Abstract
Within a single neuron the correct targeting of the diverse neurotransmitter receptor types to discrete synaptic regions is crucial for proper function. However, the molecular mechanisms that underlie neuronal receptor clustering and targeting are still largely undefined. Here we report advances in defining the mechanisms that mediate nicotinic acetylcholine receptor (nAChR) targeting to interneuronal synapses. Recent in vivo studies have demonstrated that one subunit plays a critical role in the differentiation of nicotinic cholinergic synapses on vertebrate autonomic neurons. The major cytoplasmic loop of the α3 subunit targets specific nAChR subtypes to the synapse. In contrast, nAChR complexes that lack the α3 targeting domain are excluded and are perisynaptic. Additional studies have demonstrated a greater complexity to α3-nAChR targeting due to a unique postsynaptic receptor microheterogeneity – under one presynaptic terminal, α3-nAChR clusters are separate, but proximal to, glycine receptor (GlyR) clusters in discrete postsynaptic membrane microregions. The surprising coexistence under one nerve ending of separate clusters of receptors that respond to different fast-acting transmitters with opposing functions may represent a novel mechanism for modulating synaptic activity. Overall, the receptor targeting and clustering studies reviewed in this issue suggest that a common mechanism underlies the formation of the diverse types of interneuronal synapses but differs from that responsible for neuromuscular junction assembly in vertebrates.
nAChRs mediate fast excitatory synaptic transmission in all vertebrate peripheral nervous system (PNS) neurons. In the central nervous system (CNS), nAChRs in the postsynaptic membrane of interneuronal synapses function to reinforce nicotine addiction, increase attention, and facilitate learning and memory (reviewed by Dani & Heinemann, 1996). nAChRs on the presynaptic terminal modulate neurotransmitter release (Coggan et al. 1997; Girod et al. 1999; Radcliffe et al. 1999). Abnormalities in nAChR number and distribution are associated with several neurological disorders such as familial frontal lobe epilepsy, Alzheimer's disease and schizophrenia (Lena & Changeaux, 1997; Perry et al. 1999). Despite the important physiological role of neuronal cholinergic receptors, the molecular interactions that mediate their targeting to discrete synaptic regions remain largely undefined. This review discusses recent studies that provide novel mechanistic insights into nicotinic cholinergic synapse assembly on neurons in vivo. This review also describes a novel postsynaptic receptor microheterogeneity on autonomic neurons – excitatory receptor clusters and separate inhibitory receptor clusters coexist in discrete membrane microregions under one presynaptic terminal.
Diversity of nAChR subtypes
A great diversity of nAChRs is expressed in the vertebrate nervous system. To date, sixteen nAChR subunit genes have been cloned from vertebrates (Lukas et al. 1999). This large multigene family encodes both neuronal and skeletal muscle nAChRs. The two receptor types are composed of homologous but distinct subunits. In the CNS and PNS, different nicotinic cholinergic neuron populations express particular combinations of nAChR subunit genes (Sargent, 1993; McGehee & Role, 1995). Within one neuron population, the subunits segregate and assemble into two distinct classes of neuronal nAChRs that differ in subunit composition, spatial distribution and functional properties.
One class of neuronal nAChRs is a pentameric complex that has the general composition of two α-type (ligand-binding) and three β-type subunits. The second class of neuronal nAChRs, distinguished by their sensitivity to α-bungarotoxin (α-Bgt), is composed of distinct α-type subunits that may function as a homopentamer (Vernallis et al. 1993; Conroy & Berg, 1995; Chen & Patrick, 1997; Drisdel & Green, 2000). There is great interest in defining the receptor subtypes that are expressed in specific cholinergic neuron populations as the functional properties of nAChRs are exquisitely sensitive to subunit composition (Sargent, 1993; McGehee & Role, 1995). Moreover, in autonomic neurons, it has been demonstrated that the different nAChR subtypes are spatially segregated from one another in discrete membrane microregions relative to synapses (Jacob & Berg, 1983; Loring et al. 1985; Jacob et al. 1986; Loring & Zigmond, 1987; Horch & Sargent, 1995; Shoop et al. 1999). The spatial segregation of nAChR subtypes on single neurons is likely to create functionally specialized synapse-associated microregions and to be essential for proper function (Moss & Role, 1993; Ullian et al. 1997; Chang & Berg, 1999).
Chick parasympathetic ciliary ganglion as a model system
The chick ciliary ganglion (CG) is uniquely well suited for the in vivo study of nicotinic cholinergic synapse formation. Extensively investigated, the CG is a relatively simple preparation that contains only two neuron types, which both receive nicotinic cholinergic innervation from a sole source of inputs, the accessory oculomotor nucleus of the midbrain (Martin & Pilar, 1963; Landmesser & Pilar, 1972). nAChRs mediate excitatory ganglionic transmission. In particular, individual CG neurons express two nAChR subtypes. One subtype, the α3-nAChR, consists of α3, α5 and β4 (occasionally β2) subunits, and these heteropentamers localize predominantly to the specialized postsynaptic membrane (Jacob et al. 1986; Loring & Zigmond, 1987; Vernallis et al. 1993). The second subtype, the α-Bgt-sensitive α7-nAChR, may be homopentamers of the α7 subunit (Vernallis et al. 1993; Conroy & Berg, 1995). α7-nAChRs are excluded from the postsynaptic membrane and restricted to the perisynaptic dendritic surface membrane (Jacob & Berg, 1983; Loring et al. 1985; Horch & Sargent, 1995; Shoop et al. 1999). Presynaptically released acetylcholine activates both nAChR subtypes, but their functional properties differ. α7-nAChRs show greater calcium permeability, and faster activation and desensitization kinetics as compared to α3-nAChRs (Ullian et al. 1997; Chang & Berg, 1999). These differences, in conjunction with the spatial segregation, create functionally specialized synapse-associated microregions on individual CG neurons.
