Abstract
In smooth muscle of the guinea-pig bladder, either membrane potential recordings or [Ca2+]i measurements were made simultaneously with isometric tension recordings.
Single transmural stimuli initiated excitatory junction potentials (EJPs) which triggered action potentials, transient increases in [Ca2+]i and associated contractions. These responses were abolished by α,β-methylene ATP, suggesting that they resulted from the activation of purinoceptors by neurally released ATP.
Nifedipine abolished action potentials leaving the underlying EJPs and reduced the amplitude of both nerve-evoked increases in [Ca2+]i and associated contractions. The subsequent co-application of caffeine and ryanodine inhibited the residual responses without inhibiting EJPs. These results indicate that stimulation of purinoceptors activates both Ca2+ influx through L-type Ca2+ channels and Ca2+ release from intracellular Ca2+ stores.
In the presence of α,β-methylene ATP, trains of stimuli failed to initiate EJPs but increased the frequency of action potentials. Trains of stimuli also initiated oscillatory increases in [Ca2+]i and associated contractions. These responses were abolished by hyoscine, indicating that they resulted from the activation of muscarinic receptors by neurally released ACh.
Oscillatory increases in [Ca2+]i and associated contractions were inhibited by either nifedipine or caffeine, indicating that the stimulation of muscarinic receptors activates both Ca2+ influx through L-type Ca2+ channels and Ca2+ release from intracellular Ca2+ stores.
The effect of excitatory nerve stimulation on the mechanical activity in urinary bladder smooth muscle preparations has been examined in many mammalian species. In most species studied, contractions of bladder smooth muscle were mediated by both cholinergic and non-adrenergic, non-cholinergic (NANC) excitatory mechanisms (Sibley, 1984). In small mammals, NANC contractions predominate and cholinergic contractions can be detected only when trains of stimuli are applied at high frequency (Sibley, 1984; Brading & Mostwin, 1989). In contrast, nerve-mediated contractions in the human bladder are almost solely mediated by a cholinergic mechanism and NANC transmission is observed only in pathological conditions (Sjögren et al. 1982; Sibley, 1984).
Electrophysiological studies of bladder smooth muscle in small mammals revealed that transmural stimuli initiated EJPs which triggered action potentials (Brading & Mostwin, 1989; Hashitani & Suzuki, 1995; Bramich & Brading, 1996). EJPs were abolished by α,β-methylene ATP but not atropine, indicating that they resulted from the activation of P2X purinoceptors by neurally released ATP (Hoyle & Burnstock, 1985; Fujii, 1988; Brading & Mostwin, 1989; Hashitani & Suzuki, 1995). On the other hand, the stimulation of muscarinic receptors initiated either slow depolarisations or no detectable membrane potential changes (Fujii, 1988; Creed et al. 1993; Hashitani & Suzuki, 1995). From these results together with measurements of contractile responses (Katsuragi et al. 1990; Bo & Burnstock, 1990), neurally released ATP has been suggested to increase [Ca2+]i, mainly by Ca2+ influx through L-type Ca2+ channels, which may be amplified by Ca2+-induced Ca2+ release (CICR) from intracellular Ca2+ stores, to initiate contractions (Ganitkevich & Isenberg, 1992). In contrast, cholinergic transmission is thought to largely depend on the production of inositol trisphosphate (InsP3) which stimulates Ca2+ release from intracellular Ca2+ stores (Mostwin, 1985; Iacovou et al. 1990; Andersson et al. 1991).
However, these hypotheses have not been directly examined. Electrophysiological studies have been performed with either double sucrose-gap techniques (Creed et al. 1983; Fujii, 1988) or microelectrode recordings in arrested tissues in which action potentials were blocked by calcium antagonists (Hashitani & Suzuki, 1995; Bramich & Brading, 1996) or in preparations in which contractions were prevented with high extracellular osmolarity (Brading & Mostwin, 1989). Changes in [Ca2+]i of bladder smooth muscle have mostly been examined in patch clamp studies in isolated smooth muscle cells (Ganitkevich & Isenberg, 1992; Imaizumi et al. 1998). In this study, to investigate the contribution of both the influx of Ca2+ and the release of Ca2+ from intracellular stores to nerve-evoked contractions of the guinea-pig bladder, isometric tension recordings were made simultaneously with either intracellular microelectrode recordings or Ca2+ fluorescence measurements in fura-PE3-loaded preparations.
METHODS
The procedures described have been approved by the animal experimentation ethics committee at the University of Melbourne. Guinea-pigs of either sex, weighing 150–200 g, were killed by a blow to the head followed by cervical exsanguination. The urinary bladder was removed and the ventral wall of the bladder was opened longitudinally from the top of the dome to the bladder neck. The mucosal layer and connective tissue were then removed and preparations of smooth muscle 8–10 mm long and 2–3 mm wide were prepared.
For preparations in which microelectrode recordings were performed, two or three smooth muscle bundle layers were removed in a 2–3 mm square area leaving an underlying single layer of smooth muscle bundles. A tension transducer was placed through muscle bundles that were in continuation with this area. This procedure markedly reduced the movement of this single layer of smooth muscle bundles without disrupting the generation of either spontaneous or nerve-evoked membrane potential changes and allowed the simultaneous recording of membrane potential with changes in isometric tension.
Preparations were pinned out on a Sylgard plate (silicone elastomer, Dow Corning Corporation, Midland, MI, USA) at the bottom of a recording chamber (volume, ∼1 ml) which was mounted on the stage of an inverted microscope. The preparations were superfused with warmed (38°C) physiological saline (see below) at a constant flow (2 ml min−1). Individual bladder smooth muscle cells were impaled with glass capillary microelectrodes, filled with 0.5 M KCl (tip resistance, 150–250 MΩ). Membrane potential changes were recorded using a high input impedance amplifier (Axoclamp-2B, Axon Instruments, Inc.), and displayed on a cathode-ray oscilloscope (Tektronix, Inc., Beaverton, OR, USA). After low-pass filtering (cut-off frequency, 1 kHz), membrane potential changes were digitised and stored on a personal computer for later analysis.
For simultaneous isometric tension recording, a window, 4–5 mm square, was made at the centre of the recording chamber. The appropriate muscle bundles were connected, using a fine L-shaped needle, to a tension transducer. Isometric tension changes were recorded using an amplifier, and displayed on a cathode-ray oscilloscope. After low-pass filtering (cut-off frequency, 5 kHz), changes in tension were digitised and stored on a personal computer for later analysis.
For Ca2+ fluorescence ratio measurements, bladder preparations were pinned out on the bottom of the same recording chamber as that used for microelectrode recordings, and were loaded with fluorescent dye, fura-PE3, by incubation in low Ca2+ physiological saline (Ca2+, 0.5 mM) containing 5 μM fura-PE3 AM and 0.01 % Pluronic F127 for 1 h at room temperature. After loading, preparations were warmed to 30°C for 15 min, then the recording chamber was mounted on the stage of an inverted microscope and tissues were superfused with dye-free, warmed (38°C) physiological saline at a constant flow (∼2 ml min−1) for 30 min. Changes in [Ca2+]i were determined using a ratiometric method (see Bramich & Hirst, 1999). Briefly, preparations, loaded with fura-PE3, were illuminated with two periods of light, with wavelengths of 340 and 380 nm, alternating at a frequency of 12.3 Hz. Photons, emitted at a wavelength of 510 nm, were counted during each period of illumination to give separate outputs reflecting the increase in concentration of the fura-Ca2+ complex and the decrease in free fura concentration. A measure of changes in [Ca2+]i was obtained by taking a ratio of these values. In all illustrations only the ratio of emitted light intensities produced by illumination with 340 and 380 nm light is given (F340/380). Simultaneous isometric tension recordings were made as described above.
Intramural nerves were stimulated selectively by passing brief square stimulating pulses (duration, 0.1-0.5 ms) between a pair of electrodes (platinum plate and wire) placed above and below the bladder preparations. The neural selectivity of stimulating pulses was confirmed by abolishing the evoked responses with tetrodotoxin (1 μM).
The ionic composition of the physiological saline was (mM): NaCl, 119.8; KCl, 5.0; MgCl2, 2.0; CaCl2, 2.5; NaHCO3, 25.0; NaH2PO4, 1.0; and glucose, 11.0. The solution was aerated with 95 % O2 and 5 % CO2.
Drugs used were α,β-methylene ATP, caffeine, cyclopiazonic acid (CPA), forskolin, hyoscine chloride, nifedipine, niflumic acid, physostigmine sulphate, Pluronic F127, tetrodotoxin, ryanodine (all from Sigma) and fura-PE3 AM (from Calbiochem, San Diego, CA, USA). These drugs were dissolved in distilled water, except nifedipine and niflumic acid which were dissolved in 100 % ethanol, and CPA, forskolin and fura-PE3 AM which were dissolved in DMSO. The final concentration of these solvents in the physiological saline did not exceed 1:1000. Recordings were made 20 min after applying CPA, guanethidine, niflumic acid and nifedipine, and 10 min after applying other agents. When caffeine and ryanodine were co-applied, recordings were made 20 min after applying the agents and again some 30 min after washing out the agents.
