Abstract
Using intracellular recording techniques, two distinct layers of smooth muscle were identified in the rat penile bulb. The inner muscle layer (parenchyma) exhibited spontaneous action potentials, while the outer sheet (sac) was electrically quiescent.
In the parenchyma, transmural stimulation initiated non-adrenergic, non-cholinergic (NANC) inhibitory junction potentials (IJPs) which were abolished by Nωnitro-L-arginine (LNA) or 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ). The amplitude of IJPs was reduced by ouabain, dinitrophenol or decreasing the extracellular potassium concentration ([K+]o) but not by several K+ channel blockers.
The parenchyma also received an excitatory innervation mediated by α-adrenoceptors which caused a contraction that was not associated with a membrane potential change.
In the sac, transmural stimulation initiated two component excitatory junction potentials (EJPs) mediated by α-adrenoceptors and associated action potentials. The initial component was more dramatically suppressed than the secondary component by caffeine, ryanodine or cyclopiazonic acid (CPA). Lowering of the extracellular chloride concentration ([Cl−]o) selectively inhibited the rapid component of EJPs, while niflumic acid was less potent.
These results suggest that IJPs in the parenchyma result from the release of NO which stimulates sodium pump activity following the activation of guanylate cyclase. In the sac, the activation of α-adrenoceptors initiates EJPs by releasing Ca2+ from intracellular stores which activates Ca2+-activated channels.
Penile erection and detumescence result from the activity of the parasympathetic and sympathetic nervous system, respectively (Anderson & Wagner, 1995). It has been well established that erection is mainly achieved by neurally released nitric oxide (NO) which relaxes corporeal smooth muscle through the activation of guanylate cyclase (Ignarro et al. 1990; Holmquist et al. 1992). Detumescence predominantly results from the activation of α-adrenoceptors which increases intracellular Ca2+ concentration of the corporeal smooth muscle by both Ca2+ influx and Ca2+ release from intracellular Ca2+ stores (Fovaeus et al. 1987).
The properties of corporeal smooth muscle have been investigated mostly by measuring contractile responses and there have been very few electrophysiological studies in this tissue (Samuelson et al. 1983; Kimoto & Ito, 1988; Anderson & Wagner, 1995). Recently, electrophysiological investigations using cultured corporeal smooth muscle cells showed that the cells have a high degree of coupling through gap junctions with respect to intracellular propagation of both electrical signals and second messengers, such as Ca2+ and inositol trisphosphate (InsP3) (Christ et al. 1992). These studies also indicated the presence of a variety of K+ channels, L-type Ca2+ channels and chloride channels in cultured corporeal smooth muscle cells (Christ et al. 1993). However, there has been no information about the physiological function of these ion channels in intact preparations, particularly regarding their role during neuroeffector transmission.
It has been reported that NO-containing nerves in the corpus spongiosum have very similar distribution and function to those of corpus cavernosum which play a major role in erection (Hedlund et al. 1995). In the present study, the penile bulb, which is the proximal part of corpus spongiosum, was studied using intracellular microelectrode recording techniques to investigate the membrane potential changes which occurred either in unstimulated preparations or in response to transmural nerve stimulation. The electrical coupling between smooth muscle cells was also examined using two independent microelectrodes. On some occasions, changes in membrane potential and tension were recorded simultaneously to determine the correlation between electrical and mechanical activity.
METHODS
The procedures described were approved by the animal experimentation ethics committee of the University of Melbourne. Male adult Wistar rats, weighing 150–200 g, were killed by a blow to the head followed by cervical exsanguination and the penile bulbs were rapidly removed with surrounding bulbospongiosum muscle. After removal of bulbospongiosum muscle and underlying tunica albuginea, which covered the penile bulbs, the penile bulbs were opened by making two lateral incisions. The penile bulbs were then pinned out in a dissecting chamber with the inner urethral mucosal side uppermost. The urethral mucosa was removed and then glandular tissue was carefully taken away to leave the underlying smooth muscle layer.
The smooth muscle was composed of two layers; the outer, thick smooth muscle layer, termed ‘sac’ and the inner, sponge-like smooth muscle meshwork, termed ‘parenchyma’. In most experiments, either sac or parenchyma was separated from the other and pinned out in a small shallow chamber (volume ∼1 ml) with the previously attached side uppermost and superfused with warmed physiological saline (38°C) at a constant flow rate (2 ml min−1).
After equilibration for 30 min, individual smooth muscle cells were impaled with glass capillary microelectrodes filled with 0.5 M KCl (tip resistance 100–210 MΩ). In some experiments in which electrical coupling between smooth muscle cells was studied, the preparation was impaled with two independent microelectrodes and the distance and location of two electrodes were determined with an inverted microscope. To study the passive electrical communications of the preparations, one electrode was used to deliver current and the other was used to record changes in membrane potential.
Membrane potential changes were recorded using a high input impedance amplifier (Axoclamp-2B, Axon Instruments, Inc., Foster City, CA, USA) and displayed on a cathode ray oscilloscope (5103N, Tektronix Inc., Beaverton, OR, USA). After low-pass filtering (cut-off frequency, 1 kHz), potential changes were digitised and stored on a personal computer for later analysis.
In some experiments, isometric tension recording was made either separately or simultaneously with membrane potential recording. To detect isometric tension changes of the preparations, an L-shaped fine needle, which was connected to a transducer, was inserted into the preparation.
Intramural nerves were stimulated selectively by passing brief pulses of constant current (duration 0.1-0.5 ms, intensity 3–10 mA) between a pair of electrodes (silver plate and wire) placed below and above the preparation. The neural selectivity of the stimulating pulse was confirmed by sensitivity to tetrodotoxin (1 μM).
The ionic composition of physiological saline was as follows (mM): NaCl, 119; KCl, 5.0; CaCl2, 2.5; MgCl2, 2.0; NaHCO3, 25.0; NaH2PO4, 1.0 and glucose, 11.0. The solution was bubbled with 95 % O2 and 5 % CO2 to maintain pH 7.4. Solutions containing reduced concentrations of chloride ions were prepared by replacing NaCl with an isosmotic amount of sodium isethionic acid.
The drugs used were Nω-nitro-L-arginine (LNA), 4-aminopyridine, apamin, caffeine, charybdotoxin, cyclopiazonic acid (CPA), dinitrophenol, glibenclamide, 18β-glycyrrhetinic acid (18β-GA), guanethidine sulfate, hyoscine chloride, nifedipine, niflumic acid, 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ), ouabain, phentolamine mesylate, phenylephrine hydrochloride, tetrodotoxin and ryanodine (all from Sigma). These drugs were dissolved in distilled water except dinitrophenol, nifedipine and niflumic acid which were dissolved in 100 % ethanol; and CPA, glibenclamide and 18β-GA which were dissolved in dimethyl sulfoxide (DMSO). The final concentration of these solvents in physiological saline did not exceed 1:1000.
Measured values are expressed as means ± standard deviation. The following parameters were measured: maximum amplitude; latency, measured as the time taken from the stimulus artifact to 10 % of peak amplitude of the events; rise time, measured as the time between 10 and 90 % of the peak amplitude of the events; half-width, measured as the time between 50 % peak amplitude on the rising and the falling phase of the events.
RESULTS
General observations
Using an inverted microscope, the smooth muscle layer of the penile bulb was seen to be composed of two structures, an outer smooth muscle sheet, the ‘sac’, which surrounded an inner sponge-like smooth muscle meshwork, the ‘parenchyma’, which attached to the sac and also extended networks into the glandular tissue.
Visual observations indicated that parenchyma smooth muscle spontaneously contracted, whereas sac smooth muscle was quiescent, suggesting that it was not spontaneously active and that it was not coupled to the parenchyma. When membrane potential changes were recorded from each layer in preparations where the two layers remained attached, parenchyma smooth muscle exhibited spontaneous action potentials, while sac smooth muscle was quiescent. In sac smooth muscle, transmural nerve stimulation initiated oscillatory membrane potential changes, indicating that the lack of spontaneous membrane potential changes in sac smooth muscle was not due to damage to the preparation.
Spontaneous electrical and mechanical activity in isolated preparations of the parenchyma and sac
When intracellular recordings were made from the isolated parenchyma, about 80 % of preparations studied exhibited spontaneous depolarisations (Figs 1Aa and 2A). Spontaneous depolarisations were characterised in 17 preparations; they occurred periodically with frequencies between 24 and 61 min−1 (mean 39.3 ± 14.1 min−1) and had peak amplitudes between 8.2 and 24.1 mV (15.2 ± 4 mV), rise times between 0.21 and 1.08 s (0.49 ± 0.24 s) and half-widths between 0.23 and 0.92 s (0.47 ± 0.19 s). Resting membrane potential, determined in all 38 preparations, which was defined as the most negative potential level between spontaneous depolarisations, varied between -31 and -48 mV (mean -36.4 ± 4.5 mV). Spontaneous depolarisations occurred either continuously or in a bursting pattern and sometimes triggered spike-like action potentials. They persisted in the presence of phentolamine (1 μM; n = 10), guanethidine (10 μM; n = 15), hyoscine (1 μM; n = 10) or tetrodotoxin (1 μM; n = 5), suggesting that they were myogenic in origin.
