Abstract
Heteronymous Ia excitatory projections from intrinsic hand muscles to human forearm motoneurones (MNs) were investigated. Changes in firing probability of single motor units (MUs) in the flexor carpi radialis (FCR), flexor carpi ulnaris (FCU), flexor digitorum superficialis (FDS), extensor carpi radialis (ECR), extensor carpi ulnaris (ECU) and extensor digitorum communis (EDC) were studied after electrical stimuli were applied to the median and ulnar nerve at wrist level and to the corresponding homonymous nerve at elbow level.
Homonymous facilitation, occurring at the same latency as the H reflex, and therefore attributed to monosynaptic Ia EPSPs, was found in all the sampled units. In many MUs an early facilitation was also evoked by heteronymous low-threshold afferents from intrinsic hand muscles. The low threshold (between 0.5 and 0.6 times motor threshold (MT)) and the inability of a pure cutaneous stimulation to reproduce this effect indicate that it is due to stimulation of group I muscle afferents.
Evidence for a similar central delay (monosynaptic) in heteronymous as in homonymous pathways was accepted when the difference in latencies of the homonymous and heteronymous peaks did not differ from the estimated supplementary afferent conduction time from wrist to elbow level by more than 0.5 ms (conduction velocity in the fastest Ia afferents between wrist and elbow levels being equal to 69 m s−1).
A statistically significant heteronymous monosynaptic Ia excitation from intrinsic hand muscles supplied by both median and ulnar nerves was found in MUs belonging to all forearm motor nuclei tested (although not in ECU MUs after ulnar stimulation). It was, however, more often found in flexors than in extensors, in wrist than in finger muscles and in muscles operating in the radial than in the ulnar side.
It is argued that the connections of Ia afferents from intrinsic hand muscles to forearm MNs, which are stronger and more widely distributed than in the cat, might be used to provide a support to the hand during manipulatory movements.
It has been demonstrated in the cat that monosynaptic excitation by muscle spindle Ia afferents from a given muscle is not distributed exclusively to the α motoneurones (MNs) of this muscle (homonymous projections) but also reaches the pools of MNs of other muscles (heteronymous projections) acting synergistically at the same joint or at different joints. These heteronymous Ia connections have been extensively investigated in the hindlimb (Eccles et al. 1957; Eccles & Lundberg, 1958) where their particular pattern had led to the hypothesis that they have evolved to assist simple stereotyped flexion-extension movements in feline locomotion (Eccles & Lundberg, 1958; Engberg & Lundberg, 1969; Lundberg, 1969). However, a much more complex pattern of Ia connections has been disclosed in the cat forelimb (Fritz et al. 1989), suggesting that these connections might also subserve the versatile movement repertoire of the distal forelimb.
Heteronymous monosynaptic Ia pathways in man have been submitted to a phylogenetic evolution with the appearance of bipedal stance and gait. Thus, probably because in bipedal stance the equilibrium is less stable than in quadrupeds and requires a more elaborate reflex assistance, transjoint Ia connections are much more widely distributed in the human lower limb than in the cat hindlimb (Meunier et al. 1993). Conversely, projections from elbow muscles to wrist muscles that exist in the cat and have been interpreted with respect to the locomotion function of the feline forelimb (Fritz et al. 1989) have disappeared in man (Cavallari et al. 1992). The only heteronymous connections so far described in the human upper limb are from wrist to elbow muscles (Cavallari & Katz, 1989; Mazevet & Pierrot-Deseilligny, 1994) and might contribute to provide proximal support for distal movements.
Because of the considerable development of cortico-motoneuronal monosynaptic projections to MNs of hand muscles in high primates (see Lemon, 1993), it has been assumed that hand function has been ‘encephalized’ (e.g. Phillips, 1969). In this respect, it was found that the homonymous monosynaptic Ia excitation in the long flexor of the human thumb is very weak (Inglis et al. 1997) and the Ia-mediated spinal stretch reflex very meagre with regard to the prominent long-latency (Marsden et al. 1976) transcortical (Day et al. 1991) response. Yet, in human intrinsic hand muscles, there are many muscle spindles (see Cooper, 1963) and the Ia input to the corresponding MNs, as revealed by an H reflex technique coupled with a collision method, appears to be powerful (Mazzochio et al. 1995). The present investigation was therefore undertaken to investigate whether Ia afferents from intrinsic hand muscles have significant heteronymous monosynaptic projections to human forearm MNs, as has been described from intrinsic paw muscles in the cat (Fritz et al. 1989).
METHODS
The experiments were carried out on ten healthy subjects (aged 21–63 years), all of whom gave informed consent (obtained according to the Declaration of Helsinki) to the experimental procedure which was approved by the institutional ethics committee. The subjects were comfortably seated in an armchair. The shoulder was in slight abduction (60 deg), the elbow semi-flexed (110 deg) and the forearm was pronated and supported by the arm of the chair.
Principle of the experiments
Experiments were performed on single forearm motor units (MUs), using the post-stimulus time histogram (PSTH) method. The principle of these experiments was to compare the latency of the peak of heteronymous excitation elicited by stimulation at wrist level of afferents from hand muscles with that of the peak of monosynaptic Ia excitation elicited by stimulation of the homonymous nerve at elbow level.
Recording
EMG was recorded by surface electrodes 1.5 cm apart secured to the skin over the corresponding muscle belly: flexor carpi radialis (FCR), flexor carpi ulnaris (FCU), flexor digitorum superficialis (FDS), extensor carpi radialis (ECR), extensor carpi ulnaris (ECU), and extensor digitorum communis (EDC). Motor units (MUs) were also recorded from the first dorsal interosseus (FDI) and abductor pollicis brevis (APB) to calculate the conduction velocity (CV) in the fastest Ia afferents in the median and ulnar nerve (see below). In some muscles (ECR, ECU, EDC, FDI and APB) needle electrodes were also used.
It was, of course, essential to ensure that the MUs only discharged during contraction of the muscle to which they were supposed to belong. Thus, it was ascertained that FCR and FCU MUs discharged during wrist flexion (and abduction or adduction, respectively) without associated contraction of finger flexors; ECR and ECU MUs during wrist extension (and abduction or adduction, respectively) without associated contraction of finger extensors; and FDS and EDC MUs during selective finger flexion and extension, without associated contraction of wrist flexors or extensors, respectively.
Conditioning stimuli
Electrical pulses (1 ms) were delivered through bipolar surface electrodes (1 cm diameter silver plates 1.5 cm apart, proximal cathode) to median and ulnar nerves at elbow and wrist levels. The deep radial nerve was stimulated in the spiral groove by two half-ball electrodes 2 cm apart.
The cutaneous sensation (radiating paraesthesia in the hand and fingers) evoked by median and ulnar nerve stimulations was mimicked by pure cutaneous stimuli applied through plate electrodes placed over the nerve projection area: 1st, 2nd and 3rd fingers (median) or 4th and 5th fingers (ulnar) (allowance was made for the extra peripheral conduction time).
