Abstract
To define the effects of prenatal hypoxia on the postnatal development of the chemoafferent pathway, ventilation and metabolism, pregnant rats were exposed to normobaric hypoxia (10 % oxygen) from embryonic day 5 to embryonic day 20. Offspring were studied at 1, 3 and 9 weeks of age in three separate protocols.
Prenatal hypoxia decreased the dopamine content in the carotid bodies at all ages, and decreased the utilisation rate of noradrenaline in the caudal part of the A2 (A2c), A1 and A5 noradrenergic brainstem cell groups at 3 weeks after birth. At 9 weeks of age, the level of dopamine in the carotid bodies was still reduced but the utilisation rate of noradrenaline was enhanced in A1.
Rats from dams subjected to hypoxia during pregnancy hyperventilated until 3 weeks after birth. In these rats, the biphasic hypoxic ventilatory response was absent at 1 week and the increase in minute ventilation was amplified at 3 weeks.
Prenatal hypoxia disturbed the metabolism of offspring until 3 weeks after birth. A weak or absent hypometabolism in response to hypoxia was observed in these rats in contrast to control animals.
Prenatal hypoxia impairs the postnatal development of the chemoafferent pathway, as well as the ventilatory and metabolic responses to hypoxia. These alterations were mostly evident until 3 weeks after birth.
Perinatal hypoxia can cause various short-term, long-term or life-spanning sequelae. Early postnatal hypoxic exposure within the first days of life induces adverse and long-term effects on postnatal growth (Soulier et al. 1997), neurobehavioural development (Nyakas et al. 1996), breathing and ventilatory response to hypoxia (Okubo & Mortola, 1988; Hertzberg et al. 1992) and development of central catecholaminergic areas involved in respiratory control (Seidler & Slotkin, 1990; Soulier et al. 1997). Postnatal breathing onset and respiratory control are dependent on the peripheral chemoreceptors. Chemosensitivity is low in the fetus and resets to a higher O2 level within a couple of days after birth (Hertzberg et al. 1990). However, the peripheral chemoreceptors and their integrative ventilatory response are not mature at birth and are susceptible to modulation by changes in environmental oxygen occurring during the early postnatal period. In fact, prolonged hypoxic exposure from birth increases the basal activity of the carotid bodies (Hertzberg et al. 1992; Soulier et al. 1997), delays the onset of the chemoreflex response to hypoxia (Eden & Hanson, 1987; Hertzberg et al. 1992) and elicits hyperventilation in adults (Okubo & Mortola, 1988). However, prolonged perinatal hyperoxia induces a hypoplasia of carotid bodies (Erickson et al. 1998), may accelerate the chemoreflex response to hypoxia (Eden & Hanson, 1986) and attenuates the hypoxic ventilatory response in adult rats (Ling et al. 1996).
Fetal hypoxia might result from several pathophysiological situations including maternal anaemia, reduced uteroplacental blood flow secondary to maternal hypertension, smoking or ethanol consumption, reduced placenta size or reduced oxygen inhalation by the mother at high altitude. Prenatal hypoxia elicits many disturbances which are manifest at and after birth. Reduced fetal growth (De Grauw et al. 1986), as well as cognitive and motor deficiency (Nyakas et al. 1996), results from hypoxic insult during gestation. Rats born after hypoxic gestation present, at 1 day of postnatal age, respiratory as well as metabolic disturbances characteristic of hypoxaemia of the newborn (Gleed & Mortola, 1991). The nature of these disturbances depends on the duration and severity of hypoxia, as well as the gestational age of the fetus at the time of the insult. Most of the studies in the rat have analysed the effects of hypoxic (8, 10 and 12 % O2) or asphyxic (99.9 % N2) insults administered during the final week or days of gestation at only one postnatal age between birth and adulthood. White & Lawson (1997), however, conducted a longitudinal study on the postnatal effects (from birth to 14 days of postnatal age) of prenatal hypoxia (10 % O2 on the last 3 days of gestation) on catecholaminergic expression in the respiratory regions of the medulla oblongata. Various maternal prenatal situations (cocaine, hyperoxia, high altitude, intrauterine conditions that induce prematurity or impair fetal growth) have also been reported to induce impairment in the postnatal respiratory adjustment to a noxious environment (Davey et al. 1996; Ling et al. 1996, 1998). In contrast to postnatal hypoxia, the long-term consequences of prenatal hypoxia have never been analysed with regard to the neurochemistry of the structures involved in hypoxic adaptation, ventilation and metabolism. Such a study is of interest during postnatal development in order to better understand the mechanisms underlying the consequences of prenatal hypoxia.
Most of the chemoafferent fibres arising from the carotid bodies terminate in the nucleus tractus solitarii (NTS) in the caudal part of the A2 cell group (A2c). Some of these fibres also project to the ventrolateral medulla oblongata (VLM) (Finley & Katz, 1992). The A2 and A1 subnuclei of the NTS and VLM, respectively, are intermingled with respiratory motoneurones and are important sites in medullary pathways mediating hypoxic ventilatory responses (Housley & Sinclair, 1988; Ellenberger et al. 1990). We have previously reported the involvement of the caudal portion of the A2 cell group of the NTS in the ventilatory acclimatisation to hypoxia (Soulier et al. 1992; Schmitt et al. 1994). Furthermore, the A5 cell group of the ventrolateral pons displays changes in its neural activity during hypoxia (Soulier et al. 1992) and is involved in the generation of respiratory rythmicity (Champagnat & Fortin, 1997).
The present study examines the effects of prenatal hypoxia lasting 2 weeks in the rat, from embryonic day 5 (E5) to E20, on the postnatal development of the chemoafferent pathway both neurochemically and functionally, at 1, 3 and 9 weeks of postnatal age. In the first protocol, we focused on the neurochemical activity of these neural pathways, i.e. of the carotid bodies and the brainstem noradrenergic cell groups, and in a second protocol we investigated the impact of the neurochemical disturbances on physiological function, i.e. on ventilation. The content of dopamine in carotid bodies (Hertzberg et al. 1992) and the utilisation rate of noradrenaline in cell groups of the medulla oblongata (caudal part of A2 cell group of the NTS, A1 cell group of the VLM) and of the pons (A5 cell group of the ventrolateral pons) were measured in an attempt to assess the long-term cell catecholaminergic disturbances due to prenatal hypoxia. The physiological impact of these neurochemical alterations was evaluated on resting ventilation and on the integrated ventilatory response to chemoreceptor stimulation, i.e. to acute postnatal hypoxia. The ventilatory response to hypoxia was used to determine the degree of functional integrity of the chemoreflex pathway. In a third protocol, total body metabolism was measured to analyse respiration and efficiency of ventilation.
METHODS
Animals and hypoxia
Twenty pregnant rats (Sprague-Dawley, IFFA Credo, l'Arbresle, France) were placed from E5 to E20 in a normobaric Plexiglass chamber supplied with a gas mixture consisting of 10 % O2-90 % N2 and maintained at 10 ± 0.5 % O2. They had free access to food and water. The CO2 expired by the rats was eliminated by circulating the gas mixture from the chamber through soda lime and never exceeded 0.1 %. Metabolic water contained in expiratory gases was trapped continuously into a chilled glass tank. The temperature inside the chamber was set at 26 ± 1°C. One day before delivery, pregnant rats were removed from the hypoxic chamber (10 % O2) and were housed individually under normoxia (21 % O2) in a climatised room at 26 ± 1°C with a 12 h light-dark cycle. They were allowed free access to food and water. At birth, in normoxia, pups were mixed and redistributed randomly to nursing dams. These hypoxic offspring were considered as the hypoxic group (Hypo). A normoxic group of 20 pregnant rats (pregnancy in normoxia giving normoxic offspring) was treated similarly. These normoxic offspring were considered as the control group (Cont). The experiments were performed using only male pups in order to avoid neurochemical and ventilatory gender differences (Porter, 1986; Mortola & Saiki, 1996). To ensure a common nutritional status among the various litters, each nursing group contained 10–12 pups. A total of 240 normoxic and hypoxic offspring were studied at 1, 3 and 9 weeks of age. All were raised in normoxia. In addition, five normoxic and five hypoxic pregnant rats were killed by cervical dislocation at embryonic day 19 (E19) and their placentas were taken out and weighed in order to estimate the impact of maternal hypoxia. After the experiments all animals were killed by cervical dislocation. All experiments were carried out according to the ethical principles laid down by the French (Ministère de l'Agriculture) and EU Council Directives for care of laboratory animals (No. 02889).
Tissue dissection (protocol one)
A total of 141 rats were killed by cervical dislocation at 1, 3 or 9 weeks of age. The brain and carotid bodies were rapidly removed from all animals. For the 1-week-old rats, the brains were placed on an ice-cold plate for dissection of the whole brainstem because noradrenergic cell groups are not defined at this age. For the older rats, the brainstem was cut into serial coronal slices 480 μm in thickness. The noradrenergic cell group A2, caudal subset (A2c) located in the NTS, the noradrenergic cell group A1 located in the VLM and the noradrenergic cell group A5 located in the pons were ‘punched out’ (diameter of the needle, 0.9 mm), according to the dissection procedure described by Palkovits & Brownstein (1988). The volume of each sample varied from 0.9 to 1.5 mm3.
