Abstract
The objectives of this study were: (1) to compare 2 methods of serology; (2) to compare 3 histologic techniques; and (3) to compare 2 methods of detecting shedding in pigs experimentally challenged with Lawsonia intracellularis. The sensitivities of these tests were determined by the detection of infection. Forty 5-week-old pigs were inoculated on day 0 with intestinal homogenate from pigs with proliferative enteropathy (PE). Clinical evaluation was done on day 7 and daily from day 14 to 28 postinoculation. Fecal shedding of L. intracellularis was monitored by use of polymerase chain reaction (PCR) analysis and immunoperoxidase staining at 7-day intervals. Serum was obtained on days 0 and 28 for serologic testing by glass slide and tissue culture indirect fluorescent antibody tests. At euthanasia on day 28, gross intestinal lesions were evaluated and ileum samples collected for histologic analyses. Ileal histologic sections from each animal were stained by hematoxylin and eosin, Warthin-Starry silver stain, and immunohistochemistry (IHC). Of the 40 pigs, 36 had gross lesions typical of PE at necropsy. The percentage of agreement between the 2 serologic methods was 94.4%. Immunoperoxidase stain of fecal smears was more sensitive than PCR for detecting fecal shedding, especially on day 21 (89.5% and 60.5%, respectively) and day 28 (59.4% and 37.5%, respectively) post-inoculation. The IHC stain was much more sensitive for detecting infection than the routinely used hematoxylin and eosin and Warthin-Starry silver stains. In conclusion, in experimentally infected pigs, both serologic methods were appropriate techniques for detecting infection. For fecal samples, PCR has low sensitivity. Immunohistochemistry is the best diagnostic tool for formalin-fixed samples.
Introduction
Proliferative enteropathy (PE) is an intestinal infectious disease caused by the intracellular bacterium Lawsonia intracellularis (1). Although first reported in 1931, research interest was minimal until the early 1970s. This disease has already been described in several species, including rat, guinea pig, rabbit, ferret, emu, pig, monkey, deer, and horse (2). It has been best described in swine, and it occurs worldwide. The economic losses that result from PE are caused by 2 major clinical aspects of the disease, namely acute hemorrhagic diarrhea and death in young adult pigs and chronic diarrhea and reduced growth performance in growing pigs. Despite some economic loss estimates in the literature (3,4), the prevalence of PE worldwide remains poorly known. The main explanation is the lack of availability of sensitive methods for diagnosis of the disease.
There are 3 ways to diagnose PE in live animals: serology, immunoperoxidase (IPX) staining of fecal smears, and polymerase chain reaction (PCR) of fecal samples (1,5). Jones et al (6) and McOrist et al (7) indicated that as few as 10 L. intracellularis organisms could be detected by PCR in DNA extracted from infected mucosal filtrate. However, there are many inhibitory factors within fecal specimens that could hamper the PCR amplification reaction (8). When using the indirect immunofluorescence technique with monoclonal antibodies specific for L. intracellularis in fecal smears, clinically affected animals are usually found to be excreting the agent (9,10). In contrast to the PCR technique, inhibitory factors in feces may not affect immunologic tests.
Knittel et al (11), using an indirect fluorescent antibody (IFA) serology test with pure culture of L. intracellularis as antigen in 96-well plates, detected the bacterium in 90% of experimentally inoculated pigs 3 to 4 wk postinoculation, showing IFA to be more sensitive than PCR in fecal samples for detecting L. intracellularis in inoculated pigs. The limitation of serologic tests is the need for in vitro maintenance of the bacteria as a source of antigen. Only a few laboratories in the world have established in vitro pure cultures of L. intracellularis. After in vitro cultivation, acetone-fixed cultures of L. intracellularis in 96-well plates need to be stored at −20°C until used. In contrast, glass slides coated with pure L. intracellularis can be fixed and stored at room temperature; as a result, transport and commercialization may be easier. Therefore, these coated slides would be another option to facilitate the availability of serologic tests for veterinary diagnostic laboratories. These slides would allow more economical testing of a smaller number of samples.
Proliferative enteropathy may be diagnosed postmortem from the typical macroscopic lesions, but histologic confirmation is necessary. Severe PE is diagnosed promptly by the demonstration of enterocyte proliferation in routine hematoxylin and eosin preparations; however, for visualization of the bacteria in the cytoplasm of enterocytes, special stains are necessary. Warthin-Starry silver stain allows the detection of the bacteria in histologic sections, improving the diagnostic sensitivity, but the technique has limitations when applied to autolyzed and necrotic samples (12). Immunohistochemistry (IHC) procedures with mouse monoclonal antibody to L. intracellularis (9) have been used successfully to diagnosis PE. There is no information in the literature comparing these techniques for diagnosing PE.
