Abstract
The objective was to characterize the circannual, circadian, and ultradian secretory patterns of growth hormone (GH) in intact crossbred and purebred dogs. In all experiments, blood samples were collected with minimal stress by direct peripheral venipuncture and GH was measured in plasma by a homologous radioimmunoassay. For circannual studies, samples were collected monthly from 6 male dogs between 15:00 and 17:30 h over a 1-year time span. For circadian studies, blood samples were collected at 145-minute intervals from 09:00 to 06:45 h of the following day in 14 female dogs. In ultradian experiments, blood samples were collected at 15-minute intervals for 2.5 h (15:00 to 17:30 h) in 7 males and 7 females. Plasma GH in male dogs remained without change in summer, autumn, and winter but declined (P ≤ 0.01) in spring (LSM ± SEM; 6.9 ± 0.5; 6.0 ± 0.5; 6.3 ± 0.5; 4.3 ± 0.5 ng/mL, respectively). No plasma GH circadian rhythmicity was detected. Nor was any ultradian pattern evident in either males or females. No gender-related differences were observed in ultradian GH plasma profiles. It is concluded that, while basal GH levels show seasonal fluctuations in dogs, neither circadian nor ultradian GH secretory fluctuations were present in the dogs assessed.
Introduction
In most vertebrates, neuroendocrine functions are organized in regular cycles of different periodicity, which may range from minutes to months. Cycles of approximately 12 mo are known as circannual rhythms, while periods of approximately 24 h are called circadian rhythms. Cycles that occur more frequently than once daily are termed ultradian (1,2,3,4). Patterns of hormone secretion are regulated mainly by endogenous cues, though they are also influenced by environmental factors (5,6). Seasonal variations in temperature and photoperiod have an influence on neuroendocrine circannual rhythms, particularly in wild animals (4). From a practical point of view, awareness of secretory rhythms is important, as adequate assessment of endocrine function must take into account the variability of hormone concentrations in blood, which is frequently associated with changes in pituitary responsiveness to natural or pharmacological secretagogues.
The secretory patterns of pituitary growth hormone (GH) are regulated primarily by the coordinated action of both a stimulatory and an inhibitory hypothalamic hypophysiotropic hormone, GH-releasing hormone (GHRH) and somatostatin, respectively (7). Autonomous production of GH by the mammary gland has been described in some species, including the dog (8). While the secretory patterns of GH are well characterized in humans and rodents (7), in dogs, studies on GH secretion have been focused mainly on pulsatile patterns (9,10,11,12). At present, there is no documented information on circannual patterns of GH secretion in dogs and the information on circadian GH secretory patterns in this species is only circumstantial (9). Furthermore, while ultradian pulsatile patterns of circulating GH have been described in this species, the information has been almost exclusively generated in laboratory beagles (10,11,13). Our objective was to characterize the circannual, circadian, and ultradian secretory patterns of GH in intact crossbred and purebred dogs. The present report describes our findings.
Materials and methods
Animals
Circannual studies — Four crossbred and 2 purebred (1 doberman and 1 collie) healthy, intact male dogs, 1 to 10 y of age, weighing 10 to 35 kg, were included in this experiment.
Circadian studies — Twelve healthy, anestrous, crossbred bitches, 1 to 5 of age, weighing 9 to 20 kg, were used.
Ultradian studies — Seven crossbred, healthy, intact male dogs, 1 to 10 y of age, weighing 25 to 35 kg, and 2 anestrous crossbred and 5 purebred (4 Argentine boar hounds and 1 German shepherd) female dogs, 1.5 to 5 y of age, weighing 12 to 35 kg, were used. Anestrous state in the circadian and ultradian studies was established by vulvar examination and vaginal cytology and was later confirmed by measuring plasma progesterone (P4) concentration (P4 ≤ 1 ng/mL). All the animals had been living in outdoor kennels for at least 1 y before the beginning of the studies, were fed commercial dog food, and were given water ad libitum. They were already accustomed to blood sampling procedures.
Place
Experiments were carried out in the city of La Plata (latitude 34° 55′ south , longitude 57° 17′ west), 56 km southeast from Buenos Aires, Argentina. Daylight length ranges from 10 h in winter to 15 h in summer.
During the experiments, the animals remained in their normal surroundings (kennels) except during the circadian experiments, in which the dogs were moved to indoor windowed kennels 2 d before and artificial light (100 W) was used during the dark hours.