As previously described (Soulier et al. 1992, 1997), after adjustment of the microtome blade to the plane containing the point where the pyramidal decussation appears (referred to as the zero plane), six serial slices were cut (Fig. 1). The A2 caudal subset was punched out from three slices and the A1 cell group was punched out from five slices as described in Fig. 1. After adjustment of the microtome blade to the plane containing the point where the VII cranial nerve appears, the A5 cell group was punched out from three slices (Fig. 1). All structures were dissected out bilaterally, frozen on dry ice and stored at -80°C until biochemical analyses were carried out by high performance liquid chromatography coupled with electrochemical detection (HPLC-ED).
Figure 1. Schematic diagram illustrating the location of the punched areas.
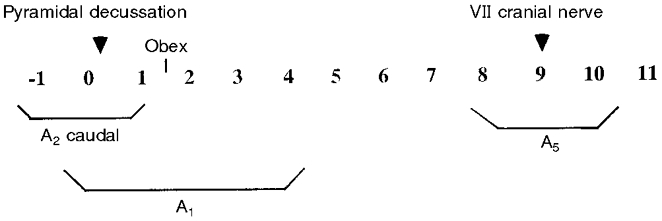
Each number (from -1 to 11) represents a consecutive slice of 480 μm coronal section. Each slice is numbered according to its position relative to that containing the pyramidal decussation (referred to as 0). The caudal part of the A2 cell group is punched out from 3 slices (-1 to 1). The A1 cell group is punched out from 5 slices (0-4) and the A5 cell group from 3 slices (8-10).
Biochemical analyses
The utilisation rate of noradrenaline was estimated in the brainstem cell groups by measuring the decrease in noradrenaline levels after block of catecholamine biosynthesis following administration of an intraperitoneal injection of α-methyl-p-tyrosine methyl ester (AMPT, 250 mg kg−1; Sigma, St Quentin Fallavier, France), as previously reported (Soulier et al. 1992, 1997; Schmitt et al. 1994). Briefly, each experimental group was divided into two subgroups, one receiving AMPT and the other receiving the same volume of vehicle (0.9 % saline) 2.5 h prior to being killed, and the noradrenaline content in each group was measured. The slope of noradrenaline decline was multiplied by the mean noradrenaline content of saline-injected rats and the product was taken as the utilisation rate expressed as picomoles per pair of structures per hour. Because of different rates of catecholamine metabolism in brainstem cell groups and carotid glomic cells, the decrease in catecholamine content after AMPT was noticeable in the brainstem neurones but was too low in the carotid body to provide a reliable estimation of the utilisation rate. Therefore, in the carotid body, only the content of dopamine was reported.
Punch brain samples and carotid bodies were disrupted by ultrasound in 100 μl 0.4 M (punches) or 0.1 M (carotid bodies) perchloric acid containing 2.7 mM EDTA-Na2. In punch samples, the excess perchloric acid was removed by addition of 8 μl 6.4 M potassium formiate. The homogenates were centrifuged (8800 g, 5 min) and a 20 μl aliquot of the supernatant was injected directly into a reverse-phase column (ODS-Hypersil, 5 mm, 150 mm × 4.6 mm; Shandon, Cheshire, UK) with a mobile phase of 27 mM citric acid, 50 mM sodium acetate, 1 mM EDTA-Na2, 0.8 mM sodium acetyl sulfate and 8 % methanol. The flow rate was 1.2 ml min−1. Dopamine (DA), noradrenaline (NA) and 3,4-dihydroxyphenylacetic acid (DOPAC), the main metabolite of DA considered as an index of dopaminergic activity (Roth et al. 1976), were measured at +0.72 V versus the Ag+-AgCl reference electrode (ELDEC 102, Chromatofield, Chateauneuf-les-Martigues, France). The detection limits calculated by doubling the noise ratios and expressed in term of picomoles of injected amounts were less than 0.03 pmol and intra-assay coefficients were 0.2 %.
Pons-medulla of 1-week-old animals was analysed as described previously (Lagercrantz et al. 1992). Samples were disrupted ultrasonically in 0.4 M perchloric acid (1.8 ml) containing 2.7 mM EDTA-Na2 and the excess perchloric acid was removed by addition of 150 μl 6.4 M potassium formiate. After centrifugation (8800 g, 5 min), two aliquots (A and B) of the supernatant were obtained. Aliquot A (500 μl) was used for the noradrenaline assay after alumina purification. Aliquot B (950 μl) was used for total (free + conjugated) 3-methoxy-4-hydroxyphenylethylene glycol (MHPG) assay after purification on a Sephadex G10 column and extraction in ethyl acetate. Total MHPG is the main metabolite of noradrenaline in brain and the ratio MHPG/NA provides an index of central noradrenergic activity (Lagercrantz et al. 1992). Noradrenaline was assayed using the HPLC system and mobile phase described above. The mobile phase for the MHPG assay consisted of 0.145 M sodium tricitrate, adjusted to pH 4.6 with orthophosphoric acid-methanol (2 ml l−1).
Catecholamine content and utilisation rate are specific markers of the catecholaminergic systems. The tissue punches included excess tissue which ensured that all catecholaminergic cells were harvested from the selected brainstem region. Thus, the expression of catecholamine utilisation rate per structure is more descriptive of the absolute value of monoamine activity in catecholaminergic tissues, which is independent of surrounding non-catecholaminergic tissue punched out together with the brainstem cell groups. On the other hand, the total protein content can fluctuate non-specifically in response to chronic hypoxia. Accordingly, expression of results per structure is more appropriate than expression per milligram of protein. This avoids artifactual variations of the catecholamine content and utilisation rate resulting from modifications of total protein levels in the structure.
Measurements of ventilation (protocol two)
Before any recordings of ventilation, additional groups of animals (Cont and Hypo of 1-, 3- and 9 weeks of age), different from those used in ventilation measurements, were used in order to record the body temperature in normoxia and during a 10 min hypoxic challenge. Each age group was composed of six Cont and six Hypo rats. Colonic temperature was monitored by a fine tungsten-constantan thermocouple (Chessel 4001). All recordings were realised at an ambient temperature of 32 ± 1°C (1 week old), 28 ± 1°C (3 weeks old) and 25 ± 1°C (9 weeks old). During the hypoxic challenge, colonic temperature was recorded after 1, 4, 7 and 10 min of hypoxia. These body temperatures have been used to calculate the tidal volume in BTPS conditions (body temperature and pressure when saturated with water vapour) for measurements of ventilation.
Ventilation was measured in the morning in 52 other 1-, 3- and 9-week-old awake unrestrained rats using a barometric plethysmograph, as described by Bartlett & Tenney (1970). The number of animals studied in the Cont and the Hypo group were, respectively, 9 and 10 for the 1-week-old, 6 and 9 for the 3-week-old and 10 and 8 for the 9-week-old rats. All recordings were obtained at 32 ± 1, 28 ± 1 and 25 ± 1°C. Plexiglass plethysmographic chambers, adapted in volume for the rat ages (volume = 0.22, 0.75 and 5.4 l for the 1-, 3- and 9-week-old rats, respectively), flushed with a humidified (and heated for the young rats) airflow, were connected to a reference box of the same size. Both boxes were saturated with water vapour. Ambient temperature, O2 and CO2 levels inside the animal chamber were monitored continuously. Before placing the animal in the box, a calibration of the chamber was obtained by injecting 10 μl of air for the two smallest boxes and 1 ml for the largest one. Immediately following chamber calibration, the animal was placed in the chamber until the respiratory pattern was stable. At this point, the gas flow was interrupted, the inlet and outlet tubes of the animal chamber were closed, and pressure fluctuations related to breathing were recorded with a differential pressure transducer (Celesco, CA, USA). Tidal volume (VT), respiratory frequency (f) and minute ventilation (V̇E) were calculated from 30–50 consecutive breath cycles (total duration approximately 30 s) by computer analysis of the spirogram. All measurements were performed in quadruplet, with measurement periods separated by intervals of 10–15 min, depending on the degree of activity of the rat. The mean of these four values was considered as the basal normoxic ventilation (baseline ventilation). The plethysmograph was then flushed with the 10 % O2 gas mixture. Different time points in the response to hypoxia were recorded at 1, 4, 7 and 10 min of hypoxia. Measurements were made on 30–50 breath cycles. Results are presented as the difference obtained between individual baseline measurements and the respective hypoxic values of each ventilatory parameter. Thus the ventilatory response to hypoxia is represented by the amplitude of response.