In vivo targeting of nAChR subtypes to the interneuronal synapse
In a recent study we tested the hypothesis that particular domains of the receptor subunits specify their in vivo localization (Williams et al. 1998). The individual subunits of the α3-nAChR and α7-nAChR complexes are highly homologous to one another and have a stereotyped structure consisting of extracellular amino (N)- and carboxy (C)-termini, four transmembrane (TM) domains, and a long intracellular loop between TM3 and TM4 (Lindstrom et al. 1996). The long cytoplasmic loop shows the greatest divergence in sequence and length between individual subunits. The study focused on the role of the long cytoplasmic loop in receptor targeting to the synapse. The approach taken was to generate chimeric subunits, express them in CG neurons during synapse formation in situ and establish their distribution relative to synapses.
Using PCR, myc-tagged chimeric subunits were constructed in which the long cytoplasmic loop of the α7 subunit immediately after TM3 and before TM4 was replaced with the homologous region of α3, α5 or β4 to determine whether the sequence substituted in could retarget perisynaptic α7 to the synapse (Fig. 1). A synaptic localization of the exogenous epitope-tagged α7 subunit could not be due to assembly with endogenous receptor subunits that target to the synapse – intersubunit assembly into receptor complexes is regulated by the N-terminus up to TM2, and α7 does not coassemble with α3, α5 or β4 subunits (Eisele et al. 1993; Vernallis et al. 1993; Conroy & Berg, 1995; Vicente-Agullo et al. 1996). This segregation has been demonstrated both for native nAChR subunits in CG neurons and for chimeric α7 with the substituted cytoplasmic loop when coexpressed with wild-type α3 and β4 subunits in reconstitution studies in Xenopus laevis oocytes (Vernallis et al. 1993; Williams et al. 1998). Thus, any change in the localization of chimeric α7 relative to endogenous α7 must be due to the substituted cytoplasmic loop sequence. In addition, the heterologous expression studies in Xenopus oocytes demonstrated that the chimeric α7 subunits form functional nAChR channels with apparently normal gating kinetics as measured by two-electrode voltage clamp (Williams et al. 1998).
Figure 1. Chimeric nAChR subunit targeting to synapses on CG neurons developing in vivo.
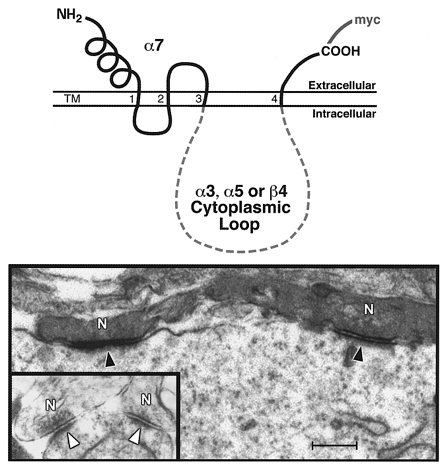
Top, schematic representation of the structure of the chimeric nAChR subunits that were overexpressed in chick CG neurons during synapse formation in vivo. Using PCR, chimeric subunits were constructed in which the large cytoplasmic loop of α7 was replaced with the corresponding region of α3, α5 or β4. Bottom, electron micrographs demonstrating that the α7/α3 chimeric subunit preferentially localizes to postsynaptic membrane microregions, whereas the α7/α5 chimera (inset) does not (filled arrowhead, labelled synapse; open arrowhead, unlabelled synapse; N, presynaptic nerve terminal). Immunolabelling for the myc-epitope tag (and visualization with horseradish peroxidase) was used to determine exogenous subunit localization relative to synapses. Scale bar, 0.7 μm. (Adapted from Figs. 1a, and 2a and f in Williams et al. 1998.)
Myc-tagged chimeric α7 subunits were expressed in CG neurons during synapse formation in vivo using avian-specific replication-competent retroviral vectors (RCASBP). RCASBP stably integrates into the genome of dividing cells and overexpresses the inserted gene in the progeny throughout development (Homburger & Fekete, 1996; Morgan & Fekete, 1996; Federspiel & Hughes, 1998). The retroviral vector constructs were injected into the mesencephalic neural tube of chick embryos in ovo at developmental stage 9–10 – determined to be the optimal time and site of injection to infect the neural crest precursor cells that give rise to CG neurons (Williams et al. 1998). After infection the embryos were allowed to develop in situ until all of the CG neurons were functionally innervated (stages 30-36), at which time the localization of the chimeric subunits relative to synapses was determined by immunolabelling for the myc-epitope tag and ultrastructural analyses.
In neurons infected with the α7/α3-loop chimera, the exogenous subunit was targeted to the synapse (Figs 1 and 2). The myc immunolabelling was concentrated in the specialized postsynaptic membrane regions that were demarcated by the postsynaptic density along their cytoplasmic face and lie opposite synaptic vesicle accumulations at active zones in the presynaptic terminal. This result demonstrates that the α3 cytoplasmic loop is sufficient to redirect the localization of perisynaptic α7 to the synapse. In addition to the α3 subunit, α5 and β4 subunits are present in the native nAChR complex in the CG. However, chimeric α7 containing the cytoplasmic loop of α5 or β4 was not targeted to the synapse (Fig. 1). On average, 90 % of the synapses were heavily labelled in α7/α3-expressing neurons. In contrast, only 10 % of synaptic sites showed only small amounts of immunolabel in neurons expressing the α7/α5 or α7/β4 chimeras, or myc-tagged wild-type α7, included as a negative control.
Figure 2. Distribution of endogenous nAChRs and chimeric subunits relative to synapses.
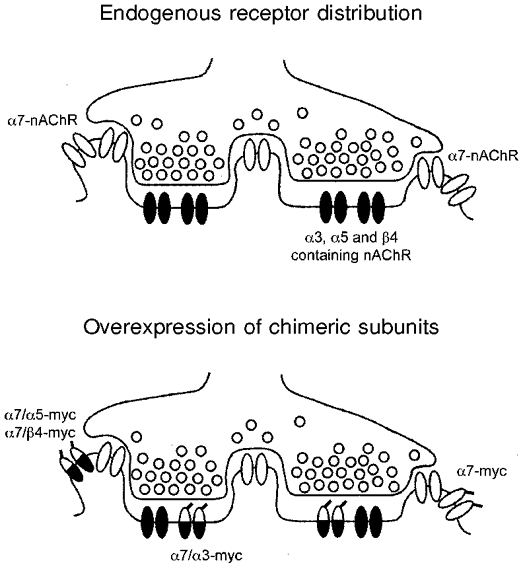
Schematic representation of the ultrastructural distribution of endogenous α3-nAChRs and α7-nAChRs (top) and exogenous chimeric subunits (bottom) in synaptic and perisynaptic membrane regions of control and infected ciliary ganglion neurons in vivo.