Measured values are expressed by the mean ± standard deviation (s.d.). Statistical significance was tested using Student's t test, and probabilities of less than 5 % were considered significant.
RESULTS
General observations
When membrane potential recordings were made simultaneously with isometric tension recordings, spontaneous action potentials and contractions were detected. However, spontaneous action potentials often failed to trigger contractions (Fig. 1A). Transmural stimuli triggered action potentials and invariably produced associated contractions (Fig. 1A). Similarly, when changes in Ca2+ fluorescence ratio were measured simultaneously with changes in isometric tension, spontaneous increases in [Ca2+]i were not always accompanied by spontaneous contractions (Fig. 1B). Single transmural stimuli triggered transient increases in [Ca2+]i and invariably produced associated contractions (Fig. 1B). These results indicated that spontaneous changes in both the membrane potential and [Ca2+]i occurred as localised events and did not cause synchronous contractions. This dissociation between spontaneous contractions and changes in membrane potential and [Ca2+]i may represent a low degree of intercellular coupling between urinary bladder smooth muscle cells (Bramich & Brading, 1996).
Figure 1. Spontaneous and nerve-evoked changes in membrane potential, Ca2+ fluorescence ratio and tension in the guinea-pig urinary bladder.
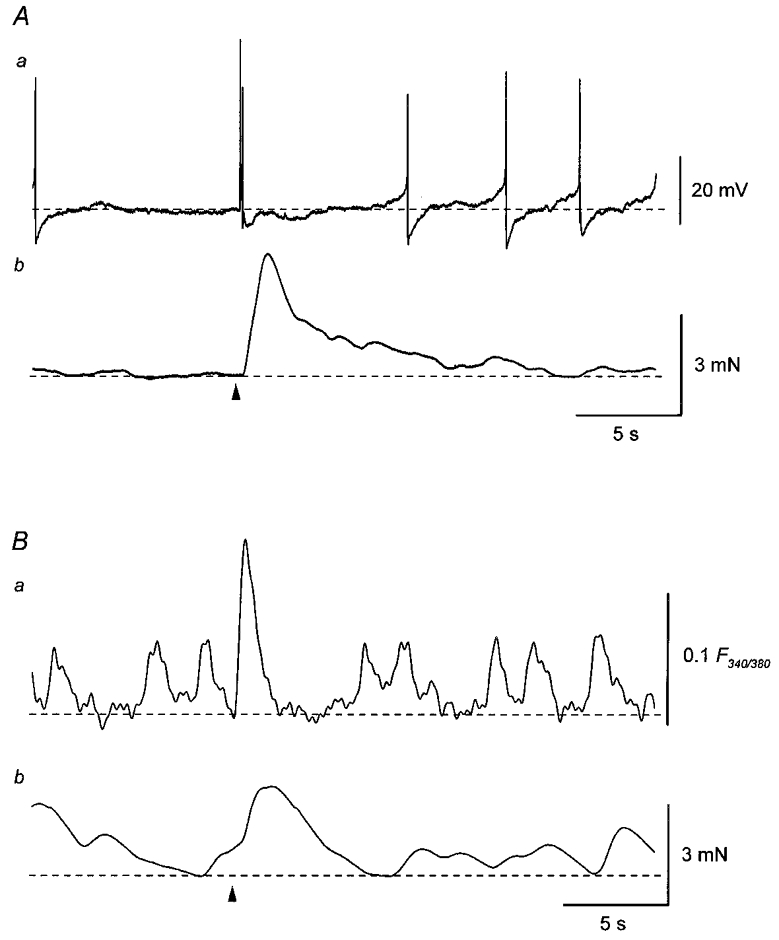
A, membrane potential recordings (a) were made simultaneously with isometric tension recordings (b) from a smooth muscle preparation of the guinea-pig urinary bladder. A single transmural impulse (arrowhead) initiated an EJP which triggered action potentials and an associated contraction. Spontaneous action potentials were associated with either smaller or no contractions. B, in another preparation, changes in Ca2+ fluorescence ratio (a) were recorded simultaneously with changes in isometric tension (b). A single impulse (arrowhead) initiated a transient increase in Ca2+ fluorescence ratio and an associated contraction. Spontaneous increases in Ca2+ fluorescence ratio were associated with either smaller or no contractions. Resting membrane potential was -37 mV in A.
Spontaneous action potentials recorded from the guinea-pig bladder
Bladder smooth muscle cells discharged spontaneous action potentials with frequencies ranging between 7.5 and 48 min−1 (mean, 24.8 ± 10.7 min−1; n = 20; Fig. 2Aa); Each action potential consisted of an initial slow depolarising phase and a subsequent regenerative depolarisation. The regenerative depolarisation was followed by a rapid repolarising phase which caused an after-hyperpolarisation (Fig. 2Ba). Action potentials were characterised in 20 preparations and had peak amplitudes of between 35.9 and 52.1 mV (mean, 45.6 ± 5.3 mV), rise times of between 32.1 and 88 ms (mean, 55.9 ± 18.6 ms) and half-widths of between 8.9 and 20.9 ms (mean, 11.5 ± 3.2 ms). Amplitudes of after-hyperpolarisations ranged between 4.5 and 20.5 mV (mean, 9.9 ± 3.9 mV). Action potentials persisted in the presence of tetrodotoxin (1 μM; n = 3), hyoscine (1 μM; n = 5), guanethidine (10 μM; n = 3) and α,β-methylene ATP (10 μM; n = 5), suggesting that they were myogenic in origin. Resting membrane potentials, which were determined at the stable, most negative potential between each action potential, ranged between -33 and -50 mV (mean, -40.8 ± 4.4 mV;n = 45).
Figure 2. Effect of co-application of caffeine and ryanodine on spontaneous action potentials in the guinea-pig urinary bladder.
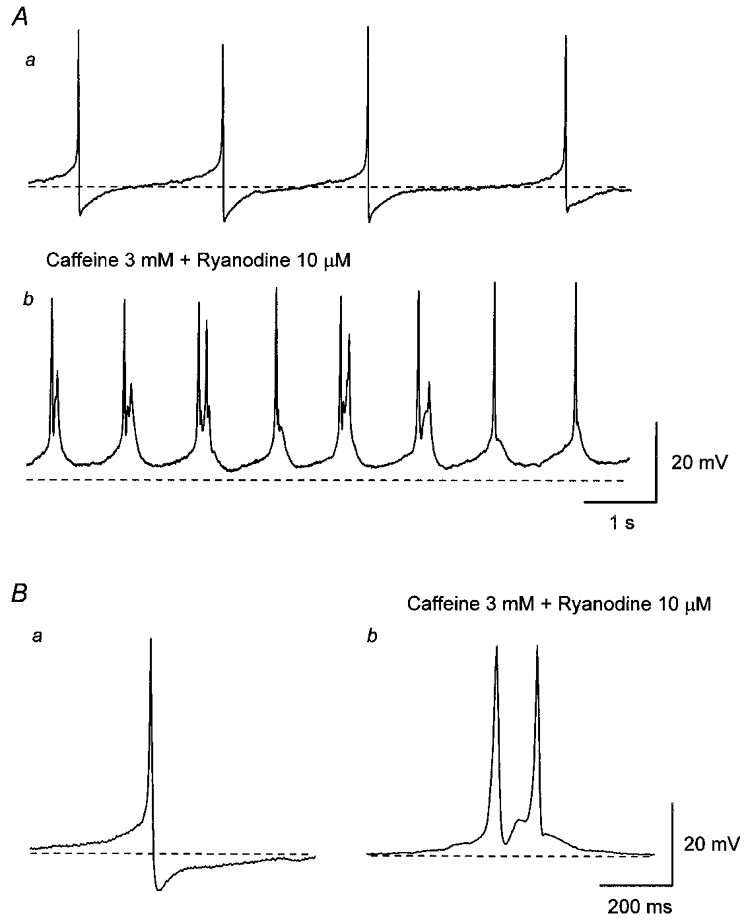
Aa, in control solution, bladder smooth muscle cells discharged spontaneous action potentials. b, co-application of caffeine (3 mM) and ryanodine (10 μM) depolarised the membrane and increased the frequency of spontaneous action potentials. Ba, in control solution, each action potential consisted of an initial slow depolarising phase and subsequent regenerative depolarisation which was followed by a rapid repolarising phase with an after-hyperpolarisation. b, in the presence of caffeine and ryanodine, each action potential had a similar amplitude and rising phase to those of control action potentials but had a longer duration. It also can be seen that action potentials lacked an after-hyperpolarisation and often multiple action potentials were discharged (Ab and Bb). All traces were recorded from the same cell. Scale bar on the right in A refers to both traces in A. Scale bar on the right in B refers to both traces in B. Resting membrane potential was -43 mV.
