Abstract
The Calgary Biofilm Device (CBD) was used to form bacterial biofilms of selected veterinary gram-negative and gram-positive pathogenic bacteria from cattle, sheep, pigs, chicken, and turkeys. The minimum inhibitory concentration (MIC) and minimum biofilm eradication concentration (MBEC) of ampicillin, ceftiofur, cloxacillin, oxytetracycline, penicillin G, streptomycin, tetracycline, enrofloxacin, erythromycin, gentamicin, tilmicosin, and trimethoprim-sulfadoxine for gram-positive and -negative bacteria were determined. Bacterial biofilms were readily formed on the CBD under selected conditions. The biofilms consisted of microcolonies encased in extracellular polysaccharide material. Biofilms composed of Arcanobacterium (Actinomyces) pyogenes, Staphylococcus aureus, Staphylococcus hyicus, Streptococcus agalactiae, Corynebacterium renale, or Corynebacterium pseudotuberculosis were not killed by the antibiotics tested but as planktonic bacteria they were sensitive at low concentrations. Biofilm and planktonic Streptococcus dysgalactiae and Streptococcus suis were sensitive to penicillin, ceftiofur, cloxacillin, ampicillin, and oxytetracycline. Planktonic Escherichia coli were sensitive to enrofloxacin, gentamicin, oxytetracycline and trimethoprim/ sulfadoxine. Enrofloxacin and gentamicin were the most effective antibiotics against E. coli growing as a biofilm. Salmonella spp. and Pseudomonas aeruginosa isolates growing as planktonic populations were sensitive to enrofloxacin, gentamicin, ampicillin, oxytetracycline, and trimethoprim/sulfadoxine, but as a biofilm, these bacteria were only sensitive to enrofloxacin. Planktonic and biofilm Pasteurella multocida and Mannheimia haemolytica had similar antibiotic sensitivity profiles and were sensitive to most of the antibiotics tested. The CBD provides a valuable new technology that can be used to select antibiotics that are able to kill bacteria growing as biofilms.
Introduction
Historically, we have studied these microorganisms by culturing them in highly enriched liquid or solid media that artificially selects for less hardy bacteria (1,2,3). However, bacteria exist within natural systems in an entirely different form from these artificially grown laboratory strains (1,2,3). In order for bacteria to survive within hostile environments such as that encountered in host tissue (antibodies, phagocytes, etc.) or on an inert surface exposed to inhospitable conditions (UV light, desiccation, heat, cold, shear forces), they have adapted by existing as adherent populations (sessile bacteria). Sessile bacteria appear to be protected in these antagonistic environments by growing as colonies encased in an extracellular matrix of carbohydrate or exopolysaccharide (1,2,3,4). A large collection of these groups of bacterial cells adhering to a surface is called a bacterial biofilm (1,2,3). When bacteria are examined in natural environments and within infected tissue, biofilms are the most predominant form. Sessile bacteria growing on surfaces have nutrient limitations and so may grow more slowly and have restricted mobility (4); planktonic forms in culture media have unnatural access to nutrients, multiply rapidly and often are highly motile. Planktonic bacteria are more susceptible to the effects of antibiotics and to environmental and host factors (1,2,3,4). Conversely, sessile bacteria are able to resist or evade such destructive factors by forming aggregates, altering their physiology, and taking advantage of deficiencies in the host clearance mechanisms (1,2,3,4).
Many common bacterial pathogens exist in animals as biofilms. Typical animal diseases where bacterial biofilms are believed to be involved based on histopathologic and ultrastructural appearance of the bacteria within tissue include: mastitis (Streptococcus agalactiae, Staphylococcus aureus), pneumonia (Mannheimia haemolytica, Pasteurella multocida), liver abscess (Fusobacterium necrophorum), lymphadenitis (Corynebacterium pseudotuberculosis, Streptococcus spp.), enteritis (Escherichia coli, Salmonella spp.) and wound infections (Staphylococcus aureus, Pseudomonas aeruginosa) (1). Infections that involve a biofilm mode of growth are generally chronic and are often difficult to treat (1,2,3,4).
