Abstract
In order to explore the potential role of the sarcoplasmic–endoplasmic reticulum Ca2+-ATPase (SERCA)-type pumps and of their modulators phospholamban (PLB) and sarcolipin (SLN) in the functional alterations of the diaphragm induced by corticosteroid treatment, expression of SERCA, PLB and SLN was assessed by RT-PCR in the diaphragm of rats treated daily for 5 days either with triamcinolone (80 mg kg−1, n = 8) or with saline (control; 0·6 ml, n = 8).
Triamcinolone treatment reduced the normalised overall amount of all SERCA mRNA in diaphragm by 70 % compared to controls (P < 0·05). This reduction was accounted for by a relatively larger decrease in the SERCA1 mRNA (-69 %, P < 0·05) whilst the decrease in SERCA2 mRNA (-49 %, P = 0·09) did not reach statistical significance. As a result the relative proportion of SERCA2 mRNA was increased from 43 ± 7 % in control diaphragm to 52 ± 4 % after triamcinolone treatment (P < 0·05).
Only the adult isoform of SERCA1 (i.e. SERCA1a) mRNA was found in the diaphragm of the 15-week-old control rats. Furthermore, triamcinolone treatment resulted in reduced levels of SERCA2a (-40 %, P < 0·05) and increased levels of SLN mRNA (+100 %, P < 0·05), while the decrease in PLB mRNA (-31 %, P = 0·277) did not reach statistical significance. SERCA1b, SERCA2b and SERCA3 mRNA levels fell below the detection limit in the diaphragm of both control and triamcinolone-treated rats.
Compared to control diaphragm, control rat heart showed a relatively high PLB/(SERCA1 + SERCA2) mRNA ratio of 7·88 while this ratio amounted only to 0·16 in control extensor digitorum longus (EDL) muscle. Remarkably, the SLN/(SERCA1 + SERCA2) mRNA ratio in normal cardiac muscle (0·96) was nearly the same as in diaphragm, but in EDL it amounted to only 0·05 that in diaphragm. This indicates the very low expression of SLN in rat EDL.
These data reveal that considerable alterations in SERCA mRNA levels accompany the functional changes seen in diaphragm after corticosteroid treatment. The relatively larger decrease in SERCA1 mRNA is in agreement with the selective type II fibre atrophy previously observed in the diaphragm of triamcinolone-treated rats, but the magnitude of SERCA alterations is more pronounced than expected on the basis of the structural changes in the diaphragm. The increase in SLN mRNA levels may represent a compensatory mechanism.
Massive doses of systemic corticosteroids are frequently administered acutely to patients to treat status asthmaticus or to prevent rejection after lung transplantation. Following such treatment, acute myopathy may develop (Douglass et al. 1992; Leatherman et al. 1996) affecting both respiratory and peripheral muscles.
When the treatment regimen normally given to patients was mimicked in rats, we showed that corticosteroid treatment was also associated with severe muscle weakness, involving the diaphragm. Indeed, pronounced functional alterations due to selective atrophy of the type II fibres were observed in the diaphragm of corticosteroid-treated rats (Nava et al. 1996). This is in agreement with other studies using ATPase staining for fibre typing where selective type II fibre atrophy with unchanged fibre proportion was demonstrated after corticosteroid treatment (Wilcox et al. 1989; Lewis et al. 1992; Dekhuijzen et al. 1993, 1995; Sieck et al. 1999). It contrasts, however, with the scanty data obtained with immunocytochemistry using antibodies directed against myosin heavy chains. In the latter studies, corticosteroid treatment was shown to affect the fibre proportion (Polla et al. 1994; van Balkom et al. 1997) and atrophy of all fibre types was observed (van Balkom et al. 1997; Prezant et al. 1997).
In rats, treatment with massive doses of corticosteroids elicited a leftward shift of the force-frequency curve and a prolongation of the relaxation time of the diaphragm that were not seen after malnutrition alone (Nava et al. 1996). Such alterations are at least in part related to a decrease in the rate of Ca2+ sequestration by the sarcoplasmic reticulum. In skeletal muscle, the key protein involved in the re-uptake of Ca2+ from the myoplasm into the sarcoplasmic reticulum is the sarcoplasmic-endoplasmic reticulum Ca2+-ATPase (SERCA) pump. Three separate genes (SERCA1, SERCA2 and SERCA3) encode the SERCA family of Ca2+ pumps (for reviews see Aubier & Viires, 1998; Wuytack et al. 1998). The SERCA1 gene is exclusively expressed in fast-twitch skeletal muscle. Developmentally regulated processing leads to alternative splicing of SERCA1 mRNA producing an adult isoform (SERCA1a)and a neonatal isoform (SERCA1b) (Brandl et al. 1986, 1987; Korczak et al. 1987). For SERCA2, four different transcripts have been found, of which the class 1 transcript is translated into SERCA2a (characteristic of slow-twitch skeletal muscle, cardiac muscle and smooth muscle) and the other classes (2-4), which differ only in their 3′ untranslated region, are translated into SERCA2b, the ubiquitous ‘housekeeping’ isoform (de la Bastie et al. 1988; Eggermont et al. 1990; Hawkins et al. 1994). Expression of the SERCA3 gene product is confined to platelets, lymphoid cells, mast cells and epithelial and endothelial cells of various organs (Bobe et al. 1994; Wuytack et al. 1994; Wu et al. 1995). Recently, alternative splicing has also been demonstrated, both in human and in mouse, resulting in three distinct isoforms (SERCA3a, SERCA3b and SERCA3c) (Dode et al. 1998). SERCA3a has been shown to be expressed in non-muscle tissues such as platelets, lymphoid cells, mast cells, endothelial cells, Purkinje neurons and pancreatic islets of Langerhans, while SERCA3b and/or SERCA3c were found to be co-expressed with SERCA3a in mouse pancreatic islets of Langerhans and in human kidney (Dode et al. 1998).
The regulation of SERCA1 catalytic activity is less well understood than that of SERCA2. However, it has recently been shown that sarcolipin (SLN), a 31-residue-long membrane protein believed to be abundantly expressed in the sarcoplasmic reticulum of fast-twitch skeletal muscle, affects SERCA1 activity in the rabbit (Odermatt et al. 1998). At low Ca2+ concentrations (< 10−6 M) it shifts the Km for Ca2+ of SERCA1 to higher values (as is also the case for the phospholamban (PLB)-SERCA2 interaction; see below) and therefore it acts as an inhibitor. However at Ca2+ concentrations saturating SERCA1, it behaves as a stimulator of the pump by increasing Vmax.
The activity of the SERCA2 pump is regulated by PLB, a small 52-residue-long transmembrane peptide. When PLB is phosphorylated (by cyclic AMP-dependent protein kinase or by calcium-calmodulin-dependent protein kinase), the affinity of SERCA2 for Ca2+ is increased (MacLennan & Toyofuku, 1992, 1996; Hawkins et al. 1994; Kimura et al. 1996).