Spontaneous action potentials in bladder were abolished by nifedipine (10 μM; n = 15), suggesting that they resulted from the activation of L-type Ca2+ channels. In other smooth muscle preparations, the contribution of Ca2+ release from intracellular Ca2+ stores to the generation of spontaneous depolarisations has been reported (Van Helden, 1993; Hashitani et al. 1996; Hashitani & Edwards, 1999). To examine the contribution of Ca2+ release from intracellular stores to the generation of spontaneous action potentials in bladder, the effect of co-application of caffeine and ryanodine was studied. Co-application of caffeine (3 mM) and ryanodine (10 μM) depolarised the membrane (mean, 6.3 ± 2.1 mV; n = 5) and increased the frequency of spontaneous action potentials (Fig. 2Ab). After prolonged application (20 min) of caffeine and ryanodine, the membrane potential gradually repolarised to the original levels. The frequency of action potentials also gradually decreased and eventually became lower than the control values. In the presence of caffeine and ryanodine, the half-width of action potentials became longer (mean, 21.1 ± 3.1 ms; n = 5, P < 0.05) without a change in either their amplitude (46.8 ± 6.2 mV, P > 0.05) or rise time (63.8 ± 10.8 ms, P > 0.05) and multiple action potentials were often observed (Fig. 2Bb). Caffeine and ryanodine also abolished the after-hyperpolarisation (Fig. 2Bb). The effects of caffeine and ryanodine could not be reversed after 1 h of washing. These results suggest that Ca2+ release from intracellular stores is not essential for the generation of spontaneous action potentials.
Responses produced by single transmural stimuli
When membrane potential changes were simultaneously recorded with isometric tension changes in bladder smooth muscle preparations, single impulses were found to initiate EJPs which triggered action potentials and caused associated contractions (Fig. 3A and D). These responses were abolished by tetrodotoxin (1 μM; n = 5) but persisted in preparations treated with hyoscine (1 μM; n = 3) or guanethidine (10 μM; n = 3). Application of α,β-methylene ATP (10 μM) produced a transient depolarisation (mean, 26.2 ± 5.1 mV; n = 6) and contraction, and dramatically increased the frequency of spontaneous action potentials during the rising phase of the depolarisation. In the continuous presence of α,β-methylene ATP, both the membrane potential and tension returned to their original levels within 3–5 min. However, single impulses failed to initiate either EJPs or nerve-evoked contractions, indicating that responses produced by single impulses were solely mediated by neurally released ATP.
Figure 3. Effects of nifedipine and co-application of caffeine and ryanodine on the mechanical and electrical responses produced by single transmural stimuli.
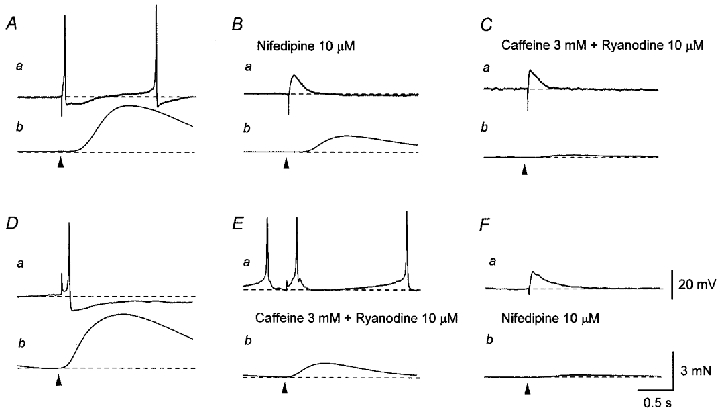
A, a single impulse (supramaximal current, 0.2 ms; arrowhead) initiated an EJP which triggered an action potential (a) and an associated contraction (b). B, nifedipine (10 μM) abolished the action potential, leaving an EJP (a), and also reduced the amplitude of the nerve-evoked contraction (b). C, in the presence of nifedipine (10 μM), co-application of caffeine (3 mM) and ryanodine (10 μM) reduced the amplitude of the residual contraction (b) without inhibiting the EJP (a). D, in another preparation, a single impulse initiated an EJP which triggered an action potential (a) and an associated contraction (b). E, co-application of caffeine (3 mM) and ryanodine (10 μM) reduced the amplitude of the nerve-evoked contraction (b) without preventing the generation of either the EJP or the action potential (b). F, the subsequent application of nifedipine (10 μM) abolished the action potential, leaving an EJP (a), and also reduced the amplitude of the residual contraction (b). A–C and D–F were recorded from two different cells. Scale bar on the right in Fa refers to all potential traces (a). Scale bar on the right in Fb refers to all tension traces (b). Resting membrane potential was -41 mV in A and -39 mV in D.
Nifedipine (10 μM) caused a membrane depolarisation (mean, 3.8 ± 1.8 mV; n = 24) and abolished action potentials leaving an underlying EJP. Nifedipine also reduced the amplitude of nerve-evoked contractions (mean, 22.1 ± 9.2 % of control; n = 21; Fig. 3B). The subsequent co-application of caffeine (3 mM) and ryanodine (10 μM) further depolarised the membrane (mean, 4.9 ± 1.8 mV; n = 4) and suppressed the residual contractions (mean, 5.3 ± 3.5 % of control; n = 4) without affecting the EJPs (Fig. 3C). The effects of co-applied caffeine and ryanodine were not reversed after 1 h of washing with physiological saline. Conversely, when caffeine (3 mM) and ryanodine (10 μM) were applied first, they depolarised the membrane (mean, 6.3 ± 2.1 mV; n = 5) but did not prevent the generation of either EJPs or action potentials (Fig. 3Ea); however, the amplitude of nerve-evoked contractions was reduced (mean, 26.1 ± 7.5 % of control; n = 5; Fig. 3Eb). Subsequently, nifedipine (10 μM) abolished action potentials, leaving EJPs, and suppressed the residual contractions (mean, 4.1 ± 3.2 % of control; Fig. 3F).
Responses produced by repetitive stimulation
Repetitive stimuli delivered at low frequency (3 impulses, 0.5 or 1 Hz) initiated EJPs which triggered action potentials and associated contractions (Fig. 4A). Co-application of caffeine (3 mM) and ryanodine (10 μM) did not prevent the generation of either EJPs or action potentials but reduced the amplitude of nerve-evoked contractions (Fig. 4B). In the presence of caffeine and ryanodine, nerve-evoked contractions had smaller amplitudes and shorter time courses than those in control solution (Fig. 4Ab and Bb). In separate preparations, repetitive stimulation applied at low frequency (3 impulses, 0.5 or 1 Hz) initiated EJPs which triggered action potentials and associated contractions (Fig. 4C). Nifedipine (10 μM) abolished the action potentials, leaving EJPs, and also reduced the amplitude of nerve-evoked contractions (Fig. 4D). In the presence of nifedipine, only the first impulse initiated contractions; the second and third impulse failed to initiate contractions (Fig. 4Db).
Figure 4. Effects of nifedipine and co-application of caffeine and ryanodine on electrical and mechanical responses produced by repetitive stimulation.
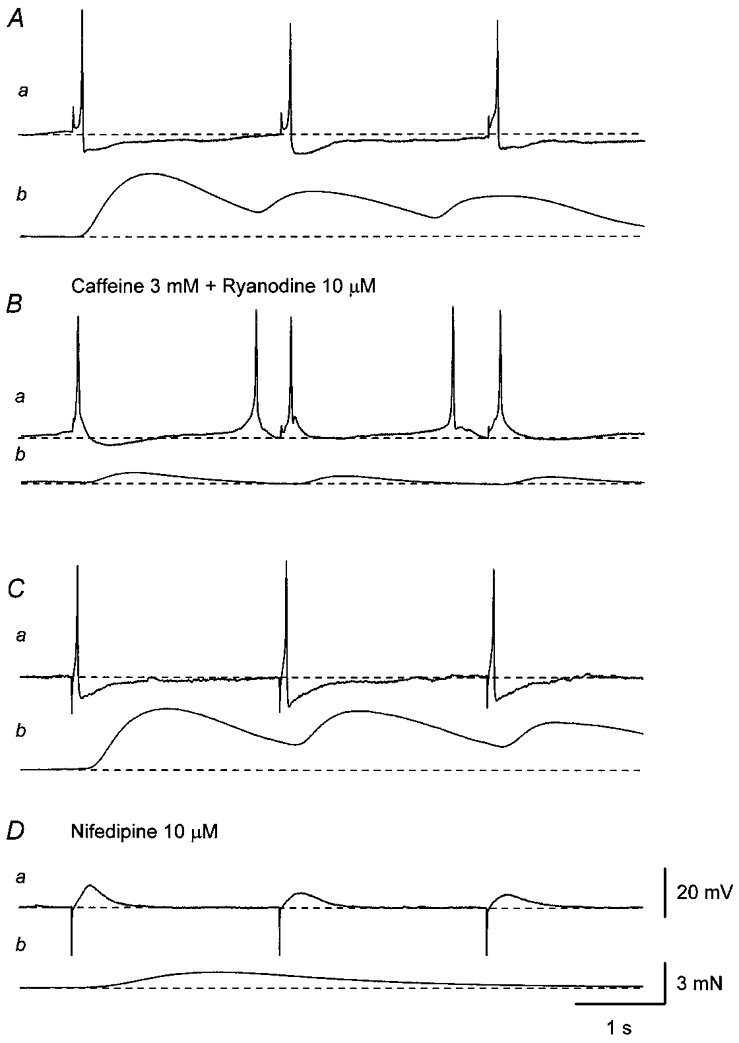
A, three impulses applied at 0.5 Hz (supramaximal current, 0.3 ms) initiated three EJPs which triggered action potentials (a) and associated contractions (b). B, co-application of caffeine (3 mM) and ryanodine (10 μM) reduced the amplitude of nerve-evoked contractions (b) without preventing the generation of either EJPs or action potentials (a). C, in another preparation, three impulses applied at 0.5 Hz (supramaximal current, 0.3 ms) initiated three EJPs which triggered action potentials (a) and associated contractions (b). Da, nifedipine (10 μM) abolished action potentials leaving three EJPs. b, in the presence of nifedipine, only the first impulse, not the second and third impulses, initiated a small contraction. A and B, and C and D were recorded from two different cells. Scale bar on the right in Da refers to all potential traces (a). Scale bar on the right in Db refers to all tension traces (b). Resting membrane potential was -38 mV in A and -40 mV in C.