Traditionally, microbiologists have evaluated the efficacy of an antibiotic by measuring the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) (5,6). In virtually all diagnostic laboratories, these measurements are made on freely floating, planktonic, laboratory phenotypes. These assays measure only the concentration of chemotherapeutic agent required to inhibit growth or kill planktonic bacteria (5,6). For some antibiotics, the concentration required to kill sessile bacteria may be greater than a thousand times that required to kill planktonic bacteria of exactly the same strain (4,7). Therefore, the use of typical laboratory planktonic bacteria for selection of chemotherapeutics may be inappropriate under some circumstances.
We have recently developed a technology to screen the effectiveness of antibiotics or biocides at eliminating sessile bacteria in vitro (8). The use of the Calgary Biofilm Device (CBD) permits rapid selection of potentially effective antibiotics for killing sessile bacteria in vivo and biocides for disinfecting contaminated inert surfaces (8). This device determines the minimum biofilm eradication concentration (MBEC), which is the concentration of an antimicrobial agent required to kill a bacterial biofilm. Recent studies conducted in our laboratory have demonstrated that selecting antibiotics that are effective for eliminating bacterial biofilms may improve the success rate in treating clinical and experimentally induced disease (9). The objective of this project was to determine culture conditions where veterinary pathogens would form biofilms on the CBD. A second objective was to evaluate the ability of antibiotics commonly used in veterinary medicine to eliminate a diverse selection of bacterial biofilms. Ultimately, the study was conducted to provide the basis of future extensive screening of the susceptibility of antibiotics to veterinary bacterial pathogens.
Materials and methods
Organisms
Bacterial isolates were obtained from clinical cases of infections of cattle, sheep, pigs, chickens and turkeys. Isolates were obtained from the Animal Health Unit of the University of Calgary in Calgary, Alberta and from Alberta Agriculture, Food and Rural Development in Edmonton, Alberta. These isolates included: Corynebacterium pseudotuberculosis, Corynebacterium renale, Mannheimia haemolytica, Pasteurella multocida, Arcanobacterium (Actinomyces), Haemophilus somnus, Pseudomonas aeruginosa, Staphylococcus aureus, Staphylococcus hyicus, Streptococcus agalactiae, Streptococcus dysgalactiae, Streptococcus suis, Escherichia coli and Salmonella spp.
Selection of antibiotics
The antibiotics evaluated were those commonly approved for the treatment of bacterial infections in animals. The antibacterial agents evaluated included: ampicillin, cloxacillin, erythromycin, gentamicin, oxytetracycline, penicillin G, streptomycin, tetracycline (Sigma Chemical Company, St. Louis, Missouri, USA), ceftiofur (The Upjohn Corporation, Kalamazoo, Michigan, USA), enrofloxacin (Bayer Animal Health, Kansas City, Kansas, USA), tilmicosin (Eli Lilly, Greenfield, Indiana, USA), and trimethoprim-sulfadoxine (Hoechst Animal Health, Regina, Saskatchewan).
Biofilm formation on the Calgary Biofilm Device
Biofilm formation and measurement of antimicrobial sensitivity of bacterial biofilms were performed on the CBD (MBEC Biofilm Technologies, Calgary, Alberta) according to previously described methods (8). The device features a microtiter plate lid with 96 pegs or projections distributed on the lid. Each peg provided the surface for bacteria to adhere, colonize and form a uniform biofilm (8). The pegs fit precisely into the wells of a standard 96-well microtiter plate. The lid was used in conjunction with special troughs for growing of bacteria, washing, and incubating. One of tryptic soy broth (BDH), tryptic soy broth with bovine serum (Sigma Chemical Company) or HS broth (Difco Laboratories, Detroit, Michigan, USA) for H. somnus was placed in the trough. The trough was inoculated with approximately 108 test bacteria (based upon McFarlane standards) obtained from colonies selected from tryptic soy agar (TSA, BDH) or brain heart infusion (BHI, BDH) agar plates. The pegged lid was placed over the troughs and the unit incubated on a rocker [Red Rocker; Hoefer Instruments, San Francisco, California, USA; 10 rpm (2.5 × g)] at 37°C and 95% relative humidity. The pegs were colonized for 4 to 24 h (depending on the specific bacterial growth rate). Selection of culture conditions for colonization of the pegs was determined in preliminary studies and the assessment of biofilm was determined by breaking several pegs from various points on the lid. The removed pegs were placed in microfuge tubes containing 200 μL of TSB, sonicated (Aquasonic, model 250; VWR Scientific, Buffalo Grove, Illinois, USA) for 5 min and plate counts of viable bacterial cells were performed on TSA or BHI agar containing 10% sheep blood. Additional pegs were fixed with 2.5% gluteraldehyde in phosphate-buffered saline (PBS), air-dried overnight, and prepared for scanning electron microscopy (SEM) (Hitachi model 450; Hitachi, Tokyo, Japan), as described previously (10).