All four exogenous subunits were localized perisynaptically on the dendritic surface membrane, but not elsewhere on the soma. However, the amount of perisynaptic myc immunolabelling was lower for α7/α3 as compared to the other exogenous subunits. These findings suggest that the α3 cytoplasmic loop is specifically and preferentially targeted to the interneuronal synapse (Fig. 2). As an important control, the observation that myc-tagged α7 is localized perisynaptically, similar to the endogenous α7-nAChR, shows that neither the epitope tag nor overexpression via the retroviral vector influenced the localization of the receptor subunits. Furthermore, chick embryonic development and CG synapse formation, including the length of the specialized postsynaptic membrane, appeared normal in all infected ganglia as compared to age-matched uninfected ganglionic neurons.
In summary, the major cytoplasmic loop of α3, but not of α5 or β4, targets exogenous α7 chimeric subunits to the synapse in vivo (Fig. 2). These findings suggest that the α3 loop of native subunits may target endogenous nAChR complexes to the synapse during normal development. Endogenous nAChR surface levels were uniquely affected by overexpression of the α7/α3 chimera, as measured by radiolabelled anti-α3-nAChR antibody binding to the surface of acutely dissociated neurons from individual infected ganglia (Williams et al. 1998). Specifically, overexpressed α7/α3 reduced native nAChR surface levels, whereas overexpression of the α7/α5 chimeric subunit did not. These results suggest that the α7/α3 chimera competes with endogenous subunits for localization to and stabilization at the synapse, suggesting that the native nAChR complex also uses the α3 cytoplasmic loop for targeting.
Altogether, our targeting studies demonstrate that the α3 long intracellular loop plays a critical role in targeting nAChRs to the synapse on autonomic neurons in vivo. The essential role of α3, and the lack of functional redundancy despite the presence of other α-type subunits in these neurons, is further supported by the α3-null mouse which shows severe deficits of fast excitatory synaptic transmission in autonomic ganglia (Xu et al. 1999a). The β4-null mouse has a similar phenotype but this nAChR subunit appears to be required for receptor complex assembly and surface expression of the wild-type α3 subunit (Xu et al. 1999b). Moreover, the normal developmental expression pattern of α3 is consistent with this subunit playing a key role in synapse differentiation in vertebrate autonomic neurons. Specifically, α3 mRNA is expressed at a higher level relative to other nAChR subunit mRNAs (α5, β4 and β2) at all stages of synapse formation and maturation in the CG (Corriveau & Berg, 1993; Levey & Jacob, 1996). α3 is a part of all CG nAChR complexes of the Bgt-insensitive type (Vernallis et al. 1993; Conroy & Berg, 1995). These nAChRs are concentrated in the postsynaptic membrane from the earliest stages of synapse formation (Jacob, 1991). In addition, the level of α3 mRNA correlates with the number of functional nAChRs on CG and sympathetic ganglion neurons both in vitro and in vivo (Listerud et al. 1991; Mandelzys et al. 1994; Levey et al. 1995; Xu et al. 1999a). In contrast to α3, the α5 loop does not target to the synapse. Although α5 is necessary for the formation of high-conductance nAChR channels (Ramirez-Latorre et al. 1996; Wang et al. 1996), it appears to be a late component of the α3-nAChR complex in CG neurons. α5 expression is developmentally delayed relative to that of α3, with α5 levels being upregulated during the conversion from low- to high-conductance channels that occurs after the CG neurons are functionally innervated (Margiotta & Gurantz, 1989; Levey et al. 1995). Moreover, α5 requires the presence of an additional α-type and β-type subunit to form functional channels in heterologous expression studies (Ramirez-Latorre et al. 1996; Wang et al. 1996).
Taken together, these data demonstrate that the intracellular domain of one particular subunit, α3, mediates the targeting of specific nAChR subtypes to the synapse. Thus, α3 plays a unique and essential role in the differentiation of nicotinic cholinergic synapses on autonomic neurons in vivo.
Postsynaptic receptor microheterogeneity
New data indicating that not all postsynaptic membrane microregions contain nAChRs at late embryonic and adult stages suggest that α3-mediated targeting to the synapse on CG neurons may be more complex than originally thought (Tsen et al. 2000). A clue as to the possible significance of synapses lacking α3-nAChRs comes from the demonstration that chick CG neurons also express functional glycine receptors (GlyRs) at late embryonic stages (Zhang & Berg, 1995). The spatial distribution and functional significance of GlyR expression on these cholinoceptive neurons is particularly interesting as the presynaptic terminals are cholinergic (Reiner et al. 1991). Moreover, the ciliary neurons are innervated by only one presynaptic terminal – a large calyx-type ending (De Lorenzo, 1960; Hess, 1965; Landmesser & Pilar, 1972). Multiple distinct synaptic microregions are present in the zone of apposition between the calyx and the postsynaptic neuron. Recent work in our laboratory has demonstrated a novel postsynaptic receptor heterogeneity – under one presynaptic calyx terminal, nAChR clusters and separate, but proximal, GlyR clusters are in discrete membrane microregions on ciliary neurons in vivo (Tsen et al. 2000). The coexistence of these two receptor types under one ending is surprising as they respond to different fast-acting transmitters that generally have opposing actions. Moreover, these data contradict the current interpretation of Dale's principle, which holds that all active zones derived from one axon release the same combination of neurotransmitters while all the underlying postsynaptic membranes on one neuron contain the same receptor types (Dale, 1935; Nicoll & Malenka, 1998).