Properties of the second component of purinergic EJPs
To investigate the mechanisms underlying EJPs, experiments were performed in the presence of nifedipine (10 μM).
In about 30 % of cells studied, single impulses initiated EJPs which had short latencies and rapid, smooth rising phases. In these cells, reducing either pulse width or stimulation intensity simply decreased the amplitude of the EJPs with little change in their time course. Furthermore, during repetitive stimulation at low frequency (0.5 or 1 Hz), the amplitude of the first EJP was almost identical to that of the second or third EJP, suggesting that they had similar properties to those of type I EJPs which have been reported by Bramich & Brading (1996).
In the remaining 70 % of preparations studied, stimulation with a single impulse also initiated EJPs that resembled type I EJPs. In these cells, however, reducing the strength of the stimulation clearly separated the EJPs into two components: a first component which had a short latency and a second regenerative component which could occur after a long latency, suggesting that they belonged to the type II or type III cells described by Bramich & Brading (1996). During delivery of trains of repetitive stimuli at low frequency (0.5 or 1 Hz), the first EJP always had a larger amplitude than the second or third EJP (see Bramich & Brading (1996).
During repetitive stimulation, the first EJP but not the second or third EJP had the second regenerative component (see Fig. 4Da). Only the first EJP was associated with a contraction; the second and third EJP failed to trigger contractions (see Fig. 4Db). These results imply the contribution of an increase in [Ca2+]i, probably resulting from the release of Ca2+ from intracellular stores, to the generation of the second component of the EJP.
To examine the contribution of Ca2+ release from intracellular stores to the generation of the second component of EJPs, the effects of CPA and co-application of caffeine and ryanodine were studied. CPA (10 μM) depolarised the membrane (mean, 5.4 ± 3.4 mV; n = 6) and abolished the second component of EJPs (Fig. 5Aa and Ba). CPA caused a sustained contraction and also abolished the nifedipine-insensitive contraction (Fig. 5Ab and Bb). Co-application of caffeine (3 mM) and ryanodine (10 μM) depolarised the membrane and abolished the second component of EJPs (Fig. 5Ca and Da). Caffeine and ryanodine caused a small relaxation and also abolished the nifedipine-insensitive contraction (Fig. 5Cb and Db). These results support the idea that Ca2+ release from intracellular Ca2+ stores is involved in the generation of the second component of EJPs.
Figure 5. Effects of CPA, co-application of caffeine and ryanodine, and niflumic acid on the second component of EJPs.
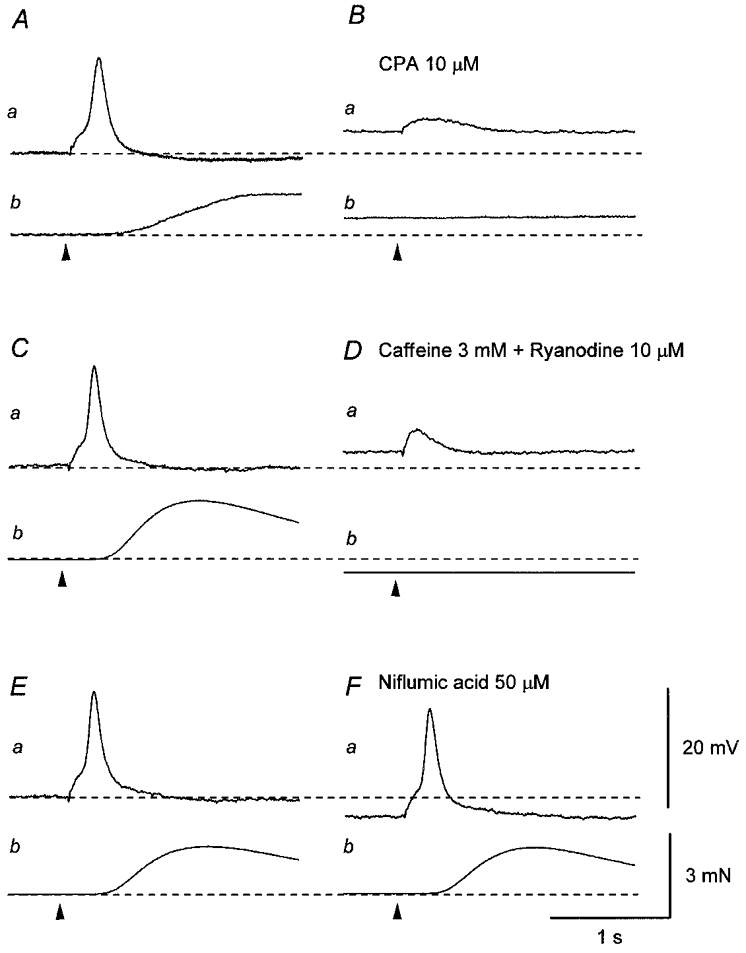
A, in the presence of nifedipine (10 μM), a single impulse (supramaximal current, 0.3 ms; arrowhead) initiated an EJP which consisted of two components (a) and an associated contraction (b). Ba, CPA (10 μM) depolarised the membrane and abolished the second component of the EJP without inhibiting the first component. b, CPA caused a sustained contraction and also abolished the nerve-evoked contraction. C, in another preparation which had been exposed to nifedipine (10 μM), a single impulse initiated an EJP which consisted of two components (a) and an associated contraction (b). Da, co-application of caffeine (3 mM) and ryanodine (10 μM) depolarised the membrane and abolished the second component of the EJP without inhibiting the first component. b, caffeine and ryanodine caused a transient contraction which turned into a relaxation and also abolished the nerve-evoked contraction. E, in a different preparation which had been exposed to 10 μM nifedipine, a single impulse initiated an EJP with two components (a) and an associated contraction (b). Fa, niflumic acid (50 μM) hyperpolarised the membrane but did not inhibit either the first or the second component of the EJPs. b, niflumic acid also did not inhibit the nerve-evoked contraction. Scale bar on the right in Fa refers to all potential traces (a). Scale bar on the right in Fb refers to all tension traces (b). Resting membrane potential was -45 mV in A, -43 mV in B and -38 mV in C.
In other smooth muscles the contribution of Ca2+-activated chloride channels to the generation of EJPs has been described (Large & Wang, 1996; Bramich & Hirst, 1999). Therefore, the effect of niflumic acid, an inhibitor of Ca2+-activated chloride channels, was examined. Niflumic acid (50 μM) hyperpolarised the membrane (4.4 ± 1.2 mV, n = 3) but did not inhibit either the first or the second component of EJPs (Fig. 5Ea and Fa). Niflumic acid did not inhibit nerve-evoked contractions (Fig. 5Eb and Fb).
Caffeine (3 mM) hyperpolarised the membrane (mean, 5.7 ± 1.9 mV; n = 7) and abolished the second component of EJPs (Fig. 6A). The inhibitory effect of caffeine alone on the second component of EJPs was very similar to that of co-application of caffeine and ryanodine (see Fig. 5B); however, the effect of caffeine was reversible in contrast to that of co-application of caffeine and ryanodine. It could be argued that the effect of caffeine on the second component of EJPs was due to the inhibition of phosphodiesterase activity and the subsequent elevation of cyclic AMP rather than stimulation of Ca2+ release from intracellular Ca2+ stores. To test this possibility, the effect of forskolin was examined. Forskolin (50 μM) caused a similar hyperpolarisation (7.3 ± 2.2 mV, n = 3) to that produced by caffeine but did not prevent the generation of the second component of EJPs (Fig. 6B). These results suggest that the inhibitory effect of caffeine on the second component of EJPs is more likely to be due to its effect on Ca2+ release from intracellular stores rather than to its inhibitory action on phosphodiesterase activity.
Figure 6. Comparative effects of caffeine and forskolin on EJPs recorded from the guinea-pig bladder.
