Abstract
P-glycoprotein (Pgp) is a transmembrane transporter causing efflux of a number of chemically unrelated drugs and is responsible for resistance to a variety of anticancer drugs during chemotherapy.
Pgp overexpression in cells is also associated with volume-activated chloride channel activity; Pgp is thought to regulate such activity.
Reversible phosphorylation is a possible mechanism for regulating the transport and chloride channel regulation functions of Pgp. Protein kinase C (PKC) is a good candidate for inducing such phosphorylation.
Hierarchical multiple phosphorylation (e.g. of different serines and with different PKC isoforms) may shuttle the protein between its different states of activity (transport or channel regulation). Cell volume changes may trigger phosphorylation of Pgp at sites causing inhibition of transport.
The possible regulation of chloride channels by Pgp and the potential involvement of reversible phosphorylation in such regulation is reviewed.
Phospho-glycoprotein (P-glycoprotein/multidrug-resistance 1 (MDR1)) is a membrane protein with transmembrane efflux activity responsible for transporting a wide variety of unrelated drugs from cells (Endicott & Ling, 1989; Gottesman & Pastan, 1993). The protein is implicated in resistance to chemotherapeutic drugs during cancer treatment and may be responsible for failure of such treatment in many instances. Pgp is the protein product of the MDR gene and is a member of the ATP binding cassette (ABC) superfamily of transporters (also known as traffic ATPases). These include bacterial histidine transporters (HisJQMP), yeast a-mating peptide exporter (STE6), human cystic fibrosis transmembrane regulator (CFTR, a plasma membrane Cl− channel that is dysfunctional during cystic fibrosis) and the sulphonylurea receptor (SUR) (Higgins, 1992). Human Pgp has an apparent molecular mass of 170 kDa and contains 1280 amino acids, consisting of two similar halves of 610 amino acids each, joined by a linker region consisting of 60 charged residues. Each of the two halves is predicted to form a total of six transmembrane domains and a cytoplasmic domain with ATPase activity that hydrolyses ATP during molecular efflux (Endicott & Ling, 1989; Gottesman & Pastan, 1993; Fig. 1). The presence of both halves (including the nucleotide binding sites) is essential for activity. The minimum functional unit for Pgp in membrane appears to be a monomer (Loo & Clarke, 1996). The mechanism of action of Pgp is unclear and its physiological function may be to protect cells from cytotoxic molecules (Gottesman & Pastan, 1993).
Figure 1. Schematic representation of Pgp.
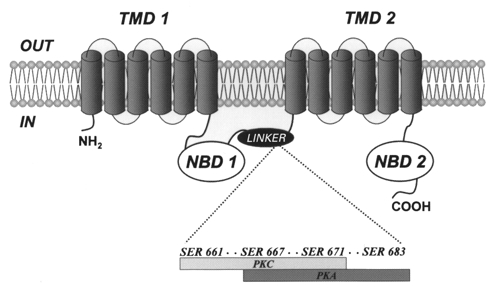
The Pgp molecule consists of two similar transmembrane domains TMD1 and TMD2, each followed by a cytoplasmic nucleotide binding domain (termed NBD1 and NBD2, respectively). The NBDs are responsible for the ATP hydrolysis that is associated with drug efflux. A short linker region joins NBD1 with TMD2. Four previously identified phosphorylation target sites for PKC and PKA within the linker region of Pgp are also shown.
Biological functions of P-glycoprotein
Drug efflux
Drug efflux was the original function described for Pgp. This was based on the observation that in MDR cells, alterations occurred in plasma membrane proteins, the major one being increased expression of Pgp. Overexpression of Pgp is sufficient to confer MDR in cells. Pgp is normally expressed in numerous human tissues as well as a great number of human cancers. It is capable of causing efflux of a broad variety of chemically unrelated hydrophobic drugs that include vinca alkaloids, anthracyclines, actinomycin D, colchicine, taxol, epipodophyllotoxins and others. Additionally, Pgp expression in MDR cells is correlated with reduced drug influx, suggesting that Pgp may directly efflux drugs from the cell membrane (Nielsen et al. 1995). Drug efflux requires a source of energy, which is derived from hydrolysis of ATP. Some degree of water solubility is required for the hydrophobic drugs to bind to Pgp and binding is only available to those compounds capable of penetrating the lipid bilayer (Safa, 1998). Drug binding/transport is affected by a number of amino acid mutations as well as N- and C-terminal deletions. MDR is also reversed by a number of drugs such as verapamil, which inhibits the efflux activity of Pgp. The transport function of Pgp is comprehensively reviewed elsewhere (Gottesman & Pastan, 1993; Germann, 1996; Gottesman et al. 1996; Sharom, 1997; Shapiro & Ling, 1998).
Cell volume regulation
Pgp was also postulated to be the swelling-induced Cl− channel. In response to cell swelling, most cells possess a variety of mechanisms for regulatory volume decrease (RVD). In cells that undergo RVD, one common mechanism is activation of plasma membrane volume-sensitive K+ and Cl− channels. The volume-sensitive anion channels are also permeable to a host of amino acids and polyols (Strange et al. 1996; Basavappa & Ellory, 1996; Okada, 1997). Valverde et al. (1992) proposed that the swelling-induced Cl− conductance is closely linked with over-expression of Pgp. Pgp is structurally related to CFTR, with a similar pattern of domain organization. The linker region in Pgp may serve to regulate Cl− conductance through phosphorylation, analogous to the R-domain in CFTR (for review see Akabas, 2000). Trezise et al. (1992, 1997) reported that an increased expression of Pgp in intestinal tissue may compensate for the loss of CFTR expression, since Pgp and CFTR exhibit complementary patterns of expression in vivo.