Serology gives information about historical exposure to L. intracellularis, histology may demonstrate the presence of L. intracellularis or of classic lesions related to the disease syndrome, and shedding may be related to the infectiousness of the disease. Therefore, the objectives of this study were: (1) to compare 2 methods of serology; (2) to compare 3 histologic techniques; and (3) to compare 2 methods of detecting shedding in pigs experimentally challenged with L. intracellularis. The sensitivities of these tests were determined by the detection of infection.
Materials and methods
Animals
All procedures were conducted in accordance with the guidelines of the Animal Care and Use Manual of the University of Minnesota and were approved by the Institutional Animal Care and Use Committee. Forty 5-week-old pigs of mixed gender (20 gilts and 20 barrows), weighing between 20 and 30 lb (9 and 13.6 kg), were obtained from a herd with no history or record of PE. The herd was serologically negative for L. intracellularis by tissue culture IFA and also serologically negative for porcine respiratory and reproductive syndrome and Actinobacillus pleuropneumoniae. It was clinically negative for Salmonella choleraesuis, transmissible gastroenteritis, and pathogenic Brachyspira species. Segregated early weaning at 16 d of age to an off-site nursery was routinely performed on the farm to help minimize any respiratory disease. All pigs were inoculated with an intestinal homogenate from PE-diseased mucosa via stomach tube on days 0 and 1.
Preparation of inoculum
The inoculum was prepared from porcine intestine affected by histologically confirmed PE, according to a previously described model (13). The scraped mucosa from affected intestines of several pigs was blended with sucrose-potassium-glutamate solution (SPG), 1:1 w/v. All pigs received 2 doses of 25 mL of the inoculum, and the total dose given per pig was approximately 3.4 × 109 L. intracellularis organisms. Bacteriologic testing of the inoculum did not detect any other enteropathogenic confounding organisms. Samples were negative for Brachyspira sp., Salmonella choleraesuis, Yersinia sp., and β-hemolytic Escherichia coli.
Quantification of inoculum
Quantification by IPX staining (DAKO K675; Dako Corporation, Carpinteria, California, USA) using a monoclonal antibody specific for L. intracellularis organisms (9) was accomplished by making serial 1:10 dilutions of the inoculum in sterile phosphate-buffered saline (PBS). A 15-well glass slide was coated with 10 μL of each dilution and dried at 37°C for 30 min. The slide was then fixed with cold acetone and stained by IPX. The numbers of L. intracellularis were counted using a light microscope.
Clinical observation
Health observations were all made by the same person on day 7 and daily from 14 to 28 d postchallenge. Fecal consistency, behavior, and body condition were observed. The fecal score was based on the following characteristics: 1 — no diarrhea; 2 — semi-solid feces with no blood; 3 — watery diarrhea without dark or bloody feces; 4 — blood-tinged feces, loose or formed; and 5 — profuse diarrhea with frank blood or dark tarry feces. Behavior and body condition were based on the following characteristics: 1 — normal; 2 — slightly to moderately gaunt and depressed, or listless, but still standing; 3 — severely gaunt, depressed, and recumbent.
Necropsy and macroscopic lesion evaluation
All pigs were euthanized 28 d after inoculation. Gross PE lesions in the jejunum, ileum, cecum, and colon were evaluated and scored separately in each pig in the following manner: 0 — normal; 1 — mild mesenteric and intestinal wall edema and hyperemia; 2 — mild to moderate edema and hyperemia of the mesentery and intestinal wall, as well as corrugated intestinal mucosa; 3 — severe mesenteric and intestinal wall edema and hyperemia, as well as necrosis of the mucosal surface with formation of pseudodiphtheric membranes (necrotic enteritis); and 4 — moderate to severe edema and hyperemia of the mesentery and intestinal wall, thick and corrugated mucosa, and blood clots in the intestinal lumen. The scored lesion length at each intestinal section was measured and recorded. Samples of ileum 2 cm from the ileocecal valve were fixed by immersion in 10% neutral buffered formalin.
Histologic tests
The formalin-fixed ileum samples were processed routinely for histology, embedded in paraffin, and sectioned (5 μm thick). Three sections of each sample were made. One section was stained with Warthin-Starry, one with hematoxylin and eosin (14), and the last by the IPX method of labeled streptavidin (DAKO K675; Dako Corporation) with mouse monoclonal antibodies to L. intracellularis (9).
