Abstract
The K+ and Cl− currents activated by hypotonic cell swelling were studied in Ehrlich ascites tumour cells using the whole-cell recording mode of the patch-clamp technique.
Currents were measured in the absence of added intracellular Ca2+ and with strong buffering of Ca2+. K+ current activated by cell swelling was measured as outward current at the Cl− equilibrium potential (ECl) under quasi-physiological gradients. It could be abolished by replacing extracellular Na+ with K+, thereby cancelling the driving force. Replacement with other cations suggested a selectivity sequence of K+ > Rb+ > NH4≈ Na+≈ Li+; Cs+ appeared to be inhibitory.
The current-voltage relationship of the volume-sensitive K+ current was well fitted with the Goldman-Hodgkin-Katz current equation between -130 and +20 mV with a permeability coefficient of around 10−6 cm s−1 with both physiological and high-K+ extracellular solutions.
The class III antiarrhythmic drug clofilium blocked the volume-sensitive K+ current in a voltage-independent manner with an IC50 of 32 μM. Clofilium was also found to be a strong inhibitor of the regulatory volume decrease response of Ehrlich cells.
Cell swelling-activated K+ currents of Ehrlich cells are voltage and calcium insensitive and are resistant to a range of K+ channel inhibitors. These characteristics are similar to those of the so-called background K+ channels.
Noise analysis of whole-cell current was consistent with a unitary conductance of 5.5 pS for the single channels underlying the K+ current evoked by cell swelling, measured at 0 mV under a quasi-physiological K+ gradient.
Following hypo-osmotic cell swelling a regulatory volume decrease (RVD) response occurs by efflux of K+, Cl− and organic osmolytes accompanied by osmotically obliged water loss (Hoffmann & Dunham, 1995). Approximately 70 % of the osmolyte loss during the RVD response is accounted for by loss of KCl (Hendil & Hoffmann, 1974) via separate conductive pathways (Hoffmann et al. 1984, 1986). In patch-clamp studies using the cell-attached or the whole-cell configuration it has been demonstrated that the proposed efflux pathways for K+ and Cl− are indeed conductive (Christensen & Hoffmann, 1992; Riquelme et al. 1998). K+ channels activated upon hypo-osmotic cell swelling include: (i) Ca2+-dependent maxi-K+ channels as seen in e.g. lacrimal gland acinar cells (Park et al. 1994), proximal tubule cells (Dubéet al. 1990; Park et al. 1994), principal cells of cortical collecting tubule (Ling et al. 1992) and embryonic hepatocytes (Pon & Hill, 1997); (ii) voltage-dependent K+ channels, including the minK channel (Busch et al. 1992), and the delayed rectifier channels Kv1.3 and Kv1.5 as seen in, for example, lymphocytes (Lewis & Cahalan, 1995); and (iii) stretch-activated K+ channels such as those in the Necturus proximal tubule (Sackin, 1989) and gallbladder (Vanoye & Reuss, 1999) epithelial cells. The field of K+ channels activated by hypo-osmotic cell swelling has recently been reviewed (Wehner, 1998). In Ehrlich cells the K+ current activated by cell swelling (IK, vol) can be distinguished from that activated by an increase in intracellular Ca2+ concentration, [Ca2+]i, (IK, Ca) in the same cells by its lack of sensitivity to charybdotoxin and by its independence of intracellular Ca2+ (Riquelme et al. 1998; Hougaard et al. 2000). This agrees with earlier observations that the volume-activated K+ net efflux is insensitive to charybdotoxin and occurs without a detectable increase in [Ca2+]i (Jørgensen et al. 1997). The Cl− current activated by cell swelling (ICl, vol) has also been found to be clearly different from that activated by an increase in [Ca2+]i (ICl, Ca) (Pedersen et al. 1998).
Several inhibitors have been tested on Ehrlich cells as potential blockers of IK, vol and also of swelling-activated K+ permeability measured with radiotracers (Jørgensen et al. 1997; Riquelme et al. 1998). These include: apamin, clotrimazole and charybdotoxin, inhibitors of Ca2+-dependent or Ca2+-activated K+ channels; kaliotoxin, margatoxin and tetraethylammonium, inhibitors of different voltage-gated K+ channels. They have all been found to be ineffective or very weak inhibitors. Ba2+, tested for its ability to block either IK, vol or cell swelling-induced K+ efflux measured with radiotracers, had only a partial inhibitory effect (Jørgensen et al. 1997; Riquelme et al. 1998).
In the present study the cell swelling-activated K+ current of Ehrlich cells was further characterised by examination of its selectivity for other ions, dependence of conductance on cell membrane potential, and single-channel conductance derived by noise analysis. In addition, we show that the class III antiarrhythmic drug clofilium is a potent inhibitor of cell swelling-activated K+ currents and of the RVD response. Clofilium does not affect concomitantly activated Cl− currents and it is therefore a convenient tool to isolate the K+-mediated component of the RVD response.
METHODS
Cells
The Ehrlich ascites tumour cell line (hyperdiploid strain) was maintained by weekly intraperitoneal inoculation into CF-1 or NMRI mice. The inoculations were well tolerated by the mice, which showed no signs of pain or discomfort when harvesting took place 6 or 7 days later. The animals were killed by cervical dislocation, according to the guidelines of the local ethical committee. Cells were harvested into saline buffer (37°C) of the following composition (mM): 150 NaCl, 5 KCl, 1 MgCl2, 1 CaCl2, 3.3 Mops, 3.3 Tes and 5 Hepes. The pH was adjusted to 7.4 with Tris. The osmolarity of the medium was 300 ± 5 mosmol l−1. The cells were discarded 2 h after harvesting.
Electrophysiological experiments
For electrophysiological experiments, cells were transferred to 22 mm coverslips, and directly mounted on the microchamber of a warming stage system (Brook, IL, USA) installed in an inverted microscope. All experiments were performed at 37°C. Solution changes were effected by complete replacement of the chamber volume (400 μl) via a gravity-fed in-flow and a peristaltic pump-suctioned out-flow mechanism so that the level in the chamber was kept constant. The specific compositions of the bath and pipette solutions are given in the legends to the figures. The bath was grounded via an agar bridge. Standard whole-cell patch-clamp recordings were performed as described elsewhere (Díaz & Sepúlveda, 1995) using EPC-7 (List Medical, Darmstadt, Germany) or Axopatch 200B (Axon Instruments) amplifiers. Patch-clamp pipettes were made from thin borosilicate (hard) glass capillary tubing with an outer diameter of 1.5 or 1.7 mm (Clark Electromedical, Reading, UK) using a BB-CH puller (Mecanex, Geneva, Switzerland). The pipettes had a resistance of 3–5 MΩ. Voltage and current signals from the amplifier were recorded on a digital tape recorder (DTR-1204, Biologic, France) and digitised using a computer equipped with a Digidata 1200 (Axon Instruments) AD/DA interface. The voltage pulse generator and analysis programs were those written by J. Dempster (University of Strathclyde, Glasgow, UK) or were obtained from Axon Instruments. Changes in liquid junction potentials, which occurred as a result of bath solution changes during an experiment, were calculated (Barry, 1994) and current-voltage relations corrected accordingly.