The main similarities between the swelling-sensitive whole-cell Cl− currents and Cl− currents in cells overexpressing Pgp are the requirement of non-hydrolysed intracellular ATP, outward rectification, inactivation at depolarizing potentials, and Eisenman I permeability sequence (SCN− > Br− > Cl− > F− > gluconate). Furthermore, verapamil, dideoxyforskolin (DDFSK) and the anti-oestrogen drug tamoxifen blocked whole-cell currents from Pgp-expressing cells as well as inhibiting drug transport by Pgp. Although tamoxifen blocked swelling-induced anion channels in many cell types, verapamil, an L-type Ca2+ channel blocker, did not affect volume-sensitive anion channels in many cell types.
Initially, Pgp was proposed to have a bimodal function such that the ATP-dependent pump during isotonic conditions switches to serve as a swelling-induced Cl− channel during hyposmotic stress. The linkage between Pgp and the swelling-induced Cl− channels was supported by the observations that: (1) cell swelling inhibited drug pump activity (Sardini et al. 1994); (2) the inclusion of intracellular drugs (in the presence of ATP), such as vincristine or colchicine, which can be transported by Pgp, activated drug efflux but prevented swelling-induced Cl− conductance (Gill et al. 1992); and (3) the anti-Pgp monoclonal antibody (C219), directed against distinct epitopes within Pgp, inhibited swelling-induced Cl− conductance (Thevenod et al. 1994; Han et al. 1996; Wu et al. 1996; Vanoye et al. 1997).
Although the above lines of evidence strongly suggest that overexpression of Pgp is closely linked with swelling-induced anion conductance, the role of Pgp in the volume-regulatory process has been controversial. Some laboratories were able to obtain results similar to those described above, implicating Pgp with volume regulation (Gill et al. 1992; Hardy et al. 1995; Wu et al. 1996), while other investigators were unable to confirm these findings (Dong et al. 1994; Wang et al. 1994; Altenberg et al. 1994b; Ehring et al. 1994; Kunzelmann et al. 1994; Rasola et al. 1994; Ambasch et al. 1995; De Greef et al. 1995; Morin et al. 1995; Tominaga et al. 1995; Weaver et al. 1996; Miwa et al. 1997; Vanoye et al. 1997; Gerard et al. 1998). In particular: (1) in a variety of cell types, many investigators reported a lack of correspondence between the level of Pgp expression (as assessed by transport assays) and the swelling-induced Cl− conductance (Rasola et al. 1994; Ehring et al. 1994; Dong et al. 1994; De Greef et al. 1995; Morin et al. 1995; Viana et al. 1995; Miwa et al. 1997; Vanoye et al. 1997; Gerard et al. 1998). Furthermore, in multidrug-resistant Chinese hamster lung fibroblast (LZ-8) cells Cl− conductance was not increased by hyposmotic exposure (Altenberg et al. 1994b). In contrast, in the human breast cancer cell line BC19/3 (multidrug resistant, transfected with the human mdr1 cDNA) cells do exhibit swelling-sensitive Cl− currents, but do not have significant Cl− loss and thus do not mediate RVD (Altenberg et al. 1994a); (2) the Pgp inhibitors verapamil or DDFSK had either varying results or no effect on swelling-induced anion conductance in many cell types (Munkonge et al. 1994; Ehring et al. 1994; Jackson et al. 1994; Tominga et al. 1995; Viana et al. 1995); (3) the intracellular inclusion of Pgp substrates, such as vinicristine, colchicine or rhodamine 123 (R123), did not inhibit the swelling-induced anion conductance in Pgp-expressing cells (Ehring et al. 1994; Jackson et al. 1994; Altenberg et al. 1994b; Rasola et al. 1994; Tominaga et al. 1995; Viana et al. 1995); (4) conversely, cell swelling failed to inhibit Pgp-mediated drug pump activity in several cell types expressing Pgp (Altenberg et al. 1994b; Ehring et al. 1994; Viana et al. 1995; Ambasch et al. 1995; Weaver et al. 1996), but did show inhibition of ATPase activity in certain cell types (Sardini et al. 1994).
Taken together, these differing results cast doubt on the linkage between Pgp and swelling-induced anion currents. Thus, the hypothesis describing the role of Pgp has been modified such that Pgp is generally accepted to not have inherent channel activity, rather that Pgp may regulate the swelling-induced anion channel (Higgins, 1995a, b; Wine & Luckie, 1996). However, Bond et al. (1998) suggest that the conflicting results regarding Pgp and the swelling-induced anion conductance may also reflect methodological differences such as cell-type differences in channel properties or differences in the period and degree of hyposmotic stress which may mask the effects of Pgp.
Regulation of the functions of P-glycoproteins
Pgp is subject to a number of post-translational modifications, two of which are N-glycosylation and phosphorylation. Glycosylation does not seem to affect the transport function of Pgp (Gottesman & Pastan, 1993); phosphorylation, however, particularly that mediated by PKC, may affect transport and the associated ATPase activity. Pgp possesses numerous potential phosphorylation sites for a number of kinases, a number of which are present in the linker region (Table 1). Early studies identified a number of serines in the linker region of human Pgp that are phosphorylated by PKC and/or PKA (serines 661, 667, 671 and 683) in vitro, with certain residues being exclusively phosphorylated by PKC (serine 661) or PKA (serine 683). Three residues (serines 661, 667 and 671) have been shown to be phosphorylated in vivo (Orr et al. 1993; Chambers et al. 1993, 1994, 1995; Chambers, 1998). Other kinases such as casein kinase II (CKII) have been shown to phosphorylate murine Pgp in vitro (Glavy et al. 1997). Phosphorylation of Pgp is likely to have a direct effect on its activity due to the prevalence of phosphorylation target sites within its primary sequence and the unlikelyhood that such a large protein functions without regulation through a common cellular regulatory modification such as reversible phosphorylation.