Measurements of metabolism (protocol three)
Energy expenditure was measured at 32 ± 1, 28 ± 1 and 26 ± 1°C for 47 other 1-, 3- and 9-week-old rats, respectively, different from those used for ventilation measurements. The number of animals studied in the Cont and the Hypo group was, respectively, 8 and 7 for the 1-week-old, 6 and 10 for the 3-week-old and 8 and 8 for the 9-week-old rats. Pups were placed inside an airtight container purged by a continuous flow of 0.3 l min−1 of atmospheric air (mass flowmeter Tylan). After a period of acclimatisation, O2 and CO2 concentrations of the outgoing air were registered every minute for 20 min. Following this, the gas supply was switched to a hypoxic gas mixture (10 % O2-90 % N2). Rats were exposed to hypoxia for 10–12 min during which O2 and CO2 concentrations were recorded every minute. A portion of the outgoing air was directed to an O2 analyser (Klogor, France) and an infrared CO2 analyser (Gascard Edinburgh Sensors Ltd, Edinburgh, UK). Air was dried through a permapure system containing calcium chloride. Total quantities of O2 consumed (V̇O2) and CO2 produced (V̇CO2) were calculated as the difference between O2 and CO2 concentrations in the container minus O2 and CO2 concentrations in normoxic air room or hypoxic gas mixture multiplied by the air flow through the sealed container. The system was calibrated daily before the experiments with pure nitrogen and with a standard mixture containing 0.5 % CO2-20.5 % O2-79 % N2. Total body metabolism was calculated from O2 consumption and CO2 production according to equations of Depocas & Hart (1957) and was expressed in joules per kilogram per minute.
Statistics
Neurochemical data are expressed as means ±s.e.m. For each structure, the effect of prenatal hypoxia was assessed using a two-way (prenatal treatment, age) analysis of variance (ANOVA) followed by Fisher's protected least significant difference test.
All data concerning ventilation and metabolism are expressed as means ±s.e.m. Because all studies were multivariate (treatment, age, hypoxic challenge), data were first subjected to global ANOVA. Where a significant effect of treatment or interaction of treatment with age or hypoxic challenge was detected, lower order ANOVAs were then carried out to determine the time of hypoxic challenge or age of effect or interaction. Therefore, a two-factor ANOVA (prenatal treatment, age) was used to determine, in resting conditions, differences in ventilatory components and metabolism between the Hypo and Cont groups. The general response to hypoxia over 10 min was analysed using a three-factor ANOVA (prenatal treatment, age, hypoxic challenge). Ventilatory values in normoxia and following 1 min of hypoxia exposure were compared using a two-way ANOVA (prenatal treatment, age). In order to compare the amplitude of the hypoxic ventilatory response over 10 min of hypoxia between the Hypo and the Cont group, the difference between resting values and the values obtained during the hypoxic test was calculated and a three-factor ANOVA (prenatal treatment, age, hypoxic challenge) was performed. When interactions were significant, a post hoc test (Fisher's protected least square difference) was used to compare individual groups. A P value of < 0.05 was considered statistically significant.
RESULTS
Viability and growth
Pregnant rats were subjected to hypoxia 5 days after impregnation in order to avoid nidation disturbances. Nevertheless, approximately 12 % of the animals resorbed their fetuses and the remainder gave birth to smaller size litters, averaging 7 pups for the Hypo group versus 13 for the Cont group. The weight of placentas and the ratio placenta/body weight at E19 was greater in the Hypo group (666 ± 22 mg and 0.304 ± 0.01, respectively) compared with the Cont group (567 ± 12 mg and 0.212 ± 0.005, respectively) (P < 0.05). The body weight of the Hypo group compared with the age-matched Cont group was significantly less at 1 week (-31 %), was not significantly different at 3 weeks, and was greater at 9 weeks of age (+19 %) (Table 1). The lung weights of the Hypo group were significantly reduced at 1 and 3 weeks of age (-20 and -14 %, respectively), as were the ratios lung weight/body weight (-15 and -11 %, respectively).
Table 1. Effect of prenatal hypoxia on body, lung and lung/body weights.
| Rats | Body weight (g) | Lung weight (g) | Lung/body weight (× 100) |
|---|---|---|---|
| 1 week | |||
| Cont | 20.61 ± 0.68 (23) | 0.34 ± 0.01 (23) | 2.0 ± 0.02 (23) |
| Hypo | 14.16 ± 0.48 (26) * | 0.27 ± 0.01 (26) * | 1.7 ± 0.03 (26) * |
| 3 weeks | |||
| Cont | 48.82 ± 4.20 (24) | 0.51 ± 0.02 (24) | 1.13 ± 0.04 (24) |
| Hypo | 45.31 ± 1.19 (21) | 0.44 ± 0.04 (21) * | 1.00 ± 0.05 (21) * |
| 9 weeks | |||
| Cont | 245 ± 10 (24) | 1.40 ± 0.05 (24) | 0.57 ± 0.03 (24) |
| Hypo | 291 ± 18 (23) * | 1.54 ± 0.10 (23) | 0.51 ± 0.03 (23) |
Data are expressed as means ±s.e.m.
P < 0.05, significant difference between the control (Cont) group of rats and the prenatal hypoxic (Hypo) group of rats.
Numbers in parentheses indicate the number of animals used.
Neurochemistry
In the carotid bodies of the Cont group, a gradual increase in the levels of dopamine and its main metabolite DOPAC was observed from 1 to 9 weeks of age. Maternal hypoxic exposure resulted in relatively lower levels of dopamine in carotid bodies compared with controls (-50, -43 and -36 % at 1, 3 and 9 weeks of age, respectively) (Table 2). The content of DOPAC was reduced by 38 % in the 3-week-old rats of the Hypo group.
Table 2. Effect of prenatal hypoxia on the dopamine and DOPAC content of the carotid body.
| Rats | Dopamine (pmol structure−1) | DOPAC (pmol structure−1) |
|---|---|---|
| 1 week | ||
| Cont | 4.76 ± 0.10 (10) | 0.76 ± 0.08 (9) |
| Hypo | 2.37 ± 0.09 (8) * | 0.67 ± 0.06 (8) |
| 3 weeks | ||
| Cont | 7.37 ± 0.82 (11) | 1.49 ± 0.15 (11) |
| Hypo | 4.21 ± 0.56 (11) * | 0.92 ± 0.06 (11) * |
| 9 weeks | ||
| Cont | 9.55 ± 0.98 (10) | 1.96 ± 0.21 (11) |
| Hypo | 6.14 ± 1.18 (10) * | 1.79 ± 0.27 (10) |
Data are expressed as means ±s.e.m.
P < 0.05, significant difference between the control (Cont) group of rats and the prenatal hypoxic (Hypo) group of rats.
Numbers in parentheses indicate the number of animals used.
In the pons-medulla of 1-week-old pups, the ratio MHPG/NA was not significantly altered in the Hypo group, (0.18 ± 0.01) compared with the Cont group (0.20 ± 0.01). In the older rats, analyses were performed on individual cell groups of the brainstem. At 3 weeks of age, prenatal hypoxia resulted in a reduced content (Table 3) and utilisation rate (Fig. 2) of noradrenaline in the A2c (-25 and -98 %), A1 (-39 and -47 %) and A5 (-42 % for utilisation rate) cell groups. At 9 weeks of age no differences in A2c and A5 were further observed between the Hypo and Cont groups. In the A1 cell group the content and utilisation rate of noradrenaline were greater (+23 and +97 %, respectively) in the Hypo group compared with the Cont group.
Table 3. Effect of prenatal hypoxia on the content of noradrenaline in brainstem cell groups.
| Rats | A2c | A1 | A5 |
|---|---|---|---|
| 3 weeks | |||
| Cont | 6.14 ± 0.74 (12) | 2.79 ± 0.41 (11) | 4.07 ± 0.31 (11) |
| Hypo | 4.57 ± 0.85 (12) * | 1.70 ± 0.08 (11) * | 4.14 ± 0.39 (11) |
| 9 weeks | |||
| Cont | 8.31 ± 0.64 (11) | 5.63 ± 0.29 (13) | 5.67 ± 0.44 (12) |
| Hypo | 7.17 ± 0.92 (11) | 6.93 ± 0.70 (10) * | 5.00 ± 0.46 (12) |
A2c, caudal part of A2 cell group of the nucleus tractus solitarii in the dorsomedial medulla oblongata; A1, cell group of the ventrolateral medulla oblongata; A5, cell group of the ventrolateral pons. Data are expressed as picomoles per structure (means ±s.e.m.)
P < 0.05, significant difference between the control (Cont) group of rats and the prenatal hypoxic (Hypo) group of rats.
Numbers in parentheses indicate the number of animals used.
Figure 2. Effect of prenatal hypoxia on noradrenaline utilisation rate in brainstem cell groups of 3- and 9-week-old rats.
