Abstract
The ATP action on spontaneous miniature glycinergic inhibitory postsynaptic currents (mIPSCs) was investigated in rat substantia gelatinosa (SG) neurons mechanically dissociated from the 2nd layer of the dorsal horn in which their presynaptic glycinergic nerve terminals remained intact.
ATP reversibly facilitated the frequency of the mIPSCs in a concentration-dependent manner without affecting their amplitude distribution. The ATP agonist, 2-methylthioATP (2MeSATP), mimicked the ATP action, while another ATP receptor agonist, αβ-methylene-ATP (α,β-meATP), had no effect on mIPSCs.
The ATP receptor antagonists, suramin (1 × 10−6 M) and pyridoxal-5-phosphate-6-azophenyl-2′,4′-disulphonic acid (PPADS) (1 × 10−5 M), completely blocked the facilitatory effect of ATP on glycine release (102·0 ± 11·2 % and 99·3 ± 16·2 %, n = 6, respectively) without altering the current amplitude distributions.
N-Ethylmaleimide (NEM), a sulphydryl alkylating agent, suppressed the inhibitory effect of adenosine on mIPSC frequency (111·2 ± 13·3 %, n = 4) without altering the current amplitude distribution. However, ATP still facilitated the mIPSC frequency (693·3 ± 245·2 %, n = 4) even in the presence of NEM.
The facilitatory effect of ATP (1 × 10−5 M) on mIPSC frequency was not affected by adding 1 × 10−4 M Cd2+ to normal external solution but was eliminated in a Ca2+-free external solution.
These results suggest that ATP enhances glycine release from nerve terminals, presumably resulting in the inhibition of SG neurons which conduct nociceptive signals to the CNS. This presynaptic P2X-type ATP receptor may function to prevent excess excitability in SG neurons, thus preventing an excessive pain signal and/or SG cell death.
Since Holton & Holton (1954) first proposed ATP as a possible neurotransmitter in the dorsal horn, it has been demonstrated that ATP can act as a fast excitatory neurotransmitter or cotransmitter in the central (Edwards et al. 1992; Jo & Schlichter, 1999) and peripheral nervous system (Evans et al. 1992; Galligan & Bertrand, 1994). Exogenous or synaptically released ATP causes a rapid depolarization of dorsal horn neurons (Jahr & Jessell, 1983; Bardoni et al. 1997) mediated by P2X receptor channel complexes in the postsynaptic membrane, which are non-selectively permeable to cations, including Ca2+ ions (Velera et al. 1994; Chen et al. 1995; Buell et al. 1996; Taschenberger et al. 1999). Seven different P2X receptor subunit genes have been cloned from various tissues (Chen et al. 1995; Lewis et al. 1995; Collo et al. 1996; Buell et al. 1996; Garcia-Guzman et al. 1997; North & Barnard, 1997). Of these cloned receptors, the ATP P2X1, P2X2, P2X3, P2X4, P2X5 and P2X6 receptor subunit RNAs are expressed in the superficial laminae of the dorsal horn (Collo et al. 1996; Vulchanova et al. 1996, 1998). Some reports have suggested that the numerous cases of neuronal colocalization of P2X4 and P2X6 or P2X2 and P2X3 subunits observed in the mammalian CNS reflect the native expression of heteromeric P2X4 + 6 or P2X2 + 3 channels (Garcia-Guzman et al. 1997; Le et al. 1998). The presence of these P2X receptor subunits in this region of the dorsal horn that receives input from slowly conducting sensory C fibres suggests that ATP may be involved in pain signalling (Li & Perl, 1995; Kennedy & Leff, 1995).
Nociceptive information arising from peripheral nociceptive receptors is transmitted to the superficial spinal cord laminae, particularly the substantia gelatinosa (SG) of the dorsal horn. Excitatory postsynaptic potentials evoked in SG neurons by primary afferent Aδ and C fibres are thought to be co-mediated by glutamate and ATP acting predominantly via non-NMDA and P2X receptors, respectively (Yoshimura & Jessell, 1990; Chen et al. 1995). It has also been reported that primary afferent Aδ fibres innervate glycinergic and/or GABAergic interneurons and that the activation of these interneurons through non-NMDA receptors evokes inhibition in nearby dorsal horn SG neurons (Yoshimura & Nishi, 1995). In neonates the same spinal interneuron may, in fact, release both GABA and glycine (Jonas et al. 1998) and GABA is co-released with ATP in cultured spinal neurons (Jo & Schlichter, 1999). The release of transmitter from these interneurons may also be affected by other neurotransmitter systems. For example, the GABAergic interneurons also possess muscarinic receptors on both axon terminals and somatodendritic sites. The activation of these muscarinic receptors enhances GABA release through a G protein-coupled mechanism (Baba et al. 1998). Interestingly, White et al. (1985) suggested that ATP might also be released from the interneurons in the dorsal horn area. Gu & MacDermott (1998) reported that ATP modulated glutamate release from the nerve terminals of sensory neurons in the dorsal horn. However, although glycine is a major inhibitory neurotransmitter in this area (Yoshimura & Nishi, 1995), little is known about the modulation of presynaptic glycine release by various neurotransmitters.
In this paper, we examine the effect of ATP on glycinergic transmission in SG neurons mechanically dissociated from the rat dorsal horn region. Such a dissociation procedure gives rise to single SG neurons isolated with intact presynaptic glycinergic terminals. Here we report that activation of ATP receptor (P2X) on these interneuronal nerve terminals increases glycine release and thus decreases the excitability of SG neurons.
METHODS
Preparation
Wistar rats, 10–14 days old, were decapitated under pentobarbitone anaesthesia (50 mg kg−1, i.p.). The spinal cord was quickly removed and was sliced at a thickness of 400 μm using a microslicer (DTK-1000, Dosaka, Kyoto, Japan). The spinal cord slices were kept in an incubation medium saturated with 95 % O2 and 5 % CO2 at room temperature (22-25°C) for at least 1 h. Thereafter, the slices were transferred into a 35 mm culture dish (Primaria3801, Becton Dickinson, NJ, USA) and the dorsal horn of the spinal cord was identified under a binocular microscope (SMZ-1, Nikon, Tokyo, Japan). A fire-polished glass pipette was placed lightly onto the surface of the dorsal horn and was vibrated horizontally. The speed and distance of pipette vibration were regulated by an AC power supplier and measured, on occasion, with the use of a digital video camera. The optimal conditions for the dissociated viable neurons were vibration of 3–5 Hz over a distance of 0.3-0.5 mm for about 2 min (Vorobjev, 1991; Rhee et al. 1999). The slices were then removed from the dish and the dissociated SG neurons left to adhere to the bottom of the dish within 10 min. These neurons, which were dissociated without using any enzymes, retained some of their original morphological features including the proximal dendritic processes.
All experiments conformed to the guiding principles for the care and use of animals approved by the Council of the Physiological Society of Japan and all efforts were made to minimize the number of animals used and their suffering.