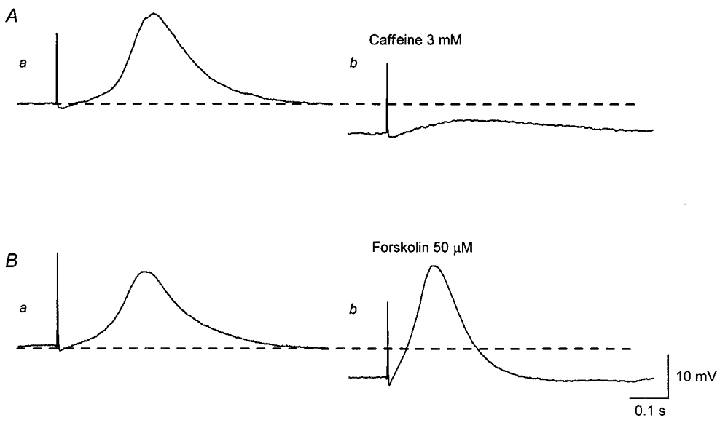
Aa, a single impulse initiated an EJP which consisted of two components. b, caffeine (3 mM) hyperpolarised the membrane and abolished the second component of the EJP without inhibiting the first component. Ba, after caffeine had been washed out, the membrane potential recovered to the original level and the second component of the EJP was restored. b, forskolin (50 μM) hyperpolarised the membrane but did not abolish the second component of the EJP. A and B were recorded from the same preparation. Scale bars on the right refer to all traces. Resting membrane potential was -42 mV.
Ca2+ responses produced by single transmural stimuli
When [Ca2+]i measurements were made simultaneously with isometric tension recordings, single impulses were found to initiate transient increases in [Ca2+]i (mean F340/380, 0.19 ± 0.03; n = 25) and cause associated contractions (Fig. 7A and C). Changes in both [Ca2+]i and tension were abolished by either tetrodotoxin (1 μM; n = 5) or α,β-methylene ATP (10 μM; n = 10) but not hyoscine (1 μM; n = 5), suggesting that they resulted from neurally released ATP. Nifedipine (10 μM) reduced the amplitude of both the increase in [Ca2+]i (mean, 19.6 ± 3.6 % of control; n = 6; Fig. 7Ba) and contraction (mean, 23.7 ± 5.3 % of control; n = 5; Fig. 7Bb). In separate experiments, caffeine (3 mM) reduced the amplitude of both the nerve-evoked increase in [Ca2+]i (mean, 32.1 ± 6.1 % of control; n = 7; Fig. 7Da) and contraction (mean, 25.4 ± 6.5 % of control; n = 5; Fig. 7Db).
Figure 7. Effects of nifedipine and caffeine on Ca2+ responses and mechanical responses produced by a single stimulation.
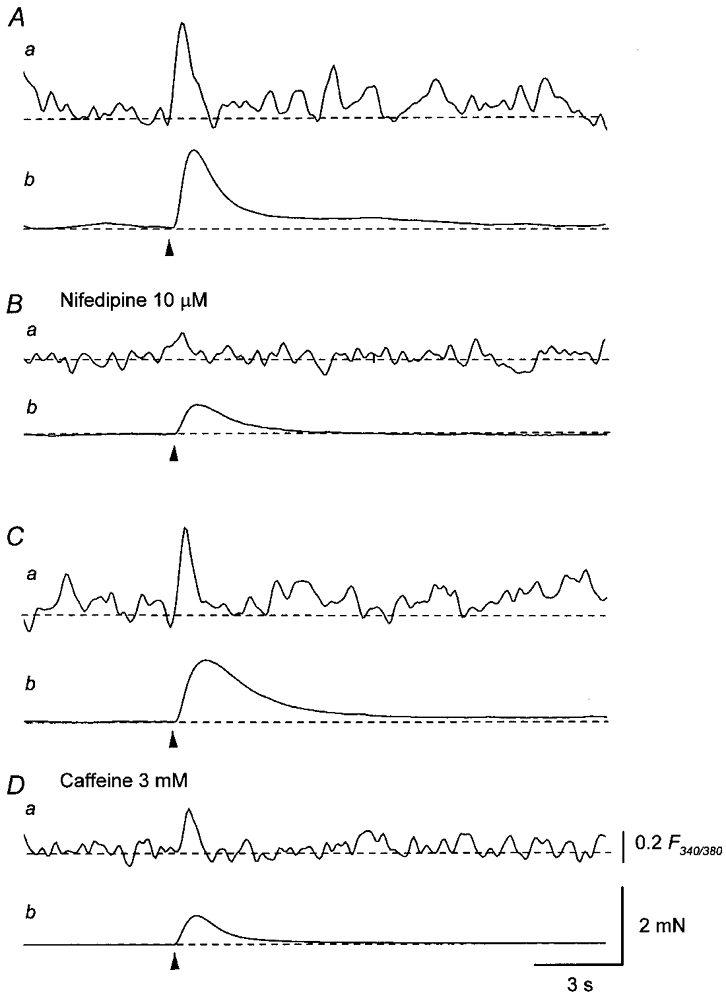
A, a single impulse (supramaximal current, 0.4 ms; arrowhead) initiated a transient increase in Ca2+ fluorescence ratio (a) and an associated contraction (b). B, nifedipine (10 μM) reduced the amplitude of the increase in Ca2+ fluorescence ratio (a) and the nerve-evoked contraction (b). C, in another preparation, a single impulse (supramaximal current, 0.5 ms; arrowhead) initiated a transient increase in Ca2+ fluorescence ratio (a) and an associated contraction (b). D, caffeine (3 mM) reduced the amplitude of the increase in Ca2+ fluorescence ratio (a) and the nerve-evoked contraction (b). Scale bar on the right in Da refers to all fluorescence ratio traces (a). Scale bar on the right in Db refers to all tension traces (b).
Responses produced by trains of transmural stimuli
When membrane potential changes were simultaneously recorded with isometric tension changes, trains of stimuli (1 s at 20 Hz) were found to initiate summed EJPs which triggered action potentials and subsequently increased the frequency of spontaneous action potentials (323 ± 117 % of control; n = 6, P < 0.05; Fig. 8Aa). These trains of impulses also initiated large, transient contractions (Fig. 8Ab). These responses were abolished by tetrodotoxin (1 μM; n = 3). In the presence of α,β-methylene ATP (10 μM), repetitive impulses failed to initiate responses during the stimulus train but increased the subsequent frequency of spontaneous action potentials occurring after the stimulus train (397 ± 139 % of control, n = 6, P < 0.05; Fig. 8Ba). Trains of stimuli also initiated delayed, prolonged contractions which often displayed oscillations (Fig. 8Bb). These residual responses were abolished by subsequent application of hyoscine (1 μM; n = 5), indicating that they resulted from the activation of muscarinic receptors by neurally released ACh. In the presence of nifedipine (10 μM), trains of impulses initiated either small (1.2 ± 0.8 mV, n = 5) or undetectable depolarisations which had a similar time course to that of the increased discharge of spontaneous action potentials observed before the addition of nifedipine (Fig. 8Ca). Nifedipine also suppressed the amplitude of cholinergic contractions (Fig. 8Cb).
Figure 8. Effects of α,β-methylene ATP and nifedipine on electrical and mechanical responses produced by trains of stimuli.
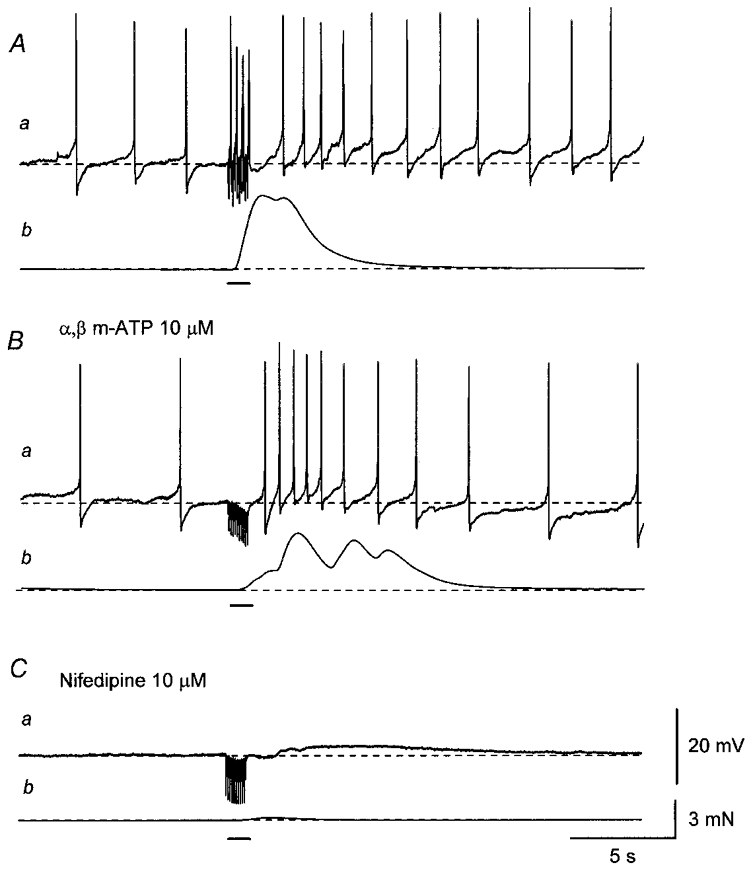
Aa, a train of stimuli applied at 20 Hz for 1 s (supramaximal current, 0.3 ms; bar) initiated summed EJPs which triggered action potentials and also increased the frequency of spontaneous action potentials.b, the stimulus train also produced a transient contraction. Ba, the desensitisation of purinoceptors with α,β-methylene ATP (α,β m-ATP; 10 μM) abolished both summed EJPs and action potentials during nerve stimulation but did not prevent the increase in the frequency of spontaneous action potentials. b, the train of stimuli initiated a contraction which had a reduced amplitude and a delayed, prolonged time course, and which often displayed oscillation. Ca, the subsequent addition of nifedipine (10 μM) abolished action potentials leaving a small, slow depolarisation. b, nifedipine also suppressed the residual contraction. A, B and C were recorded from the same cell. Scale bar on the right in Ca refers to all potential traces (a). Scale bar on the right in Cb refers to all tension traces (b). Resting membrane potential was -43 mV.