Figure 1. Differences in electrical and mechanical activity between the parenchyma and sac smooth muscle in the rat penile bulb.
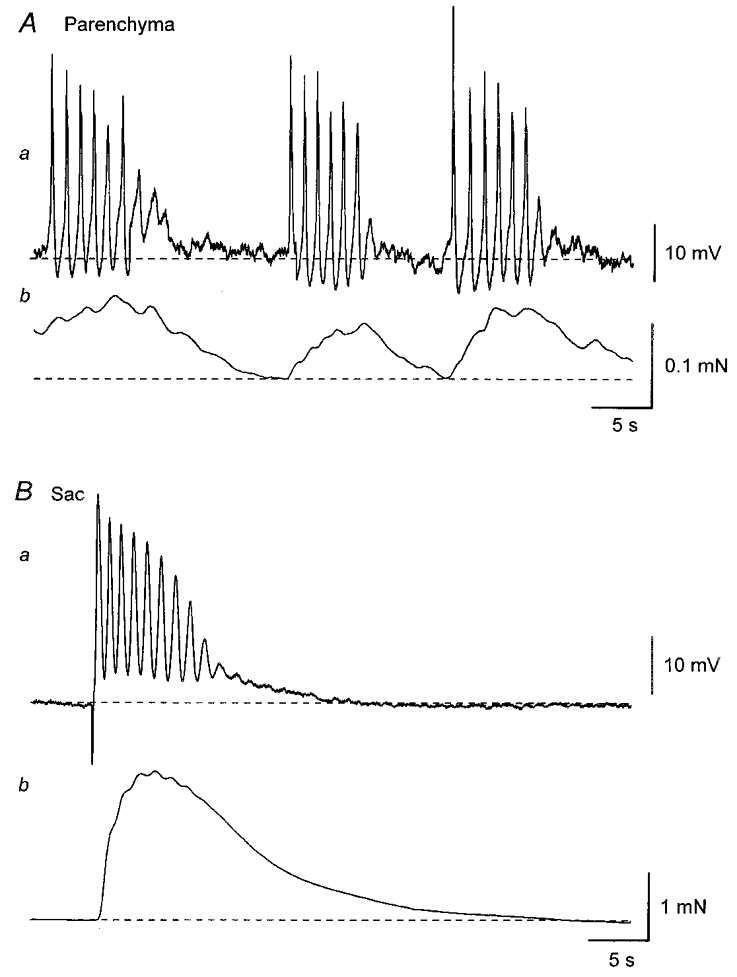
A, parenchyma smooth muscle cells exhibited bursts of spontaneous action potentials which were interrupted by quiescent periods (Aa). Each burst of action potentials was associated with a contraction (Ab). B, sac smooth muscle cells were electrically quiescent (Ba) and did not develop spontaneous contractions (Bb). Transmural nerve stimulation initiated an oscillatory membrane depolarisation in sac smooth muscle cells which triggered action potentials (Ba) and also produced a contraction (Bb). A and B were recorded from two different preparations. Resting membrane potentials were -43 mV in A and -50 mV in B.
Figure 2. Electrical coupling between smooth muscle cells in the parenchyma and sac of the rat penile bulb.
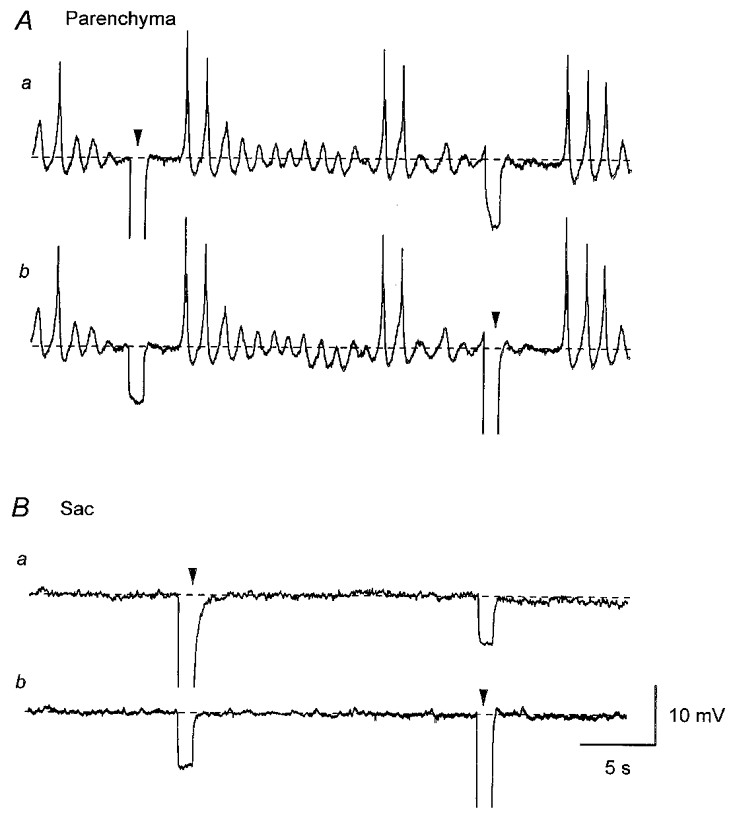
In a parenchyma preparation, membrane potential changes were simultaneously recorded from two independent microelectrodes which were separated by a distance of 200 μm. Identical resting membrane potential changes were recorded from both electrodes and hyperpolarising current of 3 nA, which was injected via the first electrode, caused membrane hyperpolarisation in the second electrode (Aa and b). In a sac preparation, membrane potential changes were simultaneously recorded from two independent microelectrodes which were separated by a distance of 250 μm. Both cells had quiescent membrane potentials (Ba and b). Hyperpolarising current of 3 nA, which was injected via the first electrode, caused hyperpolarisation in the second electrode (Ba and b). A and B were recorded from two different preparations. Resting membrane potential was -42 mV in A and -53 mV in B. Arrowheads indicate times of current injection. Scale bars in B apply to all traces.
When isometric tension was recorded at the same time as membrane potential, each depolarisation initiated an associated contraction (Fig. 1Ab). During bursts of spontaneous depolarisations, a larger contraction developed and then muscle relaxed on cessation of the discharge of spontaneous depolarisations (Fig. 1Ab). Changes in both membrane potential and tension were abolished by nifedipine (10 μM; n = 8), suggesting that spontaneous depolarisations were initiated by the opening of L-type Ca2+ channels and the Ca2+ influx triggered contractions.
In contrast, isolated sac preparation smooth muscle cells had stable membrane potentials ranging between -45 mV and -64 mV (Figs 1Ba and 2B, mean -51.4 ± 5.6 mV, n = 20). When isometric tension recording was made simultaneously, isolated sac smooth muscle preparations did not develop spontaneous contractions but contracted in response to transmural stimuli (Fig. 1Bb).
Electrical coupling between smooth muscle cells of the parenchyma or sac
In five parenchyma preparations, electrical coupling between the smooth muscle cells was examined using the two electrodes. Initially both microelectrodes, which were placed 60–250 μm apart, were used to record membrane potential. In all preparations studied, identical spontaneous depolarisations were simultaneously recorded with the two electrodes (Fig. 2A). One electrode was then switched to pass current and membrane potential changes were recorded by the other electrode. When hyperpolarising current pulses with amplitudes of 1–5 nA were injected into one cell, membrane hyperpolarisations with amplitudes of 2–12 mV were recorded from the second cell, suggesting that there is a high degree of electrical coupling between the cells in the parenchyma (Fig. 2A). The apparent input resistance in the parenchyma smooth muscle was estimated from the slope of the relationship between the amplitude of injected currents and the amplitude of induced hyperpolarisations with separation of two electrodes between 60 and 250 μm. This was found to be 3.7 ± 1.8 MΩ (n = 5).
In the same series of experiments, the effect of 18β-glycyrrhetinic acid (18β-GA), a gap junction uncoupler (Yamamoto et al. 1998), was studied in the presence of nifedipine (10 μM). 18β-GA (40 μM) reduced the amplitude of electrotonic potentials (to 24.1 ± 6.3 % of control, n = 3), indicating the electrical coupling between smooth muscle cells in the parenchyma was developed through gap junction channels which were sensitive to 18β-GA.
In seven sac preparations, electrical coupling of the smooth muscle cells was examined using the same technique. In all preparations studied, electrical coupling was better in the axial direction (along the axis of the muscle bundle) than the transverse direction (perpendicular to the axis of the muscle bundle) as reported in intestinal and bladder smooth muscle (Cousins et al. 1993; Bramich & Brading, 1996). When two electrodes were placed in the axial direction at a distance of 200 μm apart, hyperpolarising current of 1–5 nA, 1 s duration, induced membrane potential changes of approximately 5–20 mV (Fig. 2B). When two electrodes were placed in the transverse direction at a distance of 60 μm apart, the same hyperpolarising current induced membrane potential changes of 1–5 mV (not shown). Thus the apparent input resistance in sac smooth muscle was 3.6 ± 1.3 MΩ in the axial direction (n = 7) and 0.8 ± 0.1 MΩ (n = 4) in the transverse direction with separation of the two electrodes of 60–250 μm.