Fecal shedding tests (PCR and IPX)
Fecal samples collected at 14, 21, and 28 d postchallenge were analyzed by PCR, using primers described elsewhere (15), and by an IPX stain, using the method of labeled streptavidin with mouse monoclonal antibodies (9).
Serology testing (tissue culture IFA and glass slide IFA tests)
Blood samples were collected at day 0 and day 28 postchallenge. The IFA procedure used in both tests was similar to that described by Knittel et al (11). The major difference between the 2 serologic methods used in this study was the procedure used for antigen preparation. Briefly, for the tissue culture IFA test, 96-well culture plates with 5 × 103 McCoy cells per well were prepared for the serologic test. Cells were allowed to grow for 24 h prior to infection. Pure culture of L. intracellularis strain VPB4 was added to Dulbecco's modified Eagle's medium (DMEM; JRH Biosciences, Lenexa, Kansas, USA) with 5% fetal bovine serum (FBS), and 100 μL was added to each well. The plates were incubated for 5 d in 8.0% O2, 8.8% CO2, and 83.2% N2. Cold 50% acetone and 50% methanol solution was used to fix the cells.
For the glass slide IFA test, 15-well glass slides (ICN Biomedicals, Aurora, Ohio, USA) were prepared. The wells were coated with whole bacteria purified from the supernatant of T-175 cm3 flasks of the L. intracellularis pure culture. For this purpose, the supernatant from a monolayer highly infected with L. intracellularis was centrifuged for 20 min at 1250 × g and suspended in PBS 3 times. One last centrifugation was then performed for 5 min at 200 × g to spin down any nuclei or cell debris. A concentration of 106 bacteria/mL was used as antigen. The same method used to titer the inoculum was used to titer the antigen. Ten microliters of antigen was added to each well of the glass slide and then incubated at 37°C until completely dry. The coated glass slides were then fixed with acetone. Two-fold dilutions of serum, starting at 1:30, were tested, and animals with a titer of 1:30 or higher were considered positive in both tests.
Statistical analysis
All tests were performed using known positive and negative controls. All 40 animals had been challenged but not necessarily infected. There is no “gold standard” diagnostic method for detecting L. intracellularis-infected animals. As a result, we considered the pig infected when it tested positive at any time by PCR in fecal samples or by IHC in ileum sections. The combination of these 2 tests was chosen to assure positivity on the basis of the high specificity of the monoclonal antibody used in IHC (100%) (9) and the PCR used to detect fecal shedding (100%) (15). The sensitivity of each diagnostic method was evaluated using the above-described criteria for detecting infection. The agreement between the titers obtained with the 2 serologic tests was assessed using the Spearman rank correlation test. The kappa test was used to evaluate the agreement between IPX and PCR for detecting fecal shedding at each sample collection date and the agreement between fecal shedding detected by PCR and IPX on day 28 postchallenge and by IHC in ileum samples. To test association, the crude lesion length data were adjusted by multiplying the level of severity of the lesions (0 to 4) in each intestine segment by the length of the lesion in that segment. The Spearman rank correlation test was used to test association between adjusted total lesion and each serology method. The agreement between the presence of macroscopic lesions and positivity detected by the 2 serologic methods and between the presence of macroscopic lesions and IHC was determined in a 2 ×2 table. The agreement between the presence of macroscopic lesions and fecal shedding detected by PCR and IPX on days 21 and 28 postchallenge was also determined in a 2 ×2 table. The kappa test was not calculated when the agreement was lower than 60%.