Minimum biofilm eradication concentration assay
Assays were performed when pegs contained approximately 104 to 106 bacteria growing as a biofilm following conditions developed from the procedure described above. By using SEM, we have established that biofilms are produced at this level of peg colonization and we are not studying adherent bacterial cells. These biofilms can then be used for assessment of antimicrobial activities. Non-adherent bacteria on the pegs were washed from the pegs in a 96-well microtiter plate containing sterile PBSS. Each test antibiotic was placed in one lane of the microtiter plate at 2-fold dilutions of antibiotic (from 1024 μg/mL to 2 μg/mL). Seven antibiotics were evaluated on each plate and one lane served as a negative control (no antibiotic). All samples were run in duplicate. Pegs with the bacterial biofilm were secured over the test microtiter plate and the plate was incubated for 24 h at 37°C with antibiotic. The pegged lid was then removed, rinsed in PBS, then placed over a second 96-well microtiter plate containing fresh, sterile broth medium. The remaining biofilm was removed from the pegs by ultrasonic disruption for 5 min. This plate was incubated for 24 h at 37°C and the presence of viable bacteria determined by plate counts or turbidity determined at 650 nm in a 96-well plate reader (Molecular Devices; Fisher Scientific, Nepean, Ontario). Growth of bacteria in a particular well indicates regrowth of planktonic bacteria from surviving biofilm. Therefore, the MBEC value represents the lowest dilution at which bacteria fail to regrow.
Determination of minimum inhibitory concentration
The minimum inhibitory concentration (MIC), which represents the concentration of antibiotic required to inhibit growth of a planktonic bacterial population, was determined using the CBD. The MIC was determined from the bacteria that were shed from the pegs of the CBD when it was placed in the differing concentrations of antibiotics (8). The MIC values obtained using the CBD are similar to those obtained using the National Committee for Clinical Laboratory Standards (NCCLS) procedure (8).
Results
Bacterial biofilms were readily formed by most pathogens on the CBD (Table I). The biofilms consisted of microcolonies encased in extracellular polysaccharide material (1,4). Typical biofilms are illustrated in Figures 1a, 1b, and 1c. Although bacteria grew readily as planktonic organisms in liquid culture media, some bacteria would not form biofilms under standard cultural conditions. Special conditions were required for these organisms to grow as biofilms (Table I). In order to form biofilms, C. renale, C. pseudotuberculosis, M. haemolytica, P. multocida, and A. pyogenes required the addition of fetal bovine serum and incubation under 10% CO2. The duration of heavy biofilm formation on the pegs [> 104 colony-forming units (cfu) per peg] varied from 4 h for P. aeruginosa to 24 h for A. pyogenes, M. haemolytica, C. renale, and C. pseudotuberculosis. Bacteria that required these more specialized culture conditions to form biofilms also required longer culture time.
Table I.
