Abstract
Using functional co-cultures of rat carotid body (CB) O2 chemoreceptors and ‘juxtaposed’ petrosal neurones (JPNs), we tested whether ATP and ACh acted as co-transmitters.
Perforated-patch recordings from JPNs often revealed spontaneous and hypoxia-evoked (PO2≈5 mmHg) excitatory postsynaptic responses. The P2X purinoceptor blocker, suramin (50 μM) or a nicotinic ACh receptor (nAChR) blocker (hexamethonium, 100 μM; mecamylamine, 1 μM) only partially inhibited these responses, but together, blocked almost all activity.
Under voltage clamp (-60 mV), fast perfusion of 100 μM ATP over hypoxia-responsive JPNs induced suramin-sensitive (IC50 = 73 μM), slowly-desensitizing, inward currents (IATP) with time constant of activation τon = 30.6 ± 4.8 ms (n = 7). IATP reversed at 0.33 ± 3.7 mV (n = 4), and the dose-response curve was fitted by the Hill equation (EC50 = 2.7 μM; Hill coefficient ≈0.9). These purinoceptors contained immunoreactive P2X2 subunits, but their activation by α,β-methylene ATP (α,β-meATP; EC50 = 2.1 μM) suggests they are P2X2/P2X3 heteromultimers.
Suramin and nAChR blockers inhibited the extracellular chemosensory discharge in the intact rat carotid body-sinus nerve preparation in vitro. Further, P2X2 immunoreactivity was widespread in rat petrosal ganglia in situ, and co-localized in neurones expressing the CB chemo-afferent marker, tyrosine hydroxylase (TH). P2X2 labelling in the CB co-localized with nerve-terminal markers, and was intimately associated with TH-positive type 1 cells.
Thus ATP and ACh are co-transmitters during chemotransduction in the rat carotid body.
In mammals, an early step in the initiation of compensatory reflex hyperventilation during blood hypoxaemia appears to involve neurotransmitter release from O2-sensitive chemoreceptor (type 1) cells in the carotid body onto apposed sensory nerve endings (reviewed by Gonzalez et al. 1994). Excitation of these endings by released transmitter(s) is proposed to result in the increased afferent spike discharge in the carotid sinus nerve, whose chemo-afferent cell bodies are located in the petrosal ganglion. A long-standing, unresolved issue is the identity of the neurotransmitter(s) initiating this reflex (Gonzalez et al. 1994; Fitzgerald et al. 1997). Whereas the best studied carotid body neurotransmitter dopamine has recently fallen into disfavour (e.g. Donnelly, 1996), there has been a rekindling of interest in ACh as a major carotid body neurotransmitter during chemo-excitation, even though it too has had a long and controversial history (Gonzalez et al. 1994; Fitzgerald et al. 1997; Nurse & Zhang, 1999).
In order to probe these transmitter mechanisms, we recently developed an attractive co-culture preparation consisting of dispersed rat type 1 cell clusters and dissociated petrosal neurones (Zhong et al. 1997; Nurse & Zhang, 1999). The main advantages are that: (i) functional connections develop de novo; (ii) subthreshold synaptic events can be conveniently studied by recording from petrosal somas, juxtaposed to type 1 cell clusters; and (iii) drugs have relatively unrestricted access to the synaptic sites under these monolayer conditions. Using this preparation we recently provided strong evidence for ACh as a co-released transmitter from type 1 cells during chemosensory signalling (Zhong et al. 1997; Nurse & Zhang, 1999). We further characterized the properties of the nicotinic ACh receptors (nAChR) expressed by petrosal neurones using pharmacological and electrophysiological techniques (Zhong & Nurse, 1997). However, it was evident that ACh alone could not account entirely for the hypoxia-induced ‘postsynaptic’ responses recorded in co-cultured neurones. We therefore considered the possibility that other co-released transmitters might be involved.
An attractive candidate for an excitatory, sensory co-transmitter in carotid body function is ATP. First, the excitatory effects of ATP on carotid body chemosensory fibres have been recognized for some time, based on sinus nerve recordings during intracarotid injections of ATP (Jarish et al. 1952; Spergel & Lahiri, 1993). Second, ATP is frequently co-released with ACh in several neuronal preparations (Schweitzer, 1987; Bean, 1992; Zimmerman, 1994; Silinsky & Redman, 1996), and purinoceptors are widely distributed on sensory neurones and their terminals (Khakh et al. 1995; Lewis et al. 1995; Vulchanova et al. 1996; Wildman et al. 1997). Third, cation-selective P2X purinoceptor channels were first described in the peripheral nervous system, where they appear to be important for mediating fast excitatory neurotransmission at a variety of synapses (Surprenant et al. 1995).
In the present study we identified an important role for ATP as a co-transmitter in carotid body function, based largely on the sensitivity of the afferent postsynaptic responses to the P2 purinoceptor blocker suramin. However, since suramin blocks both ligand-gated P2X and G-protein-coupled P2Y purinoceptors (Dunn & Blakely, 1988; Evans et al. 1992), we carried out a more detailed characterization of the P2 receptor subtype(s) expressed by chemosensory petrosal neurones using electrophysiological, pharmacological and immunofluorescence techniques. In addition, in order to validate the results obtained from co-cultures, we compared the effects of both nicotinic cholinergic and purinoceptor blockers on the afferent discharge recorded from the intact rat carotid body sinus nerve preparation in vitro (Pepper et al. 1995; Donnelly, 1996). These combined data led to the conclusion that co-release of ATP and ACh, acting at postsynaptic P2X2-containing and nicotinic-ACh receptors, respectively, plays a dominant role in rat carotid body PO2 chemotransmission.
METHODS
Co-cultures
Co-cultures were produced by first preparing monolayers containing carotid body type 1 cell clusters from 9- to 14-day-old rats, and then adding an overlay of dissociated petrosal neurones 3–5 days later. The carotid bifurcation, with attached nodose and/or petrosal complex, was excised after pups were first rendered unconscious by a blow to the back of the head (produced by rapid deceleration) and killed immediately by decapitation. All procedures for animal handling and tissue removal were carried out according to the guidelines of the Canadian Council on Animal Care (CCAC). Details of the methods used for preparing co-cultures, or separate cultures, of dissociated rat carotid body and petrosal ganglia are described elsewhere (Stea & Nurse, 1992; Jackson & Nurse, 1995; Zhong et al. 1997). Cultures were grown at 37°C in a humidified atmosphere of 95 % air-5 % CO2 in F-12 nutrient medium (Gibco) supplemented with 10 % (v/v) fetal bovine serum (Gibco), 80 U l−1 insulin (Sigma), 0.6 % (w/v) glucose, 2 mM L-glutamine and 1 % penicillin- streptomycin (Gibco). Electrophysiological recordings from co-cultures were carried out 3–8 days after the neurones were plated.
Electrophysiology
Perforated-patch recordings
The methods for obtaining nystatin perforated-patch, whole-cell recordings of membrane potential (current clamp) and ionic currents (voltage clamp) from petrosal neurones were identical to those previously described (Stea & Nurse, 1992; Zhong et al. 1997). In order to facilitate rapid switching from normoxic (PO2∼160 mmHg) to hypoxic (PO2∼5 mmHg) solutions, a ‘fast perfusion’ system utilizing double barrelled pipettes was used (Zhong et al. 1997). Neurotransmitters (ACh and ATP) and the agonist α,β-methylene ATP (α,β-meATP) were applied either by fast perfusion or by pressure ejection from a ‘puffer’ pipette as described previously (Zhong & Nurse, 1997; Zhong et al. 1999). In some cases, drugs (e.g. antagonists) were applied to the bath by perfusion under gravity. All recordings were carried out at ∼35°C in bicarbonate/CO2-buffered extracellular fluid of the following composition (mM): NaCl, 115; NaHCO3, 24; KCl, 5; CaCl2, 2; MgCl2, 1; glucose, 10; and sucrose, 12; at pH 7.4, maintained by bubbling 95 % air-5 % CO2.
Extracellular recording from rat carotid body-sinus nerve preparation
The carotid bifurcation, including the carotid body and attached sinus nerve, was removed from postnatal 7- to 14-day-old rat pups using the procedures described above for co-culture preparations. The tissue was placed in a chilled oxygenated, bicarbonate-buffered (95 % O2-5 % CO2) saline containing (mM): NaCl, 115; NaHCO3, 24; KCl, 5; CaCl2, 2; MgCl2, 1; glucose, 10; sucrose, 12; at pH 7.4. To facilitate sinus nerve recording (Pepper et al. 1995; Donnelly, 1996), the bifurcation was partially cleaned of surrounding tissue and then placed for 8 min in bicarbonate-buffered saline, containing 0.02 % collagenase (Gibco), 0.01 % Dispase I (Boehringer Mannheim, Quebec) at 37°C. Following a few rinses in enzyme-free saline, the bifurcation was pinned on a wax-coated dish and superfused at 3 ml min−1 with oxygenated saline at ∼36°C.
Extracellular spike activity was recorded from the sinus nerve using a carbon fibre (10 μm diameter) electrode, whose tip was exposed at the end of a capillary tube. Signals were amplified with a differential AC amplifier (Model 1700; A-M Systems Inc.), filtered at 10 Hz to 1 kHz, displayed on an oscilloscope, and stored on a 486 PC with the aid of a Digidata 1200 A/D converter (Axon Instruments). In these experiments the same rapid perfusion system (see above) was used for stimulus application and drug delivery.