Electrical measurements
Electrical measurements were performed in the nystatin-perforated patch whole cell recording mode at a holding potential of -60 mV under the voltage-clamp conditions. Patch pipettes were made from borosilicate glass tubes (1.5 mm o.d., 0.9 mm i.d.; G-1.5, Narishige, Tokyo, Japan) in two stages on a vertical pipette puller (PB-7, Narishige). The neurons were visualized with phase-contrast equipment on an inverted microscope (Diaphot, Nikon). The current and voltage were measured with a patch-clamp amplifier (CEZ-2300, Nihon Kohden, Tokyo, Japan), monitored on both an oscilloscope (Tektronix 5111A, Sony, Tokyo, Japan) and a pen recorder (Recti-Horiz 8K, Nippondenki San-ei, Tokyo, Japan) and stored on videotapes after digitization with a pulse-coded modulation processor (PCM-501 ES, Sony). The membrane currents were filtered at 1 kHz (E-3201A Dicade Filter, NF Electronic Instruments, Tokyo, Japan) and data were digitized at 4 kHz. The resistance between the recording electrode filled with the internal solution and the reference electrode in the external solution was 5–7 MΩ. All experiments were performed at room temperature (22-25°C).
Data analysis
Synaptic events were counted and analysed using DETECTiVENT (Ankri et al. 1994) and IGOR Pro software (Wavemetrics, Lake Oswego, OR, USA). Analysis of these miniature inhibitory postsynaptic currents (mIPSCs) was performed using cumulative probability plots and standard all-point histograms are also displayed for comparison. Amplitude histograms were binned in 5 pA intervals. Cumulative histograms were compared using the Kolmogorov-Smirnov test for significant difference. Statistically significant difference was assumed for P < 0.05. Numerical values are provided as means ± standard error of the mean (s.e.m.) Differences in mean amplitude and frequency were tested by Student's paired two-tailed t test.
Solutions
The ionic composition of the incubation medium was (mM): 124 NaCl, 5 KCl, 1.2 KH2PO4, 24 NaHCO3, 2.4 CaCl2, 1.3 MgSO4 and 10 glucose. The pH of the incubation medium was adjusted to 7.4 with 95 % O2 and 5 % CO2. The ionic composition of the external standard solution was (mM): 150 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 10 glucose and 10 Hepes. Ca2+-free external solution contained (mM): 150 NaCl, 5 KCl, 3 MgCl2, 10 glucose, 10 Hepes and 2 EGTA. For recording mIPSCs, these solutions routinely contained 3 × 10−7 M tetrodotoxin (TTX) to block voltage-dependent Na+ channels, 3 × 10−6 M 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) and 1 × 10−5 M dl-2-amino-5-phosphonovaleric acid (AP5) to block glutamatergic responses, and 3 × 10−6 M bicuculline to block the GABAA responses.
The ionic composition of the internal (patch pipette) solution for the nystatin-perforated patch recording was (mM): 20 N-methyl-D-glucamine methanesulphonate, 20 caesium-methanesulphonate, 5 MgCl2, 100 CsCl and 10 Hepes. The pH of the internal solution was adjusted to 7.2 with Tris-OH. Nystatin was dissolved in acidified methanol at 10 mg ml−1. The stock solution was added to the internal solution just before use at a final concentration of 100–200 μg ml−1.
Drugs
Drugs used in the present study were dl-2-amino-5-phosphonovaleric acid (AP5), bicuculline, 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), EGTA, N-ethylmaleimide (NEM), nystatin, pyridoxal-5-phosphate-6-azophenyl-2′,4′-di-sulphonic acid (PPADS), suramin, αβ-methylene-ATP (α,β-meATP) and 2-methylthio-ATP (2MeSATP) (Sigma, USA), Na-ATP (Yamasa, Japan), TTX (Wako Pure Chemicals, Japan). CNQX was dissolved in dimethyl sulphoxide (DMSO) at 1 × 10−2 M as a stock solution.
Drugs were applied using a ‘Y-tube’ perfusion system (Akaike & Harata, 1994), which enables a solution exchange within 20 ms.
RESULTS
Miniature inhibitory postsynaptic currents (mIPSCs) mediated by glycine receptors
Spontaneous postsynaptic currents were recorded from acutely dissociated substantia gelatinosa (SG) neurons from the rat spinal cord dorsal horn region (layer II) whose presynaptic nerve endings remained intact (the ‘synaptic bouton preparation’). These dissociated SG neurons had oval, fusiform or triangular somata with somatic diameters between 10 and 30 μm (Rexed, 1952; Gobel, 1978). The measurements were made using the nystatin-perforated patch recording mode at a holding potential (Vh) of -60 mV. As shown in Fig. 1A, these spontaneous inhibitory currents were blocked by strychnine in a reversible and dose-dependent manner in the presence of 3 × 10−7 M TTX, 3 × 10−6 M bicuculline, 3 × 10−6 M CNQX and 1 × 10−5 M AP5, indicating that the spontaneous miniature inhibitory postsynaptic currents (mIPSCs) are glycinergic.
Figure 1. Spontaneous glycinergic miniature inhibitory postsynaptic currents (mIPSCs) recorded from acutely dissociated single substantia gelatinosa (SG) neuron.
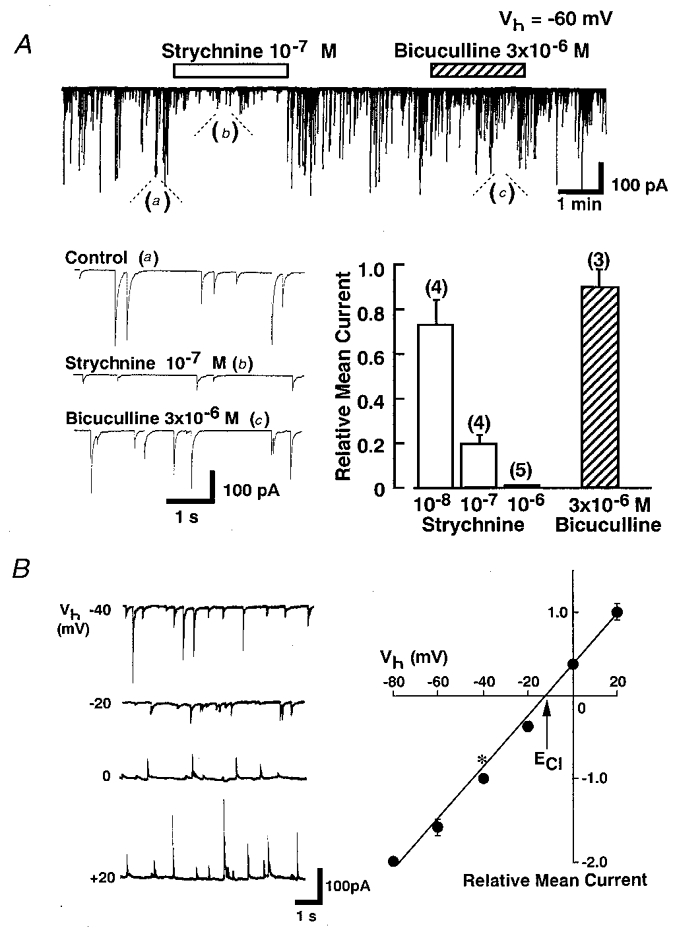
A, in the presence of TTX (3 × 10−7 M), AP5 (1 × 10−5 M) and CNQX (3 × 10−6 M), strychnine reversibly blocked the mIPSCs in a concentration-dependent manner at a holding potential (Vh) of -60 mV while bicuculline (3 × 10−6 M) had little effect on the postsynaptic currents. The relative mean current shows the amplitude ratio of mean mIPSCs before and during application of strychnine or bicuculline and the vertical bars show the s.e.m.B, the left panel shows representative recordings of mIPSCs at various Vh values while the right panel shows a plot of the mean amplitude of mIPSCs during a 3 min recorded period at various Vh values. All responses were normalized to the mean amplitude of mIPSCs at a Vh of -40 mV (*). Each point is the mean of 4 neurons. The reversal potential of mIPSCs was almost identical to the theoretical Cl− equilibrium potential (ECl). Vertical bars show ±s.e.m.
