Abstract
Intracellular current clamp recordings were performed from identified oxytocin (OT) neurones in acute hypothalamic slices taken from lactating Wistar rats at early (5th day: LD-5) and late (21st day: LD-21) lactation.
The basic electrophysiological properties of LD-21 OT neurones differed from those of LD-5 OT neurones: their resting membrane potential was more depolarised (-51·5 versus -54·9 mV); their action potential duration was longer (1·6 versus 1·2 ms); their hyperpolarising after-potential (HAP) following single spikes and after-hyperpolarisation (AHP) following a burst of action potentials had smaller amplitudes (-46 and -67 %, respectively); and they lacked spike frequency adaptation during a burst.
In LD-21 neurones bath application of 17β-oestradiol (10−7 M, 6–14 min) reversibly restored all these properties to values observed in LD-5 cells. This treatment had no effect on LD-5 neurones.
LD-21 neurones were less sensitive to kainate than LD-5 neurones. 17β-Oestradiol significantly potentiated the kainate-induced response in LD-21, but not in LD-5 neurones.
The effects of 17β-oestradiol were presumably mediated through a non-genomic mechanism since they occurred within a few minutes of administration, and disappeared within 30–40 min of washout. They were not inhibited by tamoxifen, an antagonist of the nuclear oestrogen receptor ER-α. Lastly, cholesterol, a non-active lipophilic molecule, had no effect.
Our observations demonstrate that, in the absence of 17β-oestradiol, the basic electrical properties and sensitivity to kainate of OT neurones become altered between early and late lactation. However, the rise in circulating levels of oestrogens during the late phase of lactation may contribute to maintain OT neurone reactivity as long as suckling continues.
In rats, the milk ejection reflex induced by suckling is characterised by a pulsatile release of oxytocin (OT), in which each pulse results from the synchronous bursting electrical activity of magnocellular OT neurones in the hypothalamus (reviewed in Poulain & Wakerley, 1982). Thus, once lactation is fully established, suckling-induced milk ejections occur every 3–5 min, and each burst of action potentials, reaching a frequency of 60–80 Hz, lasts about 2–4 s. The reflex, however, is not a strictly fixed neuroendocrine mechanism. It can be modulated during a suckling episode, during which the temporal pattern of milk ejections can vary greatly in relation to the level of vigilance of the mother or the number of suckling pups. Moreover, the reflex is gradually programmed at the end of gestation and undergoes a number of changes in its characteristics throughout the period of lactation, as weaning approaches. For instance, in late pregnancy, the reflex can be evoked prematurely by using a foster litter to suckle, but the incidence of bursts is low; at the other end of lactation, after weaning, both burst frequency and burst amplitude are lower than at mid-lactation (Jiang & Wakerley, 1995).
The detailed organisation of the reflex is incompletely understood, notably concerning the mechanisms by which OT neurones are activated during the bursts. In the hypothalamus, a glutamatergic innervation appears to play a major role. OT neurones receive a large number of glutamatergic synapses, the proportion of which increases at the time of lactation (El Majdoubi et al. 1996, 1997). As seen from electrophysiological recordings in slices, glutamate constitutes the main excitatory transmitter for magnocellular neurones (for a review, see Renaud & Bourque, 1991). Moreover, we recently showed that in organotypic slice cultures obtained from postnatal rats, OT neurones display periodic bursts due to volleys of glutamatergic excitatory potentials, via the activation of non-NMDA glutamate receptors (Jourdain et al. 1998).
Whatever the afferent pathways controlling the reflex directly, its modulation from pre-partum to weaning may be due to several factors, acting separately or in conjunction. Of these, ovarian steroids are of particular relevance. Just before parturition, there is a sharp and transient fall in the plasma concentration of progesterone, which occurs simultaneously with a transient, sharp rise in that of oestradiol. Thereafter, oestradiol levels, low at the beginning of lactation, rise progressively, whereas progesterone concentrations decline (Smith & Neill, 1977). These changes may contribute to the modulation of the reflex since ovariectomy and/or treatment with ovarian steroids alter milk ejection frequency and the facilitatory influence of OT itself on the reflex (Jiang & Wakerley, 1997). Other observations underline the influence of oestrogens on OT function. Thus, experiments using ovariectomy and/or substitutive treatment have shown the effects of oestrogens on plasma concentrations of OT (Yamaguchi et al. 1979), the content of OT within hypothalamic nuclei (Wang et al. 1995), the electrical activity of paraventricular nucleus neurones (Akaishi & Sakuma, 1985; Minami et al. 1990) and the morphological reorganisation of magnocellular nuclei induced by OT (Montagnese et al. 1990).
The present study was undertaken to investigate whether the electrophysiological properties of OT neurones themselves are altered throughout lactation and, if so, to examine the contribution of oestrogens to such alteration. For this purpose, we recorded intracellularly identified supraoptic OT neurones from hypothalamic slices taken at day 5 (LD-5) and day 21 (LD-21) of lactation, stages characterised by different steroid profiles, and we evaluated the effects of 17β-oestradiol on their electrical properties. We show here that OT neurones indeed exhibit changes in some of their basal electrical properties and in their sensitivity to kainate between early and late lactation, and that 17β-oestradiol, acting presumably through a rapid non-genomic effect, restores at late lactation these electrophysiological properties to their values at early lactation.
Some of these results have already appeared in abstract form (Israel et al. 1996).
METHODS
All experiments were carried out according to French/European guidelines.
Slice preparation
Lactating Wistar rats were killed by decapitation using a guillotine. Immediately after decapitation and opening of the skull, the brain was rapidly cooled with a cold perifusion medium (-1 to 0°C) for 20 s in situ. It was then quickly removed and immersed in cool (0°C) oxygenated (95 % O2-5 % CO2) medium for 1 min. The composition of the perifusion medium was (mM): NaCl, 125; KCl, 3; MgSO4, 1.24; KH2PO4, 1.3; NaHCO3, 25; CaCl2, 2; and glucose, 11. When necessary, the pH was adjusted to 7.25 with NaOH. The brain was cut with a razor blade into a small cube containing the supraoptic nucleus. The block was then fixed with cyanoacrylic glue on the top of the holder of a vibroslicer (MacIlwain, Campden Instruments Ltd). Two or three coronal sections (450 μm) were made and transferred onto a filter paper (optic lens neutral cleaner) in contact with a ramp-style interface recording chamber. The medium was perfused using a peristaltic pump (Gibson) at a rate of 0.8 ml min−1 and at constant temperature (32°C). The slices were constantly oxygenated with humidified 95 % O2-5 % CO2. The electrophysiological session began after a 2 h equilibration period.
Electrophysiology
Conventional intracellular recordings (current clamp) were made with sharp microelectrodes (o.d., 1 mm; i.d., 0.5 mm; Sutter Instruments) pulled with a horizontal puller (P-87, Flaming-Brown puller, Sutter Instruments). The microelectrodes displayed a resistance of 120–200 MΩ when filled with potassium acetate (1 M) containing 0.5 % biocytin. The microelectrode was positioned into the supraoptic nucleus with a micromanipulator (Microcontrôle, France) under visual control using a dissecting microscope. The electrode was lowered into the supraoptic nucleus with a piezoelectric microdrive (Nanostepper) in 1 μm steps. Cellular potentials were recorded and amplified with an Axoclamp-2A (Axon Instruments). Membrane potentials and intracellular currents were monitored on a digital oscilloscope (Gould DSO 420) and stored on magnetic tape after digitisation (Digital Pulse Code Modulation, Sony) through a Digidata 1200 interface (Axon) and pCLAMP 6.1 software (Axon).