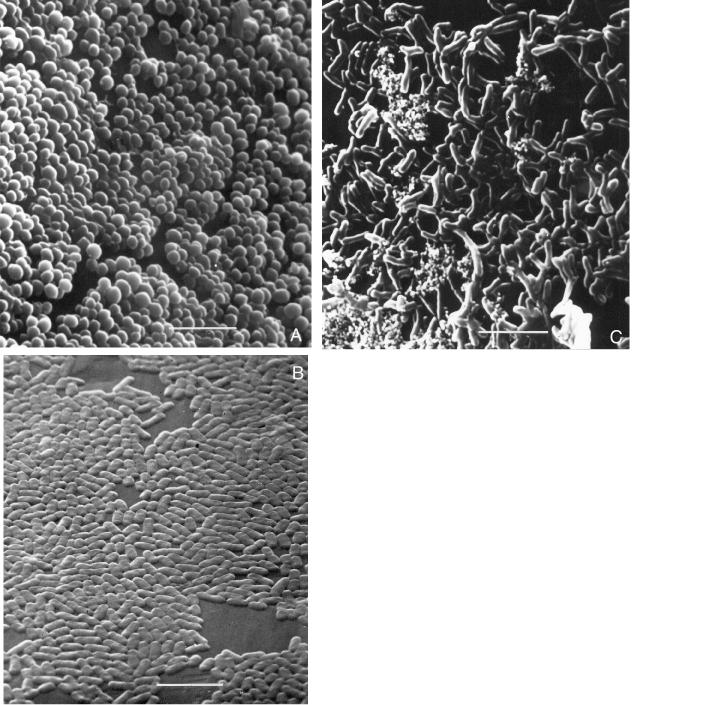
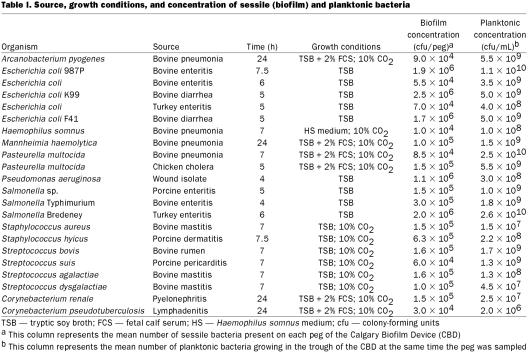
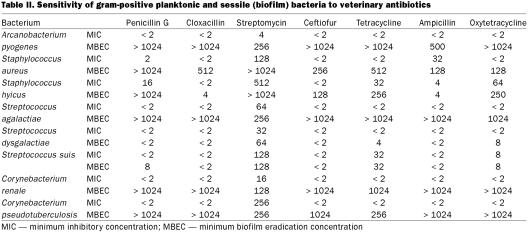
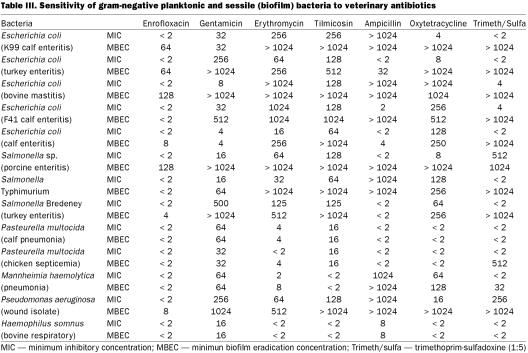
Figure 1. Representative examples of biofilm formation of veterinary pathogens. Staphylococcus aureus (mastitis isolate), Pasteurella multocida (poultry isolate), and Corynebacterium renale (bovine isolate) are demonstrated colonizing the peg of the CBD in Figure 1a, 1b, and 1c, respectively. Note how the bacteria tend to grow in clumps (microcolonies) and the exopolysaccharide that is covering the bacteria; bar = 5 μm.
The concentrations of antibiotic required to inhibit planktonic bacteria (MIC) and those required to kill biofilm bacteria (MBEC) are summarized in Tables II and III. Most antibiotics were effective in inhibiting planktonic bacterial growth at low concentrations and the bacteria would be considered sensitive based upon NCCLS breakpoints (11). Only a limited number of antibiotics were effective in killing biofilm bacteria at relatively low concentrations. In some cases, biofilm bacteria, such as A. pyogenes and S. aureus, appeared to lack sensitivity to all of the antibiotics evaluated.
Table II.
Table III.
Gram-positive organisms growing as biofilms proved to be particularly resistant to most antimicrobial agents. Most planktonically growing organisms were sensitive to virtually all of the antibiotics tested (Table II). Biofilms composed of A. pyogenes, S. aureus, S. hyicus, S. agalactiae, C. renale, and C. pseudotuberculosis were highly resistant to antimicrobial agents evaluated but sensitive to the tested agents as planktonic bacteria. Both biofilm and planktonic forms of S. dysgalactiae and S. suis were sensitive to the β-lactam drugs (penicillin, ceftiofur, cloxacillin, ampicillin) and oxytetracycline.