Immunofluorescence
Co-cultures, separate cultures of CB type 1 cells and petrosal neurones, and cryostat sections of the carotid bifurcation from 2- to 3-week-old rats were processed for immunofluorescence. In some cases animals (>2 weeks old) were first anaesthetized by a brief exposure to Fluothane vapour (Wyeth-Ayerst Canada Inc. Montreal, Quebec), and then killed by cervical dislocation prior to dissection of the carotid bifurcation. In other cases, animals (∼3 weeks old) were first anaesthetized by intraperitoneal administration of Somnotol (65 mg kg−1), before perfusion via the aorta with phosphate-buffered saline (PBS) followed by PBS containing 4 % paraformaldehyde. The following antibodies were used: (i) anti-P2X2 (Alomone Laboratories, Jerusalem, Israel), a rabbit polyclonal antibody raised against highly purified peptide (C)SQQDSTSTDPKGLAQL (P2X2457-472), corresponding to residues 457–472 of rat P2X2, with additional N-terminal cysteine; (ii) anti-SV2, a monoclonal antibody against the presynaptic marker, synaptic vesicle protein SV2 (Developmental Studies Hybridoma Bank, University of Iowa; see Buckley & Kelly, 1985); (iii) anti-neurofilament (NF), a monoclonal antibody against NF68kDa (Boehringer Mannheim, Montreal); (iv) anti-GAP-43, a monoclonal antibody against the neuronal marker GAP-43 (a generous gift from Dr D. J. Schreyer; see Jackson & Nurse, 1995); and (v) anti-tyrosine hydroxylase (TH), a monoclonal antibody against TH (Boehringer Mannheim), a cytoplasmic marker for CB chemo-afferent petrosal neurones (Katz & Black, 1986; Finley et al. 1992). Procedures for immunofluorescence labelling of the cultured cells were similar to those described in detail elsewhere (Jackson & Nurse, 1995; Zhong et al. 1997). Prior to processing of tissue sections, the carotid bifurcation together with the petrosal and/or nodose complex was excised from anaesthetized animals as described above. The tissue was then snap frozen with Frostbite (Surgipath, Winnepeg, Manitoba) and sections (thickness, 12–15 μm) were cut in a cryostat and collected on glass slides coated with 2 % silane (Sigma). After air drying for 3–5 min, unfixed sections were exposed for 10 min to 4 % paraformaldehyde in PBS at room temperature, and washed (3 × 5 min each) in PBS. Fixed sections from perfused animals were simply air dried and then washed (3 × 5 min each) in PBS. Sections were then exposed to a blocking solution containing 2 % bovine serum albumin in PBS (BSA/PBS) for 45 min at room temperature, followed by overnight incubation at 4°C with one of the following cocktails of primary antisera: (i) anti-SV2 (1:20) plus anti-P2X2 (1:800); (ii) anti-NF (1:4) plus anti GAP-43 (1:20000) plus anti-P2X2 (1:800); (iii) anti-TH (1:40) plus anti-P2X2 (1:800). The primary antiserum was diluted in PBS containing 1 % BSA, 10 % normal goat serum and 0.3 % Triton X-100. After incubation overnight at 4°C, sections were washed in PBS (3 × 10 min) and incubated for 1 h at room temperature with the secondary antibodies. These consisted of goat anti-mouse IgG conjugated to FITC (1:100; Cappel, Malvern, PA, USA) or Alexa 488 (1:400; Molecular Probes Inc., Eugene, OR, USA), and goat anti-rabbit IgG conjugated to Cy3 (1:500; Jackson Research Laboratories, Westgrove, PA, USA). Secondary antibodies were diluted in PBS containing 1 % BSA, 10 % normal goat serum and 0.3 % Triton X-100. Samples were covered with a photobleaching reagent, Mowiol (Calbiochem, La Jolla, CA, USA) containing DABCO (Lancaster Synthesis, Windham, NH, USA), before viewing under epifluorescence illumination with fluorescein and rhodamine filter sets. In control experiments, omission of the primary antiserum resulted in abolition of staining in all cases. Further, in the case of P2X2 immunostaining, pre-incubation of the primary antibody for 1 h with excess (∼80:1 w/w) blocking peptide P2X2 457-472 (corresponding to residues 457–472 of rat P2X2) resulted in complete abolition of staining.
Drugs
Mecamylamine, hexamethonium and suramin were obtained from Research Biochemicals (RBI; Natick, MA, USA). ACh, ATP, α,β-meATP, tetrodotoxin (TTX), and tetraethylammonium (TEA) were obtained from Sigma Chemical Co.
RESULTS
The appearance of co-cultures used in this study was similar to those described in detail elsewhere (Zhong et al. 1997; Nurse & Zhang, 1999). Petrosal neurones (PN), fortuitously ‘juxtaposed’ to type 1 cell clusters in co-culture (e.g. Fig. 1b in Stea & Nurse, 1992), were selected for electrophysiological investigation using perforated-patch recording (Zhong et al. 1997; Nurse & Zhang, 1999). The presence of spontaneous synaptic activity (i.e. subthreshold EPSPs and/or spikes) during recordings of membrane potential from such ‘juxtaposed’ petrosal neurones (JPNs) was taken as preliminary evidence for de novo development of functional connections (Figs 1a–e and 2Aa-c), since such activity was never seen in cultures of petrosal neurones alone (Zhong et al. 1997). In a few JPNs (n = 8) from older (3-6 days) co-cultures, subthreshold events were not evident as the spontaneous activity consisted of a rhythmic spike discharge with frequencies of 10–19 Hz (Fig. 2). Consistent with our previous studies, the spontaneous activity was partially and reversibly suppressed by nicotinic ACh receptor (nAChR) blockers, 100–200 μM hexamethonium or 0.5-2 μM mecamylamine (Figs 1c and 2Ab), suggesting it was mediated in part by spontaneous release of ACh (Zhong et al. 1997; Nurse & Zhang, 1999).
Figure 1. Effects of nicotinic and P2 purinoreceptor blockers on spontaneous synaptic activity recorded from a petrosal neurone juxtaposed to a type 1 cell cluster in co-culture.
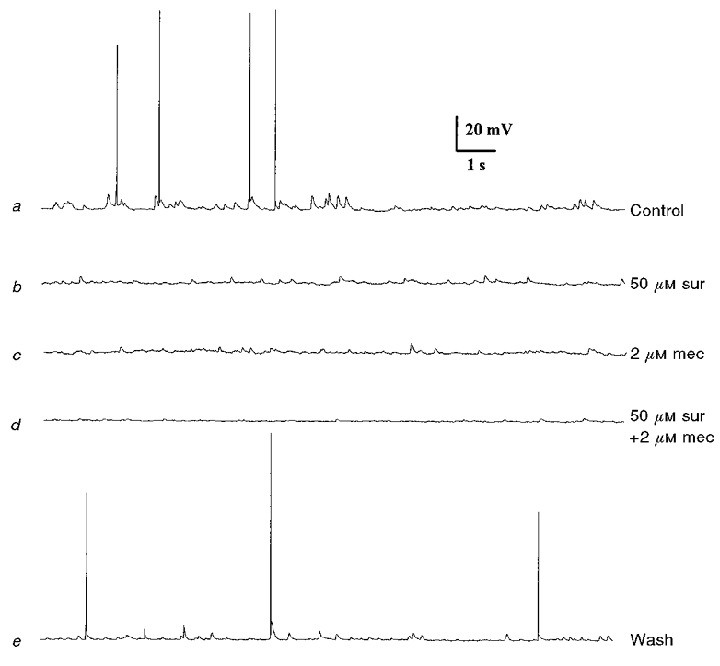
a, spontaneous subthreshold postsynaptic potentials (EPSPs) and sporadic spikes recorded under control conditions. This activity was partially inhibited during perfusion of either the P2 purinoceptor blocker suramin (50 μM; b), or the nicotinic blocker mecamylamine (2 μM; c). Combined application of the two blockers abolished almost all spontaneous activity (d). Note abolition of spike discharge when the blockers were present either separately (b and c) or in combination (d). After wash-out of the two blockers, control responses recovered (e). Resting potential -59 mV.
Figure 2. Effects of nicotinic and P2 purinoceptor blockers on spike frequency of a co-cultured petrosal neurone that showed spontaneous rhythmic discharge.
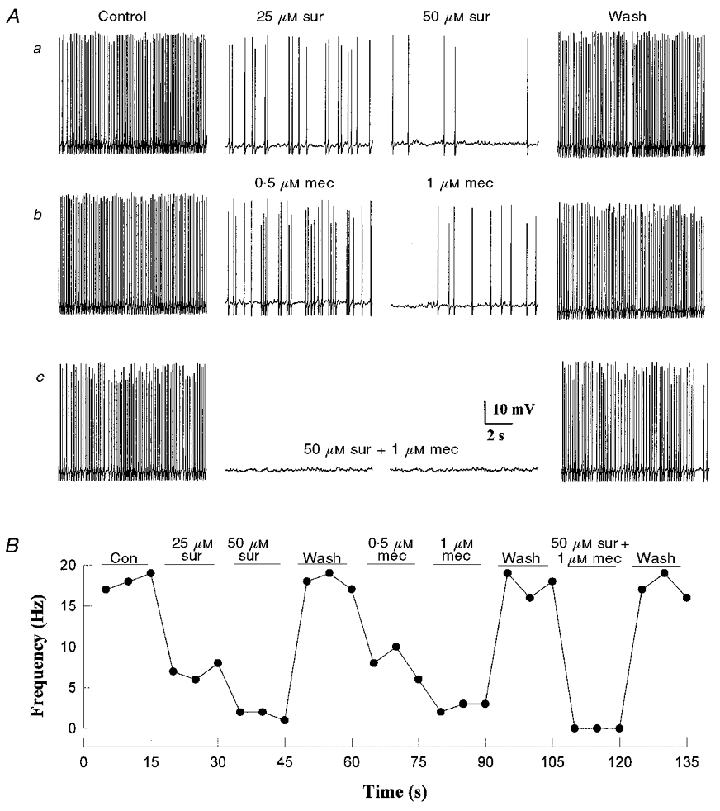
The neurone was adjacent to a large type 1 cell cluster (≈40 cells). Spontaneous spike frequency was reversibly inhibited in a dose-dependent manner by the P2 purinoceptor blocker suramin (Aa), and by the nicotinic blocker mecamylamine (Ab), at the indicated concentrations. In Ac, combined application of suramin (50 μM) and mecamylamine (1 μM) abolished the spike discharge and the effect was reversible. The time course of the effects of the drugs on spike frequency is shown for this cell in B; spike frequency was calculated from 5 s segments of the continuous record. Resting potential -62 mV.