PSTH method
PSTHs of a voluntarily activated MU were constructed for the period following a conditioning stimulation. This process extracts from the naturally occurring spike train only those changes in firing probability that are time-locked to the stimulus (Stephens et al. 1976). Details of the particular PSTH technique used in this study have been given elsewhere (Fournier et al. 1986), so it will be only briefly described here. PSTHs of MUs from various forearm (FCR, FCU, FDS, ECR, ECU and EDC) and hand (FDI and APB) muscles were constructed for the 15–55 ms following a conditioning stimulation (bin width: 0.2 or 0.5 ms). The EMG potentials were converted into standard pulses by a spike discriminator and were then used to trigger a computer which subsequently triggered the stimulator about every 1 s. Stimuli were delivered in relation to the MU discharge so as to avoid the time when the MN was refractory. Histograms of the firing probability were constructed after the conditioning stimulus (filled columns on the left of Figs 1-6) and in a control situation without stimulation (open columns on the left of Figs 1-6).
Figure 1. Conduction velocity in median Ia afferents between wrist and elbow levels.
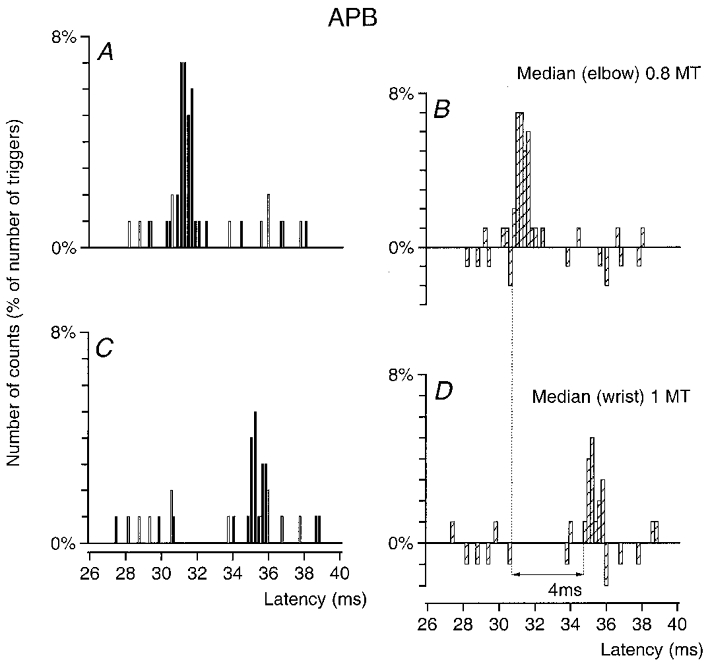
Post-stimulus time histograms (PSTHs) of an abductor pollicis brevis (APB) unit are shown after stimulation of the median nerve at elbow level (0.8MT; A–B) and at wrist level (1MT; C–D). The histograms in A and C show discharges of the voluntary activated MU in control conditions (□) and after stimulation of the nerve (▪). The differences between these two histograms, expressed as a percentage of the number of triggers are plotted in B and D (×) against the latency from the stimulation (bin width 0.2 ms). The difference between the latencies of the peak of excitation elicited by stimulation of the median at elbow (B, 30.8 ms) and wrist (D, 34.8 ms) levels was 4 ms. Distance between wrist and elbow electrodes, 26.7 cm.
Figure 6. Changes in firing probability of an EDC MU after stimulation of the homonymous and heteronymous group I afferents.
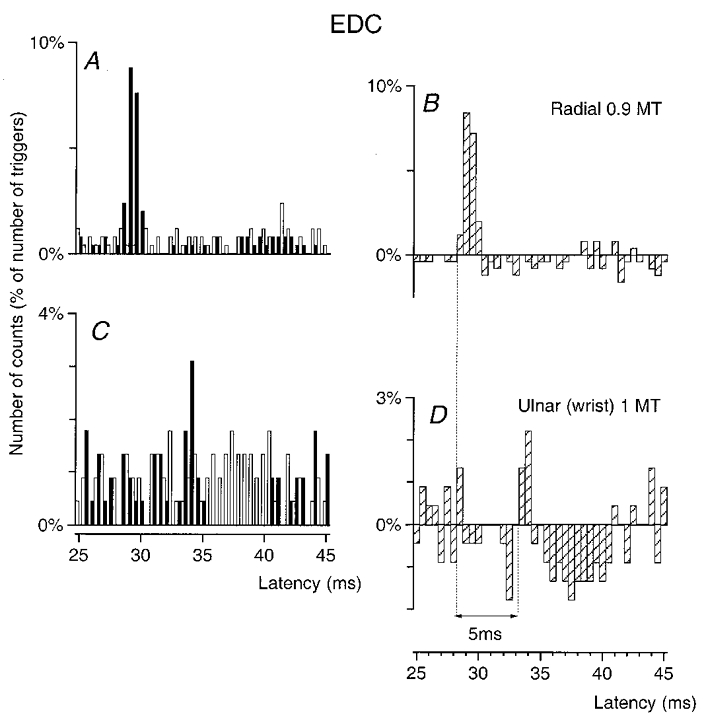
PSTHs of an EDC MU are compared after stimulation of the homonymous Ia afferents in the radial nerve in the spiral groove (0.9MT; A–B) and of the ulnar nerve at wrist level at 1MT (C–D). Abscissa and ordinate, and open, filled and hatched columns as in Fig. 1. Bin width 0.5 ms. The heteronymous peak was significant (P < 0.01). The difference between the latencies of the homonymous (B, 28.5 ms) and heteronymous (D, 33.5 ms) peaks was 5 ms. Distance between wrist and elbow electrodes, 33.5 cm.
Organisation of the experiments
A control situation without stimulation, stimulation of the homonymous nerve at the elbow level and of the median and ulnar nerves at the wrist level (and in some experiments cutaneous stimulation mimicking the cutaneous sensation evoked by median or ulnar nerve stimulation; see above) were randomly alternated in the same sequence so that the latencies of the heteronymous peaks were always compared to that of the homonymous peak recorded under the same conditions. To clarify the differences between the results obtained in the control and conditioned situations the control value in each bin was subtracted from that observed after conditioning stimulation (hatched columns on the right of Figs 1-6, in which the number of counts in each bin is expressed as a percentage of the total number of corresponding stimuli delivered during the sequence). The exceptional sequences in which a change in the control sequence significantly contributed to the differences seen between the two situations were not retained for further analysis.
Statistical analysis
A χ2 test was used within different time-interval windows to determine the extent to which the distribution of firing probability after stimulation differed from that in the control situation. A peak of excitation was accepted if there was a significant (at least P < 0.05) increase in firing probability in a group of adjacent bins (while there were at least 5 counts in each situation). The latency of the first bin of the increased firing probability was taken to be the latency of the excitation provided that the probability was significantly increased in the first group of two or three bins. Although the relation between the amplitude of a peak in the PSTH and that of the underlying EPSP is complex (see Gustafsson & McCrea, 1984), the larger the EPSP the higher the peak. Thus, the size of the peak was estimated as the sum of the differences (conditioned – control counts) in the different consecutive bins with increased firing probability contributing to a given peak: e.g. 25 % between 32 and 33 ms in Fig. 2D, or 9 % between 28.5 and 30.5 in Fig. 3D.
Figure 2. Changes in firing probability of an FCR MU after stimulation of the homonymous and heteronymous group I afferents in the median and ulnar nerve.