All the drugs used in this study were applied dissolved in the perifusion medium. The standard kainate application consisted of a bolus of 0.4 ml given over 30 s, whereas steroids were applied for 6–15 min. 17β-Oestradiol, 17α-oestradiol, the ER-α oestrogen receptor antagonist tamoxifen and cholesterol were first dissolved in absolute ethanol at 10−2 M; 10 μl of this solution was then diluted in a 9 per thousand sodium chloride solution to reach 10−4 M. The solution was stored in 10 μl aliquots at -26°C for a maximum of 30 days. Just prior to utilisation, each aliquot was dissolved in normal medium to reach a final concentration varying from 5 × 10−8 to 2 × 10−6 M. Under these conditions, the final ethanol volume never exceeded 0.01 %.
Electrophysiological parameters, expressed as means ±s.e.m., were compared using Student's t test.
Histology and immunocytochemistry
Recorded cells were identified by biocytin (0.5 %) injected during recording and then by immunocytochemistry. Just after the recording session, the slices were fixed in a mixture of paraformaldehyde (4 %) and picric acid (0.15 %) in sodium phosphate buffer for 30 min, and then stored in 4 % paraformaldehyde at 4°C.
Each slice was then sectioned on a cryostat (25 μm; Microm HM 500 M). Biocytin was revealed with streptavidin conjugated with Texas Red (Biosys, France), diluted 1:1000 in phosphate-buffered saline (PBS) containing 2 % Triton X-100. The slices were thoroughly rinsed in PBS prior to immunocytochemical staining. For this, the slices were incubated (1 h at room temperature) in PBS containing 0.5 % casein (Sigma) to reduce non-specific binding of antibodies. They were then incubated under constant agitation (48 h, 4°C) in a mixture of primary antibodies consisting of a monoclonal mouse antibody raised against OT-associated neurophysin (OT-Np; kindly provided by Dr H. Gainer, Bethesda, USA) and a polyclonal rabbit antiserum raised against vasopressin-associated neurophysin (VP-Np, kindly provided by Dr A. Robinson, Los Angeles, USA). The slices were washed in Tris-buffered saline (TBS) and incubated in a mixture of fluorescent conjugates (2 h, room temperature): goat anti-rabbit immunoglobulins conjugated to fluorescein isothiocyanate (FITC, Biosys; diluted 1:400) served to visualise OT-Np; goat anti-mouse immunoglobulins conjugated with 7-amino-4-methyl-coumarin-3-acetic acid (AMCA, Biosys; diluted 1:200) served to identify VP-Np. The cultures were mounted with fluoromount (Vectashield, Vector Laboratories Inc.) and examined under epifluorescence microscopy with appropriate filters (Leica, Leitz DMR).
RESULTS
Supraoptic neurones were recorded from slices taken on day 5 (LD-5) and day 21 (LD-21) of lactation. Immunocytochemistry for OT-Np and VP-Np showed that, out of 113 recorded cells, 67 were oxytocinergic (15 LD-5 and 52 LD-21 neurones) and 16 were vasopressinergic (3 LD-5 and 13 LD-21). Three neurones were immunoreactive for both VP-Np and OT-Np. The 27 other neurones could not be identified for technical reasons and were excluded from this study. Unless otherwise stated, all the results presented here were obtained from OT neurones.
Resting membrane potential, membrane resistance and activity of OT neurones
Resting membrane potential was slightly but significantly lower (P < 0.001) in LD-5 neurones (-54.9 ± 1.5 mV, n = 11) than in LD-21 neurones (-51.5 ± 2.5 mV, n = 22). The input membrane resistance of LD-5 neurones and LD-21 neurones was not significantly different, being 326 ± 60 MΩ (n = 7) and 286 ± 30 MΩ (n = 12), respectively. LD-5 neurones had a continuous pattern of action potential discharge of around 1–3 Hz, but during long recording periods (up to 6 h), many cells (11/15) switched spontaneously and reversibly to a bursting activity similar to the phasic activity recorded in vivo; none were silent. In contrast, most LD-21 OT neurones (38/52) were silent during long periods of recording; nine neurones had a slow irregular activity, and five had a bursting activity.
At hyperpolarised potentials more negative than -80 mV, in both LD-5 and LD-21 neurones, a depolarising current pulse of sufficient amplitude triggered action potentials with a delay caused by an initial hyperpolarising ‘notch’, due to a transient K+ current similar to IA and typical of magnocellular neurones (Fig. 1Aa and Ab). During spontaneous and current-evoked trains of action potentials, spike broadening was observed in both types of neurone (Fig. 1Ba and Bb).
Figure 1. Transient outward rectification and spike broadening in OT neurones.
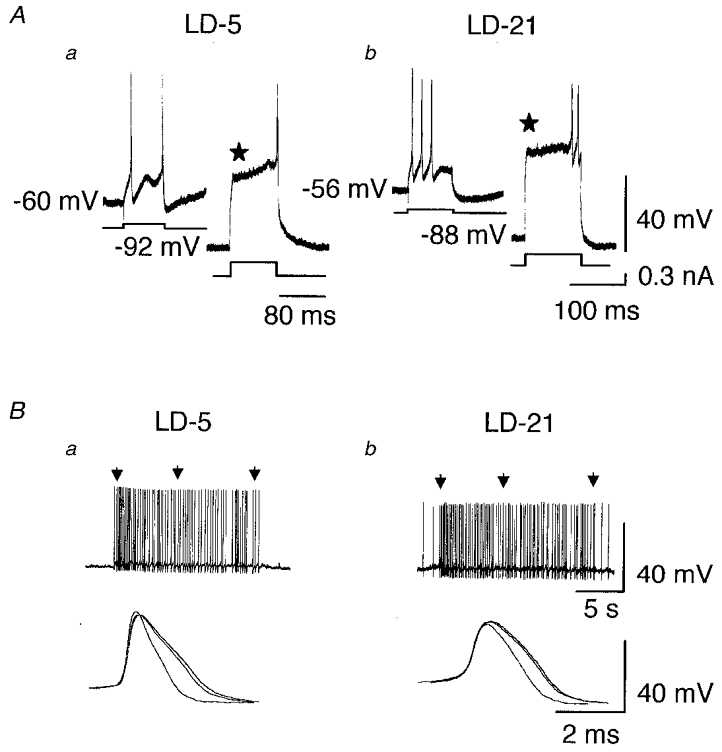
A, one LD-5 (a) and one LD-21 (b) neurone at resting potential were stimulated by a depolarising current pulse (100 pA, 80–100 ms). In both cases, the first evoked action potential appeared without any delay. In contrast, when the neurones were hyperpolarised near -90 mV, an appropriate depolarising pulse induced a transient outward rectification (⋆) which delayed the occurrence of the first action potential. B, spontaneous bursts of action potentials recorded from a LD-5 (a) and a LD-21 neurone (b). Three action potentials within each burst (indicated by arrows) were superimposed at a higher speed (a and b, lower traces) and clearly show action potential broadening.
The duration of action potentials measured at half-amplitude was significantly shorter in LD-5 than in LD-21 neurones (1.2 ± 0.2 versus 1.6 ± 0.2 ms, P < 0.001; Fig. 2A).
Figure 2. Comparison of action potentials recorded from LD-5 and LD-21 neurones and effects of 17β-oestradiol on their duration.
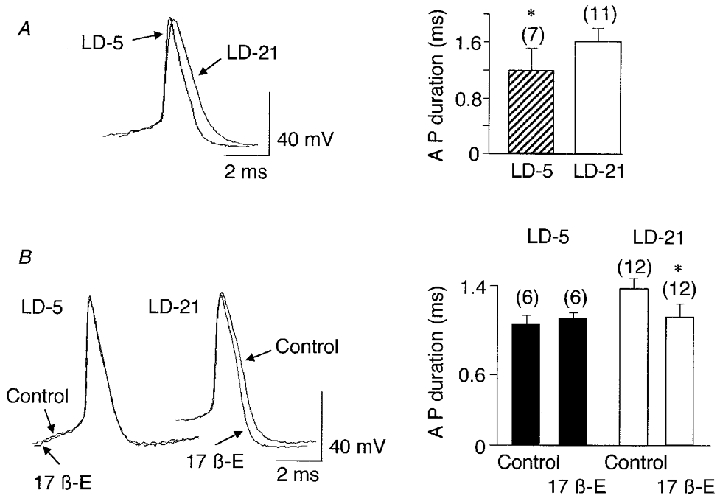
A, the superimposition of a spontaneous action potential recorded from a LD-5 neurone and one from a LD-21 neurone shows the longer duration of action potentials in the LD-21 neurone. The difference in duration was statistically significant (* P < 0.001). B, 17β-oestradiol application (17β-E, 10−7 M, 8 min) did not affect the duration of action potentials recorded from a LD-5 neurone whereas it shortened the action potentials in a LD-21 cell. The difference in duration was statistically significant between control and treatment (17β-E) only in LD-21 neurones (* P < 0.0003). Numbers in parentheses are the number of cells recorded in each group.