Our present results show that acute corticosteroid treatment in the diaphragm is associated with a relatively larger downregulation of SERCA1 mRNA than of SERCA2 mRNA, but that the SERCA1- and SERCA2-specific alternative splicing mechanisms are not affected. These alterations are in agreement with the selective type II atrophy observed in the diaphragm after corticosteroid treatment. They are, however, more pronounced than the observed diaphragm atrophy, suggesting that the functional changes induced by corticosteroid treatment are more important than expected on the basis of the structural changes alone. An upregulation of SLN might represent a compensatory mechanism for the SERCA downregulation seen after corticosteroid treatment.
METHODS
Design of the study
Sixteen male Wistar rats (15 weeks old; body weight, 300–400 g) were randomly assigned to receive, for 5 days, daily intramuscular injections in the left hindlimb of either triamcinolone (Albicort, Sanofi Winthrop, Brussels, Belgium; 80 mg kg−1, n = 8) or saline (control; 0.6 ml, n = 8). Animals were kept in individual cages and body mass was measured daily. The study was approved by the Animal Experiments Committee of the Medical Faculty of the Katholieke Universiteit, Leuven.
Experimental procedures
RNA extraction
Twenty-four hours after the last injection, animals were anaesthetised with sodium pentobarbital (Nembutal, 60 mg kg−1i.p.). The whole diaphragm was taken from triamcinolone-treated and control rats, while in the latter extensor digitorum longus (EDL) and heart were also removed. The muscles were blotted, weighed, frozen in liquid nitrogen and stored at -80°C. Total RNA was isolated using a modified guanidinium isothiocyanate-CsCl method (Chirgwin et al. 1979). Approximately 0.2 g of tissue was homogenised using an Ultra-Turrax homogeniser (Janke & Kunkel, Germany) in a solution containing 50 % (w/v) guanidinium thiocyanate, 25 mM EDTA, 0.5 % lauryl sarcosine and 0.1 M 2-mercaptoethanol. The homogenate was layered on top of a solution containing 5.7 M CsCl, 25 mM sodium acetate (pH 5.0) and 10 mM EDTA. After ultracentrifugation at 20°C in an SW41 rotor (Beckman, Germany) at 100 000g for approximately 16 h, the supernatant was removed and the RNA pellet was dissolved in water. Sodium acetate (pH 5.2) was added to the solution to a final concentration of 0.3 M and the RNA was precipitated with 2.5 volumes of absolute ethanol. The RNA was then rinsed in 70 % ethanol, vacuum dried and redissolved in water.
The quality and quantity of the RNA preparations were determined by measurement of absorbance at 260 and 280 nm.
Reverse transcription reaction
Samples of 4.5 μg of total RNA from the muscle were subjected to oligo(dT)-primed first-strand cDNA synthesis in a volume of 20 μl.
Polymerase chain reaction
A 3 μl portion of the first-strand cDNA mixture was subjected to PCR under the conditions specified in Table 1, as previously described (Wuytack et al. 1994). The number of PCR cycles was adjusted to avoid saturation of the amplification system. Amplification products were identified by their sizes. To radiolabel the PCR fragments for quantification, 5 μl of the primary PCR mixture was transferred to a new tube containing 45 μl of the same amplification buffer except that [α-32P]dCTP was added. Two additional PCR cycles were performed with the same cycle parameters used in the primary PCR.
Table 1. Oligonucleotide primers and PCR conditions used for cDNA amplification following reverse transcription.
| Primer | Name | Sequence | PCR cycles |
|---|---|---|---|
| SERCA1/SERCA2 | 8 | 5′-GAC/TGAGTTTGGGGAACAGCT-3′ | 94, 60, 72 °C/1, 1, 1 min/27 cycles |
| 9 | 5′-GAGGTGGTGATGACAGCAGG-3′ | ||
| SERCA2/SERCA3 | 12 | From Wuytack et al. (1994) | 94, 55, 72 °C/1, 1, 1 min/27 cycles |
| 13 | |||
| SERCA1a/SERCA1b | 20 | 5′-TTCCATCTGCCTGTCCATGTC-3′ | 94, 60, 72 °C/1, 1, 1 min/27 cycles |
| 23 | 5′-CTGGTTACTTCCTTCTTTCGTCTT-3′ | ||
| SERCA2a | Uf | From Van den Bosch et al. (1994) | 94, 55, 72 °C/1, 1, 1 min/26 cycles |
| C1 | |||
| SERCA2b | Uf | From Van den Bosch et al. (1994) | 94, 55, 72 °C/1, 1, 1 min/26 cycles |
| C2 | |||
| PLB | PLB+ | 5′-TGTGACGATCACAGAAGCC-3′ | 94, 56, 72 °C/1, 1, 1 min/30 cycles |
| PLB− | 5′-GCAGCAGACATATCAAGATGAG-3′ | ||
| SLN | SLN+ | 5′-GGTGTGCACTCAGAAGTCCTCC-3′ | 94, 60, 72 °C/1, 1, 1 min/23 cycles |
| SLN− | 5′-GGAGCTCGGGGCACACAGCAG-3′ | ||
| GAPDH | G+ | 5′-TCCTGCACCACCAACTGCTTAGCC-3′ | 94, 60, 72 °C/1, 1, 1 min/23 cycles |
| G− | 5′-TAGCCCAGGATGCCCTTTAGTGGG-3′ |
Strategy for PCR analysis of the fragments
Ratios of two homologous mRNA species which present stretches of identical sequences can be obtained by means of ratio RT-PCR (Wuytack et al. 1994). Hence PCR primers were designed in such a way that they exactly matched the common sequences in the reverse-transcribed cDNAs of the two mRNAs to be quantified. A few hundred base pair-long fragments of both cDNAs were then co-amplified, electrophoresed and discriminated from each other by restriction analysis. We used this principle for analysis of the ratio of SERCA1, SERCA2 and SERCA3 mRNA forms. The corresponding cDNA sequences have already been published and are available from the EMBL database (accession numbers M99223, X15635 and M30581).
Table 2 lists the enzymes and fragment sizes used to discriminate SERCA1 from SERCA2, and SERCA2 from SERCA3. The SERCA1/SERCA2 and SERCA2/SERCA3 mRNA ratios were thus assessed from the corresponding digestion fragments obtained with the enzymes listed in Table 2, as previously described (Wuytack et al. 1994).
Table 2. Amplified fragments and their identification.
| Isoform | Product size (bp) | Diagnostic restriction enzyme | Restriction products (bp) |
|---|---|---|---|
| SERCA1 | 194 | NcoI | 102, 92 |
| SERCA2 | 194 | MseI | 127, 67 |
| SERCA2 | 206 | BsaHI | 178, 28 |
| SERCA3 | 209 | StyI | 176, 33 |
| SERCA1a | 248 | AvaII* | 103, 145 |
| SERCA1b | 206 | AvaII* | 103, 103 |
| SERCA2a | 231 | HinfI* | 149, 82 |
| SERCA2b | 328 | HinfI* | 246, 82 |
| PLB | 260 | — | Completely sequenced |
| SLN | 173 | — | Completely sequenced |
| GAPDH | 377 | BstXI* | 196, 181 |
Only used for diagnostic purposes; the quantification was done using the full-length PCR fragments.
Data analysis
Statistical analysis was performed using the SAS statistical package (SAS Institute, Cary, NC, USA). Comparison between groups was performed using Student's t test. Data are expressed as means ± standard deviation (s.d.).