Isolation of ICl and IK
In order to determine K+ and Cl− currents developing during cell swelling, a two-pulse protocol was used, as explained in detail previously (Riquelme et al. 1998). Briefly, the membrane potential was clamped at either the K+ or the Cl− equilibrium potential (EK and ECl) introducing a correction where exposure to hypotonicity led to cell swelling and consequent dilution of intracellular ion concentrations. In experiments designed to measure current- voltage relations, the two-pulse stimulation protocol was alternated with ramp stimulation as described in the Results section.
Noise analysis
Noise analysis was carried out on whole-cell currents during activation of the hypotonicity-induced current or its inhibition by clofilium (Sigworth, 1980; Heinemann & Conti, 1992; Heinemann, 1995). Current recordings obtained at ECl (measured with the above-described pulse protocol) were filtered with an 8-pole Bessel filter at 5 kHz and digitised at 10 kHz. Mean current (I) and variance (σ2) were calculated within each voltage pulse for individual cells, using Clampfit 8 software (Axon Instruments). For each experiment, the variance in isotonicity was subtracted from the variance once the conductance had been activated. Data were fitted using the following equation:
where i stands for single-channel current and N for the number of channels.
Solutions
The composition of the solutions used is given in Table 1. In cation substitution experiments the extracellular solution was that given as 100 mM Cl− in Table 1, but with 91 mM NaCl replaced with equimolar amounts of KCl, RbCl, CsCl, LiCl or NH4Cl.
Table 1. Composition of solutions.
| 10 mm Cl− | 37 mm Cl− | 100 mm Cl− | |
|---|---|---|---|
| Bath solutions | |||
| NaCl | 1 | 28 | 91 |
| Sodium gluconate | 90 | 62 | 0 |
| KCl | 5 | 5 | 5 |
| MgCl2 | 1 | 1 | 1 |
| CaCl2 | 1 | 1 | 1 |
| Pipette solutions | |||
| NaCl | 2 | 2 | 2 |
| KCl | 8 | 40 | 116 |
| Potassium gluconate | 108 | 76 | 0 |
| MgCl2 | 1.2 | 1.2 | 1.2 |
| EGTA | 10 | 10 | 10 |
Values are millimolar. All solutions contained 10 mm Hepes and were titrated to pH 7.4 with Tris. Na+ bath solutions described in the text and figures had the composition given above; K+ bath solutions had all sodium salts replaced by the respective potassium salts or the salt of the cation replacement specified. Osmolarities of pipette solutions were adjusted to 295 mosmol l−1 with d-mannitol. Hyposmotic bath solutions were left as reported above, giving an osmolarity value of ∼190 mosmol l−1. For isotonic solutions the same solutions were adjusted to 300 mosmol l−1 with D-mannitol. Note that the concentrations of Cl− in the pipette solutions, given as column headings, are calculated concentrations that take into account the dilution of the intracellular contents occurring upon osmotically induced cell swelling (Riquelme et al. 1998). All pipette solutions contained 1 mm ATP and 0.1 mm GTP.
Measurement of cell volume
Cell volume was determined by electronic cell sizing as described previously (Hoffmann et al. 1984) using a Coulter multisizer II (Coulter, Luton, UK); the tube orifice was 100 μm. The cytocrit value of the experimental solution was 0.008 %. The mean cell volume was calculated as the median of the cell volume distribution curves after calibration with latex beads (diameter, 14.1 μm).
Materials
Chemicals were from Sigma, Merck (Darmstadt, Germany), JT Baker (Deventer, Holland) and BRL (Gaithersburg, MD, USA) unless otherwise stated. Clofilium was dissolved in water.
RESULTS
Voltage dependence of the cell swelling-activated K+ current IK, vol of Ehrlich cells
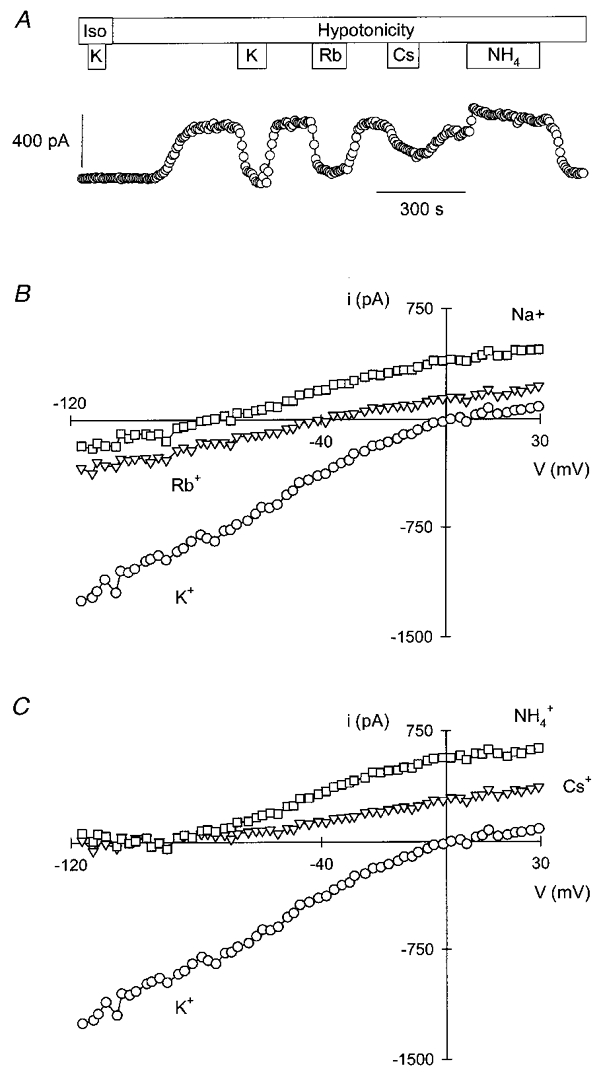
In order to isolate IK, vol from ICl, vol, advantage was taken of the fact that when low intracellular Cl− concentration and strong Ca2+ buffering are used, the latter current develops before the former upon osmotically induced cell swelling (Hougaard et al. 2000). At early times, therefore, the current-voltage relation for ICl, vol can be obtained without contamination from K+ current. This could be used to apply a correction to currents recorded once IK, vol has become activated. Experiments were therefore carried out using an intracellular Cl− concentration of 10 mM, with no added Ca2+ and with 10 mM EGTA in the pipette. To collect the information required, the voltage protocol depicted in Fig. 1A was used. Square voltage pulses to EK (-78 mV) and ECl (0 mV) were used to monitor ICl and IK, and a more complete current-voltage relation was collected by application of a ramp taking the potential from -130 to +80 mV. In Fig. 1B, the currents evoked by the square voltage pulses, ICl and IK, are plotted as a function of time. The currents were activated by decreasing the tonicity of the external medium without altering the ionic composition (removal of D-mannitol). In the experiment shown in Fig. 1B, Cl− current developed fully within 100 s of exposure to hypotonicity without any detectable K+ current, as witnessed by the lack of effect of replacing Na+ with K+ in the bath. If a K+ current had been activated this solution change would result in abolishment of outward current measured at 0 mV and inducement of an extra inward current at -78 mV (see below). In Fig. 1C, the current-voltage relations derived from measurements taken during a ramp stimulus in Na+- and K+-rich solution, depicted as C, K+ and C, Na+ in Fig. 1B, are shown. The two curves were very similar, and had the typical outwardly rectifying shape of the cell swelling-dependent anion currents described for Ehrlich cells as well as other cell types (Díaz et al. 1993; Pedersen et al. 1998). During development of ICl, vol