To further investigate the source of the Ca2+ that contributed to the cholinergic contractions, changes in [Ca2+]i were measured simultaneously with changes in isometric tension in a separate series of experiments. Trains of stimuli initiated large increases in [Ca2+]i (mean F340/380, 0.31 ± 0.05; n = 26) and large contractions (Fig. 9A). In the presence of α,β-methylene ATP (10 μM), trains of stimuli failed to initiate rapid increases in [Ca2+]i but caused delayed, prolonged increases in [Ca2+]i which often displayed oscillations (Fig. 9Ba). Trains of stimuli also initiated associated contractions (Fig. 9Bb). To quantify the oscillatory changes in [Ca2+]i and associated contractions, increases in Ca2+ fluorescence ratio and isometric tension were integrated. The integrated increase in [Ca2+]i was increased by physostigmine (0.1 μM; 179 ± 28 % of control, n = 3; Fig. 9Ca). Physostigmine also enhanced nerve-evoked contractions (196 ± 24 % of control, n = 3; Fig. 9Cb). These responses were abolished by hyoscine (1 μM; n = 7), indicating that they were mediated by cholinergic nerves (Fig. 9D).
Figure 9. Effects of α,β-methylene ATP, physostigmine and hyoscine on Ca2+ responses and mechanical responses produced by trains of stimuli.
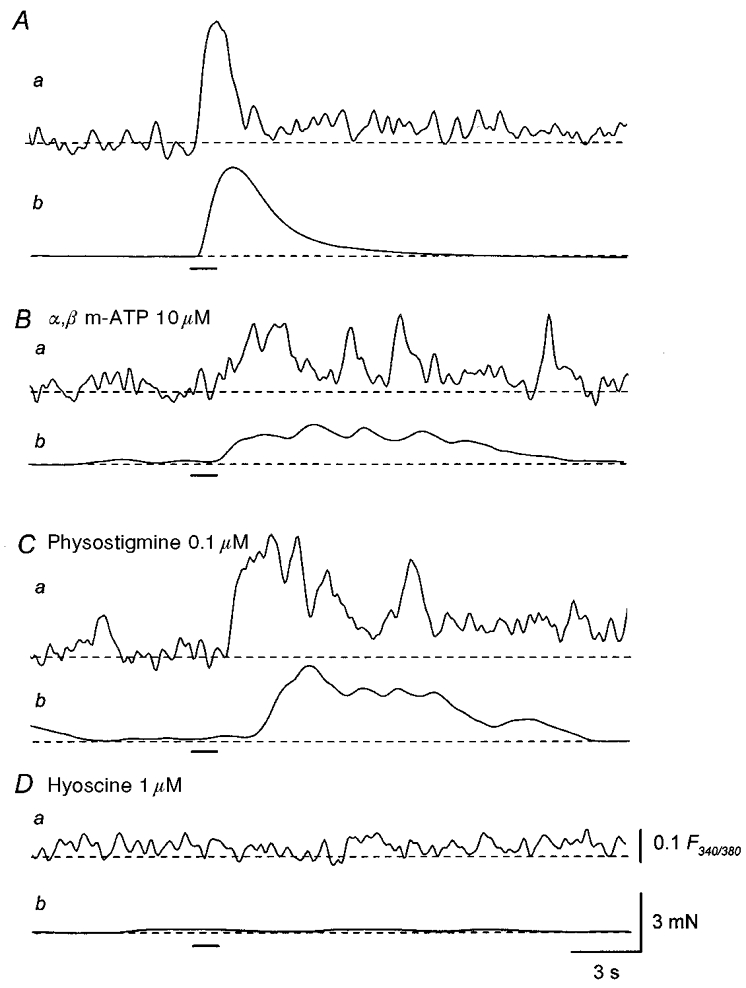
A, a train of stimuli applied at 20 Hz for 1 s (supramaximal current, 0.3 ms; bar) initiated a transient increase in Ca2+ fluorescence ratio (a) and an associated contraction (b). B, in the presence of α,β-methylene ATP (10 μM), a train of stimuli initiated an oscillatory increase in Ca2+ fluorescence ratio which had a delayed, prolonged time course (a) and an associated, oscillatory contraction (b). C, in the same preparation, physostigmine (0.1 μM) increased the amplitude of both the oscillatory increase in Ca2+ fluorescence ratio (a) and the associated contraction (b). D, the subsequent application of hyoscine (1 μM) abolished both the increase in Ca2+ fluorescence ratio (a) and the associated contraction (b). Scale bar on the right in Da refers to all fluorescence traces (a). Scale bar on the right in Db refers to all tension traces (b).
Nifedipine (10 μM) reduced the oscillatory increases in [Ca2+]i in response to trains of stimuli leaving an initial small increase in [Ca2+]i. The integrated increase in [Ca2+]i was reduced to 10.3 ± 7.1 % of control (n = 7; Fig. 10Ba). Nifedipine also inhibited the oscillatory contractions and reduced the amplitude of the integrated contraction to 14.9 ± 5.5 % of control (n = 6; Fig. 10Bb). Caffeine (3 mM) suppressed the oscillations in [Ca2+]i and reduced the integrated increase in [Ca2+]i to 34.5 ± 11.7 % of control (n = 8; Fig. 10Da). Caffeine also inhibited oscillatory contractions and reduced the amplitude of the integrated contraction to 20.4 ± 8.1 % of control (n = 6; Fig. 10Db). The effect of caffeine on both [Ca2+]i and contraction was reversible.
Figure 10. Effects of nifedipine and caffeine on Ca2+ responses and mechanical responses produced by trains of stimuli.
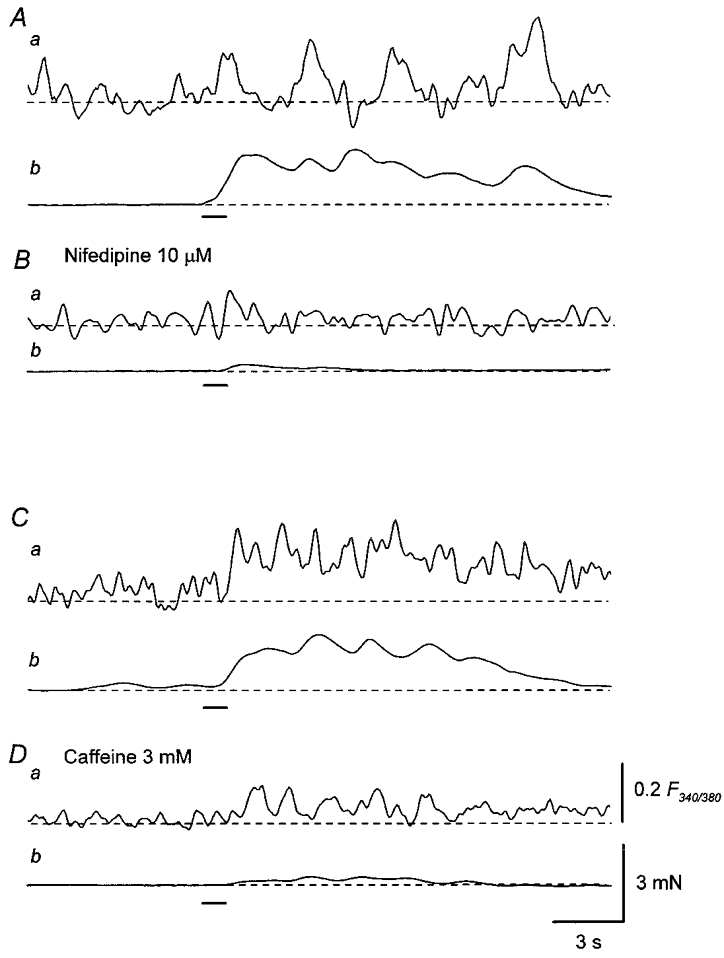
A, in the presence of α,β-methylene ATP (10 μM), a train of stimuli applied at 20 Hz for 1 s (supramaximal current, 0.2 ms; bar), initiated an oscillatory increase in Ca2+ fluorescence ratio which had a delayed, prolonged time course (a) and an associated contraction (b). B, nifedipine (10 μM) reduced the amplitude of both the oscillatory increase in Ca2+ fluorescence ratio (a) and associated contraction (b). C, in another preparation, a train of stimuli initiated an oscillatory increase in Ca2+ fluorescence ratio which had a delayed onset and a prolonged time course (a), and an associated, oscillatory contraction (b). D, caffeine (3 mM) reduced the amplitude of both the increase in Ca2+ fluorescence ratio (a) and the associated contraction (b). Scale bar on the right in Da refers to all fluorescence traces (a). Scale bar on the right in Db refers to all tension traces (b).