Table 1.
Potential phosphorylation sites within the human Pgp linker region §
| Consensus sequence* | Target residue | Protein kinase | Phosphorylation observed | Mutant residue | Effect‡ | Selected reference |
|---|---|---|---|---|---|---|
| 644SKSE647 | Ser 644 | CKII | No | N/A | ND | This paper |
| 654SSND657 | Ser 654 | CKII | No | N/A | ND | This paper |
| 655SNDS658 | Ser 658*** | CKII | No | N/A | ND | This paper |
| 654SSND657 | Ser 655** | CKII | No | N/A | ND | This paper |
| 659RSS661 | Ser 661 | PKC | Yes | Ala/Asp† | ICl, swell↓ | Vanoye et al. 1999 |
| 664RKRS667 | Ser 667 | PKC/PKA | Yes | Ala/Asp† | None | German et al. 1995 |
| 668TRR670 | Thr 668 | PKC | No | N/A | ND | This paper |
| 669RRS671 | Ser 671 | PKC | Yes | Asn/Ala | ATPase↑ | Ahmed et al. 1994 |
| 673RGS675 | Ser 675 | PKC | Yes | Ala/Asp† | None | This paper |
| 680RKLS683 | Ser 683 | PKA | Yes | Ala/Asp† | ICl, swell↓ | Vanoye et al. 1999 |
| 681KLST684 | Thr 684 | PKC/PKA | No | N/A | ND | This paper |
| 683STKE686 | Ser 683 | CKII | No | N/A | ICl, swell↑ | Vanoye et al. 1999 |
| CK II sites | (S/T XX E/DX) |
| PKA sites | (K/R XX S/T) |
| PKC sites | (S/T X R/K, R/K X S/T, K/R XX S/T) |
Secondary phosphorylation site dependent on pre-phosphorylation of serine 661.
Tertiary phosphorylation site dependent on sequential phosphorylation of serines 661 and 655.
Residues mutated simultaneously, but no data on the effect of individual mutations.
ND (not determined); arrows depict increase or decrease in activity.
633 NEVELENAADESKSEIDALEMSSNDSRSSLIRKRSTRRSVRGSQAQDRKLSTKEALDESI 692. Emboldened characters denote phosphorylation target residues.
Conflicting results emerged from studies examining the effect of such phosphorylation on transmembrane drug transport. Early reports indicated that phosphorylation affects drug efflux and ATPase activity (Germann et al. 1995; Fine et al. 1996). However, subsequent studies, mainly based on mutating the phosphorylatable serines to alanines or aspartic acids, suggested that Pgp-mediated transport and/or associated ATPase activity are unaffected by phosphorylation (Germann et al. 1996; Goodfellow et al. 1996; Szabo et al. 1997). However, all such studies suffer from the limitation of mutating a multiple of serines (3–8) simultaneously, rather than examining the effect of altering a single residue at a time. Multi-residue mutations may mask variation in the activity of Pgp resulting from a single phosphorylation, which may be negated by phosphorylation of additional sites. It would be important to determine the effect of mutating individual serines on Pgp activities. On the other hand, studies using the baculovirus system showed that co-expression of Pgp and PKC α in insect cells stimulates Pgp's ATPase activity (Fig. 2), an effect that was not observed with PKC ε (Ahmad et al. 1994; H. Idriss, V. Urquidi & S. Basavappa, manuscript in preparation). Mutation of serine 671 to aspargine prevented such stimulation (Ahmad et al. 1994), indicating that phosphorylation of a single serine is sufficient to regulate Pgp's ATPase activity. Phosphorylation at additional sites may function to alter other activities of Pgp such as its postulated regulation of volume-activated Cl− channel activity, in response to membrane stretch during volume increase (see below).
Figure 2. Regulation of ATPase activity by PKC α-mediated phosphorylation.
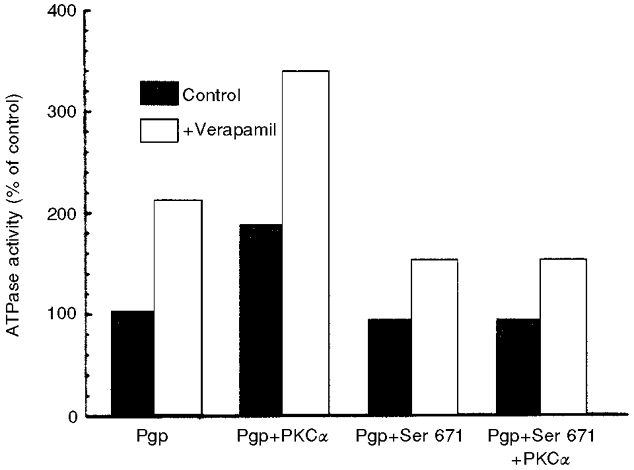
Pgp and PKC α were co-expressed in Sf9 cells as described in Ahmad et al. (1994). Phosphorylation of Pgp stimulates ATPase activity of Pgp in the presence or absence of verapamil. This stimulation is lost when serine 671 in the Pgp linker region is mutated to aspargine. Reproduced from Ahmad et al. (1994) with permission of the American Chemical Society.