Figure 1B shows typical spontaneous glycinergic mIPSCs at various Vh values. The chloride equilibrium potential (ECl) of these mIPSCs, estimated from the I–V relationship, was about -12.0 ± 0.52 mV (n = 4), which was almost identical to the theoretical Cl− equilibrium potential (-10.4 mV) calculated from the Nernst equation using extra- and intracellular Cl− concentrations (161 and 110 mM, respectively).
Modulation of mIPSCs by ATP
In the present study, we examined the effect of ATP on glycinergic mIPSCs in 217 cells having oval and triangular somata with a thick proximal dendrite of 15 to 20 μm diameter. All experiments were conducted in the presence of 3 × 10−7 M TTX, 3 × 10−6 M CNQX, 1 × 10−5 M AP5 and 3 × 10−6 M bicuculline at a Vh of -60 mV. ATP induced postsynaptic inward current without changing mIPSC frequency in only 16 of 217 cells (Fig. 2Ac), and produced an inward current along with an increase of the mIPSC frequency in 36 of 217 cells (Fig. 2Ab). In the majority of cells (115/217), ATP increased markedly the frequency of spontaneous mIPSCs without eliciting any postsynaptic inward currents (Fig. 2Aa). In the remaining 50 cells, ATP apparently had no effect (Fig. 2Ad).
Figure 2. Effect of ATP on glycinergic mIPSCs.
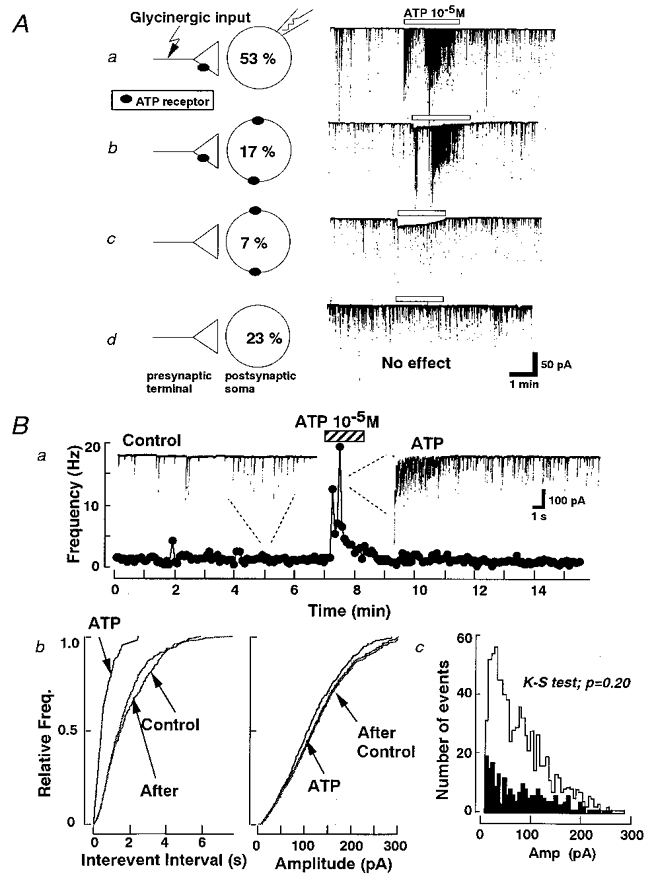
A, in the presence of TTX (3 × 10−7 M), AP5 (1 × 10−5 M), CNQX (3 × 10−6 M) and bicuculline (3 × 10−6 M), glycinergic mIPSCs were isolated. There were four types (a, b, c, d) of ATP actions observed in acutely dissociated SC neurons at a Vh of -60 mV. These actions are summarized schematically in the left hand panels (along with their incidences). Ba, time course of mIPSC frequency before, during and after the application of ATP to a single SG neuron. Each point represents mIPSC frequency counted in 10 s bins. During the 7 min control period mean IPSC frequency was 1.33 Hz. This increased to 5.55 Hz during the 2 min 30 s application of ATP without affecting the current amplitude. b, normalized cumulative probability curves show that 1 × 10−5 M ATP increased the mIPSC frequency (left) without altering the current amplitude distribution (right). c, amplitude histograms for control (▪) and ATP (□) from the same neuron.
In order to analyse the mechanism underlying the enhancement of the presynaptic glycine release by ATP, the following experiments were carried out only on the neurons exhibiting an increased mIPSC frequency without an inward postsynaptic current in the presence of ATP. Figure 2Ba shows that 1 × 10−5 M ATP significantly increased the frequency of mIPSCs from 1.13 ± 0.09 to 2.92 ± 0.20 Hz (n = 71). There was no statistically significant difference in the distribution of mIPSC amplitudes with or without ATP (P = 0.20 by the Kolmogorov-Smirnov test (K-S test), with 199 events for control and 822 for ATP) (Fig. 2B c). Figure 2Bb shows the cumulative probability plots of mIPSCs and Fig. 2B c shows the amplitude histogram for comparison. The enhancement of frequency was not accompanied by a change in the mIPSC amplitude, indicating that the probability of glycine release from the presynaptic nerve terminals was enhanced. After washing out ATP, the facilitatory effect disappeared with a variable time course.
Effect of N-ethylmaleimide (NEM)
We next examined whether the ATP-dependent increase of mIPSC frequency is mediated by a G protein. A previous report suggested that the inhibition of mIPSCs in the presence of adenosine was mediated by G protein (Li & Perl, 1994). N-Ethylmaleimide (NEM), a sulphydryl alkylating agent, itself enhanced the mIPSC frequency to 237 ± 24% of control (P < 0.05, paired two-tailed t test, n = 4) but had no effect on the distribution of their current amplitude (data not shown). Thus, we first examined whether NEM could remove an inhibitory effect of adenosine on the glycinergic mIPSCs in the present preparation. In the external solution without NEM treatment, 1 × 10−5 M adenosine significantly suppressed the mIPSC frequency to 62.4 ± 5.0 % of control (P < 0.05, paired two-tailed t test, n = 4) without affecting their amplitude distribution (110.1 ± 11.3 %, n = 4). The inhibitory effect of adenosine on mIPSC frequency was abolished by NEM treatment over 10 min (111 ± 13% of control in NEM-containing external solution, n = 4) (Fig. 3B). In contrast, such NEM treatment did not prevent the facilitatory action of 1 × 10−5 M ATP on mIPSC frequency or affect the amplitude distributions (P = 0.558 by K-S test; number of events: control, 217; and ATP, 266) (Fig. 3Ab). The ATP potentiated mIPSC frequency to 736.2 ± 171.1% (n = 4) in the control condition, and to 693.1 ± 245.0 % (n = 4) in the presence of NEM (Fig. 3B). These data clearly indicate that ATP-evoked facilitation is not mediated by metabotropic ATP receptors (P2Y receptors) coupled to G protein, but by ligand-gated purinoreceptors (P2X receptors).