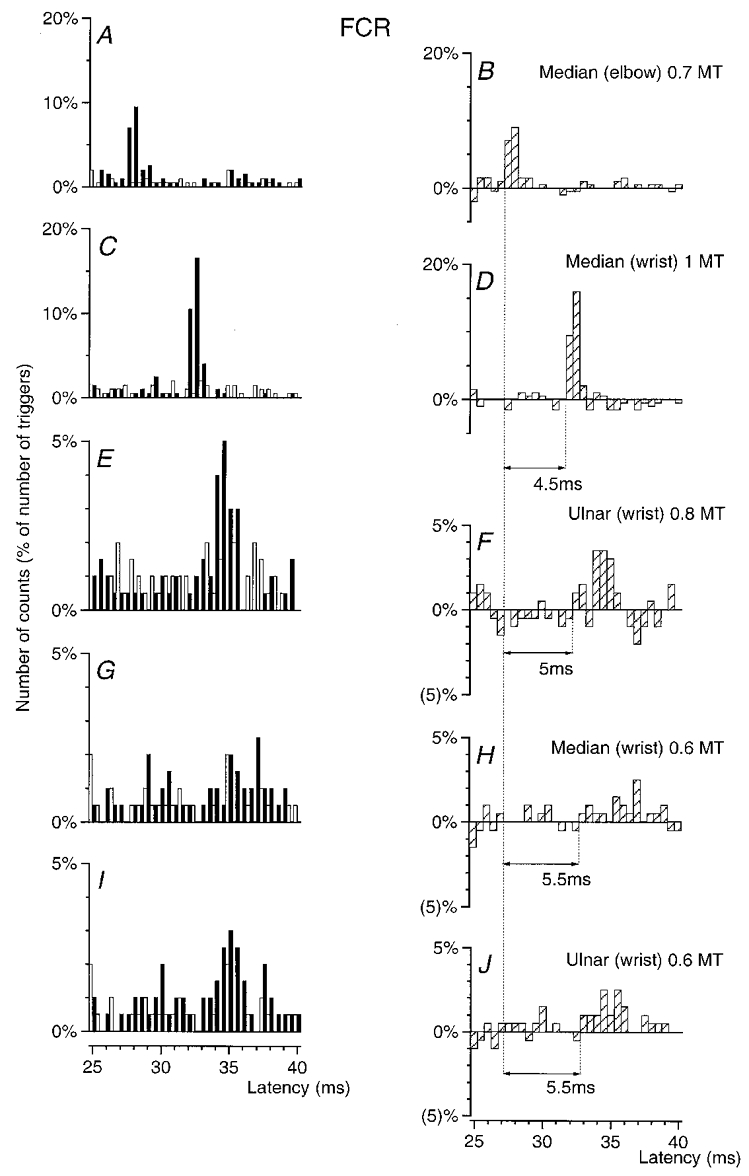
PSTHs of an FCR MU are compared after stimulation of the homonymous Ia afferents in the median nerve at elbow level (0.7MT; A–B), of the median at wrist level at 1MT (C–D) and at 0.6MT (G-H), and of the ulnar nerve at wrist level at 0.8MT (E–F) and at 0.6MT (I–J). Abscissa and ordinate, open, filled and hatched columns as in Fig. 1. Bin width 0.5 ms. Distance between wrist and elbow electrodes, 30 cm.
Figure 3. Changes in firing probability of an FCR MU after stimulation of the homonymous and heteronymous group I afferents and cutaneous afferents.
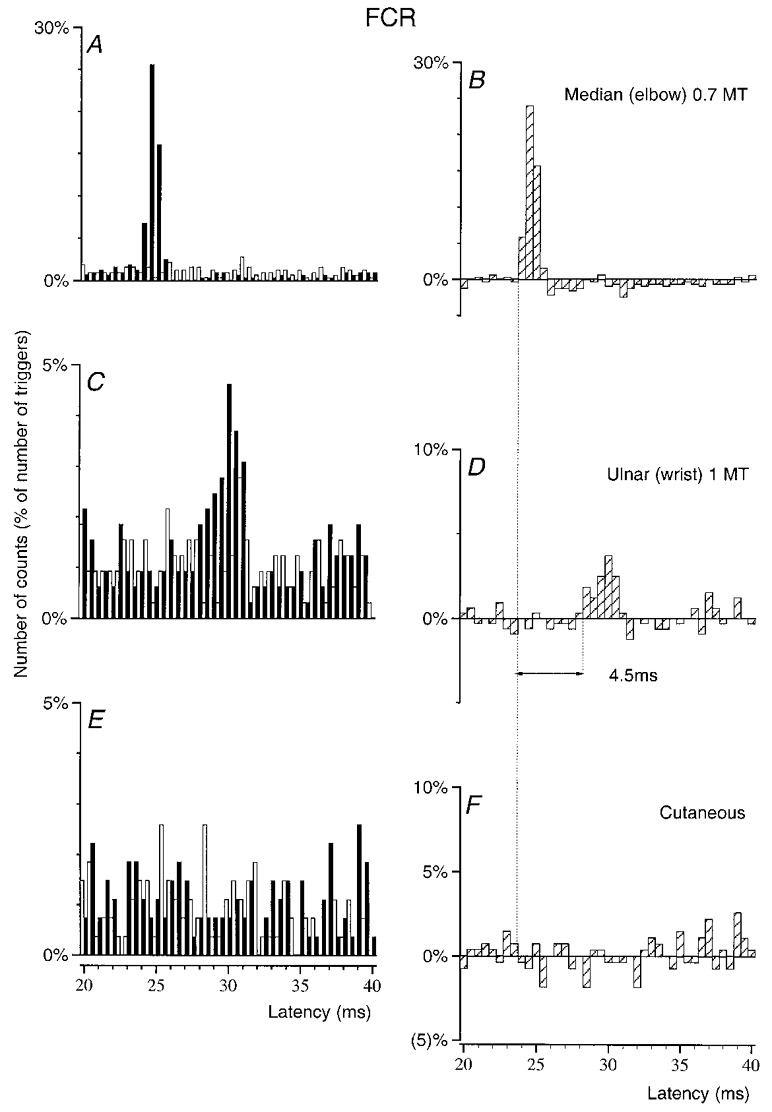
PSTHs of an FCR MU (in another subject) are compared after stimulation of the homonymous Ia afferents in the median nerve at elbow level (0.7MT; A–B), of the ulnar nerve at wrist level (1MT, C–D) and of the skin of the 4th and 5th fingers (E-F, mimicking the cutaneous sensation elicited by ulnar nerve stimulation, allowance being made for the extra peripheral conduction time). Abscissa and ordinate, and open, filled and hatched columns as in Fig. 1. Bin width 0.5 ms. The difference between the latencies of the homonymous (B, 24 ms) and heteronymous (D, 28.5 ms) peaks was 4.5 ms. Distance between wrist and elbow electrodes, 29 cm.
Homonymous Ia facilitation
Changes in firing probability were always studied after stimulation at elbow level of the nerve containing the afferents from the investigated muscle (referred to as the ‘homonymous’ nerve). Conditioning stimulation was set to be subthreshold for the compound H reflex. Stimulation of homonymous afferents from FCR and FDS were elicited by stimulation of the median nerve, those from FCU by stimulation of the ulnar nerve and those from ECR, ECU and EDC by stimulation of the deep radial nerve in the spiral groove. Stimulation of the ‘homonymous’ nerve at motor threshold (1MT), or usually below in order not to elicit a compound H reflex, always evoked an early increase in firing probability. After correction for the trigger delay, the actual latency of this early peak was identical to that of the H reflex in the corresponding muscle, obtained at rest (FCR) or during tonic voluntary contraction. This early peak can therefore be attributed to the monosynaptic Ia EPSP (Mao et al. 1984).
