Abstract
Modulation of release probability is a major factor underlying short-term synaptic plasticity in the central nervous system. We have investigated the relationship between release probability (Pr) and paired-pulse modulation at a large auditory calyceal synapse containing many transmitter release sites. Whole-cell patch electrode recordings were made of excitatory postsynaptic currents (EPSCs), evoked by stimulation of auditory nerve fibres giving rise to the endbulbs of Held.
Quantitative estimates of Pr and quantal amplitude were obtained using the recently developed variance-mean analysis technique. Release probability conditions were modulated by bath application of cadmium, elevated calcium and protein kinase C activation by phorbol esters.
Our results show that, under physiological conditions, most sites released neurotransmitter following a single presynaptic nerve impulse, with a mean Pr of 0·6. The mean quantal amplitude was 44 pA, which was consistent with the mean amplitude of miniature EPSCs (47 pA).
Under high release probability conditions with elevated calcium or phorbol esters, Pr at all sites approached 1·0. At these high Pr values, variance-mean analysis indicated a significant postsynaptic contribution to paired-pulse depression. The miniature EPSC amplitudes were decreased following stimulation in elevated calcium, confirming a postsynaptic component of paired-pulse depression at this glutamatergic connection.
A notable feature was the large variability between neurons in the relationship between paired-pulse ratio and Pr. Based on current models of vesicle release and ultrastructural evidence, we suggest that this variability may be partly due to morphological differences between endbulb specializations, particularly in the ratio of fusion-ready to reserve populations of vesicles at endbulb release sites.
Most synaptic connections between individual neurons in the central nervous system involve multiple synaptic contacts. The number of contacts between a single presynaptic fibre and a postsynaptic neuron is often in the range of tens to hundreds, but at some synaptic connections may exceed one thousand (Walmsley et al. 1998). The strength of synaptic transmission at such multisynaptic connections is determined by the total number of synaptic contacts, the release probability at all of these contacts, and the postsynaptic response to transmitter release at all sites. Previous attempts to analyse the mechanisms underlying transmission at multisynaptic connections have been largely based on conventional quantal analysis, which has serious limitations, partly due to the complications of site-to-site non-uniformities in release probability and quantal postsynaptic current amplitude (Walmsley et al. 1988; Redman, 1990; Walmsley, 1991, 1995; Rosenmund et al. 1993; Stevens, 1993; Bekkers, 1994; Stricker et al. 1994; Silver et al. 1998). These problems have been circumvented in some studies by investigating the mechanisms operating at single release sites. While these studies have produced valuable insights (Liu & Tsien, 1995; Stevens & Wang, 1995; Silver et al. 1996), the results may not be directly applicable to transmission at connections involving multiple synapses, where there may be additional factors such as presynaptic interactions between adjacent release sites, or postsynaptic effects due to accumulation of neurotransmitter released simultaneously from adjacent sites (Trussell et al. 1993; Barbour et al. 1994; Silver et al. 1998).
In order to investigate the mechanisms regulating transmission at multisynaptic connections, an alternative approach to conventional quantal analysis has been developed recently by Silver et al. (1998) and Reid & Clements (1999), based on analysis of the fluctuations of nerve-evoked synaptic current amplitudes recorded under a range of release probability conditions. This approach has been termed ‘multiple-probability analysis’ by Silver et al. (1998), and ‘variance- mean analysis’ by Reid & Clements (1999). Variance-mean analysis takes into account complicating factors such as non-uniformities in release probability, and avoids many of the problems associated with conventional quantal analysis. The method produces quantitative estimates of the average quantal amplitude (Qav), the mean release probability (Pr) and the total number of release sites (N) (Silver et al. 1998; Reid & Clements, 1999). In the present study, we have used this new experimental and analytical approach to investigate the pre- and postsynaptic factors operating at the synaptic connection between single auditory nerve fibres and bushy cells in the rat anteroventral cochlear nucleus. This is a powerful glutamatergic synaptic connection in which a single auditory nerve fibre gives rise to a large presynaptic terminal, the endbulb of Held, containing many separate but closely spaced release sites, numbering in the range of tens to hundreds (Cant & Morest, 1979; Ryugo & Sento, 1991; Ryugo et al. 1996). This connection has many advantages for the study of fast quantal synaptic transmission, as demonstrated in previous studies (Isaacson & Walmsley, 1995a,b, 1996; Bellingham et al. 1998; Bellingham & Walmsley, 1999). A major advantage is the lack of complications due to electrotonic attenuation of synaptic currents in the dendritic tree, since the endbulb of Held contacts are made with the soma of bushy cells, which lack an extensive dendritic arbor (Cant & Morest, 1979; Isaacson & Walmsley, 1995B). The excitatory postsynaptic current (EPSC) evoked in bushy cells by stimulation of an auditory nerve fibre is large, representing the synchronous release of quanta at many endbulb release sites (Isaacson & Walmsley, 1995a). However, the proportion of sites which normally release neurotransmitter and the mean probability of release at these sites is unknown. This knowledge is important to our understanding of transmission at this connection, and in the present study we have used variance-mean analysis to determine the release probability at endbulb release sites under normal conditions, and under conditions in which release was altered experimentally or modified during short-term synaptic plasticity.
Because auditory nerves are often activated naturally to produce high frequency bursts of nerve impulses, it is important to study the mechanisms underlying transmission under these conditions (Trussell, 1997). An important indicator of high frequency transmission is the response of the cell to a closely spaced pair of presynaptic nerve impulses. Our previous results show that the endbulb-bushy cell synapse normally exhibits marked paired-pulse depression at short intervals of 10–50 ms (Isaacson & Walmsley, 1995a; Bellingham & Walmsley, 1999). Under conditions of reduced release, however, paired-pulse depression is converted to paired-pulse facilitation (Bellingham & Walmsley, 1999). Previous studies of paired-pulse facilitation at different presumed single release site connections indicate that initial release probability may determine the amount of paired-pulse facilitation at a synapse (Dobrunz & Stevens, 1997). In the present study, we have investigated this relationship directly at the endbulb-bushy cell connection by manipulating the initial release probability over a wide range, at the same connection. The results provide insight into the mechanisms and modulation of transmission at the giant endbulb synapse.
METHODS
Electrophysiology
Parasaggital slices (150 μm) were made of the anterior ventral cochlear nucleus (AVCN) of 10- to 12-day-old Wistar rats, following decapitation without anaesthetic in accordance with local guidelines (Isaacson & Walmsley, 1995a,b; Bellingham & Walmsley, 1999). Whole-cell patch electrode recordings were performed at room temperature (22-25°C) from bushy cells visualized using infra-red differential interference contrast (DIC) optics. Slices were superfused with an artificial cerebrospinal fluid (ACSF) containing (mM): 130 NaCl, 3.0 KCl, 1.3 Mg2SO4, 2.0 CaCl2, 1.25 NaH2PO4, 26.2 NaHCO3 and 10 glucose, equilibrated with 95 % O2, 5 % CO2. Patch electrodes (3-6 MΩ resistance) contained (mM): 120 CsCl, 4 NaCl, 4 MgCl2, 0.001 CaCl2, 10 Hepes, 2 Mg-ATP, 0.2 GTP (Tris salt) and 10 EGTA (pH 7.3; 306 mosmol l−1). Series resistance, which was < 10 MΩ, was compensated by > 80 %.
