Abstract
Respiratory, heart rate and hindlimb vascular responses were studied in response to increasing levels of stimulation of the carotid body chemoreceptors, together with an examination of the modulation of their effects by distension of the urinary bladder in the dog anaesthetized with a mixture of chloralose and urethane.
The vascularly isolated carotid bifurcation regions were perfused with blood, stimulation of the carotid bodies being carried out by three different levels of hypoxic isocapnic blood (PO2 approximately 58, 40 and 22 mmHg) obtained from a donor animal. A vascularly isolated hindlimb was autoperfused at constant blood flow through its femoral artery.
In spontaneously breathing animals, increasingly intense hypoxic stimulation of the carotid bodies caused a progressive augmentation of respiratory minute volume. Superimposition of distension of the bladder increased ventilation further, by the same amount during hypoxic as during normoxic blood perfusion of the chemoreceptors.
Prevention of the effects of lung stretch afferent stimulation by artificial ventilation modified the heart rate and hindlimb vascular responses to excitation of the carotid bodies by revealing or accentuating the primary cardiovascular responses, bradycardia and vasoconstriction. In contrast, no such respiratory modulation was apparent in the cardiovascular responses to bladder distension.
When, under conditions of artificial ventilation and in the absence of changes in the arterial baroreceptor input, the primary cardio-inhibitory and vasoconstrictor responses to carotid chemoreceptor stimulation predominated, the heart slowed progressively as the stimulus was increased. At the same time the cardio-accelerator effects of bladder distension progressively diminished, indicating an interaction between the cardiac reflex responses evoked by the two inputs. In contrast, the reflex vascular responses resulting from stimulation of the two inputs were additive, at least for PO2 levels of carotid body perfusate down to approximately 40 mmHg.
In conclusion these experiments demonstrate the differential nature of the integration of respiratory and cardiovascular responses evoked by stimulation of the carotid chemoreceptors and bladder distension.
Distension of the urinary bladder in man and animals causes reflex vasoconstriction, hypertension and tachycardia (Mukherjee, 1957; Taylor, 1968; Daly et al. 1986, 1993; Hassan et al. 1987a,b; Ward et al. 1995). In the presence of a cervical or upper thoracic spinal cord transection the rise in blood pressure in response to bladder distension is typically large. A bradycardia then predominates due to initiation of an arterial baroreceptor reflex, mediated by the intact vagal pathways (Guttmann & Whitteridge, 1947). Further studies showed that distension of the bladder modified the relationship between the pressure in the isolated perfused carotid sinus regions and the reflex respiratory, heart rate and hindlimb vascular resistance responses (Daly et al. 1993; Ward et al. 1995) and the maximum rate of rise of left ventricular pressure (inotropic response) (Ward et al. 1995).
We now report the results of experiments carried out to determine whether the respiratory, cardiac and hindlimb vascular responses evoked by stimulation of the carotid body chemoreceptors are also affected by distension of the bladder. Some of our initial results have been reported briefly elsewhere (Daly & Wood, 1983).
METHODS
All experiments were carried out in accordance with UK legislation governing experiments on animals.
Two mongrel dogs were used in each experiment. The recipient and test animals were of either sex weighing 10·3–16·7 kg (Table 1). About 45 min after an injection of morphine hydrochloride (1 mg kg−1s.c.), they were anaesthetized by an intravenous injection of a mixture of 2 % α-chloralose (56 mg kg−1; Establishments Kuhlmann, Paris, France) and 20 % urethane (560 mg kg−1; British Drug Houses, Ltd) dissolved in 85 parts sodium chloride solution (154 mM) and 15 parts polyethylene glycol (molecular weight, 200; Carbowax, Union Carbide Ltd, Herts, UK). Supplementary doses were administered as required. Rectal temperature was monitored throughout and maintained between 36·5 and 38°C. Penicillin (1 million i.u.; Crystapen, Glaxo Laboratories Ltd, Middlesex, UK) was given intravenously to both animals at the beginning of each experiment.
Table 1.
Initial control values for the measured respiratory, cardiovascular and blood gas variables in the recipient animal at the start of the experimental period
| Variable | Spontaneously breathing | Artificially ventilated | Artificially ventilated, cervical vagotomy |
|---|---|---|---|
| No. of animals | 6 | 6 | 8 |
| Body weight (kg) | 14·2 ± 1·5 | 14·9 ± 1·2 | 13·9 ± 2·0 |
| V̇E (l min−1 kg−1) | 0·193 ± 0·049 | — | — |
| VT (ml kg−1) | 15·5 ± 1·9 | — | — |
| Rf(breaths min−1) | 12·7 ± 3·7 | — | — |
| Pa(mmHg) | |||
| Systolic | 166·0 ± 32·5 | 156 ± 24 | 177·3 ± 36·3 |
| Diastolic | 102·7 ± 32·8 | 106 ± 23·4 | 124 ± 29·1 |
| Mean | 123·7 ± 34·2 | 122·7 ± 23·5 | 141·8 ± 35·3 |
| ivc (mmHg) | 4·7 ± 0·7 | 5·2 ± 1·8 | 5·1 ± 1·9 |
| HR (beats min−1) | 130 ± 33·9 | 163 ± 45·8 | 234 ± 21·3 |
| limb (mmHg) | 104·0 ± 13·1 | 99·8 ± 9·6 | 107·9 ± 8·4 |
| Pcs(mmHg) | |||
| Systolic | 160·7 ± 10·6 | 156·7 ± 6·9 | 177·8 ± 29·5 |
| Diastolic | 95·3 ± 12·8 | 93 ± 6·7 | 102·3 ± 20·2 |
| Mean | 122·3 ± 10·6 | 120·7 ± 4·7 | 134·8 ± 23·8 |
| Arterial blood | |||
| PO2(mmHg) | 122 ± 15·3 | 115·2 ± 16·6 | 120·6 ± 14·2 |
| PCO2(mmHg) | 44·5 ± 2·9 | 41·4 ± 3·8 | 40 ± 4·2 |
| pH | 7·35 ± 0·036 | 7·381 ± 0·028 | 7·394 ± 0·047 |
| Ht(%) | 47·8 ± 3·9 | 48·3 ± 3·1 | 49·8 ± 3·8 |
Values are means ± s. d. V̇E, respiratory minute volume; VT, tidal volume; Rf, respiratory frequency Pa, arterial blood pressure; limb, hindlimb mean perfusion pressure; ivc, inferior vena caval mean pressure; HR, heart rate; Pcs, carotid sinus perfusion pressure; Ht, haematocrit. Animals are grouped as spontaneously breathing, artificially ventilated and artificially ventilated combined with division of both cervical vagosympathetic nerves.
The essential features of the experimental preparation are shown in Fig. 1.
Figure 1. Diagrammatic representation of the two-animal preparation.
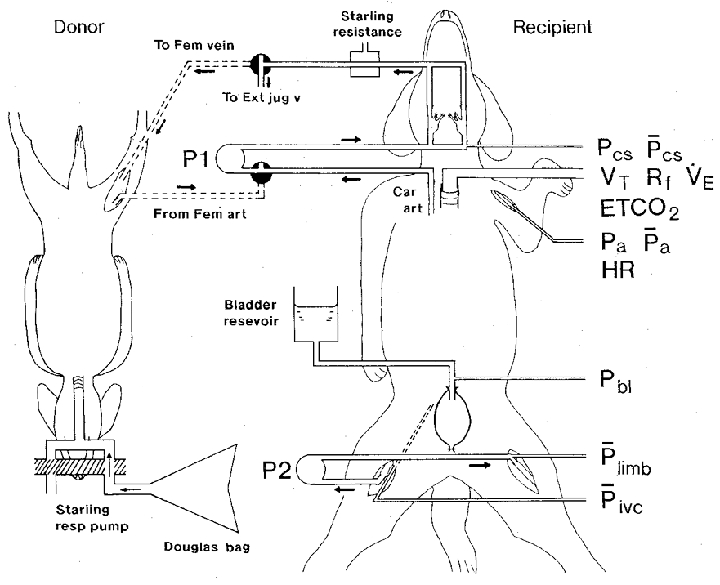
The recipient animal is shown breathing spontaneously. The carotid bifurcation regions were vascularly isolated and perfused with blood from the right common carotid artery (Car art) by means of a pump (P1) providing a pulsatile flow, via a three-way tap positioned on the input side of the pump. Blood from the cannulated external carotid arteries returned to the external jugular vein (Ext jug v) by way of a Starling resistance connected to a compressed air device for controlling the pressure, and a second three-way tap. Stimulation of the carotid bodies was achieved by turning both taps anticlockwise 90 deg so that hypoxic blood from the right femoral artery (Fem art) of the donor dog now perfused the carotid bifurcation regions and was returned to the donor's right femoral vein (Fem vein) via the Starling resistance. The donor animal was artificially ventilated by means of a Starling ‘Ideal’ respiration (resp) pump and was made hypoxic by administering appropriate gas mixtures stored in Douglas bags. The left hindlimb of the recipient animal was vascularly isolated and perfused by pump P2 at constant flow through its femoral artery with blood from the right femoral artery. The bladder was cannulated suprapubically and distended with a sodium chloride solution (154 mM) contained in a reservoir at a temperature of 37 °C, which was elevated to an appropriate height above the animal. Catheters were inserted for the measurement of carotid sinus perfusion pressure (phasic (Pcs) and mean (Pcs)), aortic blood pressure (phasic (Pa) and mean (Pa)) from which heart rate (HR) was obtained, urinary bladder pressure (Pbl), hindlimb mean perfusion pressure ({kern 0 0}Plimb) and mean inferior vena caval pressure (Pivc). Other variables measured were: tidal volume (VT), respiratory frequency (Rf), respiratory minute volume (VE) and end-tidal CO2 (ETCO2). The donor animal's arterial blood pressure was also measured (not shown). For further details, see text.