When depolarised by a current pulse, 9 out of 12 LD-5 neurones tested showed spike frequency adaptation. In contrast, none of the 13 LD-21 neurones tested displayed this property (Fig. 3A).
Figure 3. Spike frequency adaptation and HAP in LD-5 and LD-21 neurones and their modulation by 17β-oestradiol.
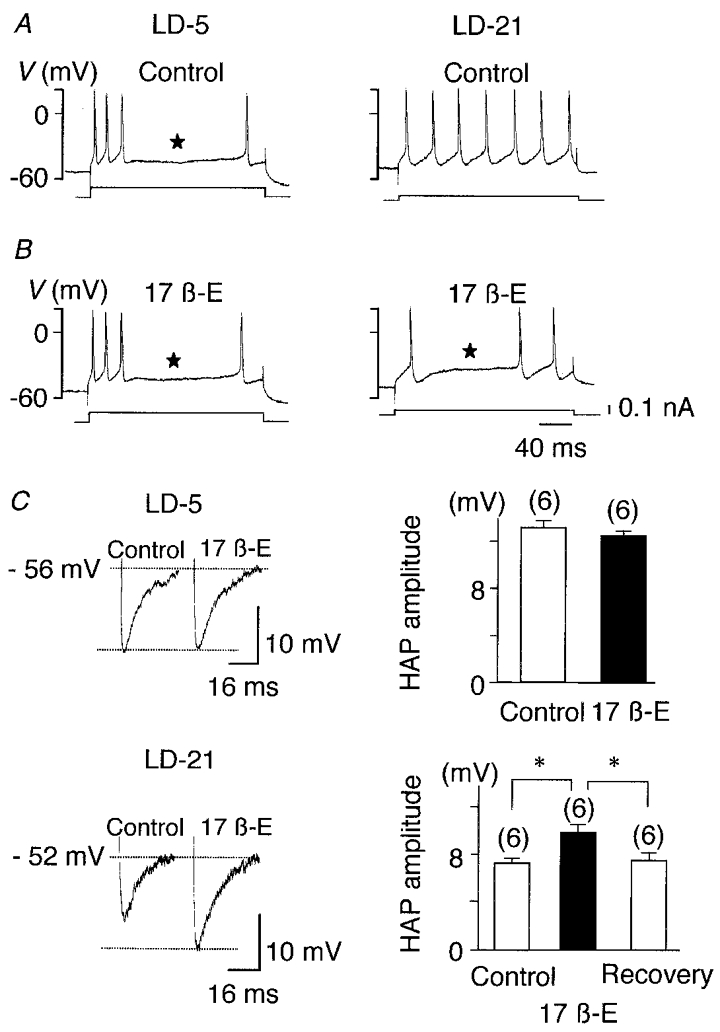
A, bursts of activity (upper traces) triggered by depolarising current pulses (lower traces) obtained from one LD-5 and one LD-21 neurone. Under control conditions firing frequency adaptation was present only in the LD-5 neurone (⋆). B, 17β-oestradiol application (10−7 M, 9 min) induced spike frequency adaptation in the LD-21 neurone whereas it had no effect on the LD-5 cell. C, when compared to control conditions, 17β-oestradiol (10−7 M, 8 min) did not alter HAP in the LD-5 neurone, but it significantly and reversibly increased the amplitude of HAP in the LD-21 cell (* P < 0.001). Numbers in parentheses are the number of cells recorded in each group.
In both types of neurone we observed a hyperpolarising after-potential (HAP) following single spikes. In LD-21 neurones, the amplitude of the HAP was significantly lower than in LD-5 neurones (-46 %, n = 6, P < 0.001; Fig. 3C). We also analysed the after-hyperpolarisation (AHP) following an evoked burst of action potentials. Because AHP is dependent on the number of action potentials within the burst (Andrew & Dudek, 1984; Bourque et al. 1985), the duration of the depolarising pulse was adjusted to trigger an identical number of action potentials. Here also, AHP amplitude was significantly lower in LD-21 neurones than in LD-5 neurones (-67 %, n = 5, P < 0.001; Fig. 4Ac and Bc).
Figure 4. Effects of 17β-oestradiol on the AHP recorded from LD-5 and LD-21 OT neurones.

A, AHP in a LD-5 neurone obtained in control conditions (a) and during 17β-oestradiol application (10−7 M, 12 min, b). The lower traces show the AHP on an expanded scale. The amplitude of the AHP did not vary significantly under the two experimental conditions (c). B, AHP recorded from a LD-21 neurone under control conditions (a) and in the presence of 17β-oestradiol (10−7 M, 8 min, b). The lower traces display the AHP on an expanded scale. The amplitude of the AHP was clearly enhanced by 17β-oestradiol at 10 min of treatment (* P < 0.0001, c). Action potentials are truncated. Numbers in parentheses are the number of cells recorded in each group.
Effects of 17β-oestradiol on the electrical properties of OT neurones
Because the plasma concentration of 17β-oestradiol increases at the end of lactation, we tested the effects of the steroid on the electrical properties of LD-5 and LD-21 neurones. Bath application of 17β-oestradiol had no effect on membrane potential and membrane resistance in either type of neurone (Figs 6A and 7A). However, Fig. 2B clearly shows that during bath application of 17β-oestradiol (10−7 M, 8 min), the action potential duration in LD-21 cells was significantly shortened (from 1.4 ± 0.1 ms, n = 12 to 1.1 ± 0.1 ms, n = 12, P < 0.0003) whereas it remained unchanged in LD-5 neurones (1.0 ± 0.1 versus 1.1 ± 0.1 ms, n = 6).
Figure 6. Effects of 17β-oestradiol on kainate-induced responses in LD-21 OT neurones.
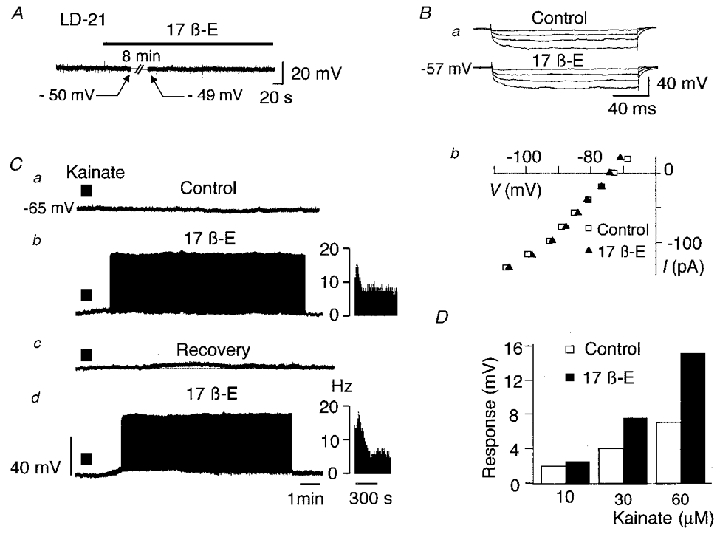
A, application of 17β-oestradiol (10−7 M, 12 min) did not modify the resting membrane potential in LD-21 neurones. Ba, downward voltage deflections induced by hyperpolarising currents (not shown) obtained under control conditions and in the presence of 17β-oestradiol in a LD-21 neurone. No significant variation was detected. Bb, current-voltage relationship obtained from another neurone under control conditions (□) and in the presence of 17β-oestradiol (▴). This neurone had previously been hyperpolarised to -73 mV to avoid triggered action potentials by depolarising current pulses. C, a LD-21 neurone was slightly hyperpolarised at -65 mV by a constant hyperpolarising current (-0.05 pA, not shown) to avoid spontaneous action potentials. Application of kainate (30 s, 30 μM, filled bar) under control conditions had no effect (a). During treatment with 17β-oestradiol (10−7 M, 10 min), another application of kainate (same parameters) strongly depolarised the cell giving rise to a sustained activity (b). This effect was reversible after a 40 min washing period (c) and was reproducible (d). In b and d, insets are sequential histograms of spike discharge per second (Hz). D, potentiation of kainate-induced responses by 17β-oestradiol. Kainate was tested on another LD-21 neurone at three different concentrations (10, 30 and 60 μM) under control conditions (□) and in the presence of 17β-oestradiol (10−7 M, 8 min, ▪).