RESULTS
Body mass and diaphragm weight
Starting body weight was similar for the two groups (controls, 315 ± 24 g; triamcinolone, 327 ± 36 g) but whereas at the end of treatment the body weight of the control animals had remained stable (323 ± 24 g), that of the triamcinolone-treated animals was significantly decreased by 23 % (251 ± 29 g, P < 0.05 vs. controls). Similarly, compared to controls, diaphragm mass was significantly reduced in triamcinolone-treated rats (-23 %, P < 0.05). In a previous study, ATPase staining, not allowing the distinction between type IIx and type IIb fibres (thus referred to as type IIx/b fibres), revealed that this triamcinolone-induced diaphragm wasting was predominantly associated with a type IIx/b fibre atrophy (type IIx/b cross-sectional area, -30 %; P < 0.05) and also with a type IIa atrophy (-19 %, P < 0.05) (Nava et al. 1996). These changes were previously shown to result in a significant leftward shift of the diaphragm force-frequency curve (Nava et al. 1996). They were also associated with a prolongation of the half-relaxation time after triamcinolone (25 ± 4 vs. 21 ± 4 ms, P < 0.05).
Changes in SERCA1/SERCA2 mRNA ratio upon triamcinolone treatment
The PCR primers designed to co-amplify the 194 bp homologous fragments of SERCA1 and SERCA2 were selected to have identical annealing sites on these two SERCA cDNAs (Zador et al. 1996). However, although the amplified SERCA1 and SERCA2 fragments were of the same length, they differed slightly in sequence (Table 2). These differences in sequence were used to distinguish SERCA1 from SERCA2 by subsequent digestion of the amplified fragments with appropriate restriction enzymes (Table 2). Nco I was used to hydrolyse the SERCA1 fragment (sum of SERCA1a and SERCA1b cDNAs) into 102 and 92 bp fragments, leaving the SERCA2 fragment intact (Fig. 1, lane 2). Similarly, the SERCA2 fragment (i.e. the sum of SERCA2a and SERCA2b cDNAs) was hydrolysed by Mse I into 127 and 67 bp fragments, this time leaving the SERCA1 fragment intact (Fig. 1, lane 3).
Figure 1. RT-PCR amplification of SERCA1 + SERCA2 mRNA species in rat diaphragm after treatment with triamcinolone.
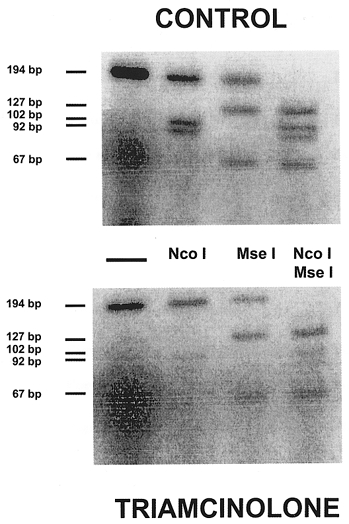
Lanes represent products from a single amplification in a representative control and triamcinolone-treated rat. In each group (from left to right): lane 1, co-amplification of SERCA1 + SERCA2; lane 2, SERCA1 amplification product cut by Nco I into 102 and 92 bp fragments (SERCA2 left intact); lane 3, SERCA2 amplification product cut by Mse I into 127 and 67 bp fragments (SERCA1 left intact); and lane 4, both enzymes added (note the complete digestion of the amplification product).
The sum of SERCA1 + SERCA2 mRNA levels was obtained from the full-length undigested bands after normalising the signals to the GAPDH signal obtained from the same samples (Fig. 1, lane 1). The individual SERCA1 and SERCA2 levels, as well as the SERCA1/SERCA2 mRNA ratio, were calculated from digestions in which both Nco I and Mse I were added (Fig. 1, lane 4). Indeed, only under these conditions could total digestion of the amplified product be ensured. These data were subsequently corrected for the CG content of the digested products since we used radiolabelled dCTP as a tracer to assess the amount of the amplified products.
Compared to control diaphragm, the combined SERCA1 + SERCA2 mRNA level was significantly reduced by 70 % after triamcinolone treatment (Fig. 2). This reduction of overall SERCA level must be mainly ascribed to a decrease in SERCA1 (-69 %, P < 0.05) and to a lesser extent in SERCA2 mRNA level (-49 %) since the latter did not reach statistical significance (P = 0.09; Fig. 3). The relative fraction of SERCA2 mRNA increased from 43 ± 7 % in control diaphragm to 52 ± 4 % after triamcinolone treatment (P < 0.05), such that the relative fraction of the SERCA1 mRNA was decreased (from 57 ± 7 to 43 ± 7 %, P < 0.05).
Figure 2. Data obtained from co-amplification of SERCA1 + SERCA2.

Data are means and s.d. (see Fig. 1, lane 1) in all animals and were corrected with GAPDH values. C, control; T, triamcinolone. * P < 0.05 vs. control. AU, arbitrary units.
Figure 3. RT-PCR amplification of SERCA1 and SERCA2 mRNA species in rat diaphragm after treatment with triamcinolone.
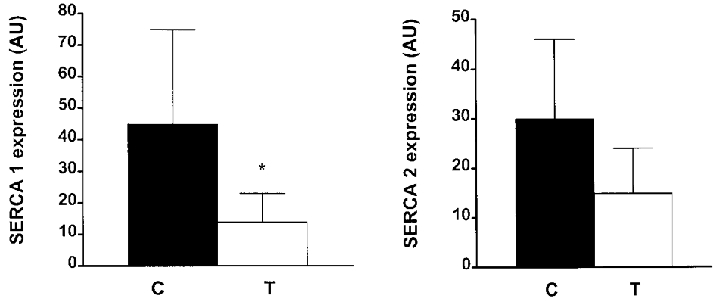
Data are means and s.d. obtained from complete digest of SERCA1 and SERCA2 (Fig. 1, lane 4) in all animals. Data were corrected for CG content. Same conventions as in Fig. 2. * P < 0.05 vs. control. Note that mainly SERCA1 expression was reduced after triamcinolone treatment.
The method used here to determine SERCA1 and SERCA2 levels did not distinguish between the splice variants (SERCA1a and SERCA1b, SERCA2a and SERCA2b), because the amplified SERCA1 and SERCA2 fragments both belong to parts of the transcripts that are common in the respective a/b splice variants (Zador et al. 1996). The next two sections describe methods to assess the splice variants of SERCA1 and SERCA2.
Only the adult SERCA1a mRNA is found in the diaphragm of control and triamcinolone-treated rats
SERCA1a and SERCA1b mRNA levels were quantified using the full-length amplified fragments. The primary SERCA1 transcript contains, towards its 3′ end, a 42 bp optional exon which is removed from the neonatal SERCA1b isoform whilst being retained in the adult SERCA1a isoform. Hence, the ratio of the two forms of transcript can be easily measured by using a couple of primers that encompass the optional exon, and thereby amplify a 248 bp adult fragment and a 206 bp neonatal fragment (Zador et al. 1996).