To test whether release of ATP contributed to the spontaneous activity, co-cultures were perfused with the P2 purinoceptor blocker, suramin (Dunn & Blakely, 1988). As shown in Figs 1b and 2Aa, perfusion of suramin (25-50 μM) alone also resulted in a partial suppression of spontaneous synaptic activity, even in the same neurones whose activity was suppressed by mecamylamine (Figs 1 and 2). In Fig. 2Aa and b inhibition of the spontaneous spike discharge in the rhythmically firing neurone was dose dependent for both suramin and mecamylamine (Fig. 2b), and the rhythmic activity recovered after wash-out of the drugs. To demonstrate that the spontaneous synaptic events arose principally from co-release of ACh and ATP, both blockers were tested in combination. As shown in Figs 1d, and 2Ac and B, the spontaneous discharge was almost completely abolished during perfusion of suramin (50 μM) plus mecamylamine (1-2 μM), and the effect was reversible. Taken together, these data suggest that the spontaneous synaptic activity recorded in co-culture is due largely to the release of ATP and ACh from type 1 cells onto afferent petrosal terminals, and is mediated via P2 purinoceptors and nicotinic AChR, respectively.
Evidence that co-release of ATP and ACh mediates hypoxic chemotransmission in co-culture
We previously showed that many complexes (∼40 %) consisting of a type 1 cell cluster and a JPN in co-culture were physiologically functional, i.e. a hypoxic stimulus was transduced and transmitted to the neurone (Zhong et al. 1997). The strength of the interaction varied from one preparation to another, depending on age of the co-culture and presumably, the number of synaptic contacts formed between petrosal terminals and type 1 cells in the cluster. Consistent with our previous observations (Zhong et al. 1997; Nurse & Zhang, 1999), even relatively high concentrations of nicotinic antagonists failed to block completely the hypoxia-induced depolarization in the JPN. An example is shown in Fig. 3a, where the initial hypoxic response was suprathreshold, leading to a burst of action potentials. Here, increasing doses of hexamethonium (100-200 μM) failed to block completely the hypoxia-induced depolarization, though spike discharge was absent at the higher dose. In other experiments where the initial hypoxic response was subthreshold (n = 16), the mean (±s.e.m.) hypoxia-induced depolarization was 4.61 ± 0.38 mV before, compared with 2.68 ± 0.32 mV during, and 4.52 ± 0.38 mV after wash-out of 200 μM hexamethonium, i.e. a suppression to ∼58 % of the initial response. In a recent study (Nurse & Zhang, 1999), another nicotinic antagonist mecamylamine (2 μM) caused a similar suppression in the hypoxia-induced depolarization (∼55 % of control). Since these concentrations of nicotinic blockers inhibit > 90 % of the ACh-induced responses in isolated petrosal somas (Zhong & Nurse, 1997; Nurse & Zhang, 1999), we tested whether the residual hypoxia-induced depolarization seen in the presence of nicotinic blockers (e.g. Fig. 3a) was mediated by ATP, acting on P2 purinoceptors. As shown in Fig. 3b, the hypoxia-induced depolarization and spike discharge were reversibly suppressed by the P2 purinoceptor blocker, suramin (50-100 μM). Acting alone, suramin (50 μM) significantly (P < 0.001; Student's t test) reduced this depolarization in JPNs to ∼45 % of control; the mean (±s.e.m.) depolarization was 4.9 ± 0.49 mV before, 2.17 ± 0.30 mV during, and 4.73 ± 0.44 mV after suramin (n = 24). These data suggest that synaptically released ATP, acting at P2 purinoceptors, may also contribute to the hypoxia-induced depolarization in JPNs.
Figure 3. Evidence that co-release of ACh and ATP mediates hypoxia-induced excitation in a co-cultured petrosal neurone.
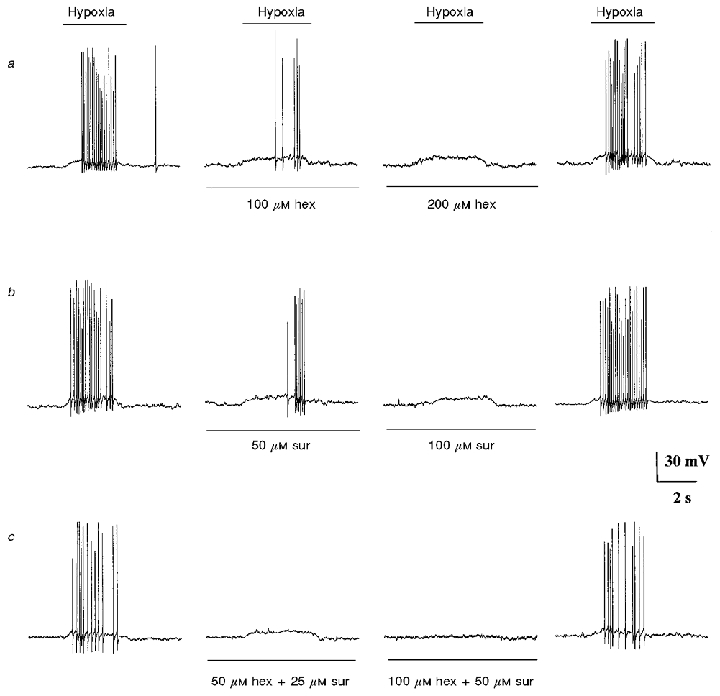
a–c, current clamp recordings from the same petrosal neurone juxtaposed to a type 1 cell cluster. For each row of traces the hypoxic stimulus was applied by fast perfusion during the period indicated by the upper horizontal bar. Also, each row shows the effects of a different pharmacological treatment, with control traces on the left, drug treatment (middle two traces), and recovery (right). a and b, effect of different concentrations of hexamethonium and suramin, respectively. c, effect of combined application of hexamethonium and suramin at the indicated concentrations. Inhibition of the hypoxia-induced depolarization and/or spike discharge by 100–200 μM hexamethonium (a) and by 50–100 μM suramin (b) was only partial when the drugs were applied separately, but complete when applied together (c; third trace from left). This cell was held for ≈2 h; resting potential -64 mV.
To determine whether co-release of ACh and ATP was the main mechanism mediating chemotransmission, we tested the effect of both blockers together. In 7/11 preparations, combined application of 100 μM hexamethonium and 50 μM suramin reversibly abolished the hypoxia-induced depolarization (e.g. Fig. 3c; same cell as Fig. 3a and b). A small residual response, 10–20 % of the initial control, was detectable in the remainder (4/11). Moreover, combinations of suramin (50 μM) and another nicotinic blocker, mecamylamine (2 μM), were similarly effective in abolishing (reversibly) the hypoxia-induced depolarization in 6/8 preparations (not shown). Taken together, these results suggest that co-release of ATP and ACh can account for 80–100 % of the hypoxia-induced depolarization in JPNs.
Evidence that petrosal neurones express distinct receptors for ACh and ATP
Nicotinic ACh receptors (nAChR) are expressed on approximately two-thirds of these cultured petrosal neurones (Zhong & Nurse, 1997). In the present study, all neurones tested under voltage or current clamp (60/60) were sensitive to ATP, suggesting that many of them express both nAChR and purinoceptors. As shown in Fig. 4a and B, the effect of puffer-applied ATP was strongly excitatory and was almost completely (and reversibly) blocked by 100 μM suramin (Fig. 4a; middle trace). However, the ATP response was unaffected by the nicotinic blocker, 2 μM mecamylamine (Fig. 4B; middle trace), which markedly inhibits (> 90 %) the depolarization induced by rapid application of ACh to the soma of isolated PN (Nurse & Zhang, 1999). Conversely, 100 μM suramin had a negligible effect on the ACh-induced excitatory response in petrosal somas, whether isolated (not shown), or juxtaposed to a type 1 cluster (Fig. 4C). Note however, in Fig. 4C, the presence of 100 μM suramin reduced the frequency and amplitude of the spontaneous subthreshold potentials, visible at the resting potential before and after application of ACh (compare baseline activity to right and left of the ACh pulse in the three traces, Fig. 4C). Under voltage clamp, 100 μM suramin almost completely abolished the inward current at -60 mV (holding potential) evoked by fast application of ATP (50 μM) over petrosal somas (e.g. Fig. 5a). The dose-response relation for suramin inhibition of the inward current (at -60 mV) evoked by 50 μM ATP in a group of four hypoxia-responsive JPNs is shown in Fig. 5b, where the IC50 is ∼73 μM suramin. Taken together, these data suggest that ACh and ATP act on separate receptors on the same cell.
Figure 4. Sensitivity of petrosal neurones to ATP and ACh under current clamp conditions.