Blood sampling
In all experiments, blood samples were collected under minimal stress by direct peripheral venipuncture (jugular/cephalic/ saphenous). Blood samples were collected in heparinized tubes and centrifuged at 4000 × g for 15 min. Plasma was separated and stored at −20°C until hormone assays were conducted.
Circannual studies — Blood samples for GH determination were collected monthly, between 15:00 and 17:30, over a 1-year time span, from December 1999 to November 2000.
Circadian studies — Experiments were performed during winter. Blood samples were collected at 145-minute intervals from 09:00 to 06:45 of the following day. A total of 10 samples (the first 5 during daylight) were taken from each bitch.
Ultradian studies — Experiments were also performed during winter. Blood samples were collected at 15-minute intervals for 2.5 h (n = 11 samples/dog) from 15:00 to 17:30.
In circadian and ultradian studies, P4 concentration was measured in the first plasma sample of each animal (n = 1 sample/bitch).
Hormone assays
Plasma GH was measured by a homologous liquid phase radioimmunoassay (RIA) with the materials provided by Dr. A.F. Parlow, Pituitary Hormones and Antisera Center, Harbor-UCLA Medical Center, Torrance, California, USA. Iodination grade canine GH (cGH) was radiolabeled by the Iodo-Gen method (14) and purified on a PD-10 Sephadex G-25M column (Pharmacia, Uppsala, Sweden) equilibrated with 0.01 M phosphosaline, pH 7.6. A 1/40 goat anti-human IgG serum in 0.05 M phosphate buffer, 1% normal rabbit serum and 8% polyethylenglycol, was used to separate bound from free hormone. Serum cGH levels were expressed in terms of cGH-RP-1. The intra- and interassay CVs for the cGH RIA were 8.2 and 13.8%, respectively. The sensitivity at 95% binding was 0.8 ng/mL. Plasma P4 concentrations were measured by RIA, using a solid phase commercial kit (Coat-A-Count, DPC; Los Angeles, California, USA). The sensitivity at 95% binding was 0.1 ng/mL and the interassay and intra-assay CVs were 5.1 and 8.8%, respectively. All samples from a given dog were determined in a single run to avoid interassay variation.
Statistical analysis
Circannual studies — Growth hormone values were analyzed by least squares analysis of variance (ANOVA) using the General Linear Model Procedure (15). The mathematical model included the main effect of season. Months were grouped in seasons as follows: spring (September, October, November), summer (December, January, February), autumn (March, April, May), and winter (June, July, August). Orthogonal contrasts were used to test differences among seasons. To characterize further GH concentrations and the number of hours of natural light, data were examined by correlation analysis (16).
Circadian studies — Two sampling time windows were studied in order to detect the presence of an eventual circadian change of plasma GH concentrations. Thus, the experimental period was divided into day [samples 1 to 5 (09:00–18:40)] and night [samples 6 to 10 (21:05–6:45)]. The GH profiles were analyzed by least squares ANOVA using the General Linear Model Procedure (15). The mathematical model included the main effect of window and bitch and the interaction of window by bitch.
Ultradian studies — Growth hormone profiles were analyzed by repeated measures ANOVA using the General Linear Model Procedure (15). The mathematical model included the main effects of gender and time and the gender by time interaction. For both experiments, data were expressed as least squares mean (LSM) ± standard error of the mean (SEM) and the level of significance was set at P ≤ 0.05.
Results
Circannual studies
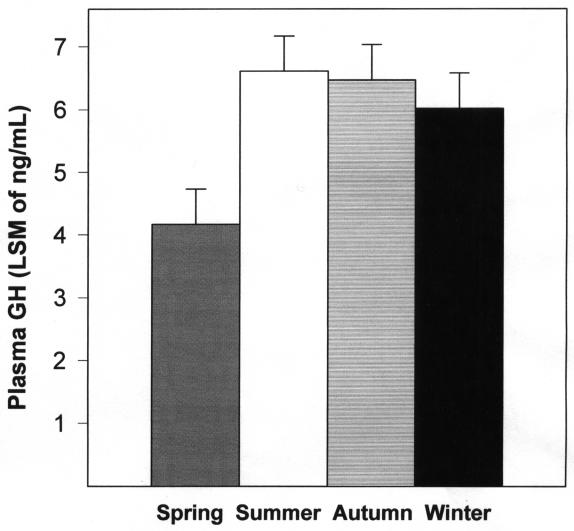
Plasma GH levels were comparable in summer, autumn, and winter but fell significantly during spring (P ≤ 0.01; Figure 1). No significant correlation was found between duration of daylight and GH concentration.