Procedure used to define coupling (mono- or oligosynaptic) of the heteronymous facilitation
The same procedure was used as in the studies in the lower limb (Meunier et al. 1990, 1993). Latencies of the early facilitation evoked in the same MU by stimulation of the homonymous and heteronymous nerves were compared. Since the efferent conduction times were identical (same MU), the difference between the two latencies must reflect the difference in the afferent conduction times and/or in the central (synaptic) delay of the Ia effects evoked by homonymous and heteronymous stimulation. If, like homonymous excitation, heteronymous excitation is mediated through a monosynaptic pathway, the difference in latencies between heteronymous and homonymous excitations should be entirely explained by the difference in afferent conduction times.
Afferent conduction times
Differences in afferent conduction times for the homonymous and heteronymous volleys were estimated from the distance between the stimulation sites of the electrodes eliciting the homonymous (at elbow level) and the heteronymous (at wrist level) volleys measured on the skin of the forearm and from the CV in Ia afferents. The CV in Ia afferents in median and ulnar nerves was calculated from the latency of the monosynaptic Ia peaks measured in the PSTH of the same APB (median) and FDI (ulnar) MU after stimulation of Ia afferents at elbow and wrist levels (see Fig. 1, and Meunier et al. 1990).
RESULTS
Conduction velocity of Ia afferents from intrinsic hand muscles
As explained in Methods, the evidence that heteronymous projections of Ia afferents from hand muscles to forearm MNs are monosynaptic relies on the fact that the difference between the latencies of the peaks of excitation elicited by stimulation of heteronymous Ia afferents at wrist level and of homonymous Ia afferents at elbow level is entirely explainable by the difference in afferent conduction times of the two volleys. It was therefore necessary to assess the CV in Ia afferents from hand muscles. To that end, homonymous monosynaptic Ia projections to MNs of hand muscles were used. The latencies of the peaks of homonymous Ia excitation elicited in the same APB or FDI MU by stimulation at wrist and elbow levels of the median and ulnar nerves, respectively, were therefore compared, while using 0.2 ms bins.
An example of the results obtained in an APB MU is shown in Fig. 1. The difference between the latencies of the peaks elicited by stimulation of the median nerve at elbow (A–B) and wrist (C–D) level was 4 ms, and the distance between the electrodes was 26.7 cm. This gives a conduction velocity of 67 m s−1 (0.267/0.004). Similar measurements were performed in six APB and six FDI MUs in four subjects. The mean values found were 69 ± 1.7 m s−1 in the median nerve and 69 ± 1.2 m s−1 in the ulnar nerve for the fastest Ia afferents, in accordance with values found in previous investigations: 68 and 70 m s−1 in the proximal part of the median nerve (in two subjects, Nielsen & Pierrot-Deseilligny, 1991) and same CV in the ulnar as in the median nerve (Malmgren & Pierrot-Deseilligny, 1988b).
Evidence for monosynaptic heteronymous excitation in single units
Evidence for monosynaptic heteronymous group I excitation of FCR MUs
The effects elicited by various stimuli in an MU from FCR, the muscle in which heteronymous projections were the most often found, are illustrated in Fig. 2. Stimulation of the median nerve at elbow level (0.7MT) elicited a highly significant (P < 0.001) peak of homonymous Ia excitation with a latency of 27.5 ms (Fig. 2a–B). After correction for the trigger delay of the MU (6.5 ms), this latency exactly corresponded to that of the FCR H reflex (21 ms), and can therefore be attributed to a monosynaptic Ia EPSP (Mao et al. 1984).
Heteronymous stimulation of the median nerve at wrist level (30 cm more distally) elicited a highly significant (P < 0.001) peak of excitation with a 32 ms latency (Fig. 2C–D), i.e. 4.5 ms later than the peak of homonymous Ia excitation. Given that in the median nerve the fastest afferents are the Ia afferents (Nielsen & Pierrot-Deseilligny, 1991), which have a CV of 69 m s−1 (see above), the supplementary peripheral conduction time from wrist to elbow level should be at least 4.35 ms (0.30/69). Since this entirely accounts for the 4.5 ms difference in the latencies of the two peaks of excitation, this strongly suggests that heteronymous and homonymous excitations had the same central delay, i.e. that the heteronymous connection also corresponds to a monosynaptic linkage.
When the stimulus intensity was decreased to 0.6MT a small but significant (P < 0.05) median-induced heteronymous excitation persisted within the 33–37 ms time window (Fig. 2G–H), indicating that the threshold of the heteronymous excitation was low (within the range found for group I afferents in the median and ulnar nerves, Malmgren & Pierrot-Deseilligny, 1988a). It should be noted that the decrease in the underlying EPSP, attested by the reduction of the peak, was then accompanied by an increase in the latency and duration of the peak in the PSTH, as described in animal experiments (Fetz & Gustafsson, 1983; Gustafsson & McCrea, 1984). Stimulation of the ulnar nerve at wrist level with low intensities (0.8MT, E–F, and 0.6MT, I and J) also evoked a significant (P < 0.01) peak of excitation.
Absence of a significant cutaneous contribution
In Fig. 3, which illustrates the results obtained in another FCR MU, the comparison is drawn between the homonymous peak (A–B), the highly significant (P < 0.001) peak elicited by ulnar nerve stimulation at wrist level (C–D, 1MT, 29 cm more distally) and the effects of a cutaneous stimulation of the 4th and 5th fingers (E and F) exactly mimicking the cutaneous sensation elicited by the ulnar nerve (see Methods). Here again, the supplementary peripheral conduction time along Ia afferents from wrist to elbow level (0.29/69 = 4.2 ms) almost entirely accounted for the difference in the latencies of the two peaks of excitation (4.5 ms, Fig. 3B–D), implying that the two excitations had a similar central linkage. Figure 3E and F shows that the pure cutaneous stimulation did not elicit any effect at the latency of the ulnar-induced peak (allowance was made for the extra peripheral conduction time). This ulnar-induced peak may therefore be attributed in all likelihood to monosynaptic excitation of the corresponding MN by rapid muscle afferents (see Discussion).
Twenty FCR MUs (from 8 subjects) were so explored. A statistically significant early increase in firing probability was found in 17 and 14 MUs after stimulation at wrist level of the median and ulnar nerve, respectively. In 11 median and 8 ulnar MUs the difference in the latencies of the homonymous and heteronymous peaks differed from the difference in afferent conduction times (calculated on the fastest afferents) by less than 0.5 ms (time resolution of the method). Accordingly, in these cases the heteronymous peak could probably be attributed to a monosynaptic connection. There were, therefore, 11/20 (55 %) (median) and 8/20 (40 %) (ulnar) FCR MUs in which there was evidence for a monosynaptic connection. This heteronymous monosynaptic group I excitation, obtained with 1MT stimuli of the median and ulnar nerves at wrist level, and assessed as explained in Methods, was on average equal to 6.7 and 5.7 %, respectively, of the number of triggers (Table 1).
Table 1.