However, we could only detect the adult isoform of SERCA1 (i.e. SERCA1a) in the diaphragm both in controls and in triamcinolone-treated animals. Thus, the reduction in SERCA1 expression observed after triamcinolone treatment described above must be entirely ascribed to a reduction in SERCA1a mRNA levels.
SERCA2b mRNA level is very low or absent in the diaphragm of control and triamcinolone-treated rats
The primary transcripts of SERCA2 are also subjected to an alternative splicing process affecting their 3′ end, which is dependent on the tissue type in contrast to SERCA1 where splicing is related to developmental stage (Zador et al. 1996). The alternative splicing of SERCA2 is, however, more complex than that of SERCA1. Indeed, in SERCA2, splicing not only involves the removal of an optional exon in muscle (generating a SERCA2a-specific mRNA, also termed the class 1 transcript) and its retention in non-muscle cells (SERCA2b-specific mRNA, known as the class 2–4 transcript), but more importantly the transcript processing generating the muscle isoform, SERCA2a, also involves cleavage and polyadenylation at a site (pAu) located in between the splice donor and acceptor sites in the class 2 mRNA. (This pAu site is not used in the class 3 or class 4 mRNAs.) Since cleavage/polyadenylation at pAu precludes the use of a single set of PCR primers to co-amplify the SERCA2a (class 1) and SERCA2b (class 2) cDNAs, we had to rely on separate sets of primers for each SERCA2 splice variant. In fact we used three PCR primers: one common forward primer (Uf), and two reverse primers, one for each class of SERCA2 mRNA (C1 and C2). A 231 bp fragment for the class 1 mRNA (SERCA2a) could be amplified with the Uf and C1 primers corresponding to parts of exon 20 and 25, respectively, while a 328 bp fragment of the non-muscle isoform (SERCA2b) could be amplified with Uf and C2 primers.
In our study, only a 231 bp fragment, pertaining to SERCA2a, was detected in control and triamcinolone-treated diaphragm. The SERCA2b mRNA level fell below the detection limit in both control and triamcinolone-treated diaphragm. SERCA2a expression was significantly decreased in the diaphragm by 61 % after triamcinolone treatment (P < 0.05 vs. controls; Fig. 4), revealing that the reduction in SERCA2 mRNA levels seen after triamcinolone treatment described above must be entirely ascribed to a downregulation of the SERCA2a mRNA, i.e. the muscle isoform of SERCA2.
Figure 4. RT-PCR amplification of SERCA2a mRNA species in rat diaphragm after treatment with triamcinolone.
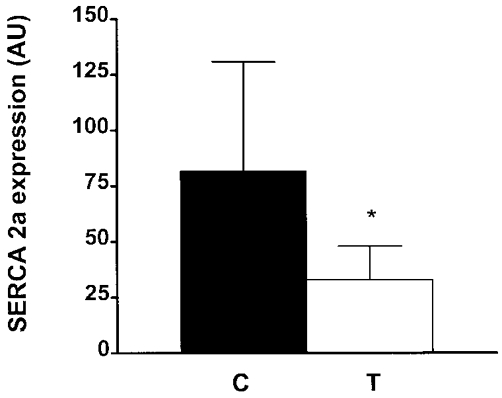
Data are means and s.d. obtained in all animals, and were corrected with GAPDH values. Same conventions as in Fig. 2. Note that SERCA2a expression was reduced by 61 % after triamcinolone treatment (* P < 0.05 vs. control).
SERCA3 mRNA level is very low or absent in the diaphragm of control and triamcinolone-treated rats
As for SERCA1 + SERCA2 co-amplification, we used a single set of primers to amplify homologous regions of SERCA2 and SERCA3 (Table 1) with an apparent fragment size of 206 and 209 bp, respectively (Table 2). SERCA2 and SERCA3 were distinguished by digesting the amplified fragments with appropriate restriction enzymes. Bsa HI was used to hydrolyse the SERCA2 fragment (sum of SERCA2a and SERCA2b cDNAs) into 178 and 28 bp fragments, leaving the SERCA3 fragment intact (Fig. 5, lane 2). The SERCA3 fragment was hydrolysed by Sty I into 176 and 33 bp fragments, leaving the SERCA2 fragment intact (Fig. 5, lane 3).
Figure 5. RT-PCR amplification of SERCA2 and SERCA3 mRNA species in rat diaphragm after treatment with triamcinolone.
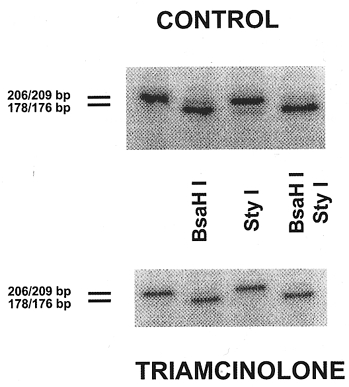
Lanes represent products from a single amplification in a representative control and triamcinolone-treated rat. In each group (from left to right): lane 1, co-amplification of SERCA2 + SERCA3; lane 2, SERCA2 amplification product cut by Bsa HI into 178 and 28 bp fragments (SERCA3 left intact); lane 3, SERCA3 amplification product cut by Sty I into 176 and 33 bp fragments (SERCA2 left intact); and lane 4, both enzymes added (note the complete digestion of the amplification product).
Compared to control diaphragm, the level of the co-amplified SERCA2 + SERCA3 cDNA (uncut fragment; see Fig. 5, lane 1) was significantly reduced by 75 % after triamcinolone treatment (P < 0.008 vs. controls). This reduction was in fact fully accounted for by a decrease in SERCA2 mRNA, because SERCA3 mRNA levels fell below the detection limit in both control and triamcinolone-treated diaphragm (Fig. 5, lane 4).
PLB and SLN mRNA are found in the diaphragm of control and triamcinolone-treated rats and SLN increases upon triamcinolone treatment
Primers were designed to amplify a 260 bp fragment of the PLB cDNA (Table 1). The identity of the amplified fragments was established by sequencing (data not shown). PLB mRNA levels, normalised to the GAPDH levels, were reduced by 31 % after triamcinolone treatment but this reduction did not reach statistical significance (P = 0.277).
Quantification of SLN levels was done using the undigested 173 bp fragment amplified using the new primers shown in Table 1. Again sequencing was used to confirm the identity of the amplified fragment (data not shown). SLN mRNA levels were significantly increased by 100 % after triamcinolone treatment (P < 0.05).
Normal rat EDL expresses low levels of SLN whereas its expression is much higher in cardiac muscle
Since it is generally assumed that fast-twitch skeletal muscle expresses both SERCA1 and SLN, whereas slow-twitch and cardiac muscle co-express SERCA2a and PLB, we compared the levels of SERCA1 and SERCA2, and of their regulators SLN and PLB, in control diaphragm with the corresponding levels in rat EDL as an example of a fast muscle, and with those in cardiac muscle. Table 3 gives the ratio of PLB and SLN to SERCA levels in rat EDL, diaphragm and heart. If we set the ratios of PLB/(SERCA1 + SERCA2) and of SLN/(SERCA1 + SERCA2) in diaphragm arbitrarily to 1, the rat heart shows a 7.88-fold higher PLB/(SERCA1 + SERCA2) ratio than diaphragm. For EDL, however, this ratio amounts to only 0.16 of that in the diaphragm. Remarkably, a similar comparison of the SLN/(SERCA1 + SERCA2) ratio in diaphragm with the corresponding ratio in the two other muscles pointed to the unexpected conclusion that the ratio of SLN to SERCA is nearly the same in diaphragm and cardiac muscle, but that typically fast-twitch EDL expresses only very low levels of SLN.