Results
Clinical observation
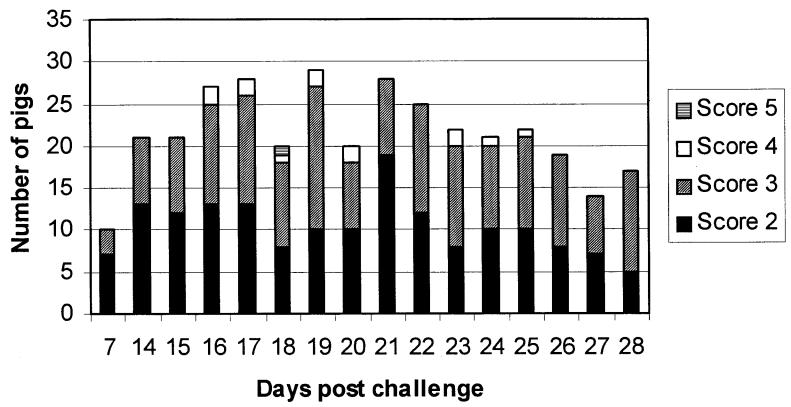
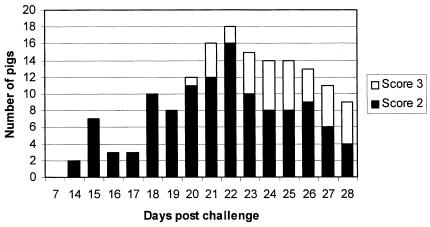
Figures 1 and 2 show the results of consistency of feces and behavior/body condition. Four pigs had to be humanely euthanized during the experiment, owing to very poor clinical conditions, on days 21, 23, 24, and 26 postchallenge. All 4 pigs had severely watery stools (score 3) for 4 or more days. Consistency of the feces and behavior/body condition were highly associated on days 21 and 28 postchallenge, rho = 0.7 and 0.82, respectively. In contrast, on day 14, the association was very poor, rho = 0.18.
Figure 1. Fecal scores on day 7 and from day 14 to 28 postchallenge. Score 2: semi-solid feces with no blood; score 3: watery diarrhea without dark or bloody feces; score 4: blood-tinged feces, loose or formed; and score 5: profuse diarrhea with frank blood or dark tarry feces. Score 1 (normal feces) was not included.
Figure 2. Behavior and body condition scores on day 7 and from day 14 to 28 postchallenge. Score 2: slightly to moderately gaunt and depressed or listless but still standing; score 3: severely gaunt, depressed and recumbent. Score 1 (normal) was not included.
Macroscopic lesions
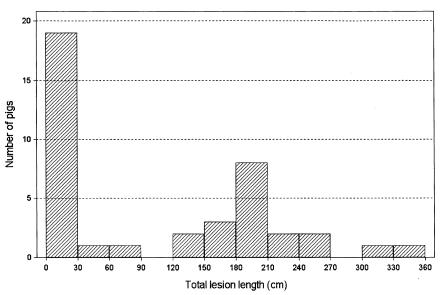
Figure 3 shows the distribution of total lesion length of the intestines of affected pigs. As the data shown in Figure 3 do not have a normal distribution, they were analyzed by non-parametric methods. Represented in the first column in Figure 3, 4 out of 40 challenged animals did not have any macroscopic lesion compatible with PE. One of those 4 animals was negative in all the diagnostic tests except the tissue culture IFA test, which was positive at the cut-off dilution (1:30). Consequently, according to the predefined criteria, this animal was considered not infected. Also represented in the first column in Figure 3, 4 other animals had small macroscopic lesions (less than 7 cm, grade 1) in the colon only, but they were also positive by PCR and both serologic methods. The remaining 11 animals that are represented in the first column had lesions of grade 1 and 2, up to 16 cm long and restricted to the ileum. Seventeen pigs had lesions between 120 and 270 cm in length; these lesions were classified mostly as grade 2 or 3, extending cranially toward the middle jejunum. The 2 animals that had the longer grade 3 lesions, affecting the ileum and jejunum, are represented in the 2 columns on the right side of Figure 3. These animals were 2 of the 4 pigs that had to be euthanized during the experiment.
Figure 3. Distribution of 40 animals based on the total lesion length (cm), including ileum, jejunum, cecum, and colon, characteristic of PE, evaluated macroscopically on day 28 postchallenge, regardless of the lesion score.
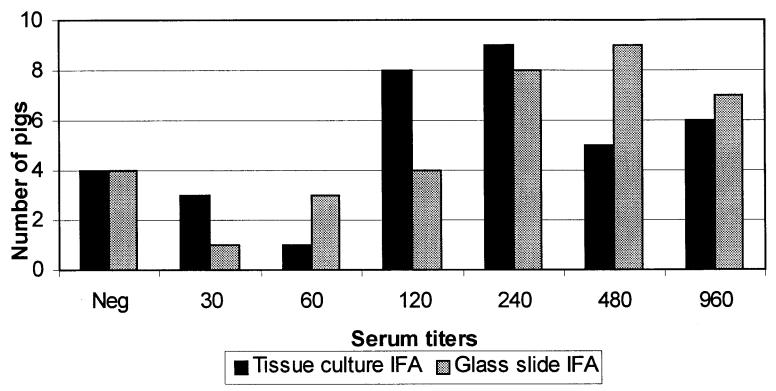
Figure 4. Tissue culture and glass slide immunofluorescent antibody titer distribution for 36 animals on day 28 postchallenge. Positive serum titers ranged from 1:30 to 1:960.