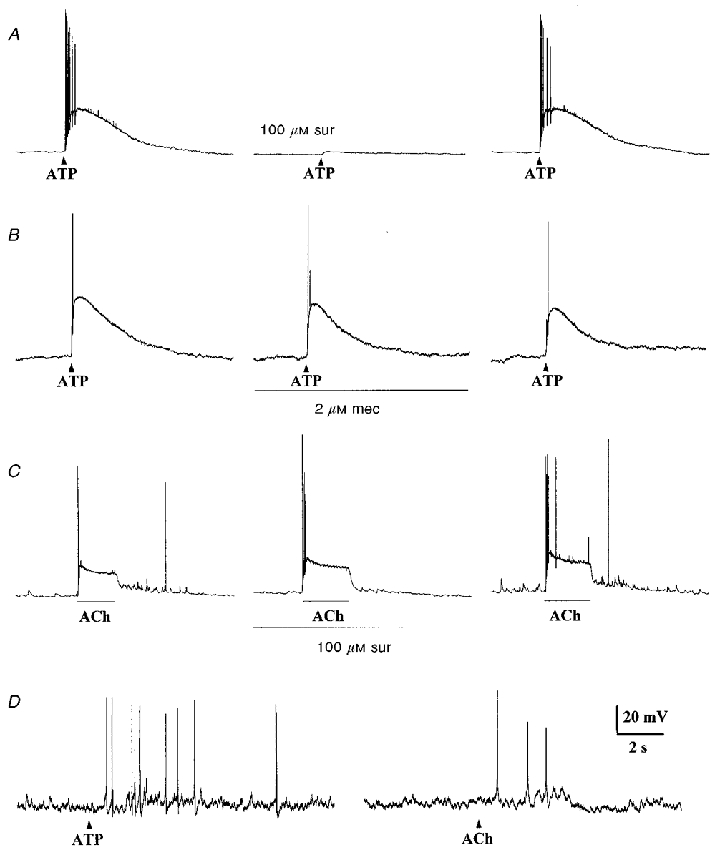
A and B, application of a ‘puff’ of ATP (1 mM; delivered at arrowhead by pressure ejection) to isolated petrosal soma elicited membrane depolarization and spike activity. In A, the ATP-induced response was almost completely abolished (reversibly) by the P2 purinoceptor blocker 100 μM suramin (middle trace). In B, the ATP-induced response was unaffected by the nicotinic cholinergic blocker 2 μM mecamylamine (middle trace). C, fast perfusion of ACh (50 μM), during the period indicated by the lower horizontal bar, over a petrosal soma (juxtaposed to a type 1 cell cluster) caused membrane depolarization and spike activity; note presence of spontaneous ‘synaptic’ potentials (upward deflections) superimposed on the resting potential of this neurone. Perfusion with 100 μM suramin had no effect on the ACh-induced depolarization (middle trace) but, consistent with spontaneous release of ATP from nearby type 1 cells, the frequency of spontaneous ‘synaptic’ potentials (occurring before and after the period of ACh application) was markedly and reversibly inhibited. D, a ‘puff’ of ATP (1 mM pipette concentration; left) or ACh (1 mM; right) to presumptive nerve terminals elicited propagating action potentials recorded in a petrosal soma located ≈100 μm away from a type 1 cell cluster; the nerve terminal region was presumed to coincide with the location of the type 1 cells, which form intimate contacts with petrosal endings in co-culture (Zhong et al. 1997; Nurse & Zhang, 1999). Resting potential was approximately -67 mV (A–C), and -57 mV (D).
Figure 5. Dose-response relation for suramin inhibition of ATP-induced inward currents in identified hypoxia-sensitive petrosal neurones in co-culture.
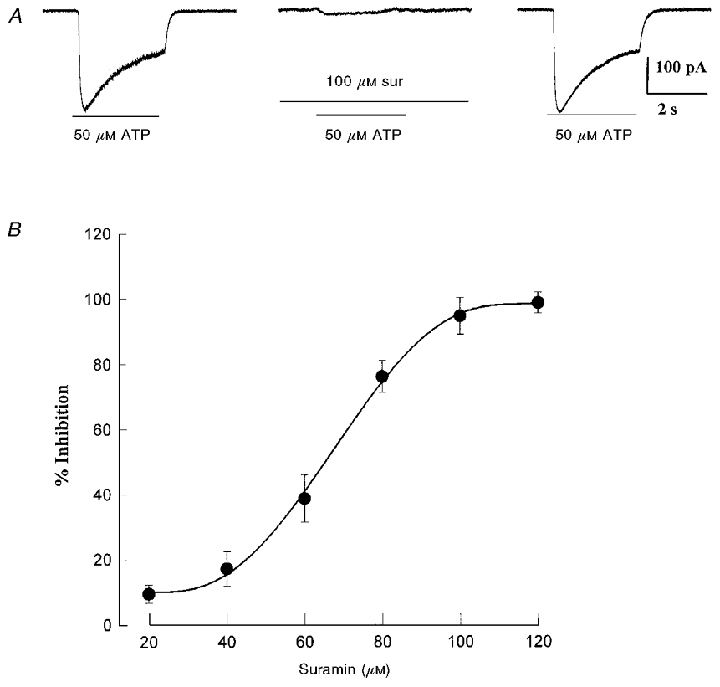
In A, 100 μM suramin almost completely blocked the inward current evoked at -60 mV by rapid application of ATP (50 μM) during the period indicated by the lower horizontal bar; note that the effect was reversible after wash-out of the drug (right trace). The dose-response relation for varying concentrations of suramin is shown in B, where the IC50 = 73 μM. Data are expressed as means ±s.e.m. for a sample of 4 cells. The ATP concentration was 50 μM and holding potential -60 mV.
To determine whether nAChR and purinoceptors might also be localized on petrosal terminals, we recorded from ‘distant’ petrosal cell bodies located >100 μm away from the nearest type 1 cell cluster in co-culture, and tested whether orthograde, propagating spikes could be elicited by ‘puffer-applied’ ACh and ATP to petrosal nerve endings. The latter are not visible in living cultures, but were presumed to be located in close proximity to type 1 cell clusters, based on our previous immunocytochemical studies (Zhong et al. 1997; Nurse & Zhang, 1999). In three examples, one of which is illustrated in Fig. 4D, both ‘terminally’ applied ATP and ACh elicited action potentials in the same petrosal soma located ∼100 μm away. Since diffusion of the applied transmitters to the recording site, i.e. soma, was negligible in these experiments, the data are consistent with the presence of both AChR and purinoreceptors at petrosal terminals. Application of ATP had no detectable effect on the resting membrane potential or whole-cell current (depolarizing steps from -50 mV) in type 1 cells (n = 23), suggesting its effects on petrosal terminals are likely to be direct. On the other hand, ACh may have dual effects since it can also depolarize type 1 cells via nicotinic AChR (Nurse & Zhang, 1999).
Electrophysiological characterization of P2X purinoceptors on petrosal neurones
The rapid response of ATP on petrosal neurones (Fig. 4A, B and D) suggested it was mediated via ligand-gated cationic P2X purinoceptors, which are oligomeric proteins assembled from a family of seven subunits, P2X1-P2X7 (Collo et al. 1996). To characterize further the purinoceptors on isolated petrosal neurones in cultures grown with or without type 1 cells, the pharmacological profile, reversal potential, activation kinetics, and desensitization properties of the native receptors were investigated under voltage clamp. As shown in Figs 5A and B, and 7A, application of varying doses of ATP by fast perfusion led to rapid activation of an inward current (at -60 mV holding potential), which showed slow desensitization over several seconds and sensitivity to suramin. Consistent with the involvement of non-selective cation channels, the ATP-induced currents reversed near 0 mV as expected for ligand-gated P2X receptors. An example of the reversal of these currents during a series of voltage clamp steps is shown in Fig. 6a and B for a JPN that was first identified as hypoxia responsive (not shown). The mean (±s.e.m.) reversal potential for a group of four JPNs investigated in this way was 0.33 ± 3.7 mV.
Figure 7. Comparison of whole-cell currents evoked by agonists ATP and α,β-meATP in petrosal neurones.