Ultrastructural immunolabelling studies demonstrated that α3-nAChRs and GlyRs are each clustered at only a subset of the specialized postsynaptic membrane microregions under the calyx (Fig. 3; Tsen et al. 2000). Double-label immunofluorescence and confocal microscopy further showed that the two receptors are not colocalized. Gephyrin, a peripheral membrane protein, was used as a positive control for the colocalization studies. Gephyrin colocalizes with GlyRs at synapses and is required for GlyR clustering on rodent CNS neurons (Kirsch et al. 1993; Colin et al. 1998; Feng et al. 1998b). We have established that gephyrin mRNA and protein are expressed in chick CG neurons and that avian and rodent gephyrin coding sequences are highly conserved. At the ultrastructural level, similar to the receptors, gephyrin was localized at only a subset of the postsynaptic membrane microregions under the calyx, where it was associated with the postsynaptic density. By confocal microscopy, gephyrin immunolabelling was seen to colocalize with GlyRs, but not with nAChRs, on CG neurons (Fig. 3). At late embryonic and adult stages, there was an approximately equal proportion of nAChR clusters and GlyR clusters (and its associated protein gephyrin). Importantly, the postsynaptic receptor microheterogeneity is not unique to calyx-type synapses. It is also present on the CG choroid neurons, which receive more typical bouton-type presynaptic terminals (Tsen et al. 2000).
Figure 3. Postsynaptic receptor microheterogeneity on chick CG neurons in vivo.
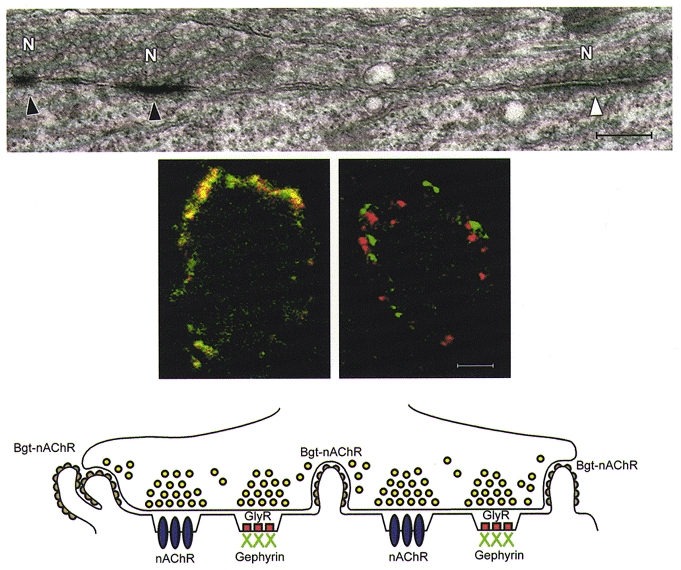
Top, electron micrograph showing that α3-nAChRs are present at only a subset of the postsynaptic membrane microregions under the presynaptic calyx terminal on the ciliary neuron (filled arrowhead, labelled synapse; open arrowhead, unlabelled synapse; N, presynaptic nerve terminal). Scale bar, 1.75 μm. Middle, confocal micrographs demonstrating by immunofluorescence double-labelling that on the surface of individual CG neurons GlyRs colocalize with gephyrin (left panel), whereas nAChRs do not (right panel). Scale bar, 13 μm. Bottom, schematic representation of the ultrastructural distribution of α3-nAChRs (nAChR), α7-nAChRs (Bgt-nAChR), GlyRs and gephyrin relative to one another and to postsynaptic membrane microregions under the presynaptic calyx terminal on the ciliary neuron. (Adapted from Figs. 1a, and 2a and b in Tsen et al. 2000.)
As independent confirmation of the colocalization results, using the yeast two-hybrid genetic assay it was shown that chick GlyRs specifically interact with chick gephyrin. This result was expected due to the presence of a conserved gephyrin binding motif in the GlyR β subunit long cytoplasmic loop (Meyer et al. 1995; Tsen et al. 2000). In contrast, chick nAChR subunits lack the gephyrin binding motif and did not interact with gephyrin in the genetic assay. Altogether, the results suggest that different protein interactions organize α3-nAChR and GlyR clusters in distinct but proximal microregions of the postsynaptic membrane under one presynaptic terminal (Fig. 3). Little is known about the proteins that interact with neuronal nAChRs and may function as nicotinic cholinergic synapse-organizing molecules (Feng et al. 1998a).
Inhibitory glycinergic synaptic transmission in the CG
GlyRs expressed on CG neurons are functional and their activation attenuates acetylcholine-mediated excitatory synaptic activity (Fig. 4; Tsen et al. 2000). Postsynaptic responses were recorded extracellularly after direct electrical stimulation of the preganglionic nerve in acutely isolated intact embryonic ganglia. Bath-applied exogenous glycine reduced the initial slope of the nAChR-mediated excitatory postsynaptic potential (EPSP) and peak-to-peak amplitude of the postganglionic compound action potential, but did not change the preganglionic action potential volley, as indicated by the electrical coupling potential (Fig. 4). The declines were completely reversed following washout of glycine. In comparison, hexamethonium, a nAChR antagonist, also decreased the initial slope of the field EPSP and the compound action potential amplitude. TTX abolished all postsynaptic responses. Thus, it appears that GlyR-mediated inhibitory currents are capable of modulating excitatory synaptic transmission through the CG.
Figure 4. GlyR activation inhibits excitatory synaptic activity in CG neurons.