Figure 1. Plasma GH concentrations in male dogs in different seasons. Data are expressed as least squares mean of GH values (ng/mL). Bars over columns represent standard error of mean.
Circadian studies
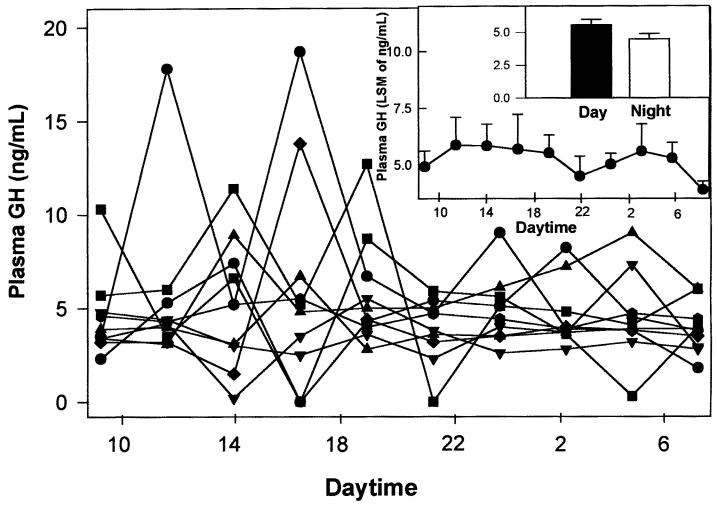
Plasma P4 concentration (expressed as LSM ± SEM of ng/mL) for all of the bitches was 0.3 ± 0.1, while overall GH concentration, similarly expressed, was 5.2 ± 0.29. Individual GH profiles were variable with occasional single-point peaks detected in some of the dogs (Figure 2, main panel). Analysis of GH values did not reveal any significant circadian fluctuation, although a trend towards trough GH concentrations was apparent at 9 and 22 h (Figure 2, large inset). No significant differences were found in plasma GH concentrations between day (9:00 to 18:40, 5.6 ± 0.4 L) and night (21:05 to 6:45, 4.9 ± 0.1 ng/mL; Figure 2, small inset).
Figure 2. Circadian GH secretory profiles in anestrous bitches. Main panel: Individual plasma GH profiles in 12 crossbred anestrous bitches. Large inset: GH plasma profiles of the 12 animals taken together. Data are expressed as least squares mean ± standard error of mean (LSM ± SEM) of GH values (ng/mL). Small inset: Plasma GH levels during day and night in the same animals. Data are expressed as above.
Ultradian studies
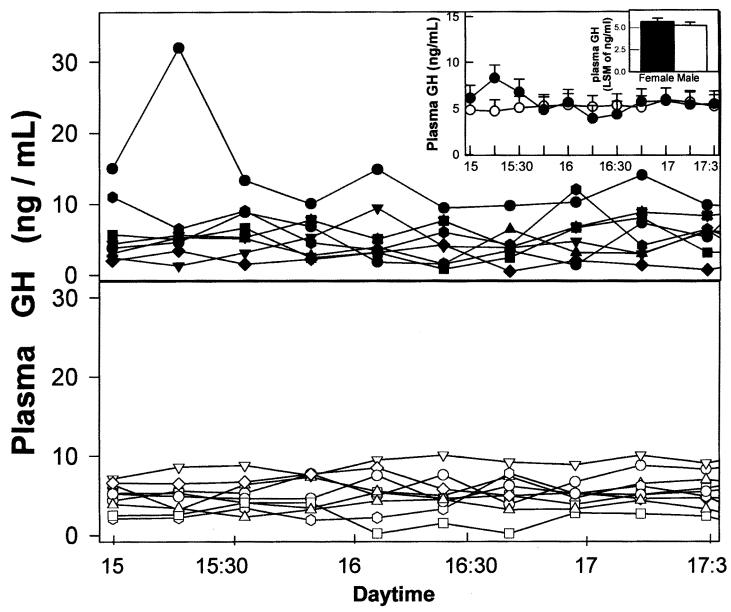
The LSM values of plasma GH concentrations for the time window assessed did not show significant differences between females and males (5.7 ± 4 vs 5.2 ± 0.4, respectively; Figure 3, small inset). For both sexes, individual plasma GH patterns were fairly homogeneous during the time-window assessed (Figure 3, main panels). Average secretory GH profiles were similar in males and females (Figure 3, large inset). Neither time nor the interaction of time by gender was significantly different. The plasma P4 concentration for the bitches used was (LSM ± SEM of ng/mL) 0.67 ± 0.25.