Additional support for a close interaction between Pgp and PKC comes from several studies demonstrating unequivocally elevation of the levels of PKC isoenzymes (especially PKC α) concomitant with the appearance of the MDR phenotype (Blobe et al. 1993; Ratnasinghe et al. 1998). In various cell culture models of drug resistance, such as MCF-7 breast cancer, HL-60 leukaemia and others, investigators have found that these MDR cell lines not only overexpress Pgp but also overexpress PKC isoforms. A study by Blobe et al. (1993) suggested transcriptional induction of PKC α in MCF-7 ADR, the MDR subline of MCF-7 cells. They also showed that this overexpression of PKC α results in greater than 20-fold enhancement of phosphorylation of Pgp in those cells. Another study by Ratnasinghe et al. (1998) also reported an increase in PKC levels (including PKC α) and a decrease in PKA levels, concomitant with MDR phenotype in MCF-7 cells. This very intimate connection between Pgp and PKC α shows that the selection pressure in the MDR phenotypes favours not only Pgp but also PKC, suggesting that PKC is required for optimal function of Pgp (most probably through direct phosphorylation). Interestingly, the phosphatase levels were also altered in MDR cells, with the levels of the tyrosine phosphatase PP1B increasing and that of the serine/threonine phosphatases PP1 and PP2A decreasing in MDR MCF-7 cells (Ratnasinghe et al. 1998). This supports the argument for a pattern consistent with the maintenance of serine and threonine residues in a phosphorylated state in MDR cells. However, such studies do not rule out an additional function for PKC α, such as enhanced viability in response to cytotoxic drugs, due to its anti-apoptotic functions (Kelly et al. 1998; Ruvolo et al. 1998). Activation of the cell volume-regulatory Cl− channel of non-MDR cells (T-lymphocytes) through activation of the tumour necrosis factor-related receptor CD95 has been postulated to participate in apoptosis (Lang et al. 1998). It is possible that Pgp may regulate apoptosis in MDR cells through regulation of Cl− channel activity.
Meanwhile, studies by Hardy et al. (1995) indicated that PKC-mediated phosphorylation of Pgp may alter the volume-sensitive Cl− channel. The observation by Hardy et al. (1995) that the PKC activator 12-O-tetradecanoylphorbol-13-acetate (TPA), prevents volume-sensitive Cl− conductance in Pgp expressing cells contradicts the findings of Tominaga et al. (1995), where a similar pretreatment of intestine 407 cells with TPA did not prevent activation of volume-sensitive anion conductance. Furthermore, Vanoye et al. (1999) suggest that phosphorylation of one or more of serine 661, serine 667 and serine 671 by PKC or serine 667, serine 671 and serine 683 by PKA on the linker region of Pgp alters the activity of the swelling-induced Cl− channel by independent mechanisms. They further observed that stimulation by PKC or PKA decreased either the rate or degree of swelling-induced Cl− current (ICl, swell) activation, respectively (Fig. 3). The authors speculate that these results may reflect phosphorylation of different target residues, such that serine 661 is phosphorylated by only PKC while serine 683 is phosphorylated by only PKA. Interestingly, the recently identified ClC-3 channel (Duan et al. 1997) is a possible candidate for regulation by PKC (Valverde, 1999), indicating the importance of this kinase in regulating channel proteins.
Figure 3. Differential effects of PKA and PKC on the swelling-induced Cl− current ICl, swell in MDR cells.
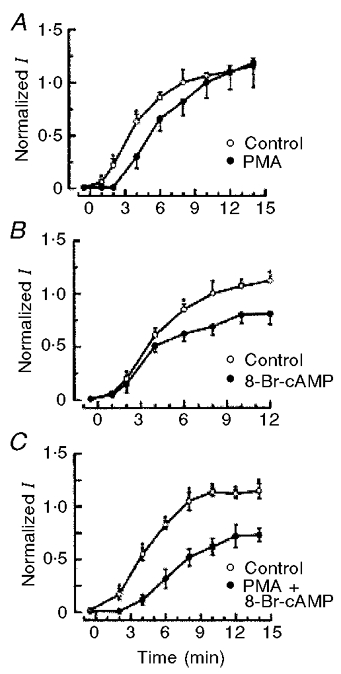
A, activation of PKC with the phorbol ester PMA reduced rate of activation of ICl, swell but not its steady-state value. B, activation of PKA with 8-bromo-cyclic AMP (8-Br-cAMP) reduced steady-state current levels. C, simultaneous activation of both PKA and PKC with 8-Br-cAMP and PMA, generated a ‘combined’ effect, such that onset of current was delayed and final current magnitude was reduced. The experimental protocol was described in Vanoye et al. (1999). Reproduced from Vanoye et al. (1999) with permission of the American Physiological Society.
Therefore, although one can argue that the different effects of PKC phosphorylation may be due to cell specificity, it is more likely that PKC-mediated phosphorylation may directly or indirectly influence the protein-to-protein interactions between the volume-sensitive anion channel and Pgp in cells overexpressing Pgp. Conflicting results regarding Pgp and swelling-induced anion conductance may also arise from the particular isoform of PKC involved.