Table 3. Expression ratio of SERCA1 or SERCA2 to total SERCA mRNA and of PLB or SLN to total SERCA mRNA in different muscles.
| EDL | Diaphragm | Heart | |
|---|---|---|---|
| SERCA1/(SERCA1 + SERCA2) | 0.99 ± 0.01 | 0.57 ± 0.07 | — |
| SERCA2/(SERCA1 + SERCA2) | 0.01 ± 0.01 | 0.43 ± 0.07 | 1.00 ± 0.00 |
| PLB/(SERCA1 + SERCA2) | 0.53 ± 0.02 (0.16) | 3.29 ± 1.33 (1.00) | 25.91 ± 3.21 (7.88) |
| (0.16) | (1.00) | (7.88) | |
| SLN/(SERCA1 + SERCA2) | 0.38 ± 0.21 (0.05) | 6.95 ± 1.04 (1.00) | 6.69 ± 7.79 (0.96) |
| (0.05) | (1.00) | (0.96) |
Values are means ± s.d. The values for PLB and SLN are arbitrary; those in parentheses are normalised to the ratio in diaphragm. The number of PCR cycles used to amplify a given fragment was similar for the three muscles (SERCA1 + SERCA2 = 17 cycles, PLB = 23 cycles, SLN = 23 cycles and GAPDH = 20 cycles). For PCR conditions, see Table 1.
DISCUSSION
The present study showed first that SERCA1a and SERCA2a mRNAs are expressed in the rat diaphragm, whereas transcripts of SERCA1b and SERCA2b fell below the detection limit with the present approach. Also, known regulators of SERCA1 and SERCA2, i.e. SLN and PLB, respectively, are expressed in this tissue. Second, functional alterations in the diaphragm previously observed after corticosteroid treatment can now be related to a downregulation of mainly SERCA1 mRNA. This is in agreement with the selective diaphragm type II fibre atrophy previously seen in this model (Nava et al. 1996). Third, SLN mRNA was significantly increased after corticosteroid treatment.
In normal animals, the expression of SERCA isoforms has been previously reported in skeletal muscles of rats (Burk et al. 1988; Schulte et al. 1993, 1994; Kandarian et al. 1994; Van der Linden et al. 1996; Zador et al. 1996), dogs (Briggs et al. 1992; Hu et al. 1995), rabbits (Brandl et al. 1987; Leberer et al. 1989; Aria et al. 1992) and cats (Talmadge et al. 1996), including the diaphragm (Sayen et al. 1992; Wu & Lytton, 1993; Wu et al. 1995; Anger et al. 1995; Viires et al. 1997). However, the reported relative levels of the two SERCA isoforms in the rat diaphragm differ between studies. Thus, SERCA1 mRNA was reported to represent 90 % (Wu & Lytton, 1993; Wu et al. 1995) or 75 % (N. Viires, personal communication) of all SERCA mRNA while Sayen et al. (1992) reported a SERCA1 fraction of 50 %, a value close to that found in our study (57 %). Similar discrepancies have already been reported for the rat soleus (Simonides et al. 1990; Wu & Lytton, 1993; Wu et al. 1995; Zador et al. 1996) and have been attributed to methodological problems. Indeed, a prerequisite for an accurate quantitative analysis of the SERCA1/SERCA2 ratio is that the efficiency of the PCR amplification must be the same for the fragments of the two isoforms. Thus, the efficiency of primer annealing for the isoforms should be similar and the length of the amplified segments should be about the same. In our study, the primers used to amplify the SERCA1 and SERCA2 isoforms were chosen in such a way that they had identical annealing sites on the two SERCAs. Only then would no differences in primer binding to either SERCA1 or SERCA2 occur.
From all earlier studies examining SERCA isoform expression in the diaphragm, only one group measured the expression of SERCA2 splice variants (Wu & Lytton, 1993; Wu et al. 1995), whereas the expression of the SERCA1 splice variants has never been investigated in the diaphragm. As in our study, Lytton's team demonstrated the presence of SERCA2a mRNA in the rat diaphragm but they furthermore found very low levels of SERCA2b mRNA (around 1 %) and of SERCA3 mRNA. The presence of SERCA3 in skeletal muscles has been suggested to result from contamination with other non-muscle cell types such as vascular endothelial cells or blood cells (Burk et al. 1988). In our experiments SERCA2b, SERCA1b and SERCA3 mRNA levels all fell below the detection limit in the diaphragm. Regenerating skeletal muscle and rat endothelial cells or platelets served as positive controls for the expression of SERCA2b/SERCA1b and SERCA3, respectively (results not shown, but see Zador et al. 1996). It should be noted that the rats used in our study were 15 weeks old, which might explain the absence of the neonatal SERCA1b form.
We noticed the presence of PLB mRNA in the diaphragm of rats in contrast to what was previously reported by Viires et al. (1997). Indeed, the ratio of PLB/(SERCA1 + SERCA2) was considerably higher in the diaphragm compared to EDL but definitely lower than in cardiac muscle. The reason for the failure to detect PLB by Viires et al. (1997) may be related to their use of the less sensitive dot blot system. PLB is a major protein regulator of the rate of Ca2+ pumping by SERCA2a in cardiac muscle, mediating amongst others its β-adrenergic effects. But although PLB is also expressed in slow-twitch skeletal muscle (MacLennan & Toyofuku, 1992; Kimura et al. 1996), at least in larger animals and to a lesser extent in some smooth muscles, its physiological role in these two muscle types remains less clear. PLB is not normally expressed in fast-twitch skeletal muscle. Since the rat diaphragm is a muscle with a large proportion of slow-twitch fibres (Delp & Duan, 1996), the presence of PLB in this muscle is not unexpected.
The present study is the first to demonstrate that SLN is expressed in the rat diaphragm. Until now, expression of SLN has mainly been reported in fast-twitch skeletal muscles but it has also been shown to be present at low levels in slow-twitch skeletal muscles (Odermatt et al. 1997). Quite unexpectedly, we found that mRNA ratios for SLN/SERCA were nearly the same in rat diaphragm and cardiac muscle, but that this ratio was very low in EDL, a typical fast-twitch muscle expressing almost only SERCA1. Clearly, a more elaborate study of the SLN/PLB/SERCA ratios in different muscles and in different animal species is needed to confirm or reject the proposed complemental expression pattern of SLN and PLB (Odermatt et al. 1997). Recent work by Odermatt et al. (1998) showed that SLN exerts a dual effect on SERCA1 in rabbit fast-twitch skeletal muscle. Thus, at low Ca2+ concentrations SLN decreased the affinity of SERCA1 for Ca2+, but at high Ca2+ concentrations it increased SERCA1 activity by enhancing its Vmax. A decrease in SLN expression in chronically stimulated fast-twitch skeletal muscle was held responsible in part for the observed partial inactivation of SERCA1. Finally, it is also interesting to note that the transmembrane helices of SLN and PLB share considerable sequence homology but that the modes of action of the two proteins are different. The effect of PLB on SERCA2 can be prevented by phosphorylation of the regulator, which is not the case for SLN.