Postsynaptic currents were evoked by focal stimulation of branches of the auditory nerve (0.1 ms, 4–60 V, 0.2 Hz), delivered via a glass microelectrode (5-10 μm tip) filled with ACSF. The evoked currents were identified as endbulb AMPA currents by their amplitude, fast kinetics and all-or-none response to graded stimulation intensities (Isaacson & Walmsley, 1995a). The synaptic currents were recorded and filtered at 10 kHz with an Axopatch 200B amplifier (Axon Instruments) before being digitized at 20 kHz. Mean peak amplitudes were measured as the mean of 30–150 single evoked responses. Rise time was calculated at 10–90 % of the peak amplitude. Excess variance in the amplitude of the synaptic currents was minimized by using a caesium chloride-based internal solution to block potassium conductances, by adding lidocaine N-ethyl bromide (QX-314) intracellularly to block sodium channels, and (n = 6 cells) by voltage clamping at -30 mV to reduce the peak amplitude and inactivate voltage-dependent conductances.
Spontaneous miniature EPSCs (mEPSCs) were recorded on videotape with a VCR (Vetter) and digitized off-line. Data acquisition and analysis were performed using Axograph 4.0 (Axon Instruments). The amplitudes of spontaneous EPSCs were measured using a semi-automated detection procedure (Axograph 4.0) in which a sliding template detects all spontaneous events with amplitudes greater than 2.5 standard deviations of the background noise. The template has a time course typical of a synaptic event and, as it slides along the current trace, it is optimally scaled to fit the trace at each position. The event detection criterion is proportional to the scaling factor and inversely proportional to the goodness-of-fit between the template and the current trace at each position. An event is detected when this criterion exceeds a specified threshold level (Clements & Bekkers, 1997). The mEPSCs were measured in the absence of TTX. Our previous results at this same synapse have shown that spontaneous events recorded in the absence of TTX are indistinguishable from mEPSCs recorded with TTX (Isaacson & Walmsley, 1996).
The phorbol esters phorbol 12-myristate 13-acetate (PMA; 0.1 μM; Sigma) and phorbol 12,13-dibutyrate (PDBu; 0.5 μM; RBI), (+)-2-amino-5-phosphonopentanoic acid (D-AP5; 30 μM; Tocris), bicuculline methochloride (10 μM; Tocris) and strychnine hydrochloride (1 μM; Sigma), were added, as indicated, to the ACSF and applied by bath perfusion. QX-314 (2 mM; RBI) was added to the patch electrode solution. Calcium is expressed as Ca2+ and magnesium is expressed as Mg2+ throughout the text. Results are expressed as means ±s.e.m. and statistical significance was determined with parametric (Student's t test) and non-parametric tests (Kendall rank correlation; Statview).
Variance-mean analysis
Three parameters can be used to describe synaptic function: the average amplitude of the postsynaptic response to a vesicle of transmitter (Qav); the average probability of vesicle release from a release site (Pr); and the number of independent release sites (N). These three parameters can be estimated from the relationship between the variance and the mean of a synaptic amplitude recorded under different release probability conditions (Silver et al. 1998; Reid & Clements, 1999). The general form of the variance- mean relationship can be understood as follows (see Fig. 1). When Pr is low, most sites do not release transmitter and the trial-to-trial variance of EPSC amplitude is low (Fig. 1A). When Pr is moderate (around 0.5), the number of sites that release transmitter varies widely from trial to trial, and EPSC amplitude variance is high. When Pr is high (approaching 1.0), almost all sites release transmitter after every stimulus and the EPSC amplitude variance is again low (Fig. 1A). Thus, the variance-mean relationship plotted over a range of release probabilities is approximately parabolic (Fig. 1B). The initial slope of the parabola provides an estimate of Qav, and the degree of curvature of the parabola provides an estimate of Pr (Reid & Clements, 1999). In this study, a simple parabola was fitted to the variance-mean data by minimizing χ2. The equation for the simple parabola is:
where y is EPSC variance and x is EPSC mean amplitude. The optimally fitted parameters A and B are used to calculate the quantal parameters:
where c.v. I is the coefficient of variation of the quantal amplitude (intrinsic quantal variability). c.v. I was estimated at 0.44 ± 0.03 (n = 12) from mEPSC amplitude distributions recorded in the same AVCN neurons that were used for the variance-mean measurements. This value of 0.44 is in good agreement with the previously published estimate of 0.4 in the AVCN preparation (Isaacson & Walmsley, 1995a,b; Bellingham & Walmsley, 1999). The calculations for Qav and Pr are not sensitive to the precise value of c.v. I, because the term appears in the equation as a minor correction, 1 + c.v. I2. For example, a 12 % error in estimating c.v. I would result in only a 4 % error in the correction term. Note that Qav and Pr are not arithmetic averages, but are weighted towards the release sites with larger release probabilities and quantal amplitudes so they will be slightly larger than the unweighted averages. The variance-mean technique does not permit a direct estimate of N, but a lower limit (Nmin) can be placed on this parameter:
Figure 1. The variance-mean analysis technique.
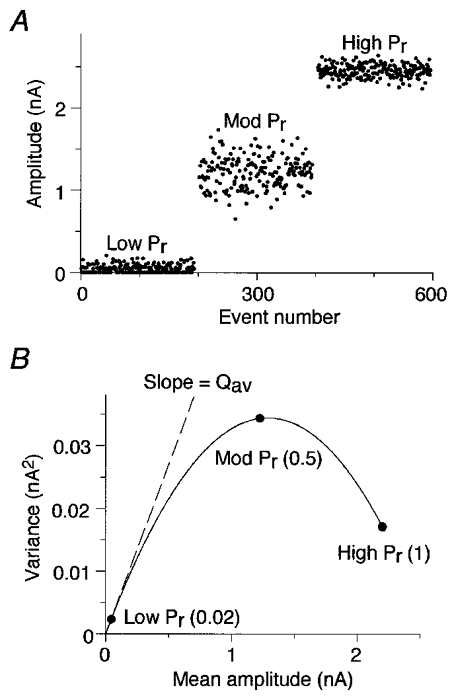
A, a series of simulated EPSC peak amplitudes were plotted against event number. The model assumed release probability (Pr) was uniform across 50 independent release sites and average quantal amplitude (Qav) was 50 pA at each site. Pr was set to three different values during three separate epochs, each of 190 events: Low Pr, 0.02; Mod Pr, 0.5; and High Pr, 1.0. B, the variance of the EPSC amplitudes was plotted against the mean amplitude for each epoch. The variance-mean plot was then fitted with a parabola. The initial slope of the fitted curve provides an estimate of Qav, and the parabolic function estimates Pr and the number of release sites (N).
The variance-mean technique outlined above represents an extension and generalization of previously reported methods for analysing synaptic amplitude fluctuations (Miyamoto, 1975; Clamann et al. 1989; Frerking & Wilson, 1996). In contrast to earlier methods, variance-mean analysis does not require the unrealistic assumption that release probability and quantal amplitude are uniform at all release sites.