There was considerable variation in the results obtained for E. coli isolates grown as sessile (biofilm) and planktonic populations (Table III). Planktonic E. coli were sensitive to enrofloxacin, gentamicin, oxytetracycline, and trimethoprim/sulfadoxine. Enrofloxacin and gentamicin were the most effective antibiotics for E. coli growing as biofilms. Salmonella growing as planktonic populations were sensitive to enrofloxacin, gentamicin, ampicillin, oxytetracycline, and trimethoprim/sulfadoxine. When Salmonella spp. were grown as biofilms, they were only sensitive to enrofloxacin and ampicillin (Salmonella Bredeny only). Planktonic and biofilm populations of P. multocida and M. haemolytica had similar antibiotic sensitivity profiles with the exception of trimethoprim/sulfadoxine. Planktonic P. aeruginosa were sensitive to enrofloxacin, erythromycin, and oxytetracycline. The sessile forms of this organism were sensitive only to enrofloxacin. Both planktonic and sessile H. somnus were sensitive to enrofloxacin, gentamicin, erythromycin, tilmicosin, ampicillin, oxytetracycline, and trimethoprim/sulfadoxine.
Discussion
The MIC has been used as a gold standard for determination of antimicrobial sensitivities for animal and human pathogenic bacteria (4,5). It is recognized that an antibiotic that is ineffective in preventing growth of a particular organism using the MIC assay will also be clinically ineffective (12). However, an organism that is sensitive in vitro may not be effective in vivo (12,13,14,15,16). For many veterinary bacterial diseases the MIC value for a particular antibiotic is not predictive of clinical efficacy. Nevertheless, up to this time, the MIC assay remains the best way to select potentially effective antimicrobial agents. The CBD and the MBEC assay were developed for rapid and reproducible antimicrobial susceptibility testing for bacterial biofilms in the anticipation that the MBEC would be more reliable for selection of clinically effective antibiotics.
In human medicine it has been estimated that 65% of nosocomial infections are biofilm associated, costing the health care system billions of dollars (1,3,15). These biofilm infections are 10 to 1000 times more resistant to the effects of antimicrobial agents (1,3,7). Indeed, many veterinary bacterial pathogens exist predominantly as adherent (also called biofilm or sessile) organisms within tissue and on inert surfaces and it is well recognized that such infections are extremely difficult to successfully treat (14,15,17,18). The mechanism for enhanced antimicrobial resistance is believed to involve alterations in gene expression leading to a phenotype difference between the planktonic and sessile forms. The sessile forms are more resistant as they produce exopolysaccharde, have different growth characteristics and take up nutrients and drugs differently from their planktonic counterpart (3,16). The CBD was developed to address the issues of enhanced antimicrobial resistance within biofilms. Determination of MBEC might, therefore, permit selection of a particular antibiotic that would more closely reflect the prognosis of antimicrobial therapy for a particular bacterial infection.
Staphylococcus biofilms have been extensively studied in human medicine and this pathogen is considered significant in both device associated infections and tissue infections such as pneumonia and osteomyelitis (1,19). The prevalence of bovine staphylococcal mastitis ranges from 7% to 40% of all dairy cattle and this infection is associated with bacterial biofilms (14,17,18,19). It is also recognized that antibiotic therapy may temporarily eliminate clinical signs of mastitis but the prognosis of a complete cure is poor (14). Although, in this study, the MIC assay clearly indicated that many antibiotics should be effective in the treatment of bovine mastitis, the MBEC values data demonstrated that the S. aureus isolate is resistant to antibiotics tested, correlating with clinical observations. The S. hyicus biofilm as measured by MBEC was sensitive to many of the antibiotics tested; indeed, S. hyicus usually responds well to antibiotic therapy (20).
There is considerable variability in therapeutic responses to streptococcal infections (20). S. suis and S. dysgalactiae infections frequently respond readily to most chemotherapeutic agents (20). Although these organisms formed biofilms, the MIC and MBEC values were similar, suggesting that most of the antibiotics evaluated would be effective as chemotherapeutic agents. The S. agalactiae isolate studied, recovered from an animal with chronic mastitis, was sensitive to most antibiotics according to the MIC, but as a biofilm, it was resistant. S. agalactiae mastitis is highly infectious and usually responds to treatment. As this isolate was recovered from an animal with chronic unresponsive mastitis, it may be genotypically and phenotypically altered to be resistant as a biofilm (3,16).