Distribution of monosynaptic Ia excitation from hand muscles to forearm MNs
| FCR | FCU | FDS | ECR | ECU | EDC | |
|---|---|---|---|---|---|---|
| Median | 11/20 (55%) | 4/20 (20%) | 1/12 (8%) | 8/23 (35%) | 2/10 (20%) | 2/11 (18%) |
| 6.7% | 6.4% | 4% | 3% | 1.6% | 2.25% | |
| Ulnar | 8/20 (40%) | 2/20 (10%) | 2/12 (36%) | 7/23 (30%) | 0/10 (0%) | 2/11 (18%) |
| 5.7% | 2.2% | 12.3%± 1.8 | 2.7% | 0% | 3.7% |
Data in the upper row of are raw values of the frequency of occurrence, i. e. the number of MUs in which a significant monosynaptic Ia excitation (P < 0.05 at least) was evoked and the number of units tested, with the former as a percentage of the latter in parentheses; data in the lower row are the mean values of the absolute size of the heteronymous peak (assessed as explained in Methods and expressed as a percentage of the number of triggers) calculated in all experiments with a significant heteronymous excitation.
Evidence for monosynaptic coupling for various muscle-nerve combinations
In MUs of the other muscles tested, the same criteria were applied to provide evidence for a heteronymous monosynaptic group I excitation: (i) difference in latencies of homonymous and heteronymous peaks ≤ 0.5 ms when the intensity of the heteronymous stimulation was equal to 1MT; (ii) low threshold; (iii) absence of effects evoked in the same muscle-nerve combination by pure cutaneous stimuli mimicking the cutaneous sensation elicited by stimulation of the median or ulnar nerve at wrist level.
This is exemplified in an FCU MU in Fig. 4. The difference between the latencies of the peak of homonymous Ia excitation (A–B) and the highly significant (P < 0.001) peaks of heteronymous excitations elicited by 1MT stimuli to ulnar (C–D) and median (G–H) nerves at wrist level (27.5 cm more distally) was 4 ms, corresponding exactly to the supplementary peripheral conduction time along the fastest Ia afferents (0.275/69 = 3.98 ms). Figure 4E, F, I and J shows that a pure cutaneous stimulation applied to the 4th and 5th fingers or the 2nd and 3rd fingers and mimicking the cutaneous sensation elicited by ulnar (E–F) and median (I–J) nerves did not modify the firing probability of the MU at the latency of the ulnar- and median-induced peaks.
Figure 4. Changes in firing probability of an FCU MU after stimulation of the homonymous and heteronymous group I afferents and cutaneous afferents.
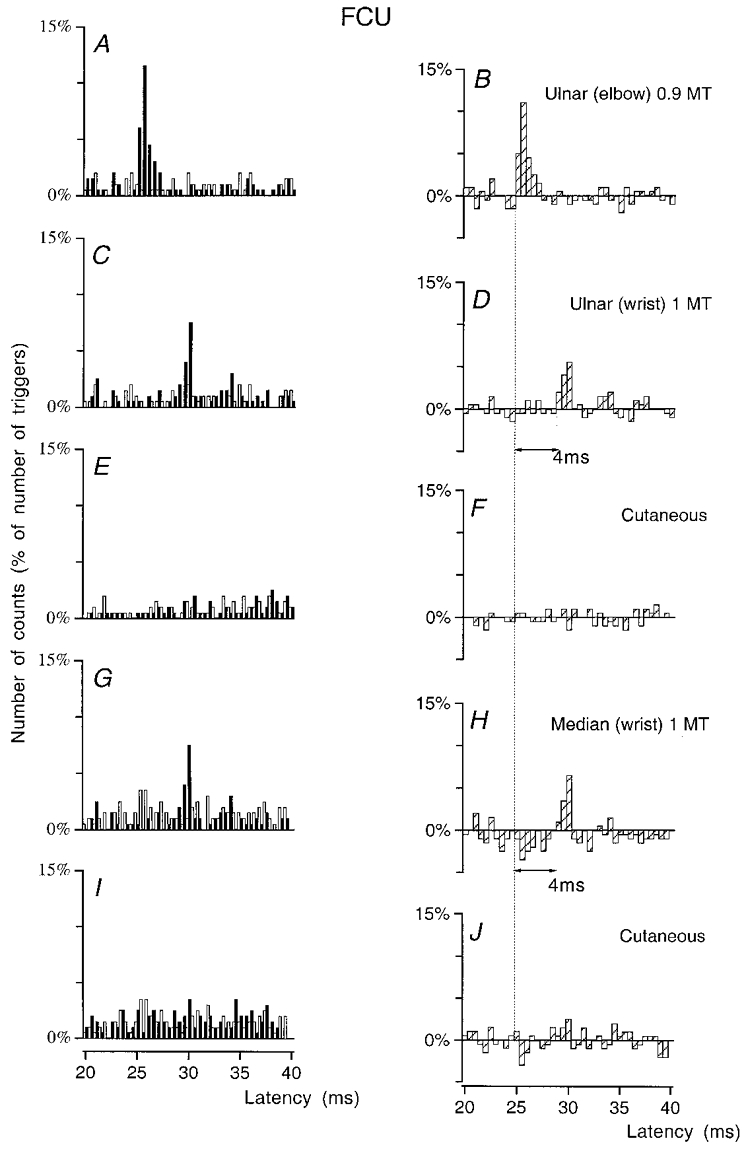
PSTHs of an FCU MU are compared after stimulation of the homonymous Ia afferents in the ulnar nerve at elbow level (0.9MT; A-B), of the ulnar nerve at wrist level (1MT; C-D), of the skin of the 4th and 5th fingers (E-F), of the median nerve at wrist level at 1MT (G–H) and of the skin of the 2nd and 3rd fingers (I–J). Abscissa and ordinate, and open, filled and hatched columns as in Fig. 1. Bin width 0.5 ms. The difference between the latencies of the homonymous (B, 25 ms) and heteronymous (D and H, 29 ms) peaks was 4 ms. Distance between wrist and elbow electrodes, 27.5 cm.
Heteronymous group I excitation of FCU MNs
In the population of 20 FCU MUs explored (7 subjects), evidence for a monosynaptic group I excitation was much more rarely found than in the FCR: only 4/20 and 2/20 MUs, after stimulation of the median and ulnar nerves, respectively. However, when it existed, the mean value of the median-induced monosynaptic excitation was very similar to that found in the FCR (6.4 %, Table 1).
Heteronymous group I excitation of FDS MNs
Median- and ulnar-induced excitations were investigated in 12 MUs of the FDS (11 supplying the head for the 4th finger, 1 the head for the 3rd finger). Evidence for monosynaptic group I excitation was still more rare, since it was found in only 1 MU after stimulation of the median nerve at wrist level. It was also very rare after ulnar nerve stimulation (2 MUs) but the mean value of the excitation was then very high (12.3 %).
Heteronymous group I excitation of ECR MNs
In Fig. 5 the peak (P < 0.05) elicited by stimulation of the ulnar nerve (C–D) applied 30.5 cm more distally than that of the radial nerve at elbow level is seen to occur 4.5 ms later than the peak of radial-induced homonymous excitation (A–B). Assuming that the CV in the fastest Ia afferents is the same in the radial as in the median and ulnar nerves (see Discussion), this fits perfectly the estimated 4.42 ms (0.305/69) supplementary peripheral conduction time. In the population of 23 MUs explored (8 subjects), evidence for a monosynaptic Ia excitation was almost as frequent as in FCR MUs – 8 (35 %) and 7 (30 %) MUs after stimulation of the median and ulnar nerves, respectively – but the mean values of these peaks was much smaller (3 and 2.7 %, respectively).
Figure 5. Changes in firing probability of an ECR MU after stimulation of the homonymous and heteronymous group I afferents.