there was an increase in the absolute magnitude of the current but no change in the shape of the current-voltage relation (not shown). Within the next 100 s of hypotonic exposure significant activation of IK, vol took place and replacement of the external Na+-rich solution with the K+-rich solution, as expected, markedly decreased outward current and evoked an extra inward current (Fig. 1B). Current-voltage relations were measured under these conditions at the times depicted in Fig. 1B as D, K+ and D, Na+ and the data were corrected for the contribution of ICl, vol. This correction resulted in the curves shown in Fig. 1D, where it can be seen that in Na+-rich medium the current was moderately outwardly rectifying with a reversal potential of around -80 mV. In K-rich medium, the current appeared ohmic in the -130 to +20 mV range, with some tailing off at more depolarised potentials and a reversal potential at around 0 mV. Similar results were obtained in seven separate experiments. The mean current- voltage relations for these experiments are shown in Fig. 2. Also shown in Fig. 2 are fits of the data to the Goldman-Hodgkin-Katz (GHK) current equation for the voltage range -130 to +20 mV. In this interval both sets of data were reasonably well described by the GHK equation with potassium permeability coefficient (PK) values of 1.04 × 10−6 and 0.96 × 10−6 cm s−1 for the Na+- and K+-rich solutions, respectively. At more depolarised potentials there were marked deviations from GHK behaviour, as illustrated by projecting the fits beyond +20 mV.
Figure 1. Current-voltage relation for swelling-activated K+ current (IK, vol) of Ehrlich cells.
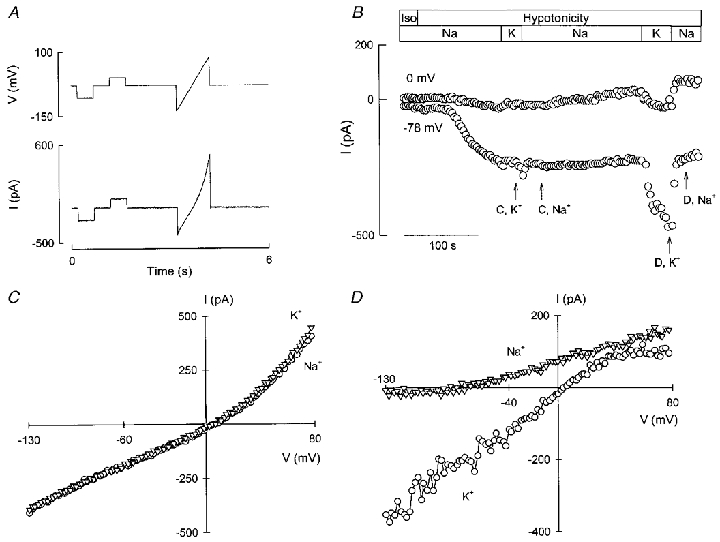
Currents were measured in the whole-cell recording mode of the patch-clamp technique, using the voltage protocol illustrated in the upper part of A, i.e. using a holding potential of -30 mV and pulsing to EK or ECl in square pulses of 500 ms followed by a ramp stimulus. The period of this two pulses + ramp protocol was 6 s. An example of a current trace during swelling is shown in the lower part of A. In B, the development of IK, vol at ECl (0 mV) and ICl, vol at EK (-78 mV) during swelling is depicted. The horizontal bars indicate the tonicity and the ionic composition of the solutions. C shows current-voltage relations taken before the development of IK, vol (C, K+ and C, Na+ in B). The current-voltage relations shown in D were taken at the points indicated in B (D, K+ and D, Na+) and were corrected for the contribution of ICl, vol as explained in the text. Solutions as in Table 1 (10 mM Cl−).
Figure 2. Current as a function of membrane potential for IK, vol of Ehrlich cells.
Data were obtained as described in the legend to Fig. 1 and are means of seven experiments. The lines shown are fits of the data to the GHK current equation, which gave PK values of 1.04 × 10−6 and 0.96 × 10−6 cm s−1 in Na+- and K+-rich hypotonic solutions, respectively. The average capacitance of the cells in these experiments was 15.5 pF.
Selectivity of IK, vol of Ehrlich cells
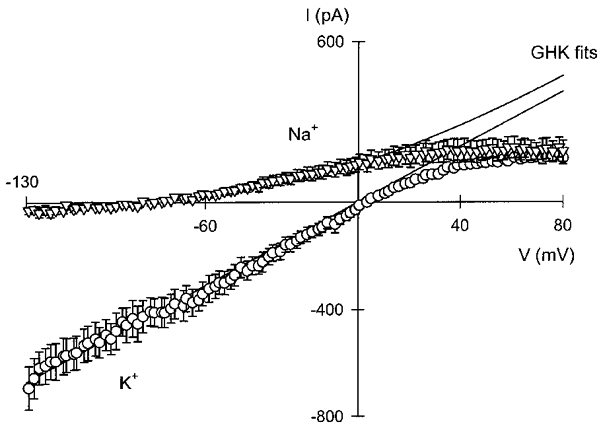
Figure 3 shows a typical ion replacement experiment designed to gauge the selectivity of IK, vol. In order to look for K+ and Cl− conductances activated by cell swelling, a 33 % decrease in the tonicity of the extracellular medium was effected without altering the ionic composition (removal of D-mannitol). The solutions used are shown in Table 1 as 100 mM Cl−. As seen from Fig. 3A, upon hypotonic cell swelling, after a delay, an outward current developed at ECl. Replacing the bathing solution with one in which the only monovalent cation was K+ abolished the current. This is consistent with the outward current seen in external Na+-rich solution being carried by K+. When Na+ in the extracellular solution was replaced by an equimolar amount of Rb+, there was also a prompt decrease in outward current, albeit of a lesser magnitude than that seen with K+, which was rapidly reversed upon re-addition of Na+. A similar manoeuvre using Cs+ as the replacement cation produced a decrease in the current whilst replacement with NH4+ did not decrease, and appeared slightly to increase, outward current. Replacement with Li+ was without effect (not illustrated). Similar results were obtained in three to six separate experiments for the different ion replacements mentioned. In the experiment illustrated, voltage ramps were also applied with a protocol similar to that illustrated in Fig. 1A. The current-voltage relations obtained in the different cation solutions are compared in Fig. 3B and C. Because IK, vol developed with a faster onset in the present high-Cl− experiments it was not possible to obtain a current-voltage curve directly. Consequently, ICl, vol used in the correction to resolve IK, vol was obtained from an early time ramp scaled to the value of current at EK from the same episode. Of the cations tested, the only one to show some inward current was Rb+. The reversal potential in the presence of this ion shifted about 40 mV in the depolarised direction. In three separate experiments the shift in reversal potential was consistent with a PRb/PK ratio of 0.18 ± 0.02. The reversal potential in Cs+ and NH4+ was indistinguishable from that in Na+, but Cs+ depressed outward current. Both cations blocked the small inward current seen in the 5 mM K+-containing, Na+-rich medium. The effect of NH4+ is interpreted with caution as possible cell loading with this cation can profoundly affect intracellular pH. This type of effect might be responsible for the current depression observed in Fig. 3A after the return to Na+-rich solution.