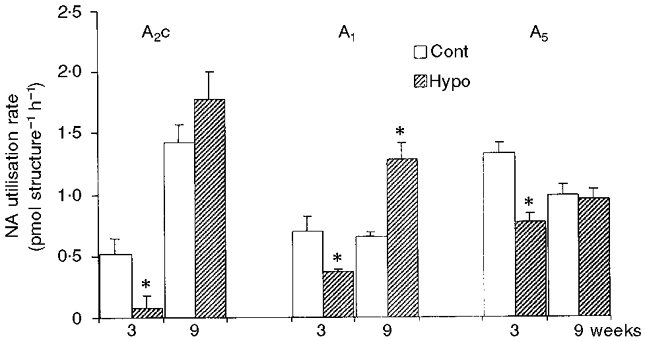
Bars represent means ±s.e.m. of noradrenaline utilisation rate in the different brainstem cell groups (A2c, A1 and A5) from control rats (Cont) at 3 (n = 24) and 9 (n = 24) weeks of age, and rats at 3 (n = 21) and 9 (n = 23) weeks of age from dams subjected to hypoxia during pregnancy (Hypo). *P < 0.05, significant difference between Cont and Hypo group.
Body temperature
No differences, at any age, in the body temperature were observed in resting conditions between the Cont and the Hypo groups (respectively, 36.6 ± 0.4 and 37.0 ± 0.5°C for the 1-week-old; 37.1 ± 0.2 and 37.3 ± 0.2°C for the 3-week-old; 37.7 ± 0.1 and 37.8 ± 0.1°C for the 9-week-old rats). During the 10 min hypoxic challenge the body temperature of the 1-week-old rats dropped significantly but no significant difference was observed between the Cont and the Hypo group. After 10 min of hypoxia, body temperature in the Cont and Hypo groups dropped, respectively, by 1.2 ± 0.2 and 1.4 ± 0.2°C. The body temperature of 3- and 9-week-old rats did not drop significantly after 10 min of hypoxia and no difference was observed between the Cont and Hypo groups.
Resting ventilatory data
In the Cont group, resting ventilatory parameters (respiratory frequency, tidal volume and minute ventilation normalised to body weight) decreased with advancing age (Table 4). The 1-week-old Hypo group of rats showed an arrhythmic pattern compared with the Cont group (Fig. 3). The coefficient of variation was higher in the Hypo group than in the Cont group (0.35 ± 0.02 and 0.24 ± 0.04, respectively). Compared with the age-matched Cont group, the Hypo group exhibited a significant increase in resting minute ventilation until 3 weeks of age (+33 % in the 1-week-old and +65 % in the 3-week-old rats) (Table 4). This increase was mainly due to a greater respiratory frequency at 1 week (+13 %) and a greater tidal volume at 3 weeks of age (+68 %). At 9 weeks of age, although minute ventilation did not differ between the two groups of animals, the respiratory frequency of the Hypo group was greater compared with the Cont group (Table 4). In the 1-week-old rats, CO2 production was greater in the Hypo group than in the Cont group (+14 %) (Table 4). Although ventilation and metabolism data were obtained from different groups of rats, an estimation of the ratio V̇E/V̇CO2 was calculated and expressed without s.e.m. values. The estimated V̇E/V̇CO2 ratio was higher in the Hypo group than in the Cont group until 3 weeks of age (respectively, 70.0 and 59.6 for the 1-week-old, 65.4 and 47.1 for the 3-week-old rats).
Table 4. Effect of prenatal hypoxia on ventilatory parameters in resting conditions and in response to postnatal hypoxia (10% O2).
| 1 week | 3 weeks | 9 weeks | ||||
|---|---|---|---|---|---|---|
| Cont | Hypo | Cont | Hypo | Cont | Hypo | |
| V̇E (ml min−1 (100 g)−1) | ||||||
| Baseline | 120.2 ± 11.4 (9) | 160.1 ± 18.6 (10) * | 90.05 ± 3.7 (6) | 137.1 ± 23.18 (9) * | 51.65 ± 2.7 (10) | 47.2 ± 2.1 (8) |
| 1 min | 170 ± 25.4 (9) † | 196 ± 26.4 (10) | 147.1 ± 5.4 (6)† | 243.4 ± 38.9 (9) *† | 78.5 ± 4.8 (10) † | 79.1 ± 6.9 (8) † |
| 10 min | 132.7 ± 23.7 (9) | 182 ± 38.7 (10) | 149.5 ± 10.9 (6) | 235 ± 39 (9) | 102.1 ± 8.9 (10) | 74.7 ± 3.9 (8) * |
| f (breaths min−1) | ||||||
| Baseline | 145 ± 5.5 (9) | 165 ± 6.4 (10) * | 145 ± 11.5 (6) | 136 ± 8.6 (9) | 92 ± 2.9 (10) | 102 ± 4.1 (8) * |
| 1 min | 179 ± 7.5 (9) † | 198 ± 6.4 (10) † | 203 ± 11.1 (6) † | 177 ± 8.6 (9) † | 125 ± 6.2 (10) † | 162 ± 12 (8) *† |
| 10 min | 158.5 ± 5.3 (9) | 165 ± 7.4 (10) | 205.2 ± 11.4 (6) | 184 ± 7.1 (9) | 160 ± 7.5 (10) | 153.5 ± 7.3 (8) |
| VT (ml (100 g)−1) | ||||||
| Baseline | 0.83 ± 0.08 (9) | 1.01 ± 0.14 (10) | 0.64 ± 0.04 (6) | 1.07 ± 0.14 (9) * | 0.57 ± 0.04 (10) | 0.47 ± 0.03 (8) |
| 1 min | 0.97 ± 0.14 (9) † | 1.04 ± 0.19 (10) | 0.73 ± 0.04 (6) † | 1.39 ± 0.17 (9) *† | 0.64 ± 0.04 (10) | 0.52 ± 0.05 (8) |
| 10 min | 0.85 ± 0.17 (9) | 1.30 ± 0.31 (10) | 0.73 ± 0.05 (6) | 1.27 ± 0.17 (9) | 0.65 ± 0.08 (10) | 0.5 ± 0.04 (8) |
| V̇CO2 (ml min−1 (100 g)−1) | ||||||
| Baseline | 2.01 ± 0.06 (8) | 2.29 ± 0.05 (7) * | 1.91 ± 0.16 (6) | 2.09 ± 0.10 (10) | 2.12 ± 0.11 (8) | 2.20 ± 0.10 (8) |
| 3 min | 2.17 ± 0.15 (8) | 2.12 ± 0.18 (7) | 2.42 ± 0.12 (6) † | 2.91 ± 0.15 (10) *† | 2.86 ± 0.11 (8) † | 3.05 ± 0.09 (8) † |
| 10 min | 1.71 ± 0.12 (8) | 2.01 ± 0.15 (7) | 1.97 ± 0.16 (6) | 1.90 ± 0.11 (10) | 2.11 ± 0.17 (8) | 2.11 ± 0.05 (8) |
Data are expressed as means ±s.e.m.V̇E, minute ventilation; f, respiratory frequency; VT, tidal volume; V̇CO2, CO2 production.
P < 0.05, significant difference between the control group (Cont) and the prenatal hypoxic group (Hypo)
P < 0.05, significant difference between values in resting conditions (baseline) and either 1 min of postnatal hypoxia for ventilation measurements (V̇E, f, VT) or 3 min of postnatal hypoxia for metabolism (V̇CO2) measurements.
Numbers in parentheses indicate the number of animals used. In both Cont and Hypo groups, FI,O2 (inspired O2 fraction) was 0.21 under baseline conditions and 0.10 at 1, 3 and 10 min of hypoxia.
Figure 3. Integrated minute ventilation.
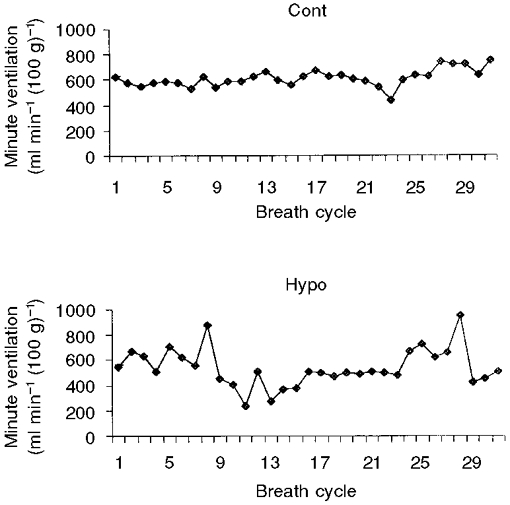
Each graph shows data from a typical animal and represents the minute ventilation integrated breath by breath in a 1-week-old control rat (Cont; top) and in a 1-week-old rat from a dam subjected to hypoxia during pregnancy (Hypo; bottom). Thirty-one consecutive breath cycles are represented and numbered 1–29. Note the irregular ventilation in the Hypo rat compared with the Cont rat. Coefficients of variation in the Cont and Hypo group of rats are 0.24 ± 0.04 and 0.35 ± 0.02, respectively.