Although C. renale, C. pseudotuberculosis and A. pyogenes grew readily as planktonic bacteria in enriched broth, they required specific culture conditions, such as addition of serum to the media and culturing under increased carbon dioxide concentration, to induce formation of biofilms. This suggests that for some microorganisms simulation of the growth conditions that exist in the host may be required. These organisms were sensitive to all antimicrobial agents tested (except streptomycin) according to the MICs, but they were highly resistant according to the MBEC values. The MBEC values appear to be more predictive, as infections caused by C. renale, C. pseudotuberculosis, and A. pyogenes require prolonged antimicrobial therapy and are frequently unresponsive to treatment (20). The ability of biofilm bacteria to avoid phagocytosis by macrophages and neutophils may also account for the abscessation observed within these infections (1). The accumulation of pus and the associated encapsulation of the infection site also inhibits the antimicrobial penetration and pathogen destruction.
Most gram-negative livestock pathogens readily form bacterial biofilms and these biofilms have been previously described in livestock infections such as neonatal colibacillosis (21) and pneumonic pasteurellosis (18). The veterinary E. coli isolates tested readily formed biofilms on the CBD and with the exception of enrofloxacin, gentamicin, and ampicillin, these biofilms were resistant to the antibiotics tested. This suggests that once E. coli biofilms have been established, they may be difficult to treat with some antibiotics. This has been observed in some clinical cases in cattle, swine and poultry (20,22). There was considerable variability among the MICs of the Salmonella spp. isolates in this study: similar variability between the MIC and the MBEC values was observed. This observation may reflect the complexity in prediction of chemotherapeutic agents for treatment of different Salmonella isolates. Bovine, porcine, and avian Pasteurella spp., as well as the H. somnus and M. haemolytica isolate tested, formed biofilms, but in most cases there was no difference between the MIC and the MBEC values. Indeed, animals with pasteurellosis or hemophilosis respond well to most antimicrobial agents provided that a secondary pathogen (A. pyogenes, S. aureus) is not involved (20).
Pseudomonas aeruginosa has been recognized in human medicine to form antibiotic resistant biofilms on implanted devices and within tissues (3). Pseudomonas aeruginosa infections in animals are similarly difficult to treat (23). The planktonic Pseudomonas isolate was resistant to most antibiotic agents, but biofilm cells were more resistant and only enrofloxacin demonstrated reasonable clinical activity. Fluorinated quinolones have been shown to be effective in treatment of most Pseudomonas infections (1,3).
Planktonic bacterial sensitivity, pharmacokinetics, drug penetration, local activity, and drug inactivation all influence the clinical efficacy of an antibacterial agent, but to date, the efficacy of veterinary antibiotics in elimination of bacterial biofilms has not been evaluated. The CBD and the MBEC assay provide a new technology that can be used to select antibiotics that are effective in killing biofilm bacteria. This new technology can also be used in the pharmaceutical industry for developing new antimicrobial agents with efficacy against bacteria growing as biofilms (8). Recently, we have used the MBEC assay to predict clinical failure and clinical success of certain antibiotics used to treat peritonitis due to device-associated infections in humans (24). It may be possible to apply this technology in veterinary bacterial infections that are difficult to treat. This study was conducted to demonstrate the diversity of organisms that could form biofilms that were resistant to common veterinary antibiotics. Further studies are required to document variations within a specific species or from a defined bacterial disease.
Footnotes
Acknowledgments
This research was supported by the Alberta Agriculture Research Institute and the Natural Sciences and Engineering Research Council. The authors acknowledge the technical support of Carol Ann Stremick and Liz Middlemiss.
Address correspondence and reprint requests to Dr. Merle E. Olson, telephone: 403-220-6836, fax: 403-270-0954, e-mail: molson@ucalgary.ca
Received July 27, 2001. Accepted November 29, 2001.
References
- 1.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science 1999;284:318–322. [DOI] [PubMed]
- 2.Rosser BT, Taylor PA, Cix PA, Cluland R. Methods for evaluating antibiotics on bacterial biofilms. Antimicrob Agents Chemother 1987;31:1502–1506. [DOI] [PMC free article] [PubMed]
- 3.Mah T-FC, O'Toole GA. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol 2001;9:34–39. [DOI] [PubMed]
- 4.Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappin-Scott HM. Microbial biofilms. Ann Rev Microbiol 1995; 49:711–745. [DOI] [PubMed]
- 5.Prescott JF, Baggott JD. Antimicrobial susceptibility testing and antimicrobial drug dosage. J Am Vet Med Assoc 1985;187: 363–368. [PubMed]
- 6.Sahm DF, Washington JA. Antibacterial susceptibility tests: dilution methods. In: Laboratory Procedures in Clinical Microbiology. New York: Springer-Verlag, 1991:1105–1116.