Future prospects
An attractive model would be that phosphorylation of Pgp is multifunctional and shuttles the protein between various activities. This would indicate that Pgp may conduct different functions in different cells and phosphorylation may dictate the role that Pgp performs depending on the kinase(s)/isoform(s) available and the residue phosphorylated. Pgp may perform the transport function in all cell types. Phosphorylation of a single serine (e.g. serine 671) stimulates Pgp's ATPase activity/transport (Ahmad et al. 1994) and phosphorylation at a second site (e.g. serine 667) may suppress Cl− channel activity. Cell volume increase may lead to phosphorylation at a site, which suppresses ATPase activity as well as activates Cl− channels. Phosphorylation at other sites may enhance specificity of binding for a specific substrate or signal for degradation of the protein. Different kinases or different isoforms of the same kinase, in response to appropriate cellular stimuli, may conduct such hierarchical multisite phosphorylation. Such differential regulation may be important for regulating Pgp's functions and suggests that both activities of Pgp are mutually exclusive by virtue of phosphorylation. Such hypotheses can be tested through individual mutations of the known phosphorylatable residues. However, the general idea is supported by the observation that cell swelling inhibits Pgp's ATPase activity (Sardini et al. 1994) and hence, phosphorylation may be required to modulate the ‘on’ and ‘off’ modes of drug transport and Cl− channel regulation. Additionally, the MDR phenotype is associated with differential expression of PKC isoforms (Blobe et al. 1993). Most recently, differential phosphorylation of Pgp's linker region peptide with various PKC isoforms was demonstrated (Sachs et al. 1999). Interestingly, PKC α was demonstrated to phosphorylate serines 661, 667 and 671, whilst PKC ε preferentially phosphorylated serine 667. This is in agreement with earlier observations suggesting that PKC α, but not PKC ε, stimulates Pgp's ATPase activity (most probably through phosphorylating serine 671), whilst both isoforms regulate iodide efflux from cells in response to TPA (H. Idriss, V. Urquidi & S. Basavappa, manuscript in preparation). This latter regulation may occur through phosphorylation of serine 667.
Phosphorylation of Pgp may yet prove to be a subtle mechanism to regulate the functions of this protein and may have implications on the whole phenomenon of multidrug resistance. Protein kinases are increasingly becoming attractive targets for designing drugs to selectively inhibit tumour growth (Glazer, 1998). Further studies are required to decipher the precise role(s) of phosphorylating individual serines in the linker region of Pgp.
Acknowledgments
H.T.I. thanks Hoechst Marion Roussel for financial support during writing of this article. S.B. was supported by the National Kidney Foundation.
References
- Ahmad S, Safa AR, Glazer RI. Modulation of P-glycoprotein by protein kinase C alpha in a baculovirus expression system. Biochemistry. 1994;33:10313–10318. doi: 10.1021/bi00200a011. [DOI] [PubMed] [Google Scholar]
- Akabas MH. Cystic Fibrosis Transmembrane Conductance Regulator: structure and function of an epithelial chloride channel. Journal of Biological Chemistry. 2000;275:3729–3732. doi: 10.1074/jbc.275.6.3729. [DOI] [PubMed] [Google Scholar]
- Altenberg GA, Deitmer JW, Glass DC, Reuss L. P-glycoprotein-associated Cl− currents are activated by cell swelling but do not contribute to cell volume regulation. Cancer Research. 1994a;54:618–622. [PubMed] [Google Scholar]
- Altenberg GA, Vanoye CG, Han ES, Deitmer JW, Reuss L. Relationships between rhodamine 123 transport, cell volume, and ion-channel function of P-glycoprotein. Journal of Biological Chemistry. 1994b;269:7145–7149. [PubMed] [Google Scholar]
- Ambasch K, Cabantchik ZI, Slotki IN. Effects of hypotonic and hypoionic media on drug pumping by P-glycoprotein expressed in epithelial and nonepithelial cell lines. Journal of Cellular Physiology. 1995;164:117–122. doi: 10.1002/jcp.1041640115. [DOI] [PubMed] [Google Scholar]
- Basavappa S, Ellory JC. The role of swelling-induced anion channels during neuronal volume regulation. Molecular Neurobiology. 1996;13:137–153. doi: 10.1007/BF02740638. [DOI] [PubMed] [Google Scholar]
- Blobe GC, Sachs CW, Khan WA, Fabbro D, Stabel S, Wetsel WC, Obeid LM, Fine RL, Hannun YA. Selective regulation of expression of protein kinase C (PKC) isoenzymes in multidrug-resistant MCF-7 cells. Functional significance of enhanced expression of PKC alpha. Journal of Biological Chemistry. 1993;268:658–664. [PubMed] [Google Scholar]
- Bond TD, Higgins CF, Valverde MA. P-glycoprotein and swelling-activated chloride channels. Methods in Enzymology. 1998;292:359–370. doi: 10.1016/s0076-6879(98)92028-6. [DOI] [PubMed] [Google Scholar]
- Chambers TC. Identification of phosphorylation sites in human MDR1 P-glycoprotein. Methods in Enzymology. 1998;292:328–442. doi: 10.1016/s0076-6879(98)92026-2. [DOI] [PubMed] [Google Scholar]