Figure 3. NEM does not block the facilitatory action of ATP on mIPSC frequency.

Aa, mIPSCs recorded in the presence of NEM (left) and in the presence of NEM and ATP (right). Ab, normalized cumulative amplitude distributions (left) and cumulative frequency distributions (right) of mIPSCs in external solution with NEM, with or without ATP. Insets show the standard histograms of amplitude and inter-event intervals for control (thin line) and ATP (thick line) from the same neuron. B, summary of mean data illustrating ATP and adenosine actions on mIPSCs in the presence or absence of NEM. The relative iGly (left) shows the amplitude ratio of mean glycinergic mIPSCs before and during application of ATP or adenosine. The relative frequency (right) shows the frequency ratio of mean glycinergic mIPSCs before and during application of ATP or adenosine (**P < 0.001, n = 4). In the presence of NEM, ATP still potentiated mIPSC frequency without altering the current amplitude distribution. On the other hand, NEM completely blocked the inhibitory effect of adenosine on mIPSC frequency.
Effects of ATP agonists and antagonists
To identify the subtypes of ATP receptors participating in the increase of mIPSC frequency, the effects of various concentrations of ATP and of alternative agonists and antagonists were examined. ATP increased the frequency of mIPSCs in a concentration-dependent manner without significantly changing their amplitude distribution (P = 0.15by K-S test; number of events: control, 309; and 1 × 10−5 M ATP, 651) (Fig. 4Ab). 2-MethylthioATP (2MeSATP) mimicked the ATP action and also increased mIPSC frequency in a concentration-dependent manner without affecting the amplitude distribution (Fig. 4B). The potency of 2MeSATP for enhancing mIPSC frequency was, however, smaller than that of ATP. Another ATP receptor agonist, αβ-methylene-ATP (α,β-meATP), had no effect on mIPSC freqeuncy or amplitude. Figure 4B summarizes the dose-response curves for ATP, 2MeSATP and α,β-meATP with each point representing the mean from 30 cells.
Figure 4. Facilitatory effects of ATP and its agonist on mIPSC frequency.

A, ATP reversibly increased the mIPSC frequency in a concentration-dependent manner without altering the current amplitude distributions. a, representative traces of mIPSCs at various concentration of ATP. b, the normalized cumulative probability curves of mIPSC frequency (right) was shifted dose dependently to higher frequency values without changing the amplitude cumulative probability curves (left). These data were obtained from the same neuron. B, normalized concentration-response relationships for ATP (^, •) and 2-methylthio-ATP (□, ▪) (2MeSATP). 2MeSATP mimicked the ATP action. • and ▪ represent mean mIPSC frequency while ^ and □ represent mean current amplitude. Each point is the mean ±s.e.m. of 30 neurons.
The effects of ATP receptor antagonists, suramin and pyridoxal-5-phosphate-6-azophenyl-2′,4′-disulphonic acid (PPADS), were also examined. In the presence of 1 × 10−6 M suramin, the facilitatory effect of ATP on the mIPSC frequency distribution was completely abolished (102.3 ± 11.2 % of control, n = 6) (P = 0.30by K-S test; number of events: control, 96; and 1 × 10−5 M ATP, 78) without affecting the amplitude distribution (109.4 ± 11.3 % of control, n = 6) (P = 0.58 by K-S test; Fig. 5Ab). PPADS at 1 × 10−5 M also blocked ATP action on mIPSC frequency (99.2 ± 16.0 % of control, n = 6) (Fig. 5B). The 1 × 10−5 M PPADS itself did not change the mIPSC frequency and current amplitude distribution (84.2 ± 8.3 % and 96.3 ± 6.2 %, respectively; n = 9) (data not shown). After washing out these blockers, the inhibitory effect of suramin was completely reversed within 15 min while the PPADS action gradually disappeared over 40 min.
Figure 5. Effect of ATP antagonists.
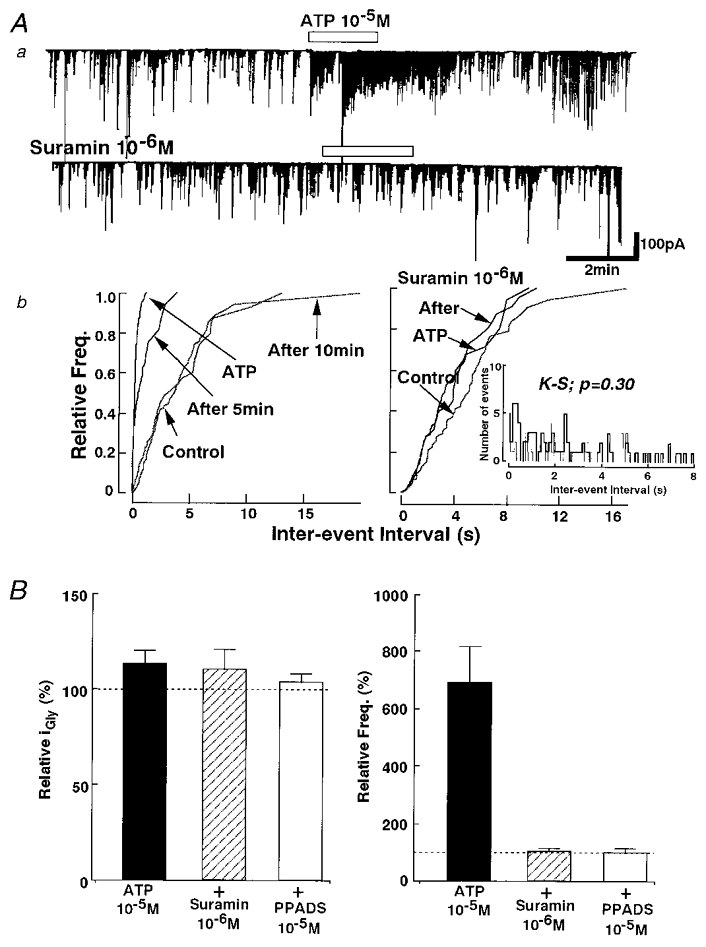
Aa, suramin (1 × 10−6 M), an ATP antagonist, abolished the facilitatory effect of ATP on glycinergic mIPSC frequency without affecting the current amplitude distribution. b, the normalized cumulative probability curves of mIPSC amplitude (left) and frequency (right) (inset shows inter-event interval histograms for control (thin line) and ATP (thick line) from the same neuron). B, complete block of ATP action on the mIPSC frequency was obtained by adding 1 × 10−6 M suramin or 1 × 10−5 M pyridoxal-5-phosphate-6-azophenyl-2′,4′-disulphonic acid (PPADS). Each column is the mean of the same 6 neurons in which both antagonists were tested.