Ventilatory response to hypoxia
Response after 1 min of hypoxia
The 1-week-old control rats showed a significant increase in minute ventilation (+41 %) compared with the Hypo group (Table 4). Both groups of animals responded to hypoxia by increasing respiratory frequency (+23 % in the Cont group and +20 % in the Hypo group). Only the Cont group showed an increase in tidal volume (+16 %). At 3 weeks of age, both groups of rats responded to hypoxia with an increased minute ventilation at 1 min (+63 % in the Cont group and +73 % in the Hypo group), resulting from an increase in respiratory frequency (+40 % in the Cont group and +30 % in the Hypo group). The tidal volume was significantly greater in the Hypo group (+30 % in the Hypo group compared with +14 % in the Cont group). At 9 weeks of age, both groups of animals responded to hypoxia by increasing minute ventilation (+51 % in the Hypo group and +68 % in the Cont group) due to an increase in respiratory frequency (+57 % in the Hypo group and +36 % in the Cont group) without significant change in tidal volume.
Response over 10 min of hypoxia
The minute ventilation of rats of the Cont group was affected by 10 min of hypoxic exposure (Fig. 4). In the 3- and 9-week-old rats, minute ventilation increased until 10 min of hypoxic exposure. In contrast, in the 1-week-old rats, the minute ventilation response to hypoxia was biphasic. It returned to baseline levels at the end of 1 min of hypoxia due to a drop in respiratory frequency and tidal volume (Table 4, Fig. 4). In the 1-week-old-rats of the Hypo group, hypoxia failed to alter significantly the minute ventilation. Although respiratory frequency declined, the absence of the biphasic response was mainly due to tidal volume which did not decrease. In contrast, at 3 and 9 weeks of age, minute ventilation increased in response to hypoxia. The components of this hypoxic ventilatory response were an increase in both respiratory frequency and tidal volume at 3 weeks, although only respiratory frequency increased at 9 weeks. Comparison of the response of the Hypo group with the response of the age-matched Cont group showed that the ventilatory response of the Hypo group was amplified at 3 weeks, due to a greater increase in the tidal volume (Fig. 4). At 9 weeks, the increase in minute ventilation in response to hypoxia was less for the Hypo group (+67 % at 7 min and +58 % at 10 min) compared with the Cont group (+88 % at 7 min and +95 % at 10 min). The production of CO2 was greater in the 3-week-old Hypo group of rats during the 10 min hypoxic test compared with the Cont group (+20 % at 3 min of hypoxia; Table 4). The estimated V̇E/V̇CO2 ratio after 10 min of hypoxia was higher in the Hypo group than in the Cont group until 3 weeks of age (respectively, 90.2 and 77.6 for the 1-week-old and 123.7 and 75.7 for the 3-week-old rats).
Figure 4. Effect of prenatal hypoxia on the amplitude of the hypoxic ventilatory response to 10 % O2 hypoxia of 1-, 3- and 9-week-old rats.
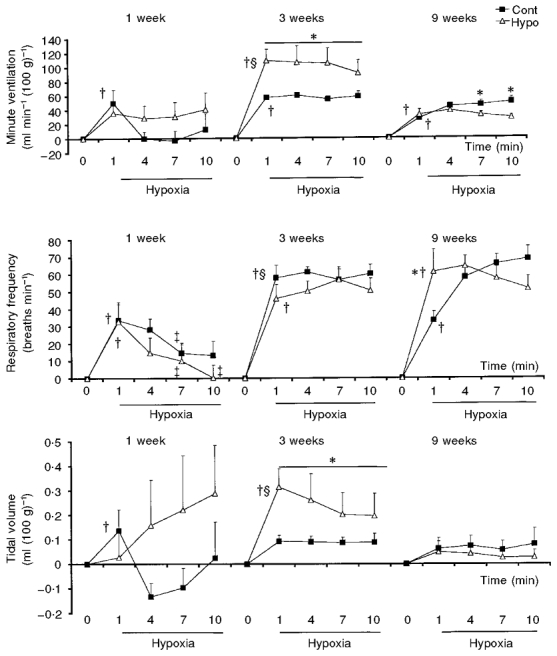
Graphs represent means ±s.e.m. of the differences between resting values and the data obtained during the hypoxic test, after 1, 4, 7 and 10 min of exposure, and therefore represent the response amplitude. Open triangles represent data obtained from rats from dams placed under hypoxia during pregnancy (Hypo) and filled squares represent data obtained from the control group of rats (Cont). In each graph: the left panel represents 1-week-old rats, the middle panel 3-week-old rats and the right panel 9-week-old rats. †P < 0.05, significant difference between hypoxic data and normoxic data within Cont or Hypo group; ‡P < 0.05, significant difference between hypoxic values at 4, 7 and 10 min compared with 1 min; *P < 0.05, significant difference between Cont and Hypo group; §P < 0.05, significant difference between data of 1- or 9-week-old rats compared with data of 3-week-old rats.
Metabolic data
In the Cont group, total body metabolism normalised to body weight slowly decreased with advancing age (Fig. 5). In resting conditions, metabolism of the 1-week-old rats of the Hypo group was significantly greater than that of the Cont group. No differences between the metabolism of the groups of age-matched rats were observed at 3 and 9 weeks of age. Immediately following exposure to hypoxia a significant decrease in metabolism was recorded in all animals (Fig. 5) except for the 3-week-old rats of the Hypo group. The decrease in metabolism was significantly less for the 1-week-old pups of the Hypo group compared with the decrease recorded for the age-matched Cont group. Thus, at 1 and at 3 weeks of age, the metabolism of rats exposed to hypoxia was greater for the Hypo group compared with the Cont group (Fig. 5). At 9 weeks, the metabolic response to hypoxia did not differ between the two groups.
Figure 5. Effect of prenatal hypoxia on metabolism in resting conditions and during a 10 % O2 hypoxic test of 1-, 3- and 9-week-old rats.
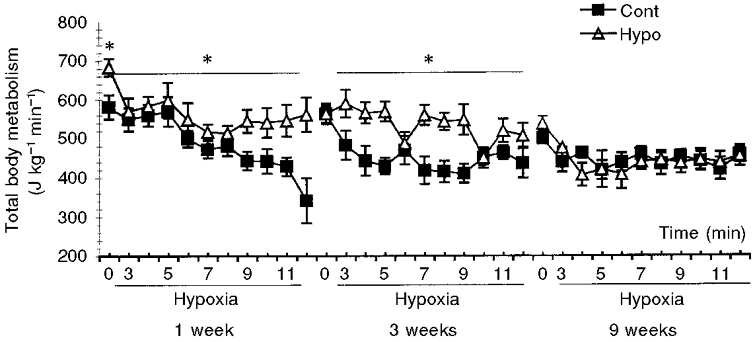
The graph represents means ±s.e.m. of metabolic data obtained in resting conditions (time 0) and in hypoxic conditions (10 % O2-90 % N2) for 12 min. The graph on the left represents 1-week-old rats (Cont, n = 8; Hypo, n = 7), the middle graph 3-week-old rats (Cont, n = 6; Hypo, n = 10) and the right graph 9-week-old rats (Cont, n = 8; Hypo, n = 8). ▵, rats from dams placed in hypoxia during pregnancy (Hypo); ▪, control animals (Cont). *P < 0.05, significant difference between Cont and Hypo group.
DISCUSSION
The findings of this study indicate that prenatal hypoxia lasting for 2 weeks pre-term results in: (1) a long-term postnatal alteration in neurochemical activity of carotid bodies and brainstem noradrenergic cell groups; and (2) an enhanced postnatal resting ventilation and metabolism and an impaired development of the ventilatory and metabolic response to hypoxia. The neurochemical and functional alterations of the chemoafferent pathway were particularly marked at 1 and 3 weeks of postnatal age, and were attenuated at 9 weeks.
Critique of experimental approach
Our approach – birth in normoxia, use of normoxic nursing dams in order to discard the noxious effect of hypoxia on the nutritional quality of milk – aimed to analyse the effects of prenatal hypoxia on the postnatal development. In order to eliminate the gender differences in ventilatory response and sensitivity to hypoxia (Mortola & Saiki, 1996), only male pups were used.
The pregnant rats remained awake, behaved normally and were not visibly stressed by hypoxic exposure. However, in addition to hypoxia itself, a number of factors might participate in the described postnatal effects. Indeed, caloric intake and hormonal status are altered as a consequence of long-term hypoxic exposure. However, food-restricted pregnant rats, used as controls for the reduced food intake due to hypoxia (Gleed & Mortola, 1991), showed a reduced placental weight compared with that of the ad libitum fed controls (De Grauw et al. 1986). In contrast, in our study, we found an increase in the placental weight and in the placenta/fetal body ratio of the pups exposed prenatally to hypoxia, this being a specific index of maternal hypoxia and not of maternal low protein diet (De Grauw et al. 1986). In agreement with these data, the size of the placenta of humans at high altitude varies inversely with oxygen availability (Kruger & Arias-Stella, 1970). Therefore, the lower weight of the 1-week-old rats that we report in our study does not appear to result only from the restricted maternal food intake but is rather related to maternal hypoxic exposure. In conclusion, although the degree of fetal hypoxaemia caused by exposure of the pregnant rats to hypoxia has not been evaluated in this study, our data on growth (Table 1) agree with previous reports (De Grauw et al. 1986; Gleed & Mortola, 1991; Harvey et al. 1993; White & Lawson, 1997) and strongly support the presence of fetal hypoxaemia during embryonic development.