In the same series of experiments, the effect of 18β-GA (40 μM), was also investigated. 18β-GA reduced the amplitudes of electrotonic potentials (to 24.5 ± 6.0 % of control, n = 4, not shown), suggesting that gap junctions, similar to those in parenchyma, existed in the sac.
Membrane potential changes evoked by transmural nerve stimulation in the parenchyma
Transmural nerve stimulation initiated transient hyperpolarisations in isolated parenchyma preparations (Fig. 3Aa and Ca). In preparations which exhibited spontaneous action potentials, transmural nerve stimulation inhibited the generation of spontaneous action potentials either with or without detectable hyperpolarisations (Fig. 3Ca). The hyperpolarisations were abolished by tetrodotoxin (1 μM; n = 5, not shown), indicating that they were inhibitory junction potentials (IJPs). IJPs initiated by single impulses had latencies ranging between 210 and 510 ms (mean 349 ± 85 ms, n = 19). As the number of stimuli was increased, the peak amplitude of IJPs increased; the mean amplitude of IJPs was 3.7 ± 1.7 mV (1 impulse, n = 19), 6.8 ± 2.8 mV (3 impulses, n = 32) and 7.1 ± 4.2 mV (5 impulses, n = 27). Increasing the number of stimuli, increased the half-width of IJPs; the mean half-width of IJPs was 1.5 ± 1.5 s (1 impulse), 2.0 ± 1.4 s (3 impulses) and 2.5 ± 1.3 s (5 impulses). The rise times of IJPs were not significantly different: 0.5 ± 0.2 s (1 impulse), 0.5 ± 0.2 s (3 impulses) and 0.4 ± 0.1 s (5 impulses).
Figure 3. Effects of LNA and ODQ on IJPs and relaxations recorded from the parenchyma of the rat penile bulb.
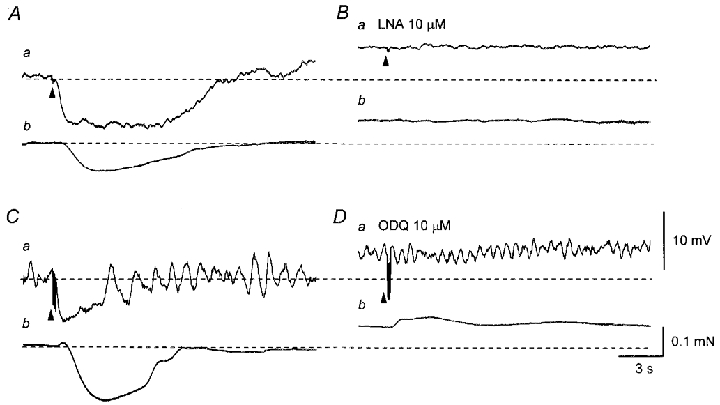
A, in the presence of phenylephrine (3 μM), three impulses delivered at 20 Hz initiated an IJP (a) and an associated relaxation (b). B, LNA (10 μM) depolarised the membrane and abolished the IJP (a). LNA caused a contraction and also abolished the nerve-evoked relaxation (b). C, in another preparation, five impulses delivered at 20 Hz evoked an IJP (a) and an associated relaxation (b). D, ODQ (10 μM) depolarised the membrane and abolished the IJP (a). ODQ caused a contraction and also abolished nerve-evoked relaxation (b). A and B were recorded from one preparation, and C and D from another preparation. Resting membrane potentials were -35 mV in A and B and -37 mV in C and D. Scale bars in D apply also to all corresponding traces in A–C. Here and in subsequent figures arrowheads indicate the time of nerve stimulation.
IJPs persisted in the presence of either guanethidine or hyoscine (not shown), indicating that IJPs were predominantly non-adrenergic, non-cholinergic (NANC) nerve-mediated responses, although guanethidine and hyoscine modified either the time course or the amplitude of IJPs. Guanethidine (10 μM) increased the half-width of IJPs (to 187 ± 77 % of control, n = 8) without significantly changing their amplitude and rise time. Hyoscine (1 μM) decreased the amplitude of IJPs (to 69 ± 6.1 % of control, n = 6) without affecting their time course. Nifedipine (10 μM) also reduced the amplitude of IJPs (71.4 ± 14.2 % of control, n = 6) with little change in their time course.
When isometric tension was recorded at the same time as membrane potential, transmural nerve stimulation evoked a relaxation, which was preceded by an initial small contraction in most preparations studied (Fig. 3Cb). When recordings were made in the presence of phenylephrine (3 μM), an α-adrenergic agonist, which increased basal tension, transmural nerve stimulation invariably caused a large relaxation (Fig. 3Ab). The relaxations had similar onsets to those of IJPs but often had more prolonged time courses than those of IJPs (Fig. 3C).
Properties of inhibitory junction potentials in the parenchyma
LNA (10 μM) depolarised the membrane (5.2 ± 2.9 mV, n = 10) and abolished IJPs, suggesting IJPs were initiated by NO released from NANC nerves (Fig. 3Aa and Ba). When isometric tension recording was simultaneously made, LNA caused a sustained contraction and abolished nerve-evoked relaxations (Fig. 3Bb). ODQ (10 μM), an inhibitor of guanylate cyclase, depolarised the membranes (5 ± 1.8 mV, n = 5) and abolished IJPs (Fig. 3Da). ODQ also caused a sustained contraction and abolished nerve-evoked relaxations (Fig. 3Db), suggesting that NO-sensitive guanylate cyclase was involved in the generation of IJPs.
In other types of smooth muscle in which IJPs have been recorded, IJPs result from the opening of potassium channels (Bywater & Taylor, 1986; Cayabyab & Daniel, 1995; Hashitani et al. 1998) and therefore the effects of a range of potassium channel blockers on IJPs were studied. IJPs persisted in the presence of charybdotoxin (CTX, 50 nM); the amplitude of IJPs was 103.6 ± 4.6 % of control values in CTX (n = 4). In the presence of apamin (0.1 μM) the amplitude of IJPs was 106.5 ± 5.9 % of control values (n = 3), and in a combination of CTX (50 nM) and apamin (0.1 μM) the amplitude of IJPs was 105.1 ± 4.8 % of control values (n = 4). In the presence of glibenclamide (1 μM) the amplitude of IJPs was 109.3 ± 8.0 % of control values (n = 3), and in 4-aminopyridine (4-AP, 1 mM) the amplitude of IJPs was 98.4 ± 5.1 % of control (n = 3). Finally in the presence of Ba2+ (0.1 mM) the amplitude of IJPs was 94.9 ± 8.8 % of control (n = 4).
If potassium channels which were insensitive to the potassium channel blockers contributed to the generation of IJPs the amplitude of IJPs would be expected to increase when [K+]o was reduced. The amplitude of IJPs was, however, reduced when [K+]o was reduced; in five preparations, the IJP amplitude was 6.9 ± 1.0 mV in control solution (5 mM ), 1.8 ± 0.6 mV in 0.5 mM , 4.2 ± 0.8 mV in 2.5 mM and 7.1 ± 1.2 mV in 10 mM (Fig. 4A). The membrane potential was depolarised by 6.4 ± 1.1 mV in 0.5 mM , 2.4 ± 0.8 mV in 2.5 mM and 5.2 ± 1.1 mV in 10 mM . The relationship between [K+]o and the amplitude of the IJPs is summarised in Fig. 4B.
Figure 4. Relationship between [K+]o and the amplitude of IJPs recorded from parenchyma of the rat penile bulb.