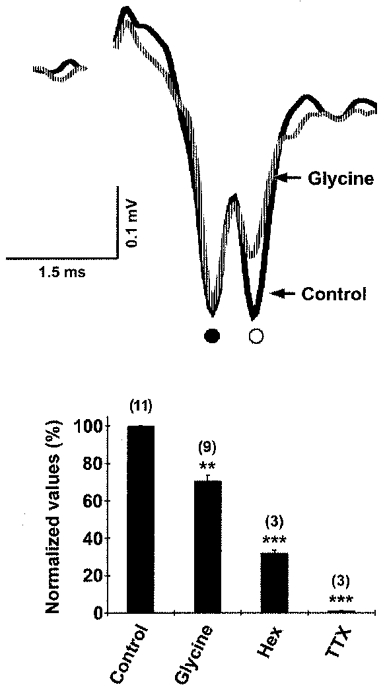
Top, representative traces of postsynaptic responses recorded extracellularly in acutely isolated intact ganglia following direct electrical stimulation of the presynaptic nerve. Bath-applied glycine reduced the initial slope of the nAChR-mediated field EPSP (○), with no effect on the coupling potential (•; due to the direct electrical connection between the presynaptic and postsynaptic cells). Bottom, histogram of EPSP initial slope values expressed as the ratio of test over control (means ±s.e.m.; the number of postsynaptic responses measured is given in parentheses). Similar to glycine, hexamethonium (Hex), a nAChR antagonist, decreased the initial slope of the field EPSP and TTX abolished all postsynaptic responses. ** P < 0.005, *** P < 0.001, Student's t test. (Adapted from Fig. 3b and c in Tsen et al. 2000.)
The functional significance of inhibitory GlyR clusters at these largely cholinergic synapses is determined by the endogenous source of glycine and conditions that lead to its release. One possible endogenous glycine source is the blood, where levels are high in vertebrates (McGale et al. 1977). Glycine transporters (GlyTs) function to take up glycine, but can also operate in reverse to release glycine under certain physiological conditions such as strong depolarization and high intracellular sodium levels (Attwell et al. 1993). Immunofluorescence labelling and uptake studies have revealed that two distinct GlyTs are expressed in the CG and have different distributions (Tsen et al. 2000). GlyT-1 is predominantly present in patches on the presynaptic terminal surface membrane and at lower levels on neuronal cell bodies, but not on Schwann cells. In contrast, GlyT-2 is detected only on Schwann cells.
Autoradiography studies showed that radiolabelled glycine is accumulated predominantly in the presynaptic terminals and Schwann cells of acutely isolated intact embryonic CG (Tsen et al. 2000). Uptake into the presynaptic terminals was dramatically reduced in a dose-dependent manner by sarcosine, a specific GlyT-1 inhibitor, whereas there was no apparent change in Schwann cell label density. In contrast, co-incubation with excess unlabelled glycine blocked all uptake. Depolarization with a high concentration of K+ caused preloaded CGs to release the radiolabelled glycine into the extracellular medium. Release was calcium independent and was blocked by the selective GlyT-1 antagonist sarcosine or by excess unlabelled glycine, consistent with a non-vesicular carrier-mediated efflux. Altogether, these data suggest that GlyT-1 on the cholinergic presynaptic terminal mediates depolarization-induced glycinergic synaptic transmission in the chick CG. We propose that two distinct release mechanisms, vesicle-mediated acetylcholine release and non-vesicular, transport-mediated glycine efflux, both occur at CG synapses in vivo and jointly regulate ganglionic transmission.
Support for this model comes from studies which demonstrate that depolarization causes amino acid transporters to release sufficiently high levels of neurotransmitter on a fast enough time scale to contribute to synaptic transmission (Schwartz, 1987). Strikingly similar to the CG model is the report that retinal starburst amacrine cells co-release ACh and GABA via vesicular and transport-mediated mechanisms, respectively (O'Malley et al. 1992). The two release mechanisms differ in their extracellular Ca2+ requirements and in the range of membrane voltages over which they are active (Schwartz, 1987; O'Malley et al. 1992; Attwell et al. 1993). These differences suggest that in the CG there are likely to be dynamic shifts in the release of fast-acting transmitters with opposing actions from single terminals.
The coexistence of separate clusters of excitatory receptors and inhibitory receptors under one presynaptic terminal represents an unexpected degree of complexity and diversity of functionally specialized synaptic microregions on CG neurons. Postsynaptic receptor microheterogeneity may be a widespread phenomenon with relevance to the mammalian CNS as well, as suggested by the recent demonstration that single dorsal horn neurons co-release fast-acting excitatory and inhibitory transmitters, possibly at the same synapse, in vitro (Jo & Schlichter, 1999). The presence under one ending of receptors that mediate opposing functions provides a novel mechanism for modulating synaptic activity in vivo.
Conclusions
There is still much that is not known about the targeting and clustering of neurotransmitter receptors. The work reviewed here provides novel mechanistic insights into interneuronal synapse formation and suggests an important difference in the mechanisms that govern assembly of nicotinic cholinergic synapses on neurons as compared to skeletal muscle of vertebrates. Localization to the neuromuscular junction seems to occur via the interaction of any of the muscle-type nAChR subunits with rapsyn, an important synapse-organizing molecule (Maimone & Merlie, 1993; Yu & Hall, 1994; Apel et al. 1997). Thus all muscle nAChR subunits appear to share a redundant targeting function that may optimize formation of the characteristic high-density clusters at the single endplate. In contrast, a single neuron assembles a greater number of synapses on its surface and expresses several different types of neurotransmitter receptors. Our in vivo studies on neuronal nicotinic cholinergic receptors and work on the glycine receptor and glutamate receptor family (Kim & Huganir, 1999; Lee & Sheng, 2000; see review by Kneussel & Betz, 2000, in this issue) all suggest that a common mechanism underlies the formation of the diverse types of interneuronal synapses. As a general principle, the intracellular domain of one particular subunit is responsible for targeting specific receptor subtypes to discrete synaptic regions of a single neuron. The presence under one presynaptic terminal of heterogeneous postsynaptic microregions that contain receptors with opposing actions further underscores the importance of receptor targeting for proper synaptic function.
Acknowledgments
We gratefully acknowledge the expert technical assistance of Brian M. Williams. This work was supported by NIH grant 21725 to M.H.J.