[Ca2+]o dependency of ATP action
We examined whether voltage-dependent Ca2+ channels in the glycinergic nerve terminals participated in the facilitatory effect of ATP on the mIPSC frequency. In the presence of 1 × 10−4 M Cd2 +, a concentration which completely blocks high-voltage activated Ca2+ channels, mIPSC frequency was reduced by 58.1 ± 9.2 % of control solution (P < 0.01; n = 8) without any effects on the current amplitude distribution (99.1 ± 5.3 %) (Fig. 6B). This suggests that the existence of voltage-dependent Ca2+ channels contributes to spontaneous glycine release from these glycinergic presynaptic nerve terminals. Figure 6 shows the actions of ATP on mIPSCs in the presence of the Cd2+. ATP could still potentiate the mIPSC frequency (154.2 ± 0.3 %, P < 0.05, n = 8) without changing the distribution of current amplitudes (96.3 ± 3.1%, n = 8) (P = 0.184 by K-S test; number of events: control, 96; and 1 × 10−5 M ATP, 234) (Fig. 6Ab). When the relative potentiation rates of mIPSC frequency in the presence of ATP with or without Cd2+ were calculated, the values were 309.2 ± 56.3 % and 316.1 ± 67.2 % of control, respectively. The data show that even when the voltage-dependent Ca2+ channels are blocked, ATP still has a strong facilitatory effect on mIPSC frequency.
Figure 6. Voltage-dependent Ca2+ channels do not participate in ATP action on mIPSCs.
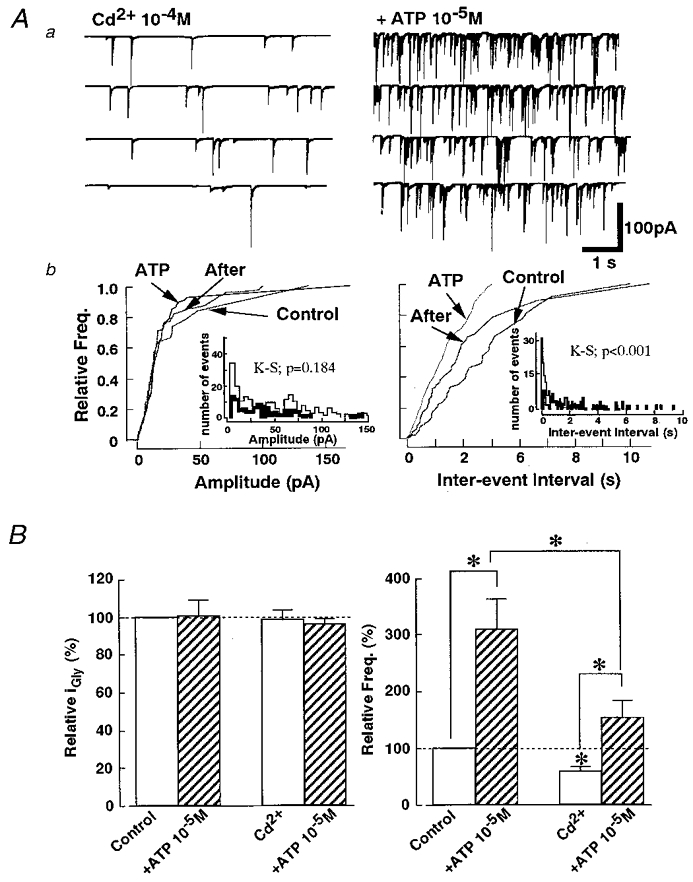
Aa, representative recordings of mIPSCs before and during application of ATP in the presence of 1 × 10−4 M Cd2+. b, cumulative probability curves show that 1 × 10−5 M ATP could increase the mIPSC frequency (right) without altering the current amplitude distribution (left) in the external solution containing Cd2+. Insets show the standard histograms of amplitude and inter-event intervals for control (thin line) and ATP (thick line) from the same neuron. B, the addition of Cd2+ significantly decreased the mIPSC frequency, but ATP still potentiated the mIPSC frequency. Each column is the mean data from 8 neurons (*P < 0.05).
The possibility remains that the ATP gated receptors may themselves be permeable to Ca2+ and this Ca2+ influx may directly contribute to the facilitation of glycine release. To examine this possibility, we investigated the effects of ATP in a Ca2+-free external solution. Ca2+-free external solution itself also reduced the mIPSC frequency to 56.1 ± 11.2 % of control (P < 0.05; n = 6) without affecting the amplitude distribution. This decrease in mIPSC frequency was almost the same as that observed in the Cd2+ solution. In the Ca2+-free solution, however, the facilitatory effect of ATP on the mIPSC frequency was completely abolished (P = 0.592 by K-S test; number of events: control, 208; and 1 × 10−5 M ATP, 176) (Fig. 7A). These results indicate that the increase in mIPSC frequency during ATP application is due to direct Ca2+ influx through the ATP-gated P2X receptor channel complexes present in the presynaptic nerve terminals of these glycinergic interneurons.
Figure 7. ATP action in Ca2+-free external solution.
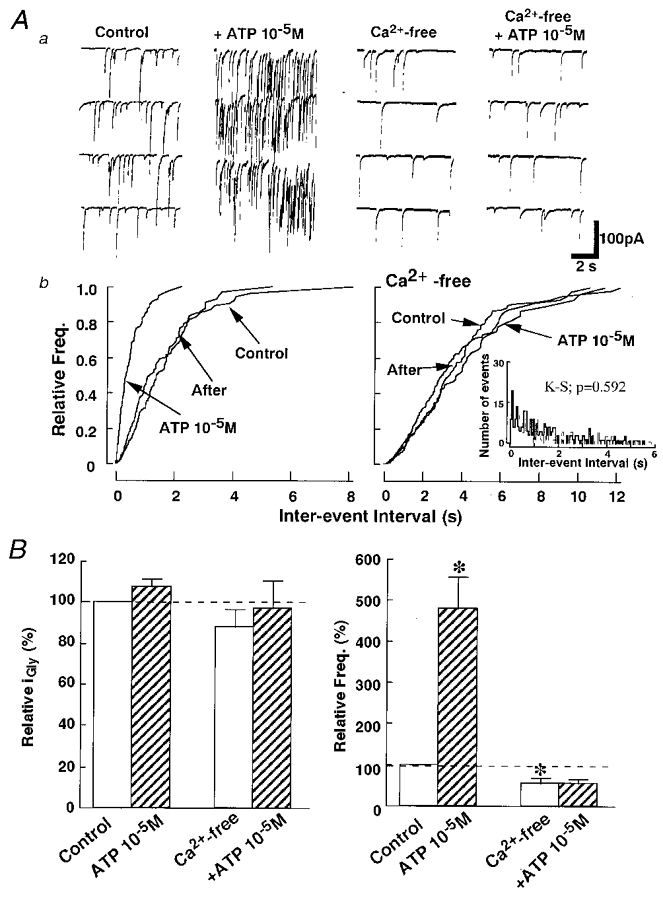
A, in a Ca2+-free external solution including 2 mM EGTA, ATP had no effect on mIPSCs. a, representative traces in control and Ca2+-free conditions. b, cumulative probability curves show that 1 × 10−5 M ATP did not alter the mIPSC frequency (right) in the absence of extracellular Ca2+ but facilitated glycine release in the standard external solution (left). The data were all obtained from the same neuron. Inset shows the standard histograms of inter-event intervals for control (thin line) and ATP (thick line) in Ca2+-free external solution. B, bar graphs showing summary of group data. Ca2+-free external solution had no significant effect on mean IPSC amplitude (left) but decreased the mIPSC frequency significantly and abolished the facilitatory effect of ATP. The reduced ratio of frequency compared to control in the standard solution was the same as that in 1 × 10−4 M Cd2+ external solution. Each column is the mean of 6 neurons (*P < 0.05).