Figure 3. Effect of cation replacement on IK, vol in Ehrlich cells.
Currents were measured in the whole-cell recording mode of the patch-clamp technique, using the same voltage protocol as described in the legend to Fig. 1. In A, a record of the current measured at ECl is shown. At the times indicated, all NaCl in the solution was replaced by the Cl− salt of the indicated cation. In B and C, the current-voltage relations measured with the cations indicated as the main extracellular monovalent cation are shown. The composition of the solutions used is given in Table 1 (100 mM Cl−).
The search for a blocker of IK, vol in Ehrlich cells
Figure 4 shows the effect of clofilium, a class III antiarrhythmic drug, on ion currents elicited by hypotonic cell swelling in Ehrlich cells. After an inward current had developed at EK, a K+ current became activated as seen by a current increase at ECl. Replacing the bathing solution with one in which the only monovalent cation was K+, abolished outward current reversibly. When steady IK, vol became established, 100 μM clofilium was added to the bathing medium. A large inhibition of outward current took place, without any discernible effect on inward current. Figure 4B shows a separate experiment designed to test the ability of clofilium to block inwardly directed IK, vol. After currents had been activated by cell swelling, inward current was monitored at -78 mV. In Na+-rich medium the current at this potential is ICl, vol, but after switching to a high-K+ solution the extra inward current corresponds to IK, vol. Addition of 50 μM clofilium reduced inward current by more than 50 %, with a further addition increasing the inhibitor concentration to 100 μM producing complete blockade. That blockade of IK, vol was indeed complete was further suggested by the lack of effect of re-addition of the Na+-rich medium, implying that the current remaining in 100 μM clofilium corresponds to ICl, vol only. Finally, Fig. 4C shows the concentration dependence of clofilium inhibition of cell swelling-activated K+ current. The values for inhibition were calculated from experiments performed in K+-rich, Na+-free medium. As there was no voltage dependence of the inhibitor effect, values were obtained using current data from the complete voltage range applied in -130 to +80 mV ramps. Inhibition (percentage) was expressed as follows: inhibition = 100(1 –IK(xμM clof)/IK), where IK is the uninhibited K+ current defined as IK=I–I100 μM clof with I and I100 μM clof being total current in control and 100 μM clofilium, respectively. IK(xμM clof) stands for K+ current at a given (xμM) concentration of clofilium, calculated as IxμM clof–I100 μM clof. The data could be described by a Hill equation with an IC50 of 32 μM and nH value of 2.05.
Figure 4. Effect of clofilium on swelling-elicited currents in Ehrlich cells.
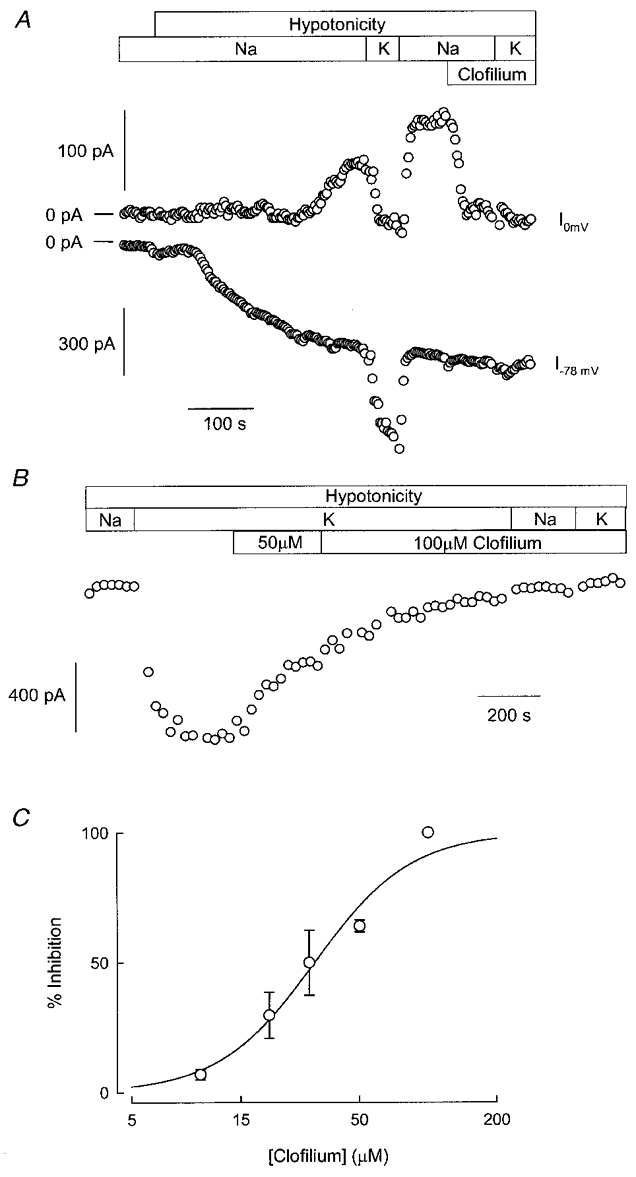
In A, the activation of IK, vol and ICl, vol during exposure to a hypotonic solution is shown. After full activation of both currents was achieved, 100 μM clofilium was added to the solution perfusing the cells. Changes in the composition of the bathing solutions are shown by the horizontal bars. B shows a recording from a separate experiment. Once IK, vol and ICl, vol had reached full activation in hypotonic solution, the inward current at -78 mV was followed. The horizontal bars show changes in solution composition, including the addition of clofilium at two different concentrations. C, concentration dependence of IK, vol inhibition by clofilium. The inhibition at 100 μM clofilium was taken as 100 %. Data are means ±s.e.m. from four to five separate experiments. The line represents the best fit to a Hill equation. The solutions used are given in Table 1 (37 mM Cl−).