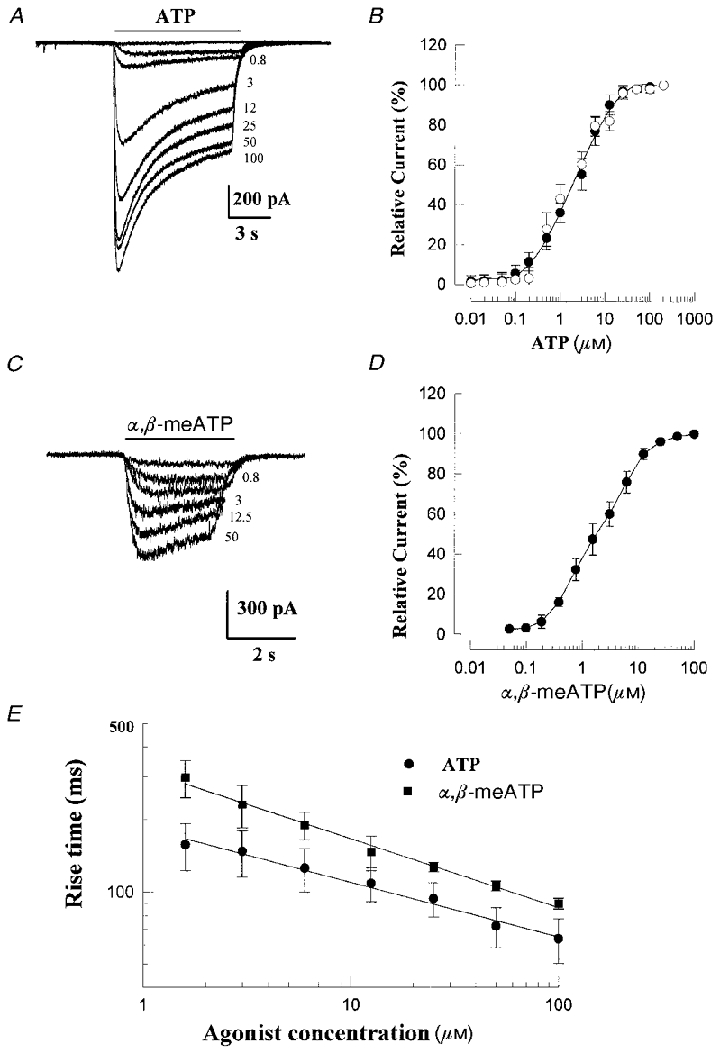
Examples of currents evoked at -60 mV by various concentrations (indicated on right in μM) of ATP and α,β-meATP are shown in A and C; data from two different identified, hypoxia-sensitive neurones in co-culture. Dose-response curves of peak current, relative to that evoked by 100 μM concentration of agonist, for a population of similar hypoxia-sensitive neurones are shown in B for ATP (•; means ±s.e.m., n = 5), and D for α,β-meATP (• means ±s.e.m., n = 4). Smooth curves were fitted to these data points by the Hill equation, with EC50 = 2.7 μM and Hill slope = 0.9 for ATP in B, and EC50 = 2.1 μM and Hill slope = 0.8 for α,β-meATP in D. In B, ○ represent data points from a separate group of neurones (n = 3) sampled randomly from petrosal-alone cultures. A double-logarithmic plot of rise time (measured as time from 10–90 % of peak amplitude; compare Fig. 4 of Khakh et al. 1995) versus agonist concentration is shown in E; note more rapid rise times for ATP (•; means ±s.e.m., n = 7) relative to α,β-meATP (▪; means ±s.e.m., n = 5) at any given concentration.
Figure 6. Reversal potential of ATP-induced currents in an identified hypoxia-sensitive petrosal neurone in co-culture.
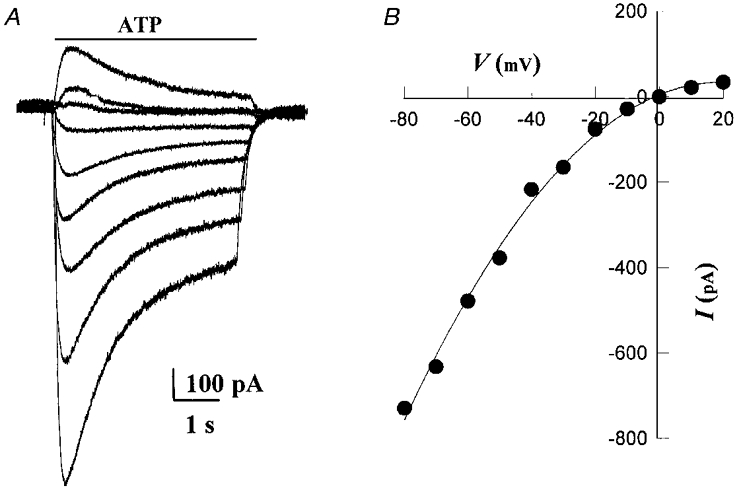
Whole-cell currents, evoked by rapid perfusion of 100 μM ATP over the soma during the period indicated by the upper horizontal bar, are shown in A. The I–V relation for this cell is shown in B; note the prominent inward rectification of the I–V curve and the reversal potential near 0 mV. Current measurements were taken at 2 ms after the beginning of ATP application. The mean reversal potential for a group of 4 identified hypoxia-sensitive neurones in co-culture was 0.33 ± 3.7 mV. The bath contained 1 μM TTX and 5 mM TEA.
The rate of activation of the inward current varied with ATP concentration (Fig. 7a and E), and at higher concentrations the current often showed a rapid transient phase followed by a slower desensitization phase (Figs 5A and 7A). The dose-response curve for the peak current, relative to that evoked by 100 μM ATP, is shown in Fig. 7b for a group of isolated neurones in petrosal-alone cultures (n = 3; open circles), and for identified hypoxia-responsive JPNs in co-culture (n = 5; filled circles). The smooth curve represents a fit of the Hill equation (EC50 = 2.7 μM; Hill coefficient = 0.9) to the data points obtained from the identified hypoxia-responsive JPNs (filled circles; Fig. 7b). The kinetics of onset of the ATP-induced current was determined from exponential fits to the initial phase of the current and from rise time measurements. In hypoxia-responsive JPNs, the activation phase was well described by a single exponential with a mean time constant (±s.e.m.) of activation τon = 30.6 ± 4.8 ms (n = 7; 100 μM ATP). A double logarithmic plot of the rise times (measured as time from 10–90 % peak amplitude) against ATP concentration is shown in Fig. 7E for a group of four hypoxia-responsive JPNs; the kinetics of onset of these ATP-induced currents appears similar to that reported for rat nodose neurones (e.g. Fig. 4 of Khakh et al. 1995). Taken together, these properties are consistent with the presence of P2X2 subunits in chemosensory petrosal neurones, as previously described for other rat peripheral sensory neurones (Lewis et al. 1995; Khakh et al. 1995; Ueno et al. 1999).
Purinoceptors on identified chemosensory neurones are activated by α,β-meATP
Homomeric P2X2 purinoceptors show slow desensitization kinetics and inhibition by suramin (Lewis et al. 1995; Khakh et al. 1995), as do native receptors on the petrosal neurones encountered in this study. However, since α,β-meATP fails to activate homomeric P2X2 purinoceptors, but does activate native receptors on a variety of peripheral sensory neurones (Lewis et al. 1995; Khakh et al. 1995; Ueno et al. 1999), we investigated the effects of α,β-meATP on identified, hypoxia-responsive JPNs in co-culture. As shown in Figs 7C and D, and 8, identified hypoxia-responsive JPNs were sensitive to both ATP and α,β-meATP, applied by fast perfusion over the soma. The mean (±s.e.m.) time constant of activation τon of the α,β-meATP-induced currents was 41.2 ± 5.1 ms (n = 4). The dose-response curve was well fitted by the Hill equation with an EC50 = 2.1 μM, and Hill slope = 0.8 (Fig. 7D). Though the EC50 values for ATP and α,β-meATP were comparable (Fig. 7b and D), τon and rise time at a given agonist concentration appeared faster for ATP (Fig. 7E) on native receptors present on identified chemosensory neurones. The excitatory effects of ATP and α,β-meATP, applied to the soma of a functional JPN in co-culture, are compared with the effect of hypoxia during current clamp recordings in Fig. 8. In this case the excitatory response was robust, resulting in a burst of action potentials during application of each of the three stimuli; in other cases (n = 3), the response was weaker and remained subthreshold for the three stimuli (not shown). Taken together, these properties of the α,β-meATP-activated purinoceptors on ‘functional’ JPNs in co-culture are similar to those reported for other rat peripheral sensory neurones, e.g. nodose (Khakh et al. 1995; Lewis et al. 1995) and dorsal root (Ueno et al. 1999) ganglia. In addition, they appear similar to heteromultimeric purinoceptors assembled from co-expressed P2X2 and P2X3 subunits (Lewis et al. 1995; Ueno et al. 1999).
Figure 8. Comparison of the effects of hypoxia, ATP and α,β-meATP on membrane potential of the same petrosal neurone in co-culture.
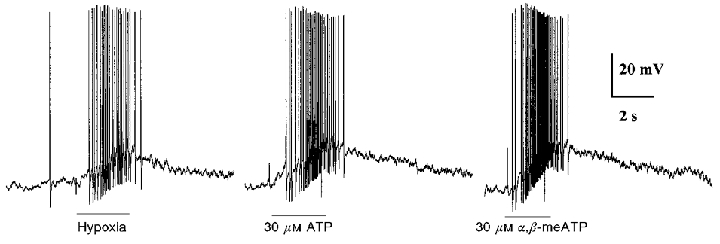
In this example, the interaction between the type 1 cell cluster and juxtaposed PN was strong, resulting in membrane depolarization and a burst of action potentials during application of the hypoxic stimulus (left trace; PO2≈5 mmHg during period indicated by lower horizontal bar). A similar strong excitatory response was elicited in the same neurone during rapid perfusion of ATP (middle trace), and α,β-meATP (right trace) over the soma. The resting potential was -56 mV, and the neurone was in co-culture for 4 days.