Respiration
The trachea of the recipient animal was cannulated just below the larynx and the animal breathed spontaneously room air enriched with oxygen, the inspired oxygen fraction being approximately 0·4, until the surgical procedures were completed. Then the animals were connected to a heated respiratory valve (Bacon et al. 1982), the inspiratory gas mixture being 30 % O2 in N2. Tidal volume was recorded by means of a balanced spirometer connected to a bag-in-the-box system (Bacon et al. 1962).
In some experiments the animals were ventilated artificially by means of a Starling ‘Ideal’ pump with 30 % O2 in N2, at a respiratory frequency of 19 cycles min−1. An open pneumothorax was created by inserting into the thorax a 2 cm diameter plastic tube through the fifth intercostal space on each side close to the sternum. The lungs collapsed against an expiratory resistance of 3–5 cmH2O. The tidal volume was adjusted to give an arterial PCO2 of about 40 mmHg. The end-tidal CO2 concentration was recorded via a catheter, the tip of which lay in the tracheal cannula. In these experiments, neuromuscular blockade was achieved by decamethonium iodide (10 mg kg−1i.v.; Allen and Hanbury). Adequate anaesthesia was maintained by supplementary doses of the anaesthetic, which were administered at regular intervals, the dose being determined from observations on the spontaneously breathing animals. In addition, the depth of anaesthesia was assessed by the stability of the arterial blood pressure, heart rate and hindlimb perfusion pressure.
Arterial blood pressure
Systemic blood pressure was measured from the aortic arch via a catheter inserted through the left brachial artery.
Perfusion of the carotid bodies
The method of perfusion and stimulation of the carotid chemoreceptors was similar to that described previously (Daly & Scott, 1958). Both carotid bifurcation regions were vascularly isolated and perfused through the rostral ends of the common carotid arteries with blood from the caudal end of a carotid artery, at a constant pulsatile flow by means of a modified pump (type MHRE 200; Watson Marlow Ltd, Cornwall, UK; Bacon et al. 1976). The internal carotid, occipital, ascending pharyngeal and pharyngeal arteries were tied, together with any other small arteries that could be found. The veins draining the carotid bodies were preserved. The perfused blood drained from the bifurcation regions via both cannulated external carotid arteries and though a Starling resistance, by means of which the perfusion pressure was controlled; it was returned to the animal via an external jugular vein. The pressure was measured via a sidearm of the inflow perfusion cannulae and was set at about the same level as the mean arterial blood pressure.
The carotid chemoreceptors were stimulated temporarily by hypoxic isocapnic blood obtained from a donor dog anaesthetized using the same regime as for the recipient animal. The donor dog was artificially ventilated with a gas mixture containing 30 % O2 in N2. The lungs collapsed against an expiratory pressure of 3–5 cmH2O. Three different levels of hypoxia were used to stimulate the carotid bodies of the recipient animal and were provided by ventilating the donor animal with 16, 12·5 or 5·5 % O2 in N2, prepared in Douglas bags. In the donor animal a bilateral pneumothorax was provided to prevent spontaneous respiratory efforts associated with the administration of the gases interfering with lung ventilation by the pump. The donor's femoral artery was connected via a cannula and a three-way tap to the input side of the perfusion pump; the donor's femoral vein was connected via a second three-way tap to the distal side of the Starling resistance (Fig. 1). Thus stimulation of the recipient's carotid bodies was achieved by turning the two three-way taps to appropriate positions, whereby the hypoxic blood from the donor's femoral artery perfused the recipient's carotid bifurcation regions and was returned to a femoral vein of the donor. No exchange of blood occurred, therefore, between the two animals.
In practice, a low oxygen gas mixture was first administered to the donor animal for a period of 3 min. The carotid bodies of the recipient were then stimulated temporarily by turning the two three-way taps appropriately, that is, from autoperfusion to donor perfusion. On completion of the test, the taps were turned to their original positions for perfusion of the chemoreceptors with arterialized blood from the recipient, and the donor's ventilation was returned to 30 % O2 in N2.
Perfusion of the left hindlimb
The left hindlimb of the recipient animal was vascularly isolated and perfused at constant flow through the femoral artery with arterial blood from the cannulated contralateral femoral artery by means of a roller pump (type MHRE 200; Watson Marlow Ltd), as described by Daly et al. (1993). To ensure that the venous drainage of the limb remained unobstructed during distention of the bladder, the left femoral venous blood was transferred into the inferior vena cava via a rigid wide-bore polyethylene tube. The tube was tied in the femoral vein and was advanced in the vein rostrally into the inferior vena cava so that its tip lay rostral to the level of the bladder. Inferior vena caval pressure was measured at the same site. With constant-flow perfusion of the limb, and in the absence of any observed changes in inferior vena caval pressure, alterations in femoral artery mean perfusion pressure were used as an index of changes in vascular resistance.
The total priming volume of the extracorporeal perfusion circuits was approximately 25 ml.
Distension of the urinary bladder
A wide-bore cannula, with a sidearm for pressure measurement, was tied into the wall of the bladder at its apex, and connected to a reservoir containing sodium chloride solution (154 mM) at a temperature of 37°C. To prevent escape of fluid through the urethra in male dogs, the urethra was occluded by tying in a blocked cannula; in females, a solid tightly fitting vaginal speculum of appropriate size was inserted. Tests of distension of the bladder were carried out by raising the height of the reservoir equivalent to about 50 mmHg so that the same pressure was used in each series of tests.
Blood gas analysis
At regular intervals during each experiment 0·2 ml samples of the recipient animal's arterial blood and of the carotid body perfusate were withdrawn anaerobically and were analysed immediately for PO2, PCO2 and pH using calibrated blood gas electrodes and analyser (model 413; Instrumentation Laboratory Ltd, UK). Metabolic acidosis was corrected with an intravenous infusion and/or bolus injections of molar sodium bicarbonate solution.
After completion of the operative procedures, and before connection to the perfusion circuits, heparin (Pularin, 1500 i.u. kg−1i.v.; Evans Medical Ltd, Poole, Dorset, UK) was given to the two animals to prevent coagulation.
Measurement of variables
All variables were recorded on a direct-writing ultraviolet light recorder (model 2100; SE Laboratories Ltd, Middlesex, UK). The pressures were measured using strain gauges (model P23Gb; Statham Inc., Puerto Rico). The frequency response of the catheter-manometer system measuring arterial blood pressure was flat (±5 %) up to 20 Hz. Mean pressures were obtained by passing the carrier amplifier output through resistance-capacity networks with a time constant of 1 s. Zero pressures were obtained post mortem with the catheter tips exposed to air in situ.
Experimental procedures
Tests of distension of the urinary bladder for a period of 30 s were performed under controlled conditions during arterialized blood perfusion of the carotid bodies.
During experimental states, the bladder was distended on a background of stimulation of the carotid chemoreceptors by three different levels of hypoxic isocapnic blood chosen at random. For this purpose, perfusion of the carotid bodies with appropriate hypoxic blood from the donor animal was carried out for about 1 min until a steady state had been reached. Then with continued stimulation of the chemoreceptors the bladder was temporarily distended for a period of 30 s. The peak responses to distension of the bladder were compared with the average of the control values taken before and after distension. Finally, the carotid chemoreceptor drive was withdrawn by substituting arterialized for hypoxic blood perfusion of the carotid bifurcation regions.
The experiments were performed in three groups of animals: spontaneously breathing, artificially ventilated and cervical vagosympathectomized artificially ventilated animals.
The animals were killed by exanguination and the creation of a bilateral pneumothorax in the case of spontaneously breathing animals. With artificially ventilated animals, exanguination was combined with stopping the respiratory pump.
Statistical analysis
Data for control and experimental values are expressed as means ±s.e.m. unless otherwise stated. Where appropriate, Student's t test was used to evaluate the difference between two sets of paired data. Changes from the control values to the peak experimental values are given in absolute units and also expressed as a percentage of the mean control values. Values were considered significant when P < 0·05.
RESULTS
The weights of the recipient animals and the initial control values for the respiratory and cardiovascular variables and arterial blood gases in the three groups of animals are shown in Table 1. Table 2 shows the values for the gaseous composition of the normoxic blood and three levels of hypoxic isocapnic blood used for perfusing the carotid bodies.
Table 2.