To generate the experimental variance-mean relationship, Pr was modulated by adding cadmium (4 or 8 μM), or by increasing extracellular calcium concentration (4 or 6 mM) and adding phorbol esters PDBu (0.5 μM) or PMA (0.1 μM). The mean EPSC amplitude and variance were calculated over a stable epoch of 30–150 events after wash-in of each extracellular solution. Solutions were changed by bath perfusion, and complete exchange typically required 5 min. Regular presynaptic stimulation continued during wash-in. After solution exchange was complete, the EPSC amplitude remained stable throughout the subsequent analysis epoch in most cases. In a few cases, obvious outliers (possibly due to stimulus failure) were removed from the list of EPSC amplitude measurements. The variance attributable to recording noise was estimated in the region prior to the test pulse, and was subtracted from the EPSC variance. A zero point was included in each variance-mean plot to indicate that the noise variance was subtracted. In approximately 15 % of epochs the synaptic response decreased during the recording period, and the variance was calculated after subtracting a fitted regression line. The linear fit removed one degree of freedom so the calculated variance was multiplied by np/(np– 1), where np was the number of points in the epoch. This rundown adjustment was more likely to be required under conditions where Pr was high. If the decrease was > 25 %, the data were not used. The validity of the rundown correction procedure was tested as follows. A stable epoch of 100 amplitude measurements (a typical number) was simulated and the mean and variance were calculated in the standard manner, and after applying the rundown correction procedure. The means measured with the two procedures were identical, and the variances were not significantly different at 33 699 ± 500 pA2 for the standard measure, versus 33 714 ± 500 pA2 for the rundown-corrected measure (n = 99, paired t test, P > 0.05). As an additional test, simulated data were generated with either 20 % presynaptic or 20 % postsynaptic rundown, and for a range of release probability conditions. After applying the rundown correction procedure, variance-mean analysis accurately estimated the synaptic parameters (results not shown).
RESULTS
Release probability following a single presynaptic nerve impulse
Whole-cell patch recordings were made from bushy cells in 150 μm thick AVCN slices (n = 13) for > 2 h, allowing an extensive modulation of transmitter release for each cell. Following focal stimulation, glutamatergic AMPA currents were isolated by addition of strychnine, bicuculline and D-AP5 to block glycinergic, GABAergic and glutamatergic NMDA currents, respectively (Fig. 2A). The amplitude of the AMPA currents in physiological calcium and magnesium conditions (2 mM Ca2+, 1.3 mM Mg2+) at -60 mV varied from 0.4 to 6.1 nA with a mean of 2.1 ± 0.4 nA (n = 13). The mean rise time was 0.37 ± 0.02 ms and the mean half-width was 0.98 ± 0.07 ms (n = 13). The evoked current was all-or-none at the stimulation threshold and remained stable for stimulation intensities 2 times the threshold (Fig. 2B). Stimulation intensity was set at 1.5 times threshold for the experiments.
Figure 2. Probability of transmitter release at a giant terminal using variance-mean analysis.
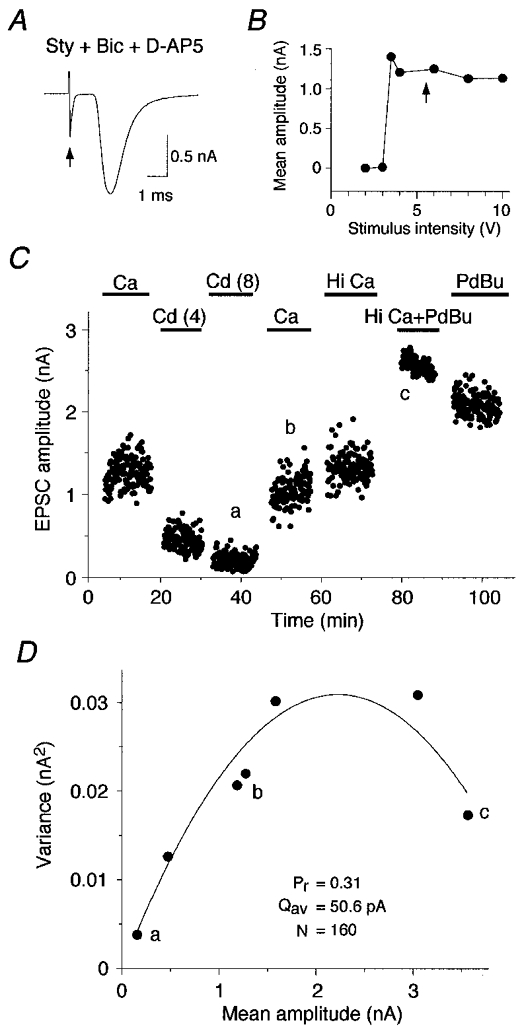
A, glutamatergic AMPA current evoked by focal stimulation (arrow) and isolated by addition of strychnine (Sty; 1 μM), bicuculline (Bic; 10 μM) and D-AP5 (30 μM). Mean amplitude of 2.0 nA (from 185 traces). B, AMPA current responded in an all-or-none fashion to a graded stimulus. Stimulation intensity was set at 1.5 times threshold for all experiments (arrow). C, Pr was altered by bath application of cadmium (Cd; 4 or 8 μM), normal calcium (Ca; 2 mM), high calcium (Hi Ca; 4 mM), and phorbol ester (PDBu; 0.5 μM). Mean amplitude and variance of evoked EPSC was measured during a stable epoch of 30–150 responses for each condition (a, b and c). D, variance-mean relationship fitted with a simple parabola estimates Qav (50.6 pA) and Nmin (160). Estimates of Pr were 0.04 in cadmium (a), 0.31 under physiological conditions (b; 2 mM Ca2+, 1.3 mM Mg2+) and 0.88 in the presence of elevated calcium (4 mM) and phorbol ester PDBu (c). Same cell as in C.
Variance-mean analysis was used to estimate the average quantal amplitude (Qav), the release probability (Pr) and the number of release sites (N) in AVCN neurons. These three parameters can be estimated from the relationship between the variance and the mean EPSC amplitude recorded under different release probability conditions (see Methods). These conditions were achieved by bath application of cadmium, elevated calcium or phorbol ester (Fig. 2C). The variance and the mean amplitude of the synaptic current were calculated during a stable epoch under each condition. Variance-mean plots were fitted with a simple parabola and the fitted function was used to estimate Qav and Pr under each recording condition (Fig. 2D see Methods). Qav varied from 11.3 to 98.4 pA with a mean of 44.4 ± 7.4 pA at a holding potential of -60 mV (n = 13; Table 1). The estimate was derived from the initial slope of the simple parabola fitted to the variance-mean plot for each cell (see Methods).
Table 1. Quantal parameters of the giant endbulb—bushy cell connection.
| Pr | Qav (pA) | Nmin | mEPSC (pA) | |
|---|---|---|---|---|
| Mean | 0.55 | 44.4 | 142.1 | 47.2 |
| s.e.m. | 0.08 | 7.4 | 48.6 | 8.0 |
| n | 10 | 13 | 10 | 7 |
Under physiological conditions (2 mm Ca2+, 1.3 mm Mg2+), more than half of the sites release neurotransmitter following a single presynaptic nerve impulse (Pr= 0.55). Qav estimated from the variance—mean analysis was in good agreement with the quantal amplitude measured directly from spontaneous miniature EPSCs.
In normal ACSF (2 mM Ca2+, 1.3 mM Mg2+), Pr varied from 0.30 to 0.95 with a mean of 0.55 ± 0.08 (n = 10; Table 1). When the bath solution was switched from control solution to a solution containing 8 μM cadmium to reduce neurotransmitter release, Pr decreased by 81 % to a mean of 0.09 ± 0.02 (n = 12). Under high release probability conditions, Pr increased to a mean of 0.59 ± 0.08 in elevated calcium (4 mM; n = 10) and further increased to a mean of 0.83 ± 0.07 with phorbol ester (2-6 mM Ca2+; n = 4).