Effect of blockers on chemosensory discharge in the isolated rat carotid body-sinus nerve preparation in vitro
It was of interest to determine whether the neurotransmitter mechanisms uncovered in the co-culture preparation were operative in the ‘intact’ rat carotid body in situ. To address this, we recorded the spontaneous and hypoxia-induced extracellular spike discharge from the isolated rat carotid body-sinus nerve preparation in vitro, in the presence and absence of P2X and nicotinic blockers. A detailed, quantitative study of the effects of blockers on the hypoxic response in the sinus nerve was not undertaken, since diffusion of blockers into and out of the synaptic complex is a potential problem with this preparation, and in addition, subthreshold events are not recorded thereby limiting quantification of the efficacy of synaptic transmission. We considered acceptable preparations which showed an obvious increase in sinus nerve discharge during perfusion of the hypoxic stimulus, and those which showed at least partial (>60 %) recovery of the control response after wash-out of the drugs. In three preparations, one of which is illustrated in Fig. 9a, suramin caused a dose-dependent inhibition of the spontaneous sinus nerve discharge; the spike frequency was ∼0.6 Hz before (control), 0.2 and ∼0 Hz following 25 μM and 50 μM suramin, respectively, and 0.4 Hz following wash-out of the drugs. The control baseline frequency was within the range of values previously reported for this preparation (Donnelly & Doyle, 1994). In nine preparations, application of 25–50 μM suramin inhibited the extracellular, hypoxia-induced spike discharge, recorded from one or a few chemosensory units in the attached sinus nerve. An example is shown in Fig. 9Bb where superfusion of a hypoxic solution caused an increase in discharge frequency to ∼6 Hz (control); this response was reduced to ∼0.5 and 0.1 Hz in the presence of 25 μM and 50 μM suramin, respectively, and recovered to ∼4 Hz after wash-out of the drugs. In this same preparation, application of the nicotinic blocker, mecamylamine (1-2 μM) also produced a graded and reversible inhibition of the hypoxia-induced discharge, though the latter diminished over the time course of the experiment (Fig. 9Bb). A similar trend was found in the remaining eight preparations exposed to both suramin and one of the two nicotinic blockers, i.e. mecamylamine (1-2 μM) or hexamethonium (100 μM). In addition, combined application of both suramin (50 μM) and mecamylamine (2 μM) virtually eliminated the hypoxia-induced discharge (not shown). These results raise the possibility that co-release of ACh and ATP may also mediate hypoxic chemotransmission in the intact carotid body.
Figure 9. Effects of receptor blockers on spontaneous and hypoxia-evoked extracellular spike discharge recorded from chemosensory units in the isolated rat carotid body-sinus nerve preparation in vitro.
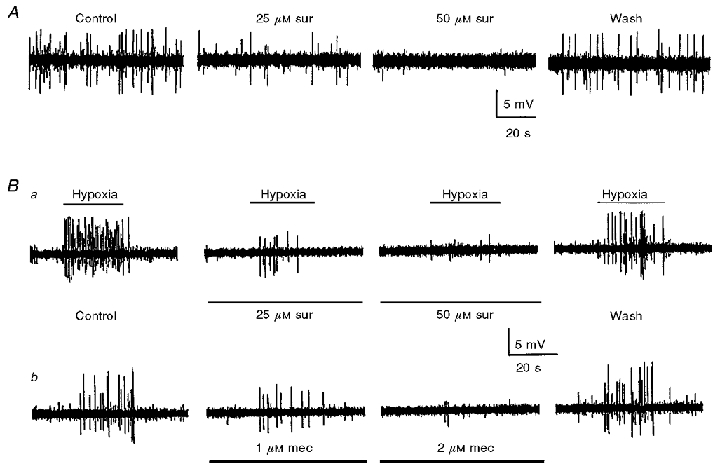
A, one of three examples where the spontaneous discharge in the carotid sinus nerve was (reversibly) inhibited in a dose-dependent manner by suramin; spike frequency was ≈0.6 Hz (control), 0.2 Hz (25 μM suramin), ≈0 Hz (50 μM suramin), and 0.4 Hz (wash). Ba and b, the effects of increasing doses of suramin (25-50 μM) and mecamylamine (1-2 μM) on the hypoxia-evoked extracellular spike discharge in the same preparation. Note that each blocker, acting alone, produced a graded reduction in the frequency of hypoxia-induced discharge and the effect was reversible. The hypoxia-induced spike frequency in Ba was ≈6 Hz (control), 0.5 Hz (25 μM suramin), 0.1 Hz (50 μM suramin), and 4 Hz (wash); in Bb the frequency was ≈4 Hz (control), 0.4 Hz (1 μM mecamylamine), 0.1 Hz (2 μM mecamylamine), and 4 Hz (wash). The combined presence of 50 μM suramin and 2 μM mecamylamine also abolished (reversibly) the hypoxic response in this preparation (not shown). For each row of traces in Ba and b, the hypoxic stimulus (PO2 = 5 mmHg) was applied during the period indicated by the upper horizontal bars.
Immunofluorescence localization of P2X2 subunits in rat petrosal neurones and carotid body
The electrophysiological studies above strongly suggest that ATP, released from type 1 cells and acting on postsynaptic P2X2-containing purinoceptors, is involved in carotid body (CB) chemotransmission. If so, P2X2 subunits should be expressed in chemo-afferent petrosal neurones and most importantly, in CB afferent nerve terminals apposed to type 1 cells in situ. We therefore applied immunofluorescence techniques to localize P2X2 subunits in rat petrosal ganglion and CB using a rabbit polyclonal antiserum raised against a highly purified peptide corresponding to residues 457–472 of the rat P2X2 subunit (see Methods). To confirm cellular and subcellular localization of P2X2 staining, antisera against a number of other neuronal and neuroendocrine markers were used on the same section. These included: (i) a monoclonal antibody against the highly conserved presynaptic marker, i.e. synaptic vesicle antigen (SV2; Buckley & Kelly, 1985); (ii) a cocktail of monoclonal antibodies against neurofilament (68 kDa) and GAP-43, so as to enhance labelling of nerve processes; and (iii) a monoclonal antibody against tyrosine hydroxylase (TH), a known phenotypic marker for both CB chemo-afferent petrosal neurones (Katz & Black, 1986; Finley et al. 1992) and type 1 cells (Gonzalez et al. 1994; Jackson & Nurse, 1995). The majority of neuronal cell bodies in petrosal ganglion stained positively for antisera against SV2 (Fig. 10a), P2X2 (Fig. 10b), and neurofilament (NF; not shown); indeed, many neurones were positive for both SV2 and P2X2 (Fig. 10A–C). The intensity of P2X2 staining was variable from one neurone to another (Fig. 10b and E), suggesting a broad distribution in density of purinoceptors containing P2X2 subunits. Moreover, a substantial proportion of neurones located in the distal region of the petrosal ganglion, and proximal to the exit of the glossopharyngeal nerve, was positive for both TH and P2X2 immunoreactivity (Fig. 10D and E); indeed, many neurones expressing TH immunoreactivity showed the strongest P2X2 staining (e.g. arrows in Fig. 10D and E). Petrosal neurones, strongly positive for P2X2 immunoreactivity, were found in all sections examined including those obtained from 3-week-old animals whose carotid body chemoreceptor response is fully mature (Donnelly & Doyle, 1994). Consistent with our electrophysiological data, almost all petrosal neurones in culture were positive for P2X2 immunoreactivity (e.g. Fig. 10F)
Figure 10. Immunofluorescence localization of P2X2 subunits and neuronal/neuroendocrine markers in petrosal ganglia and carotid body in situ and dispersed cells in vitro.
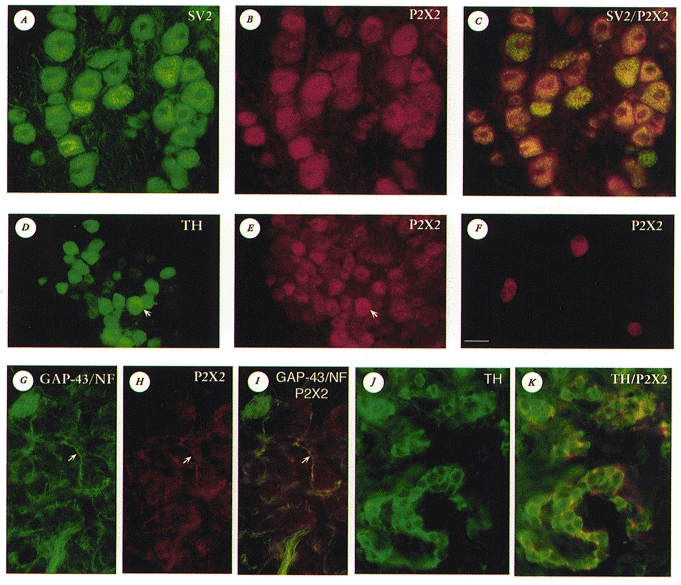
A-C, the same microscopic field from a tissue section of rat petrosal ganglion after immunostaining for SV2 (A; FITC fluorescence) and P2X2 subunits (B; Cy3 fluorescence); in C, dual exposure shows coincident staining for SV2 and P2X2 (yellow-orange fluorescence) in the majority of neurones. D and E, the same microscopic field from a tissue section of rat petrosal ganglion (near exit of the glossopharyngeal nerve) after immunostaining for TH (D; FITC fluorescence) and P2X2 subunits (E; Cy3 fluorescence). Note that many TH-positive neurones, the majority of which innervate carotid body type 1 cells, showed strong P2X2 immunoreactivity, e.g. neurone indicated by arrow in D and E; also, several P2X2-positive neurones were TH-negative (e.g. neurones in top right corner of E). F, petrosal neurones from a 4-day-old culture stained positive for P2X2 immunoreactivity; background cells, not visible in this dark field micrograph, are unstained. G–I, the same microscopic field from a tissue section of rat carotid body after immunostaining for NF/GAP-43 (G; FITC fluorescence) and P2X2 subunits (H; Cy3 fluorescence); note prominent nerve fibre staining for both NF/GAP-43 and P2X2 (e.g. arrows in G–I). Coincident staining is revealed in I by the occurrence of yellow fluorescence. J and K, the same microscopic field from a tissue section of rat carotid body after immunostaining for TH (J; Alexa 488 fluorescence) and P2X2 subunits (Cy3 fluorescence); dual exposure reveals both Alexa 488 and Cy3 fluorescence (K). In K, strong P2X2-positive nerve terminals (red) are intimately associated with TH-positive type 1 cells (green). Note type 1 cells are negative for P2X2 in K. The calibration bar shown in F (lower left) represents: 25 μm in A-C, F, J and K; 30 μm in D, E and G–I.
