Abstract
The biophysical and pharmacological properties of an oxygen-sensitive background K+ current in rat carotid body type-I cells were investigated and compared with those of recently cloned two pore domain K+ channels.
Under symmetrical K+ conditions the oxygen-sensitive whole cell K+ current had a linear dependence on voltage indicating a lack of intrinsic voltage sensitivity.
Single channel recordings identified a K+ channel, open at resting membrane potentials, that was inhibited by hypoxia. This channel had a single channel conductance of 14 pS, flickery kinetics and showed little voltage sensitivity except at extreme positive potentials.
Oxygen-sensitive current was inhibited by 10 mM barium (57 % inhibition), 200 μM zinc (53 % inhibition), 200 μM bupivacaine (55 % inhibition) and 1 mM quinidine (105 % inhibition).
The general anaesthetic halothane (1.5 %) increased the oxygen-sensitive K+ current (by 176 %). Halothane (3 mM) also stimulated single channel activity in inside-out patches (by 240 %). Chloroform had no effect on background K+ channel activity.
Acidosis (pH 6.4) inhibited the oxygen-sensitive background K+ current (by 56 %) and depolarised type-I cells.
The pharmacological and biophysical properties of the background K+ channel are, therefore, analogous to those of the cloned channel TASK-1. Using in situ hybridisation TASK-1 mRNA was found to be expressed in type-I cells. We conclude that the oxygen- and acid-sensitive background K+ channel of carotid body type-I cells is likely to be an endogenous TASK-1-like channel.
The primary sensory cells of the carotid body respond to hypoxia or acidosis with depolarisation which initiates electrical activity, calcium entry and neurosecretion (Buckler & Vaughan-Jones, 1994a,b; Ureña et al. 1994; Montoro et al. 1996). A key ionic current involved in mediating these responses in rat type-I cells is an oxygen-sensitive background K+ current (Buckler, 1997). The molecular nature of such background currents has long remained a mystery; however, a new family of K+ channels has recently been cloned (Lesage et al. 1996; Fink et al. 1996, 1998; Duprat et al. 1997; Reyes et al. 1998; Chavez et al. 1999; Pountney et al. 1999; Salinas et al. 1999). These channels have a unique structure (4 transmembrane segments and 2 pore domains) and little or no conventional voltage sensitivity making them ideal candidates for generating background K+ currents. Here we show that the oxygen- and acid-sensitive background K+ channel of the rat carotid body type-I cell is comparable biophysically and pharmacologically to the tandem-P-domain K+ channel TASK-1 and that TASK-1 mRNA is expressed in type-I cells.
METHODS
Cell isolation
Carotid bodies were excised from anaesthetised (4 % fluothane) Sprague-Dawley rat pups (10-16 days old) and enzymically dispersed using collagenase (0.4 mg ml−1, type-1 Worthington) and trypsin (0.15-0.2 mg ml−1, Sigma) as previously described (Buckler, 1997). The rats were then killed by decapitation whilst still anaesthetised. Isolated cells were maintained in Ham's F-12 (supplemented with: 10 % heat-inactivated fetal calf serum, 100 i.u. ml−1; penicillin-streptomycin, 100 μg ml−1; and insulin, 84 U l−1) at 35°C with 5 % CO2 in air until use (2-12 h).
Electrophysiology
Perforated patch whole cell recordings were performed with simultaneous measurement of intracellular calcium as previously described (see Buckler, 1997). Voltage clamp commands were generated using an analog-to-digital and digital-to-analog converter (CED1401), consequently all voltage ramps are staircases with a step amplitude of 0.78 mV. Membrane potential and current were filtered at 1 kHz (3 dB) and digitised at 2–4 kHz. Cells were subjected to repetitive voltage ramps and the resultant current records from each cell were averaged. Then, since the ramps were essentially staircases, current was averaged for each step. Oxygen-sensitive currents were determined by subtracting the current- voltage (I–V) relation obtained under hypoxic conditions from that obtained under control conditions. Mean current-voltage relationships were calculated by averaging the I–V relationships from each cell in the study. Membrane conductance was determined by fitting a linear regression to the current-voltage relationship over the range -60 to -70 mV.
Single channel recordings were performed using an Axopatch 200B. Data were acquired using an Axon Digidata 1200 interface and either pCLAMP 6 or pCLAMP 7 software. Patch current was filtered at 2–5 kHz (3 dB) and digitised at 10–20 kHz.