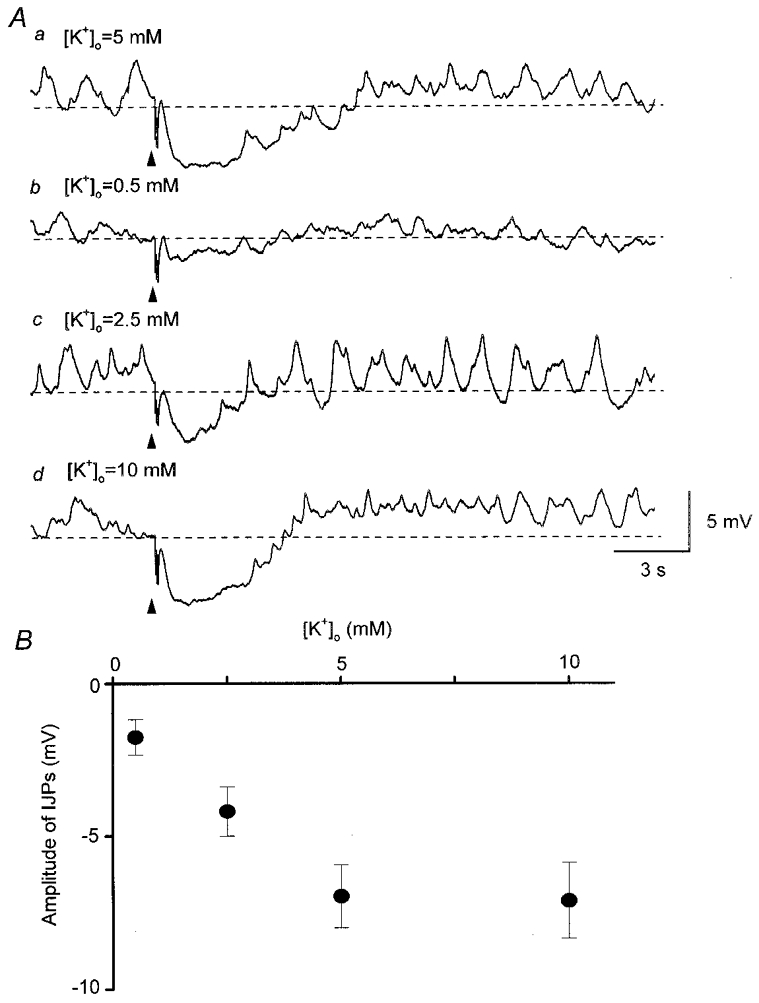
Five impulses delivered at 20 Hz initiated an IJP in control solution containing 5 mM (Aa). In solution containing 0.5 mM K+, the same stimulation initiated a small IJP (Ab). In solution containing 2.5 mM K+, the same stimulation initiated an IJP with a reduced amplitude (Ac). In solution containing 10 mM K+, the same stimulation initiated an IJP which had a similar amplitude to that of control IJPs (Ad). B, relationship between [K+]o and amplitude of IJPs. Symbols and bars represent means and standard deviations. All traces in A were recorded from the same cell. Resting membrane potential was -33 mV. Scale bars in Ad apply to all traces.
Effects of the inhibition of sodium pump activity on IJPs
Sodium pump activity has been shown to be suppressed in a range of tissues when [K+]o is reduced (Gadsby, 1980). The possibility that IJPs resulted from activation of the sodium pump was considered. Ouabain (0.1 mM) depolarised the membrane (6 ± 3.6 mV, n = 9) and reduced the amplitude of IJPs (to 24.9 ± 9.6 % of control, n = 9, Fig. 5Aa and b). Dinitrophenol (0.1 mM) which inhibits oxidative phosphorylation, hyperpolarised the membrane (4.4 ± 3.8 mV, n = 5) and reduced the amplitude of IJPs (to 28.2 ± 8.7 % of control, n = 5, Fig. 5Ba and b).
Figure 5. Comparison of the effects of ouabain, dinitrophenol and decreasing [K+]o on IJPs and nerve-evoked relaxations recorded from parenchyma of the rat penile bulb.
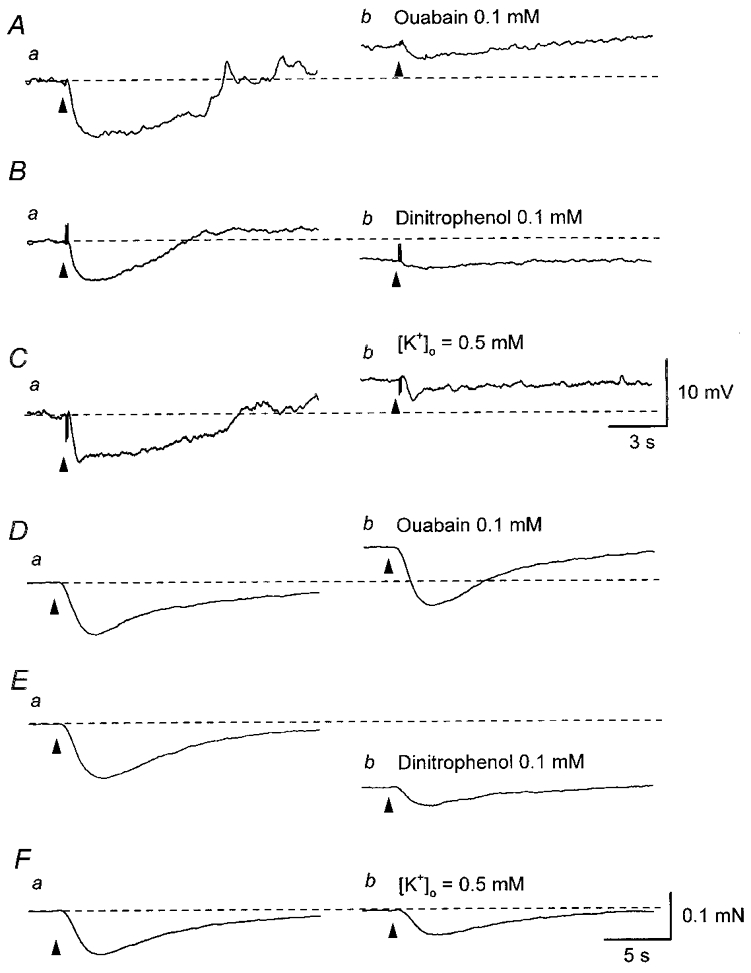
Three impulses delivered at 20 Hz initiated an IJP in parenchyma (Aa). Ouabain (0.1 mM) depolarised the membrane and reduced the amplitude of the IJP (Ab). In another parenchyma preparation, dinitrophenol (0.1 mM) hyperpolarised the membrane and reduced the amplitude of the IJP initiated by two impulses delivered at 20 Hz (Ba and b). In the other parenchyma preparation, decreasing [K+]o to 0.5 mM depolarised the membrane and reduced the amplitude of IJPs initiated by two impulses delivered at 20 Hz (Ca and b). In a separate series of experiments, five impulses delivered at 20 Hz invariably caused relaxations (Da, Ea and Fa). Ouabain (0.1 mM) caused a contraction but did not reduce the amplitude of the nerve-evoked relaxation (Db). Dinitrophenol (0.1 mM) caused a relaxation and reduced the amplitude of the nerve-evoked relaxation (Eb). Reducing [K+]o to 0.5 mM did not change the basal tension but reduced the amplitude of the nerve-evoked relaxation (Fb). Resting membrane potentials were -38 mV in A, -36 mV in B and -40 mV in C. D–F were recorded from the same preparation. Scale bars in C refer to all traces in A–C. Scale bars in F refer to all traces in D–F.
To examine the contribution of IJPs to nerve-evoked relaxations, the effect of ouabain, dinitrophenol and decreasing [K+]o on nerve-evoked relaxations were separately tested in preparations precontracted with phenylephrine (3 μM). Phenylephrine (3 μM) caused a sustained contraction which was associated with a small membrane depolarisation (3.6 ± 1.7 mV, n = 5). In the presence of phenylephrine (3 μM), transmural stimulation invariably initiated large relaxations. Ouabain (0.1 mM) caused a further increase in tension and did not alter the amplitude of the relaxations (97.9 ± 4.4 % of control values, n = 3, Fig. 5Da and b). Dinitrophenol (0.1 mM) partially relaxed the preparations and reduced the amplitude of nerve-evoked relaxations (54.5 ± 4.9 % of control values, n = 3, Fig. 5Ea and b). Changing the physiological saline to one containing 0.5 mM K+ did not change basal tension but reduced the amplitude of nerve-evoked relaxations (64.3 ± 6.5 % of control values, n = 4, Fig. 5Fa and b). Together the results indicate that nerve-evoked relaxations persist after IJPs have been much reduced in amplitude and that an additional pathway contributes to mechanical inhibition.
Excitatory transmission in the parenchyma
When isometric tension was recorded simultaneously with membrane potential recordings, nerve-evoked relaxations were always accompanied by IJPs (Fig. 6A). After the inhibitory responses had been abolished by LNA (10 μM), transmural stimulation triggered contractions. These responses were never associated with a detectable change in membrane potential (Fig. 6B). The subsequent application of phentolamine (10 μM; n = 5) abolished the contractions (Fig. 6C). In separate preparations exposed to LNA (10 μM), nerve-evoked contractions were inhibited by either CPA (10 μM; the amplitude of contractions was reduced to 16.1 ± 11.7 % of control, n = 3) or caffeine (3 mM; the amplitude of contractions was reduced to 10.3 ± 5.2 % of control, n = 3). These results indicate that neurally released noradrenaline stimulates α-adrenoceptors located on the parenchyma smooth muscle to initiate contractions without changing membrane potentials, presumably through Ca2+ release from intracellular Ca2+ stores.
Figure 6. Difference in excitatory responses produced by transmural nerve stimulation between the parenchyma and sac of the rat penile bulb.