We observed a pronounced downregulation of SERCA1 and also of SERCA2a mRNA after triamcinolone treatment, resulting in an increase of the relative proportion of SERCA2. This decrease in SERCA1 expression correlates with the selective type II fibre atrophy we previously reported in the rat diaphragm after corticosteroid treatment (Nava et al. 1996). Surprisingly, SERCA2a expression was decreased after corticosteroid treatment while no changes in type I fibres occurred in triamcinolone-treated diaphragm. This can be related to two potential mechanisms. First, it may signal that corticosteroid treatment induces functional changes that are not expected on the basis of the observed structural changes. Second, although it has been claimed that SERCA2 is mainly expressed in slow-twitch fibres, we cannot rule out the possibility that in the rat diaphragm a certain proportion of the fast fibres may coexpress SERCA2 and SERCA1.
Viires et al. (1995, 1997) demonstrated a significant reduction in SERCA1 mRNA (Viires et al. 1995; as in our study) and in SERCA1 protein levels (Viires et al. 1997); they also found that this reduction was associated with a slight increase in SERCA2 protein (Viires et al. 1997). These changes in SERCA pumps were associated with an increase in myosin heavy chain β/slow and a concomitant decrease in myosin heavy chain 2X (Viires et al. 1995). It should, however, be noted that no control group was used in the study. Indeed, corticosteroid-treated rats were compared to sham-treated rats under the same degree of caloric restriction. No adlibitum-fed control group was used. It is therefore difficult to compare our data where an adlibitum-fed control group was used with those of Viires et al. (1995, 1997).
Associated with the SERCA2a downregulation in the diaphragm of triamcinolone-treated rats, there was also a decrease in the PLB mRNA level that failed to reach statistical significance. Such a co-ordinated regulation of SERCA and PLB expression is, in fact, not always seen. Thus, in rabbits, during fast-twitch skeletal muscle development, SERCA2a was highly expressed, but PLB remained undetectable (Aria et al. 1992). In contrast, in chronically stimulated fast-twitch skeletal muscle in dogs, complete upregulation of both SERCA2a and PLB was observed, but even in this case upregulation of SERCA2a occurred before PLB (Hu et al. 1995). Finally, while in the present study SERCA1 mRNA levels were severely reduced in the diaphragm after corticosteroid treatment, the expression of its potential regulatory protein, SLN, was markedly increased. Therefore, as for PLB and SERCA2, SLN and SERCA1 expression is unlikely to be co-ordinated. Nevertheless, SLN upregulation may serve as a compensatory mechanism for the reduction in SERCA1 mRNA. Such compensatory mechanisms are also suggested by the fact that the pronounced reduction in Ca2+ pump mRNA expression was not matched by an equal increase in half-relaxation time.
Indeed, half-relaxation time was prolonged by 19 % after corticosteroid treatment (Nava et al. 1996) while SERCA isoform expression was reduced by 69 and 49 % for SERCA1 (P < 0.05) and SERCA2 (P = 0.09), respectively. Thus, the changes in Ca2+ pump mRNA were more pronounced than the changes in muscle function as reflected by prolonged half-relaxation time or muscle atrophy (-30 % reduction in type IIx/b cross-sectional area). The reason for such a discrepancy in magnitude is not known but it suggests that functional changes were greater than expected on the basis of the observed structural changes. Such a discrepancy has, however, been previously observed in rat soleus after unweighting where pronounced changes in fast calcium pump gene expression were reported while changes in myofibrillar gene expression were modest (Schulte et al. 1993).
The conclusion that the prolonged half-relaxation time after triamcinolone treatment is probably related to changes in fibre dimensions remains valid because it seems reasonable to assume that a selective atrophy of fast-twitch fibres induces a shift towards a slow profile in the diaphragm after corticosteroid treatment. Along the same lines, it seems obvious to expect the alterations in SERCA pump expression in the diaphragm after corticosteroid treatment, which we demonstrated in the present study. We cannot exclude the possibility that other mechanisms, beyond the aim of the present study, are involved. In any event, the dramatic changes in SERCA pump expression compared to the changes in fibre atrophy suggest that the functional changes induced by corticosteroid treatment are greater than expected on the basis of the structural changes alone.
In conclusion, our data reveal that considerable alterations in SERCA mRNA levels accompany the functional changes seen in diaphragm after corticosteroid treatment. The relatively larger decrease in SERCA1 mRNA is in agreement with the selective type II fibre atrophy previously observed in the diaphragm of triamcinolone-treated rats. The increase in SLN levels might represent a compensatory mechanism.
Acknowledgments
The excellent technical assistance of E. Broeckhoven is sincerely acknowledged. This work was supported by grants G.0175.99 and G.0189.97 from the ‘Fonds voor Wetenschappelijk Onderzoek Vlaanderen’ (F.W.O.), by the interuniversity Poles of Attraction Programme, Belgian State, Prime Minister's Federal Office for Scientific, Technical and Cultural affairs IUAP P4/23 and by Astra Pharmaceuticals. Ghislaine Gayan-Ramirez is a postdoctoral fellow of the ‘Fonds voor Wetenschappelijk Onderzoek-Vlaanderen’.