Nminranged from 26 to 547 with a mean value of 142 ± 49 (n = 10), which is consistent with the number of synaptic specializations observed in ultrastructural studies of endbulbs in the rat (M. J. Nicol & B. Walmsley, unpublished observations).
Release probability is correlated with paired-pulse ratio
Two consecutive stimuli were delivered 10 ms apart. The amplitude of the second response (S2) was compared to that of the first (S1) to calculate the paired-pulse ratio. The paired-pulse ratio (S2/S1) changed when release probability conditions were altered (Fig. 3A, inset). In normal ACSF (2 mM Ca2+, 1.3 mM Mg2+), the paired-pulse ratio was quite variable from cell to cell, ranging from 0.13 to 1.84 (mean, 1.09 ± 0.23; n = 8). Some neurons showed paired-pulse depression (PPD) in normal ACSF (Fig. 3Ab) while others showed paired-pulse facilitation (PPF). Bath application of cadmium (8 μM) enhanced PPF and gave a mean paired-pulse ratio of 2.0 ± 0.4 (n = 7; Fig. 3Aa). Application of elevated calcium (4 mM) enhanced PPD and gave a mean paired-pulse ratio of 0.68 ± 0.17 (n = 8). In some experiments, the viability of the recording allowed progressively higher concentrations of calcium (6 mM) to be tested with a resulting increase in PPD with a ratio of 0.56 ± 0.27 (n = 4; Fig. 3Ac). Phorbol ester application in 2–6 mM calcium enhanced PPD further with a mean paired-pulse ratio of 0.43 ± 0.12 (n = 5).
Figure 3. Probability of transmitter release is correlated with paired-pulse ratio.
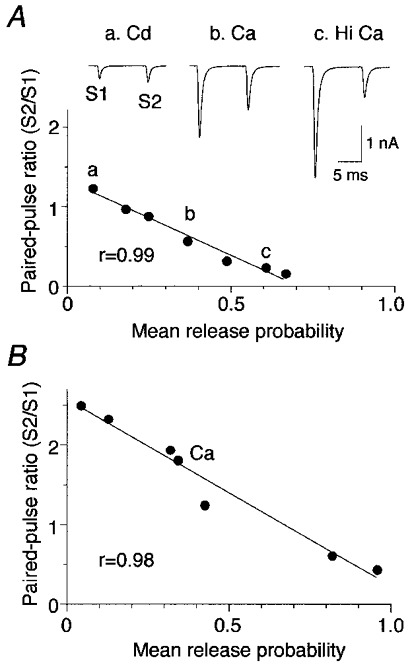
A, paired AMPA responses were evoked by two consecutive stimuli at an interval of 10 ms. The paired-pulse ratio (S2/S1) was altered when release probability conditions were changed (inset). Cadmium (Cd; 8 μM) induced PPF (a), physiological calcium (Ca; 2 mM) evoked PPD (b), and elevated calcium (Hi Ca; 6 mM) enhanced PPD (c). Pr was estimated under different release conditions (a, b and c) using variance-mean analysis. A linear correlation was observed between Pr and the paired-pulse ratio. Pr was 0.4 at physiological calcium concentration for this neuron. B, second example of a linear relationship between Pr and paired-pulse ratio in an individual neuron. Pr was 0.3 at physiological calcium concentration.
The mean Pr was estimated for each of the different release probability conditions using variance-mean analysis. The relationship between Pr and the paired-pulse ratio was investigated for individual neurons (Fig. 3A). The relationship was different between neurons (compare Fig. 3A and B). However, within individual neurons, a statistically significant linear correlation between Pr and the paired-pulse ratio was observed (Fig. 3A and B). The linear correlation was observed in all cells (mean correlation coefficient, r = 0.97; P < 0.05; n = 8; Fig. 4A). Only those cells with four or more data points were used. Figure 4A illustrates the relationship between Pr and paired-pulse ratio for all cells, showing that the parameters of the linear fit varied greatly between cells (see Discussion). The slope of the linear fit to the relationship varied from -1.01 to -3.63 with a mean slope of -2.22 ± 0.37 (n = 8). Thus, the amount of PPF or PPD observed in a particular cell is not a reliable indicator of mean Pr. However, within the same cell, relative changes in the paired-pulse ratio appear to be a good indicator of relative changes in mean Pr.
Figure 4. Relationship between Pr and paired-pulse ratio varies between neurons.
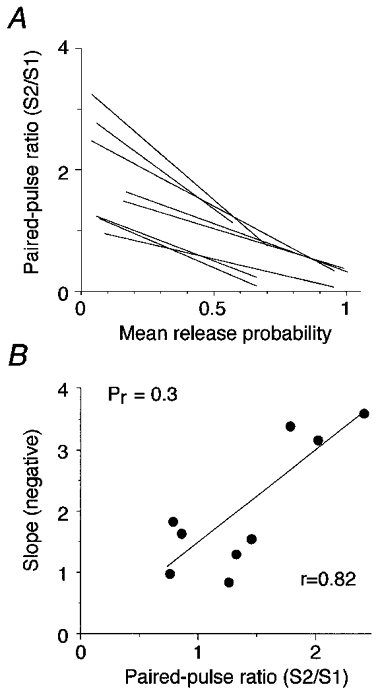
A, relationship between Pr and paired-pulse ratio for all neurons. For a given Pr value, some neurons show PPF while others show PPD. The slope of the linear fit between Pr and the paired-pulse ratio varied between neurons. B, direct relationship between the slope of the linear relationship and the paired-pulse ratio. The slopes of the linear fits in A were plotted against the paired-pulse ratio for a particular release probability (Pr= 0.3).
To further investigate the relationship between Pr and paired-pulse ratio, the slope of the linear correlation fit was plotted against the paired-pulse ratio for a particular release probability. A low release probability was chosen (Pr= 0.3) to avoid the complications of postsynaptic depression due to desensitization at high Pr values (see below). (Without postsynaptic depression, the Prversus paired-pulse ratio relationship would obviously deviate from linearity at high Pr values.) For Pr= 0.3, the paired-pulse ratio varied from 0.74 to 2.4 between cells (Fig. 4B). A direct relationship was observed between the slope of the linear relationship and the paired-pulse ratio (r = 0.82).
Mechanism of paired-pulse depression
Paired AMPA responses were evoked under a range of release probability conditions (Fig. 5A, inset). Variance-mean plots were constructed for both the first and second responses (Fig. 5A). The variance-mean relationship of the first response conformed to the simple parabolic shape expected from theory (see Methods). In contrast, the variance-mean relationship of the second response often exhibited a distinct form which was clearly non-parabolic, and curled back under itself when Pr was high (Fig. 5A and B). The non-parabolic variance-mean relationship of the second pulse was observed in 6 out of 12 cells, and only occurred for cells with a maximum Pr greater than 0.7 for the first pulse. The mean paired-pulse ratio for these six cells in normal ACSF (2 mM Ca2+, 1.3 mM Mg2+) was 1.0 ± 0.3, which decreased to 0.6 ± 0.2 in elevated calcium (6 mM). At normal calcium concentrations and under conditions of low Pr (8 μM cadmium), the variance-mean relationship of the second response did not deviate significantly from the variance-mean relationship of the first response in 8 out of 12 cells (Fig. 5B).
Figure 5. Variance-mean analysis of paired-pulse responses.