Figure 7. 17β-Oestradiol did not potentiate the kainate-induced response obtained in LD-5 OT neurones.

A, application of 17β-oestradiol (10−7 M, 16 min) did not modify the resting membrane potential in LD-5 neurones. Ba, under control conditions, kainate application (10 μM, 30 s, filled bar) induced a slight depolarisation in a LD-5 OT neurone (the dotted line indicates the resting membrane potential). After 17β-oestradiol treatment (10−7 M, 13 min), no potentiation of the kainate-induced response was observed. Bb, histogram showing the lack of effect of 17β-oestradiol treatment (10−7 M, 10 min, ▪) on the kainate-induced response at three different concentrations (10, 20 and 40 μM kainate) compared to control (□), in the same neurone.
Spike frequency adaptation was not altered by 17β-oestradiol treatment in LD-5 neurones (Fig. 3A and B), but it was induced in 7 out 13 LD-21 neurones tested (Fig. 3B). The amplitudes of the HAP and AHP were not affected by 17β-oestradiol treatment in LD-5 cells (Figs 3C, and 4Ab and Ac). In contrast, both parameters were significantly increased by 17β-oestradiol in LD-21 neurones (n = 6, P < 0.001; Figs 3C, and 4Bb and Bc).
In summary, 17β-oestradiol had no effect on the basal electrical properties of LD-5 neurones. In contrast, in LD-21 neurones, 17β-oestradiol shortened action potential duration, increased the amplitude of the HAP and AHP, and induced spike frequency adaptation during a train of action potentials, bringing each of these parameters close to the values observed in LD-5 neurones.
Responses to kainate in LD-5 and LD-21 neurones
Since glutamate exerts its excitatory effect on OT neurones mainly through non-NMDA receptors (for a review, see Renaud & Bourque, 1991), we tested the effect of kainate, an agonist of AMPA/kainate receptors, on the neurones at the two stages of lactation. As expected, both LD-5 and LD-21 OT neurones were depolarised by kainate. However, LD-5 neurones were more responsive than LD-21 neurones. With 10 μM kainate, 90 % of LD-5 neurones (n = 12) showed a depolarisation of 3–10 mV (Fig. 5A), while 81 % of the LD-21 neurones (22 out of 27) were unaffected (Fig. 5B). At higher doses, OT LD-5 neurones always gave a larger response than LD-21 neurones (Fig. 5A–C). For both groups, the maximum response was obtained at 100 μM kainate. Kainate effects were abolished by its antagonist, 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX; not shown).
Figure 5. Sensitivity to kainate of LD-5 and LD-21 neurones.
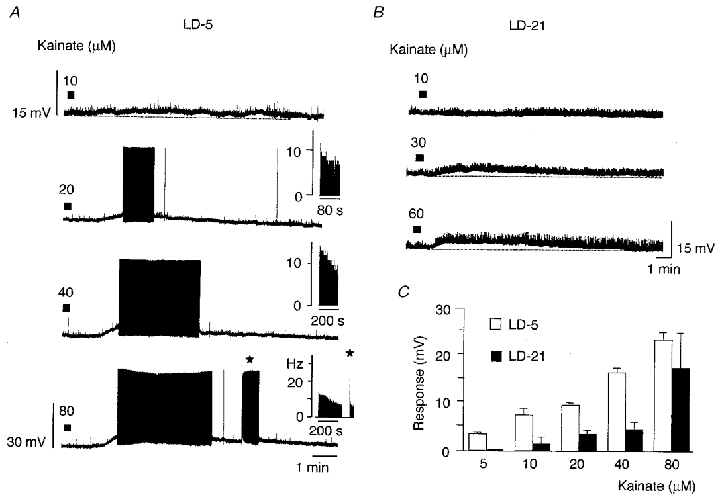
A, effect of successive applications of kainate (filled bars) at different concentrations on a LD-5 neurone. At 10 μM, kainate slightly depolarised the cell, whereas for higher concentrations the response was accompanied by a sustained generation of action potentials. Insets, sequential histograms of spike discharge per second (Hz). Note the fast frequency discharge at the onset of the second burst (⋆). B, recordings obtained from a LD-21 neurone showing that higher concentrations of kainate were needed for this neurone to be depolarised. C, amplitude of responses to kainate of LD-5 (n = 5, □) and LD-21 neurones (n = 11, ▪). At low concentrations of kainate (5 μM), only LD-5 neurones were depolarised.
Effects of steroids on the kainate-induced response
Kainate-induced responses in LD-5 and LD-21 neurones were compared before and during 17β-oestradiol treatment. In this set of experiments, kainate was first applied for 30 s while perifusing the slice with normal medium (test response with 5 μM kainate for LD-5 OT neurones, 10 μM kainate for LD-21 neurones). Bath application of 17β-oestradiol at 10−7 M was then performed for 10 min, before kainate was applied again.
During 17β-oestradiol treatment, we observed no significant variation of the membrane potential of OT neurones (LD-5, n = 6; LD-21, n = 17; Figs 6A and 7A). For the great majority of the LD-21 neurones, the membrane resistance was not modified (Fig. 6Ba and Bb), although it increased by up to 7–13 % in 7 % of the cells.
In 14 out of 25 LD-21 cells tested, the kainate-induced response was greatly increased during 17β-oestradiol treatment as illustrated in Fig. 6C. In most cases, this potentiation led to a sustained firing activity. This effect was totally reversed after a 30–40 min washing period. 17β-Oestradiol-induced potentiation increased with kainate concentration (Fig. 6D), varying between cells from 30 to 200 %.
In six LD-5 OT neurones similarly treated with 17β-oestradiol, the kainate-induced response was not potentiated by 17β-oestradiol (Fig. 7Ba and Bb).
To study the specificity of the action of 17β-oestradiol, we also tested the effects of 17α-oestradiol, a poorly active isomer of 17β-oestradiol. In 5 out of 8 LD-21 OT cells which previously showed a kainate-induced response potentiated by 17β-oestradiol, 17α-oestradiol (10−7 M) had no effect (Fig. 8A). In the three other neurones, it potentiated the kainate-induced response (Fig. 8B).
Figure 8. Effects of 17α-oestradiol on the kainate-induced response in LD-21 neurones.