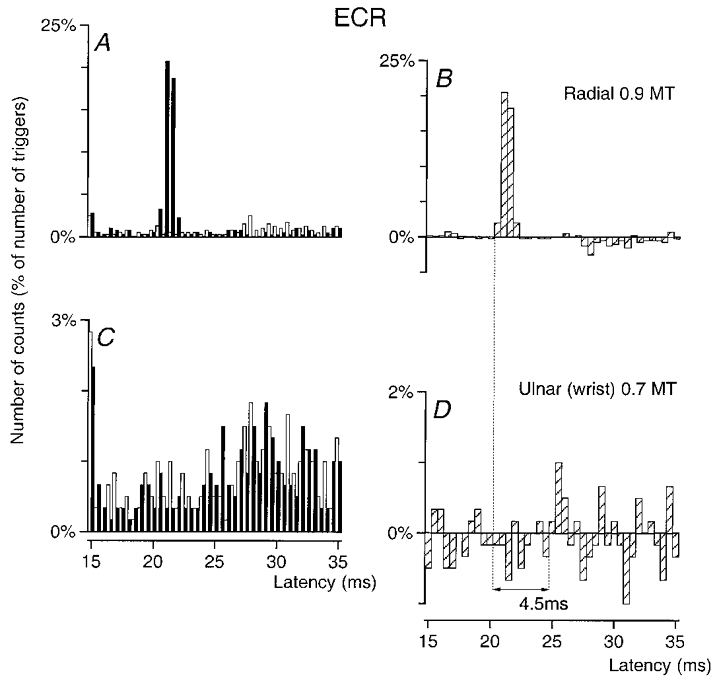
PSTHs of an ECR MU are compared after stimulation of the homonymous Ia afferents in the radial nerve in the spiral groove (0.9MT, A–B) and of the ulnar nerve at wrist level (0.7MT, C–D). Abscissa and ordinate, and open, filled and hatched columns as in Fig. 1. Bin width 0.5 ms. The difference between the latencies of the homonymous (B, 20.5 ms) and heteronymous (D, 25 ms) peaks was 4.5 ms. Distance between wrist and elbow electrodes, 30.5 cm.
Heteronymous group I excitation of ECU MNs
Ten ECU MUs were explored (4 subjects). A significant monosynaptic excitation was found in only 2 MUs after median stimulation and none was found in any MU after ulnar stimulation.
Heteronymous group I excitation of EDC MNs
Figure 6 shows an example of ulnar-induced excitation of an EDC MU. The difference between the latencies of the peaks of homonymous and heteronymous excitations (5 ms) exactly corresponded to the expected latency (0.335/69 = 4.9 ms) for a monosynaptic excitation elicited by a stimulation applied 33.5 cm more distally. Figure 6C and D also shows that this excitation was followed by a clear and relatively short-lasting inhibition, the threshold of which was 0.7MT. Evidence for monosynaptic excitation was found in only 2/11 EDC MUs after median and ulnar nerve stimulation.
Strength of the different monosynaptic heteronymous group I connections
The strength of the monosynaptic group I connections in the different motor nucleus-nerve combinations is compared in Table 1. The comparison involves heteronymous peaks which occurred at monosynaptic latency and were statistically significant. Two factors were considered in estimating the strength of these heteronymous Ia connections: (i) the frequency of occurrence (upper row of data for each nerve), with the number of MUs where heteronymous Ia excitation reached statistical significance (at least P < 0.05) (numerator), the number of explored MUs (divisor) and the former as a percentage of the latter; (ii) the absolute size of the heteronymous peak (lower row) assessed as explained in Methods and expressed as a percentage of the number of triggers was calculated in all experiments with a significant heteronymous excitation, and the mean value of these heteronymous peaks is indicated. Several points should be noted.
(i) The number of MUs with a monosynaptic heteronymous group I excitation was in all likelihood underestimated, since in many MUs (e.g. 5 in both the FCU and the FDS) there was a small peak of excitation occurring with a central delay which exceeded that of the homonymous Ia excitation by only 0.5-1 ms. This could be attributed to the small underlying EPSP (see above), but since there was some doubt that the excitation was monosynaptic the data were not included in Table 1.
(ii) Even using these severe criteria, monosynaptic group I projections from hand muscles to forearm MNs were found in all six motor nuclei explored.
(iii) When they existed, these projections were stronger in flexors than in extensors, in wrist than in finger muscles and in muscles operating in the radial than in the ulnar side.
(iv) Even when statistically significant, the mean values for heteronymous excitation were generally small, below 5 % of the number of triggers, and thus far below the size of the homonymous peak, yet they were evoked with lower stimulus intensities (set at 0.7-0.8MT in order to be below the threshold of the compound H reflex) and reached 30–50 % of the number of triggers. However, in all motor nuclei (except the EDC), there were MUs with a peak above 10 % revealing a rather large underlying heteronymous EPSP.
(v) In some units (3 in both the FCR and the ECR) evidence for a monosynaptic excitation was found after stimulation of both nerves. In most MUs, however, a significant monosynaptic excitation was only found after stimulation of either the median or the ulnar nerve at wrist level.
DISCUSSION
Evidence for monosynaptic Ia connections from hand muscles to forearm MNs
Changes in the firing probability of voluntarily activated MUs from various forearm muscles were investigated after stimulation of the median and ulnar nerve at wrist level. Conditioning volleys to these two nerves were shown to elicit a statistically significant early increase in the firing probability of MUs belonging to all muscles tested (FCR, FCU, FDS, ECR, ECU and EDC).
Peripheral pathway
Excitation had an electrical threshold between 0.5 and 0.6MT, i.e. as low as that of Ia afferents (see Malmgren & Pierrot-Deseilligny, 1988a). The latency of the effect was so early that, even when assuming a mediation through the most rapid central pathway (monosynaptic; see below), the CV of the responsible afferents was compatible with that of the most rapid Ia afferents in the median and ulnar nerve (69 m s−1). Any significant contribution from cutaneous afferents to the excitation elicited by the stimuli used in the present investigation was eliminated by control experiments (Figs 3E–F and 4E–F and I–J). The low threshold and high CV of afferents responsible for the excitation, as well as the inability of a pure cutaneous stimulation to reproduce this effect, indicate that it is due to stimulation of group I muscle afferents.
Central pathway
The comparison was drawn between the difference in the latencies of excitations evoked by heteronymous afferent volleys elicited at wrist level and by the homonymous volley at elbow level. This difference was then compared to the supplementary afferent conduction time from wrist to elbow level, calculated from CVs in the fastest Ia fibres from hand muscles and from the distance between stimulation sites at elbow and wrist levels. Evidence for a similar central delay in homonymous and heteronymous pathways was accepted when the difference in latencies of the peaks did not differ from the estimated supplementary afferent conduction time by more than 0.5 ms. Since the onset of the homonymous peak, occurring with the same latency as the H reflex, is known to be monosynaptic (Mao et al. 1984), a monosynaptic transmission was admitted in such cases. The validity of this conclusion depends on the time resolution of the method and on the reliability of both latency measurements and estimates concerning peripheral afferent conduction times.
Time resolution of the method
The earliest disynaptic group I effects in humans start to manifest themselves at least 0.8 ms after the earliest monosynaptic Ia excitation (Pierrot-Deseilligny et al. 1981; Day et al. 1984; Hultborn et al. 1987). One can therefore estimate that a disynaptic effect should have a central delay exceeding by more than 0.5 ms (the time resolution of the method) the estimated monosynaptic group I-induced central delay.