In the CB, nerve processes were labelled with a cocktail of monoclonal antibodies directed against 68 kDa NF and GAP-43 (Fig. 10G), and interestingly, this labelling pattern showed substantial overlap with that of P2X2 immunoreactivity (Fig. 10H and I). Furthermore, P2X2-positive processes and terminals (Fig. 10H, I and K) were found in close apposition to clusters of CB type 1 cells, which were immunopositive for TH (Fig. 10J and K) and SV2 (not shown). Type 1 cells were, however, immunonegative for P2X2 subunits and NF/GAP-43 (Fig. 10G, H, I and K; see also Jackson & Nurse, 1995). In control experiments, omission of the primary antiserum resulted in no positive staining above background; in addition, pre-incubation of the P2X2 antiserum with excess peptide (P2X2457-472; see Methods) led to the abolition of P2X2 staining (not shown). These data are consistent with the proposal that both in situ and in vitro, ATP released from type 1 cells can act on afferent terminals expressing purinoceptors that contain P2X2 subunits.
DISCUSSION
This study provides the first demonstration that co-release of ATP and ACh from type 1 chemoreceptor cells is largely responsible for hypoxic signalling in the rat carotid body. The main conclusion was based on the observation that in co-culture, as well as in the intact rat carotid body-sinus nerve preparation in vitro, combined application of a P2 purinceptor and a nicotinic blocker was necessary to inhibit most or all of the spontaneous and hypoxia-induced postsynaptic responses. Though release of ACh has long been considered, and sometimes rejected, as the basis of carotid body chemotransmission (Gonzalez et al. 1994; Fitzgerald et al. 1997; Zhong et al. 1997; Nurse & Zhang, 1999), the possible participation of ATP has received less attention. Nevertheless, the excitatory effects of ATP on carotid body chemosensory fibres have been recognized for some time, based on sinus nerve recordings during intra-carotid injections of ATP (Jarish et al. 1952; Spergel & Lahiri, 1993). More recently, it was concluded that P2X purinoreceptors were present in the rat carotid body, based on the rapid onset of chemo-excitation in response to P2X agonists (McQueen et al. 1998). In concert with these findings, we found that many isolated petrosal neurones were strongly excited by ATP, and that the effects were inhibited by the P2 purinoceptor blocker suramin. However, in contrast to our electrophysiological results which were based on cells obtained from 7- to 14-day-old rats, McQueen et al. (1998) found that the purinoceptors in the carotid body of the anaesthetized adult rat were insensitive to suramin. At present it is unclear whether this difference is dose or age related, though our immunocytochemical studies (see below) revealed expression of P2X2 purinoceptor subunits in rat petrosal neurones in situ at 3 weeks of age, when the CB chemo-afferent response is fully mature (Donnelly & Doyle, 1994).
The demonstration that co-release of ATP and ACh mediates carotid body O2 chemotransmission provides a satisfactory solution to the long-standing problem posed by the ‘cholinergic hypothesis’ of chemoreception, i.e. the inability of cholinergic blockers to abolish completely the hypoxia-induced chemosensory discharge (Gonzalez et al. 1994; Fitzgerald et al. 1997). ATP is frequently co-released with ACh at cholinergic synapses (Schweitzer, 1987; Bean, 1992; Zimmerman, 1994; Silinsky & Redman, 1996), and within carotid body type 1 cells the cholinergic storage vesicles provide one of several potential sources of synaptically released ATP. We recently reported that rat type 1 cell clusters are immunopositive for vesicular ACh transporter (VAChT), a cholinergic vesicle marker that shares a common gene locus with the biosynthetic enzyme, choline acetyltransferase (Nurse & Zhang, 1999). Additional sources of ATP in type 1 cells include the mono-aminergic dense cored vesicles (Zimmerman, 1994), which release catecholamines (especially dopamine) and probably 5-HT during hypoxia (Gonzalez et al. 1994). Indeed, given the rapid excitatory response of ATP when applied to petrosal somas and their terminals (see Fig. 4), it is plausible that regardless of the physiological effects of dopamine itself, secretion from dopaminergic vesicles could cause an initial rapid excitation due to co-released ATP, even though the latter is present in lower amounts (Zimmerman, 1994). Thus, ATP may well be the dominant player in hypoxic chemotransmission across species, where there appears to be broad variability in content and proportion of the classical transmitters within type 1 cells (Gonzalez et al. 1994). In the present study, the ATP component accounted for ∼55 % of the hypoxia-induced depolarization in petrosal neurones, and the corresponding P2 purinoreceptors were present on the soma of all neurones tested and apparently their terminals as well. The fact that the isolated, but intact, rat carotid body-sinus nerve preparation behaved qualitatively similarly to the co-culture preparation, with respect to sensitivity of the hypoxic response to the same cholinergic and purinoceptor blockers, suggests that our main conclusions were not biased by the foreign culture conditions.
A significant finding in these co-cultures was that simultaneous blockade of nicotinic receptors and P2 purinoceptors resulted in complete inhibition of the hypoxia-induced depolarization in ∼68 % (13/19) of the petrosal neurone/type 1 cell complexes. It is presently unclear whether the small residual response (10-20 % of initial control) in the unblocked preparations was due to insufficiently high drug concentrations, or whether a third co-transmitter (or other process, e.g. electrical transmission) was involved. In this regard, our previous studies on transmitter sensitivities of isolated rat petrosal somas revealed that ∼68 % of the neurones expressed nicotinic receptors (Zhong & Nurse, 1997), and ∼43 % expressed MDL72222-sensitive 5-HT3 receptors (Zhong et al. 1999). Since 5-HT is another transmitter expressed in type 1 cells, it is plausible that in some cases 5-HT may contribute slightly to hypoxic chemotransmission. Indeed, we have identified a few petrosal neurones which expressed receptors for all three neurotransmitters ACh, ATP and 5-HT (Nurse & Zhang, 1999). Thus, the particular response in any one chemosensory fibre will ultimately depend on the spectrum of receptors expressed on their terminals that are in direct synaptic contact with type 1 cells.
Identified chemosensory petrosal neurones express P2X2 subunits
The demonstration that ATP is a co-transmitter in CB chemosensory signalling led to an investigation of the subtype(s) of purinoceptors mediating the response. A combination of electrophysiological, pharmacological and immunohistochemical studies indicated that these purinoceptors contained P2X2 subunits. For example, the ATP-induced currents in identified hypoxia-responsive neurones in co-culture showed slow desensitization kinetics, reversed near 0 mV, and were inhibited by suramin (IC50∼73 μM). These properties appear similar to those described in P2X2-expressing nodose neurones (Khakh et al. 1995; Lewis et al. 1995), which share a similar developmental origin with petrosal neurones. Further, for native receptors on petrosal neurones the EC50 for ATP was ∼2.7 μM, Hill coefficient ∼1, and time constant of activation τon was ∼30 ms, values similar to those reported for rat nodose neurones (EC50∼3 μM, Hill coefficient ∼1; Khakh et al. 1995, and τon∼24 ms; Lewis et al. 1995).
Immunofluorescence studies confirmed the presence of P2X2 subunits on the majority of petrosal neurones in culture, and in tissue sections of the petrosal ganglia. Interestingly, in these sections most of the tyrosine hydroxylase (TH)-positive neurones, which comprise mainly the CB chemo-afferent population in the distal region of the ganglion (Katz & Black, 1986; Finley et al. 1992), displayed strong P2X2 immunoreactivity. In concert with this staining pattern, peripheral chemo-afferent nerve processes and terminals apposed to CB type 1 cell clusters also expressed strong P2X2 immunoreactivity. This P2X2 labelling in the CB coincided with that of nerve fibre markers, i.e. neurofilament/GAP-43, and was routinely observed in nerve terminals that formed an intimate association with TH-positive type 1 cells. Since the central terminations of the nodose/petrosal complex are known to express P2X2 purinoceptor subunits in situ, as detected by immunocytochemistry (Vulchanova et al. 1996), it appears there is a bidirectional transport of P2X2 receptors from the soma. A highly conserved synaptic vesicle marker SV2, that is expressed in a variety of neurones and neuroendocrine cells (Buckley & Kelly, 1985), was also found to be expressed in most (if not all) petrosal neurones, as well as in type 1 cells (not shown).
Despite the strong evidence presented above that the purinoceptors expressed by chemo-afferent petrosal neurones contain P2X2 subunits, they are likely to be heteromultimeric receptors. This conclusion is based on the finding that α,β-meATP was a potent agonist (EC50∼2.1 μM) for native receptors on identified hypoxia-responsive neurones in co-culture, but is known to have little or no agonist function at homomeric P2X2 receptors (Khakh et al. 1995; Lewis et al. 1995). Indeed, the combined kinetic and pharmacological profile of the receptors on identified chemosensitive neurones in co-culture suggests the native receptor is a heteromultimer of P2X2 and P2X3 subunits, as reported for rat nodose (Lewis et al. 1995) and medium-sized dorsal root ganglion (Ueno et al. 1999) neurones. It will be of interest to determine whether P2X3 subunits (as well as specific nicotinic ACh receptor subunits) are similarly co-localized to petrosal chemo-afferent neurones and their terminals in situ.