DISCUSSION
In the present study, either membrane potential recordings or [Ca2+]i measurements were made simultaneously with isometric tension recordings from intact smooth muscle preparations of the guinea-pig urinary bladder. The activation of purinoceptors by neurally released ATP initiated EJPs which triggered action potentials. The resultant influx of Ca2+ through L-type Ca2+ channels and the release of Ca2+ from intracellular stores produced contraction of the bladder smooth muscle. The activation of muscarinic receptors by neurally released ACh increased the frequency of action potentials, initiated oscillatory increases in [Ca2+]i and caused oscillatory contractions. Both the influx of Ca2+ through L-type Ca2+ channels and the release of Ca2+ from intracellular stores appeared to be involved in the generation of these oscillatory responses.
Spontaneous action potentials recorded from bladder smooth muscle had similar amplitudes, time courses and frequencies to those observed in preparations in which contractions were prevented with high extracellular osmolarity (Mostwin, 1986; Fujii, 1988). As reported previously, spontaneous action potentials were myogenic in origin and were abolished by nifedipine, suggesting that they resulted from the activation of L-type Ca2+ channels (Mostwin, 1986; Bramich & Brading, 1996). In other smooth muscle, including urethral smooth muscle of both rabbit and guinea-pig and tracheal smooth muscle of guinea-pig, spontaneous activity results from both Ca2+ release from intracellular stores and the activation of L-type Ca2+ channels (Van Helden, 1993; Hashitani et al. 1996; Hashitani & Edwards, 1999; Bramich, 2000). Thus the discharge of action potentials is often abolished both by blocking Ca2+ channels and by depleting intracellular Ca2+ stores. In the guinea-pig bladder, however, neither the co-application of caffeine and ryanodine nor buffering of [Ca2+]i to low levels with BAPTA AM prevented the generation of spontaneous action potentials (H. Hashitani, unpublished observations). However, both the co-application of caffeine and ryanodine and loading with BAPTA increased the half-width of action potentials and also abolished the after-hyperpolarisation (H. Hashitani, unpublished observations). This suggests that release of Ca2+ from intracellular Ca2+ stores may contribute to the opening of two sets of Ca2+-activated K+ channels contributing to the repolarising and after-hyperpolarising phases of the action potential (Fujii et al. 1990; Heppner et al. 1997).
In control solutions purinergic EJPs triggered action potentials. However, the resultant Ca2+ influx appeared to be small, causing only small contractions unless it was amplified by CICR. This has been reported previously for other tissues (Ganitkevich & Isenberg, 1992). In the presence of nifedipine, purinergic EJPs continued to trigger an increase in [Ca2+]i, probably in part through the release of Ca2+ from intracellular stores, and caused small contractions. Intracellular Ca2+ stores in smooth muscle contain both ryanodine and InsP3 receptors (Berridge, 1993), therefore Ca2+ could be released as a consequence of either CICR or the production of InsP3. Purinoceptors have been reported to couple with G-proteins and stimulate the production of InsP3, but unlike P2X receptors in the guinea-pig urinary bladder, these types of purinoceptors are not readily desensitised with α,β-methylene ATP (Tawada, 1987). Thus the activation of P2X receptors is more likely to increase [Ca2+]i through CICR which may be stimulated by Ca2+ influx through non-selective cation channels underlying EJPs (Inoue & Brading, 1990). This is consistent with previous reports which showed that the ATP-induced increase in [Ca2+]i was sensitive to co-application of caffeine and ryanodine but not heparin (Pacaude et al. 1994). Furthermore, in the guinea-pig urethra, the stimulation of P2X receptors by neurally released ATP has been reported to initiate depolarisation by stimulating regenerative CICR (Hashitani & Edwards, 1999).
Two types of purinergic EJPs can be recorded from guinea-pig bladder, i.e. EJPs consisting of single or two components (Bramich & Brading, 1996). Single-component EJPs probably result from the opening of non-selective cation channels (Inoue & Brading, 1990). Two-component EJPs were composed of an initial depolarisation and a secondary regenerative depolarisation. The initial depolarisation of a two-component EJP, like that of a single-component EJP, was insensitive to caffeine. However, the regenerative depolarisation was abolished by caffeine, co-application of caffeine and ryanodine, and CPA, suggesting that it resulted from Ca2+ release from intracellular Ca2+ stores and the subsequent activation of Ca2+-sensitive channels. In other types of smooth muscle, the contribution of Ca2+-activated chloride channels to the generation of α-adrenergic EJPs has been reported (Van Helden, 1988; Bramich & Hirst, 1999). However, the regenerative components of EJPs in the guinea-pig bladder were not inhibited by niflumic acid, a blocker of Ca2+-activated chloride channels (Large & Wang, 1996). Perhaps, Ca2+-activated cation channels, which have been described in other smooth muscles, may contribute to the generation of regenerative components of EJPs in urinary bladder (Loirand et al. 1991).
In the presence of α,β-methylene ATP to desensitise purinoceptors, the neural activation of muscarinic receptors triggered oscillatory increases in [Ca2+]i and associated contractions. In other types of smooth muscle, the stimulation of muscarinic receptors initiates oscillations in [Ca2+]i that are sensitive to both ryanodine and heparin but not nifedipine, suggesting a primary role for InsP3-induced Ca2+ release (Komori & Bolton, 1991). In the presence of nifedipine, the stimulation of muscarinic receptors caused initial small responses but failed to initiate oscillatory responses. Thus InsP3-induced Ca2+ release may contribute to an initial small but not large oscillatory response. The oscillatory responses were also suppressed by caffeine, suggesting that both the influx of Ca2+ through L-type Ca2+ channels and the release of Ca2+ from intracellular stores are required to generate these responses. The stimulation of muscarinic receptors increased the frequency of spontaneous action potentials with either small or no underlying depolarisations. Increases in the frequency of spontaneous action potentials would produce periodical Ca2+ influx which may trigger CICR to initiate oscillatory changes in [Ca2+]i. The influx of Ca2+ through L-type Ca2+ channels may also contribute to the refilling of Ca2+ stores so that repetitive CICR could occur (Rivera et al. 1998; Wu et al. 1998). Oscillatory changes in [Ca2+]i may result from Ca2+-induced positive and negative feedback to the subsequent release of Ca2+ and thus CICR could occur in a regenerative way but a preceding increase in [Ca2+]i would inhibit the following Ca2+ release (Berridge, 1993). Similarly, trains of stimuli never initiated oscillatory responses in control solutions. The initial stimulation of purinoceptors may activate regenerative CICR to increase [Ca2+]i to a level that prevents the further release of Ca2+ or may cause depletion of intracellular Ca2+ stores resulting in a refractory period until they are refilled.
Most studies that have shown the contribution of InsP3 production to cholinergic contractions examined the effects of bath-applied muscarinic agonists at relatively high concentrations (Mostwin, 1985; Iacovou et al. 1990; Andersson et al. 1991). It may be that the concentration of neurally released ACh which acts on muscarinic receptors of urinary bladder smooth muscle is not sufficiently high to stimulate InsP3 production. In the circular smooth muscle of the guinea-pig stomach, it has been reported that high concentrations (0.1 mM) but not low concentrations (0.1 μM) of carbachol caused InsP3-mediated contractions (Parekh & Brading, 1991). Thus M3 muscarinic receptors, which stimulate the production of InsP3, may operate at high concentrations of muscarinic agonist, whereas M2 muscarinic receptors, which do not trigger the formation of InsP3, may be activated by lower concentrations of agonist (Hegde & Eglen, 1999). Alternatively, bladder smooth muscle might have a localisation of muscarinic receptors as reported in other smooth muscle (Hirst et al. 1996), in that junctional receptors are probably M2 receptors while extra-junctional receptors are probably M3 receptors.
In conclusion, the activation of purinoceptors by neurally released ATP increased [Ca2+]i by both Ca2+ influx through L-type Ca2+ channels and CICR from intracellular stores to contract bladder smooth muscle. Ca2+ release from intracellular stores also appears to contribute to the generation of the second component of EJPs. The activation of muscarinic receptors by neurally released ACh initiated oscillatory increases in [Ca2+]i and associated contractions. Again, both the Ca2+ influx through L-type Ca2+ channels and CICR from intracellular stores seemed to contribute to the generation of these oscillatory responses.
Acknowledgments
This project was supported by a research grant from National Health and Medical Research Council of Australia.