Trigger delay
Because of the trigger delay, i.e. the delay between the exact onset of the EMG potential and the moment at which the computer was triggered by the rapidly rising phase of the potential, there was some uncertainty about the absolute latency of the peaks. However, the trigger delay was the same for homonymous and heteronymous peaks in a given MU, since they were investigated in the same sequence, and a greater trigger delay would not alter the difference in latencies of the two peaks, the critical measurement in these experiments.
Estimate of the supplementary afferent conduction time
Another source of uncertainty concerns the estimates in peripheral afferent conduction times. In the case of forearm flexors, there is little doubt about their reliability: the CV found for the fastest Ia afferents between wrist and elbow levels was identical to that calculated for the fastest Ia afferents in the median nerve between elbow and shoulder levels (69 m s−1, Nielsen & Pierrot-Deseilligny, 1991; identical in ulnar Ia afferents, Malmgren & Pierrot-Deseilligny, 1988b). In addition, the distances measured on the skin were quite reliable, since: (i) the electrodes eliciting homonymous and heteronymous volleys were placed in the same position on the limb; (ii) the course of the two nerves in the forearm is not winding; (iii) heteronymous and homonymous volleys run along the same nerves between the elbow level and the spinal cord. In the case of forearm extensors, the comparison between the afferent conduction times of heteronymous and homonymous volleys in the radial nerve is more uncertain because: (i) electrodes eliciting conditioning and test volleys were not placed in the same position on the limb, thus making the measurement of the distance between electrodes less certain; (ii) homonymous and heteronymous volleys run along different nerves; (iii) the course of the radial nerve in the spiral groove is winding; (iv) there are no definite data concerning the CV of radial Ia afferents.
However, it has been shown that the peak of radial-induced monosynaptic Ia excitation in a given biceps MU occurs 0.5-0.6 ms earlier than that evoked by stimulation of the median nerve (Pauvert et al. 1998). Given that the radial nerve was stimulated some 5–7 cm more proximally than the median nerve, one would have expected, in view of the similar CV and the parallel course of the two nerves in the arm, that the radial-induced excitation would occur about 1 ms earlier than the median-induced peak. Assuming a similar CV in the two nerves, the winding course of the radial nerve could account for the slightly shorter difference observed.
Since motor columns supplying intrinsic hand muscles are located in C8-Th1 (Kendall et al. 1971), the more rostral Ia afferents from these muscles enter the spinal cord in C8, i.e. possibly (FDS and FCU MNs located between C7 and Th1) or probably (FCR, ECR, ECU and EDC MNs located between C6 and C8) more caudally than the forearm MNs explored here. An intraspinal conduction time should therefore be added to the afferent conduction time of the heteronymous Ia volley. However, because this is compensated for by a longer extra spinal conduction time for the homonymous afferent volley in the brachial plexus, this factor can be neglected.
It is therefore concluded that the onset of the heteronymous excitation, like that of the homonymous one, reflects a monosynaptic connection of group I afferents, i.e. of Ia afferents, since they are the only ones to have monosynaptic projections to MNs. Of course it cannot be excluded that oligosynaptic group I EPSPs (Jankowska & McCrea, 1983) contribute to the late bins of the increase in firing probability (Burke et al. 1984). Nonetheless, peaks longer than 1 ms do not necessarily imply a contribution of oligosynaptic pathways, since a small underlying monosynaptic EPSP during its decay phase, added to the synaptic noise, can still cause the MN to fire (Fetz & Gustafsson, 1983).
Following inhibition
Increasing the conditioning stimulus intensity resulted in an inhibition. This inhibition was particularly pronounced in extensor MNs, in which it appeared with intensities as low as 0.7MT and was strong enough at 1MT to completely suppress the ‘spontaneous’ firing of the MU after the presumed monosynaptic excitation (Fig. 6C and D). The very brief duration (1 ms) and the abrupt termination of the preceding presumed monosynaptic excitation suggest that this low-threshold depression is evoked with only a slightly longer central delay than the early facilitation, the initial EPSP being truncated by IPSPs mediated through pathways involving only one more synapse. Ib interneurones, also fed by Ia afferents (Jankowska & McCrea, 1983), are good candidates for this inhibition.
Comparison with animal data and functional significance
In the cat, it has been shown that Ia afferents from intrinsic paw muscles supplied by the median and the ulnar nerve have monosynaptic projections to different forelimb muscles (Fritz et al. 1989). Thus afferents in the median nerve project onto FCR MNs and afferents in both nerves project onto ECU MNs, but there are no projections from intrinsic hand muscles onto FCU and ECR MNs. The projections described here in humans are much more widely distributed, since monosynaptic Ia projections from intrinsic hand muscles supplied by both median and ulnar nerves were found on MNs belonging to all six motor nuclei tested: FCR, FCU, FDS, ECR, ECU (but without ulnar projections) and EDC. In addition to the phylogenetic evolution from felines to primates (see below), a possible cause for these differences may arise from the fact that different MNs were investigated in the two species: with the small voluntary contractions used in human PSTH experiments, all MUs from which recordings were taken were in the low-threshold range, whereas intracellular recordings in the cat favour the largest MNs. Finally, as described in the cat (Fritz et al. 1989), although the mean value of these projections was small, in all motor nuclei tested (except the EDC) there were MUs with a peak above 10 %, revealing a rather large underlying EPSP.
Since it was not possible in the present investigation to stimulate Ia afferents from a given muscle, it is difficult to propose a precise function for these projections. Although a more precise analysis might have revealed specific projections, the main feature characterising the projections described in this study is their diffuse distribution, since they reach both flexors and extensors and cross the radio-ulnar plane. This diffuse distribution and the finding that the connections are stronger on muscles operating at the wrist than on long flexors and extensors of the fingers suggest that these projections might be used to fix the wrist so as to provide a firm support for hand muscles during manipulatory movements. Their development in man could then simply reflect the greater development of intrinsic hand muscles in higher primates. This postural interpretation would be reinforced if, as is the case between wrist and elbow muscles (see Introduction), it was possible to demonstrate that projections from hand to wrist muscles are not paralleled by projections from proximal to distal muscles. Because of the difficulty of stimulating the nerves of wrist muscles without encroaching upon afferents from hand muscles in the median and ulnar nerves, along their course in the forearm, this was not possible with the non-invasive methods used in the present investigation.
Acknowledgments
The authors wish to express their gratitude to Drs L. Jami and L. Mazières for reading and commenting upon the manuscript. Our thanks are also due to Annie Rigaudie and Michèle Dodo for excellent technical assistance. This work was supported by grants from Assistance Publique-Hôpitaux de Paris (PHRC AOM 95078), Institut National de la Santé et de la Recherche Médicale (INSERM, CRI 96037), Ministère de l'Enseignement Supérieur et de la Recherche (EA 2393) and Institut pour la Recherche sur la Moelle Épinière (IRME). V. Marchand-Pauvert was supported by a grant from Institut Lilly.