References
- Apel ED, Glass DJ, Moscoso LM, Yancopoulos GD, Sanes JR. Rapsyn is required for MuSK signaling and recruits synaptic components to a MuSK-containing scaffold. Neuron. 1997;18:623–635. doi: 10.1016/s0896-6273(00)80303-7. [DOI] [PubMed] [Google Scholar]
- Attwell D, Barbour B, Szatkowski M. Nonvesicular release of neurotransmitter. Neuron. 1993;11:401–407. doi: 10.1016/0896-6273(93)90145-h. [DOI] [PubMed] [Google Scholar]
- Chang KT, Berg DK. Nicotinic acetylcholine receptors containing alpha7 subunits are required for reliable synaptic transmission in situ. Journal of Neuroscience. 1999;19:3701–3710. doi: 10.1523/JNEUROSCI.19-10-03701.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Patrick JW. The α-bungarotoxin-binding nicotinic acetylcholine receptor from rat brain contains only the α-7 subunit. Journal of Biological Chemistry. 1997;272:24024–24029. doi: 10.1074/jbc.272.38.24024. [DOI] [PubMed] [Google Scholar]
- Coggan JS, Paysan J, Conroy WG, Berg DK. Direct recording of nicotinic responses in presynaptic nerve terminals. Journal of Neuroscience. 1997;17:5798–5806. doi: 10.1523/JNEUROSCI.17-15-05798.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colin I, Rostaing P, Augustin A, Triller A. Localization of components of glycinergic synapses during rat spinal cord development. Journal of Comparative Neurology. 1998;398:359–372. [PubMed] [Google Scholar]
- Conroy WG, Berg DK. Neurons can maintain multiple classes of nicotinic acetylcholine receptors distinguished by different subunit compositions. Journal of Biological Chemistry. 1995;270:4424–4431. doi: 10.1074/jbc.270.9.4424. [DOI] [PubMed] [Google Scholar]
- Corriveau RA, Berg DK. Coexpression of multiple acetylcholine receptor genes in neurons: quantification of transcripts during development. Journal of Neuroscience. 1993;13:2662–2671. doi: 10.1523/JNEUROSCI.13-06-02662.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale H. Pharmacology and nerve-endings. Proceedings of the Royal Society of Medicine. 1935;28:319–332. [PMC free article] [PubMed] [Google Scholar]
- Dani JA, Heinemann S. Molecular and cellular aspects of nicotine abuse. Neuron. 1996;16:905–908. doi: 10.1016/s0896-6273(00)80112-9. [DOI] [PubMed] [Google Scholar]
- De Lorenzo AJ. The fine structure of synapses in the ciliary ganglion of the chick. Journal of Biophysics, Biochemistry and Cytology. 1960;7:31–36. doi: 10.1083/jcb.7.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drisdel RC, Green WN. Neuronal α-bungarotoxin receptors are 7 subunit homomers. Journal of Neuroscience. 2000;20:133–139. doi: 10.1523/JNEUROSCI.20-01-00133.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisele JL, Bertrand S, Galzi JL, Devillers-Thiery A, Changeux JP, Bertrand D. Chimaeric nicotinic-serotonergic receptor combines distinct ligand binding and channel specificities. Nature. 1993;366:479–483. doi: 10.1038/366479a0. [DOI] [PubMed] [Google Scholar]
- Federspiel MJ, Hughes SH. Retroviral gene delivery. Methods in Cell Biology. 1998;52:179–214. [PubMed] [Google Scholar]
- Feng G, Steinbach JH, Sanes JR. Rapsyn clusters neuronal acetylcholine receptors but is inessential for the formation of an interneuronal cholinergic synapse. Journal of Neuroscience. 1998a;18:4166–4176. doi: 10.1523/JNEUROSCI.18-11-04166.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng G, Tintrup H, Kirsch J, Nichol MC, Kuhse J, Betz H, Sanes JR. Dual requirement for gephyrin in glycine receptor clustering and molybdoenzyme activity. Science. 1998b;282:1321–1324. doi: 10.1126/science.282.5392.1321. [DOI] [PubMed] [Google Scholar]
- Girod R, Crabtree G, Ernstrom G, Ramirez-Latorre J, McGehee D, Turner J, Role L. Heteromeric complexes of alpha 5 and/or alpha 7 subunits. Effects of calcium and potential role in nicotine-induced presynaptic facilitation. Annals of the New York Academy of Sciences. 1999;868:578–590. doi: 10.1111/j.1749-6632.1999.tb11331.x. [DOI] [PubMed] [Google Scholar]
- Hess A. Developmental changes in the structure of the synapse on the myelinated cell bodies of the chicken ciliary ganglion. Journal of Cell Biology. 1965;25(suppl.):1–19. doi: 10.1083/jcb.25.3.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homburger SA, Fekete DM. High efficiency gene transfer into the embryonic chicken CNS using B-subgroup retroviruses. Developmental Dynamics. 1996;206:112–120. doi: 10.1002/(SICI)1097-0177(199605)206:1<112::AID-AJA10>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Horch HL, Sargent PB. Perisynaptic surface distribution of multiple classes of nicotinic acetylcholine receptors on neurons in the chicken ciliary ganglion. Journal of Neuroscience. 1995;15:7778–7795. doi: 10.1523/JNEUROSCI.15-12-07778.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob MH. Acetylcholine receptor expression in developing chick ciliary ganglion neurons. Journal of Neuroscience. 1991;11:1701–1712. doi: 10.1523/JNEUROSCI.11-06-01701.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob MH, Berg DK. The ultrastructural localization of α-bungarotoxin binding sites in relation to synapses on chick ciliary ganglion neurons. Journal of Neuroscience. 1983;3:260–271. doi: 10.1523/JNEUROSCI.03-02-00260.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob MH, Lindstrom JM, Berg DK. Surface and intracellular distribution of a putative neuronal nicotinic acetylcholine receptor. Journal of Cell Biology. 1986;103:205–214. doi: 10.1083/jcb.103.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo YH, Schlichter R. Synaptic corelease of ATP and GABA in cultured spinal neurons. Nature Neuroscience. 1999;2:241–245. doi: 10.1038/6344. [DOI] [PubMed] [Google Scholar]