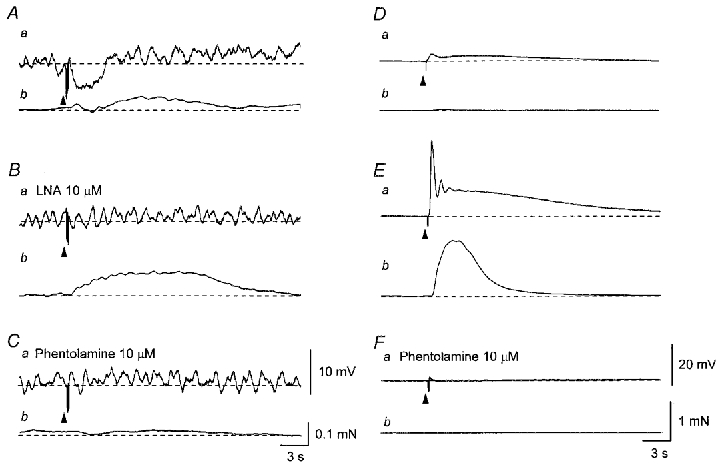
In a parenchyma preparation, five impulses delivered at 20 Hz initiated an IJP (Aa) and a contraction which was interrupted by a relaxation (Ab). In the presence of LNA (10 μM) five impulses delivered at 20 Hz triggered an enhanced contraction without detectable membrane potential changes (Ba and b). Subsequent addition of phentolamine (10 μM) abolished the contraction (Ca and b). In the sac, a single impulse initiated an initial depolarisation which was followed by a slow depolarisation (Da) and also produced a tiny contraction (Db). Three impulses delivered at 20 Hz initiated an initial depolarisation which trigged an action potential and a slow depolarisation (Ea). The action potential caused a large contraction (Eb). Phentolamine (10 μM) abolished both the depolarisation and contraction (Fa and b). Traces in A–C and D–F were recorded from two different preparations. Resting membrane potentials were -36 mV in A–C and -56 mV in D–F. Scale bars in C also refer to corresponding traces in A and B. Scale bars in F also refer to corresponding traces in D and E.
Changes in membrane potential and tension evoked by transmural nerve stimulation in the sac
In contrast to the parenchyma, transmural nerve stimulation with single impulses initiated transient depolarisations which were followed by slow depolarisations in the sac (Fig. 6Da). The initial depolarisations often triggered action potentials and caused associated contractions (Fig. 7Da and b). If the initial depolarisations failed to trigger an action potential, they caused either no or a small contraction (Fig. 6Da and b). On some occasions, even a single impulse triggered several action potentials during the secondary slow depolarisations and this was associated with an oscillatory contraction (not shown). When trains of impulses were applied, the initial depolarisations invariably triggered action potentials and multiple action potentials were usually observed on slow depolarisations (Figs 6Ea and 7Ca). Again this sequence of membrane potential changes was accompanied by a large oscillatory contraction (Figs 6Eb and 7Cb). Nifedipine (10 μM) abolished the action potentials leaving a depolarisation or excitatory junction potential (EJP) which consisted of two components (Fig. 7Ba and Da). Nifedipine also reduced the amplitude of the associated contractions (Fig. 7Bb and Db). All residual membrane potential changes were abolished by tetrodotoxin (1 μM; n = 5), indicating that the responses resulted from nerve stimulation. EJPs, initiated by single impulses, had latencies ranging between 185 and 365 ms (mean 248 ± 60 ms, n = 15). As the number of stimuli was increased, the peak amplitude of the initial component increased more dramatically than that of the slow component. The mean amplitude of the initial, fast, component was 5.9 ± 2.6 mV (1 impulse, n = 15) and 18.3 ± 7.2 mV (3 impulses, n = 15), the mean amplitude of the secondary, slow, component was 4.3 ± 1.8 mV (1 impulse) and 7.8 ± 4.3 mV (3 impulses). Increasing the number of stimuli shortened the rise times of EJPs (mean 0.41 ± 0.15 s for 1 impulse, 0.25 ± 0.13 s for 3 impulses) but the half-width of EJPs did not change obviously (mean 12.8 ± 2.3 s for 1 impulse, 13.7 ± 2.7 s for 3 impulses).
Figure 7. Effects of nifedipine on responses produced by transmural nerve stimulation in the sac of the rat penile bulb.
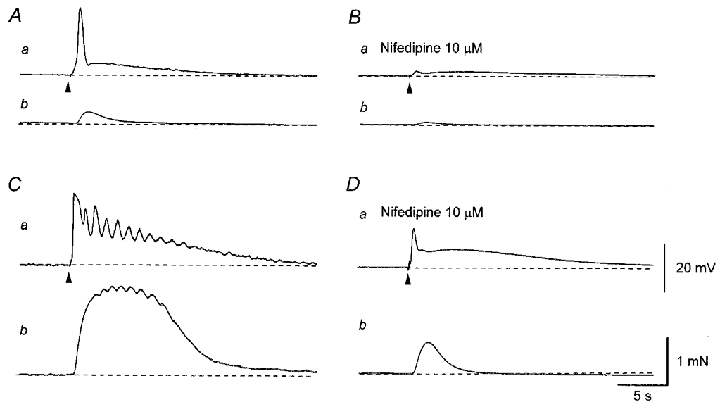
A single impulse evoked an initial depolarisation which triggered an action potential and a slow depolarisation (Aa). The action potential caused a small contraction (Ab). Nifedipine (10 μM) abolished an action potential leaving an EJP, which consisted of two components (Ba). Nifedipine also inhibited the nerve-evoked contraction (Bb). Three impulses delivered at 20 Hz initiated a larger depolarisation and triggered multiple action potentials (Ca) and an associated larger, oscillatory contraction (Cb). Nifedipine (10 μM) abolished action potentials leaving two components of the EJP (Da). Nifedipine also inhibited the nerve-evoked contraction (Db). All traces were recorded from the same cell. Resting membrane potential was -52 mV. Scale bars in D refer to corresponding traces in A–C.
EJPs were abolished by phentolamine (10 μM; Fig. 6Fa, n = 6) or guanethidine (10 μM; n = 5) but not hyoscine (1 μM; n = 5), suggesting that they were initiated through the activation of α-adrenoceptors by neurally released noradrenaline. Phentolamine (10 μM) also abolished associated contractions (Fig. 6Fb). Bath-applied phenylephrine (3 μM) depolarised the membrane and triggered large oscillatory membrane potential changes on the rising phase which were very similar to those induced by neural activation of α-adrenoceptors (not shown). The mean amplitude of the phenylephrine-induced depolarisations was 24 ± 4 mV (n = 5) which was associated with smaller oscillations in membrane potential. In preparations in which transmural stimulation initiated EJPs and triggered action potentials, the same stimulation failed to initiate any membrane potential changes in the presence of phenylephrine (3 μM), suggesting the sac smooth muscle did not receive an inhibitory innervation of NO-containing nerves or did not respond to NO.
Role of intracellular Ca2+ stores in the generation of excitatory junction potentials
The following experiments were performed in the presence of nifedipine (10 μM). To investigate the possible contribution of the intracellular Ca2+ stores to the generation of EJPs, the effects of caffeine on EJPs were studied. Caffeine (3 mM) depolarised the membrane (12.1 ± 3.2 mV, n = 10, Fig. 8Aa and Ba) and reduced the amplitude of both components of EJPs (fast: 15.6 ± 4.6 % of control, slow: 31.2 ± 4.2 % of control, n = 10). When isometric tension recording was simultaneously made, caffeine (3 mM) initiated a transient contraction which was converted into a small relaxation during application of caffeine. Caffeine (3 mM) also inhibited nerve-evoked contractions (Fig. 8Ab and Bb), suggesting this concentration of caffeine was sufficient to stimulate Ca2+ release from intracellular Ca2+ stores.
Figure 8. Effects of caffeine, ryanodine and CPA on EJPs and contractions recorded from the sac of the rat penile bulb.