Evaluation of sensitivity
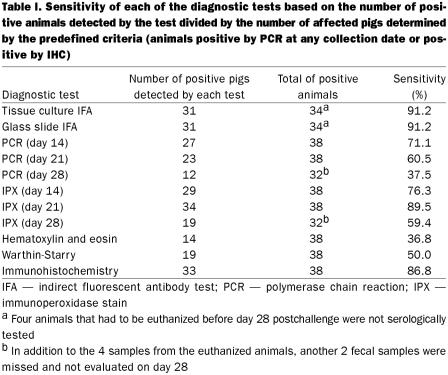
According to the criteria set previously, 2 of the 40 challenged animals were considered not infected (i.e., not shedding L. intracellularis in the feces at any collection date and negative for L. intracellularis antigen by IHC at necropsy) and were excluded from the sensitivity evaluation. These 2 animals were negative by IHC and by all PCR and IPX tests of fecal samples. One was seropositive at the cut-off dilution (1:30) by the tissue culture IFA test and negative by the glass slide IFA test on day 28 postchallenge. It did not have any macroscopic lesions. The other was seronegative by the tissue culture IFA test but positive by the glass slide IFA test (1:30) and was classified as having 6 cm of grade 1 macroscopic lesion in the ileum. Table I summarizes the sensitivity results of each test.
Table I.
Tissue culture and glass slide IFA serology tests
All 40 animals were negative on day 0 by both tests. Out of 36 animals bled on day 28, 31 were positive by both tests, titers ranging from 1:30 to 1:960 (Figure 4). Three samples were negative by both tests. The remaining 2 animals were negative by all the diagnostic tests, except that one pig was positive only by the tissue culture IFA test at the cut-off dilution (1:30), and the other pig was positive only by the slide IFA test, also at the cut-off dilution (1:30). These 2 animals were considered non-affected on the basis of defined criteria, as previously described. The agreement between the 2 IFA tests, using a 2 × 2 table, was 94.4%. The Spearman rank correlation coefficient (rho), considering the serum titers, was 0.72.
Adjusted total lesion had poor association with both tissue culture and glass slide IFA serology results: rho = 0.47 and 0.58, respectively. However, the levels of agreement between the presence of macroscopic lesions characteristic of PE and positivity by tissue culture and glass slide IFA serology tests were 78% and 83%, respectively.
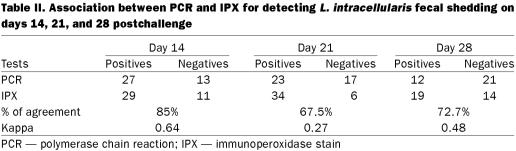
Fecal shedding detected by PCR and IPX
Table II summarizes the fecal PCR and IPX results at each sample collection date. The levels of agreement between PCR and IPX and the kappa coefficient on days 14, 21, and 28 postchallenge were 85% and 0.64, 67.5% and 0.27, and 72.7% and 0.48, respectively.
Table II.
There was very low agreement between the presence of any macroscopic lesion characteristic of PE and fecal shedding detected by PCR and IPX on day 28 postchallenge (36% and 56%, respectively). In contrast, the agreement between PE macroscopic lesions and fecal shedding detected by PCR and IPX on day 21 postchallenge was very high (67% and 90%, respectively). Kappa was not calculated for these comparison owing to very small numbers in some cells of the 2 × 2 tables.
Different histologic stain techniques
The IHC test was the most sensitive histologic staining technique as shown in Table I. The 14 animals positive by hematoxylin and eosin were also positive by Warthin-Starry staining, and the 19 pigs positive by Warthin-Starry were among the 33 positive by IHC.
The agreement between positive antigen label by IHC and presence of macroscopic lesions characteristic of PE was 82.5%. Kappa was not calculated because of the very small number in some cells of the 2 × 2 table. IHC showed very poor association with both PCR and IPX for detecting fecal shedding on day 28 postchallenge (kappa coefficient 0.28 and 0.19, respectively).