- Kim JH, Huganir RL. Organization and regulation of proteins at synapses. Current Opinion in Cell Biology. 1999;11:248–254. doi: 10.1016/s0955-0674(99)80033-7. [DOI] [PubMed] [Google Scholar]
- Kirsch J, Wolters I, Triller A, Betz H. Gephyrin antisense oligonucleotides prevent glycine receptor clustering in spinal neurons. Nature. 1993;366:745–748. doi: 10.1038/366745a0. [DOI] [PubMed] [Google Scholar]
- Kneussel M, Betz H. Receptors, gephyrin and gephyrin-associated proteins: novel insights into the assembly of inhibitory postsynaptic membrane specializations. The Journal of Physiology. 2000;525:1–9. doi: 10.1111/j.1469-7793.2000.t01-4-00001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landmesser L, Pilar G. The onset and development of transmission in the chick ciliary ganglion. The Journal of Physiology. 1972;222:691–713. doi: 10.1113/jphysiol.1972.sp009822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Sheng M. Development of neuron-neuron synapses. Current Opinion in Neurobiology. 2000;10:125–131. doi: 10.1016/s0959-4388(99)00046-x. [DOI] [PubMed] [Google Scholar]
- Lena C, Changeux JP. Pathological mutations of nicotinic receptors and nicotine-based therapies for brain disorders. Current Opinion in Neurobiology. 1997;7:674–682. doi: 10.1016/s0959-4388(97)80088-8. [DOI] [PubMed] [Google Scholar]
- Levey MS, Brumwell CL, Dryer SE, Jacob MH. Innervation and target tissue interactions differentially regulate acetylcholine receptor subunit mRNA levels in developing neurons in situ. Neuron. 1995;14:153–162. doi: 10.1016/0896-6273(95)90249-x. [DOI] [PubMed] [Google Scholar]
- Levey MS, Jacob MH. Changes in the regulatory effects of cell-cell interactions on neuronal AChR subunit transcript levels after synapse formation. Journal of Neuroscience. 1996;16:6878–6885. doi: 10.1523/JNEUROSCI.16-21-06878.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstrom J, Anand R, Gerzanich V, Peng X, Wang F, Wells G. Structure and function of neuronal nicotinic acetylcholine receptors. Progress in Brain Research. 1996;109:125–137. doi: 10.1016/s0079-6123(08)62094-4. [DOI] [PubMed] [Google Scholar]
- Listerud M, Brussaard AB, Devay P, Colman DR, Role LW. Functional contribution of neuronal AChR subunits revealed by antisense oligonucleotides. Science. 1991;254:1518–1521. doi: 10.1126/science.1720573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loring RH, Dahm LM, Zigmond RE. Localization of alpha-bungarotoxin binding sites in the ciliary ganglion of the embryonic chick: an autoradiographic study at the light and electron microscopic level. Neuroscience. 1985;14:645–660. doi: 10.1016/0306-4522(85)90316-1. [DOI] [PubMed] [Google Scholar]
- Loring RH, Zigmond RE. Ultrastructural distribution of 125I-toxin F binding sites on chick ciliary neurons: synaptic localization of a toxin that blocks ganglionic nicotinic receptors. Journal of Neuroscience. 1987;7:2153–2162. doi: 10.1523/JNEUROSCI.07-07-02153.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas RJ, Changeux JP, Le Novere N, Albuquerque EX, Balfour DJ, Berg DK, Bertrand D, Chiappinelli VA, Clarke PB, Collins AC, Dani JA, Grady SR, Kellar KJ, Lindstrom JM, Marks MJ, Quik M, Taylor PW, Wonnacott S. International Union of Pharmacology. XX. Current status of the nomenclature for nicotinic acetylcholine receptors and their subunits. Pharmacological Reviews. 1999;51:397–401. [PubMed] [Google Scholar]
- McGale EH, Pye IF, Stonier C, Hutchinson EC, Aber GM. Studies of the inter-relationship between cerebrospinal fluid and plasma amino acid concentrations in normal individuals. Journal of Neurochemistry. 1977;29:291–297. doi: 10.1111/j.1471-4159.1977.tb09621.x. [DOI] [PubMed] [Google Scholar]
- McGehee DS, Role LW. Physiological diversity of nicotinic acetylcholine receptors expressed by vertebrate neurons. Annual Review of Physiology. 1995;57:521–546. doi: 10.1146/annurev.ph.57.030195.002513. [DOI] [PubMed] [Google Scholar]
- Maimone MM, Merlie JP. Interaction of the 43 kd postsynaptic protein with all subunits of the muscle nicotinic acetylcholine receptor. Neuron. 1993;11:53–66. doi: 10.1016/0896-6273(93)90270-2. [DOI] [PubMed] [Google Scholar]
- Mandelzys A, Pie B, Deneris ES, Cooper E. The developmental increase in ACh current densities on rat sympathetic neurons correlates with changes in nicotinic ACh receptor alpha-subunit gene expression and occurs independent of innervation. Journal of Neuroscience. 1994;14:2357–2364. doi: 10.1523/JNEUROSCI.14-04-02357.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margiotta JF, Gurantz D. Changes in the number, function, and regulation of nicotinic acetylcholine receptors during neuronal development. Developmental Biology. 1989;135:326–339. doi: 10.1016/0012-1606(89)90183-8. [DOI] [PubMed] [Google Scholar]
- Martin AR, Pilar G. Dual mode of synaptic transmission in the avian ciliary ganglion. The Journal of Physiology. 1963;168:443–463. doi: 10.1113/jphysiol.1963.sp007202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer G, Kirsch J, Betz H, Langosch D. Identification of a gephyrin binding motif on the glycine receptor beta subunit. Neuron. 1995;15:563–572. doi: 10.1016/0896-6273(95)90145-0. [DOI] [PubMed] [Google Scholar]
- Morgan BA, Fekete DM. Manipulating gene expression with replication-competent retroviruses. Methods in Cell Biology. 1996;51:185–218. doi: 10.1016/s0091-679x(08)60629-9. [DOI] [PubMed] [Google Scholar]