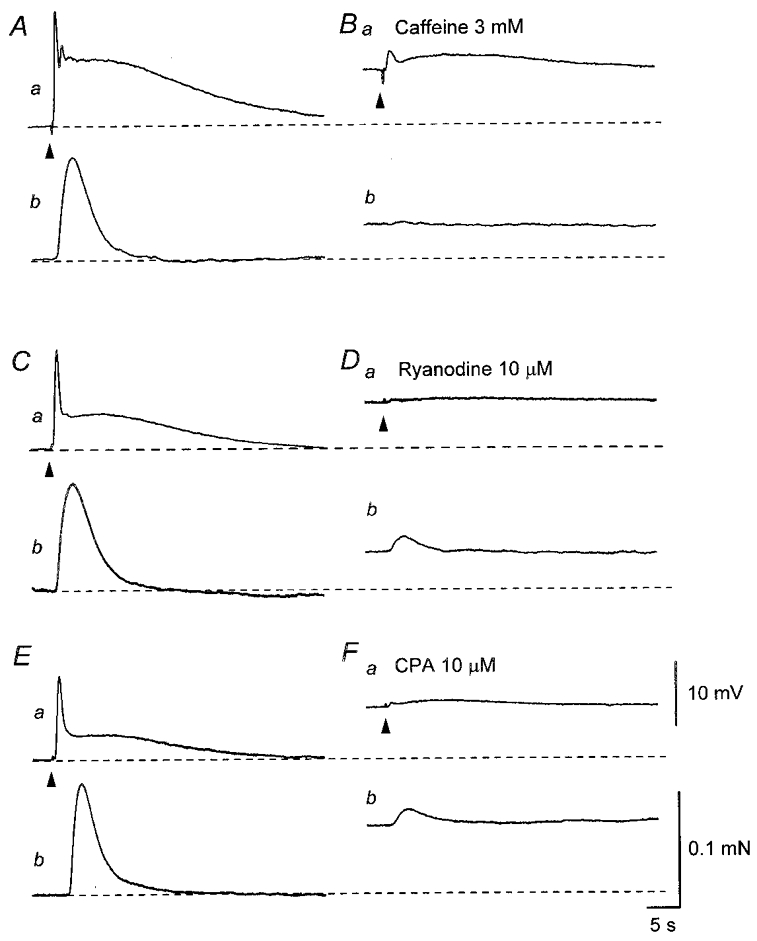
In the presence of nifedipine (10 μM), three impulses delivered at 20 Hz initiated an EJP which consisted of two components and an associated contraction (Aa and b). Caffeine (3 mM) depolarised the membrane and reduced the amplitude of the EJP (Ba). Caffeine (3 mM) caused a transient contraction and also reduced the amplitude of the nerve-evoked contraction (Bb). In a different preparation exposed to nifedipine (10 μM), three impulses delivered at 20 Hz initiated an EJP, which consisted of two components and an associated contraction (Ca and b). Ryanodine (10 μM) depolarised the membrane and reduced the amplitude of the EJP (Da). Ryanodine caused a sustained contraction and also inhibited the nerve-evoked contraction (Db). In another preparation, three impulses initiated an EJP, which consisted of two components and an associated contraction (Ea and b). CPA (10 μM) depolarised the membrane and reduced the amplitude of the EJP (Fa). CPA caused a sustained contraction and also inhibited the nerve-evoked contraction (Fb). A and B,C and D, and E and F were recorded from three different preparations. Resting membrane potentials were -53 mV in A and B, -46 mV in C and D and -50 mV in E and F. Scale bars in F refer to corresponding traces in A–E.
To investigate further the role of intracellular Ca2+ stores in the initiation of EJPs, the effects of ryanodine and CPA were studied. Ryanodine (10 μM) induced a slowly developed sustained depolarisation of the membrane (19.3 ± 4.3 mV, n = 3) and reduced the amplitudes of both components of EJPs (fast: 6.7 ± 2.6 % of control; slow: 20.2 ± 5.9 % of control, n = 3, Fig. 8Ca and Da). Ryanodine initiated sustained contractions and also inhibited nerve-evoked contractions (Fig. 8Cb and Db), suggesting ryanodine stimulated Ca2+ release from intracellular Ca2+ stores and eventually depleted the stores.
CPA (10 μM) depolarised the membrane (14.3 ± 4.3 mV, n = 8) and reduced the amplitudes of both components of EJPs (fast, 6.6 ± 3.9 % of control; slow, 18.2 ± 8.1 % of control, n = 8, Fig. 8Ea and Fa). CPA (10 μM) caused sustained contractions and also inhibited nerve-evoked contractions, suggesting that CPA increased [Ca2+]i by inhibiting Ca2+ uptake into the intracellular Ca2+ stores and eventually depleted the stores (Fig. 8Eb and Fb).
Identification of ion channels which contribute to the generation of EJPs
To examine the involvement of a chloride conductance in the generation of EJPs, the effect of lowering extracellular chloride concentration ([Cl−]o) was studied. Switching the physiological saline to a solution containing a reduced chloride concentration hyperpolarised the membrane (3.4 ± 1.5 mV, n = 4) and selectively inhibited the initial components of EJPs (24.5 ± 9.6 % of control) without inhibiting the slow components of EJPs (84.8 ± 7.2 % of control, Fig. 9Aa and Ba). Low chloride solution caused some contractions and also inhibited nerve-evoked contractions (Fig. 9Ab and Bb).
Figure 9. Effects of low chloride solution and niflumic acid on EJPs and contractions recorded from the sac of the rat penile bulb.
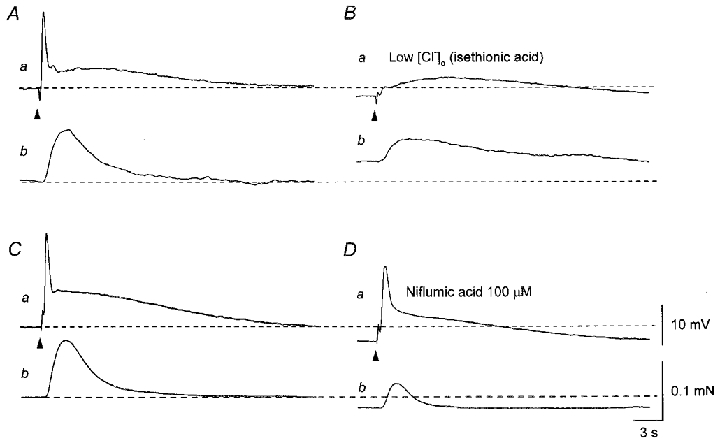
In the presence of nifedipine (10 μM), three impulses delivered at 20 Hz initiated an EJP which consisted of two components and an associated contraction (Aa and b). Low chloride solution hyperpolarised the membrane and selectively inhibited the amplitude of the rapid component of the EJP without inhibiting the slow component (Ba). Low chloride solution caused a contraction and also inhibited the nerve-evoked contraction (Bb). In a different preparation exposed to nifedipine, three impulses delivered at 20 Hz initiated an EJP which consisted of two components and an associated contraction (Ca and b). Niflumic acid (100 μM) hyperpolarised the membrane and reduced the amplitude of the EJP (Da). Niflumic acid (100 μM) caused relaxation and also inhibited the nerve-evoked contraction (Db). A and B, and C and D were recorded from two different cells. Resting membrane potentials were -47 mV in A and B and -54 mV in C and D. Scale bars in D refer to corresponding traces in A–C.
To further investigate the contribution of Ca2+-activated chloride channels to the generation of EJPs, the effects of niflumic acid, a blocker of Ca2+-activated chloride channels (Large & Wang, 1996), were studied. Niflumic acid (100 μM) caused hyperpolarisations of the membrane (7.5 ± 2.8 mV, Fig. 9Ca and Da) and inhibited both components of EJPs (fast to 65.1 ± 9.7 % of control, slow to 72.2 ± 7.3 % of control, n = 5, Fig. 9Ca). Thus, the effect of niflumic acid on the rapid component was less than that of low chloride solution. When isometric tension recordings were simultaneously made, niflumic acid (100 μM) caused relaxation and also reduced the amplitude of nerve-evoked contractions (Fig. 9Cb and Db), suggesting that niflumic acid (100 μM) may inhibit Ca2+ release from intracellular stores.
DISCUSSION
In the rat penile bulb two distinct layers of smooth muscle were identified. The parenchyma smooth muscle layer, an inner sponge-like meshwork of cells, exhibited spontaneous action potentials and associated contractions, while the sac, the outer sheet of muscle, was electrically and mechanically quiescent. The parenchyma layer received both a NANC inhibitory innervation and an excitatory innervation mediated by α-adrenoceptors. The NANC inhibitory nerves release NO and appeared to initiate IJPs by stimulating sodium pump activity following the activation of guanylate cyclase. The stimulation of α-adrenoceptors contracted parenchyma smooth muscle without an associated change in membrane potential. As well as being quiescent, the sac failed to receive an inhibitory innervation. However, it received an adrenergic excitatory innervation that stimulated Ca2+ release from intracellular Ca2+ stores and the increase in [Ca2+]i subsequently activated Ca2+-activated channels to give rise to an EJP.
Spontaneous contractions have been recorded from corporeal preparations obtained from various mammals, including human, but the electrophysiological basis for them is unclear (Andersson & Wagner, 1995). In the bovine retractor penis muscle, Samuelson (1983) demonstrated a correlation between electrical waves and contractions using sucrose gap recordings. In the dog corpus spongiosum smooth muscle, slow membrane potential changes recorded using intracellular microelectrodes were suggested to trigger spontaneous contractions (Kimoto & Ito, 1988). In the present study, spontaneous action potentials and associated contractions were recorded simultaneously. Each action potential triggered a small contraction and a burst of action potentials initiated a larger contraction. Spontaneous action potentials were abolished by nifedipine but not by tetrodotoxin, phentolamine, guanethidine or hyoscine, indicating that they result from the myogenic activation of L-type Ca2+ channels. As parenchyma smooth muscle cells were well coupled to their neighbouring cells via gap junctions, which are sensitive to 18 β-GA, myogenic activity could readily propagate to nearby cells and maintain synchronous contraction of the parenchyma during the flaccid state of the penis. Smooth muscle cells in the sac were well coupled to their neighbours through similar types of gap junctions to those distributed in the parenchyma. In sac smooth muscle, the high degree of electrical coupling may contribute to the spread of electrical signals during the excitation of adrenergic nerves thus synchronising contraction. This synchronisation may relate to the physiological function of the sac, i.e. ejaculation and terminating erection.