Discussion
The evolution and presentation of clinical signs observed in this study were similar to what was obtained in trials using the same diseased mucosa homogenate in a challenge model (1,13). This model reproduces mostly the chronic form of the disease, characterized by mild diarrhea and poor growth. As shown in Figure 1, some animals developed diarrhea at the end of the first week postchallenge, but it was only after the second week that the clinical signs were more evident, with a peak at the third week. However, even during this most clinically severe phase of the disease, less than 75% of the challenged animals presented any clinical signs. And after this peak, animals tended to recover until the end of the trial (28 d postchallenge). In field conditions, sick pigs with mild diarrhea are easily missed; and, even if they are detected, diarrhea is a non-specific clinical presentation. Then, sample collection and further tests are necessary. Therefore, a better understanding about the capabilities and limitations of laboratory tests for detecting PE will help to evaluate the importance of this disease in the field.
Necropsy and macroscopic evaluation of lesions are extremely important steps toward detection of PE. Severe lesions, such as those observed in pigs with affected intestinal segments longer than 30 cm, are easy to detect grossly. As a result, the real challenge in PE diagnosis is detecting those animals that have mild lesions located in the ileum. In this study, these animals represented almost half of the total studied.
Both serology methods were sensitive (91.2%) in diagnosing PE among challenged pigs. In one study, a similar sensitivity (90%) was observed using the same tissue culture IFA test 3 wk after experimental infection of pigs with pure culture of L. intracellularis (11). In addition to their high sensitivities, the 2 tests corroborate each other with an agreement of 94.4% and a Spearman rank correlation coefficient (rho) of 0.72. As glass slides coated with L. intracellularis antigen can be stored at room temperature for long periods, and 96-well plates with eukaryotic cells infected with L. intracellularis have to be stored at −20°C, the former may be a better option for a broad distribution of PE serologic testing. The major drawback for the glass slide IFA test is the excessive manipulation involved in the procedure and the reduced number of samples that can be run per slide: a maximum of 13 plus negative and positive controls. Both methods require some practice and expertise to be read under the fluorescent microscope, as some background fluorescence could be wrongly interpreted as positive.
The agreement between positive tissue cultures and glass slide IFA tests and the presence of macroscopic lesions characteristic of PE was fairly good in this study, at 78% and 83%, respectively. Thus, both serology tests could be used as predictors of the presence of macroscopic lesion of PE. Conversely, the poor association between the serum titers and the adjusted total lesion length showed that high or low titers do not correspond to more or less severe macroscopic lesions. Looking at the systemic humoral immune response from a different perspective, we would not expect serum IgG levels to correlate with protection, because L. intracellularis is an obligate intracellular organism that resides in the cytoplasm of enterocytes. Local mucosa IgA levels and cell-mediated immune responses are probably more involved in protection against infection. Thus, oral or intranasal modified-live vaccines against L. intracellularis are more likely than parentally administered ones to be protective. At this point, detectable serum IgG against L. intracellularis should be interpreted as indicating previous exposure to the bacteria and gross lesions in the intestines.
More than 90% of the pigs in this study seroconverted, titers ranging from l:30 to l:960. This probably does not represent what happens in the field. The spread of the infection among growing- finishing pigs is likely more sporadic and the infective dose ingested by susceptible animals much smaller in the field than in this study. Consequently, the percentage of seropositive pigs and the titers in farms with PE are probably much lower than those seen in challenge trials. More field studies have to be carried out for us to better understand the epidemiology of the disease.
Both pigs that were excluded from the evaluation of sensitivity on the basis of the pre-defined criteria were positive at the cut-off serum dilution in one or other serologic test. There are 2 possible explanations. One is that both results were false positive. The other is that a very weak humoral immune response was still present 4 wk after oral exposure to L. intracellularis antigen and represented merely exposure but not necessarily infection.
Knittel et al (11), using PCR in fecal samples, could detect shedding of L. intracellularis 3 wk postinfection in only 39% of the pigs challenged. In another study (16), PCR testing of fecal samples from a group of experimentally infected animals detected the bacterium in only 38% (3 out of 8 pigs) and 25% (2 out of 8 pigs) on days 21 and 24 postchallenge, respectively. In our study, the higher sensitivity of PCR was observed on days 14 (71.05%) and 21 (60.5%) postchallenge. The sensitivity then decreased to 37.5% on day 28 postchallenge. The higher sensitivity observed on days 14 and 21, in comparison with the results in both papers by Knittel et al (11,16), might be attributed to the different challenge models. Pigs experimentally infected with diseased intestinal mucosa, as in the present study, usually develop more severe clinical signs and gross lesions than animals challenged with pure culture, as in the studies of Knittel et al (11,16). Nevertheless, sensitivity levels varying between 37.5% and 70%, as observed in our study, and ranging between 25% and 39%, as shown by Knittel et al, are considered low for diagnostic tests. The specificity of PCR for detecting fecal shedding of L. intracellularis at the individual animal level is close to 100% (15); however, the sensitivity of the test is likely hampered by the presence of fecal inhibitors (8).