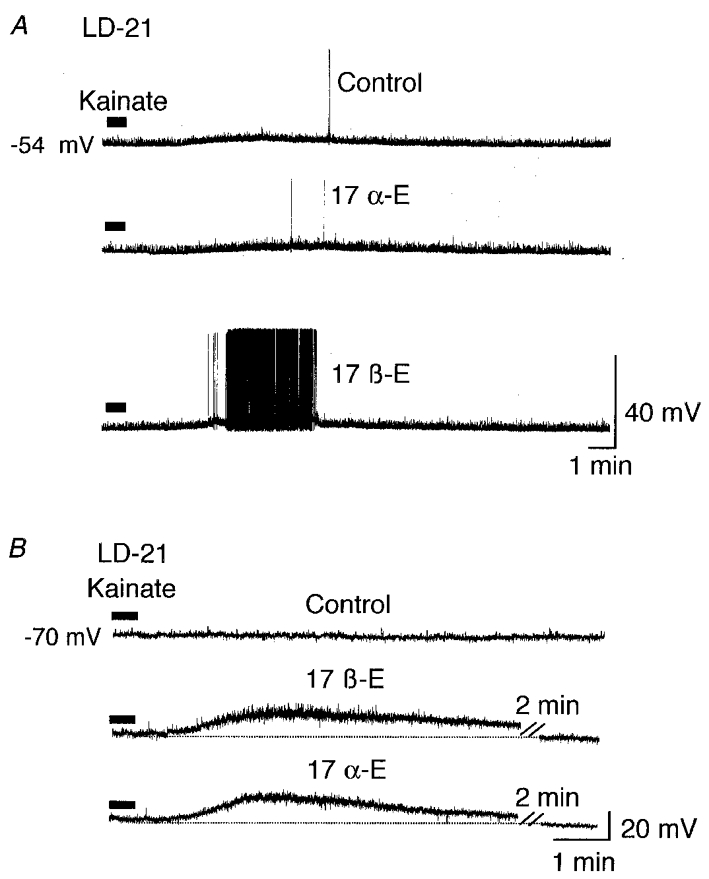
A, in the majority of LD-21 OT neurones, 17α-oestradiol did not mimic the 17β-oestradiol-induced potentiation of the response to kainate. Upper trace, kainate (10 μM, 30 s, filled bar) induced a slight depolarisation in a LD-21 neurone. Middle trace, this response was not potentiated by 17α-oestradiol (17α-E, 10−7 M, 15 min). Lower trace, the kainate-induced response was, however, potentiated by 17β-oestradiol (10−7 M, 6 min). B, in a minority of LD-21 OT neurones, 17α-oestradiol did mimic the 17β-oestradiol-induced potentiation. Upper trace, kainate (10 μM, 30 s) induced no significant depolarisation. Middle trace, this response was potentiated by 17β-oestradiol (10−7 M, 8 min; the dotted line represents the resting membrane potential). Lower trace, the kainate-induced response was also potentiated by 17α-oestradiol (10−7 M, 10 min). The cell had previously been hyperpolarised to -70 mV.
Because steroids can act at genomic and non-genomic levels, two LD-21 neurones were tested with tamoxifen, an antagonist of the ER-α nuclear receptor of 17β-oestradiol. Tamoxifen was first applied alone in the bath at 10−6 M for 10 min, then together with 17β-oestradiol (10−7 M) for another 10 min. Application of kainate was then performed in the presence of tamoxifen and 17β-oestradiol. In both neurones, tamoxifen did not modify action potential duration or membrane input resistance (Fig. 9B and C), and did not prevent potentiation by 17β-oestradiol of the kainate-induced response (Fig. 9A).
Figure 9. Tamoxifen did not prevent the 17β-oestradiol-induced potentiation of the kainate response.
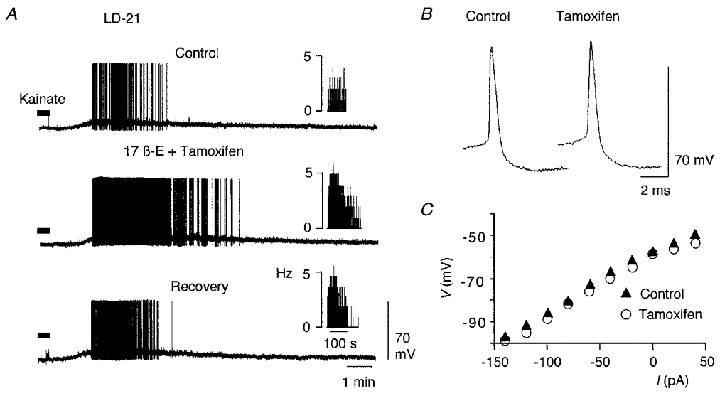
A, application of kainate (30 μM, 30 s, filled bar) induced a depolarisation in a LD-21 neurone (Control) accompanied by action potential discharge. Subsequently, the cell was first treated with tamoxifen (10−6 M, 10 min), then with tamoxifen (10−6 M) and 17β-oestradiol (10−7 M) for another 10 min before a new standard application of kainate. Under these conditions (middle trace), tamoxifen treatment did not prevent the 17β-oestradiol-induced potentiation of the kainate response. Insets, sequential histograms of spike discharge per second (Hz). In another LD-21 neurone, tamoxifen did not alter the amplitude or duration of action potentials (B), and did not significantly change input membrane resistance (C).
Finally, since steroids are lipophilic and can dissolve into the plasma membrane, it was of interest to examine the effects of a non-active lipophilic molecule such as cholesterol. Cholesterol, applied at 2 × 10−7 M for 12 min to two LD-21 neurones had no effect on the resting membrane potential, the membrane resistance (Fig. 10A) or the kainate-induced response (Fig. 10B).
Figure 10. Cholesterol did not potentiate the kainate-induced response.
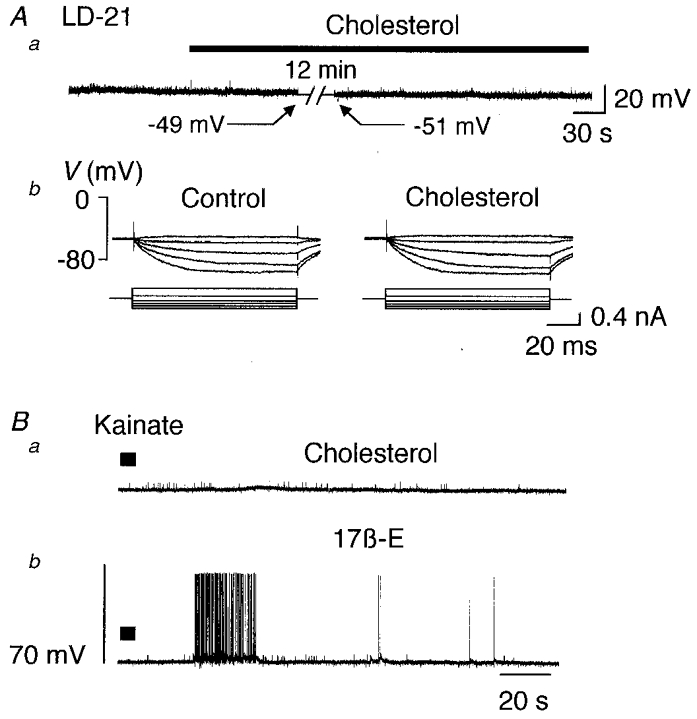
Aa, a LD-21 cell at resting potential (-49 mV) was treated by addition of cholesterol to the perifusion medium (2 × 10−7 M, 12 min, filled bar). No significant variation in membrane potential was observed. Ab, the membrane resistance was not affected by this treatment. Ba, in the presence of cholesterol, kainate (10 μM, 30 s, filled bar) slightly depolarised the neurone. Bb, in the presence of 17β-oestradiol (10−7 M, 6 min) another application of kainate induced a more pronounced response giving rise to a burst of action potentials.
DISCUSSION
Several lines of evidence indicate that the characteristics of the milk ejection reflex and the underlying electrical activity of OT neurones change during the course of lactation, and that they are influenced by circulating levels of sexual steroids (Jiang & Wakerley, 1997). Many sites of action are probably involved along the afferent pathway from the mammary gland to the OT neurones in the hypothalamus. As seen from the present observations, oestrogens can directly influence OT neurones during lactation. The basal electrical properties of identified OT neurones differ between early and late lactation and their sensitivity to kainate decreases at the end of lactation. Administration of 17β-oestradiol at day 21, however, restored both the electrical properties and the kainate-induced responses of OT neurones to their levels at day 5, through a rapid, non-genomic effect.