Clofilium appears to be a specific inhibitor of IK, vol of Ehrlich cells. It should, therefore, be possible to use it to isolate this current from the total current elicited by hypotonic cell swelling, which should be made up of the sum of IK, vol and ICl, vol. That this is indeed the case is suggested by the experiment illustrated in Fig. 5. Current-voltage relations are shown in Fig. 5A for the currents produced in response to hypotonic cell swelling. Triangles show results for a Na+-rich bathing medium and circles those for a high-K+ medium. The squares illustrate equivalent data obtained in a Na+-rich medium containing 100 μM clofilium. Subtraction of the current remaining in the presence of clofilium from the total current gave the results illustrated in Fig. 5B. As expected these current-voltage relations were similar to those obtained in Fig. 1 for the K+ current, confirming the specificity of clofilium as a blocker of IK, vol. In Na+-rich medium the clofilium-inhibitable current was outwardly rectifying with a reversal potential near -80 mV. In K+-rich medium the current-voltage curve was linear between -130 and +30 mV and tailed off at more depolarised potentials. An experiment with 30 μM clofilium produced an intermediate blockade and allowed derivation of the current-voltage relation of the remaining IK. Despite the difference in magnitude, the shape of this curve was similar to that for uninhibited IK, consistent with the lack of voltage dependence. Similar results were obtained in nine separate experiments.
Figure 5. Resolution of IK, vol from total currents activated by cell swelling in Ehrlich cells based upon clofilium inhibition.
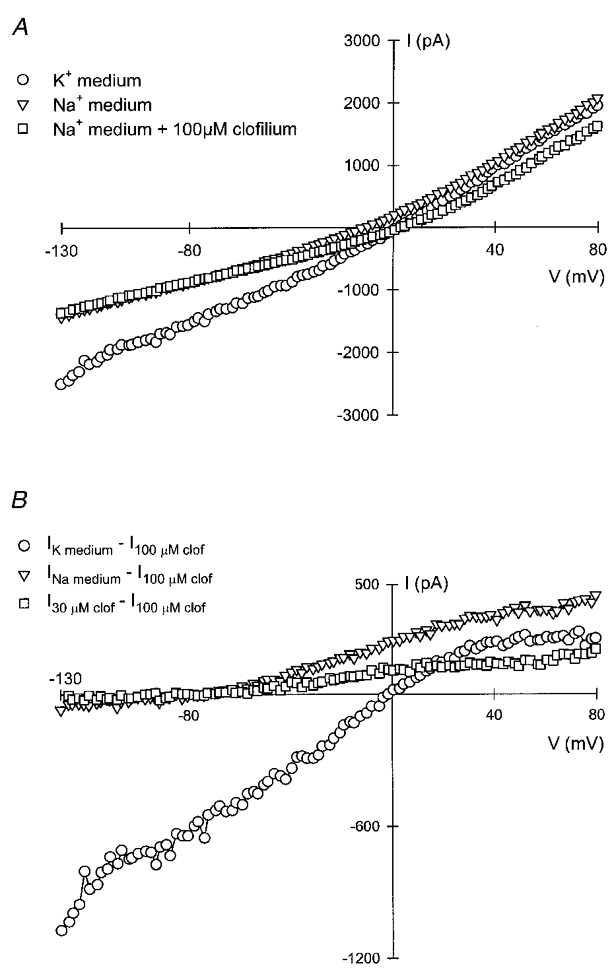
Currents were measured by the protocol described in the legend to Fig. 1 with the pipette and external solutions described in Table 1 (37 mM Cl−). In A, total currents measured in high-Na+ or high-K+ external solution, or after addition of 100 μM clofilium to the bathing medium are shown. In B, IK, vol was calculated by subtraction on the assumption that all IK, vol was inhibited by 100 μM clofilium (see Results).
If clofilium is an effective blocker of IK, vol it would be expected to inhibit the RVD response in Ehrlich cells. This was investigated by measuring the cell volume recovery after exposing the Ehrlich cells to hypo-osmotic medium in the presence or absence of 100 μM clofilium (Fig. 6). Cell volume did not vary when cells were suspended in isotonic medium. Exposure to a hypotonic medium was followed by a rapid (< 1 min) quasi-osmometric increase in cell volume, followed by rapid volume recovery. This RVD has been shown to depend upon the activation of K+, Cl− and organic osmolyte efflux. As shown in the inset to Fig. 6, the initial rate of RVD was markedly decreased from 328 fl min−1 under control conditions to 82 fl min−1 in the presence of 100 μM clofilium.
Figure 6. Effect of clofilium on the RVD response in Ehrlich cells.
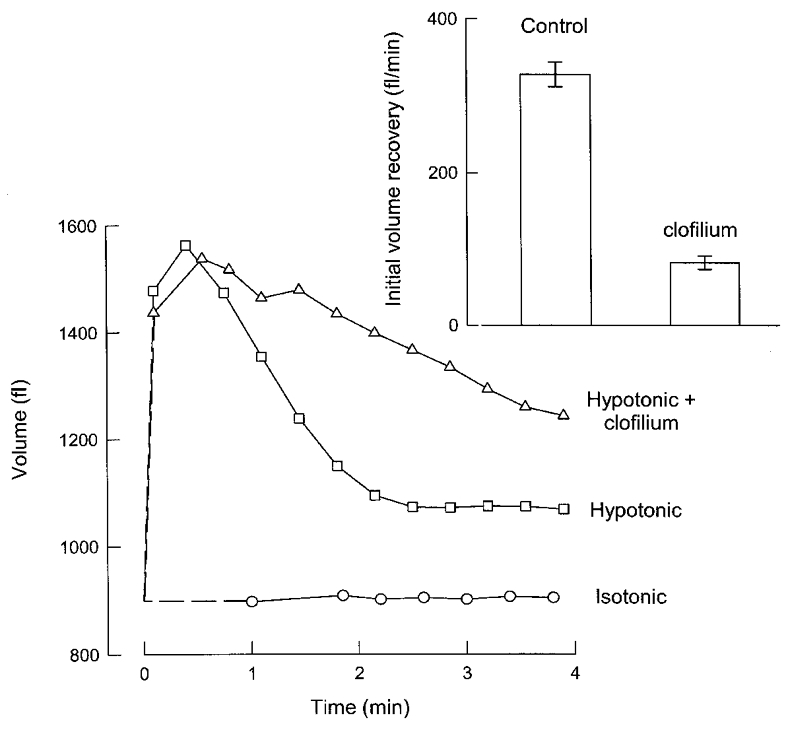
Clofilium (100 μM), when used, was added at time 0. The figure shows a single experiment representative of three independent experiments. The inset shows the initial volume recovery of Ehrlich cells suspended in a hypotonic solution in the presence or absence of 100 μM clofilium (means ±s.e.m. of three experiments).
Single-channel conductance estimated from noise analysis
Figure 7A shows the development of the swelling-activated K+ current, recorded at ECl, as a function of time. Each point corresponds to mean current measured during 400 ms current pulses. Variance was determined within each pulse for all episodes before and after the hypotonic challenge. In Fig. 7B, current records corresponding to the episodes indicated by the arrows in Fig. 7A are shown. The whole-cell current remained steady during the 400 ms pulse, because of the slow rate of increase of the swelling-activated K+ current. The corresponding plot of variance, corrected for variance before current activation, against mean current is shown in Fig. 7C. Data were fitted to the equation described in Methods (indicated by the continuous line in the graph), giving a single-channel amplitude of 0.48 pA and the number of channels, N, as 395. The single-channel current corresponds to a unitary conductance, γ, of 5.5 pS at 0 mV. Similar analysis in 13 independent experiments yielded a mean ±s.e.m. for i of 0.43 ± 0.03 pA.