Figure 3. Ultradian GH secretory patterns in dogs. Main panels: Individual ultradian plasma GH profiles for females (lower panel) and males (upper panel). Large inset: Average ultradian GH profiles in male (●) and female (○) dogs. Small inset: Comparison between integrated (pooled data for each gender) plasma GH levels in male and female dogs. Bars over columns represent values for standard error of mean.
Discussion
The present study provides an integrated view of canine GH secretory patterns in different time frames. For ultradian studies we were able to compare plasma GH profiles between sexes. In general, basal GH plasma concentrations were higher than those reported by others (10,12). This discrepancy may be due to variations in laboratory methodology or to breed-related differences in hormone levels.
The possible influence of temperature and photoperiod was investigated in this paper. In line with reports in heifers (17), no significant correlation between daylight span and plasma GH concentration was found in our male dogs. The significantly lower GH concentrations found in spring might be due to the fact that this time of the year is characterized by a relatively rapid increase in photoperiod length. The documented information about circadian GH secretory patterns in dogs is scarce. Studies in male dogs failed to detect any significant circadian rhythmicity for circulating GH (9), which is consistent with the present findings for GH in anestrous bitches. We have reported previously that in anestrous bitches, plasma luteinizing hormone also lacks a circadian rhythmicity; however, in the same study, a subpopulation of anestrous bitches with high plasma prolactin levels was found to display a surge in PRL secretion around 18:00 h (18). Whether the trend towards the occurrence of plasma GH minima at 09:00 and 22:00 h observed in our anestrous bitches represents a true physiological situation remains to be confirmed. During the circadian studies, bitches slept intermittently throughout the experiments, a behavior not distant from that of domestic pets, which usually have short naps during the day and a split sleep at night. Therefore, our results appear to be representative of what happens in pet dogs. Nevertheless, the fact that our dogs were not under a natural light-dark cycle during the experiments represents an admitted limitation. In male dogs, it has been reported that basal GH concentrations were not affected by sleep-wake cycles (9).
In dogs, sex-related differences in circulating GH profiles have not been systematically assessed. The present study is the first, to our knowledge, to assess sex-associated differences for the ultradian secretory patterns of GH in dogs. Unlike rats (19,20), cattle (21), and humans (22), where a distinct sexual dimorphism has been demonstrated for ultradian GH secretory patterns, dogs appear to lack this sex-associated difference. Nevertheless, it should be pointed out that our ultradian studies were carried out only in anestrous bitches. Since the secretory pattern of GH in the bitch is influenced by P4 (12), the possibility exists that differences may occur between males and bitches during either the follicular or luteal phase.
It is concluded that, while basal GH levels showed seasonal fluctuations in crossbred and purebred dogs, neither circadian nor ultradian GH secretory fluctuations were present in the dogs assessed.
Footnotes
Acknowledgments
The authors thank Ms. Yolanda Sosa for technical help and the breeding kennel Los Cinco Montes for providing some of the dogs. This study was supported in part by grants M065 and M089 to RGG from the National University of La Plata. RLS and RGG are career scientists of the Argentinean Research Council (CONICET).
Address correspondence and reprint requests to Dr. Cristina Gobello, tel: +54-221 4252155, fax: +54-221 4250924, e-mail: cgobello@netverk.com.ar
Received August 23, 2001. Accepted January 18, 2002.
References
- 1.Reichlin S. Neuroendocrinology. In: Wilson JD, Foster DW, eds. Textbook of Endocrinology. 8th ed. Philadelphia: WB Saunders, 1992:135–220.
- 2.Van Cauter E. Diurnal and ultradian rhythms in human endocrine function: a minireview. Horm Res 1990;34:45–53. [DOI] [PubMed]
- 3.Van Cauter E, Turek FW. Endocrine and other biological rhythms. In: De Groot LJ, ed. Endocrinology. 3rd ed. Philadelphia: WB Saunders, 1995:2497–2548.