Immunoperoxidase testing of fecal smears showed sensitivity close to 90% at the peak of the disease, 21 d postchallenge. The IPX technique is not affected by fecal inhibitors, as is PCR; conversely, non-specific background could increase the number of false positives. The IFA testing of fecal smears using the L. intracellularis monoclonal antibody was also successfully used to detect fecal shedding in naturally infected pigs (9,10). The advantage of IPX over IFA in fecal smears is the reduction of background and clear visualization of the bright red positive antigen-labeled bacteria using IPX. The high specificity of the monoclonal antibody against L. intracellularis (9) and the use of a red chromogen, aminoethyl-carbazole (AEC), in the IPX of fecal smears increased the confidence in detecting positive results.
The higher sensitivity of IHC over Warthin-Starry silver stain and hematoxylin and eosin can be explained by the specific binding of the monoclonal antibody to the 21 kD outer membrane protein of L. intracellularis (17). Jensen et al (12) used Warthin-Starry and IHC to examine 65 intestines from pigs with macroscopic PE lesions and 22 intestines from pigs with suspected PE but no apparent macroscopic lesions. They found 62 (95%) and 63 (98%) positive cases in the group with characteristic lesions and 4 (18%) and 14 (64%) positive cases in the group suspected to have PE by Warthin-Starry and IHC, respectively. Use of hematoxylin and eosin for diagnosis is restricted to cases that show evident enterocyte proliferation, present in moderate and severe cases of PE. Warthin-Starry silver stain is not specific for L. intracellularis, and a positive diagnosis is determined by the presence of bacteria in the apical cytoplasm of proliferated enterocytes. Using IHC, L. intracellularis positive antigen can be detected even in cases of severe necrosis, in which the mucosa is completely destroyed, or during recovery, when the bacterial antigen is found only in the cytoplasm of mononuclear cells in the lamina propria. As demonstrated in Figures 1 and 2, the pigs in this study tended to recover during the 4th week; and the number of animals with small macroscopic lesions at necropsy was significant (4 pigs with grade 1, with small lesions in the colon, and 11 pigs with grade 1 or 2, with lesions smaller than 16 cm). The resolving lesions may have contributed to the low sensitivity of the hematoxylin and eosin and Warthin-Starry silver stains at necropsy. The high agreement (82.5%) between IHC and the presence of macroscopic lesions 28 d after infection emphasizes the importance of IHC for the diagnosis of PE.
The poor association observed between IHC in ileum sections and detection of fecal shedding by PCR and IPX may also be explained by the resolution of lesions on day 28. In other words, IHC staining may still detect L. intracellularis antigen in some glands and lamina propria of ileum sections; however, one would expect fecal shedding to be drastically reduced, as bacteria are not multiplying or being released from enterocytes. A similar argument could be used to explain the reduction of agreement between the presence of macroscopic lesions at necropsy and fecal shedding detected on days 21 and 28 postchallenge.
Currently, most veterinary diagnostic laboratories are using only hematoxylin and eosin and Warthin-Starry silver stains for histologic samples and PCR for fecal or fresh intestine samples in the diagnosis of PE. These techniques can be very easily applied anywhere in the world. Conversely, the use of immunologic techniques such as IHC for histologic samples and IPX for fecal material is limited by the availability of the monoclonal antibody against L. intracellularis (9). Production of rabbit polyclonal or new monoclonal antibodies specific for L. intracellularis would overcome this limitation. Serologic tests have recently become more available for PE diagnosis, but more studies must be done to validate them and to interpret serologic profiles in field conditions.
Our findings showed the importance of the immunologic tests for diagnosing PE. Serology seems to be a promising technique for assessing a herd's epidemiologic status. The IHC stain using the L. intracellularis monoclonal antibody was much more sensitive for detecting infection than the routinely used Warthin-Starry silver stain. The IPX staining, also using L. intracellularis monoclonal antibody, had better sensitivity than PCR in fecal samples, but IPX specificity in feces needs to be investigated. The low sensitivity of PCR in fecal samples suggests that testing pooled samples may not be useful. More studies are necessary to improve the sensitivity of PCR for detecting L. intracellularis in fecal samples.