Gaseous composition of the blood perfusing the isolated carotid body chemo receptors of the recipient animals under control conditions (normoxic recipient blood) and during three levels of hypoxia (hypoxic isocapnic blood from the donor animal)
| Recipient animal, carotid body perfusate | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Spontaneously breathing (10/6) | Artificially ventilated (9/6) | Artifically ventilated cervical vagotomy (16/8) | |||||||
| Donor ventilating gas | PO2 (mmHg) | PCO2 (mmHg) | pH | PO2 (mmHg) | PCO2 (mmHG) | pH | PO2 (mmHg) | PCO2 (mmHg) | pH |
| Normoxia | |||||||||
| 30% O2 in N2 | 125 ± 16·4 | 45·8 ± 2·8 | 7·319 ± 0·049 | 118·2 ± 18·6 | 40·0 ± 5·1 | 7·393 ± 0·042 | 119·1 ± 14·2 | 40·5 ± 4·5 | 7·40 ± 0·051 |
| Hypoxia | |||||||||
| 16% O2 in N2 | 60·7 ± 9·0 | 41·6 ± 2·5 | 7·350 ± 0·037 | 58·9 ± 3·7 | 42·8 ± 5·1 | 7·352 ± 0·046 | 50·6 ± 5·9 | 42·7 ± 5·1 | 7·345 ± 0·042 |
| 12·5% O2 in N2 | 39·9 ± 8·7 | 41·0 ± 3·8 | 7·350 ± 0·038 | 44·8 ± 3·1 | 39·3 ± 4·0 | 7·374 ± 0·048 | 37·1 ± 6·4 | 42·7 ± 5·6 | 7·352 ± 0·05 |
| 5·5% O2 in N2 | 23·2 ± 3·5 | 40·3 ± 3·5 | 7·366 ± 0·04 | 21·8 ± 2·1 | 38·8 ± 4·7 | 7·390 ± 0·063 | 19·5 ± 3·5 | 40·5 ± 4·7 | 7·401 ± 0·04 |
Values are means ± s.d. Numbers in parentheses are the number of sets of observations/number of recipient animals in each group of experiments: spontaneously breathing, artificially ventilated and artificially ventilated combined with division of both cervical vagosympathetic nerves.
Spontaneously breathing animals
Selective stimulation of the carotid bodies
The results of ten sets of observations in six animals are shown in Fig. 2. Stimulation of the carotid bodies by reducing in steps the PO2 of the blood perfusing the carotid bifurcation regions (Pcb, O2) from 125 mmHg to 61, 40 and then to 23 mmHg resulted in a progressive increase in respiratory minute volume (P < 0·025, P < 0·001 and P < 0·001, respectively), tidal volume and respiratory frequency. Minute volume increased from a control value of 0·152 ± 0·011 l min−1 kg−1 to a maximum of 0·420 ± 0·052 l min−1 kg−1 or by 176 % There was, however, no effect on arterial blood pressure or heart rate except at the lowest value of Pcb, O2 when a significant bradycardia occurred (P < 0·025). Neither the inferior vena caval pressure nor the hindlimb perfusion pressure was affected at any level of stimulation of the carotid bodies.
Figure 2. The respiratory and cardiovascular effects of stimulation of the vascularly isolated perfused carotid bodies and of superimposition of distension of the bladder in spontaneously breathing animals.
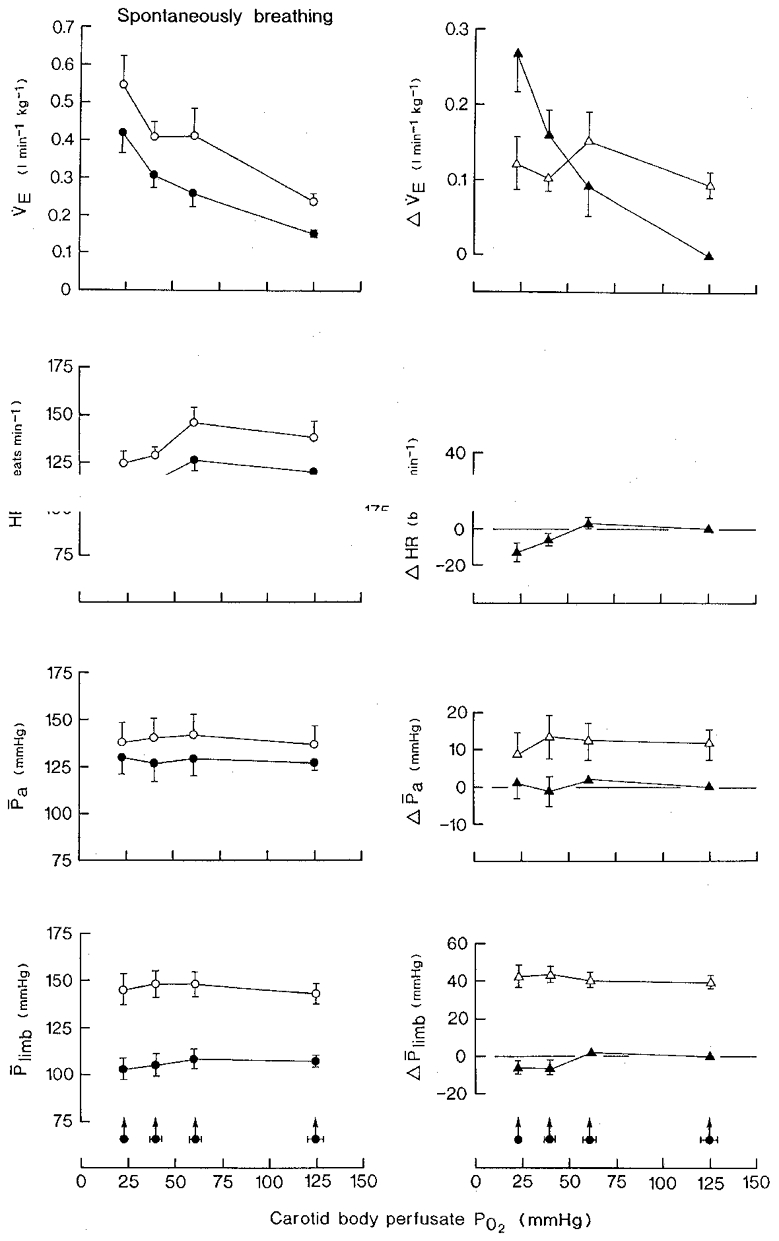
Bladder distension pressure, 45·8 ± 1·6 mmHg (±s.d.); volume, 15·1 ± 4·5 ml (kg body wt)−1 (±s.d.). Carotid sinus perfusion pressure was maintained constant. Control mean PCO2 of recipient animal's arterial blood, 44·5 ± 1·2 mmHg (±s.d.). Left-hand panels: •, hypoxic isocapnic blood stimulation of the carotid bodies at zero bladder pressure; ^, superimposition of distension of the bladder at different levels of PO2 of the carotid body perfusate. Right-hand panels: ▴, differences between the control (normoxic blood) and experimental (hypoxic blood) values during the stimulation of the carotid bodies; ▵, change in variable resulting from distension of the bladder at each level of carotid body perfusate PO2. Values are means ±s.e.m. for ten series of observations in six animals. Arrowed filled circles with horizontal bars are the mean values ±s.e.m. for the PO2 of the carotid body perfusate. Where no s.e.m. bar is given, the s.e.m. is smaller than the size of the symbol. VE, respiratory minute volume; HR, heart rate; Pa, mean arterial blood pressure; Plimb, hindlimb mean perfusion pressure; Δ, change in variable.
Bladder distention
The typical responses to distension of the bladder during normoxic blood perfusion of the carotid bodies are shown in Fig. 3. There was an increase in respiratory minute volume and tidal volume, tachycardia, and little change in arterial blood pressure in this experiment, but an increase in hindlimb perfusion pressure indicating vasoconstriction. The changes in individual variables are summarized in Fig. 2 (left-hand panels). Overall, respiratory minute volume increased by 0·093 ± 0·020 l min−1 kg−1 from a control value of 0·150 ± 0·013 l min−1 kg−1 or by 62 % (P < 0·005), heart rate increased by 18 ± 2·9 beats min−1 (P < 0·001) and blood pressure increased by 13·4 ± 3·9 mmHg (P < 0·01). The hindlimb perfusion pressure rose by 39·3 ± 3·3 mmHg from a control value of 106·4 ± 4·4 mmHg (36·9 %; P < 0·001).
Figure 3. The respiratory and cardiovascular effects of distension of the urinary bladder in the spontaneously breathing animal.
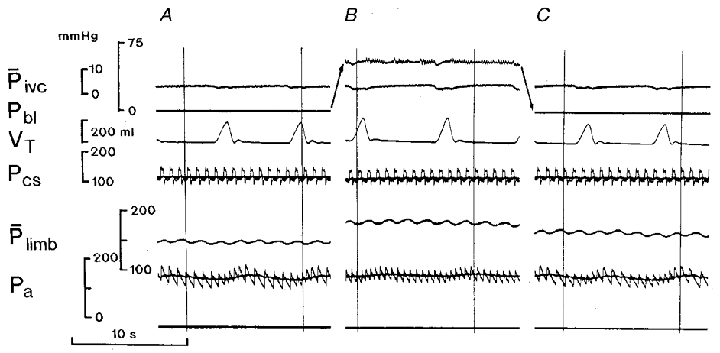
Arterialized blood perfusion of the vascularly isolated carotid bifurcation regions. Carotid sinus pressures (phasic and mean) were maintained constant. A and C, control records taken at zero urinary bladder pressure. B, distension of the bladder. Records from top to bottom: Pivc, inferior vena caval mean pressure; Pbl, urinary bladder pressure; VT, tidal volume (inspiration upwards); Pcs, phasic and mean carotid sinus perfusion pressure; Plimb, hindlimb mean perfusion pressure; Pa, phasic and mean arterial blood pressure. Note: the arrows are used for clarity and indicate the movements of the record for bladder pressure, from A to B and from B to C.