IJPs, evoked in the parenchyma, were mediated by NO released from NANC nerves. In most other smooth muscle preparations IJPs result from the opening of K+ channels regardless of the transmitter released (Bywater & Taylor, 1986; Cayabyab & Daniel, 1995; Bridgewater et al. 1995; Hashitani et al. 1998). Furthermore NO or NO donors open a variety of types of K+ channels in many smooth muscle preparations (Murphy & Brayden, 1995; Koh et al. 1995; Hashitani et al. 1998). However, IJPs in the parenchyma were not sensitive to a variety of K+ channel blockers. Moreover the amplitudes of IJPs were reduced when [K+]o was reduced and the relationship between [K+]o and IJPs amplitude (Fig. 4) was similar to that observed for [K+]o and the amplitude of sodium pump current (a half-activation [K+]o= 1 mM, Gadsby, 1980). Furthermore both ouabain and dinitrophenol, an inhibitor for oxidative phosphorylation, reduced the amplitudes of IJPs. The simplest explanation for these findings is that, unlike IJPs recorded from many other smooth muscles, the activation of a sodium pump underlies the IJPs recorded from parenchyma. However, the possibility cannot be ruled out that the inhibitory effects of chemicals which inhibit the sodium pump on IJPs are due to the inhibition of transmitter release.
IJPs were abolished by LNA and by ODQ, indicating that NO followed by guanylate cyclase stimulation was involved in the activation of sodium pump. In other tissues, including human corpus cavernosum, NO-induced stimulation of sodium pump activity has been reported (Gupta et al. 1995; Yaktubay et al. 1999). Clearly inhibition included other pathways than that resulting in the initiation of an IJP. Transmural stimulation often inhibited the generation of spontaneous action potentials without detectable hyperpolarisations, indicating that NO could directly inhibit L-type Ca2+ channels perhaps through the activation of a cyclic GMP pathway (Ishikawa et al. 1993). Moreover much of the mechanical inhibition persisted after the amplitude of IJPs had been dramatically reduced (Fig. 5).
The excitatory innervation in the parenchyma was predominantly adrenergic with the activation of α-adrenoceptors initiating contractions. Unlike other smooth muscle preparations described to date, the activation of α-adrenergic receptors in parenchyma by neurally released transmitter did not cause an obvious depolarisation. Contractions mediated by α-adrenoceptors in the parenchyma were greatly inhibited by either caffeine or CPA, suggesting that the stimulation of α-adrenoceptors by neurally released noradrenaline contracts parenchyma smooth muscle mainly by Ca2+ release from intracellular stores probably through InsP3 production (Andersson & Wagner, 1995). If this is the case, parenchyma smooth muscle cells may well lack Ca2+-activated channels of any description. Alternatively, Ca2+ released from intracellular stores may not have access to such channels.
In contrast to the parenchyma, transmural nerve stimulation initiated α-adrenergic EJPs which are composed of two components in the sac; the initial, rapid component and the second slow component which lasted for over 10 s. Similar sequences of membrane potential change occur following sympathetic nerve stimulation in the rat anococcygeus muscle (Bramich & Hirst, 1999). EJPs often triggered action potentials and caused oscillatory contractions. Nerve-evoked contractions were strongly inhibited by nifedipine, suggesting that α-adrenergic contractions in the sac largely depend on Ca2+ influx through L-type Ca2+ channels, although Ca2+-induced Ca2+ release (CICR) mechanism may amplify entered Ca2+.
In the presence of nifedipine, the rapid, but not the slow, component of EJPs was associated with contractions. Furthermore, caffeine, ryanodine and CPA more dramatically reduced the amplitudes of the rapid component than those of the slow components. These results suggested that Ca2+ release from intracellular Ca2+ stores contributes to the generation of the rapid component. It is difficult to determine whether or not the slow component is sensitive to Ca2+ release from intracellular stores. The amplitude of the slow component was apparently reduced by caffeine, ryanodine and CPA; however, these chemicals caused large depolarisations which often exceeded the peak membrane potential of the slow components.
In sac smooth muscle, the initial components of EJPs were obviously Ca2+ dependent as in the case of EJPs in the rat anococcygeus and mesenteric veins (Van Helden, 1988a, 1988b; Bramich & Hirst, 1999). In the sac, reducing the extracellular chloride ion concentration selectively inhibited the rapid components of EJPs, suggesting the involvement of Ca2+-activated chloride channels in the generation of the rapid components of EJPs. However, niflumic acid, a blocker of Ca2+-activated chloride channels (Large & Wang, 1996), had much weaker effects. Niflumic acid has been shown to produce marked inhibition of Ca2+-activated chloride currents in various isolated smooth muscle cells (Large & Wang, 1996) and has also been reported to abolish spontaneous depolarisations which are thought to be initiated by the opening of Ca2+-activated chloride channels in intact urethral smooth muscle preparations of rabbit and guinea-pig (Hashitani et al. 1996; Hashitani & Edwards, 1999). Therefore, if the rapid component of EJPs results from the opening of Ca2+-activated chloride channels, niflumic acid (100 μM) should have inhibited the components more greatly than it did. It is possible that some chloride channels other than Ca2+-activated chloride channels contribute to the rapid components. However, this seems unlikely because the rapid components were obviously Ca2+ dependent. Alternatively, niflumic acid did not block this particular type of Ca2+-activated chloride channel present in the sac.
Low chloride solution and niflumic acid may indirectly inhibit the rapid components of EJPs through the inhibition of Ca2+ release from intracellular stores; as both procedures inhibited nerve-evoked contractions. Both low chloride solution and niflumic acid have been reported to inhibit noradrenaline-induced contraction in the rat aortic smooth muscle (Lamb & Barna, 1998). Isethionic acid has also been reported to inhibit Ca2+ uptake into intracellular stores and so selectively inhibit the initial phasic contractions induced by muscarinic agonist in the guinea-pig ileal smooth muscle (Rangachari & Triggle, 1986). Niflumic acid has been reported not to inhibit Ca2+ uptake in smooth muscle (Pollock et al. 1998) but has been shown to inhibit Ca2+ release from intracellular Ca2+ stores in skeletal muscle (Oba et al. 1996).
In other smooth muscle, the activation of α-adrenergic receptors have been reported to open both Ca2+-activated Cl− channels and Ca2+-activated cation channels (Loirand et al. 1991; Large & Wang, 1996). In the sac, as both components of EJPs persisted in the presence of niflumic acid, they could result from the opening of Ca2+-activated cation channels rather than Ca2+-activated Cl− channels. However, in the presence of nifedipine, the rapid, but not the slow, components triggered contractions. This would imply that Ca2+ released during the first, but not the second, component could access the contractile protein, implying there are two separate internal Ca2+ stores. Alternatively, different types of channel may contribute to the initial and slow components of EJPs. Noradrenaline-activated cation channels, which were insensitive to caffeine, have been reported in rabbit portal vein (Wang & Large, 1991). These channels were also reported to be activated by a diacylglycerol analogue but not by inositol trisphosphate (Helliwell & Large, 1997). Therefore, it may be that the rapid component of EJPs results from the activation of Ca2+-activated cation channels, whereas the slow component results from the opening of Ca2+-independent cation channels. A third possibility is that the slow component results from a closing of potassium channels as was suggested in the guinea-pig mesenteric vein (Suzuki, 1981; Van Helden, 1988b).
In conclusion, the penile bulb of the rat is composed of two layers of smooth muscle which exhibit different patterns of electrical activity. Parenchyma smooth muscle cells generated spontaneous action potentials which appeared to account for the maintenance of the resting tone. IJPs in parenchyma resulted from the release of NO from NANC nerves and the apparent activation of a sodium pump following stimulation of guanylate cyclase. Neurally released noradrenaline activated α-adrenoceptors and contracted parenchyma smooth muscle without changing the membrane potential, presumably due to the release of Ca2+ from intracellular stores. The sac was electrically quiescent at rest and generated EJPs mediated by α-adrenoceptors in response to transmural stimulation. The rapid component of EJPs probably results from Ca2+ release from intracellular Ca2+ stores and subsequent activation of Ca2+-activated channels. The slow components appeared to be initiated by Ca2+-independent mechanisms.
Acknowledgments
The author wishes to thank Dr N. J. Bramich for her critical reading of the manuscript. The author is also grateful to Professor G. D. S. Hirst for his helpful comments on the manuscript. This project was supported by a research grant from the National Health and Medical Research Council of Australia.