References
- Anger M, Lambert F, Chemla D, Desché P, Scalbert E, Lompré A-M, Lecarpentier Y. Sarcoplasmic reticulum Ca2+ pumps in heart and diaphragm of cardiomyopathic hamster: effects of perindopril. American Journal of Physiology. 1995;268:H1947–1953. doi: 10.1152/ajpheart.1995.268.5.H1947. [DOI] [PubMed] [Google Scholar]
- Aria M, Otsu K, MacLennan DH, Periasamy M. Regulation of sarcoplasmic reticulum gene expression during cardiac and skeletal muscle development. American Journal of Physiology. 1992;262:C614–620. doi: 10.1152/ajpcell.1992.262.3.C614. [DOI] [PubMed] [Google Scholar]
- Aubier M, Viires N. Calcium ATPase and respiratory muscle function. European Respiratory Journal. 1998;11:758–766. [PubMed] [Google Scholar]
- Bobe R, Bredoux R, Wuytack F, Quarck R, Kovàcs T, Papp B, Corvazier E, Magnier C, Enouf J. The rat platelet 97-kDa Ca2+ ATPase isoform is the sarcoendoplasmic reticulum Ca2+ ATPase 3 protein. Journal of Biological Chemistry. 1994;269:1417–1424. [PubMed] [Google Scholar]
- Brandl CJ, Deleon S, Martin DR, MacLennan DH. Adult forms of the Ca2+ ATPase of sarcoplasmic reticulum. Expression in developing skeletal muscle. Journal of Biological Chemistry. 1987;262:3768–3774. [PubMed] [Google Scholar]
- Brandl CJ, Green NM, Korczak B, MacLennan DH. Two Ca2+ ATPase genes: homologies and mechanistic implications of deduced amino acid sequences. Cell. 1986;44:597–607. doi: 10.1016/0092-8674(86)90269-2. [DOI] [PubMed] [Google Scholar]
- Briggs FN, Lee KF, Wechsler AW, Jones LR. Phospholamban expressed in slow-twitch and chronically stimulated fast-twitch muscles minimally affects calcium affinity of sarcoplasmic reticulum Ca2+-ATPase. Journal of Biological Chemistry. 1992;267:26056–26061. [PubMed] [Google Scholar]
- Burk SE, Lytton J, MacLennan DH, Shull GE. cDNA cloning, functional expression, and mRNA tissue distribution of a third organellar Ca2+ pump. Journal of Biological Chemistry. 1988;263:15032–15040. [PubMed] [Google Scholar]
- Chirgwin JM, Przybyla AE, McDonald RJ, Rutter WJ. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979;18:5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Dekhuijzen PNR, Gayan-Ramirez G, Bisschop A, de Bock V, Dom R, Decramer M. Corticosteroid treatment and nutritional deprivation cause a different pattern of atrophy in rat diaphragm. Journal of Applied Physiology. 1995;78:629–637. doi: 10.1152/jappl.1995.78.2.629. [DOI] [PubMed] [Google Scholar]
- Dekhuijzen PNR, Gayan-Ramirez G, de Bock V, Dom R, Decramer M. Triamcinolone and prednisolone affect contractile properties and histopathology of rat diaphragm differently. Journal of Clinical Investigation. 1993;92:1534–1542. doi: 10.1172/JCI116732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Bastie D, Wisnewsky C, Schwartz K, Lompré A-M. (Ca2++ Mg2+)-dependent ATPase mRNA from smooth muscle sarcoplasmic reticulum differs from that in cardiac and fast skeletal muscles. FEBS Letters. 1988;229:45–48. doi: 10.1016/0014-5793(88)80794-4. [DOI] [PubMed] [Google Scholar]
- Delp MD, Duan C. Composition and size of type I, IIA, IID/X and IIB fibers and citrate synthase activity of rat muscle. Journal of Applied Physiology. 1996;80:261–270. doi: 10.1152/jappl.1996.80.1.261. [DOI] [PubMed] [Google Scholar]
- Dode L, de Greef C, Mountian I, Attard M, Town MM, Casteels R, Wuytack F. Structure of the human sarco/endoplasmic reticulum Ca2+-ATPase 3 gene. Journal of Biological Chemistry. 1998;273:13982–13994. doi: 10.1074/jbc.273.22.13982. [DOI] [PubMed] [Google Scholar]
- Douglass JA, Tuxen DV, Horne M, Scheinkestel CD, Weinmann M, Czarny D, Bowes G. Myopathy in severe asthma. American Review of Respiratory Disease. 1992;146:517–519. doi: 10.1164/ajrccm/146.2.517. [DOI] [PubMed] [Google Scholar]
- Eggermont JA, Wuytack F, Casteels R. Characterization of the mRNAs encoding the gene 2 sarcoplasmic/endoplasmic-reticulum Ca2+ pump in pig smooth muscle. Biochemical Journal. 1990;266:901–907. [PMC free article] [PubMed] [Google Scholar]
- Hawkins C, Xu A, Narayanan N. Sarcoplasmic reticulum calcium pump in cardiac and slow twitch skeletal muscle but not fast twitch skeletal muscle undergoes phosphorylation by endogenous and exogenous Ca2+/calmodulin-dependent protein kinase. Journal of Biological Chemistry. 1994;269:31198–31206. [PubMed] [Google Scholar]
- Hu P, Yin C, Zhang K-M, Wright LD, Nixon TE, Wechsler AS, Spratt JA, Briggs FN. Transcriptional regulation of phospholamban gene and translational regulation of SERCA2 gene produces coordinate expression of these two sarcoplasmic reticulum proteins during skeletal muscle phenotype switching. Journal of Biological Chemistry. 1995;270:11619–11622. doi: 10.1074/jbc.270.19.11619. [DOI] [PubMed] [Google Scholar]
- Kandarian SC, Peters DG, Taylor JA, Williams JH. Skeletal muscle overload upregulates the sarcoplasmic reticulum slow calcium pump gene. American Journal of Physiology. 1994;266:C1190–1197. doi: 10.1152/ajpcell.1994.266.5.C1190. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Kurzydlowski K, Tada M, MacLennan DH. Phospholamban regulates the Ca2+-ATPase through intramembrane interactions. Journal of Biological Chemistry. 1996;271:21726–21731. doi: 10.1074/jbc.271.36.21726. [DOI] [PubMed] [Google Scholar]
- Korczak B, Zarain-Herzberg A, Brandl CJ, Ingles CJ, Green NM, MacLennan DH. Structure of the rabbit fast-twitch skeletal muscle Ca2+-ATPase gene. Journal of Biological Chemistry. 1987;262:3768–3774. [PubMed] [Google Scholar]
- Leatherman JW, Fluegel WL, David WS, Davies SF, Iber C. Muscle weakness in mechanically ventilated patients with severe asthma. American Journal of Respiratory and Critical Care Medicine. 1996;153:1686–1690. doi: 10.1164/ajrccm.153.5.8630621. [DOI] [PubMed] [Google Scholar]
- Leberer E, Hartner K-T, Brandl CJ, Fujii J, Tada M, MacLennan DH, Pette D. Slow/cardiac sarcoplasmic reticulum Ca2+-ATPase and phospholamban mRNAs are expressed in chronically stimulated rabbit fast-twitch muscle. Journal of Biochemistry. 1989;185:51–54. doi: 10.1111/j.1432-1033.1989.tb15080.x. [DOI] [PubMed] [Google Scholar]
- Lewis MI, Monn SA, Sieck GC. Effect of corticosteroids on diaphragm fatigue, SDH activity, and muscle fiber size. Journal of Applied Physiology. 1992;72:293–301. doi: 10.1152/jappl.1992.72.1.293. [DOI] [PubMed] [Google Scholar]
- MacLennan DH, Toyofuku T. Structure-function relationships in sarcoplasmic or endoplasmic reticulum type Ca2+ pumps. Annals of the New York Academy of Sciences. 1992;671:1–10. doi: 10.1111/j.1749-6632.1992.tb43779.x. [DOI] [PubMed] [Google Scholar]