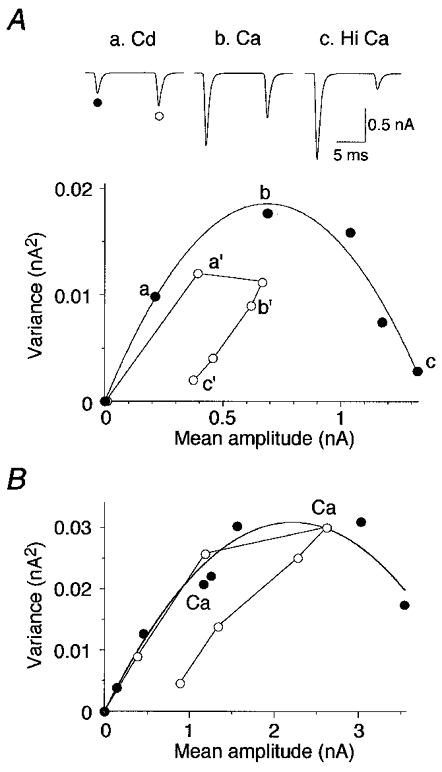
A, paired AMPA EPSCs were evoked under a range of release probability conditions following bath application of cadmium (a, Cd; 8 μM), physiological calcium (b, Ca; 2 mM) and elevated calcium (c, Hi Ca; 6 mM) (inset). Variance-mean analysis was applied to both the first (•) and the second EPSCs (^). The variance-mean relationship of the first response followed a simple parabola while the variance-mean relationship of the second response was unexpectedly non-parabolic, curling back on itself when Pr was elevated in high calcium (c′). B, in most neurons, the second EPSC did not deviate from the first EPSC under conditions of low to normal (Ca; 2 mM) Pr, as shown for this individual neuron.
Simulations of a fluctuating synaptic response revealed that the distinctive form of the variance-mean relationships seen in Fig. 5 is expected when PPD is due to a mixture of pre- and postsynaptic mechanisms, but not when PPD is purely presynaptic or purely postsynaptic (Fig. 6). Simulated variance-mean relationships are plotted in Fig. 6A for both the first response (filled circles) and the second response (open circles) to a paired-pulse paradigm. Three different models were used in the simulations. All three models incorporated both PPF and PPD. PPF was modelled as a 100 % increase in Pr at every release site (for example, due to presynaptic calcium accumulation). The maximum potentiation was limited so that Pr at an individual site never exceeded 1.0. In the first model (Fig. 6A, Presynaptic), the mechanism of PPD was purely presynaptic. If release occurred at a given site in response to the first stimulus, then the Pr at that site was reduced for the second stimulus (for example, due to vesicle depletion). In the third model (Fig. 6A, Postsynaptic), the mechanism of PPD was purely postsynaptic. If release occurred at a given site in response to the first stimulus, then the quantal amplitude at that site was reduced for the second stimulus (for example, due to postsynaptic receptor desensitization). In the second model (Fig. 6A, Mixed), these pre- and postsynaptic mechanisms contributed equally to PPD. As Pr for the first pulse approached 1.0, PPF became ineffective and was dominated by PPD which reached 70 % in all three models. The variance-mean plot for the second response followed a characteristic trajectory depending on the mechanism underlying PPD (Fig. 6A). It did not curl under when the PPD mechanism was purely presynaptic, but curled down almost to the x-axis when the PPD mechanism was purely postsynaptic (Fig. 6A). Thus, the detailed shape of the variance-mean plot for the second response may be useful in distinguishing between a pre- or postsynaptic mechanism of PPD, at least under conditions in which there is a large deviation from the expected parabolic relationship.
Figure 6. Simulations indicate that variance-mean plots can distinguish between different mechanisms of PPD.
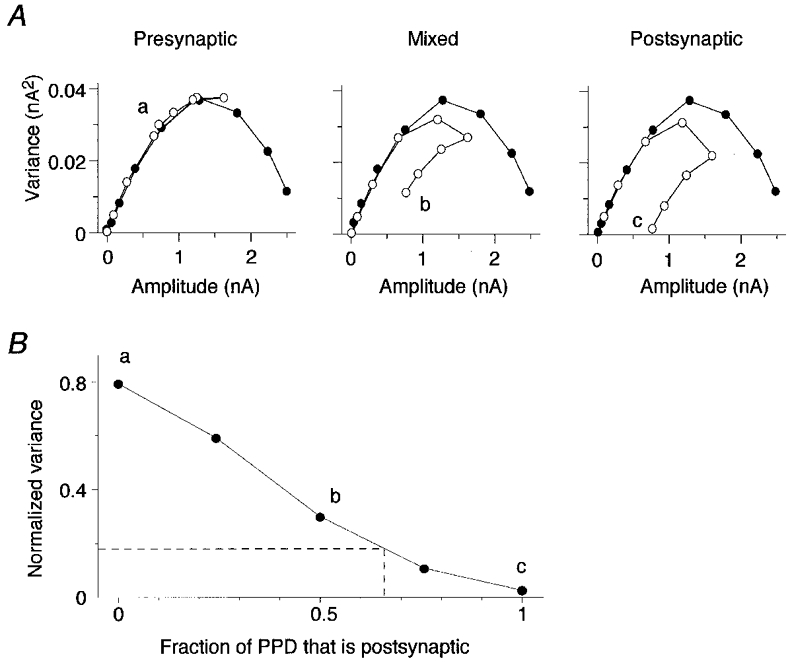
A, EPSC amplitudes were simulated under a range of release probability conditions, and the variance-mean relationship was plotted for both the first response (•) and the second response (^) to a paired-pulse paradigm. Three different models were used in the simulations (see Results). In the first model (Presynaptic), the mechanism of PPD was purely presynaptic (vesicle depletion); in the second model (Mixed), pre- and postsynaptic mechanisms contributed equally to PPD; and in the third model (Postsynaptic), the mechanism of PPD was purely postsynaptic (AMPA receptor desensitization). B, for each PPD model, the variance of the second response recorded at maximum Pr was normalized with respect to the maximum variance of the first response (a, b and c). The normalized variance was plotted against the fraction of PPD that was due to a postsynaptic mechanism. This fraction was varied from 0 to 1 in a series of different models. Models assumed that Pr was uniform. Dashed line indicates values for experimental results (see Results).
For each PPD model, the variance of the second response recorded at maximum Pr (Fig. 6Aa, b and c) was normalized with respect to the maximum variance of the first response. The normalized variance was plotted against the fraction of PPD that was due to a postsynaptic mechanism (Fig. 6B). Using this graphical representation, a given value for normalized variance can estimate the fraction of the PPD that is due to postsynaptic mechanisms. For the cells that showed the non-parabolic variance-mean relationship of the second pulse, the mean normalized variance was 0.17 ± 0.05 (n = 5), under high release probability conditions. The fraction of the PPD that was due to postsynaptic mechanisms at these high release probability conditions was approximately 66 % in these cells (Fig. 6B).
