994.74.3.723. [DOI] [PubMed] [Google Scholar]
- Mayer ML, Westbrook GL, Guthrie PB. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984;309:261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- Mendez F, Penner R. Near-visible ultraviolet light induces a novel ubiquitous calcium-permeable cation current in mammalian cell lines. The Journal of Physiology. 1998;507:365–377. doi: 10.1111/j.1469-7793.1998.365bt.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morliere P, Moysan A, Santus R, Huppe G, Maziere JC, Dubertret L. UVA-induced lipid peroxidation in cultured human fibroblasts. Biochimica et Biophysica Acta. 1991;1084:261–268. doi: 10.1016/0005-2760(91)90068-s. [DOI] [PubMed] [Google Scholar]
- Nowak L, Bregestovski P, Ascher P, Herbet A, Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984;307:462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Ozawa S, Kamiya H, Tsuzuki K. Glutamate receptors in the mammalian central nervous system. Progress in Neurobiology. 1998;54:581–618. doi: 10.1016/s0301-0082(97)00085-3. [DOI] [PubMed] [Google Scholar]
- Paoletti P, Ascher P. Mechanosensitivity of NMDA receptors in cultured mouse central neurons. Neuron. 1994;13:645–655. doi: 10.1016/0896-6273(94)90032-9. [DOI] [PubMed] [Google Scholar]
- Paoletti P, Ascher P, Neyton J. High-affinity zinc inhibition of NMDA NR1-NR2A receptors. Journal of Neuroscience. 1997;17:5711–5725. doi: 10.1523/JNEUROSCI.17-15-05711.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry VH. The ganglion cell layer of the retina of the rat: a Golgi study. Proceedings of the Royal Society. 1979;B 204:363–375. doi: 10.1098/rspb.1979.0033. [DOI] [PubMed] [Google Scholar]
- Ransom RW, Stec NL. Cooperative modulation of [3H]MK-801 binding to the N-methyl-D-aspartate receptor-ion channel complex by L-glutamate, glycine, and polyamines. Journal of Neurochemistry. 1988;51:830–836. doi: 10.1111/j.1471-4159.1988.tb01818.x. [DOI] [PubMed] [Google Scholar]
- Schiller J, Schiller Y, Clapham DE. NMDA receptors amplify calcium influx into dendritic spines during associative pre- and postsynaptic activation. Nature Neuroscience. 1998;1:114–118. doi: 10.1038/363. [DOI] [PubMed] [Google Scholar]
- Tang CM, Dichter M, Morad M. Modulation of the N-methyl-D-aspartate channel by extracellular H+ Proceedings of the National Academy of Sciences of the USA. 1990;87:6445–6449. doi: 10.1073/pnas.87.16.6445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang LH, Aizenman E. The modulation of N-methyl-D-aspartate receptors by redox and alkylating reagents in rat cortical neurones in vitro. The Journal of Physiology. 1993;465:303–323. doi: 10.1113/jphysiol.1993.sp019678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tingley WG, Ehlers MD, Kameyama K, Doherty C, Ptak JB, Riley CT, Huganir RL. Characterization of protein kinase A and protein kinase C phosphorylation of the N-methyl-D-aspartate receptor NR1 subunit using phosphorylation site-specific antibodies. Journal of Biological Chemistry. 1997;272:5157–5166. doi: 10.1074/jbc.272.8.5157. [DOI] [PubMed] [Google Scholar]
- Traynelis SF, Burgess MF, Zheng F, Lyuboslavsky P, Powers J. Control of voltage-independent zinc inhibition of NMDA receptors by the NR1 subunit. Journal of Neuroscience. 1998;18:6163–6175. doi: 10.1523/JNEUROSCI.18-16-06163.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traynelis SF, Cull-Candy SG. Proton inhibition of N-methyl-D-aspartate receptors in cerebellar neurons. Nature. 1990;345:347–350. doi: 10.1038/345347a0. [DOI] [PubMed] [Google Scholar]
- Villarroel A, Regalado MP, Lerma J. Glycine-independent NMDA receptor desensitization: localization of structural determinants. Neuron. 1998;20:329–339. doi: 10.1016/s0896-6273(00)80460-2. [DOI] [PubMed] [Google Scholar]
- Williams K. Pharmacological properties of recombinant N-methyl-D-aspartate (NMDA) receptors containing the epsilon 4 (NR2D) subunit. Neuroscience Letters. 1995;184:181–184. doi: 10.1016/0304-3940(94)11201-s. [DOI] [PubMed] [Google Scholar]
- Williams K. Separating dual effects of zinc at recombinant N-methyl-D-aspartate receptors. Neuroscience Letters. 1996;215:9–12. doi: 10.1016/s0304-3940(96)12924-4. [DOI] [PubMed] [Google Scholar]
- Wu X, Christensen BN. Proton inhibition of the NMDA-gated channel in isolated catfish cone horizontal cells. Vision Research. 1996;36:1521–1528. doi: 10.1016/0042-6989(95)00254-5. [DOI] [PubMed] [Google Scholar]
- Yang SN, Tang YG, Zucker RS. Selective induction of LTP and LTD by postsynaptic [Ca2+]i elevation. Journal of Neurophysiology. 1999;81:781–787. doi: 10.1152/jn.1999.81.2.781. [DOI] [PubMed] [Google Scholar]
- Zheng F, Gingrich MB, Traynelis SF, Conn PJ. Tyrosine kinase potentiates NMDA receptor currents by reducing tonic zinc inhibition. Nature Neuroscience. 1998;1:185–191. doi: 10.1038/634. [DOI] [PubMed] [Google Scholar]
- Zhong W, Gallivan JP, Zhang Y, Li L, Lester HA, Dougherty DA. From ab initio quantum mechanics to molecular neurobiology: a cation-pi binding site in the nicotinic receptor. Proceedings of the National Academy of Sciences of the USA. 1998;95:12088–12093. doi: 10.1073/pnas.95.21.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]