At each of the three levels of hypoxic blood perfusion of the carotid bodies, the bladder was distended and again caused an increase in respiratory minute volume, heart rate, arterial blood pressure and hindlimb perfusion pressure (Fig. 2, left-hand panels). Statistically, for each variable there was no difference between the size of the response evoked during normoxic blood perfusion of the carotid bodies and that during perfusion with blood at any of three levels of hypoxia (Fig. 2, right-hand panels).
Artificially ventilated animals
Selective stimulation of the carotid bodies
The results of nine sets of observations in six animals are summarized in Fig. 4 (left-hand panels). Increasing intensities of stimulation of the carotid bodies caused a progressive bradycardia. At the lowest level of Pcb, O2 heart rate fell by 20 ± 9·6 beats min−1 from a control value of 146·7 ± 18·2 beats min−1 (P < 0·05). The same stimulus caused a rise in hindlimb mean perfusion pressure of 9·6 ± 3·0 mmHg from a control value of 102·9 ± 3·3 mmHg (P < 0·01). At the two milder levels of Pcb, O2, there was no significant change in the pressure compared with the value during normoxia. Arterial blood pressure remained unchanged at all three levels of stimulation of the chemoreceptors.
Figure 4. The cardiovascular effects of hypoxic isocapnic blood stimulation of the vascularly isolated perfused carotid bodies and of superimposition of distension of the bladder in artificially ventilated animals with open pneumothorax.
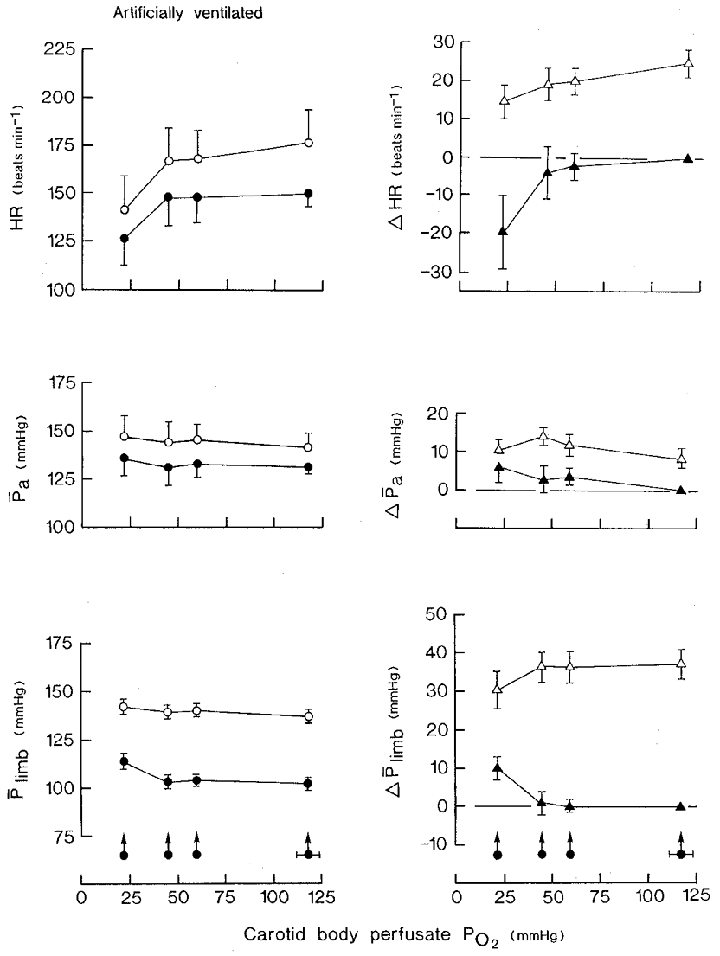
Neuromuscular blockade. Bladder distension pressure, 47·9 ± 2·9 mmHg (±s.d.); volume, 12·8 ± 4·3 ml (kg body wt)−1 (±s.d.). Carotid sinus perfusion pressure was maintained constant. Left-hand panels: •, stimulation of the carotid bodies at zero bladder pressure; ^, superimposition of distension of the bladder at different levels of PO2 of the carotid body perfusate. Right-hand panels: ▴, difference between the control (normoxic blood) and experimental (hypoxic blood) values during stimulation of the carotid bodies; ▵, change in variable resulting from distension of the bladder at each level of carotid body perfusate PO2. Values are means ±s.e.m. for nine series of observations in six animals. Arrowed filled circles with horizontal bars are the mean values ±s.e.m. for the PO2 of the carotid body perfusate. Where no s.e.m. bar is given, the s.e.m. is smaller than the size of the symbol. Abbreviations and symbols as in Fig. 2.
Bladder distension
The results are summarized in Fig. 4. As in the spontaneously breathing group of animals, distension of the bladder during normoxic blood perfusion of the carotid bodies caused tachycardia, heart rate increasing by 25·0 ± 3·6 beats min−1 from a control value of 151·0 ± 17·3 beats min−1 (16·6 %; P < 0·001), and a rise in blood pressure of 9·3 ± 3·1 mmHg from a control value of 131·2 ± 5·7 mmHg (7·1 %; P < 0·02). Hindlimb mean perfusion pressure increased by 37·1 ± 3·9 mmHg from a control value of 99·8 ± 3·4 mmHg (37·2 %; P < 0·001) (Fig. 4).
When the bladder was distended during stimulation of the carotid bodies, the heart invariably accelerated but the size of the response progressively decreased from 25·0 ± 3·6 beats min−1 without stimulation of the carotid bodies to 20·0 ± 3·7 beats min−1 (P < 0·05) and 14·7 ± 5·6 beats min−1 (P < 0·05) during stimulation at perfusate Pcb, O2 of 58·9 and 21·8 mmHg, respectively (Fig. 4, right-hand panel). The size of the increase in hindlimb perfusion pressure also decreased but only at the most intense level of Pcb, O2. Thus, the increase in perfusion pressure of 37·1 ± 3·9 mmHg on distension of the bladder during normoxic blood perfusion of the carotid bodies was reduced to an increase of 29·8 ± 4·8 mmHg at a Pcb, O2 of 21·8 mmHg (P < 0·05). Arterial blood pressure increased by 9·3 ± 3·1 mmHg on distension of the bladder during normoxic blood perfusion of the carotid bodies, the response remaining unchanged at all three levels of Pcb, O2.
Artificially ventilated animals with cervical vagosympathectomy
Both cervical vagosympathetic and accompanying aortic nerves were cut so that in these animals with constant pressure perfusion of the carotid sinuses, the input from the arterial baroreceptors remained constant in the face of changes in arterial blood pressure. The averaged results obtained in 16 sets of observations in eight animals are shown in Fig. 5.
Figure 5. The cardiovascular effects of hypoxic isocapnic blood stimulation of the vascularly isolated perfused carotid bodies and of superimposition of distension of the bladder in artificially ventilated cervical vagosympathectomized animals with open pneumothorax.
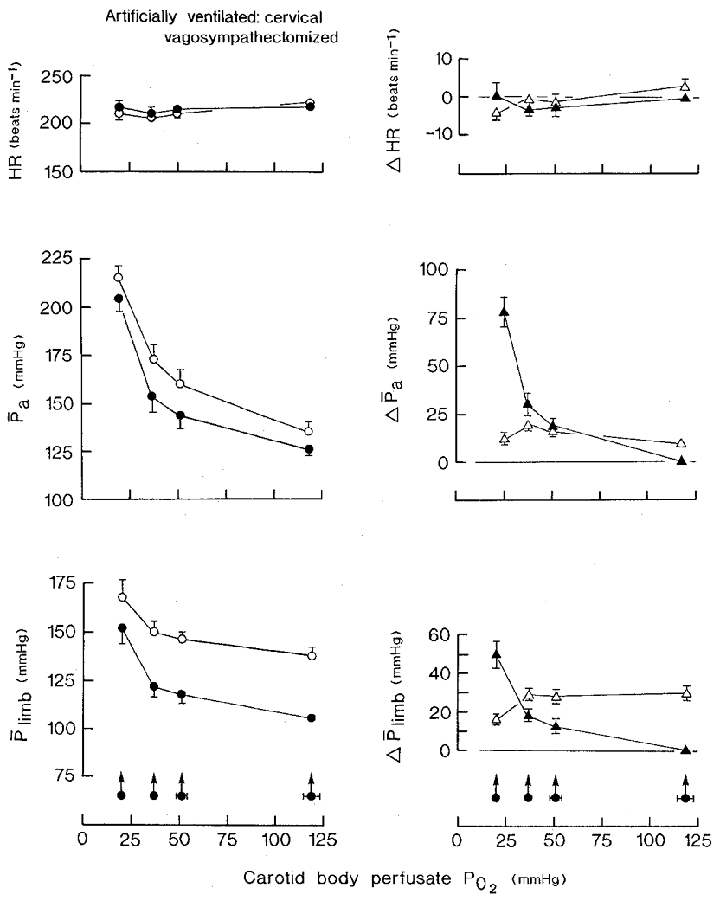
Neuromuscular blockade. Bladder distension pressure, 48·4 ± 3·1 mmHg (±s.d.); volume, 9·9 ± 4·2 ml (kg body wt)−1 (±s.d.). Carotid sinus perfusion pressure was maintained constant. Left-hand panels: •, stimulation of the carotid bodies at zero bladder pressure; ^, superimposition of distension of the bladder at different levels of PO2 of the carotid body perfusate. Right-hand panels: ▴, difference between the control (normoxic blood) and experimental (hypoxic blood) values during stimulation of the carotid bodies; ▵, change in variable resulting from distension of the bladder at each level of carotid body perfusate PO2. Values are means ±s.e.m. for sixteen series of observations in eight animals. Arrowed filled circles with horizontal bars are the mean values ±s.e.m. for the PO2 of the carotid body perfusate. Where no s.e.m. bar is given, the s.e.m. is smaller than the size of the symbol. Abbreviations and symbols as in Fig. 2.