- Moss BL, Role LW. Enhanced ACh sensitivity is accompanied by changes in ACh receptor channel properties and segregation of ACh receptor subtypes on sympathetic neurons during innervation in vivo. Journal of Neuroscience. 1993;13:13–28. doi: 10.1523/JNEUROSCI.13-01-00013.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll RA, Malenka RC. A tale of two transmitters. Science. 1998;281:360–361. doi: 10.1126/science.281.5375.360. [DOI] [PubMed] [Google Scholar]
- O'Malley DM, Sandell JH, Masland RH. Co-release of acetylcholine and GABA by the starburst amacrine cells. Journal of Neuroscience. 1992;12:1394–1408. doi: 10.1523/JNEUROSCI.12-04-01394.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry E, Walker M, Grace J, Perry R. Acetylcholine in mind: a neurotransmitter correlate of consciousness? Trends in Neurosciences. 1999;22:273–280. doi: 10.1016/s0166-2236(98)01361-7. [DOI] [PubMed] [Google Scholar]
- Radcliffe KA, Fisher JL, Gray R, Dani JA. Nicotinic modulation of glutamate and GABA synaptic transmission of hippocampal neurons. Annals of the New York Academy of Sciences. 1999;868:591–610. doi: 10.1111/j.1749-6632.1999.tb11332.x. [DOI] [PubMed] [Google Scholar]
- Ramirez-Latorre J, Yu CR, Qu X, Perin F, Karlin A, Role L. Functional contributions of alpha5 subunit to neuronal acetylcholine receptor channels. Nature. 1996;380:347–351. doi: 10.1038/380347a0. [DOI] [PubMed] [Google Scholar]
- Reiner A, Erichsen JT, Cabot JB, Evinger C, Fitzgerald ME, Karten HJ. Neurotransmitter organization of the nucleus of Edinger-Westphal and its projection to the avian ciliary ganglion. Visual Neuroscience. 1991;6:451–472. doi: 10.1017/s0952523800001310. [DOI] [PubMed] [Google Scholar]
- Sargent PB. The diversity of neuronal nicotinic acetylcholine receptors. Annual Review of Neuroscience. 1993;16:403–443. doi: 10.1146/annurev.ne.16.030193.002155. [DOI] [PubMed] [Google Scholar]
- Schwartz EA. Depolarization without calcium can release gamma-aminobutyric acid from a retinal neuron. Science. 1987;238:350–355. doi: 10.1126/science.2443977. [DOI] [PubMed] [Google Scholar]
- Shoop RD, Martone ME, Yamada N, Ellisman MH, Berg DK. Neuronal acetylcholine receptors with α-7 subunits are concentrated on somatic spines for synaptic signaling in embryonic chick ciliary ganglia. Journal of Neuroscience. 1999;19:692–704. doi: 10.1523/JNEUROSCI.19-02-00692.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsen G, Williams B, Allaire P, Zhou Y, Ikonomov O, Kondova I, Jacob MH. Receptors with opposing functions are in postsynaptic microdomains under one presynaptic terminal. Nature Neuroscience. 2000;3:126–132. doi: 10.1038/72066. [DOI] [PubMed] [Google Scholar]
- Ullian EM, McIntosh JM, Sargent PB. Rapid synaptic transmission in the avian ciliary ganglion is mediated by two distinct classes of nicotinic receptors. Journal of Neuroscience. 1997;17:7210–7219. doi: 10.1523/JNEUROSCI.17-19-07210.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernallis AB, Conroy WG, Berg DK. Neurons assemble acetylcholine receptors with as many as three kinds of subunits while maintaining subunit segregation among receptor sites. Neuron. 1993;10:451–464. doi: 10.1016/0896-6273(93)90333-m. [DOI] [PubMed] [Google Scholar]
- Vicente-Agullo F, Rovira JC, Campos-Caro A, Rodriguez-Ferrer C, Ballesta JJ, Sala S, Sala F, Criado M. Acetylcholine receptor subunit homomer formation requires compatibility between amino acid residues of the M1 and M2 transmembrane segments. FEBS Letters. 1996;399:83–86. doi: 10.1016/s0014-5793(96)01291-4. [DOI] [PubMed] [Google Scholar]
- Wang F, Gerzanich V, Wells GB, Anand R, Peng X, Keyser K, Lindstrom J. Assembly of human neuronal nicotinic receptor alpha5 subunits with alpha3, beta2, and beta4 subunits. Journal of Biological Chemistry. 1996;271:17656–17665. doi: 10.1074/jbc.271.30.17656. [DOI] [PubMed] [Google Scholar]
- Williams BM, Temburni MK, Levey MS, Bertrand S, Bertrand D, Jacob MH. The long internal loop of the α3 subunit targets nAChRs to subdomains within individual synapses in vivo. Nature Neuroscience. 1998;1:557–562. doi: 10.1038/2792. [DOI] [PubMed] [Google Scholar]
- Xu W, Gelber S, Orr-Urtreger A, Armstrong D, Lewis RA, Ou C, Patrick J, Role L, de Biasi M, Beaudet AM. Megacystis, mydriasis and ion channel deficits in mice lacking the α3 neuronal nicotinic acetyl choline receptor. Proceedings of the National Academy of Sciences of the USA. 1999a;96:5746–5751. doi: 10.1073/pnas.96.10.5746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Orr-Urtreger A, Nigro F, Gelber S, Sutcliffe CB, Armstrong D, Patrick JW, Role LW, Beaudet AL, De Biasi M. Multiorgan autonomic dysfunction in mice lacking the beta2 and the beta4 subunits of neuronal nicotinic acetylcholine receptors. Journal of Neuroscience. 1999b;19:9298–9305. doi: 10.1523/JNEUROSCI.19-21-09298.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu XM, Hall ZW. The role of the cytoplasmic domains of individual subunits of the acetylcholine receptor in 43 kDa protein-induced clustering in COS cells. Journal of Neuroscience. 1994;14:785–795. doi: 10.1523/JNEUROSCI.14-02-00785.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZW, Berg DK. Patch-clamp analysis of glycine-induced currents in chick ciliary ganglion neurons. The Journal of Physiology. 1995;487:395–405. doi: 10.1113/jphysiol.1995.sp020888. [DOI] [PMC free article] [PubMed] [Google Scholar]