- Chambers TC, Germann UA, Gottesman MM, Pastan I, Kuo JF, Ambudkar SV. Bacterial expression of the linker region of human MDR1 P-glycoprotein and mutational analysis of phosphorylation sites. Biochemistry. 1995;34:14156–14162. doi: 10.1021/bi00043a021. [DOI] [PubMed] [Google Scholar]
- Chambers TC, Pohl J, Glass DB, Kuo JF. Phosphorylation by protein kinase C and cyclic AMP-dependent protein kinase of synthetic peptides derived from the linker region of human P-glycoprotein. Biochemical Journal. 1994;299:309–315. doi: 10.1042/bj2990309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers TC, Pohl J, Raynor RL, Kuo JF. Identification of specific sites in human P-glycoprotein phosphorylated by protein kinase C. Journal of Biological Chemistry. 1993;268:4592–4595. [PubMed] [Google Scholar]
- De Greef C, Seherer J, Viana F, Acker KV, Eggermont J, Mertens L, Raeymakers L, Droogmans G, Nilius B. Volume-activated chloride currents are not correlated with P-glycoprotein expression. Biochemical Journal. 1995;307:713–718. doi: 10.1042/bj3070713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Chen G, Duran GE, Kouyama K, Chao AC, Sikic BI, Gollapudi SV, Gupta S, Gardner P. Volume-activated chloride current is not related to P-glycoprotein overexpression. Cancer Research. 1994;54:5029–5032. [PubMed] [Google Scholar]
- Duan D, Winter C, Cowley S, Hume JR, Horowitz B. Molecular identification of a volume-regulated chloride channel. Nature. 1997;390:417–421. doi: 10.1038/37151. [DOI] [PubMed] [Google Scholar]
- Ehring GR, Osipchuk YV, Cahalan MD. Swelling-activated chloride channels in multidrug-sensitive and -resistant cells. Journal of General Physiology. 1994;104:1129–1161. doi: 10.1085/jgp.104.6.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endicott JA, Ling V. The biochemistry of P-glycoprotein-mediated multidrug resistance. Annual Review of Biochemistry. 1989;58:137–171. doi: 10.1146/annurev.bi.58.070189.001033. [DOI] [PubMed] [Google Scholar]
- Fine RL, Chambers TC, Sachs CW. P-glycoprotein, multidrug resistance and protein kinase C. Stem Cells. 1996;14:47–55. doi: 10.1002/stem.140047. [DOI] [PubMed] [Google Scholar]
- Gerard V, Rouzaire-Doubois B, Dilda P, Dubois JM. Alterations of ionic membrane permeabilities in multidrug-resistant neuroblastoma × glioma hybrid cells. Journal of Experimental Biology. 1998;201:21–31. doi: 10.1242/jeb.201.1.21. [DOI] [PubMed] [Google Scholar]
- Germann UA. P-glycoprotein – a mediator of multidrug resistance in tumour cells. European Journal of Cancer. 1996;32A:927–944. doi: 10.1016/0959-8049(96)00057-3. [DOI] [PubMed] [Google Scholar]
- Germann UA, Chambers TC, Ambudkar SV, Licht T, Cardarelli CO, Pastan I, Gottesman MM. Characterization of phosphorylation-defective mutants of human P-glycoprotein expressed in mammalian cells. Journal of Biological Chemistry. 1996;271:1708–1716. doi: 10.1074/jbc.271.3.1708. [DOI] [PubMed] [Google Scholar]
- Germann UA, Chambers TC, Ambudkar SV, Pastan I, Gottesman MM. Effects of phosphorylation of P-glycoprotein on multidrug resistance. Journal of Bioenergetics and Biomembranes. 1995;27:53–61. doi: 10.1007/BF02110331. [DOI] [PubMed] [Google Scholar]
- Gill DR, Hyde SC, Higgins CF, Valverde MA, Mintenig GM, Sepulveda FV. Cell. 1992;71:23–32. doi: 10.1016/0092-8674(92)90263-c. [DOI] [PubMed] [Google Scholar]
- Glavy JS, Horwitz SB, Orr GA. Identification of the in vivo phosphorylation sites for acidic-directed kinases in murine mdr1b P-glycoprotein. Journal of Biological Chemistry. 1997;272:5909–5914. doi: 10.1074/jbc.272.9.5909. [DOI] [PubMed] [Google Scholar]
- Glazer RI. The protein kinase ABC's of signal transduction as targets for drug development. Current Pharmaceutical Design. 1998;4:277–290. [PubMed] [Google Scholar]
- Goodfellow HR, Sardini A, Ruetz S, Callaghan R, Gros P, McNaughton PA, Higgins CF. Protein kinase C-mediated phosphorylation does not regulate drug transport by the human multidrug resistance P-glycoprotein. Journal of Biological Chemistry. 1996;271:13668–13674. doi: 10.1074/jbc.271.23.13668. [DOI] [PubMed] [Google Scholar]
- Gottesman MM, Pastan I. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annual Review of Biochemistry. 1993;62:385–427. doi: 10.1146/annurev.bi.62.070193.002125. [DOI] [PubMed] [Google Scholar]
- Gottesman MM, Pastan I, Ambudkar SV. P-glycoprotein and multidrug resistance. Current Opinion in Genetics and Development. 1996;6:610–617. doi: 10.1016/s0959-437x(96)80091-8. [DOI] [PubMed] [Google Scholar]
- Han ES, Vanoye CG, Altenberg GA, Reuss L. P-glycoprotein-associated chloride currents revealed by specific block by an anti-P-glycoprotein antibody. American Journal of Physiology. 1996;270:C1370–1378. doi: 10.1152/ajpcell.1996.270.5.C1370. [DOI] [PubMed] [Google Scholar]