References
- Andersson K-E, Holmquist F, Fovaeus M, Hedlund H, Sundler R. Muscarinic receptor stimulation of phosphoinositide hydrolysis in the human isolated urinary bladder. Journal of Urology. 1991;146:1156–1159. doi: 10.1016/s0022-5347(17)38030-8. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Inositol trisphosphate and calcium signalling. Nature. 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Bo XN, Burnstock G. The effects of Bay K 8644 and nifedipine on the responses of rat urinary bladder to electrical field stimulation, β,γ-methylene ATP and acetylcholine. British Journal of Pharmacology. 1990;101:494–498. doi: 10.1111/j.1476-5381.1990.tb12736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brading AF, Mostwin JL. Electrical and mechanical responses of guinea-pig bladder muscle to nerve stimulation. British Journal of Pharmacology. 1989;98:1083–1090. doi: 10.1111/j.1476-5381.1989.tb12651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramich NJ. Electrical behaviour of guinea-pig tracheal smooth muscle. American Journal of Physiology – Lung Cellular and Molecular Physiology. 2000;278:L320–328. doi: 10.1152/ajplung.2000.278.2.L320. [DOI] [PubMed] [Google Scholar]
- Bramich NJ, Brading AF. Electrical properties of smooth muscle in the guinea-pig urinary bladder. The Journal of Physiology. 1996;492:185–198. doi: 10.1113/jphysiol.1996.sp021300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramich NJ, Hirst GDS. Sympathetic neuroeffector transmission in the rat anococcygeus muscle. The Journal of Physiology. 1999;516:101–115. doi: 10.1111/j.1469-7793.1999.101aa.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creed KE, Ishikawa S, Ito Y. Electrical and mechanical activity recorded from rabbit urinary bladder in response to nerve stimulation. The Journal of Physiology. 1983;338:149–164. doi: 10.1113/jphysiol.1983.sp014666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii K. Evidence for adenosine triphosphate as an excitatory transmitter in guinea-pig, rabbit and pig urinary bladder. The Journal of Physiology. 1988;404:39–52. doi: 10.1113/jphysiol.1988.sp017277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii K, Foster CD, Brading AF, Parekh AB. Potassium channel blockers and the effects of cromakalim on the smooth muscle of the guinea-pig bladder. British Journal of Pharmacology. 1990;99:779–785. doi: 10.1111/j.1476-5381.1990.tb13006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganitkevich VY, Isenberg G. Contribution of Ca2+-induced Ca2+ release to the [Ca2+]i transients in myocytes from guinea-pig urinary bladder. The Journal of Physiology. 1992;458:119–137. doi: 10.1113/jphysiol.1992.sp019409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashitani H, Edwards FR. Spontaneous and neurally activated depolarizations in smooth muscle cells of the guinea-pig urethra. The Journal of Physiology. 1999;514:459–470. doi: 10.1111/j.1469-7793.1999.459ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashitani H, Suzuki H. Electrical and mechanical responses produced by nerve stimulation in detrusor smooth muscle of the guinea-pig. European Journal of Pharmacology. 1995;284:177–183. doi: 10.1016/0014-2999(95)00386-y. [DOI] [PubMed] [Google Scholar]
- Hashitani H, Van Helden DF, Suzuki H. Properties of spontaneous depolarizations in circular smooth muscle cells of rabbit urethra. British Journal of Pharmacology. 1996;118:1627–1632. doi: 10.1111/j.1476-5381.1996.tb15584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde SS, Eglen RM. Muscarinic receptor subtypes modulating smooth muscle contractility in the urinary bladder. Life Sciences. 1999;64:419–428. doi: 10.1016/s0024-3205(98)00581-5. [DOI] [PubMed] [Google Scholar]
- Heppner TJ, Bonev AD, Nelson MT. Ca2+-activated K+ channels regulate action potential repolarization in urinary bladder smooth muscle. American Journal of Physiology. 1997;273:C110–117. doi: 10.1152/ajpcell.1997.273.1.C110. [DOI] [PubMed] [Google Scholar]
- Hirst GDS, Choate JK, Cousins HM, Edwards FR, Klemm MF. Transmission by post-ganglionic axons of the autonomic nervous system: the importance of the specialized neuroeffector junction. Neuroscience. 1996;73:7–23. doi: 10.1016/0306-4522(96)00031-0. [DOI] [PubMed] [Google Scholar]
- Hoyle CHV, Burnstock G. Atropine-resistant excitatory junction potentials in rabbit bladder are blocked by α,β-methylene ATP. European Journal of Pharmacology. 1985;114:239–240. doi: 10.1016/0014-2999(85)90635-1. [DOI] [PubMed] [Google Scholar]
- Iacovou JW, Hill SJ, Birmingham AT. Agonist-induced contraction and accumulation of inositol phosphates in the guinea-pig detrusor: evidence that muscarinic and purinergic receptors raise intracellular calcium by different mechanisms. Journal of Urology. 1990;144:775–779. doi: 10.1016/s0022-5347(17)39590-3. [DOI] [PubMed] [Google Scholar]
- Imaizumi Y, Torii Y, Ohi Y, Nagano N, Atsuki K, Yamamura H, Muraki K, Watanabe M, Bolton TB. Ca2+ images and K+ current during depolarization in smooth muscle cells of the guinea-pig vas deferens and urinary bladder. The Journal of Physiology. 1998;510:705–719. doi: 10.1111/j.1469-7793.1998.705bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue R, Brading AF. The properties of the ATP-induced depolarization and current in single cells isolated from the guinea-pig urinary bladder. British Journal of Pharmacology. 1990;100:619–625. doi: 10.1111/j.1476-5381.1990.tb15856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuragi T, Usune S, Furukawa T. Antagonism by nifedipine of contraction and Ca2+-influx evoked by ATP in guinea-pig urinary bladder. British Journal of Pharmacology. 1990;100:370–374. doi: 10.1111/j.1476-5381.1990.tb15811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komori S, Bolton TB. Inositol trisphosphate releases stored calcium to block voltage-dependent calcium channels in single smooth muscle cells. Pflügers Archiv. 1991;418:437–441. doi: 10.1007/BF00497770. [DOI] [PubMed] [Google Scholar]
- Large WA, Wang Q. Characteristics and physiological role of the Ca2+ activated Cl− conductance in smooth muscle. American Journal of Physiology. 1996;271:C435–454. doi: 10.1152/ajpcell.1996.271.2.C435. [DOI] [PubMed] [Google Scholar]
- Loirand G, Pacaud P, Baron A, Mironneau C, Mironneau J. Large conductance calcium-activated non-selective cation channel in smooth muscle cells isolated from rat portal vein. The Journal of Physiology. 1991;437:461–475. doi: 10.1113/jphysiol.1991.sp018606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostwin JL. Receptor operated intracellular calcium stores in the smooth muscle of the guinea pig bladder. Journal of Urology. 1985;133:900–905. doi: 10.1016/s0022-5347(17)49277-9. [DOI] [PubMed] [Google Scholar]
- Mostwin JL. The action potential of guinea pig bladder smooth muscle. Journal of Urology. 1986;135:1299–1303. doi: 10.1016/s0022-5347(17)46079-4. [DOI] [PubMed] [Google Scholar]
- Pacaud P, Gregoire G, Loirand G. Release of Ca2+ from intracellular store in smooth muscle cells of rat portal vein by ATP-induced Ca2+ entry. British Journal of Pharmacology. 1994;113:457–462. doi: 10.1111/j.1476-5381.1994.tb17011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh AB, Brading AF. The sources of calcium for carbachol-induced contraction in the circular smooth muscle of the guinea-pig. British Journal of Pharmacology. 1991;104:412–418. doi: 10.1111/j.1476-5381.1991.tb12444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera L, McMurray G, Brading AF. The role of calcium stores in the action of muscarinic agonists on mammalian urinary bladder. The Journal of Physiology. 1998;507.P:21–22P. [Google Scholar]
- Sibley GNA. A comparison of spontaneous and nerve-mediated activity in bladder muscle from man, pig and rabbit. Ca2+ images and K+ current during depolarization in smooth muscle cells of the guinea-pig vas deferens and urinary bladder. The Journal of Physiology. 1984;354:431–443. doi: 10.1113/jphysiol.1984.sp015386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjögren C, Andersson K-E, Husted S, Mattiasson A, Møller-Madsen B. Atropine resistance of transmurally stimulated isolated human bladder muscle. Journal of Urology. 1982;128:1368–1371. doi: 10.1016/s0022-5347(17)53509-0. [DOI] [PubMed] [Google Scholar]
- Tawada Y, Furukawa K, Shigekawa M. ATP-induced calcium transient in cultured rat aortic smooth muscle cells. Journal of Biochemistry. 1987;102:1499–1509. doi: 10.1093/oxfordjournals.jbchem.a122197. [DOI] [PubMed] [Google Scholar]
- van Helden DF. Electrophysiology of neuromuscular transmission in guinea-pig mesenteric veins. The Journal of Physiology. 1988;401:469–488. doi: 10.1113/jphysiol.1988.sp017173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Helden DF. Pacemaker potentials in lymphatic smooth muscle of the guinea-pig mesentery. The Journal of Physiology. 1993;471:465–479. doi: 10.1113/jphysiol.1993.sp019910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Sui G, Fry CH. The role of the membrane potential in filling functional intracellular Ca2+ stores in guinea-pig detrusor smooth muscle. The Journal of Physiology. 1998;507.P:22P. doi: 10.1113/jphysiol.2001.013191. [DOI] [PMC free article] [PubMed] [Google Scholar]