Hypoxia in adults induces adaptative hormonal and neural changes leading to an improvement of oxygen supply. The levels of circulating catecholamine (Johnson et al. 1983) and glucocorticoids (Raff et al. 1983) are increased under long-term exposure to hypoxia. Although most maternal catecholamines and glucocorticoids are inactivated by the placenta, catecholamines might reduce the umbilical blood flow, thus leading to a decrease in the oxygen supply for the fetus and glucocorticoids might reduce the fetal body weight (Lindsay et al. 1996). Nevertheless, these factors, if they occurred, are consequences of exposure of the mother to hypoxia during gestation regardless of the mechanisms.
Prenatal hypoxia altered the chemical activity of carotid bodies and of the brainstem noradrenergic cell groups
In our first protocol, the effect of prenatal hypoxia on the chemoafferent pathway was analysed in four areas of importance for hypoxic adaptation.
Carotid bodies
Following prenatal hypoxia, the content of dopamine in the carotid bodies was significantly reduced until 9 weeks of postnatal age, which might contribute to alteration of chemosensitivity. Evidence generally suggests that, within days after birth, the peripheral chemoreceptors play a key role in maintaining and regulating breathing efforts. In order to play this role, carotid bodies have to reset their chemosensitivity at birth. The individual coming from a lower FI,O2 (inspired O2 fraction) environment needs to adapt to a new extra-uterine life. One index of this chemosensitivity resetting is the drop of dopamine content in carotid bodies followed by an increased catecholaminergic activity (Hertzberg et al. 1990; Gauda et al. 1996) within the first days of extra-uterine life. In the Hypo group of rats, the carotid bodies never gained the normal dopamine content. This situation could contribute to alteration of their chemosensitivity.
Central noradrenergic cell groups
In central regions, the noradrenergic activity of the brainstem areas was reduced within the first 3 postnatal weeks and was quite normalised at 9 weeks, or even enhanced for the A1 cell group. These medullary noradrenergic regions are part of a network involved in cardiorespiratory control, especially the caudal portion of the A2 cell group which is specifically relevant to the chemoreflex pathway (Soulier et al. 1992; Guyenet et al. 1993). However, punch removal of samples corresponds to a wide dissection that includes catecholaminergic neurones involved in cardiovascular as well as respiratory regulation. Thus our results might also have cardiovascular significance.
We conclude that prenatal hypoxia impairs the development of peripheral and central areas involved in cardiorespiratory control. The next question is whether these neurochemical changes have an impact on physiological function.
Prenatal hypoxia altered the resting minute ventilation and the ventilatory response to postnatal hypoxia
In our second protocol the resting minute ventilation and the hypoxic ventilatory response (reactivity index of the chemoafferent pathway) were measured in conscious 1-, 3- and 9-week-old rats of the Cont and Hypo groups using non-invasive barometric plethysmography (Bartlett & Tenney, 1970), adapted for pups.
Resting ventilation
Our baseline Cont group data are within the same range of values reported for neonates, prepubertal and adult rats of similar weights (Eden & Hanson, 1987; Mortola & Saiki, 1996). In the Hypo group, a greater ventilatory variability was noticeable in the 1-week-old rats compared with the control animals (as measured by the coefficients of variation in breath-to-breath ventilation), thus suggesting a less organised ventilatory control in these pups.
Offspring exposed to hypoxia during gestation displayed a greater resting ventilation than controls during the 3 postnatal weeks and a greater production of carbon dioxide at 1 week of age. The ratio V̇E/V̇CO2 was therefore enhanced in the Hypo group compared with the Cont until 3 weeks of age, indicating true ventilatory disturbances. By 9 weeks of age in both groups, resting ventilation was similar. Hyperventilation of this sort has been reported in 1-day-old pups born after entire gestation at high altitude (Gleed & Mortola, 1991). In our study, this increased resting minute ventilation was due to an increased respiratory frequency at 1 week of age, and to an increased tidal volume at 3 weeks of age, suggesting that age-dependent mechanisms are involved in determining the components of ventilation.
Ventilation during 10 min of hypoxia
Measuring the response to sustained hypoxia provides information about the time-dependent components which determine the overall ventilatory response, i.e.the initial step reflects the carotid body chemosensitivity and central processing, whilst the second phase of the response reflects the integration of peripheral chemosensory inputs and subsequent central adaptative events.
The 1-week-old pups of the Cont group exhibited a biphasic hypoxic ventilatory response. The ventilatory response to hypoxia changes with maturation, and is characteristically biphasic during the first days of postnatal life (Lahiri et al. 1978; Eden & Hanson, 1987) and can be recorded until 5, 7 or 14 days of postnatal age following exposure to hypoxic mixtures of 15, 12 or 8 % O2, respectively. This is thought to reflect, first, a stimulatory effect of hypoxia acting via the carotid chemoreceptors (Lahiri et al. 1978) and, second, a depressant effect arising from inhibitory mechanisms of brainstem origin (Nolan & Waldrop, 1996; Waites et al. 1996). The biphasic response to hypoxia is therefore not an ‘all-or-nothing’ phenomenon, and the pattern of hypoxic ventilatory response is dependent on maturational processes (Eden & Hanson, 1987). In contrast, 1-week-old rats exposed to hypoxia during gestation did not exhibit a significant initial increase in ventilation in response to 10 % O2. In the Hypo rats, the reduced dopamine content in carotid bodies might contribute to the lack of significant hypoxic response thus suggesting that these rats were less sensitive than the controls. In rats, the functional development of carotid bodies continues for up to 2 weeks after birth and this process of maturation has been previously reported to be altered by early postnatal hypoxia (Hertzberg et al. 1992). The present study indicates that prenatal hypoxia also altered the functional development of carotid bodies. In humans, the negative effects of hypoxia on carotid body sensitivity have been described in chronically hypoxic adults and children, and in persons born at a high altitude, all of whom develop long-lasting insensitivity to acute hypoxia (Lahiri, 1984).
At 3 weeks of age, the Hypo as well as the Cont group responded to hypoxia with a sustained increase in ventilation from the first minute of exposure. The response of the Hypo group was amplified compared with the response of the age-matched Cont group, due principally to a larger but variable tidal volume response. In addition, the enhanced ratio V̇E/V̇CO2 in the Hypo group indicates true ventilatory disturbances. Taken together, these data suggest that regulation of breathing remained altered during the 10 min test. By 9 weeks of age, the differences between the hypoxic ventilatory response of the control and the Hypo rats had largely disappeared. These results are consistent with those of Ling et al. (1996, 1998), who reported that the functional impairment of the ventilatory and phrenic responses of rats to hypoxia following perinatal hyperoxia was attenuated with advancing age, and was virtually eliminated by 15 months of age.
Correlation between neurochemistry and ventilation
Several neurotransmitters and modulators have been proposed as candidates for mediating hyperventilation following long-term hypoxia. Among these factors, ventilation is regulated by excitatory (glutamate, substance P), inhibitory (adenosine, GABA, enkephalin, opioid peptides, serotonin) and regulatory (monoamines) components. Any or all of these may be altered as a consequence of prenatal hypoxia. Our results indicate that this is the case for noradrenaline, the utilisation rate of which was reduced in the noradrenergic cell groups of the brainstem of the 3-week-old rats of the Hypo group. Since catecholamines depress respiration both at the carotid body and brainstem levels (Hilaire et al. 1993; Champagnat & Fortin, 1997), the reduced catecholaminergic activity in carotid bodies (indicated by lowered dopamine content) and in A1, A2c and A5 noradrenergic cell groups (indicated by lowered noradrenaline utilisation rate) until 3 weeks of age might contribute to the resting hyperventilation and the altered hypoxic ventilatory response during the first 3 weeks of life. Neurochemical alterations were still observed in carotid bodies and in the A1 cell group at 9 weeks of age together with discrete disturbances in the hypoxic ventilatory response indicating that the rats have not fully recovered at this age. Neurotrophic factors (brain derived neurotrophic factor, neurotrophin-4/5) located in the carotid bodies and petrosal ganglia (Hertzberg et al. 1992; Erickson et al. 1998) may also play a role, in view of the influence of these factors on breathing and chemosensory drive and on the expression of normal respiratory behaviour in newborn animals (Erickson et al. 1996).