DISCUSSION
In the present study, by using the novel ‘synaptic bouton’ preparation, we have demonstrated that glycine release from the nerve terminals of glycinergic interneurons projecting onto substantia gelatinsa (SG) neurons in layer II of the rat dorsal horn contain P2X-type ATP receptors and that activation of these receptors facilitates the release of glycine. ATP has been suggested to be a neurotransmitter in the dorsal horn (Jahr & Jessell, 1983; Salter & Hicks, 1994), and Gu & MacDormott (1998) have demonstrated that ATP mediates a fast synaptic current within the dorsal horn. However, some investigators have also suggested a role for ATP in the dorsal horn as a neuromodulator (Li & Perl, 1995). In this report, we have shown that ATP may have two functional roles: first, a direct action as an excitatory neurotransmitter on SG neurons; and second, an indirect action as a neuromodulator of presynaptic glycinergic interneurons, resulting in the inhibition of the SG neurons as illustrated in Fig. 8.
Figure 8. Proposed scheme for the facilitatory action of ATP on glycine release from the nerve terminals of glycinergic interneurons.
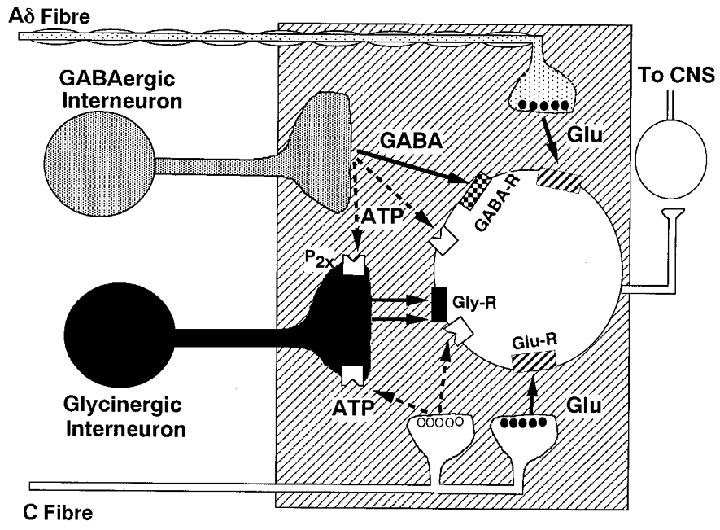
ATP released from C-fibre and/or GABAergic interneuron might activate P2X receptors existing on the presynaptic nerve terminals of glycinergic interneurons and/or the postsynaptic soma membrane of SG neurons.
P2X2 receptor modulating mIPSCs
We present here evidence for the presynaptic modulation of glycine release from glycinergic inhibitory interneurons by ATP. The ATP action on the glycinergic nerve endings seems to be mediated by ionotropic P2X receptor−channel complexes because: (1) the increase of spontaneous glycine release by ATP required extracellular Ca2+ (Fig. 7) but not the operation of the voltage-gated Ca2+ channels (Fig. 6); (2) the ATP effect was completely removed by suramin and PPADS; and (3) the ATP action was not affected by NEM, which blocks the function of G proteins (Fig. 3).
Collo et al. (1996) have reported that P2X2, P2X4 and P2X6 subunits of ATP receptors exist in the superficial dorsal horn using cDNA isolation. In situ hybridization experiments showed that the P2X1 and P2X2 receptors are found at the presynaptic sites of dorsal horn and nucleus of the solitary tract (Vulchanova et al. 1996). A pharmacological difference among ATP receptor subunits is that P2X2 subunits are sensitive to antagonists such as suramin and PPADS (Nakazawa et al. 1995), while homomeric P2X4 or P2X6 subunits are relatively insensitive. Moreover, the homomeric P2X2 receptors are sensitive to 2MeSATP but not to α,β-meATP (Buell et al. 1996; Collo et al. 1996). Furthermore, Le et al. (1998) suggested that heteromeric P2X receptors containing P2X4 subunits and P2X6 subunits, when expressed in Xenopus oocytes were sensitive to PPADS, suramin, 2MeSATP and αβ-Met-ATP. The pharmacological properties of our present data, showing that glycinergic mIPSC frequency was potentiated by 2MeSATP, but not by α,β-meATP, and that both suramin and PPADS effectively blocked the facilitatory effect of ATP on mIPSC frequency, suggest that the purinorecptors involved are likely to be P2X2 homomers. This result is also consistant with immunohistochemical results showing the presence of P2X subunits in dorsal horn (Kanjhan et al. 1999).
Functional role of P2X receptors in the glycinergic presynaptic nerve terminals
There are a number of papers demonstrating direct excitatory ATP responses in various peripheral and central sensory nuclei including neurons of nodose and trigeminal ganglia, dorsal root ganglia (DRG) and spinal dorsal horn (Holton, 1959; Jahr & Jessell, 1983; Salt & Hill, 1983; Krishtal et al. 1993). Exogenous ATP and other ATP analogues induce a slow onset pain sensation in the human blister base preparation (Biechen & Keele, 1977; Salter & Hicks, 1994; Lewis et al. 1995; Burnstock & Wood, 1996) and that activation of ATP receptors on small diameter (capsaicin-sensitive), primary afferent neurons in rat hindpaw mediates the behaviour indicative of acute nociception (Bland-Ward & Humphrey, 1997). Previous reports also showed that the ATP released from primary sensory nerve terminals excited dorsal horn neurons directly and also that ATP activated P2X receptors on glutamatergic nerve terminals (Vulchanova et al. 1996; Gu & MacDermott, 1998). These reports suggest that ATP might have an important excitatory role in mediating and/or modulating pain signalling.
In addition to these peripheral excitatory effects of ATP in nociception, there are some reports that ATP plays a central inhibitory role in nociception (Koizumi & Inoue, 1997). ATP may be released not only from sensory afferents, but also from interneurons (White et al. 1985) (Fig. 8). It is not unreasonable to expect that the released ATP might act on the nerve endings of inhibitory glycinergic and GABAergic interneurons, and in light of the present results, thereby increase the release of these inhibitory neurotransmitters from their nerve terminals, resulting in the inhibition of the attached SG neuron. Interestingly, a direct facilitatory postsynaptic inward current action was found, in the present study, in only 24 % of the neurons tested. In contrast, the enhancement of glycine release was observed in 70 % of neurons, suggesting that this indirect inhibitory action of ATP on SG neurons may dominate the direct excitatory action.
Jo & Schlichter (1999) have shown that GABA and ATP are co-released in the spinal cord. In the present study, it was unlikely that glycine and ATP were co-released from glycinergic presynaptic nerve terminals as PPADS itself did not alter the mIPSC frequency and amplitude despite blocking the action of exogenous ATP. These results suggested that the ATP might not be co-released with glycine.