Footnotes
Acknowledgments
The authors thank Evelyn Townsend and Debra Swanson for technical assistance. R.M.C. Guedes is a recipient of a graduate studentship from the Conselho Nacional de desenvolvimento CientÍfico e Tecnológico (CNPq) from Brazil.
Address correspondence and reprint requests to Dr. Roberto M.C. Guedes, 1971 Commonwealth Avenue, Room 205, Veterinary Science Building, Saint Paul, Minnesota 55108 USA, tel: 612-625-8110, fax: 612-625-5203, e-mail: gued0001@tc.umn.edu
Received August 29, 2001. Accepted December 7, 2001.
Preliminary data from this study were presented at the 80th Annual Meeting of the Conference of Research Workers in Animal Diseases (CRWAD), in Chicago, Illinois, USA, 1999.
References
- 1.McOrist S, Gebhart CJ. Porcine proliferative enteropathies. In: Straw BE, D'Allaire S, et al., eds. Disease of Swine, 9th ed. Ames: Iowa State University Press, 1999:521–534.
- 2.Cooper DM, Gebhart CJ. Comparative aspects of proliferative enteritis. J Am Vet Med Assoc 1998;212:1446–1451. [PubMed]
- 3.Lawson GHK, McOrist S. The enigma of the proliferative enteropathies: a review. J Comp Pathol 1993;108:41–46. [DOI] [PubMed]
- 4.Winkelman NL, Dee SD. Ileitis: an update. Compend Cont Educ Pract Vet 1996;19:519–525.
- 5.Lawson GHK, Gebhart CJ. Proliferative enteropathy. J Comp Pathol 2000;122:77–100. [DOI] [PubMed]
- 6.Jones GF, Ward GE, Murtaugh MP, Lin G, Gebhart CJ. Enhanced detection of intracellular organism of swine proliferative enteritis, Ileal symbiont intracellularis, in feces by polymerase chain reaction. J Clin Microbiol 1993;31:2611–2615. [DOI] [PMC free article] [PubMed]
- 7.McOrist S, Gebhart CJ, Lawson GHK. Polymerase chain reaction for diagnosis of porcine proliferative enteropathy. Vet Microbiol 1994;41:205–212. [DOI] [PubMed]
- 8.Wilde J, Ieden J, Yolken R. Removal of inhibitory substances from human fecal specimens for detection of group A rotaviruses by reverse transcriptase and polymerase chain reactions. J Clin Microbiol 1990;28:1300–1307. [DOI] [PMC free article] [PubMed]
- 9.McOrist S, Boid R, Lawson GHK, McConell I. Monoclonal antibodies to intracellular Campylobacter-like organisms of the porcine proliferative enteropathies. Vet Rec 1987;121:421–422. [DOI] [PubMed]
- 10.McOrist S, Lawson GHK. Failure to demonstrate Campylobacter-like organisms of the proliferative enteropathies in pigs excreting Campylobacter sp. Vet Rec 1989;124:41.2916314
- 11.Knittel JP, Jordan DM, Schwartz KJ, et al. Evaluation of antemortem polymerase chain reaction and serologic methods for detection of Lawsonia intracellularis-exposed pigs. Am J Vet Res 1998;59:722–726. [PubMed]
- 12.Jensen TK, Møller K, Leser TD, Jorsal SE. Comparison of histology, immunohistochemistry and polymerase chain reaction for detection of Lawsonia intracellularis in natural porcine proliferative enteropathy. Eur J Vet Pathol 1997;3:115–123.
- 13.Winkelman NL, Pauling GE, Bagg RN, et al. Use of a challenge model to measure the impact of subclinical porcine proliferative enteritis on growth performance in pigs. Proc Am Assoc Swine Pract 1998:209–211.
- 14.Luna LC, ed. Manual of Histologic Staining. Methods of the Armed Forces Institute of Pathology. 3rd ed. New York: McGraw, 1968.
- 15.Jones GF, Davies PR, Rose R, Ward GE, Murtaugh MP. Comparison of techniques for diagnosis of proliferative enteritis of swine. Am J Vet Res 1993;54:1980–1985. [PubMed]
- 16.Knittel JP, Roof M, Schwartz KJ, Jordan DM, Haris DL, McOrist S. Diagnosis of porcine proliferative enteritis. Compend Cont Educ Pract Vet 1997;19:S26–S29.
- 17.McOrist S, Boid R, Lawson GHK. Antigenic analysis of Campylobacter species and an intracellular Campylobacter-like organism associated with porcine proliferative enteropathies. Infect Immun 1989;57:957–962. [DOI] [PMC free article] [PubMed]