- 7.Nickel JC, Ruseska I, Wright JB, Costerton JW. Tobramycin resistance of Pseudomonas aeruginosa cells growing as a biofilm on urinary catheter material. Antimicrob Agents Chemother 1985;27:619–624. [DOI] [PMC free article] [PubMed]
- 8.Ceri H, Olson ME, Stremick C, Morck DW, Read RR, Buret AG. The Calgary Biofilm Device: Measurement of antimicrobial sensitivity of bacterial biofilms. J Clin Microbiol 1999;37: 1771–1776. [DOI] [PMC free article] [PubMed]
- 9.Morck DW, Lam K, McKay SG, Olson ME, Costerton JW. Comparative evaluation of fleroxacin, ampicillin, trimethoprim-sulfamethoxazole and gentamicin as treatments of catheter-associated urinary tract infection in a rabbit model. Inter J Antimicrob Agents 1994;4:S21–27. [DOI] [PubMed]
- 10.Marie TJ, Costerton JW. Scanning and transmission electron microscopy of in situ bacterial colonization of intravenous and intraarterial catheters. J Clin Microbiol 1984;19:687–693. [DOI] [PMC free article] [PubMed]
- 11.NCCLS. Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals; approved standard. NCCLS document M31A. Wayne, Pennsylvania: National Committee for Clinical Laboratory Standards.
- 12.Langston VC. Antimicrobial use in food animals. In: Howard JL, Smith RA, eds. Current Veterinary Therapy 4: Food Animal Practice. Philadelphia: WB Saunders, 1999:17–32.
- 13.Owens WE, Ray CH, Watts JL, et al. Comparison of success of antibiotic therapy during lactation and results of antimicrobial susceptibility tests for bovine mastitis. J Dairy Sci 1997;80: 313–317. [DOI] [PubMed]
- 14.Sandholm M, Kaartinen L, Pyorala S. Bovine mastitis — Why does antibiotic therapy not always work? An overview. J Vet Pharm Ther 1990:13:248–260. [DOI] [PubMed]
- 15.Potera C. Forging a link between biofilms and disease. Science 1999;283:1837–1838. [DOI] [PubMed]
- 16.Pratt LA, Kolter R. Genetic analysis of biofilm formation. Curr Opin Microbiol 1999;2:598–603. [DOI] [PubMed]
- 17.Baselga R, Albizu I, De la Cruz M, Del Cacho E, Barberan M, Amorena B. Phase variation of slime production in Staphylococcus aureus: implications in colonization and virulence. Infect Immun 1993;61:4857–4862. [DOI] [PMC free article] [PubMed]
- 18.Morck DW, Olson ME, Acres SD, Daoust PY, Costerton JW. Presence of bacterial glycocalyx and fimbriae on Pasteurella haemolytica in feedlot cattle with pneumonic pasteurellosis. Can J Vet Res 1989;53:167–171. [PMC free article] [PubMed]
- 19.Bezek DM. Genus identification and antibiotic susceptibility patterns of bacterial isolates from cows with acute mastitis in a practice population. J Am Vet Med Assoc 1998;212:404–406. [PubMed]
- 20.Radostits OM, Gay CC, Blood DC, Hinchcliff KW. Veterinary Medicine. Philadelphia: WB Saunders, 2000:701–996.
- 21.Chan R, Acres SD, Costerton JW. Morphological examination of cell surface structures of enterotoxigenic strains of Escherichia coli. Can J Microbiol 1984;30:451–60. [DOI] [PubMed]
- 22.Fairbrother JM. Escherichia coli infections in farm animals. In: Howard JL, Smith RA, eds. Current Veterinary Therapy 4: Food Animal Practice. Philadelphia: WB Saunders, 1999:328–330.
- 23.Gyles CL. Pseudomonas; Moraxella. In: Gyles CL, Thoen C, eds. Pathogenesis of Bacterial Infections in Animals. Ames: Iowa State University Press, 1986:172–180.
- 24.Ceri H, Sepandj F, Gibb AP, Read RR, Olson ME. Comparison of standard minimal inhibitory concentration (MIC) versus minimal biofilm eradication concentration (MBEC) antibiotic sensitivity of coagulase-negative Staphylococcus sp. from peritoneal catheter-related peritonitis. Proc Am Soc Microbiol 1999;99:245.