- Hardy SP, Goodfellow HR, Valverde MA, Gill DR, Sepulveda FV, Higgins CF. Protein kinase C-mediated phosphorylation of the human multidrug resistance P-glycoprotein regulates cell volume-activated chloride channels. EMBO Journal. 1995;14:68–75. doi: 10.1002/j.1460-2075.1995.tb06976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins CF. ABC transporters: from microorganisms to man. Annual Review in Cell Biology. 1992;8:67–113. doi: 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- Higgins CF. Volume-activated chloride currents associated with the multidrug resistance P-glycoprotein. The Journal of Physiology. 1995a;482.P:21–36S. doi: 10.1113/jphysiol.1995.sp020562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins CF. P-glycoprotein and cell volume-activated chloride channels. Journal of Bioenergetics and Biomembranes. 1995b;27:63–70. doi: 10.1007/BF02110332. [DOI] [PubMed] [Google Scholar]
- Jackson PS, Morrison R, Strange K. The volume-sensitive organic osmolyte channel VSOAC is regulated by non-hydrolytic ATP binding. American Journal of Physiology. 1994;267:C1203–1209. doi: 10.1152/ajpcell.1994.267.5.C1203. [DOI] [PubMed] [Google Scholar]
- Kelly ML, Tang Y, Rosensweig N, Clejan S, Beckman BS. Granulocyte-macrophage colony-stimulating factor rescues TF-1 leukemia cells from ionizing radiation-induced apoptosis through a pathway mediated by protein kinase C α. Blood. 1998;92:416–424. [PubMed] [Google Scholar]
- Kunzelmann K, Slotki IN, Klein P, Koslowsky T, Ausiello DA, Greger R, Cabantchik ZI. Effects of P-glycoprotein expression on cyclic AMP and volume-activated ion fluxes and conductances in HT-29 colon adenocarcinoma cells. Journal of Cellular Physiology. 1994;161:393–406. doi: 10.1002/jcp.1041610302. [DOI] [PubMed] [Google Scholar]
- Lang F, Lepple-Wienhues A, Paulmichl M, Szabo I, Siemen D, Gulbins E. Ion channels, cell volume, and apoptotic cell death. Cellular Physiology and Biochemistry. 1998;8:285–292. doi: 10.1159/000016290. [DOI] [PubMed] [Google Scholar]
- Loo TW, Clarke DM. The minimum functional unit of human P-glycoprotein appears to be a monomer. Journal of Biological Chemistry. 1996;271:27488–27492. doi: 10.1074/jbc.271.44.27488. [DOI] [PubMed] [Google Scholar]
- Miwa A, Ueda K, Okada Y. Protein kinase C-independent correlation between P-glycoprotein expression and volume sensitivity of Cl− channel. Journal of Membrane Biology. 1997;157:63–69. doi: 10.1007/s002329900216. [DOI] [PubMed] [Google Scholar]
- Morin XK, Bond TD, Loo TW, Clarke DM, Bear CE. Failure of P-glycoprotein (MDR1) in Xenopus oocytes to produce swelling-activated chloride channel activity. The Journal of Physiology. 1995;486:707–714. doi: 10.1113/jphysiol.1995.sp020846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munkonge FM, Osborne LR, Geddes DM, Alton EW. Lack of inhibition by dideoxy-forskolin and verapamil of DIDS-sensitive volume-activated Cl− secretion in human squamous lung carcinoma epithelial cells. Biochimica et Biophysica Acta. 1994;1224:342–348. doi: 10.1016/0167-4889(94)90266-6. [DOI] [PubMed] [Google Scholar]
- Nielsen D, Maare C, Skovsgaard T. Influx of daunorubicin in multidrug resistant Ehrlich ascites tumour cells: correlation to expression of P-glycoprotein and efflux. Influence of verapamil. Biochemical Pharamacology. 1995;50:443–450. doi: 10.1016/0006-2952(95)00172-v. [DOI] [PubMed] [Google Scholar]
- Okada Y. Volume expansion-sensing outward-rectifier Cl− channel: fresh start to the molecular identity and volume sensor. American Journal of Physiology. 1997;273:C755–789. doi: 10.1152/ajpcell.1997.273.3.C755. [DOI] [PubMed] [Google Scholar]
- Orr GA, Han EK, Browne PC, Nieves E, O'Connor BM, Yang CP, Horwitz SB. Identification of the major phosphorylation domain of murine mdr1b P-glycoprotein. Analysis of the protein kinase A and protein kinase C phosphorylation sites. Journal of Biological Chemistry. 1993;268:25054–25062. [PubMed] [Google Scholar]
- Pearson RB, Kemp BE. Protein kinase phosphorylation site sequences and consensus specificity motifs: tabulations. Methods in Enzymology. 1991;200:62–81. doi: 10.1016/0076-6879(91)00127-i. [DOI] [PubMed] [Google Scholar]
- Rasola A, Galietta LJV, Gruenert DC, Romeo G. Volume-sensitive chloride currents in four epithelial cell lines are not directly correlated to the expression of MDR-1 gene. Journal of Biological Chemistry. 1994;269:1432–1436. [PubMed] [Google Scholar]
- Ratnasinghe D, Phang JM, Yeh GC. Differential expression and activity of phosphatases and protein kinases in adriamycin sensitive and resistant human breast cancer MCF-7 cells. International Journal of Oncology. 1998;13:79–84. doi: 10.3892/ijo.13.1.79. [DOI] [PubMed] [Google Scholar]
- Ruvolo PP, Deng XM, Carr BH, May WS. A functional role for mitochondrial protein kinase Ca in Bcl2 phosphorylation and suppression of apoptosis. Journal of Biological Chemistry. 1998;273:25436–25442. doi: 10.1074/jbc.273.39.25436. [DOI] [PubMed] [Google Scholar]
- Sachs CW, Chambers TC, Fine RL. Differential phosphorylation of sites in the linker region of P-glycoprotein by protein kinase C isozymes alpha, betaI, betaII, gamma, delta, epsilon, eta, and zeta. Biochemical Pharmacology. 1999;58:1587–1592. doi: 10.1016/s0006-2952(99)00240-3. [DOI] [PubMed] [Google Scholar]