The PPD models illustrated in Fig. 6 assume that Pr was uniform. Additional simulations were performed based on the assumption that Pr was highly non-uniform. This substantial change in the model assumptions introduced skew into the variance-mean relationship for the first pulse (not shown; see also Silver et al. 1998), but had only a small effect on the relationship, shown in Fig. 6B, between the normalized variance and the fraction of PPD that was due to a presynaptic mechanism.
mEPSC measurements support a partly postsynaptic mechanism for PPD at high release probabilities
The variance-mean analysis indicated a partially postsynaptic mechanism of PPD. A postsynaptic mechanism such as receptor desensitization might result in a reduction of the mean quantal amplitude of the mEPSCs in the period following an evoked response (Otis et al. 1996a). To investigate this possibility, mEPSCs were recorded in AVCN bushy cells (Fig. 7A). A low rate of occurrence of mEPSCs did not permit analysis of mEPSCs for most of the cells which showed a non-parabolic variance-mean relationship for the second pulse. However, adequate numbers of mEPSCs were obtained for seven cells under high release probability conditions (4 mM Ca2+). The mean amplitude of mEPSCs for these seven cells was 47.2 ± 8.0 pA (Table 1). This value is in good agreement with the Qav estimated from variance-mean analysis (44.4 ± 7.4 pA; Table 1). mEPSCs were collected in a time period prior to the paired-pulse stimulation and termed control mEPSCs. Control mEPSCs were compared to mEPSCs collected in a time period of 30 ms following the second pulse in the paired-pulse stimulation (test mEPSCs). The comparison of control mEPSCs versus test mEPSCs was performed at both low and high release probabilities (Fig. 7A). In the presence of cadmium (4 or 8 μM), the test mEPSCs were not significantly different from control mEPSCs (Fig. 7B). However, in high calcium conditions (4 mM), the test mEPSCs were significantly depressed by 20.9 ± 2.7 % (n = 5; P < 0.01). This finding supports the results from variance-mean analysis which also showed a postsynaptic contribution to PPD under high release probability conditions at the endbulb-bushy cell connection.
Figure 7. mEPSCs support a postsynaptic mechanism for PPD at high release probabilities.
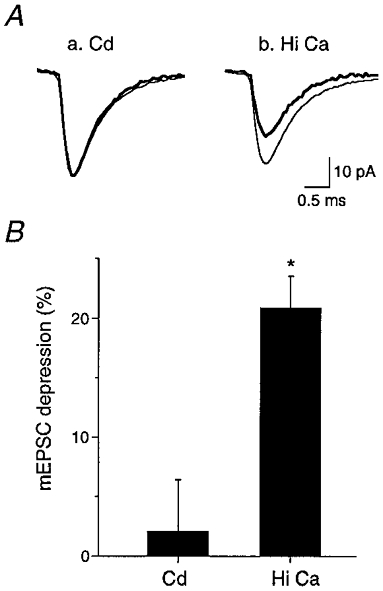
A, control AMPA mEPSCs were collected in a time period prior to paired-pulse stimulation (thin trace) and test mEPSCs were collected 30 ms following paired-pulse stimulation (thick trace). The comparison of control mEPSCs versus test mEPSCs was performed at both low (a, Cd; 4 or 8 μM) and high Pr (b, Hi Ca; 4 mM). A difference was observed under elevated calcium conditions (b). B, the test mEPSCs were not significantly depressed compared to control at low Pr in the presence of cadmium (Cd; n = 7). However, at high Pr (Hi Ca), the test mEPSCs were significantly depressed by 20.9 ± 2.7 % (n = 5; * P < 0.01).
DISCUSSION
Release probability is a major determinant of synaptic strength, and modulation of release probability is likely to play an important role in various types of synaptic plasticity, such as short-term facilitation or depression. Transmission at the endbulb-bushy cell connection in the anteroventral cochlear nucleus is fast and reliable, in keeping with its role in auditory processing and sound localization (Oertel, 1997; Trussell, 1997). The present study provides a number of key observations on release probability and its role in short-term modulation of synaptic efficacy at this powerful multisynaptic connection.
Our results demonstrate that, following the arrival of a nerve impulse at the endbulb, most sites release neurotransmitter (mean Pr= 0.6). However, there is a range of mean Pr values between endbulbs, with some connections exhibiting a low mean Pr value (< 0.3), and others exhibiting a Pr value close to 1.0. The reason for this difference between endbulbs is not known, but significant morphological differences between endbulbs have been observed (Ryugo et al. 1996). In particular, the number and size of endbulb release sites appear to differ between endbulbs, and this difference may be related to auditory nerve activity (Ryugo et al. 1996). Endbulbs arising from auditory nerves with high spontaneous activity rates are larger, with more but smaller synaptic specializations (Ryugo et al. 1996). Our previous studies have shown that the amplitude of quantal currents is developmentally regulated at endbulb synapses (Bellingham et al. 1998). Since young animals (10-12 days) were used in the present experiments, some of the observed variability in release properties between endbulb-bushy cell connections may be due to different states of synaptic maturation. In addition, different endbulb connections may be regulated to different final levels of mean Pr. In general, our results indicate that mean Pr is moderately high at endbulb connections. A similar conclusion was reached in a study of the climbing fibre-Purkinje cell connection by Silver et al. (1998). This is another powerful synaptic connection consisting of hundreds of release sites, although the contacts are made via conventional synaptic boutons, in contrast to the calyceal endbulb-bushy cell contacts. Mean Pr at the climbing fibre-Purkinje cell connection was found to be exceptionally high (0.9) under low frequency stimulation (0.033 Hz) (Silver et al. 1998). In the present study, some endbulb connections were found to have a similarly high mean Pr.
Auditory signals are often relayed as bursts of nerve impulses in auditory nerve fibres, and the properties of transmission at the endbulb connection under these conditions are obviously important to an understanding of auditory processing (Zucker, 1989; O'Donovan & Rinzel, 1997; Oertel, 1997; Trussell, 1997). In the present study we have examined the probabilistic nature of transmission at this connection following two closely spaced nerve impulses. As previously demonstrated (Isaacson & Walmsley, 1995a; Bellingham & Walmsley, 1999), endbulb-bushy cell transmission may be modulated experimentally to exhibit a range of responses, from PPF to pronounced PPD. We have taken advantage of this property and used the variance-mean technique to estimate Pr at endbulb release sites, and to explore its relationship with PPF and depression. Our results demonstrate that there is a remarkably linear relationship between initial mean Pr and the amount of PPF or PPD at a given connection. Under conditions of low to moderate Pr, PPF is entirely due to a presynaptic change in mean Pr. This supports and extends our previous observations on PPF at this connection, based on direct counting of individual quanta at extremely low Pr (Isaacson & Walmsley, 1995a). This result is consistent with experimental evidence on PPF obtained at putative single release sites in hippocampal slices (Dobrunz & Stevens, 1997). However, the single release site data also showed PPD to be an entirely presynaptic phenomenon, with high release probability sites more likely to exhibit PPD. Recent evidence at the endbulb indicates that, under normal conditions, PPD is due to a presynaptic reduction in Pr mostly due to a novel calcium-mediated presynaptic inhibitory process (Bellingham & Walmsley, 1999).