Stimulation of the carotid bodies
Heart rate responses were now minimal, the only significant change being a slowing of 3.4 ± 1·6 beats min−1 from a control value of 210·4 ± 3·2 beats min−1, which occurred at a stimulus Pcb, O2 of 37·1 mmHg (P < 0·05). Arterial blood pressure rose progressively at each decreasing level of Pcb, O2 (P < 0·01) to a maximum of 205·5 ± 6·7 mmHg, or by 79·5 ± 7·6 mmHg, from a control value of 126 ± 5 mmHg (63·1 %; P < 0·001). Hindlimb mean perfusion pressure also rose progressively at each stage of increasing hypoxia of the carotid bodies to a maximum of 151·8 ± 7·7 mmHg from a control value of 107·9 ± 2·2 mmHg, a rise of 43·9 ± 7·2 mmHg (40·7 %; P < 0·001).
Bladder distention
The tachycardia that occurred on distension of the bladder during normoxic blood perfusion of the carotid bodies in animals with intact vagus nerves was abolished by bilateral cervical vagosympathectomy (Fig. 5). In the vagotomized animals, during normoxia, bladder distension caused an increase in arterial blood pressure of 9·5 ± 2·7 mmHg, from a control value of 126 ± 5 mmHg (7·5 %; P < 0·005), and an increase in the hindlimb mean perfusion pressure of 29·8 ± 3·6 mmHg, from 107·9 ± 2·2 mmHg (27·6 %; P < 0·001).
Bladder distension during stimulation of the carotid bodies caused a slight bradycardia, not tachycardia, the response only being significant at the lowest perfusate Pcb, O2 (P < 0·05) (Fig. 5). Arterial blood pressure, however, increased (P < 0·001). Although the size of the response at the Pcb, O2 of 19·5 mmHg did not differ significantly from that during normoxic blood perfusion, the responses at Pcb, O2 of 50·6 and 37·1 mmHg were increased (P < 0·05> and P < 0·01, respectively). The hindlimb mean perfusion pressure increased at each of the three levels of hypoxic blood stimulation of the chemoreceptors, the size of the response being the same at a Pcb, O2 of 50·6 and 37·1 as at 119 mmHg (Fig. 5). It was significantly reduced to 15·0 ± 3·2 mmHg, however, at the perfusate Pcb, O2 of 19·5 mmHg (P < 0·01) (Fig. 5, right-hand panel).
Further tests were made in an attempt to discover the possible mechanism responsible for the reduction in the size of the vasoconstrictor response to distension of the bladder that occurred during the period of the strongest stimulus to the carotid bodies. The following experiments were therefore designed to show whether the hindlimb vessels were capable of constricting further on a background vasoconstriction evoked by distension of the bladder during stimulation of the carotid chemoreceptors.
The results from eleven series of observations in 4 animals are summarized in Fig. 6A–C. During normoxic blood perfusion of the carotid bodies, bladder distension caused a significant increase in hindlimb perfusion pressure of 22·3 ± 1·9 mmHg (P < 0·001; Fig. 6A). In confirmation of the results shown in Fig. 5, this response was attenuated when the test was repeated during stimulation of the carotid bodies, with bladder distension causing an increase in hindlimb perfusion pressure of only 6·5 ± 2·9 mmHg (P < 0·001; Fig. 6C).
Figure 6. The effects on hindlimb mean perfusion pressure of distension of the bladder, unloading the carotid baroreceptors under control conditions (A and B) and during hypoxic isocapnic blood perfusion of the carotid bodies (C) in artificially ventilated cervical vagosympathectomized animals with open pneumothorax.
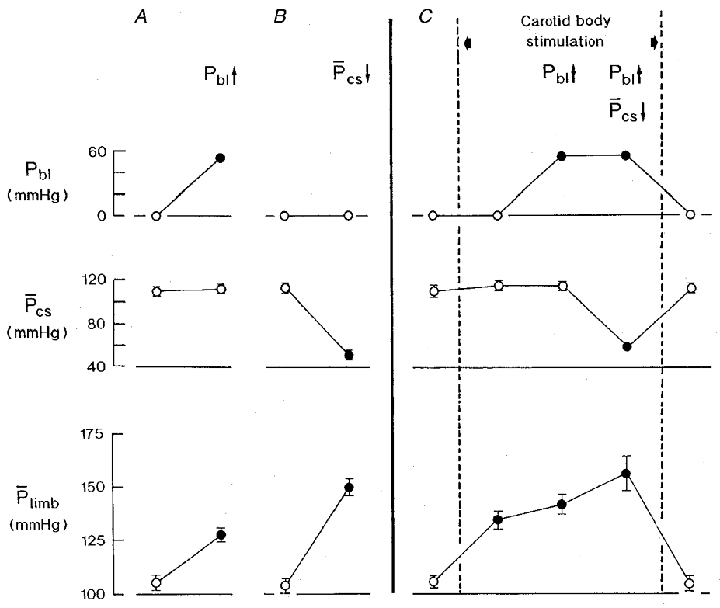
Neuromuscular blockade. Carotid sinus perfusion pressure was maintained constant except during specified tests. A and B, separate tests of distension of the bladder at a pressure of 54·4 ± 1·4 mmHg (A) and of lowering the carotid sinus mean perfusion pressure by 62·7 ± 1·6 mmHg (B) during normoxic blood perfusion of the carotid bodies. C, stimulation of the carotid bodies with hypoxic isocapnic blood (PO2, 23·8 ± 1·8 mmHg; PCO2, 41·2 ± 1·3 mmHg; pH 7·351 ± 0·021) between the vertical dashed lines. During chemoreceptor stimulation (C), bladder distension (Pbl↑; 54·4 ± 1·4 mmHg) was maintained during the subsequent period of lowering the carotid sinus mean perfusion pressure by 56·6 ± 1·7 mmHg (Pcs↓). ^, control values; •, experimental values. Eleven series of observations in four animals. Values are means ±s.e.m. Where no s.e.m. bar is given, the s.e.m. is smaller than the size of the symbol. Pbl, bladder pressure; Pcs, carotid sinus mean perfusion pressure; Plimb, hindlimb mean perfusion pressure. Note: (1), the reduced rise in hindlimb perfusion pressure to bladder distension during stimulation of the carotid bodies (C) compared with the response in A; and (2), the further rise in hindlimb perfusion pressure on lowering the carotid sinus mean perfusion pressure during stimulation of the carotid bodies combined with bladder distension.
On a background of stimulation of the carotid bodies combined with distension of the bladder, the carotid sinus baroreceptors were partially unloaded by lowering the carotid sinus mean perfusion pressure by 56·5 ± 1·7 mmHg to assess the potential for a further increase in sympathetic activity to the hindlimb vessels. As shown in Fig. 6, this resulted in a further significant increase in hindlimb perfusion pressure of 18·5 ± 7·5 mmHg (P < 0·05; Fig. 6C) but this response was nevertheless reduced compared with the control (Fig. 6B).
DISCUSSION
The results of these experiments have shown that in dogs breathing spontaneously, distension of the urinary bladder caused an increase in respiratory minute volume, tachycardia and an increase in hindlimb vascular resistance, with similar cardiovascular responses occurring in artificially ventilated animals. These findings confirm previously reported results (for references, see Introduction). The new information is as follows. (1) In spontaneously breathing animals, stimulation of the carotid bodies and distension of the bladder both evoked increases in respiratory minute volume which, when the separate stimuli were combined, resulted in responses that were additive. (2) Artificial ventilation modified the heart rate and hindlimb vascular responses to hypoxic blood stimulation of the carotid bodies revealing or accentuating the primary cardiac and vascular responses, bradycardia and vasoconstriction, respectively, confirming previous findings (see Daly, 1986, 1997; Marshall, 1994). The cardio-accelerator and vasoconstrictor responses to bladder distension during normoxic blood perfusion of the carotid bodies were of similar magnitude in spontaneously breathing and artificially ventilated animals. (3) In artificially ventilated animals, as the primary cardio-inhibitory response increased progressively on lowering of the Pcb, O2, the size of the cardio-accelerator effect of distension of the bladder gradually decreased, indicating an interaction between the two reflex responses evoked by the two inputs. (4) The reflex vascular effects resulting from stimulation of the two inputs were additive, at least over the physiological range of Pcb, O2 down to approximately 40 mmHg.