Some reports have suggested that ATP may mediate the pain relief in response to mechano-sensory stimulation (Fyffe & Perl, 1984; Cook et al. 1997). ATP may mediate synaptic transmission from tactile mechano-receptors via fine-diameter afferent fibres to dorsal horn neurons (Salter & Henry, 1985). Since Aδ fibres innervate not only SG neurons, but also both GABAergic and glycinergic interneurons, the impulses evoked by mechano-stimulation could be conducted to these dorsal horn interneurons through Aδ fibres, increasing the release of inhibitory transmitters which may counteract the excitatory nociceptive transmission mediated directly by ATP and by glutamate (Yoshimura & Nishi, 1995). Released ATP may further facilitate glycine and GABA release via presynaptic receptors. Therefore, the mechano-stimulation of the area near the pain-generating spot may ameliorate the pain and ATP may contribute to a partial relief.
In conclusion, we have found that ATP increases glycine release from the nerve terminals of glycinergic interneurons in the dorsal horn region (Fig. 8). This may be an additional and important functional role of ATP providing a negative feedback system, to protect against excessive excitation of SG neurons in response to nociceptive input. In addition this ATP action may mediate the modulation of pain transmission by other afferent sensory neurons in the spinal cord.
Acknowledgments
The authors wish to thank Dr M. Brodwick for comments and Drs D. Sanes and Andrew Moorhouse for critically reading the manuscript and correcting the English. This study was supported by Grants-in-Aid for Scientific Research to J. S. Rhee and N. Akaike from the Japan Health Sciences Foundation, and N. Akaike (Nos 10044301 and 10470009) from the Ministry of Education, Science and Culture, Japan.
References
- Akaike N, Harata N. Nystatin perforated patch recording and its applications to analysis of intracellular mechanisms. Japanese Journal of Physiology. 1994;44:433–473. doi: 10.2170/jjphysiol.44.433. [DOI] [PubMed] [Google Scholar]
- Ankri N, Legendre P, Faber DS, Korn H. Automatic detection of spontaneous synaptic response in central neurons. Journal of Neuroscience Methods. 1994;52:87–100. doi: 10.1016/0165-0270(94)90060-4. [DOI] [PubMed] [Google Scholar]
- Baba H, Kohno T, Okamura M, Goldstein PA, Shimoji K, Yoshimura M. Muscarinic facilitation of GABA release in substantia gelatinosa of the rat spinal dorsal horn. The Journal of Physiology. 1998;508:83–93. doi: 10.1111/j.1469-7793.1998.083br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardoni R, Goldstein PA, Lee CJ, Gu JG, MacDermott AB. ATP P2X receptors mediate fast synaptic transmission in the dorsal horn of the rat spinal cord. Journal of Neuroscience. 1997;17:5297–5304. doi: 10.1523/JNEUROSCI.17-14-05297.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biechen T, Keele CA. Observations on the algogenic actions of adenosine compounds on the human blister base preparation. Pain. 1977;3:367–377. doi: 10.1016/0304-3959(77)90066-5. [DOI] [PubMed] [Google Scholar]
- Bland-Ward PA, Humphrey PP. Acute nociception mediated by hindpaw P2X receptor activation in the rat. British Journal of Pharmacology. 1997;122:365–371. doi: 10.1038/sj.bjp.0701371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buell G, Lewis C, Collo G, North RA, Surprenant A. An antagonist-insensitive P2X receptor expressed in epithelia and brain. EMBO Journal. 1996;15:55–62. [PMC free article] [PubMed] [Google Scholar]
- Burnstock G, Wood JN. Purinergic receptors: their role in nociception and primary afferent neurotransmission. Current Opinion in Neurobiology. 1996;6:526–532. doi: 10.1016/s0959-4388(96)80060-2. [DOI] [PubMed] [Google Scholar]
- Chen CC, Akopian AN, Silvilotti L, Colquhoun D, Burnstock G, Wood JN. A P2X purinoceptor expressed by a subset of sensory neurons. Nature. 1995;377:428–431. doi: 10.1038/377428a0. [DOI] [PubMed] [Google Scholar]
- Collo G, North RA, Kawashima E, Merio-Pich E, Neidhart S, Surprenant A, Buell G. Cloning of P2X5 and P2X6 receptors and the distribution and properties of an external family of ATP-gated ion channels. Journal of Neuroscience. 1996;16:2495–2507. doi: 10.1523/JNEUROSCI.16-08-02495.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook SP, Vulchanova L, Hargreaves KM, Elde R, McCleskey EW. Distinct ATP receptors on pain-sensing and stretch-sensing neurons. Nature. 1997;387:505–508. doi: 10.1038/387505a0. [DOI] [PubMed] [Google Scholar]
- Edwards FA, Gibb AJ, Colquhoun D. ATP receptor-mediated synaptic currents in the central nervous system. Nature. 1992;359:144–147. doi: 10.1038/359144a0. [DOI] [PubMed] [Google Scholar]
- Evans RJ, Derkach V, Surprenant A. ATP mediates fast synaptic transmission in mammalian neurons. Nature. 1992;357:503–505. doi: 10.1038/357503a0. [DOI] [PubMed] [Google Scholar]
- Fyffe RE, Perl ER. Is ATP a central synaptic mediator for certain primary afferent fibers from mammalian skin? Proceedings of the National Academy of Sciences of the USA. 1984;81:6890–6893. doi: 10.1073/pnas.81.21.6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galligan JJ, Bertrand PP. ATP mediates fast synaptic potentials in enteric neurons. Journal of Neuroscience. 1994;14:7563–7571. doi: 10.1523/JNEUROSCI.14-12-07563.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Guzman M, Stuhmer W, Soto F. Molecular characterization and pharmacological properties of the human P2X3 purinoceptor. Molecular Brain Research. 1997;47:59–66. doi: 10.1016/s0169-328x(97)00036-3. [DOI] [PubMed] [Google Scholar]
- Gobel S. Golgi studies of neurons in layer II of the dorsal horn of medulla (Trigeminal nucleus caudals) Journal of Comparative Neurology. 1978;180:395–414. doi: 10.1002/cne.901800213. [DOI] [PubMed] [Google Scholar]
- Gu JG, MacDermott AB. Activation of ATP P2X receptors elicits glutamate release from sensory neuron synapses. Nature. 1998;389:749–753. doi: 10.1038/39639. [DOI] [PubMed] [Google Scholar]
- Holton P. The liberation of adenosine triphosphate on antidromic stimulation of sensory nerves. The Journal of Physiology. 1959;145:124–140. doi: 10.1113/jphysiol.1959.sp006157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holton FA, Holton PJ. The capillary dilator substances in dry powders of spinal roots; a possible role of adenosine triphosphate in chemical transmission from nerve endings. The Journal of Physiology. 1954;126:124–140. doi: 10.1113/jphysiol.1954.sp005198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahr CE, Jessell TM. ATP excites a subpopulation of rat dorsal horn neurones. Nature. 1983;304:730–733. doi: 10.1038/304730a0. [DOI] [PubMed] [Google Scholar]