Figure 7. Noise analysis of IK, vol.
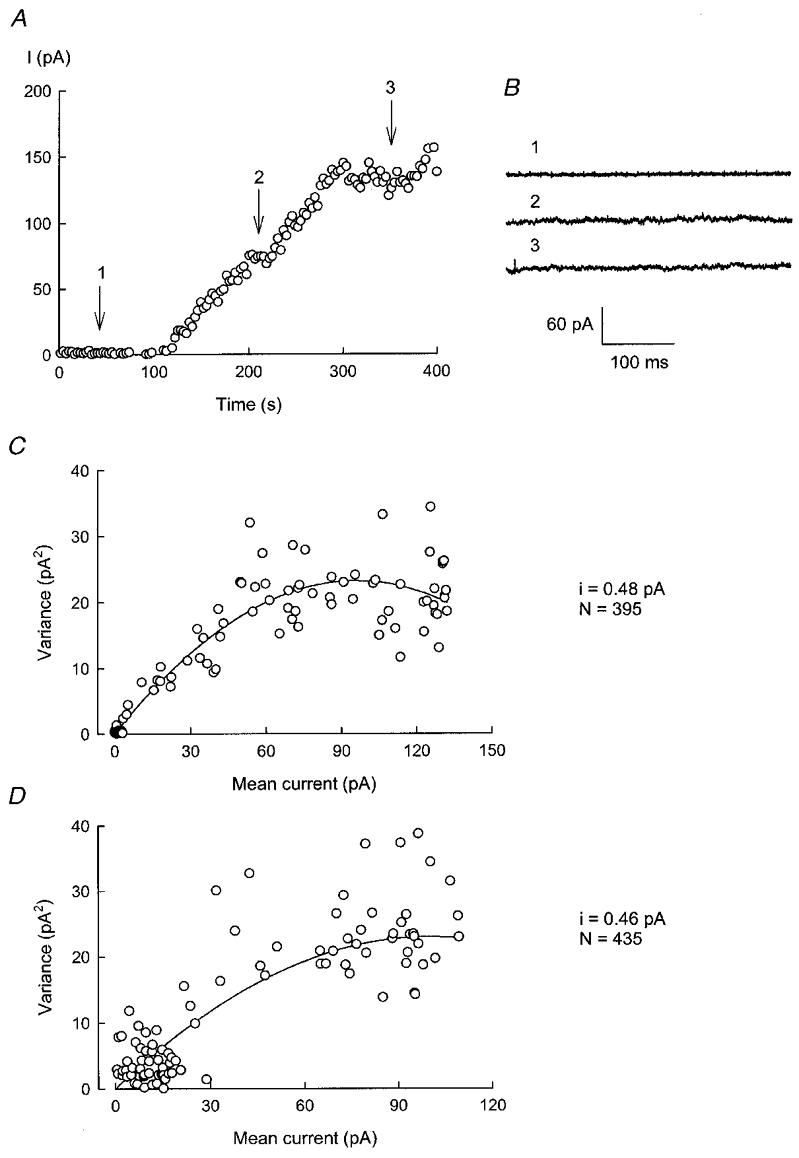
A shows the development of IK, vol as a function of time. Currents were recorded in isotonic solution and at various times after exposure to hypotonic medium, by pulsing to ECl as described in the legend to Fig. 1. In B, current traces taken at the points identified in A (1–3) are shown. C depicts the variance against mean current for the increase in IK, vol for the experiment shown in A. A similar analysis of data for the 100 μM clofilium-induced decrease in current is shown in D.
The noise associated with the decrease in current produced by increasing clofilium concentration to 10 and then to 100 μM was also analysed. The plot of variance against mean current for one such experiment is shown in Fig. 7D. The fit illustrated was obtained with respective i and N values of 0.46 pA and 435. In four separate experiments, the mean ±s.e.m. for i was 0.42 ± 0.01 pA.
DISCUSSION
Previous work on Ehrlich cells has shown that volume-sensitive K+ and Cl− channels are distinct from the Ca2+-activated K+ and Cl− channels also present in these cells (Jørgensen et al. 1997; Riquelme et al. 1998; Pedersen et al. 1998; Hougaard et al. 2000). The Ca2+-dependent K+ channel in Ehrlich cells has been characterised (Christensen & Hoffmann, 1992) as an intermediate conductance inwardly rectifying channel which has many similarities to the hIK1 channel recently cloned (Ishii et al. 1997; Jensen et al. 1998). On the other hand, the molecular counterpart of the volume-sensitive K+ channel is unknown. In the present report we have characterised further the K+ current activated by hypotonic swelling of Ehrlich cells with respect to selectivity, voltage dependence, apparent single-channel conductance and inhibitor profile.
One major problem encountered in studying IK, vol has been its occurrence together with a very large ICl, vol, making the distinction between the two currents difficult. In order to study the dependence of IK, vol upon voltage in isolation, we took advantage of the fact that under certain experimental conditions this current activates with a delay relative to that of ICl, vol. Except for potentials positive to +20 mV, the current-voltage relation of IK, vol appears to follow GHK behaviour for asymmetric K+ concentrations. In symmetrical K+, the current-voltage curve is nearly linear, which is also consistent with the constant field equation. This suggests that the channels underlying IK, vol lack intrinsic voltage dependence.
The K+ permeability pathway activated in Ehrlich cells upon hypotonic cell swelling, studied as current in patch-clamp experiments or from flux measurements (Jørgensen et al. 1997; Riquelme et al. 1998), has proved rather insensitive to a number of known K+ channel inhibitors. Among the potential inhibitors tested were those blocking different classes of Ca2+-dependent and voltage-gated K+ channels. These include the toxins charybdotoxin, kaliotoxin, margatoxin and apamin, TEA and clotrimazole. Ba2+ at millimolar concentrations acts as a partial inhibitor.
The lack of voltage dependence and peculiar channel blocker pharmacology of the volume-sensitive K+ current are reminiscent of the properties of members of a two-pore K+ channel family of recent discovery (Goldstein et al. 1998). The TRAAK channel, a member of the two-pore channel family, is, like the volume-sensitive K+ channel in Ehrlich cells, insensitive to TEA and only partially inhibited by Ba2+ (Fink et al. 1998). Likewise, the current-voltage relationships of the TRAAK-related currents also follow the GHK equation (Fink et al. 1998) and have been described to be mechano-sensitive (Maingret et al. 1999). However, unlike the TRAAK channel, the volume-sensitive K+ channel in Ehrlich cells is not stimulated, but rather is inhibited by arachidonic acid (M. I. Niemeyer, unpublished results). In addition, the single-channel conductance of the channels underlying IK, vol in Ehrlich cells, estimated from noise analysis, appears to be smaller by one order of magnitude than that described for the TRAAK channel. Another K+ channel insensitive to membrane voltage is the stretch-activated K+ channel located in the basolateral membrane of Necturus gallbladder epithelial cells (Vanoye & Reuss, 1999). This channel is quite permeable to Rb+, is insensitive to TEA but can be fully inhibited by Ba+ and Gd3+. Gd3+ has previously been shown to inhibit the RVD response in Ehrlich cells, although the non-selective stretch-activated cation channel described in these cells is insensitive to Gd3+ (Christensen & Hoffmann, 1992). The current-voltage curve (in Na+ medium) of the cell swelling-activated K+ current described here resembles that for a similarly activated conductance in an amphibian cell line of kidney origin (Nilius et al. 1995), of which there has been no further characterisation.