Solutions and reagents for electrophysiology
The filling solution for perforated patch recordings contained (mM): K2SO4 55, KCl 30, MgCl2 2, EGTA 1, Hepes 10; pH adjusted to 7.2-7.3 with NaOH. Amphotericin B, 120–240 μg ml−1, was added to this from a stock solution of 60 mg ml−1 in DMSO. Data are presented without correction for liquid junction potentials (approx. 3 mV). Filling solutions for single channel recording contained (mM): KCl 140, MgCl2 4, EGTA 1, Hepes 10; pH 7.4 (with KOH, final [K+]= 146 mM).
The standard HCO3−-buffered saline contained (mM): NaCl 117, KCl 4.5, NaHCO3 23, MgCl2 1.0, CaCl2 2.5, glucose 11; pH 7.4-7.45. Acidic HCO3−-buffered saline was as above except for 2.3 mM NaHCO3 and 137.7 mM NaCl; pH 6.4. Potassium- and calcium-free Tyrode solution (140 mM; K+,Ca2+-free; Fig. 1a and B) contained (mM): K2SO4 58.5, KHCO3 23, NaCl 21, MgCl2 1, NiCl2 2.5, glucose 10, tetraethylammonium-Cl (TEA) 10, 4-aminopyridine (4-AP) 5. All of the above bicarbonate-buffered solutions were equilibrated with 5 % CO2 in air (control) or nitrogen (hypoxia). Bathing solution for inside-out patch recordings contained (mM): KCl 130, MgCl2 5, EGTA 10, Hepes 10, glucose 10; pH 7.2 (with KOH). In independent experiments during perfusion with hypoxic solutions bath PO2 typically fell to about 5 Torr (∼0.665 kPa) within 1–2 min (measured with a miniature Clake-type electrode).
Figure 1. Biophysical properties of background K+ channels.
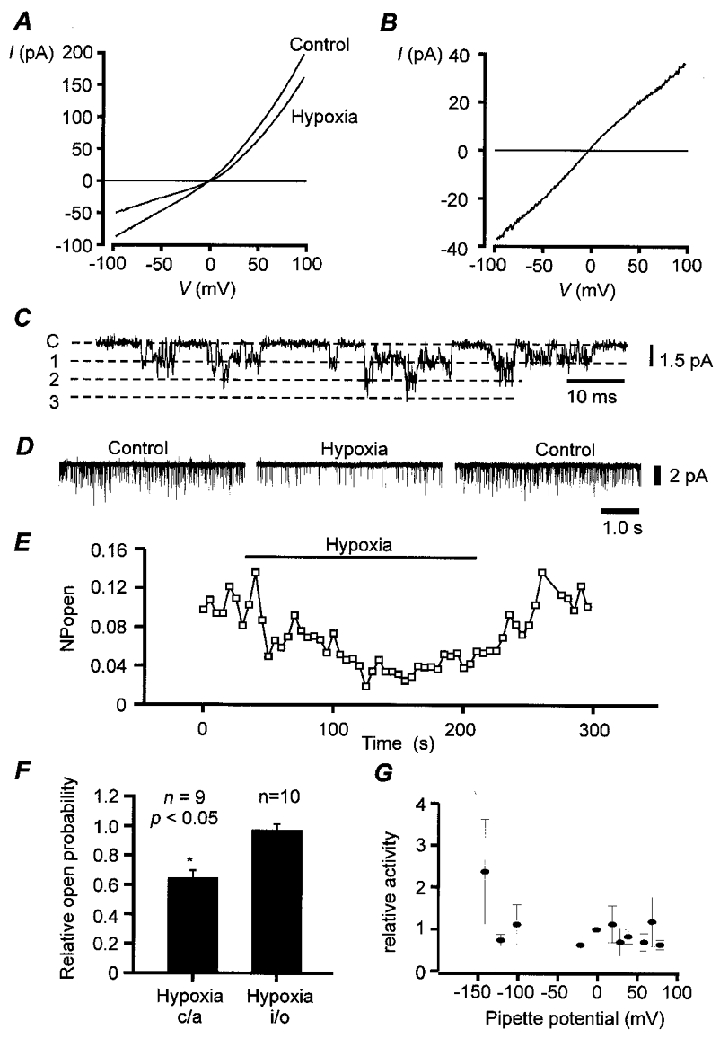
A, mean whole cell current-voltage relationship (I–V) for type-I cells bathed in 140 mM K+,Ca2+-free Tyrode solution containing 2.5 mM Ni2+, 10 mM TEA and 5 mM 4-AP, under normoxic and hypoxic conditions (n = 10). Hypoxia was induced by gassing saline solutions with 5 % CO2-95 % N2. Hypoxia reduced membrane conductance (over voltage range -70 to -60 mV) from 0.84 ± 0.12 nS control to 0.49 ± 0.09 nS hypoxia (n = 10; P < 0.001). Mean currents at -50 mV were 46.4 ± 0.6 pA control, and 26.4 ± 3.0 pA hypoxia. B, mean oxygen-sensitive current determined by subtraction of I–V obtained under hypoxic conditions from that obtained under normoxic conditions (from data in A). C, single channel recording from a cell-attached patch. Cell bathed in standard Tyrode solution, pipette solution contained 10 mM TEA, pipette potential 0 mV. D and E, hypoxia reversibly reduces channel activity in cell-attached patch. Cells were bathed in 100 mM potassium bicarbonate-buffered Tyrode solution (K+ substituted for Na+). Pipette potential was 70 mV (membrane potential approx. -70 to -80 mV). F, effects of hypoxia on single channel activity in cell-attached (c/a) patches and inside-out (i/o) patches. Hypoxia significantly reduced channel activity in cell-attached but not inside-out patches (*). Inside-out patches were clamped at -100 mV. Data expressed as fraction of control NPopen. G, channel activity, relative to that observed at the resting potential (0 mV pipette potential), as a function of pipette potential for cell-attached patch (n = 5). Pipette solution contained 10 mM TEA and 5 mM 4-AP. Cells bathed in standard bicarbonate Tyrode solution.