The question as to the evidence that is required to demonstrate an interaction between two reflexes has been discussed in detail previously in relation to the effects of bladder distension on carotid baroreceptor reflexes (Daly et al. 1993, and references therein). In that paper, the term interaction was used in such a way as to exclude non-algebraic summation of responses that are due to non-linearities of the S-shaped stimulus-response curves. In the present paper, the curves relating the stimulus to the carotid bodies (Pcb, O2) to the primary cardiac and vascular responses are hyperbolic so that with intense stimulations of the carotid chemoreceptors the heart rate is very slow and the arterioles are near maximum constriction. The intense stimulation of the cardiac vagal preganglionic neurones under these conditions might be expected to reduce the inhibitory response due to distention of the bladder if the effects of carotid chemoreceptor stimulation and bladder distension share the same cardiac vagal neurone pool, as appears to be the case. Again, with the systemic vessels being near maximal constriction during intense carotid body stimulation, the vasoconstrictor response to bladder distension would be reduced or abolished, particularly as the two effects share the same sympathetic neurone pool (see Daly et al. 1993). In order to define the nature of the reflex interaction, we therefore consider the important observations to be those on the carotid body stimulus- response curve in the range of Pcb, O2 of 120–40 mmHg where the possibility of maximal stimulation is avoided. Thus a comparison of the slopes of the control curve of carotid chemoreceptor stimulation alone and the experimental curve depicting the responses to superimposition of distension of the bladder provides the best indication of the nature of any interaction between the two reflexes.
Urinary bladder reflexes
The distension pressure of about 50 mmHg selected for use in the present study is within the physiological range of pressures found during micturition in man (Denny-Brown & Robertson, 1933; Walter et al. 1979). It lies on the linear part of the relationship between distension pressure and heart rate or hindlimb vascular resistance (Ward, 1988) and therefore gives rise to submaximal responses.
The tachycardia and hindlimb vasoconstriction occurring on distension of the bladder are reflex in nature, being abolished by selective denervation of the bladder (Daly et al. 1993). The efferent pathways for the cardiac response are by way of the vagus nerves (Hassan et al. 1987b; Daly et al. 1993) and sympathetic nerves (Hassan et al. 1987a; Daly et al. 1993). Our inability to demonstrate an increase in heart rate after cutting the vagus nerves may have been due to the high background level of heart rate and cardiac sympathetic nerve activity that prevailed following this denervation procedure. The hindlimb vasoconstrictor responses are abolished by cutting the sciatic and femoral nerves (Daly et al. 1993). All the changes in heart rate and vascular resistance occurred independently of alterations in arterial blood pressure and therefore of the arterial baroreceptor inputs (Ward et al. 1995).
Carotid body chemoreceptor reflexes
The cardiac and vascular responses evoked by selective stimulation of the carotid bodies in spontaneously breathing animals are variable (Daly & Scott, 1958, 1963), and this was evident in the present study in which heart rate and hindlimb perfusion pressure either increased, decreased or remained unchanged. There was, however, a trend towards cardio-inhibitory responses of vagal origin with the stronger stimuli applied to the carotid bodies. The reason for these variable responses is that stimulation of the carotid chemoreceptors gives rise to a number of secondary mechanisms, important amongst which are those initiated by the concomitant increase in respiratory minute volume. When these respiratory mechanisms and systemic blood pressure are controlled, stimulation of the carotid bodies causes bradycardia due to an increase in vagal efferent nerve activity (Daly & Scott, 1958; Jewett, 1964; Neil, 1979; Hassan et al. 1987b) and a decrease in cardiac sympathetic nerve activity (Daly & Scott, 1958; Linden et al. 1982; Hassan et al. 1987a). Under the same conditions vasoconstriction occurs in most vascular territories (see Marshall, 1994; Daly, 1997) associated with an increase in activity in sympathetic efferent nerve fibres (Dontas, 1955; Linden et al. 1981; Drinkhill et al. 1989). These responses of bradycardia and vasoconstriction represent, therefore, the direct or primary carotid chemoreceptor reflex cardiac and vascular effects. The term ‘respiratory modulation’ is used to indicate that the primary reflex responses to stimulation of a specific group of receptors are facilitated or inhibited by a concomitant change in pulmonary ventilation.
There are two important neurogenic mechanisms by which an increase in pulmonary ventilation suppresses or even reverses these primary cardiac and vascular responses evoked by stimulation of the carotid bodies. The first is an increase in central respiratory drive, and the second is an increased activity of slowly adapting pulmonary stretch receptors driven by lung inflation. With regard to the vagal control of heart rate, these two respiratory mechanisms are responsible for phasically altering the excitability of the cardiac vagal preganglionic motoneurones situated in the region of the nucleus ambiguus. During the inspiratory phase of the respiratory cycle, the cardiac vagal motoneurones are refractory to incoming excitatory inputs from baroreceptors and chemoreceptors; it is only during the phase of expiration, when their excitability returns, that the full expression of these excitatory inputs is seen, the cardio-inhibitory response being mediated via vagal efferent small myelinated fibres (McAllen & Spyer, 1978).
The peripheral vascular effects to stimulation of the carotid bodies are also respiratory modulated. There are several groups of neurones in the subretrofacial nucleus in the rostral ventrolateral medulla that project to the spinal cord and terminate in the intermediolateral cell column. These sympathetic premotor neurones constitute the major, although not exclusive, descending source of activity that maintains vasomotor tone via sympathetic efferent pathways (Dampney et al. 1985; McAllen, 1986, 1987). The sympathetic premotor neurones are stimulated by central respiratory activity (McAllen, 1987; Haselton & Guyenet, 1989) and, by inference, inhibited by lung stretch afferents (Lipski et al. 1977; Gootman et al. 1980). In addition, they are inhibited by the input from arterial baroreceptors and excited by stimulation of the carotid bodies (Sun & Spyer, 1991; McAllen, 1992) and the defence area of the hypothalamus (McAllen et al. 1982; Hilton et al. 1983). Thus, in the absence of secondary respiratory mechanisms, vasoconstrictor responses predominate on stimulation of the carotid bodies (Daly & Ungar, 1966; Angell James & Daly, 1969; Daly et al. 1986) as found in the present study (Fig. 5).
The variable cardiac and vascular responses evoked by stimulation of the carotid bodies in the spontaneously breathing animal, dependent as they are on a balance between primary and secondary mechanisms, had a bearing on the design of the present experiments. It was important to examine the effects of bladder distension under two different conditions: first, in the spontaneously breathing animal in which the cardiovascular effects of stimulation of the carotid bodies are dominated by mechanisms secondary to the increase in respiration, and second, in the artificially ventilated animal where their primary cardiac and vascular effects tend to predominate (see Daly, 1986, 1997).
Modification of carotid body chemoreceptor reflexes by bladder distension
Respiratory responses
The increase in ventilation occurring on distension of the bladder during normoxic blood perfusion of the carotid bodies was unaffected by their stimulation at all three levels of hypoxia. The respiratory response to a combination of bladder distension and carotid chemoreceptor stimulation was a simple algebraic sum of the separate responses to excitation of the two sets of receptors. This is indicated by the resetting of the curve relating respiratory minute volume to the hypoxic stimulus towards an increase in ventilation without any change in gain (Fig. 2).
Heart rate responses
In spontaneously breathing animals there was no difference in the size of the cardio-accelerator responses to bladder distension at each level of hypoxic blood stimulation of the carotid bodies compared with the control value without stimulation (Fig. 2). This finding was apparent despite the progressively increasing respiratory minute volume, by a maximum of 176 % associated with the strongest stimulus to the chemoreceptors. In effect therefore, bladder distension simply reset the Pcb, O2-heart rate relationship to a higher level of heart rate without any change in gain.
When, however, the primary cardio-inhibitory response to stimulation of the carotid bodies predominated in artificially ventilated animals, bladder distension caused tachycardia, the size of the response diminishing progressively as the Pcb, O2 was lowered (Fig. 4). This indicates that there is an interaction between the primary cardiac chronotropic responses evoked by stimulation of the carotid chemoreceptors and distension of the bladder. The exact nature of the integration between the cardiac reflex evoked by stimulation of the carotid bodies and that resulting from distension of the bladder is not entirely clear and could be influenced by a number of factors that have to be taken into account. First, the evidence already cited indicates that both reflexes involve cardiac vagal efferent fibres with their cell bodies in the cardiac vagal motoneurones in the region of the nucleus ambiguus. Furthermore, there is convergence on cardiac efferent pathways of the effects of both reflexes, that is, a group of vagal efferent nerve fibres exists that show decreased activity on distension of the bladder and also respond to stimulation of the carotid bodies by an increase in activity (Hassan et al. 1987b). These chronotropic actions of the vagus are mediated by myelinated B fibres (Heinbecker & Bishop, 1935; Jones et al. 1995).
Second, the chronotropic responses to stimulation of the carotid bodies (bradycardia) and distension of the bladder (tachycardia) are due, at least in part, to changes in activity of cardiac sympathetic efferents. There is convergence of the projections of receptors in the carotid bodies and those which respond to bladder distension onto the same efferent sympathetic nerves (Hassan et al. 1987a).