Our present results extend these observations to show that, at high levels of release, there is a significant postsynaptic contribution to PPD. The variance-mean analysis revealed a non-parabolic curve for the second response, which corresponded to a two-thirds postsynaptic contribution to PPD under high release probability conditions. A postsynaptic component was also observed independently through a 20 % depression of mEPSC amplitude after stimulation in elevated calcium. The variance-mean analysis provides a semi-quantitative estimate of the fraction of PPD that can be attributed to a postsynaptic mechanism, while the mEPSC analysis can only provide a lower limit for this parameter due to the difficulty of detecting small spontaneous events. A postsynaptic contribution to PPD was reported at the chick endbulb connection (Trussell et al. 1993). This postsynaptic effect is most probably due to desensitization of postsynaptic receptors, caused by accumulation of glutamate in the synaptic cleft under high release probability conditions (Hestrin, 1992; Trussell et al. 1993; Barbour et al. 1994; Mosbacher et al. 1994; Clements, 1996; Jones & Westbrook, 1996; Otis et al. 1996a,b). Pr at the endbulb-bushy cell connection appears to be normally regulated near the level at which a postsynaptic component to PPD becomes evident. Thus, setting Pr below this level may help to preserve the postsynaptic response to transmitter release during high frequency activation. It is interesting to compare this result with the observations on synaptic depression at the climbing fibre-Purkinje cell connection. There, Silver et al. (1998) found synaptic depression to be entirely explained by a presynaptic reduction in Pr, despite an initial probability close to one (Pr= 0.9). An obvious difference between these two connections is in the multiple bouton contacts of the climbing fibre versus the calyceal contact of the endbulb. In the case of the endbulb, there may be a greater accumulation of transmitter in the synaptic cleft under elevated release probability conditions in which most sites release transmitter, whereas this is less likely to occur for the separated climbing fibre boutons.
Mechanism of paired-pulse release differences between endbulb connections
Our results demonstrate considerable differences between endbulb-bushy cell connections in the paired-pulse ratio versus mean Pr relationship. Both the slope of this relationship and the magnitude of the paired-pulse ratio for a particular value of Pr may differ greatly between connections (Figs 3 and 4). Our results also show that, between connections at the same particular value of Pr, there is a positive relationship between paired-pulse ratio and the slope of the paired-pulse ratio versusPr plot (Fig. 4B). We have explored a variety of different models of paired-pulse release (to be described in detail elsewhere) to gain insight into the potential mechanism(s) underlying these observations, in particular the reason for the large differences between endbulb connections. Basically, our models assume that each individual release site has an associated population of ‘fusion-ready’ vesicles, Nr, and a population of reserve, or ‘not fusion-ready’ vesicles, Nnr. When an impulse arrives at the nerve terminal, calcium entry activates vesicle fusion and transmitter release. An individual vesicle in the fusion-ready state may be released with probability Pr, remain in the fusion-ready state, or be transformed into a not fusion-ready state (e.g. due to the depressing mechanisms underlying ‘adaptation’ of release (Hsu et al. 1996), or calcium-mediated inhibition of release (Bellingham & Walmsley, 1999)). Pr defines the fundamental processes for an individual vesicle in a fusion-ready state, and is obviously dependent on a variety of factors, including calcium concentration at the release site. The number of vesicles released following the arrival of a single nerve impulse is simply PrNr. If a second nerve impulse arrives, then the number of vesicles available for release is determined by the number of fusion-ready vesicles remaining after the first impulse, minus the number transformed to a not fusion-ready state, plus the number recruited from the not fusion-ready population by all processes (including facilitatory processes dependent on residual calcium, etc.). Under this scheme, the ratio of the number of vesicles released on the second impulse to the number released on the first is dependent not only on all of the fundamental underlying processes, but also on the ratio of Nnr to Nr. Similarly, the derivative of the paired-pulse ratio with respect to Pr (i.e. the slope) is also dependent on the Nnr to Nr ratio. These observations apply to an individual release site, but for a connection with many release sites, there will undoubtedly be a mixture of sites with different Nnr/Nr ratios. In addition, there may be differences in other factors such as the number and density of calcium channels, resulting in differences in the concentration of calcium between sites. Therefore, the paired-pulse ratio and Pr values for a particular connection will represent a weighted average of the properties of all release sites.
Assuming the fundamental mechanisms of release are the same at all synapses, then, under identical conditions (e.g. same calcium entry and same time interval between nerve impulses), our models predict that differences between connections in the paired-pulse ratio and in the slope of the paired-pulse ratio versusPr relationship will be directly influenced by differences in the Nnr/Nr ratio. That is, for a given value of Pr, the models indicate that the greater the paired-pulse ratio value, the greater the slope of the paired-pulse ratio versusPr relationship. Such a relationship is supported by our experimental data at different endbulb connections (Fig. 4B). These observations imply that there may be morphological differences between endbulbs in the average number of docked and undocked vesicles at synaptic specializations, and significant morphological differences between endbulb synapses have been reported in electron-microscopic studies (Ryugo et al. 1996). As these morphological differences are related to the activity of the auditory nerve fibres (Ryugo et al. 1996), our results raise the possibility of a relationship between auditory nerve activity and the properties of release at endbulb terminals.
Acknowledgments
This research was supported by grants from the Australian National Health and Medical Research Foundation (B.W. and S.O.) and the Australian Research Council (J.C.).
References
- Barbour B, Keller BU, Llano I, Marty A. Prolonged presence of glutamate during excitatory synaptic transmission to cerebellar Purkinje cells. Neuron. 1994;12:1331–1343. doi: 10.1016/0896-6273(94)90448-0. [DOI] [PubMed] [Google Scholar]
- Bekkers JM. Quantal analysis of synaptic transmission in the central nervous system. Current Opinion in Neurobiology. 1994;4:360–365. doi: 10.1016/0959-4388(94)90097-3. [DOI] [PubMed] [Google Scholar]
- Bellingham MC, Lim R, Walmsley B. Developmental changes in EPSC quantal size and quantal content at a central glutamatergic synapse in rat. The Journal of Physiology. 1998;511:861–869. doi: 10.1111/j.1469-7793.1998.861bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellingham MC, Walmsley B. A novel presynaptic inhibitory mechanism underlies paired pulse depression at a fast central synapse. Neuron. 1999;23:1925–1945. doi: 10.1016/s0896-6273(00)80762-x. [DOI] [PubMed] [Google Scholar]
- Cant NB, Morest DK. The bushy cells in the anteroventral cochlear nucleus of the cat. A study with the electron microscope. Neuroscience. 1979;4:1925–1945. doi: 10.1016/0306-4522(79)90066-6. [DOI] [PubMed] [Google Scholar]
- Clamann HP, Mathis J, Luscher HR. Variance analysis of excitatory postsynaptic potentials in cat spinal motoneurons during posttetanic potentiation. Journal of Neurophysiology. 1989;61:403–416. doi: 10.1152/jn.1989.61.2.403. [DOI] [PubMed] [Google Scholar]