Basic electrophysiological properties
The basic electrical properties of magnocellular VP and OT neurones have been extensively studied (for a review, see Armstrong, 1995). A number of them are thought to confer characteristic electrical profiles on magnocellular cells. These are the hyperpolarisation after-potential (HAP; Bourque, 1988), the after-hyperpolarisation (AHP; Andrew & Dudek, 1984), the depolarisation after-potential (Andrew & Dudek, 1984; Andrew, 1987) and spike broadening during a train of action potentials (Andrew & Dudek, 1985; Oliet & Bourque, 1992). In addition, the majority of OT neurones, when current clamped at a depolarised potential above -50 mV, display a sustained outward rectification and a rebound depolarisation which could be considered as a specific signature of OT neurones (Stern & Armstrong, 1995, 1996, 1997). We did not look systematically for such a property, but, under our conditions, 20 % of the identified OT neurones clearly displayed such a rectification.
Our study has shown that some of these characteristics change throughout lactation and are influenced by 17β-oestradiol. Interestingly, similar changes have been observed in OT neurones recorded between dioestrus and lactation (Stern & Armstrong, 1996). Action potentials in magnocellular neurones depend on Na+/K+ conductances, but also on a voltage-dependent Ca2+ conductance which is responsible for the shoulder on the repolarising phase and is particularly conspicuous during spike broadening. That the action potential duration was longer in LD-21 neurones than in LD-5 neurones suggests, therefore, that voltage-dependent Ca2+ currents increase as the lactation period proceeds. Since 17β-oestradiol reduced spike duration, one can speculate that oestradiol reduces this Ca2+ conductance, as has been reported for rat neostriatal neurones (Mermelstein et al. 1996). However, if Ca2+ currents were increased at LD-21, one should expect that HAP and spike frequency adaptation, both of which depend on Ca2+-dependent K+ conductances, would also be more pronounced at LD-21. This was not observed here. An alternative explanation is that a reduction in spike duration is due, paradoxically, to an increase in Ca2+ currents. Kirkpatrick & Bourque (1991) have shown that increasing internal Ca2+ activates a Ca2+-dependent K+ current (IK(Ca)) which enhances the rate of spike repolarisation. In that case, increased spike duration, decreased HAP amplitude and the disappearance of spike frequency adaptation at LD-21 may all be due to a decrease in Ca2+ currents at this stage. 17β-Oestradiol would restore all these properties to their levels at LD-5, either by activating the voltage-dependent Ca2+ conductance during the action potential or by triggering Ca2+ release from internal stores (Beyer & Raab, 1998). Lastly, it has recently been reported that oestradiol could interact directly with voltage-gated K+ channels in vascular smooth muscle (Joëls & Karst, 1995). Clearly, further experiments are still necessary to determine the precise effect of oestrogen on Ca2+ and K+ channels in OT neurones.
Responses to kainate
Glutamate constitutes the dominant excitatory transmitter in the regulation of magnocellular neurones (reviewed in Renaud & Bourque, 1991; Armstrong, 1995). OT neurones receive an abundant glutamatergic innervation which increases at the time of parturition and lactation (El Majdoubi et al. 1996, 1997). Moreover, in OT neurones in organotypic slice cultures, bursts of action potentials analogous to the in vivo high frequency discharges were shown to be due to volleys of afferent glutamatergic excitatory postsynaptic potentials (EPSPs; Jourdain et al. 1998). Since at resting potential the majority of EPSPs are due to activation of AMPA/kainate-type receptors (Wuarin & Dudek, 1993; Jourdain et al. 1996), we here examined the effect of kainate, a non-NMDA ionotropic glutamate receptor agonist. Nevertheless, this does not preclude the role of NMDA receptors present on these cells (Hu & Bourke, 1992) and their activation may further enhance the response triggered by activation of AMPA/kainate receptors once the cells are depolarised.
As shown here, OT neurones at a late stage of lactation were less sensitive to kainate than at the beginning of lactation. It is possible that towards the end of lactation, the decrease in the effect of glutamate is linked to a decrease in the number of postsynaptic receptor sites for glutamate. However, the number of synapses on OT neurones does not diminish visibly before at least 1 month after weaning (Theodosis & Poulain, 1984; Montagnese et al. 1987). Another possibility is that, like GABAA receptors on OT neurones (Brussaard et al. 1997, 1999), the expression pattern of glutamate receptor subunits is altered, leading to a progressive decrease in their efficacy.
A striking observation was that 17β-oestradiol at LD-21 restored the efficiency of glutamate to the levels observed at LD-5. This corroborates earlier reports on the potentiation by oestrogens of glutamate-evoked excitation in Purkinje cells (Smith et al. 1987a,b, 1988) and of kainate-induced currents in CA1 neurones (Gu & Moss, 1996). However, since the effect that we observed was reversible within 30–40 min of washout, this implies that glutamate sensitisation of the neurones by 17β-oestradiol is a rapid and short-lived phenomenon.
Mode of action of 17β-oestradiol
Circulating concentrations of oestrogens during the oestrous cycle, parturition and lactation in the rat vary from 10−10 to 10−7 M. The concentration we used in this study (10−7 M), comparable with those used by others (Wang et al. 1995; Gu & Moss, 1996), overlaps the physiological range. Nevertheless, it is unlikely that the effects were unspecific since cholesterol, even at higher concentrations (2 × 10−6 M) did not interact with the kainate-induced response. Also, 17α-oestradiol generally had no effect. Finally, the lack of 17β-oestradiol-induced potentiation of the kainate response in either LD-5 OT neurones or 21-day-old VP neurones (authors’ unpublished observations) reduces the possibility of an artefact.
The question then arises as to the mode of action of 17β-oestradiol. In the rat, OT neurones lack the nuclear oestrogen receptor ER-α which mediates long-term effects at the genomic level (Burbach et al. 1990; Simonian & Herbison, 1997). That we detected no effect of the ER-α receptor antagonist tamoxifen is in agreement with these data. Recently, however, the β nuclear receptor (ER-β) has been located within the supraoptic nuclei (Shughrue et al. 1996; Li et al. 1997). Nevertheless, it is unlikely that the electrophysiological effects observed in the present study were due to an action of 17β-oestradiol at the genomic level since the steroid effects on OT neurones occurred within a few minutes, disappeared upon washout within 30–40 min, and were reproducible. A similar time course was observed in Purkinje cells (Smith et al. 1987a,b, 1988) and CA1 neurones (Gu & Moss, 1996). It is more likely, therefore, that our results can be explained in terms of rapid membrane effects, 17β-oestradiol interacting presumably with a membrane receptor. Such a concept has been substantiated by the use of conjugated active molecule and protein synthesis inhibitor (see Schumacher, 1990; Ramirez & Zheng, 1996). Activation of the membrane receptor would lead to activation of an intracellular second messenger system such as the cyclic AMP cascade in concert with an action on GS protein-coupled receptors (Gu & Moss, 1996, 1998). This in turn could activate either particular conductances and/or glutamate receptors. Finally, it is also possible that, as seen in vascular smooth muscle cells, the hormone interacts directly with a voltage-gated K+ channel (Valverde et al. 1999).
If there are membrane receptors for 17β-oestradiol on OT neurones, the question then arises why 17β-oestradiol did not affect neurones at LD-5. One possibility is that full expression of the mechanisms controlling spike discharge and sensitivity to glutamate cannot be further enhanced at that stage. Another possibility is that such receptors are progressively expressed or become progressively more efficient as lactation progresses. In this respect, one cannot exclude the possibility that, in addition to its rapid membrane effects, oestradiol has a long-term genomic effect which would prime the system to its membrane action. Oestradiol has been shown to increase Ca2+ currents in hippocampal CA1 neurones through a genomic action (Joëls & Karst, 1995), and there is evidence that the electrical activity of paraventricular neurones can be modulated by long-term manipulation of oestradiol levels (castration, substitutive treatment) (Akaishi & Sakuma, 1985).