In conclusion, it remains to be determined whether co-release of ATP and ACh plays a similar dominant role in mediating hypoxic signalling in the carotid body of other species. Our data do not rule out the involvement of other carotid body transmitters in the hypoxic response, though it would appear that such contributions are likely to be mediated mainly via neuromodulation of the chemosensory discharge. With respect to the initiation of fast excitatory postsynaptic responses during hypoxia, the classical transmitter (e.g. ACh) may well vary from one species to another, but ATP, present in apparently all synaptic vesicles (Zimmerman, 1994), is likely to remain a common co-transmitter in all cases. The special properties of purinoceptors containing P2X2 subunits are well suited to carotid body physiology. These include slow desensitization and the novel property of P2X channel ‘pore dilation’ during prolonged exposure (> 10 s) to agonist (Virginio et al. 1999). In rat chemo-afferent neurones, these properties may help compensate for the rapidly desensitizing responses during nicotinic AChR stimulation (Zhong & Nurse, 1997), and ensure continued robust signalling during periods of sustained hypoxia.
Acknowledgments
We thank Dr Ellis Cooper and Dr Stephen Sims for insightful comments and suggestions. In addition we thank Evi Pertens and Mike Holmes for assistance with the perfusion, and Robert Tatlock (Audio Visual Services) for assistance in preparation of Fig. 10. The SV2 monoclonal antibody, developed by K. Buckley and R. Kelly (1985), was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by the University of Iowa, Department of Biological Sciences, Iowa City, IA 52242, USA. This work was supported by an operating grant from the Medical Research Council of Canada (MOP 12037), and an equipment grant from the Natural Sciences and Engineering Research Council of Canada (NSERC).
References
- Bean BP. Pharmacology and electrophysiology of ATP-activated ion channels. Trends in Pharmacological Science. 1992;s13:87–90. doi: 10.1016/0165-6147(92)90032-2. [DOI] [PubMed] [Google Scholar]
- Buckley KM, Kelly RB. Identification of a transmembrane glycoprotein specific for secretory vesicles of neural and endocrine cells. Journal of Cell Biology. 1985;100:1284–1294. doi: 10.1083/jcb.100.4.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collo G, North RA, Kawashima E, Merlo-Pich E, Neidhart S, Surprenant A, Buell G. Cloning of P2X5 and P2X6 receptors and the distribution and properties of an extended family of ATP-gated ion channels. Journal of Neuroscience. 1996;16:2495–2507. doi: 10.1523/JNEUROSCI.16-08-02495.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly DF. Chemoreceptor nerve excitation may not be proportional to catecholamine secretion. Journal of Applied Physiology. 1996;81:657–664. doi: 10.1152/jappl.1996.81.2.657. [DOI] [PubMed] [Google Scholar]
- Donnelly DF, Doyle TP. Developmental changes in hypoxia-induced catecholamine release from rat carotid body, in vitro. The Journal of Physiology. 1994;475:267–275. doi: 10.1113/jphysiol.1994.sp020067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn PM, Blakely AGH. Suramin: a reversible P2-purinoceptor antagonist in the mouse vas deferens. British Journal of Pharmacology. 1988;93:243–245. doi: 10.1111/j.1476-5381.1988.tb11427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans RJ, Derkach V, Surprenant A. ATP mediates fast synaptic transmission in mammalian neurons. Nature. 1992;357:503–505. doi: 10.1038/357503a0. [DOI] [PubMed] [Google Scholar]
- Finley JCW, Polak J, Katz DM. Transmitter diversity in carotid body afferent neurons: dopaminergic and peptidergic phenotypes. Neuroscience. 1992;51:973–987. doi: 10.1016/0306-4522(92)90534-9. [DOI] [PubMed] [Google Scholar]
- Fitzgerald RS, Shirahata M, Ide T. Further cholinergic aspects of carotid body chemotransduction of hypoxia in cats. Journal of Applied Physiology. 1997;82:819–827. doi: 10.1152/jappl.1997.82.3.819. [DOI] [PubMed] [Google Scholar]
- Gonzalez C, Almaraz L, Obeso A, Rigual R. Carotid body chemoreceptors: from natural stimuli to sensory discharges. Physiologial Reviews. 1994;74:829–898. doi: 10.1152/physrev.1994.74.4.829. [DOI] [PubMed] [Google Scholar]
- Jackson A, Nurse CA. Plasticity in cultured carotid body chemoreceptors: environmental modulation of GAP-43 and neurofilament. Journal of Neurobiology. 1995;26:485–496. doi: 10.1002/neu.480260403. [DOI] [PubMed] [Google Scholar]
- Jarisch A, Landgren S, Neil E, Zotterman G. Impulse activity in the carotid sinus nerve following intracarotid injection of potassium chloride, veratrine, sodium citrate, adenosine triphosphate and α-dinitrophenol. Acta Physiologica Scandinavica. 1952;25:195–211. doi: 10.1111/j.1748-1716.1952.tb00872.x. [DOI] [PubMed] [Google Scholar]
- Lewis C, Neldhart S, Holy C, North RA, Buell G, Surprenant A. Coexpression of P2X2 and P2X3 receptor subunits can account for ATP-gated currents in sensory neurons. Nature. 1995;377:432–435. doi: 10.1038/377432a0. [DOI] [PubMed] [Google Scholar]
- Katz DM, Black IB. Expression and regulation of catecholaminergic traits in primary sensory neurons; relationship to target innervation in vivo. Journal of Neuroscience. 1986;6:983–989. doi: 10.1523/JNEUROSCI.06-04-00983.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakh BS, Humphrey PPA, Surprenant A. Electrophysiological properties of P2x purinoceptors in rat superior cervical, nodose and guinea-pig coeliac neurones. The Journal of Physiology. 1995;484:385–395. doi: 10.1113/jphysiol.1995.sp020672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQueen DS, Bond SM, Moores C, Chessell I, Humphrey PPA, Dowd E. Activation of P2X receptors for adenosine triphosphate evokes cardiorespiratory reflexes in anaesthetized rats. The Journal of Physiology. 1998;507:843–855. doi: 10.1111/j.1469-7793.1998.843bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurse CA, Zhang M. Acetylcholine contributes to hypoxic chemotransmission in co-cultures of rat type 1 cells and petrosal neurons. Respiration Physiology. 1999;115:189–199. doi: 10.1016/s0034-5687(99)00017-1. [DOI] [PubMed] [Google Scholar]
- Pepper DR, Landauer RC, Kumar P. Postnatal development of CO2-O2 interaction in the rat carotid body in vitro. The Journal of Physiology. 1995;485:531–541. doi: 10.1113/jphysiol.1995.sp020749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweitzer E. Coordinated release of ATP and ACh from cholinergic synaptosomes and its inhibition by calmodulin antagonists. Journal of Neuroscience. 1987;7:2948–2956. doi: 10.1523/JNEUROSCI.07-09-02948.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silinsky EM, Redman RS. Synchronous release of ATP and neurotransmitter within milliseconds of a motor nerve impulse in the frog. The Journal of Physiology. 1996;492:815–822. doi: 10.1113/jphysiol.1996.sp021348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spergel D, Lahiri S. Differential modulation by extracellular ATP of carotid chemosensory responses. Journal of Applied Physiology. 1993;74:3052–3056. doi: 10.1152/jappl.1993.74.6.3052. [DOI] [PubMed] [Google Scholar]
- Surprenant A, Buell G, North RA. P2X receptors bring new structure to ligand-gated ion channels. Trends in Neurosciences. 1995;18:224–229. doi: 10.1016/0166-2236(95)93907-f. [DOI] [PubMed] [Google Scholar]
- Stea A, Nurse CA. Whole-cell currents in two sub-populations of cultured rat petrosal neurons with different tetrodotoxin sensitivities. Neuroscience. 1992;47:727–736. doi: 10.1016/0306-4522(92)90180-a. [DOI] [PubMed] [Google Scholar]
- Ueno S, Tsuda M, Iwanaga T, Inoue K. Cell type-specific ATP-activated responses in rat dorsal root ganglion neurons. British Journal of Pharmacology. 1999;126:429–436. doi: 10.1038/sj.bjp.0702319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virginio C, MacKenzie A, Rassendren FA, North RA, Surprenant A. Pore dilation of neuronal P2X receptor channels. Nature Neuroscience. 1999;2:315–321. doi: 10.1038/7225. [DOI] [PubMed] [Google Scholar]
- Vulchanova L, Arvidsson U, Riedl M, Wang J, Buell G, Surprenant A, North RA, Elde R. Differential distribution of two ATP-gated ion channels (P2x receptors) determined by immmunocytochemistry. Proceedings of the National Academy of Sciences of the USA. 1996;93:8063–8067. doi: 10.1073/pnas.93.15.8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildman SS, King BF, Burnstock G. Potentiation of ATP-responses at a recombinant P2X2 receptor by neurotransmitters and related substances. British Journal of Pharmacology. 1997;120:221–224. doi: 10.1038/sj.bjp.0700903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong H, Nurse CA. Nicotinic acetylcholine sensitivity of rat petrosal sensory neurons in dissociated cell culture. Brain Research. 1997;766:153–161. doi: 10.1016/s0006-8993(97)00526-x. [DOI] [PubMed] [Google Scholar]
- Zhong H, Zhang M, Nurse CA. Synapse formation and hypoxic signalling in co-cultures of rat petrosal neurones and carotid body type 1 cells. The Journal of Physiology. 1997;503:599–612. doi: 10.1111/j.1469-7793.1997.599bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong H, Zhang M, Nurse CA. Electrophysiological characterization of 5-HT receptors on rat petrosal neurons in dissociated cell culture. Brain Research. 1999;816:544–553. doi: 10.1016/s0006-8993(98)01232-3. [DOI] [PubMed] [Google Scholar]
- Zimmerman H. Signalling via ATP in the nervous system. Trends in Neurosciences. 1994;17:420–426. doi: 10.1016/0166-2236(94)90016-7. [DOI] [PubMed] [Google Scholar]