- Clements JD. Transmitter timecourse in the synaptic cleft: its role in central synaptic function. Trends in Neurosciences. 1996;19:163–171. doi: 10.1016/s0166-2236(96)10024-2. [DOI] [PubMed] [Google Scholar]
- Clements JD, Bekkers JM. Detection of spontaneous synaptic events with an optimally scaled template. Biophysical Journal. 1997;73:220–229. doi: 10.1016/S0006-3495(97)78062-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrunz LE, Stevens CF. Heterogeneity of release probability, facilitation, and depletion at central synapses. Neuron. 1997;18:995–1008. doi: 10.1016/s0896-6273(00)80338-4. [DOI] [PubMed] [Google Scholar]
- Frerking M, Wilson M. Effects of variance in mini amplitude on stimulus-evoked release: a comparison of two models. Biophysical Journal. 1996;70:2078–2091. doi: 10.1016/S0006-3495(96)79774-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hestrin S. Activation and desensitization of glutamate-activated channels mediating fast excitatory synaptic currents in the visual cortex. Neuron. 1992;9:991–999. doi: 10.1016/0896-6273(92)90250-h. [DOI] [PubMed] [Google Scholar]
- Hsu SF, Augustine GJ, Jackson MB. Adaptation of Ca2+-triggered exocytosis in presynaptic terminals. Neuron. 1996;17:501–512. doi: 10.1016/s0896-6273(00)80182-8. [DOI] [PubMed] [Google Scholar]
- Isaacson JS, Walmsley B. Counting quanta: direct measurements of transmitter release at a central synapse. Neuron. 1995a;15:875–884. doi: 10.1016/0896-6273(95)90178-7. [DOI] [PubMed] [Google Scholar]
- Isaacson JS, Walmsley B. Receptors underlying excitatory synaptic transmission in slices of the rat anteroventral cochlear nucleus. Journal of Neurophysiology. 1995b;73:964–973. doi: 10.1152/jn.1995.73.3.964. [DOI] [PubMed] [Google Scholar]
- Isaacson JS, Walmsley B. Amplitude and time course of spontaneous and evoked excitatory postsynaptic currents in bushy cells of the anteroventral cochlear nucleus. Journal of Neurophysiology. 1996;76:1566–1571. doi: 10.1152/jn.1996.76.3.1566. [DOI] [PubMed] [Google Scholar]
- Jones MV, Westbrook GL. The impact of receptor desensitization on fast synaptic transmission. Trends in Neurosciences. 1996;19:96–101. doi: 10.1016/s0166-2236(96)80037-3. [DOI] [PubMed] [Google Scholar]
- Liu G, Tsien RW. Properties of synaptic transmission at single hippocampal synaptic boutons. Nature. 1995;375:404–408. doi: 10.1038/375404a0. [DOI] [PubMed] [Google Scholar]
- Miyamoto MD. Binomial analysis of quantal transmitter release at glycerol treated frog neuromuscular junctions. The Journal of Physiology. 1975;250:121–142. doi: 10.1113/jphysiol.1975.sp011045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosbacher J, Schoepfer R, Monyer H, Burnashev N, Seeburg PH, Ruppersberg JP. A molecular determinant for submillisecond desensitization in glutamate receptors. Science. 1994;266:1059–1062. doi: 10.1126/science.7973663. [DOI] [PubMed] [Google Scholar]
- O'Donovan MJ, Rinzel J. Synaptic depression: a dynamic regulator of synaptic communication with varied functional roles. Trends in Neurosciences. 1997;20:431–433. doi: 10.1016/s0166-2236(97)01124-7. [DOI] [PubMed] [Google Scholar]
- Oertel D. Encoding of timing in the brain stem auditory nuclei of vertebrates. Neuron. 1997;19:959–962. doi: 10.1016/s0896-6273(00)80388-8. [DOI] [PubMed] [Google Scholar]
- Otis T, Zhang S, Trussell LO. Direct measurement of AMPA receptor desensitization induced by glutamatergic synaptic transmission. Journal of Neuroscience. 1996a;16:7496–7504. doi: 10.1523/JNEUROSCI.16-23-07496.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otis TS, Wu YC, Trussell LO. Delayed clearance of transmitter and the role of glutamate transporters at synapses with multiple release sites. Journal of Neuroscience. 1996b;16:1634–1644. doi: 10.1523/JNEUROSCI.16-05-01634.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman S. Quantal analysis of synaptic potentials in neurons of the central nervous system. Physiological Reviews. 1990;70:165–198. doi: 10.1152/physrev.1990.70.1.165. [DOI] [PubMed] [Google Scholar]
- Reid CA, Clements JD. Postsynaptic expression of long-term potentiation in the rat dentate demonstrated by variance-mean analysis. The Journal of Physiology. 1999;518:121–130. doi: 10.1111/j.1469-7793.1999.0121r.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenmund C, Clements JD, Westbrook GL. Nonuniform probability of glutamate release at a hippocampal synapse. Science. 1993;262:754–757. doi: 10.1126/science.7901909. [DOI] [PubMed] [Google Scholar]
- Ryugo DK, Sento S. Synaptic connections of the auditory nerve in cats: relationship between endbulbs of held and spherical bushy cells. Journal of Comparative Neurology. 1991;305:35–48. doi: 10.1002/cne.903050105. [DOI] [PubMed] [Google Scholar]
- Ryugo DK, Wu MM, Pongstaporn T. Activity-related features of synapse morphology: a study of endbulbs of held. Journal of Comparative Neurology. 1996;365:141–158. doi: 10.1002/(SICI)1096-9861(19960129)365:1<141::AID-CNE11>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Silver RA, Cull-Candy SG, Takahashi T. Non-NMDA glutamate receptor occupancy and open probability at a rat cerebellar synapse with single and multiple release sites. The Journal of Physiology. 1996;494:231–250. doi: 10.1113/jphysiol.1996.sp021487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver RA, Momiyama A, Cull-Candy SG. Locus of frequency-dependent depression identified with multiple- probability fluctuation analysis at rat climbing fibre-Purkinje cell synapses. The Journal of Physiology. 1998;510:881–902. doi: 10.1111/j.1469-7793.1998.881bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens CF. Quantal release of neurotransmitter and long-term potentiation. Cell. 1993;72(suppl.):55–63. doi: 10.1016/s0092-8674(05)80028-5. [DOI] [PubMed] [Google Scholar]
- Stevens CF, Wang Y. Facilitation and depression at single central synapses. Neuron. 1995;14:795–802. doi: 10.1016/0896-6273(95)90223-6. [DOI] [PubMed] [Google Scholar]
- Stricker C, Redman S, Daley D. Statistical analysis of synaptic transmission: model discrimination and confidence limits. Biophysical Journal. 1994;67:532–547. doi: 10.1016/S0006-3495(94)80513-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trussell LO. Cellular mechanisms for preservation of timing in central auditory pathways. Current Opinion in Neurobiology. 1997;7:487–492. doi: 10.1016/s0959-4388(97)80027-x. [DOI] [PubMed] [Google Scholar]
- Trussell LO, Zhang S, Raman IM. Desensitization of AMPA receptors upon multiquantal neurotransmitter release. Neuron. 1993;10:1185–1196. doi: 10.1016/0896-6273(93)90066-z. [DOI] [PubMed] [Google Scholar]
- Walmsley B. Central synaptic transmission: studies at the connection between primary afferent fibres and dorsal spinocerebellar tract (DSCT) neurones in Clarke's column of the spinal cord. Progress in Neurobiology. 1991;36:391–423. doi: 10.1016/0301-0082(91)90017-u. [DOI] [PubMed] [Google Scholar]
- Walmsley B. Interpretation of ‘quantal’ peaks in distributions of evoked synaptic transmission at central synapses. Proceedings of the Royal Society. 1995;B 261:245–250. doi: 10.1098/rspb.1995.0144. [DOI] [PubMed] [Google Scholar]
- Walmsley B, Alvarez FJ, Fyffe RE. Diversity of structure and function at mammalian central synapses. Trends in Neurosciences. 1998;21:81–88. doi: 10.1016/s0166-2236(97)01170-3. [DOI] [PubMed] [Google Scholar]
- Walmsley B, Edwards FR, Tracey DJ. Nonuniform release probabilities underlie quantal synaptic transmission at a mammalian excitatory central synapse. Journal of Neurophysiology. 1988;60:889–908. doi: 10.1152/jn.1988.60.3.889. [DOI] [PubMed] [Google Scholar]
- Zucker RS. Short-term synaptic plasticity. Annual Review of Neuroscience. 1989;12:13–31. doi: 10.1146/annurev.ne.12.030189.000305. [DOI] [PubMed] [Google Scholar]