Our ventilation study shows that respiratory frequency and tidal volume components are differently affected by prenatal hypoxia, thus suggesting that different mechanisms are involved in their regulation. The respiratory frequency is known to be modulated by the pontine A5 noradrenergic cell group which exerts a depressant effect via the α2 receptors (Errchidi et al. 1991). The tidal volume is dependent on the development of brainstem areas (Borday et al. 1997) and is considered to be a relatively direct index of the output of the brainstem control system to the respiratory motoneurones (Clark & von Euler, 1972; Blanco et al. 1984). The stimulation of tidal volume by hypoxia is markedly dependent upon precollicular structures (Martin-Body, 1988). At 3 weeks of age, the amplified increase and variability in tidal volume of the Hypo group (Fig. 4) might be associated with dysmaturity of the central network. In addition, this variability probably reflects a less organised ventilatory control.
In summary, neurochemical alterations resulting from prenatal hypoxia are probably implicated in the dysfunction of ventilation. Because the decrease in metabolic rate under acute hypoxia represents an important component of the respiratory drive, the next question is whether prenatal hypoxia affects the metabolic rate.
Prenatal hypoxia altered the resting metabolism and the metabolic response to postnatal hypoxia
In the additional experiment on body temperature, 10 min of hypoxic exposure decreased body temperature in the 1-week-old rats of the Hypo and the Cont groups. However, this drop is small compared with the metabolic drop in the 1-week-old animals. At 3 and 9 weeks of age, the drop in body temperature is not significant. No differences were observed between the two groups at any age. In fact, in some species, usually the largest, the drop in body temperature can be apparent only after long exposure, presumably because of the long time constant for passive heat exchange (Mortola & Gautier, 1995). For instance in kittens, V̇O2 can be 50 % of the control after 15–30 min of 10 % O2, while body temperature decreases by 1–1.5°C (Mortola & Rezzonico, 1988). Our 10 min hypoxic test was certainly not long enough to produce a significant body temperature drop.
In the Cont group used for metabolism measurements, metabolism normalised to body weight declined from 1 to 9 weeks. The most noticeable finding was that, in the Hypo group, the hypometabolism elicited by postnatal hypoxia was less than that of the Cont group at 1 week of age, and was absent at 3 weeks of age. The magnitude of the hypometabolic response to hypoxia depends on thermogenic components which are depressed by hypoxic exposure (Mortola & Gautier, 1995; Saiki & Mortola, 1997). In fact, hypothermia has been reported to attenuate the ventilatory response and increase the hypometabolic response to hypoxia (Saiki et al. 1994) in adult rats. In the Hypo rats, particularly those aged 3 weeks, we observed an opposite effect. Thus, the weak or absent hypometabolic response at 1 and 3 weeks of age suggests a disturbed thermogenesis as a consequence of prenatal hypoxia. We had expected a lower body temperature drop in the Hypo group than in the Cont group during the hypoxic challenge. A longer hypoxic test might have produced significant body temperature differences between the two groups of rats.
Conclusion
In conclusion, our neurochemical and ventilatory observations indicate that prenatal hypoxia impairs the postnatal development of: (1) the chemoafferent pathway participating in the establishment and regulation of resting ventilation and metabolism, and (2) the chemosensory defence mechanisms against an hypoxic challenge. Prenatal hypoxia may in fact lead to a long-term inability to modulate respiration and metabolism during postnatal hypoxia. The attenuated neurochemical and respiratory alterations which we observed at 9 weeks suggest, however, a remarkable plasticity of the developing chemoafferent pathway following long-lasting prenatal hypoxia but indicate that, at this age, the rats still exhibited some deficiencies when compared with control animals.
Acknowledgments
This study was supported by CNRS (UMR CNRS 5578) and by Région Rhône-Alpes (grant Souffrance Foetale et Maturation neuronale). J. C. Roux held a fellowship from the Région Rhône-Alpes. We gratefully acknowledge Dr Gary Cohen for English correction of the manuscript, and J. Pequignot, C. Perrin and S. Prevost for their skilful technical assistance.
References
- Bartlett D, Jr, Tenney SM. Control of breathing in experimental anemia. Respiration Physiology. 1970;10:384–395. doi: 10.1016/0034-5687(70)90056-3. [DOI] [PubMed] [Google Scholar]
- Blanco CE, Hanson MA, Johnson P, Rigatto H. Breathing pattern of kittens during hypoxia. Journal of Applied Physiology. 1984;56:12–17. doi: 10.1152/jappl.1984.56.1.12. [DOI] [PubMed] [Google Scholar]
- Borday V, Fortin G, Champagnat J. Early ontogeny of rhythm generation and control of breathing. Respiration Physiology. 1997;110:245–249. doi: 10.1016/s0034-5687(97)00089-3. [DOI] [PubMed] [Google Scholar]
- Champagnat J, Fortin G. Primordial respiratory-like rhythm generation in the vertebrate embryo. Trends in Neurosciences. 1997;20:119–124. doi: 10.1016/s0166-2236(96)10078-3. [DOI] [PubMed] [Google Scholar]
- Clark FJ, Von Euler C. On the regulation of depth and rate of breathing. The Journal of Physiology. 1972;222:267–295. doi: 10.1113/jphysiol.1972.sp009797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey MG, Moss TJ, McCrabb GJ, Harding R. Prematurity alters hypoxic and hypercapnic ventilatory responses in developing lambs. Respiration Physiology. 1996;105:57–67. doi: 10.1016/0034-5687(96)00038-2. [DOI] [PubMed] [Google Scholar]
- De Grauw TJ, Myers RE, Scott WJ. Foetal growth retardation in rats from different levels of hypoxia. Biology of the Neonate. 1986;49:85–89. doi: 10.1159/000242515. [DOI] [PubMed] [Google Scholar]
- Depocas F, Hart JS. Use of the Pauling oxygen analyser for measurement of oxygen consumption of animals in open-circuit and in a short lag closed-circuit apparatus. Journal of Applied Physiology. 1957;10:388–392. doi: 10.1152/jappl.1957.10.3.388. [DOI] [PubMed] [Google Scholar]
- Eden GJ, Hanson MA. Effect of hyperoxia from birth on the carotid chemoreceptor and ventilatory response to acute hypoxia. The Journal of Physiology. 1986;374:24P. doi: 10.1113/jphysiol.1987.sp016766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eden GJ, Hanson MA. Maturation of the respiratory response to acute hypoxia in the newborn rat. The Journal of Physiology. 1987;392:1–9. doi: 10.1113/jphysiol.1987.sp016765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellenberger HH, Feldman JL, Zhan WZ. Subnuclear organisation of the lateral tegmental field of the rat. II: Catecholamine neurones and ventral respiratory group. Journal of Comparative Neurology. 1990;294:212–222. doi: 10.1002/cne.902940206. [DOI] [PubMed] [Google Scholar]
- Erickson JT, Conover JC, Borday V, Champagnat J, Barbacid M, Yancopoulos G, Katz DM. Mice lacking brain-derived neurotrophic factor exhibit visceral sensory neurone losses distinct from mice lacking NT4 and display a severe developmental deficit in control of breathing. Journal of Neuroscience. 1996;16:5361–5371. doi: 10.1523/JNEUROSCI.16-17-05361.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson JT, Mayer C, Jawa A, Ling L, Olson EB, Jr, Vidruk EH, Mitchell GS, Katz DM. Chemoafferent degeneration and carotid body hypoplasia following chronic hyperoxia in newborn rats. The Journal of Physiology. 1998;509:519–526. doi: 10.1111/j.1469-7793.1998.519bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Errchidi S, Monteau R, Hilaire G. Noradrenergic modulation of the medullary respiratory rhythm generator in the newborn rat: an in vitro study. The Journal of Physiology. 1991;443:477–498. doi: 10.1113/jphysiol.1991.sp018846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley JC, Katz DM. The central organisation of carotid body afferent projections to the brainstem of the rat. Brain Research. 1992;572:108–116. doi: 10.1016/0006-8993(92)90458-l. [DOI] [PubMed] [Google Scholar]
- Gauda EB, Bamford O, Gerfen CR. Developmental expression of tyrosine hydroxylase, D2-dopamine receptor and substance P genes in the carotid body of the rat. Neuroscience. 1996;75:969–977. doi: 10.1016/0306-4522(96)00312-0. [DOI] [PubMed] [Google Scholar]