References
- Burke D, Gandevia SC, McKeon B. Monosynaptic and oligosynaptic contributions to human ankle jerk and H-reflex. Journal of Neurophysiology. 1984;52:435–448. doi: 10.1152/jn.1984.52.3.435. [DOI] [PubMed] [Google Scholar]
- Cavallari P, Katz R. Pattern of projections of group I afferents from forearm muscles to motoneurones supplying biceps and triceps muscles in man. Experimental Brain Research. 1989;78:465–478. doi: 10.1007/BF00230235. [DOI] [PubMed] [Google Scholar]
- Cavallari P, Katz R, Pénicaud A. Pattern of projections of group I afferents from elbow muscles to motoneurones supplying wrist muscles in man. Experimental Brain Research. 1992;91:311–319. doi: 10.1007/BF00231664. [DOI] [PubMed] [Google Scholar]
- Cooper S. Muscle spindles in man; their morphology in the lumbricals and deep muscles of the neck. Brain. 1963;86:563–586. doi: 10.1093/brain/86.3.563. [DOI] [PubMed] [Google Scholar]
- Day BL, Marsden CD, Obeso JA, Rothwell JC. Reciprocal inhibition between the muscles of the human forearm. The Journal of Physiology. 1984;349:519–534. doi: 10.1113/jphysiol.1984.sp015171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day BL, Riescher H, Struppler A, Rothwell JC, Marsden CD. Changes in the response to magnetic and electrical stimulation of the motor cortex following muscle stretch in man. The Journal of Physiology. 1991;433:41–57. doi: 10.1113/jphysiol.1991.sp018413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JC, Eccles RM, Lundberg A. The convergence of monosynaptic excitatory afferents onto many different species of alpha motoneurones. The Journal of Physiology. 1957;137:22–50. doi: 10.1113/jphysiol.1957.sp005794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles RM, Lundberg A. Integrative pattern of Ia synaptic actions on motoneurones of hip and knee muscles. The Journal of Physiology. 1958;144:271–298. doi: 10.1113/jphysiol.1958.sp006101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engberg I, Lundberg A. An electromyographic analysis of muscular activity in the hindlimb of the cat during unrestrained locomotion. Acta Physiologica Scandinavica. 1969;75:105–122. doi: 10.1111/j.1748-1716.1969.tb04415.x. [DOI] [PubMed] [Google Scholar]
- Fetz EE, Gustafsson B. Relation between shapes of post-synaptic potentials and changes in firing probability of cat motoneurones. The Journal of Physiology. 1983;341:387–410. doi: 10.1113/jphysiol.1983.sp014812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier E, Meunier S, Pierrot-Deseilligny E, Shindo M. Evidence for interneuronally mediated Ia excitatory effects to human quadriceps motoneurones. The Journal of Physiology. 1986;377:143–169. doi: 10.1113/jphysiol.1986.sp016179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz N, Illert M, de la Motte S, Reeh P, Saggau P. Pattern of monosynaptic Ia connections in the cat forelimb. The Journal of Physiology. 1989;419:321–351. doi: 10.1113/jphysiol.1989.sp017875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson B, McCrea D. Influence of stretch-evoked synaptic potentials on firing probability of cat spinal motoneurones. The Journal of Physiology. 1984;347:431–451. doi: 10.1113/jphysiol.1984.sp015074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H, Meunier S, Morin C, Pierrot-Deseilligny E. Assessing changes in presynaptic inhibition of Ia fibres: a study in man and the cat. The Journal of Physiology. 1987;389:729–756. doi: 10.1113/jphysiol.1987.sp016680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglis JT, Meunier S, Leeper JB, Burke D, Gandevia SC. Weak short-latency spinal projections to the long flexor of the human thumb. Experimental Brain Research. 1997;115:165–168. doi: 10.1007/pl00005677. [DOI] [PubMed] [Google Scholar]
- Jankowska E, McCrea D. Shared reflex pathways from Ib tendon organ afferents and Ia muscle spindle afferents in the cat. The Journal of Physiology. 1983;338:99–111. doi: 10.1113/jphysiol.1983.sp014663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall HO, Kendall FP, Wadsworth GE. Muscles. Testing and Function. Baltimore, USA: The William & Wilkins Company; 1971. [Google Scholar]
- Lemon RN. Cortical control of the primate hand. The 1992 G. L. Brown Prize Lecture. Experimental Physiology. 1993;78:263–301. doi: 10.1113/expphysiol.1993.sp003686. [DOI] [PubMed] [Google Scholar]
- Lundberg A. Oslo: Universiteitsforlaget; 1971. Reflex control of stepping. The Nansen Memorial Lecture; pp. 1–42. [Google Scholar]
- Malmgren K, Pierrot-Deseilligny E. Evidence for non-monosynaptic Ia excitation of wrist flexor motoneurones, possibly via propriospinal neurones. The Journal of Physiology. 1988a;405:747–764. doi: 10.1113/jphysiol.1988.sp017359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmgren K, Pierrot-Deseilligny E. Inhibition of neurones transmitting non-monosynaptic Ia excitation to human wrist flexor motoneurones. The Journal of Physiology. 1988b;405:765–783. doi: 10.1113/jphysiol.1988.sp017360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao CC, Ashby P, Wang M, McCrea D. Synaptic connections from large muscle afferents to the motoneurones of various leg muscles in man. Experimental Brain Research. 1984;56:341–350. doi: 10.1007/BF00236290. [DOI] [PubMed] [Google Scholar]
- Marsden CD, Merton PA, Morton HB. Stretch reflex and servo action in a variety of human muscles. The Journal of Physiology. 1976;259:531–560. doi: 10.1113/jphysiol.1976.sp011481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazevet D, Pierrot-Deseilligny E. Pattern of descending excitation of presumed propriospinal neurones at the onset of voluntary movement in man. Acta Physiologica Scandinavica. 1994;150:27–38. doi: 10.1111/j.1748-1716.1994.tb09656.x. [DOI] [PubMed] [Google Scholar]
- Mazzocchio R, Rothwell JC, Rossi A. Distribution of Ia effects onto human hand muscle motoneurones as revealed using an H reflex technique. The Journal of Physiology. 1995;489:263–273. doi: 10.1113/jphysiol.1995.sp021048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier S, Pénicaud A, Pierrot-Deseilligny E, Rossi A. Monosynaptic Ia excitation and recurrent inhibition from quadriceps to ankle flexors and extensors in man. The Journal of Physiology. 1990;423:661–675. doi: 10.1113/jphysiol.1990.sp018046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier S, Pierrot-Deseilligny E, Simonetta M. Pattern of monosynaptic heteronymous Ia connections in the human lower limb. Experimental Brain Research. 1993;96:533–544. doi: 10.1007/BF00234121. [DOI] [PubMed] [Google Scholar]
- Nielsen J, Pierrot-Deseilligny E. Pattern of cutaneous inhibition of the propriospinal-like excitation to human upper limb motoneurones. The Journal of Physiology. 1991;434:169–182. doi: 10.1113/jphysiol.1991.sp018464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauvert V, Pierrot-Deseilligny E, Rothwell JC. Role of spinal premotoneurones in mediating corticospinal input to forearm motoneurones in humans. The Journal of Physiology. 1998;508:301–312. doi: 10.1111/j.1469-7793.1998.301br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips CG. Motor apparatus of the baboon's hand. Proceedings of the Royal Society. 1969;B 173:141–174. doi: 10.1098/rspb.1969.0044. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E, Morin C, Bergego C, Tankov N. Pattern of group I fibre projections from ankle flexor and extensor muscles in man. Experimental Brain Research. 1981;42:337–350. doi: 10.1007/BF00237499. [DOI] [PubMed] [Google Scholar]
- Stephens JA, Usherwood TP, Garnett R. Technique for studying synaptic connections of single motoneurones in man. Nature. 1976;263:343–344. doi: 10.1038/263343a0. [DOI] [PubMed] [Google Scholar]