Physiological significance
At the beginning of lactation, the electrophysiological properties of OT neurones seem to be most efficient for the secretory activity of the cells. In comparison to late lactation, the high sensitivity of OT neurones to glutamate makes them highly responsive to their afferent input. In addition, during a high frequency discharge which facilitates hormone release, the large HAP and AHP exert a limitation on firing frequency and burst duration that may prevent secretory ‘fatigue’ and protect neurones against deleterious effects of Ca2+ accumulation consecutive to a sustained electrical activity. As lactation proceeds, if there was no or little 17β-oestradiol secretion, these characteristics would be altered, rendering the neurones progressively less efficient.
However, levels of circulating 17β-oestradiol during lactation, although low at the beginning, start to increase towards the 8th to 10th day of lactation (Smith & Neill, 1977). Since at LD-21, the characteristics of action potentials and kainate responses were restored to their LD-5 values by 17β-oestradiol treatment, this implies that in vivo, these electrophysiological properties may remain relatively stable because of increased 17β-oestradiol levels.
From our experiments, however, it appears that the effects of 17β-oestradiol are short-lived, since they disappear within 30–40 min of washout. In vivo, therefore, any fluctuation in 17β-oestradiol levels would have rapid consequences, particularly towards the end of lactation. In early lactation, suckling by the pups is almost continuous. Suckling inhibits luteinising hormone release and oestrogen levels are low. When the litter grows, it relies more and more on solid food, and suckling becomes more intermittent. As intervals between suckling episodes increase, one can imagine that luteinising hormone released during these intervals enhances oestrogen production. This higher level of oestradiol would compensate, up to a point, for the alteration of the electrophysiological properties of OT neurones and of their sensitivity to glutamate. Such a mechanism could explain how lactation can be prolonged for 3–4 times its normal duration when regularly substituting a young litter for the growing one.
Finally, our observations emphasise that the short-term effects of oestradiol should be taken into consideration when studying electrophysiological properties in acute brain slices. One generally assumes the physiological state of the slice is that of the animal at the time of the experiment. The transient effect of 17β-oestradiol, which disappears rapidly from the perifusion medium, indicates that this is not necessarily the case.
Acknowledgments
The authors would like to express their gratitude to Dr S. H. R. Oliet and Dr D. T. Theodosis for helpful criticism and comments. The technical skills of R. Bonhomme made this work possible. This work was supported by a research grant from the Conseil Régional d'Aquitaine (no. 9803001214).
References
- Akaishi T, Sakuma Y. Estrogen excites oxytocinergic, but not vasopressinergic cells in the paraventricular nucleus of female rat hypothalamus. Brain Research. 1985;335:302–305. doi: 10.1016/0006-8993(85)90481-0. [DOI] [PubMed] [Google Scholar]
- Andrew RD. Endogenous bursting by rat supraoptic neuroendocrine cells is calcium dependent. The Journal of Physiology. 1987;384:451–465. doi: 10.1113/jphysiol.1987.sp016463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew RD, Dudek FE. Intrinsic inhibition in magnocellular neuroendocrine cells of rat hypothalamus. The Journal of Physiology. 1984;353:171–185. doi: 10.1113/jphysiol.1984.sp015330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew RD, Dudek FE. Spike broadening in magnocellular neuroendocrine cells of rat hypothalamic slices. Brain Research. 1985;334:176–179. doi: 10.1016/0006-8993(85)90583-9. [DOI] [PubMed] [Google Scholar]
- Armstrong WE. Morphological and electrophysiological classification of hypothalamic supraoptic neurons. Progress in Neurobiology. 1995;47:291–339. [PubMed] [Google Scholar]
- Beyer C, Raab H. Nongenomic effects of oestrogen: embryonic mouse midbrain neurones respond with a rapid release of calcium from intracellular stores. European Journal of Neuroscience. 1998;10:255–262. doi: 10.1046/j.1460-9568.1998.00045.x. [DOI] [PubMed] [Google Scholar]
- Bourque CW. Transient calcium-dependent potassium current in magnocellular neurosecretory cells of the rat supraoptic nucleus. The Journal of Physiology. 1988;397:331–347. doi: 10.1113/jphysiol.1988.sp017004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourque CW, Randle JCR, Renaud LP. Calcium dependent potassium conductance in rat supraoptic nucleus neurosecretory neurons. Journal of Neurophysiology. 1985;54:1375–1382. doi: 10.1152/jn.1985.54.6.1375. [DOI] [PubMed] [Google Scholar]
- Brussaard AB, Devay P, Leytig-Vermeulen JL, Kits KS. Changes in properties and neurosteroid regulation of GABAergic synapses in the supraoptic nucleus during the mammalian female reproductive cycle. The Journal of Physiology. 1999;516:513–524. doi: 10.1111/j.1469-7793.1999.0513v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brussaard AB, Kits KS, Baker RE, Willems WPA, Leytig-Vermeulen JW, Voorn P, Smit AB, Bicknell RJ, Herbison AE. Plasticity in fast synaptic inhibition of adult oxytocin neurons caused by switch in GABA-A receptor subunit expression. Neuron. 1997;19:1103–1114. doi: 10.1016/s0896-6273(00)80401-8. [DOI] [PubMed] [Google Scholar]
- Burbach JPH, Adan RAH, van Tol HHM, Verbeeck MAE, Axelson JF, van Leeuwen FW, Beekman JM, Ab G. Regulation of the rat oxytocin gene by estradiol. Journal of Neuroendocrinology. 1990;2:633–639. doi: 10.1111/j.1365-2826.1990.tb00458.x. [DOI] [PubMed] [Google Scholar]
- El Majdoubi M, Poulain DA, Theodosis DT. The glutamatergic innervation of oxytocin- and vasopressin-secreting neurons in the rat supraoptic nucleus and its contribution to lactation-induced synaptic plasticity. European Journal of Neuroscience. 1996;8:1377–1389. doi: 10.1111/j.1460-9568.1996.tb01600.x. [DOI] [PubMed] [Google Scholar]
- El Majdoubi M, Poulain DA, Theodosis DT. Lactation-induced plasticity in the supraoptic nucleus augments axodendritic and axosomatic GABAergic and glutamatergic synapses: an ultrastructural analysis using the disector method. Neuroscience. 1997;80:1137–1147. doi: 10.1016/s0306-4522(97)00193-0. [DOI] [PubMed] [Google Scholar]
- Gu Q, Moss RL. 17β-Estradiol potentiates kainate-induced currents via activation of the cAMP cascade. Journal of Neuroscience. 1996;16:3620–3629. doi: 10.1523/JNEUROSCI.16-11-03620.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Q, Moss RL. Novel mechanism for non-genomic action of 17β-oestradiol on kainate-induced currents in isolated rat CA1 hippocampal neurones. The Journal of Physiology. 1998;506:745–754. doi: 10.1111/j.1469-7793.1998.745bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B, Bourque CW. NMDA receptor-mediated rhythmic bursting activity in rat supraoptic nucleus neurones in vitro. The Journal of Physiology. 1992;458:667–687. doi: 10.1113/jphysiol.1992.sp019440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel JM, Breton H, Theodosis DT, Poulain DA. Steroids modulate kainate-induced electrical responses in oxytocinergic supraoptic neurons throughout lactation in rats. Society for Neuroscience Abstracts. 1996;22:608.14. [Google Scholar]