Third, the cardio-inhibitory response to vagal stimulation is hyperbolic, reaching a plateau at the higher frequencies of stimulation (Levy & Zieske, 1969). Thus, a given reduction in vagus nerve activity brought about by distension of the bladder from a high background level of vagal tone, i.e. at low levels of Pcb, O2, would result in a smaller cardio-accelerator response than the same reduction in activity from a lower level of vagal tone corresponding to normoxic blood perfusion of the carotid bodies. Although this could explain the present results, the vagal effects may be complicated by concomitant changes in activity in cardiac sympathetic efferent fibres. The opposing influences of the vagal and sympathetic responses are not algebraically additive; instead, complicated interactions exist (Levy & Zieske, 1969).
Fourth, the cardiac reflex responses resulting from stimulation of the carotid bodies and mediated via vagal efferent fibres are respiratory modulated (see references above). So too are those mediated via cardiac sympathetic efferent fibres (Davis et al. 1977). In contrast the responses to distension of the bladder are not respiratory modulated. In this connection, afferent fibres from the bladder responding to distension of the organ are mainly myelinated with a varying proportion of non-myelinated C fibres (Floyd et al. 1976; Bahns et al. 1986; Sengupta & Gebhart, 1994). The non-respiratory-modulated nature of the cardiac response to bladder distension could be explained were C fibre afferents to involve the dorsal vagal motor neurones, projecting cardiac C fibre efferents, as well as the cardiac vagal motoneurones in the region of the nucleus ambiguus that project cardiac B fibre efferents. Preganglionic neurones with C fibre axons fire tonically (Ford et al. 1990; Jones et al. 1995) whereas preganglionic neurones with B fibre axons fire phasically with both beat-by-beat and breath-by-breath rhythms (McAllen & Spyer, 1978). It is possible that an integration between the two projections occurs at the level of the cardiac ganglia and/or postganglionic nerve endings, such as has been postulated by Jones et al. (1994, 1995). If so, this could account for the cardiac response to distension of the bladder not being respiratory modulated.
And finally, both excitation of the carotid bodies and distension of the bladder, by virtue of a noxious stimulus, can give rise to stimulation of defence areas in the brainstem (Hilton, 1966). The nature of the interaction may, therefore, be more complicated. However, the use of chloralose-urethane anaesthesia is known to prevent, partly or wholly, activation of these areas from afferent inputs (see Marshall, 1987), so that there must be some doubt about their participation in the present experiments.
Clearly further studies are required to establish the underlying mechanisms.
Hindlimb vascular responses
In spontaneously breathing animals stimulation of the carotid bodies caused either a fall or no change in hindlimb perfusion pressure. This result is in keeping with earlier observations that demonstrated the respiratory modulation of the primary vasoconstrictor reflex from the chemoreceptors (Angell James & Daly, 1969). Superimposition of tests of distention of the bladder resulted in increases in hindlimb perfusion pressure, the size of the response being the same at all levels of stimuli applied to the carotid bodies as in the control state without stimulation (Fig. 2). The vasoconstrictor response to bladder distension is not, therefore, respiratory modulated, as found previously (Daly et al. 1986). In artificially ventilated animals in which changes in the arterial baroreceptor input were prevented, the primary vasoconstrictor response to stimulation of the carotid bodies predominated (Angell James & Daly, 1969), the size of the response increasing progressively as the hypoxic stimulus to the carotid bodies was augmented (this paper, Fig. 5). When tests of bladder distension were superimposed at each level of Pcb, O2 the magnitude of the response was the same as the control during normoxic blood perfusion of the carotid bodies. Over the physiological range of Pcb, O2, taken as 126–39 mmHg, the relationship between Pcb, O2 and hindlimb perfusion pressure was linear and distension of the bladder caused a parallel shift of the plot towards a higher level of perfusion pressure. This indicates that the hindlimb vascular responses to stimulation of the carotid bodies and to bladder distension are additive with no interaction between the reflexes evoked by the two inputs.
With the strongest stimulus applied to the carotid bodies, however, a significant reduction in the size of the vasoconstrictor response to bladder distension occurred. At this level of stimulation of the chemoreceptors the slope of the plot relating Pcb, O2 and hindlimb perfusion pressure increased towards full vasoconstriction. The available range for further vasoconstriction is thereby diminished and this probably accounts for the reduction in the response to bladder distension, particularly if the effects of the two inputs share the same sympathetic neurone pool. This appears to be the case, for afferents from the bladder converge at some point on sympathetic neurones which also receive an excitatory input from the carotid chemoreceptors (Drinkhill et al. 1989). In the present study it was found that on a background of hindlimb vasoconstriction produced by a combination of distension of the bladder and the strongest stimulus applied to the carotid bodies, further reflex constriction was still possible as indicated by unloading the carotid sinus baroreceptors, albeit this response, too, was considerably reduced in size compared with the control (Fig. 6).
In conclusion, the results of these experiments have shown that the respiratory, cardiac and vascular responses evoked by stimulation of the carotid bodies can be appreciably modified by distending the bladder with pressures that are within the physiological range. The nature of the integration of the responses elicited by the two inputs is clearly complex. Quite apart from the fact that the inputs differ in the way in which they are respiratory modulated, there are also differences in the mechanisms that are involved in the integration of the primary cardiac and vascular responses evoked by inputs resulting from stimulation of the carotid bodies and distension of the bladder.
Acknowledgments
We wish to express our thanks to Mr D. R. Bacon for expert technical assistance. This work was supported by grants from the Medical Research Council and British Heart Foundation to M.deB.D.
References
- Angell James JE, Daly MdeB. Cardiovascular responses in apnoeic asphyxia: role of arterial chemoreceptors and the modification of their effects by a pulmonary vagal inflation reflex. The Journal of Physiology. 1969;201:87–104. doi: 10.1113/jphysiol.1969.sp008744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacon DR, Daly C, de B, Daly M, de B, Scott RW. A modified roller pump with improved haemodynamic characteristics and temperature control of perfusate. Laboratory Practice. 1976;25:464–466. [PubMed] [Google Scholar]
- Bacon DR, Daly MdeB, Ead HW, Scott RW. Low resistance, low dead space heated respiratory valves for use in animals. International Research Communications System Medical Sciences. 1982;10:738–739. [Google Scholar]
- Bacon DR, Daly MdeB, Scott MJ. A method for continuously recording respiration quantitatively during administration of various gas mixtures in the cat. The Journal of Physiology. 1962;161:2–3P. [Google Scholar]
- Bahns E, Ernsberger U, Jänig W, Nelke A. Functional characteristics of lumbar visceral afferent fibres from the urinary bladder and the urethra in the cat. Pflügers Archiv. 1986;407:510–518. doi: 10.1007/BF00657509. [DOI] [PubMed] [Google Scholar]
- Daly MdeB. Interactions between respiration and circulation. In: Cherniack NS, Widdicombe JG, editors. Handbook of Physiology, section 3, The Respiratory System, vol. 2, Control of Breathing, part II. Bethesda, MD, USA: American Physiological Society; 1986. pp. 529–594. [Google Scholar]
- Daly MdeB. Peripheral Arterial Chemoreceptors and Respiratory-Cardiovascular Integration. Physiological Society Monograph no. 46. Oxford, UK: Clarendon Press; 1997. [Google Scholar]
- Daly MdeB, Scott MJ. The effects of stimulation of the carotid body chemoreceptors on heart rate in the dog. The Journal of Physiology. 1958;144:148–166. doi: 10.1113/jphysiol.1958.sp006092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly MdeB, Scott MJ. The cardiovascular responses to stimulation of the carotid body chemoreceptors in the dog. The Journal of Physiology. 1963;165:179–197. doi: 10.1113/jphysiol.1963.sp007051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly MdeB, Ungar A. Comparison of the reflex responses elicited by stimulation of the separately perfused carotid and aortic body chemoreceptors in the dog. The Journal of Physiology. 1966;182:379–403. doi: 10.1113/jphysiol.1966.sp007828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly MdeB, Ward J, Wood LM. Modification by lung inflation of the vascular responses from the carotid body chemoreceptors and other receptors in dogs. The Journal of Physiology. 1986;378:13–30. doi: 10.1113/jphysiol.1986.sp016205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly MdeB, Ward J, Wood LM. Effects of distension of the urinary bladder on the cardiovascular reflexes from the carotid baroreceptors in the dog. The Journal of Physiology. 1993;463:545–564. doi: 10.1113/jphysiol.1993.sp019610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly MdeB, Wood LM. Effects of distension of the urinary bladder on the respiratory and cardiovascular responses elicited by stimulation of the carotid body chemoreceptors in the dog. The Journal of Physiology. 1983;341:47P. [Google Scholar]