We report here that IK, vol is efficiently inhibited, however, by the quaternary ammonium derivative clofilium in the micromolar concentration range. The inhibition appears to be specific for IK, vol and independent of voltage. Subtraction of clofilium-insensitive currents from total current activated by cell swelling yields the same type of current-voltage curve for IK, vol as is obtained by using the activation delay approach explained above. Noise analysis performed on the progressive inhibition of IK, vol by clofilium was consistent with a slow blockade. This class III antiarrhythmic drug has been shown to block various types of K+ channel including Kv1.5, Kir, HERG and KvLQT1 (Malayev et al. 1995; Bhattacharyya et al. 1997; Yang et al. 1997; Suessbrich et al. 1997). All these channels seem, however, to be voltage dependent and sensitive to clofilium in the nanomolar to low micromolar range. We do not regard these channels, therefore, as candidates for the swelling-activated K+ channel in Ehrlich cells.
In accordance with the inhibitory effects of clofilium on the volume-sensitive K+ current in Ehrlich cells, clofilium is shown here to also be a potent inhibitor of RVD in Ehrlich cells, with the rate of volume recovery being inhibited by about 80 % with 100 μM clofilium. One question arising from these experiments is the cause of the residual RVD observed at a concentration of clofilium that blocks IK, vol completely. Some proportion of RVD, near 30 % (Hendil & Hoffmann, 1974), is due to efflux of organic osmolytes and is not expected to be inhibited by clofilium (Sakai & Tosaka, 1999). There is also a discrepancy between the short delay for the onset of the RVD response (30–60 s) and the relatively longer delay for activation of IK, vol in the present experiments. The onset time for IK, vol and the delay between the onset of activation of IK, vol and ICl, vol is shorter in cells with normal intracellular Ca2+ and high Cl− concentrations (Riquelme et al. 1998), and these differences might account for the discrepancy.
In conclusion, we have described a small-conductance swelling-activated K+ channel in Ehrlich cells, which is voltage independent and has a permeability sequence of K+ > Rb+ > NH4+ ≡ Na+≡ Li+; Cs+ appeared to be inhibitory. In addition, this conductance was found to be blocked specifically by clofilium. The current described here shares similarities with that associated with two-pore background channel activity, but its molecular identity remains unknown.
Acknowledgments
We are grateful to M.-A. Valverde for suggesting to us the use of clofilium and for helpful discussions. This work was supported by grants from Fondecyt, Chile (1980718), the Volkswagen Stiftung, Germany, the Carlsberg Foundation, Denmark (970438-40-1256) and the Danish Research Councils (9502762). Institutional support to the Centro de Estudios Científicos (CECS) from a group of Chilean private companies (AFP Protección, CGE, Codelco, Copec, Empresas CMPC, Gener S.A., Minera Collahuasi, Minera Escondida, Novagas, Business Design Associates, Xerox Chile), Fuerza Aérea de Chile and Municipalidad de Las Condes is also acknowledged. The research of F.V.S. was supported in part by an International Research Scholars grant from the Howard Hughes Medical Institute and a Cátedra Presidencial en Ciencias. CECS is a Millennium Science Institute.
References
- Barry PH. JPCalc, a software package for calculating liquid junction potential corrections in patch-clamp, intracellular, epithelial and bilayer measurements and for correcting junction potential measurements. Journal of Neuroscience Methods. 1994;51:107–116. doi: 10.1016/0165-0270(94)90031-0. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya ML, Sarker S, Mull KP, Debnam Q. Clofilium-induced block of delayed rectifier type K+ current in atrial tumor cells (AT-1 cells) Journal of Molecular and Cellular Cardiology. 1997;29:301–307. doi: 10.1006/jmcc.1996.0275. [DOI] [PubMed] [Google Scholar]
- Busch AE, Varnum M, Adelman JP, North RA. Hypotonic solution increases the slowly activating potassium current IsK expressed in Xenopus oocytes. Biochemical and Biophysical Research Communications. 1992;184:804–810. doi: 10.1016/0006-291x(92)90661-4. [DOI] [PubMed] [Google Scholar]
- Christensen O, Hoffmann EK. Cell swelling activates K+ and Cl− channels as well as nonselective, stretch-activated cation channels in Ehrlich ascites tumor cells. Journal of Membrane Biology. 1992;129:13–36. doi: 10.1007/BF00232052. [DOI] [PubMed] [Google Scholar]
- Díaz M, Sepúlveda FV. Characterisation of Ca2+-dependent inwardly rectifying K+ currents in HeLa cells. Pflügers Archiv. 1995;430:168–180. doi: 10.1007/BF00374647. [DOI] [PubMed] [Google Scholar]
- Díaz M, Valverde MA, Higgins CF, Rucareanu C, Sepúlveda FV. Volume-activated chloride channels in HeLa cells are blocked by verapamil and dideoxyforskolin. Pflügers Archiv. 1993;422:347–353. doi: 10.1007/BF00374290. [DOI] [PubMed] [Google Scholar]
- Dubé L, Parent L, Sauvé R. Hypotonic shock activates a maxi K+ channel in primary cultured proximal tubule cells. American Journal of Physiology. 1990;259:F348–356. doi: 10.1152/ajprenal.1990.259.2.F348. [DOI] [PubMed] [Google Scholar]
- Fink M, Lesage F, Duprat F, Heurteaux C, Reyes R, Fosset M, Lazdunski M. A neuronal two P domain K+ channel stimulated by arachidonic acid and polyunsaturated fatty acids. EMBO Journal. 1998;17:3297–3308. doi: 10.1093/emboj/17.12.3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein SA, Wang KW, Ilan N, Pausch MH. Sequence and function of the two P domain potassium channels: implications of an emerging superfamily. Journal of Molecular Medicine. 1998;76:13–20. doi: 10.1007/s001090050186. [DOI] [PubMed] [Google Scholar]
- Heinemann SH. Guide to data acquisition and analysis. In: Sakmann B, Neher E, editors. Single-Channel Recording. New York and London: Plenum Press; 1995. pp. 53–91. [Google Scholar]