References
- Anderson KE, Wagner G. Physiology of penile erection. Physiological Reviews. 1995;75:191–236. doi: 10.1152/physrev.1995.75.1.191. [DOI] [PubMed] [Google Scholar]
- Bramich NJ, Brading AF. Electrical properties of smooth muscle in the guinea-pig urinary bladder. The Journal of Physiology. 1996;492:185–198. doi: 10.1113/jphysiol.1996.sp021300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramich NJ, Hirst GDS. Sympathetic neuroeffector transmission in the rat anococcygeus muscle. The Journal of Physiology. 1999;516:101–115. doi: 10.1111/j.1469-7793.1999.101aa.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridgewater M, Cunnane TC, Brading AF. Characteristic features of inhibitory junction potentials evoked by single stimuli in the isolated guinea-pig taenia caeci. The Journal of Physiology. 1995;485:145–155. doi: 10.1113/jphysiol.1995.sp020719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bywater RA, Taylor GS. Non-cholinergic excitatory and inhibitory junction potentials in the circular smooth muscle of the guinea-pig ileum. The Journal of Physiology. 1986;374:153–164. doi: 10.1113/jphysiol.1986.sp016072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayabyab FS, Daniel EE. K+ channel opening mediates hyperpolarizations by nitric oxide donors and IJPs in opossum esophagus. American Journal of Physiology. 1995;268:G831–842. doi: 10.1152/ajpgi.1995.268.5.G831. [DOI] [PubMed] [Google Scholar]
- Christ GJ, Brink PR, Melman A, Spray DC. The role of gap junctions and ion channels in the modulation of electrical and chemical signals in human corpus cavernosum smooth muscle. International Journal of Impotence Research. 1993;5:77–96. [PubMed] [Google Scholar]
- Christ GJ, Moreno AP, Melman A, Spray DC. Gap junction-mediated intercellular diffusion of Ca2+ in cultured human corporal smooth muscle cells. American Journal of Physiology. 1992;263:C373–383. doi: 10.1152/ajpcell.1992.263.2.C373. [DOI] [PubMed] [Google Scholar]
- Cousins HM, Edwards FR, Hirst GDS, Wendt IR. Cholinergic neuromuscular transmission in the longitudinal muscle of the guinea-pig ileum. The Journal of Physiology. 1993;471:61–86. doi: 10.1113/jphysiol.1993.sp019891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fovaeus M, Andersson K-E, Hedlund H. Effects of some calcium channel blockers on isolated human penile erectile tissues. Journal of Urology. 1987;138:1267–1272. doi: 10.1016/s0022-5347(17)43582-8. [DOI] [PubMed] [Google Scholar]
- Gadsby DC. Activation of electrogenic Na+/K+ exchange by extracellular K+ in canine cardiac Purkinji fibers. Proceedings of the National Academy of Sciences of the USA. 1980;77:4035–4039. doi: 10.1073/pnas.77.7.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Moreland RB, Munarriz R, Daley J, Goldstein I, Saenz de Tejada I. Possible role of Na+,K+-ATPase in the regulation of human corpus cavernosum smooth muscle contractility by nitric oxide. British Journal of Pharmacology. 1995;116:2201–2206. doi: 10.1111/j.1476-5381.1995.tb15054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashitani H, Edwards FR. Spontaneous and neurally activated depolarizations in smooth muscle cells of the guinea-pig urethra. The Journal of Physiology. 1999;514:459–470. doi: 10.1111/j.1469-7793.1999.459ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashitani H, van Helden DF, Suzuki H. Properties of spontaneous depolarizations in circular smooth muscle cells of rabbit urethra. British Journal of Pharmacology. 1996;118:1627–1632. doi: 10.1111/j.1476-5381.1996.tb15584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashitani H, Windle A, Suzuki H. Neuroeffector transmission in arterioles of the guinea-pig choroid. The Journal of Physiology. 1998;510:209–223. doi: 10.1111/j.1469-7793.1998.209bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedlund P, Larsson B, Alm P, Andersson KE. Distribution and function of nitric oxide-containing nerves in canine corpus cavernosum and spongiosum. Acta Physiologica Scandinavica. 1995;155:445–455. doi: 10.1111/j.1748-1716.1995.tb09994.x. [DOI] [PubMed] [Google Scholar]
- Helliwell RM, Large WA. α1-Adrenoceptor activation of a non-selective cation current in rabbit portal vein by 1,2-diacyl-sn-glycerol. The Journal of Physiology. 1997;499:417–428. doi: 10.1113/jphysiol.1997.sp021938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmquist H, Hedlund H, Andersson K-E. Characterization of inhibitory neurotransmission in the isolated corpus cavernosum from rabbit and man. The Journal of Physiology. 1992;449:295–311. doi: 10.1113/jphysiol.1992.sp019087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignarro LJ, Bush PA, Buga GM, Wood KS, Fukuto JM, Rajfer J. Nitric oxide and cyclic GMP formation upon electrical field stimulation cause relaxation of corpus cavernosum smooth muscle. Biochemical and Biophysical Research Communications. 1990;170:843–850. doi: 10.1016/0006-291x(90)92168-y. [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Hume JR, Keef KD. Regulation of Ca2+ channels by cAMP and cGMP in vascular smooth muscle cells. Circulation Research. 1993;73:1128–1137. doi: 10.1161/01.res.73.6.1128. [DOI] [PubMed] [Google Scholar]
- Kimoto Y, Ito Y. Functional innervation patterns in the corpus spongiosum and glans in the dog. British Journal of Urology. 1988;62:597–602. doi: 10.1111/j.1464-410x.1988.tb04435.x. [DOI] [PubMed] [Google Scholar]
- Koh SD, Campbell JD, Carl A, Sanders KM. Nitric oxide activates multiple potassium channels in canine colonic smooth muscle. The Journal of Physiology. 1995;489:735–743. doi: 10.1113/jphysiol.1995.sp021087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb FS, Barna TJ. Chloride ion currents contribute functionally to norepinephrine-induced vascular contraction. American Journal of Physiology. 1998;275:H151–160. doi: 10.1152/ajpheart.1998.275.1.H151. [DOI] [PubMed] [Google Scholar]
- Large WA, Wang Q. Characteristics and physiological role of the Ca2+-activated Cl− conductance in smooth muscle. American Journal of Physiology. 1996;217:C435–454. doi: 10.1152/ajpcell.1996.271.2.C435. [DOI] [PubMed] [Google Scholar]
- Loirand G, Pacaud P, Baron A, Mironneau C, Mironneau J. Large conductance calcium-activated non-selective cation channel in smooth muscle cells isolated from rat portal vein. The Journal of Physiology. 1991;437:461–475. doi: 10.1113/jphysiol.1991.sp018606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy ME, Brayden JE. Nitric oxide hyperpolarizes rabbit mesenteric arteries via ATP-sensitive potassium channels. The Journal of Physiology. 1995;486:47–58. doi: 10.1113/jphysiol.1995.sp020789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oba T, Koshita M, van Helden DF. Modulation of frog skeletal muscle Ca2+ release channel gating by anion channel blockers. American Journal of Physiology. 1996;271:C819–824. doi: 10.1152/ajpcell.1996.271.3.C819. [DOI] [PubMed] [Google Scholar]
- Pollock NS, Kargacin ME, Kargacin GJ. Chloride channel blockers inhibit Ca2+ uptake by the smooth muscle sarcoplasmic reticulum. Biophysical Journal. 1998;75:1759–1766. doi: 10.1016/S0006-3495(98)77617-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangachari PK, Triggle CR. Chloride removal and excitation-contraction coupling in guinea pig ileal smooth muscle. Canadian Journal of Physiology and Pharmacology. 1986;64:1521–1527. doi: 10.1139/y86-256. [DOI] [PubMed] [Google Scholar]
- Samuelson U, Sjöstrand NO, Klinge E. Correlation between electrical and mechanical activity in myogenic and neurogenic control of the bovine retractor penis muscle. Acta Physiologica Scandinavica. 1983;119:335–345. doi: 10.1111/j.1748-1716.1983.tb07349.x. [DOI] [PubMed] [Google Scholar]
- Suzuki H. Effects of endogenous and exogenous noradrenaline on the smooth muscle of guinea-pig mesenteric vein. The Journal of Physiology. 1981;321:495–512. doi: 10.1113/jphysiol.1981.sp013999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Helden DF. Electrophysiology of neuromuscular transmission in guinea-pig mesenteric veins. The Journal of Physiology. 1988a;401:469–488. doi: 10.1113/jphysiol.1988.sp017173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Helden DF. An α-adrenoceptor-mediated chloride conductance in mesenteric veins of the guinea-pig. The Journal of Physiology. 1988b;401:489–501. doi: 10.1113/jphysiol.1988.sp017174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Large WA. Noradrenaline-evoked cation conductance recorded with the nystatin whole-cell method in rabbit portal vein cells. The Journal of Physiology. 1991;435:21–39. doi: 10.1113/jphysiol.1991.sp018496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaktubay N, Ogulener N, Onder S, Baysal F. Possible stimulation of Na+,K+-ATPase by NO produced from sodium nitrite by ultraviolet light in mouse gastric fundal strip. General Pharmacology. 1999;32:159–162. doi: 10.1016/s0306-3623(98)00067-6. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Fukuta H, Nakahira Y, Suzuki H. Blockade by 18β-glycyrrhetinic acid of intercellular electrical coupling in guinea-pig arterioles. The Journal of Physiology. 1998;511:501–508. doi: 10.1111/j.1469-7793.1998.501bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