- Jo YH, Schlichter R. Synaptic corelease of ATP and GABA in cultured spinal neurons. Nature Neuroscience. 1999;2:241–245. doi: 10.1038/6344. [DOI] [PubMed] [Google Scholar]
- Jonas P, Bischofberger J, Sandkuhler J. Corelease of two fast neurotransmitters at a central synapse. Science. 1998;281:419–424. doi: 10.1126/science.281.5375.419. [DOI] [PubMed] [Google Scholar]
- Kanjhan R, Housley GD, Burton LD, Christie DL, Kippenberger A, Thorne PR, Luo L, Ryan AF. Distribution of the P2X2 receptor subunit of the ATP-gated ion channels in the rat central nervous system. Journal of Comparative Neurology. 1999;407:11–32. [PubMed] [Google Scholar]
- Kennedy C, Leff P. Painful connection for ATP. Nature. 1995;377:385–386. doi: 10.1038/377385a0. [DOI] [PubMed] [Google Scholar]
- Koizumi S, Inoue K. Inhibition by ATP of calcium oscillations in rat cultured hippocampal neurones. British Journal of Pharmacology. 1997;122:51–58. doi: 10.1038/sj.bjp.0701344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishtal OA, Marchenko SM, Pidolplichko VI. Receptor for ATP in the membrane of mammalian sensory neurones. Neuroscience Letters. 1993;35:41–45. doi: 10.1016/0304-3940(83)90524-4. [DOI] [PubMed] [Google Scholar]
- Le KK, Badinski K, Seguela P. Central P2X4 and P2X6 channel subunits coassemble into a novel heteromeric ATP receptor. Journal of Neuroscience. 1998;18:7152–7159. doi: 10.1523/JNEUROSCI.18-18-07152.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis C, Neidhart S, Holy C, North RA, Buell G, Surprenant A. Coexpression of P2X2 and P2X3 receptor subunits can account for ATP-gated currents in sensory neurons. Nature. 1995;377:432–435. doi: 10.1038/377432a0. [DOI] [PubMed] [Google Scholar]
- Li J, Perl ER. Adenosine inhibition of synaptic transmission in the substantia gelatinosa. Journal of Neurophysiology. 1994;72:1611–1621. doi: 10.1152/jn.1994.72.4.1611. [DOI] [PubMed] [Google Scholar]
- Li J, Perl ER. ATP modulation of synaptic transmission in the spinal substantia gelatinosa. Journal of Neuroscience. 1995;15:3357–3365. doi: 10.1523/JNEUROSCI.15-05-03357.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa K, Inoue K, Ito K, Koizumi S, Inoue K. Inhibition by suramin and reactive blue 2 of GABA and glutamate receptor channels in rat hippocampal neurons. Naunyn-Schmiedeberg's Archives of Pharmacology. 1995;351:202–208. doi: 10.1007/BF00169334. [DOI] [PubMed] [Google Scholar]
- North RA, Barnard EA. Nucleotide receptors. Current Opinion in Neurobiology. 1997;7:346–357. doi: 10.1016/s0959-4388(97)80062-1. [DOI] [PubMed] [Google Scholar]
- Rexed B. The cytoarchitectonic organization of the spinal cord in the cat. Journal of Comparative Neurology. 1952;96:415–496. doi: 10.1002/cne.900960303. [DOI] [PubMed] [Google Scholar]
- Rhee JS, Ishibashi H, Akaike N. Calcium channels in the GABAergic presynaptic nerve terminals projecting to meynert neurons of the rat. Journal of Neurochemistry. 1999;72:800–807. doi: 10.1046/j.1471-4159.1999.0720800.x. [DOI] [PubMed] [Google Scholar]
- Salt TE, Hill RG. Excitation of single sensory neurones in the rat caudal trigeminal nucleus by iontophoretically applied adenosine 5′-triphosphate. Neuroscience Letters. 1983;35:53–57. doi: 10.1016/0304-3940(83)90526-8. [DOI] [PubMed] [Google Scholar]
- Salter MW, Hicks JL. ATP-evoked increases in intracellular calcium in neurons and glia from the dorsal spinal cord. Journal of Neuroscience. 1994;14:1563–1575. doi: 10.1523/JNEUROSCI.14-03-01563.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter MW, Henry JL. Effects of adenosine 5′-monophosphate and adenosine 5′-triphosphate on functionally identified units in the cat spinal dorsal horn. Evidence for a differential effect of adenosine 5′-triphosphate on nociceptive vs. non-nociceptive units. Neuroscience. 1985;15:815–825. doi: 10.1016/0306-4522(85)90080-6. [DOI] [PubMed] [Google Scholar]
- Taschenberger H, Juttner R, Grantyn R. Ca2+-permeable P2X receptor channels in cultured rat retinal ganglion cells. Journal of Neuroscience. 1999;19:3353–3366. doi: 10.1523/JNEUROSCI.19-09-03353.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valera S, Hussy N, Evans RJ, Adami N, North RA, Surprenant A, Buell G. A new class of ligand-gated ion channels defined by P2X receptor for extracellular ATP. Nature. 1994;371:516–519. doi: 10.1038/371516a0. [DOI] [PubMed] [Google Scholar]
- Vorobjev VS. Vibrodissociation of sliced mammalian nervous tissue. Journal of Neuroscience Methods. 1991;38:145–150. doi: 10.1016/0165-0270(91)90164-u. [DOI] [PubMed] [Google Scholar]
- Vulchanova L, Arvidsson U, Riedl M, Buell G, Surprenant A, North RA. Differential distribution of two ATP-gated ion channels (P2X receptors) determined by immunocytochemistry. Proceedings of the National Academy of Sciences of the USA. 1996;93:8063–8067. doi: 10.1073/pnas.93.15.8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vulchanova L, Riedl MS, Shuster SJ, Buell G, Surprenant A, North RA, Elde R. Immunohistochemical study of the P2X2 and P2X3 receptor subunits in rat and monkey sensory neurons and their central terminals. Neuropharmacology. 1997;36:1229–1242. doi: 10.1016/s0028-3908(97)00126-3. [DOI] [PubMed] [Google Scholar]
- Vulchanova L, Riedl MS, Shuster SJ, Stone LS, Hargreaves KM, Buell G, Surprenant A, North RA, Elde R. P2X3 is expressed by DRG neurons that terminate in inner lamina II. European Journal of Neuroscience. 1998;10:3470–3478. doi: 10.1046/j.1460-9568.1998.00355.x. [DOI] [PubMed] [Google Scholar]
- White TD, Downie JW, Leslie RA. Characteristics of K+- and veratridine-induced release of ATP from synaptosomes prepared from dorsal and ventral spinal cord. Brain Research. 1985;334:372–374. doi: 10.1016/0006-8993(85)90235-5. [DOI] [PubMed] [Google Scholar]
- Yoshimura M, Jessell T. Amino acid-mediated EPSPs at primary afferent synapses with substantia gelatinosa neurones in the rat spinal cord. The Journal of Physiology. 1990;430:315–335. doi: 10.1113/jphysiol.1990.sp018293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura N, Nishi S. Primary afferent-evoked glycine- and GABA-mediated IPSPs in substantia gelatinosa neurones in the rat spinal cord in vitro. The Journal of Physiology. 1995;482:29–38. doi: 10.1113/jphysiol.1995.sp020497. [DOI] [PMC free article] [PubMed] [Google Scholar]