- Dampney RAL, Goodchild AK, Tan E. Vasopressor neurons in the rostral ventrolateral medulla of the rabbit. Journal of the Autonomic Nervous System. 1985;14:239–254. doi: 10.1016/0165-1838(85)90113-4. [DOI] [PubMed] [Google Scholar]
- Davis AL, McCloskey DI, Potter EK. Respiratory modulation of baroreceptor and chemoreceptor reflexes affecting heart rate through the sympathetic nervous system. The Journal of Physiology. 1977;272:691–703. doi: 10.1113/jphysiol.1977.sp012067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny-Brown D, Robertson EG. On the physiology of micturition. Brain. 1933;56:149–190. [Google Scholar]
- Dontas AS. Effects of protoveratrine, serotonin and ATP on afferent and splanchnic nerve activity. Circulation Research. 1955;3:363–373. doi: 10.1161/01.res.3.4.363. [DOI] [PubMed] [Google Scholar]
- Drinkhill MJ, Mary DASG, Ramadan MRM, Vacca G. The effect of distension of the urinary bladder on activity in efferent renal fibres in anaesthetized dogs. The Journal of Physiology. 1989;409:357–369. doi: 10.1113/jphysiol.1989.sp017502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd K, Hick VE, Morrison JFB. Mechanosensitive afferent units in the hypogastric nerve of the cat. The Journal of Physiology. 1976;259:457–471. doi: 10.1113/jphysiol.1976.sp011476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford TW, Bennett JA, Kidd C, McWilliam PN. Neurones in the dorsal motor vagal nucleus of the cat with non-myelinated axons projecting to the heart and lungs. Experimental Physiology. 1990;75:459–473. doi: 10.1113/expphysiol.1990.sp003423. [DOI] [PubMed] [Google Scholar]
- Gootman PM, Feldman JL, Cohen MI. Pulmonary afferent influences on respiratory modulation of sympathetic discharge. In: Koepchen HP, Hilton SM, Trzebski A, editors. Central Interaction between Respiratory and Cardiovascular Control Systems. Berlin: Springer Verlag; 1980. pp. 172–178. [Google Scholar]
- Guttmann L, Whitteridge D. Effects of bladder distension on autonomic mechanisms after spinal cord injury. Brain. 1947;70:361–404. doi: 10.1093/brain/70.4.361. [DOI] [PubMed] [Google Scholar]
- Haselton JR, Guyenet PG. Central respiratory modulation of medullary sympathoexcitatory neurons in rat. American Journal of Physiology. 1989;256:R739–750. doi: 10.1152/ajpregu.1989.256.3.R739. [DOI] [PubMed] [Google Scholar]
- Hassan AAM, Hicks MN, Walters GE, Mary DASG. Effect of distension of the urinary bladder on efferent cardiac sympathetic nerve fibres which respond to stimulation of atrial receptors. Quarterly Journal of Experimental Physiology. 1987a;72:1–11. doi: 10.1113/expphysiol.1987.sp003035. [DOI] [PubMed] [Google Scholar]
- Hassan AAM, Hicks MN, Walters GE, Mary DASG. Effect on efferent cardiac vagal nerve fibres of distension of the urinary bladder in the dog. Quarterly Journal of Experimental Physiology. 1987b;72:473–481. doi: 10.1113/expphysiol.1987.sp003089. [DOI] [PubMed] [Google Scholar]
- Heinbecker P, Bishop GH. Studies on the extrinsic and instrinsic nerve mechanisms of the heart. American Journal of Physiology. 1935;114:212–223. [Google Scholar]
- Hilton SM. Hypothalamic regulation of the cardiovascular system. British Medical Bulletin. 1966;22:243–248. doi: 10.1093/oxfordjournals.bmb.a070481. [DOI] [PubMed] [Google Scholar]
- Hilton SM, Marshall JM, Timms RJ. Ventral medullary relay neurones in the pathway from the defence areas of the cat and their effect on blood pressure. The Journal of Physiology. 1983;345:149–166. doi: 10.1113/jphysiol.1983.sp014971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewett DL. Activity of single efferent fibres in the cervical vagus nerve of the dog, with special reference to possible cardioinhibitory fibres. The Journal of Physiology. 1964;175:321–357. doi: 10.1113/jphysiol.1964.sp007520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JFX, Wang Y, Jordan D. Activity of cardiac vagal preganglionic neurones during the pulmonary chemoreflex in the anaesthetized cat. In: O'Regan RG, Nolan P, McQueen DS, Paterson DJ, editors. Arterial Chemoreceptors: Cell to Systems. New York: Plenum Press; 1994. pp. 301–303. [DOI] [PubMed] [Google Scholar]
- Jones JFX, Wang Y, Jordan D. Heart rate responses to selective stimulation of cardiac vagal C fibres in anaesthetized cats, rats and rabbits. The Journal of Physiology. 1995;489:203–214. doi: 10.1113/jphysiol.1995.sp021042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy MN, Zieske H. Autonomic control of cardiac pacemaker activity and atrioventricular transmission. Journal of Applied Physiology. 1969;27:465–470. doi: 10.1152/jappl.1969.27.4.465. [DOI] [PubMed] [Google Scholar]
- Linden RJ, Mary DASG, Weatherill D. The responses in renal nerves to stimulation of atrial receptors, carotid sinus baroreceptors and carotid chemoreceptors. Quarterly Journal of Experimental Physiology. 1981;66:179–191. doi: 10.1113/expphysiol.1981.sp002544. [DOI] [PubMed] [Google Scholar]
- Linden RJ, Mary DASG, Weatherill D. The response in efferent cardiac sympathetic nerves to stimulation of atrial receptors, carotid sinus baroreceptors and carotid chemoreceptors. Quarterly Journal of Experimental Physiology. 1982;67:151–163. doi: 10.1113/expphysiol.1982.sp002609. [DOI] [PubMed] [Google Scholar]
- Lipski J, Coote JH, Trzebski A. Temporal patterns of antidromic invasion latencies of sympathetic preganglionic neurones related to central inspiratory activity and pulmonary stretch receptor reflex. Brain Research. 1977;135:162–166. doi: 10.1016/0006-8993(77)91061-7. [DOI] [PubMed] [Google Scholar]
- McAllen RM. Action and specificity of ventral medullary vasopressor neurones in the cat. Neuroscience. 1986;18:51–59. doi: 10.1016/0306-4522(86)90178-8. [DOI] [PubMed] [Google Scholar]
- McAllen RM. Central respiratory modulation of subretrofacial bulbospinal neurones in the cat. The Journal of Physiology. 1987;388:533–545. doi: 10.1113/jphysiol.1987.sp016630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllen RM. Actions of carotid chemoreceptors on subretrofacial bulbospinal neurons in the cat. Journal of the Autonomic Nervous System. 1992;40:181–188. doi: 10.1016/0165-1838(92)90199-q. [DOI] [PubMed] [Google Scholar]
- McAllen RM, Neil JJ, Loewy AD. Effects of kainic acid applied to the ventral surface of the medulla oblongata on vasomotor tone, the baroreceptor reflex and hypothalamic autonomic responses. Brain Research. 1982;238:65–76. doi: 10.1016/0006-8993(82)90771-5. [DOI] [PubMed] [Google Scholar]
- McAllen RM, Spyer KM. Two types of vagal preganglionic motoneurones projecting to the heart and lungs. The Journal of Physiology. 1978;282:353–364. doi: 10.1113/jphysiol.1978.sp012468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall JM. Contribution to overall cardiovascular control made by the chemoreceptor-induced alerting/defence response. In: Taylor EW, editor. Neurobiology of the Cardiorespiratory System. Manchester: Manchester University Press; 1987. pp. 222–240. [Google Scholar]
- Marshall JM. Peripheral chemoreceptors and cardiovascular regulation. Physiological Reviews. 1994;74:543–594. doi: 10.1152/physrev.1994.74.3.543. [DOI] [PubMed] [Google Scholar]
- Mukherjee SR. Effect of bladder distension on arterial blood pressure and renal circulation: role of splanchnic and buffer nerves. The Journal of Physiology. 1957;138:307–325. doi: 10.1113/jphysiol.1957.sp005853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neil E. Cardiac vagal efferent activity. In: Hainsworth R, Kidd C, Linden RJ, editors. Cardiac Receptors. Cambridge: Cambridge University Press; 1979. pp. 361–374. [Google Scholar]
- Sengupta JN, Gebhart GF. Mechanosensitive properties of pelvic nerve afferent fibers innervating the urinary bladder of the rat. Journal of Neurophysiology. 1994;72:2420–2430. doi: 10.1152/jn.1994.72.5.2420. [DOI] [PubMed] [Google Scholar]
- Sun M-L, Spyer KM. Responses of rostroventrolateral medulla spinal vasomotor neurones to chemoreceptor stimulation in rats. Journal of the Autonomic Nervous System. 1991;33:79–84. doi: 10.1016/0165-1838(91)90020-4. [DOI] [PubMed] [Google Scholar]
- Taylor DEM. Afferent pathways and efferent mechanisms in the bladder viscero-vascular reflex. Quarterly Journal of Experimental Physiology. 1968;53:262–272. doi: 10.1113/expphysiol.1968.sp001969. [DOI] [PubMed] [Google Scholar]
- Walter S, Olesen KP, Nordling J, Hald T. Bladder function in urologically normal middle aged females. A urodynamic and radiological investigation. Scandinavian Journal of Urology and Nephrology. 1979;13:249–258. doi: 10.3109/00365597909179533. [DOI] [PubMed] [Google Scholar]
- Ward J. University of London; 1988. Cardiovascular responses to distension of the urinary bladder.3 PhD Thesis. [Google Scholar]
- Ward J, Daly MdeB, Wood LM. Urinary bladder distension: its effects on carotid baroreceptor reflex left ventricular inotropic response in the dog. The Journal of Physiology. 1995;489:857–868. doi: 10.1113/jphysiol.1995.sp021098. [DOI] [PMC free article] [PubMed] [Google Scholar]