- Gleed RD, Mortola JP. Ventilation in new-born rats after gestation at simulated high altitude. Journal of Applied Physiology. 1991;70:1146–1151. doi: 10.1152/jappl.1991.70.3.1146. [DOI] [PubMed] [Google Scholar]
- Guyenet PG, Koshiya N, Huangfu D, Verberne AJ, Riley TA. Central respiratory control of A5 and A6 pontine noradrenergic neurons. American Journal of Physiology. 1993;264:R1035–1044. doi: 10.1152/ajpregu.1993.264.6.R1035. [DOI] [PubMed] [Google Scholar]
- Harvey LM, Gilbert RD, Longo LD, Ducsay CA. Changes in ovine foetal adrenocortical responsiveness after long-term hypoxemia. American Journal of Physiology. 1993;264:E741–747. doi: 10.1152/ajpendo.1993.264.5.E741. [DOI] [PubMed] [Google Scholar]
- Hertzberg T, Hellström S, Holgert H, Lagercrantz H, Pequignot JM. Ventilatory response to hyperoxia in newborn rats born in hypoxia – possible relationship to carotid body dopamine. The Journal of Physiology. 1992;456:645–654. doi: 10.1113/jphysiol.1992.sp019358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertzberg T, Hellström S, Lagercrantz H, Pequignot JM. Development of the arterial chemoreflex and turnover of carotid body catecholamines in the newborn rat. The Journal of Physiology. 1990;425:211–225. doi: 10.1113/jphysiol.1990.sp018099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilaire G, Morin D, Lajard A-M, Monteau R. Changes in serotonin metabolism may elicit obstructive apnoea in the newborn rat. The Journal of Physiology. 1993;466:367–382. [PMC free article] [PubMed] [Google Scholar]
- Housley GD, Sinclair JD. Localisation by kainic acid lesions of neurones transmitting the carotid chemoreceptor stimulus for respiration in rat. The Journal of Physiology. 1988;406:99–114. doi: 10.1113/jphysiol.1988.sp017371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TS, Young JB, Landsberg L. Sympathoadrenal responses to acute and chronic hypoxia in the rat. Journal of Clinical Investigation. 1983;71:1263–1272. doi: 10.1172/JCI110876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger H, Arias-Stella J. The placenta and the newborn infant at high altitudes. American Journal of Obstetrics and Gynecology. 1970;106:586–591. doi: 10.1016/0002-9378(70)90045-1. [DOI] [PubMed] [Google Scholar]
- Lagercrantz H, Pequignot J, Pequignot JM, Peyrin L. The first breaths of air stimulate noradrenaline turnover in the brain of the newborn rat. Acta Physiologica Scandinavica. 1992;144:433–438. doi: 10.1111/j.1748-1716.1992.tb09317.x. [DOI] [PubMed] [Google Scholar]
- Lahiri S. Respiratory control in Andean and Himalayan high-altitude natives. In: West JB, Lahiri S, editors. High Altitude and Man. Bethesda, MD, USA: American Physiological Society; 1984. pp. 147–162. [Google Scholar]
- Lahiri S, Brody JS, Motoyama EK, Velasquez TM. Regulation of breathing in new-borns at high altitude. Journal of Applied Physiology. 1978;44:673–678. doi: 10.1152/jappl.1978.44.5.673. [DOI] [PubMed] [Google Scholar]
- Lindsay RS, Lindsay RM, Edwards CR, Seckl JR. Inhibition of 11-beta-hydroxysteroid dehydrogenase in pregnant rats and the programming of blood pressure in the offspring. Hypertension. 1996;27:1200–1204. doi: 10.1161/01.hyp.27.6.1200. [DOI] [PubMed] [Google Scholar]
- Ling L, Olson EB, Jr, Vidruk EH, Mitchell GS. Attenuation of the hypoxic ventilatory response in adult rats following one month of perinatal hyperoxia. The Journal of Physiology. 1996;495:561–571. doi: 10.1113/jphysiol.1996.sp021616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling L, Olson EB, Jr, Vidruck EH, Mitchell GS. Slow recovery of impaired phrenic responses to hypoxia following perinatal hyperoxia in rats. The Journal of Physiology. 1998;511:599–603. doi: 10.1111/j.1469-7793.1998.599bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Body RL. Brain transections demonstrate the central origin of hypoxic ventilatory depression in carotid body-denervated rats. The Journal of Physiology. 1988;407:41–52. doi: 10.1113/jphysiol.1988.sp017402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortola JP, Gautier H. Interaction between metabolism and ventilation: Effects of respiratory gases and temperature. In: Dempsey JA, Pack AI, editors. Regulation of Breathing. New York, Basel, Hong Kong: Marcel Dekker, Inc.; 1995. pp. 1011–1063. [Google Scholar]
- Mortola JP, Rezzonico R. Metabolic and ventilatory rates in newborn kittens during acute hypoxia. Respiration Physiology. 1988;73:55–67. doi: 10.1016/0034-5687(88)90127-2. [DOI] [PubMed] [Google Scholar]
- Mortola JP, Saiki C. Ventilatory response to hypoxia in rats: gender differences. Respiration Physiology. 1996;106:21–34. doi: 10.1016/0034-5687(96)00064-3. [DOI] [PubMed] [Google Scholar]
- Nolan PC, Waldrop TG. Ventrolateral medullary neurons show age-dependent depolarizations to hypoxia in vitro. Developmental Brain Research. 1996;91:111–120. doi: 10.1016/0165-3806(95)00166-2. [DOI] [PubMed] [Google Scholar]
- Nyakas C, Buwalda B, Luiten PG. Hypoxia and brain development. Progress in Neurobiology. 1996;49:1–51. doi: 10.1016/0301-0082(96)00007-x. [DOI] [PubMed] [Google Scholar]
- Okubo S, Mortola JP. Long-term respiratory effects of neonatal hypoxia in the rat. Journal of Applied Physiology. 1988;64:952–958. doi: 10.1152/jappl.1988.64.3.952. [DOI] [PubMed] [Google Scholar]
- Palkovits M, Brownstein MJ. Maps and Guide to Microdissection of the Rat Brain. Amsterdam: Elsevier; 1988. [Google Scholar]
- Porter JC. Relationship of age, sex, and reproductive status to the quantity of tyrosine hydroxylase in the median eminence and superior cervical ganglion of the rat. Endocrinology. 1986;118:1426–1432. doi: 10.1210/endo-118-4-1426. [DOI] [PubMed] [Google Scholar]
- Raff H, Shinsako J, Keil LC, Dallman MF. Vasopressin, ACTH, and corticosteroids during hypercapnia and graded hypoxia in dogs. American Journal of Physiology. 1983;244:E453–458. doi: 10.1152/ajpendo.1983.244.5.E453. [DOI] [PubMed] [Google Scholar]
- Roth RH, Murrin LC, Walters JR. Central dopaminergic neurons: effects of alterations in impulse flow on the accumulation of dihydroxyphenylacetic acid. European Journal of Pharmacology. 1976;36:163–171. doi: 10.1016/0014-2999(76)90268-5. [DOI] [PubMed] [Google Scholar]
- Saiki C, Matsuoka T, Mortola JP. Metabolic-ventilatory interaction in conscious rats: effect of hypoxia and ambient temperature. Journal of Applied Physiology. 1994;76:1594–1599. doi: 10.1152/jappl.1994.76.4.1594. [DOI] [PubMed] [Google Scholar]
- Saiki C, Mortola JP. Effect of 2,4-dinitrophenol on the hypometabolic response to hypoxia of conscious adult rats. Journal of Applied Physiology. 1997;83:537–542. doi: 10.1152/jappl.1997.83.2.537. [DOI] [PubMed] [Google Scholar]
- Schmitt P, Soulier V, Péquignot JM, Pujol JF, Denavit-Saubié M. Ventilatory acclimatization to chronic hypoxia: relationship to noradrenaline metabolism in the rat solitary complex. The Journal of Physiology. 1994;477:331–337. doi: 10.1113/jphysiol.1994.sp020194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler FJ, Slotkin TA. Effects of acute hypoxia on neonatal rat brain: regionally selective, long-term alterations in catecholamine levels and turnover. Brain Research Bulletin. 1990;24:157–161. doi: 10.1016/0361-9230(90)90200-j. [DOI] [PubMed] [Google Scholar]
- Soulier V, Cottet-Emard JM, Pequignot J, Hanchin F, Peyrin L, Pequignot JM. Differential effects of long-term hypoxia on norepinephrine turnover in brain stem cell groups. Journal of Applied Physiology. 1992;73:1810–1814. doi: 10.1152/jappl.1992.73.5.1810. [DOI] [PubMed] [Google Scholar]
- Soulier V, Dalmaz Y, Cottet-Emard JM, Lagercrantz H, Pequignot JM. Long-term influence of neonatal hypoxia on catecholamine activity in carotid bodies and brainstem cell groups of the rat. The Journal of Physiology. 1997;498:523–530. doi: 10.1113/jphysiol.1997.sp021878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waites BA, Ackland GL, Noble R, Hanson MA. Red nucleus lesions abolish the biphasic respiratory response to isocapnic hypoxia in decerebrate young rabbits. The Journal of Physiology. 1996;495:217–225. doi: 10.1113/jphysiol.1996.sp021586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White LD, Lawson EE. Effects of chronic prenatal hypoxia on tyrosine hydroxylase and phenylethanolamine N-methyltransferase messenger RNA and protein levels in medulla oblongata of postnatal rat. Pediatric Research. 1997;42:455–462. doi: 10.1203/00006450-199710000-00006. [DOI] [PubMed] [Google Scholar]