- Jiang Q, Wakerley JB. The milk-ejection reflex in the peri-partum rats: effects of oestradiol and progesterone on basal milk-ejection frequency and the facilitatory response to central oxytocin. Journal of Neuroendocrinology. 1997;9:9–16. doi: 10.1046/j.1365-2826.1997.00602.x. [DOI] [PubMed] [Google Scholar]
- Jiang QB, Wakerley JB. Analysis of bursting responses of oxytocin neurones in the rat in late pregnancy, lactation and after weaning. The Journal of Physiology. 1995;486:237–248. doi: 10.1113/jphysiol.1995.sp020806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joëls M, Karst H. Effects of estradiol and progesterone on voltage-gated calcium and potassium conductances in rat CA1 hippocampal neurons. Journal of Neuroscience. 1995;15:4289–4297. doi: 10.1523/JNEUROSCI.15-06-04289.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourdain P, Israel JM, Dupouy B, Oliet SHR, Allard M, Vitiello S, Theodosis DT, Poulain DA. Evidence for a hypothalamic oxytocin-sensitive pattern-generating network governing oxytocin neurons in vitro. Journal of Neuroscience. 1998;18:6641–6649. doi: 10.1523/JNEUROSCI.18-17-06641.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourdain P, Poulain DA, Theodosis DT, Israel JM. Electrical properties of oxytocin neurons in organotypic cultures from postnatal rat hypothalamus. Journal of Neurophysiology. 1996;76:2772–2785. doi: 10.1152/jn.1996.76.4.2772. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick K, Bourque CW. Dual role for calcium in the control of spike duration in rat supraoptic neuroendocrine cells. Neuroscience Letters. 1991;133:271–274. doi: 10.1016/0304-3940(91)90586-i. [DOI] [PubMed] [Google Scholar]
- Li X, Schwartz PE, Rissman EF. Distribution of estrogen receptor-beta-like immunoreactivity in rat forebrain. Neuroendocrinology. 1997;66:63–67. doi: 10.1159/000127221. [DOI] [PubMed] [Google Scholar]
- Mermelstein PG, Becker JB, Surmeier DJ. Estradiol reduces calcium currents in rat neostriatal neurons via a membrane receptor. Journal of Neuroscience. 1996;15:595–604. doi: 10.1523/JNEUROSCI.16-02-00595.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami T, Oomura Y, Nabekura J, Fukuda A. 17β-Estradiol depolarization of hypothalamic neurons is mediated by cyclic AMP. Brain Research. 1990;519:301–307. doi: 10.1016/0006-8993(90)90092-p. [DOI] [PubMed] [Google Scholar]
- Montagnese C, Poulain DA, Theodosis DT. Influence of ovarian steroids on the ultrastructural plasticity of the adult supraoptic nucleus induced by central administration of oxytocin. Journal of Neuroendocrinology. 1990;2:225–231. doi: 10.1111/j.1365-2826.1990.tb00855.x. [DOI] [PubMed] [Google Scholar]
- Montagnese C, Poulain DA, Vincent JD, Theodosis DT. Structural plasticity in the rat supraoptic nucleus during gestation, post-partum lactation and suckling-induced pseudogestation and lactation. Journal of Endocrinology. 1987;115:97–105. doi: 10.1677/joe.0.1150097. [DOI] [PubMed] [Google Scholar]
- Oliet SHR, Bourque CW. Properties of supraoptic magnocellular neurones isolated from the adult rat. The Journal of Physiology. 1992;455:291–306. doi: 10.1113/jphysiol.1992.sp019302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulain DA, Wakerley JB. Electrophysiology of hypothalamic magnocellular neurones secreting oxytocin and vasopressin. Neuroscience. 1982;7:773–808. doi: 10.1016/0306-4522(82)90044-6. [DOI] [PubMed] [Google Scholar]
- Ramirez VD, Zheng J. Membrane sex-steroid receptors in the brain. Frontiers in Neuroendocrinology. 1996;17:402–439. doi: 10.1006/frne.1996.0011. [DOI] [PubMed] [Google Scholar]
- Renaud LP, Bourque CW. Neurophysiology and neuropharmacology of hypothalamic magnocellular neurons secreting vasopressin and oxytocin. Progress in Neurobiology. 1991;36:131–169. doi: 10.1016/0301-0082(91)90020-2. [DOI] [PubMed] [Google Scholar]
- Schumacher M. Rapid membrane effects of steroid hormones: an emerging concept in neuroendocrinology. Trends in Neurosciences. 1990;13:359–362. doi: 10.1016/0166-2236(90)90016-4. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Komm B, Merchenthaler I. The distribution of estrogen receptor-beta mRNA in the rat hypothalamus. Steroids. 1996;61:678–681. doi: 10.1016/s0039-128x(96)00222-x. [DOI] [PubMed] [Google Scholar]
- Simonian SX, Herbison AE. Differential expression of estrogen receptor alpha and beta immunoreactivity by oxytocin neurons of rat paraventricular nucleus. Journal of Neuroendocrinology. 1997;9:803–806. doi: 10.1046/j.1365-2826.1997.00659.x. [DOI] [PubMed] [Google Scholar]
- Smith MS, Neill JD. Inhibition of gonadotropin secretion during lactation in the rat: relative contribution of suckling and ovarian steroids. Biology of Reproduction. 1977;17:255–261. doi: 10.1095/biolreprod17.2.255. [DOI] [PubMed] [Google Scholar]
- Smith SS, Waterhouse BD, Woodward DJ. Sex steroid effects on extrahypothalamic CNS. I. Estrogen augments neuronal responsiveness to iontophoretically applied glutamate in the cerebellum. Brain Research. 1987a;422:40–51. doi: 10.1016/0006-8993(87)90538-5. [DOI] [PubMed] [Google Scholar]
- Smith SS, Waterhouse BD, Woodward DJ. Sex steroid effects on extrahypothalamic CNS. II. Progesterone, alone and in combination with estrogen, modulates cerebellar responses to amino acid neurotransmitters. Brain Research. 1987b;422:52–62. doi: 10.1016/0006-8993(87)90539-7. [DOI] [PubMed] [Google Scholar]
- Smith SS, Waterhouse BD, Woodward DJ. Locally applied estrogens potentiate glutamate-evoked excitation of cerebellar Purkinje cells. Brain Research. 1988;475:272–282. doi: 10.1016/0006-8993(88)90615-4. [DOI] [PubMed] [Google Scholar]
- Stern JE, Armstrong WE. Electrophysiological differences between oxytocin and vasopressin neurones recorded from female rats in vitro. The Journal of Physiology. 1995;488:701–708. doi: 10.1113/jphysiol.1995.sp021001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern JE, Armstrong WE. Changes in the electrical properties of supraoptic nucleus oxytocin and vasopressin neurons during lactation. Journal of Neuroscience. 1996;16:4861–4871. doi: 10.1523/JNEUROSCI.16-16-04861.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern JE, Armstrong WE. Sustained outward rectification of oxytocinergic neurones in the rat supraoptic nucleus: ionic dependence and pharmacology. The Journal of Physiology. 1997;500:497–508. doi: 10.1113/jphysiol.1997.sp022036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodosis DT, Poulain DA. Evidence for structural plasticity in the supraoptic nucleus of the rat hypothalamus in relation to gestation and lactation. Neuroscience. 1984;11:183–193. doi: 10.1016/0306-4522(84)90222-7. [DOI] [PubMed] [Google Scholar]
- Valverde MA, Rojas P, Amigo J, Cosmelli D, Orio P, Bahamonde MI, Mann GE, Vergara C, Latorre R. Acute activation of maxi-K channels (hSlo) by estradiol binding to the β subunit. Science. 1999;285:1929–1931. doi: 10.1126/science.285.5435.1929. [DOI] [PubMed] [Google Scholar]
- Wang H, Ward AR, Morris JF. Oestradiol acutely stimulates exocytosis of oxytocin and vasopressin from dendrites and somata of hypothalamic magnocellular neurons. Neuroscience. 1995;68:1179–1188. doi: 10.1016/0306-4522(95)00186-m. [DOI] [PubMed] [Google Scholar]
- Wuarin JP, Dudek FE. Patch-clamp analysis of spontaneous synaptic currents in supraoptic neuroendocrine cells of the rat hypothalamus. Journal of Neuroscience. 1993;13:2323–2331. doi: 10.1523/JNEUROSCI.13-06-02323.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi K, Akaishi T, Negoro H. Effects of estrogen treatment on plasma oxytocin and vasopressin in ovariectomised rats. Endocrinologia Japonica. 1979;26:197–205. doi: 10.1507/endocrj1954.26.197. [DOI] [PubMed] [Google Scholar]