- Heinemann SH, Conti F. Nonstationary noise analysis and application to patch clamp recordings. Methods in Enzymology. 1992;207:131–148. doi: 10.1016/0076-6879(92)07009-d. [DOI] [PubMed] [Google Scholar]
- Hendil KB, Hoffmann EK. Cell volume regulation in Ehrlich ascites tumor cells. Journal of Cellular Physiology. 1974;84:115–125. doi: 10.1002/jcp.1040840113. [DOI] [PubMed] [Google Scholar]
- Hoffmann EK, Dunham PB. Membrane mechanisms and intracellular signalling in cell volume regulation. International Review of Cytology. 1995;161:173–262. doi: 10.1016/s0074-7696(08)62498-5. [DOI] [PubMed] [Google Scholar]
- Hoffmann EK, Lambert IH, Simonsen LO. Separate, Ca2+-activated K+ and Cl− transport pathways in Ehrlich ascites tumor cells. Journal of Membrane Biology. 1986;91:227–244. doi: 10.1007/BF01868816. [DOI] [PubMed] [Google Scholar]
- Hoffmann EK, Simonsen LO, Lambert IH. Volume-induced increase of K+ and Cl− permeabilities in Ehrlich ascites tumor cells. Role of internal Ca2+ Journal of Membrane Biology. 1984;78:211–222. doi: 10.1007/BF01925969. [DOI] [PubMed] [Google Scholar]
- Hougaard C, Niemeyer MI, Hoffmann EK, Sepúlveda FV. Pflügers Archiv. 2000. K+ current activated by leukotriene D4 or osmotic swelling of Ehrlich ascites tumour cells. in the Press. [DOI] [PubMed] [Google Scholar]
- Ishii TM, Silvia C, Hirschberg B, Bond CT, Adelman JP, Maylie J. A human intermediate conductance calcium-activated potassium channel. Proceedings of the National Academy of Sciences of the USA. 1997;94:11651–11656. doi: 10.1073/pnas.94.21.11651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen BS, Strøbæk D, Christophersen P, Jørgensen TD, Hansen C, Silahtaroglu A, Olesen SP, Ahring PK. Characterization of the cloned human intermediate-conductance Ca2+-activated K+ channel. American Journal of Physiology. 1998;275:C848–856. doi: 10.1152/ajpcell.1998.275.3.C848. [DOI] [PubMed] [Google Scholar]
- Jørgensen NK, Christensen S, Harbak H, Brown AM, Lambert IH, Hoffmann EK, Simonsen LO. On the role of calcium in the regulatory volume decrease (RVD) response in Ehrlich mouse ascites tumor cells. Journal of Membrane Biology. 1997;157:281–299. doi: 10.1007/s002329900236. [DOI] [PubMed] [Google Scholar]
- Lewis RS, Cahalan MD. Potassium and calcium channels in lymphocytes. Annual Review of Immunology. 1995;13:623–653. doi: 10.1146/annurev.iy.13.040195.003203. [DOI] [PubMed] [Google Scholar]
- Ling BN, Webster CL, Eaton DC. Eicosanoids modulate apical Ca2+-dependent K+ channels in cultured rabbit principal cells. American Journal of Physiology. 1992;263:F116–126. doi: 10.1152/ajprenal.1992.263.1.F116. [DOI] [PubMed] [Google Scholar]
- Maingret F, Fosset M, Lesage F, Lazdunski M, Honoré E. TRAAK is a mammalian neuronal mechano-gated K+ channel. Journal of Biological Chemistry. 1999;274:1381–1387. doi: 10.1074/jbc.274.3.1381. [DOI] [PubMed] [Google Scholar]
- Malayev AA, Nelson DJ, Philipson LH. Mechanism of clofilium block of the human Kv1.5 delayed rectifier potassium channel. Molecular Pharmacology. 1995;47:198–205. [PubMed] [Google Scholar]
- Nilius B, Sehrer J, De Smet P, Van Driessche W, Droogmans G. Volume regulation in a toad epithelial cell line: role of coactivation of K+ and Cl− channels. The Journal of Physiology. 1995;487:367–378. doi: 10.1113/jphysiol.1995.sp020886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KP, Beck JS, Douglas IJ, Brown PD. Ca2+-activated K+ channels are involved in regulatory volume decrease in acinar cells isolated from the rat lacrimal gland. Journal of Membrane Biology. 1994;141:193–201. doi: 10.1007/BF00238253. [DOI] [PubMed] [Google Scholar]
- Pedersen SF, Prenen J, Droogmans G, Hoffmann EK, Nilius B. Separate swelling- and Ca2+-activated anion currents in Ehrlich ascites tumor cells. Journal of Membrane Biology. 1998;163:97–110. doi: 10.1007/s002329900374. [DOI] [PubMed] [Google Scholar]
- Pon DC, Hill CE. Existence, properties, and functional expression of “Maxi- K”-type, Ca2+-activated K+ channels in short-term cultured hepatocytes. Journal of Cellular Physiology. 1997;171:87–94. doi: 10.1002/(SICI)1097-4652(199704)171:1<87::AID-JCP10>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Riquelme G, Sepúlveda FV, Jørgensen F, Pedersen S, Hoffmann EK. Swelling-activated potassium currents of Ehrlich ascites tumour cells. Biochimica et Biophysica Acta. 1998;1371:101–106. doi: 10.1016/s0005-2736(98)00006-6. [DOI] [PubMed] [Google Scholar]
- Sackin H. A stretch-activated K+ channel sensitive to cell volume. Proceedings of the National Academy of Sciences of the USA. 1989;86:1731–1735. doi: 10.1073/pnas.86.5.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai S, Tosaka T. Analysis of hyposmolarity-induced taurine efflux pathways in the bullfrog sympathetic ganglia. Neurochemistry International. 1999;34:203–212. doi: 10.1016/s0197-0186(99)00004-2. [DOI] [PubMed] [Google Scholar]
- Sigworth FJ. The variance of sodium current fluctuations at the node of Ranvier. The Journal of Physiology. 1980;307:97–129. doi: 10.1113/jphysiol.1980.sp013426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suessbrich H, Schönherr R, Heinemann SH, Lang F, Busch AE. Specific block of cloned Herg channels by clofilium and its tertiary analog LY97241. FEBS Letters. 1997;414:435–438. doi: 10.1016/s0014-5793(97)01030-2. [DOI] [PubMed] [Google Scholar]
- Vanoye CG, Reuss L. Stretch-activated single K+ channels account for whole-cell currents elicited by swelling. Proceedings of the National Academy of Sciences of the USA. 1999;96:6511–6516. doi: 10.1073/pnas.96.11.6511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehner F. Cell volume-regulated cation channels. Contributions in Nephrology. 1998;123:8–20. doi: 10.1159/000059918. [DOI] [PubMed] [Google Scholar]
- Yang WP, Levesque PC, Little WA, Conder ML, Shalaby FY, Blanar MA. KvLQT1, a voltage-gated potassium channel responsible for human cardiac arrhythmias. Proceedings of the National Academy of Sciences of the USA. 1997;94:4017–4021. doi: 10.1073/pnas.94.8.4017. [DOI] [PMC free article] [PubMed] [Google Scholar]