- MacLennan DH, Toyofuku T. Regulatory interactions between calcium ATPases and phospholamban. Society of General Physiologist Series. 1996;51:89–103. [PubMed] [Google Scholar]
- Nava S, Gayan-Ramirez G, Rollier H, Bisschop A, Dom R, de Bock V, Decramer M. Effects of acute steroid administration on ventilatory and peripheral muscles in rats. American Journal of Respiratory and Critical Care Medicine. 1996;153:1888–1896. doi: 10.1164/ajrccm.153.6.8665051. [DOI] [PubMed] [Google Scholar]
- Odermatt A, Becker S, Khanna VK, Kurzydlowski K, Leisner E, Pette D, MacLennan DH. Sarcolipin regulates the activity of SERCA1, the fast-twitch skeletal muscle sarcoplasmic reticulum Ca2+-ATPase. Journal of Biological Chemistry. 1998;273:12360–12369. doi: 10.1074/jbc.273.20.12360. [DOI] [PubMed] [Google Scholar]
- Odermatt A, Taschner PEM, Scherer SW, Beatty B, Khanna VK, Cornblath DR, Chaudhry V, Yee WC, Schrank B, Karpati G, Breuning MH, Knoers N, MacLennan DH. Characterization of the gene encoding human sarcolipin (SLN), a proteolipid associated with SERCA1: absence of structural mutations in five patients with Brody disease. Genomics. 1997;45:541–553. doi: 10.1006/geno.1997.4967. [DOI] [PubMed] [Google Scholar]
- Polla B, Bottinelli R, Sandoli D, Sardi C, Reggiani C. Cortisone-induced changes in myosin heavy chain distribution in respiratory and hindlimb muscles. Acta Physiologica Scandinavica. 1994;151:353–361. doi: 10.1111/j.1748-1716.1994.tb09754.x. [DOI] [PubMed] [Google Scholar]
- Prezant DJ, Karwa ML, Richner B, Maggiore D, Gentry EI, Cahill J. Gender-specific effects of dexamethasone treatment on rat diaphragm structure and function. Journal of Applied Physiology. 1997;82:125–133. doi: 10.1152/jappl.1997.82.1.125. [DOI] [PubMed] [Google Scholar]
- Sayen MR, Rohrer DK, Dillmann WH. Thyroid hormone response of slow and fast sarcoplasmic reticulum Ca2+ ATPase mRNA in striated muscle. Molecular and Cellular Endocrinology. 1992;87:87–93. doi: 10.1016/0303-7207(92)90236-y. [DOI] [PubMed] [Google Scholar]
- Schulte L, Peters D, Taylor J, Navarro J, Kandarian SC. Sarcoplasmic reticulum Ca2+ pump expression in denervated skeletal muscle. American Journal of Physiology. 1994;567:C617–622. doi: 10.1152/ajpcell.1994.267.2.C617. [DOI] [PubMed] [Google Scholar]
- Schulte LM, Navarro J, Kandarian SC. Regulation of sarcoplasmic reticulum calcium pump gene expression by hindlimb unweighting. American Journal of Physiology. 1993;264:C1308–1315. doi: 10.1152/ajpcell.1993.264.5.C1308. [DOI] [PubMed] [Google Scholar]
- Sieck GC, van Balkom RHH, Prakash YS, Zhan WZ, Dekhuijzen PNR. Corticosteroid effects on diaphragm neuromuscular junctions. Journal of Applied Physiology. 1999;86:114–122. doi: 10.1152/jappl.1999.86.1.114. [DOI] [PubMed] [Google Scholar]
- Simonides WS, Van der Linden CG, van Harneveld C. Thyroid hormone differentially affects mRNA levels of Ca-ATPase isozymes of sarcoplasmic reticulum in fast and slow skeletal muscle. FEBS Letters. 1990;274:73–76. doi: 10.1016/0014-5793(90)81332-i. [DOI] [PubMed] [Google Scholar]
- Talmadge RJ, Roy RR, Chalmers GR, Edgerton VR. MHC and sarcoplasmic reticulum protein isoforms in functionally overloaded cat plantaris muscle fibers. Journal of Applied Physiology. 1996;80:1296–1303. doi: 10.1152/jappl.1996.80.4.1296. [DOI] [PubMed] [Google Scholar]
- van Balkom RHH, Dekhuijzen PNR, Folgering H, Veerkamp JH, Fransen JAM, van Herwaarden CLA. Effects of long-term low-dose methylprednisolone on rat diaphragm function and structure. Muscle and Nerve. 1997;20:983–990. doi: 10.1002/(sici)1097-4598(199708)20:8<983::aid-mus8>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Van den Bosch L, Eggermont JA, De Smedt H, Mertens L, Wuytack F, Casteels R. Regulation of splicing is responsible for the expression of the muscle-specific 2a isoform of the sarco/endoplasmic-reticulum Ca2+-ATPase. Biochemical Journal. 1994;302:559–566. doi: 10.1042/bj3020559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Linden CG, Simonides WS, Muller A, Van der Laarse WJ, Vermeulen JL, Zuidwijk MJ, Moorman AF, Van Harneveld C. Fiber-specific regulation of Ca2+-ATPase isoform expression by thyroid hormone in rat skeletal muscle. American Journal of Physiology. 1996;271:C1908–1919. doi: 10.1152/ajpcell.1996.271.6.C1908. [DOI] [PubMed] [Google Scholar]
- Viires N, D'Albis A, Lompré A-M, Aubier M. Chronic steroid administration modifies myosin heavy chain composition and Ca2+-ATPase expression in the rat diaphragm. American Journal of Respiratory and Critical Care Medicine. 1995;151:A479. [Google Scholar]
- Viires N, Govela M, Zedda C, Danialou G, Aubier M. Corticosteroids modify the sarcoplasmic reticulum Ca2+-ATPase (SERCA) expression in the rat diaphragm. American Journal of Respiratory and Critical Care Medicine. 1997;155:A921. [Google Scholar]
- Wilcox PG, Hards JM, Bockhold K, Bressler B, Pardy RL. Pathologic changes and contractile properties of the diaphragm in corticosteroid myopathy in hamsters: comparison to peripheral muscle. American Journal of Respiratory Cell and Molecular Biology. 1989;1:191–199. doi: 10.1165/ajrcmb/1.3.191. [DOI] [PubMed] [Google Scholar]
- Wu K-D, Lee W-S, Wey J, Bungard D, Lytton J. Localization and quantification of endoplasmic reticulum Ca2+-ATPase isoform transcripts. American Journal of Physiology. 1995;269:C775–784. doi: 10.1152/ajpcell.1995.269.3.C775. [DOI] [PubMed] [Google Scholar]
- Wu K-D, Lytton J. Molecular cloning and quantification of sarcoplasmic reticulum Ca2+-ATPase isoforms in rat muscles. American Journal of Physiology. 1993;264:C333–341. doi: 10.1152/ajpcell.1993.264.2.C333. [DOI] [PubMed] [Google Scholar]
- Wuytack F, Papp B, Verboomen H, Raeymaekers L, Dode L, Bobe R, Enouf J, Bokkala S, Authi KS, Casteels R. A sarco/endoplasmic reticulum Ca2+-ATPase 3-type Ca2+ pump is expressed in platelets, in lymphoid cells, and in mast cells. Journal of Biological Chemistry. 1994;269:1410–1416. [PubMed] [Google Scholar]
- Wuytack F, Raeymaekers L, Eggermont JA, Van den Bosch L, Verboomen H, Mertens L. Isoform diversity and regulation of organellar-type Ca2+-transport ATPases. In: Andersen JP, editor. Advances in Molecular and Cell Biology. Greenwich, CT, USA: JAI Press Inc.; 1998. pp. 205–248. [Google Scholar]
- Zador E, Mendler L, ver Heyen M, Dux L, Wuytack F. Changes in mRNA levels of the sarcoplasmic/endoplasmic-reticulum Ca2+-ATPase isoforms in the rat soleus muscle regenerating from netoxin-induced necrosis. Biochemical Journal. 1996;320:107–113. doi: 10.1042/bj3200107. [DOI] [PMC free article] [PubMed] [Google Scholar]