- Safa AR. Photoaffinity labels for characterizing drug interaction sites of P-glycoprotein. Methods in Enzymology. 1998;292:289–307. doi: 10.1016/s0076-6879(98)92023-7. [DOI] [PubMed] [Google Scholar]
- Sardini A, Mintenig GM, Valverde MA, Sepulveda FV, Gill DR, Hyde SC, Higgins CF, McNaughton PA. Drug efflux mediated by the human multidrug resistance P-glycoprotein is inhibited by cell swelling. Journal of Cell Science. 1994;107:3281–3290. doi: 10.1242/jcs.107.12.3281. [DOI] [PubMed] [Google Scholar]
- Shapiro AB, Ling V. The mechanism of ATP-dependent multidrug transport by P-glycoprotein. Acta Physiologica Scandinavica. 1998;(suppl. 643):227–234. [PubMed] [Google Scholar]
- Sharom FJ. The P-glycoprotein efflux pump: how does it transport drugs? Journal of Membrane Biology. 1997;160:161–175. doi: 10.1007/s002329900305. [DOI] [PubMed] [Google Scholar]
- Strange K, Emma F, Jackson PS. Cellular and molecular physiology of volume-sensitive anion channels. American Journal of Physiology. 1996;270:C711–730. doi: 10.1152/ajpcell.1996.270.3.C711. [DOI] [PubMed] [Google Scholar]
- Szabo K, Bakos E, Welker E, Muller M, Goodfellow HR, Higgins CF, Varadi A, Sarkadi B. Phosphorylation site mutations in the human multidrug transporter modulate its drug-stimulated ATPase activity. Journal of Biological Chemistry. 1997;272:23165–23171. doi: 10.1074/jbc.272.37.23165. [DOI] [PubMed] [Google Scholar]
- Thevnod F, Anderie I, Schulz I. Monoclonal antibodies against MDR1 P-glycoprotein inhibit chloride conductance and label a 65-kDa protein in pancreatic zymogen granule membranes. Journal of Biological Chemistry. 1994;269:24410–24417. [PubMed] [Google Scholar]
- Tominaga M, Tominaga T, Miwa A, Okada Y. Volume-sensitive chloride channel activity does not depend on endogenous P-glycoprotein. Journal of Biological Chemistry. 1995;270:27887–27893. doi: 10.1074/jbc.270.46.27887. [DOI] [PubMed] [Google Scholar]
- Trezise AE, Ratcliff R, Hawkins TE, Evans MJ, Freeman TC, Romano PR, Higgins CF, Colledge WH. Co-ordinate regulation of the cystic fibrosis and multidrug resistance genes in cystic fibrosis knockout mice. Human Molecular Genetics. 1997;6:527–537. doi: 10.1093/hmg/6.4.527. [DOI] [PubMed] [Google Scholar]
- Trezise AE, Romano PR, Gill DR, Hyde SC, Sepulveda FV, Buchwald M, Higgins CF. The multidrug resistance and cystic fibrosis genes have complementary patterns of epithelial expression. EMBO Journal. 1992;11:4291–4303. doi: 10.1002/j.1460-2075.1992.tb05528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valverde MA. CIC channels: leaving the dark ages on the verge of a new millennium. Current Opinion in Cell Biology. 1999;11:509–516. doi: 10.1016/s0955-0674(99)80074-x. [DOI] [PubMed] [Google Scholar]
- Valverde MA, Hyde SC, Sepulveda FV, Gill DR, Hyde SC, Higgins CF. Volume-regulated chloride channels associated with the human multidrug-resistance P-glycoprotein. Nature. 1992;355:830–833. doi: 10.1038/355830a0. [DOI] [PubMed] [Google Scholar]
- Vanoye CG, Altenberg GA, Reuss L. P-glycoprotein is not a swelling-activated Cl− channel; possible role as a Cl− channel regulator. The Journal of Physiology. 1997;502:249–258. doi: 10.1111/j.1469-7793.1997.249bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanoye CG, Castro AF, Pourcher T, Reuss L, Altenberg GA. Phosphorylation of P-glycoprotein by PKA and PKC modulates swelling-activated Cl− currents. American Journal of Physiology. 1999;276:C370–378. doi: 10.1152/ajpcell.1999.276.2.C370. [DOI] [PubMed] [Google Scholar]
- Viana F, Acker V, De Greef C, Eggermont J, Raeymaekers L, Droogmans G, Nilius B. Drug-transport and volume-activated chloride channel functions in human erythroleukemia cells: Relation to expression level of P-glycoprotein. Journal of Membrane Biology. 1995;145:87–89. doi: 10.1007/BF00233309. [DOI] [PubMed] [Google Scholar]
- Wang X, Wall DM, Parkin JD, Zaloberg JR, Kemm RE. P-glycoprotein expression in classical multi-drug resistant leukemia cells does not correlate with enhanced chloride channel activity. Clinical and Experimental Pharmacology and Physiology. 1994;21:101–108. doi: 10.1111/j.1440-1681.1994.tb02475.x. [DOI] [PubMed] [Google Scholar]
- Weaver JL, McKinney L, Schoenlein PV, Goldenberg S, Gottesman MM, Aszalos A. MDR1/P-glycoprotein function. I. Effect of hypotonicity and inhibitors on rhodamine 123 exclusion. American Journal of Physiology. 1996;270:C1447–1452. doi: 10.1152/ajpcell.1996.270.5.C1447. [DOI] [PubMed] [Google Scholar]
- Wine JJ, Luckie DB. Cell-volume regulation: P-glycoprotein – a cautionary tale. Current Biology. 1996;6:1410–1412. doi: 10.1016/s0960-9822(96)00744-0. [DOI] [PubMed] [Google Scholar]
- Wu J, Zhang JJ, Koppel H, Jacob TJ. P-glycoprotein regulates a volume-activated chloride current in bovine non-pigmented ciliary epithelial cells. The Journal of Physiology. 1996;491:743–755. doi: 10.1113/jphysiol.1996.sp021254. [DOI] [PMC free article] [PubMed] [Google Scholar]