- 4.Touitou Y, Bogdan A, Reinberg A, Haus E. Les rythmes annueles endocriniens chez l'homme. Pathol Biol (Paris) 1996;44:654–665. [PubMed]
- 5.Johnson MS. Activity and distribution of certain wild mice in relation to biotic communities. J Mammal 1926;7:245–277.
- 6.Aschoff J. Circadian rhythms in man. Science 1965;148:1427–1432. [DOI] [PubMed]
- 7.Thorner MO, Vance ML, Laws RE, Horvath E, Kovath K. The anterior pituitary. In: Wilson JD, Foster DW, Kronenberg HM, Larsen PR, eds. Textbook of Endocrinology. 9th ed. Philadelphia: WB Saunders, 1998:249–340.
- 8.Selman PJ, Mol JA, Rutteman GR, Rijnberk AD. Canine growth hormone regulation: new insights. Tijdschr Diergeneeskd. 1993;118(Suppl 1):36S–37S. [PubMed]
- 9.Takahashi Y, Ebihara S, Nakamura Y, Takahashi K. A model of human sex related growth hormone secretion in dogs: effects of 3, 6, and 12 hours of forced wakefulness on plasma growth hormone, cortisol and sleep stages. Endocrinology 1981;109: 262–272. [DOI] [PubMed]
- 10.Cowan JS, Gaul P, Moor BC, Kraicer J. Secretory burst of growth hormone secretion in the dog may be initiated by somatostatin withdrawal. Can J Physiol Pharmacol 1984;62:189–197. [DOI] [PubMed]
- 11.French MB, Vaitkus P, Cukerman E, Sirek A, Sirek OV. Secretory pattern of canine growth hormone. Am J Physiol Endocrinol Metab 1987;15:268–272. [DOI] [PubMed]
- 12.Kooistra HS, den Hertog E, Okkens AC, Mol JA, Rijnberk A. Pulsatile secretion pattern of growth hormone during the luteal phase and mid-anestrous in beagle bitches. J Reprod Fertil 2000;119:217–222. [PubMed]
- 13.Kooistra H. Adenohypophyseal function in healthy dogs and dogs with pituitary disease [PhD Thesis]. University of Utrecht, 2000:247.
- 14.Fraker PJ, Speck JC. Protein and cell membrane iodinations with a sparingly soluble chloramide, 1,3,4,6-tetrachloro-3a,6a-diphenylglycoluril. Biochem Biophys Res Commun 1978;80: 849–857. [DOI] [PubMed]
- 15.SAS/STAT User's guide. v. 6, 4th ed. Cary, NC: SAS Institute, 1989:189.
- 16.SAS/STAT User's guide. v. 5, 4th ed. Cary, NC: SAS Institute, 1988:189.
- 17.Borromeo V, Cremonesi F, Perucchetti E, Berrini A, Secchi C. Circadian and circannual plasma secretory patterns of growth hormone and prolactin in fresian heifers: hormonal profiles and signal analysis. Comp Biochem Physiol 1994;107:313–321. [DOI] [PubMed]
- 18.Gobello MC, Bolognani F, de la Sota RL, Goya RG. Twenty four hour profiles of prolactin and luteinizing hormone in anestrous crossbred bitches. Reprod Domestic Anim 2001;36:41–45. [DOI] [PubMed]
- 19.Jansson JO, Eden S, Isaksson O. Sexual dimorphism in the control of GH secretion. Endocrine Rev 1985;6:128–150. [DOI] [PubMed]
- 20.Carlsson L, Eden S, Jansson J. The plasma pattern of growth hormone in conscious rats during late pregnancy. J Endocrinol 1990;124:191–198. [DOI] [PubMed]
- 21.Anfinson MS, Davis SL, Christian E, Everson DO. Episodic secretion of growth hormone in steers and bulls: an analysis of frequency and magnitude of secretory spikes occuring in a 24-hr period. Proc West Sect Am Soc Anim Sci 1975;26:175–177.
- 22.Eriksson L, Frankenne F, Eden S, Hennen G, Von Schoultz B. Growth hormone 24 hr serum profiles during pregnancy — lack of pulsatility for the secretion of the placental variant. Br J Obstet Gynecol 1989;96:949–953. [DOI] [PubMed]