The following drugs/chemicals were added to the saline/filling solutions as indicated; BaCl2, ZnCl2 from a 1 M stock in H2O; TEA, 4-AP, bupivacaine, quinidine as solids. Halothane and chloroform were added as liquids in Hepes saline for single channel recordings (these solutions were prepared freshly every 2–3 h, and kept in stoppered borosilicate bottles between recordings). For whole cell recordings halothane was included in the gas mixture used to continuously bubble the HCO3−-buffered salines (at 1.5 % by vol.). This concentration is approximately twice the minimum alveolar concentration required for anaesthesia of 50 % of human subjects (MAC50; 0.8 %). Whole cell recordings were conducted at 33–37°C. Single channel recordings were conducted at 30–33°C except for those recordings utilising halothane or chloroform which were done at room temperature.
In situ hybridisation
Carotid body cells from 11-day-old to 3-week-old Wistar or Sprague-Dawley rats were prepared using the protocol above. Cells were plated on poly-D-lysine-coated glass culture chamber slides and maintained in Ham's F12 for 2–3 h in a 37°C 5 % CO2 incubator before fixation with ice-cold 4 % (w/v) paraformaldehyde in 0.1 M sodium phosphate-buffered solution (PBS, pH 7.4) for 30 min. The slides were then washed twice in PBS and stored in 70 % ethanol at -4°C. Digoxigenin- or fluorescein-labelled RNA sense and anti-sense TASK probes (nucleotides 521-838; TASK accession number: AF006824) were generated with an in vitro transcription kit (Boehringer Mannheim) which incorporates one modified digoxigenin-labelled UTP every 20-25th position in the transcript. Pre-hybridization consisted of: 3 min wash in PBS; permeabilization for 5 min in PBS + 0.1 % Triton; 2 × 5 min washes in PBS; 10 min citric acid-sodium citrate microwave irradiation; 2 × 5 min washes in PBS. Hybridization was performed at 75°C overnight with 10 ng probe μl−1 (in 4 × saline-sodium citrate buffer (SSC) with 50 % formamide, 10 % dextran sulfate, 1 % Denhardt's solution, 5 % sarcosyl, 500 mg ml−1 denatured salmon sperm DNA, 250 μg ml−1 yeast tRNA, 20 mM dithiothreitol and 20 mM NaPO4). Slides were washed briefly in 4 × SSC, followed by 30 min in SSC + RNase A (40 μg ml−1) at 37°C, 30 min at room temperature in 0.1 × SSC, and finally 30 min at room temperature in 0.01 × SSC. Immunological detection of labelled mRNA was carried out using anti- digoxigenin-alkaline phosphatase. Type-I cells were stained using a mouse monoclonal anti-tyrosine hydroxylase (diluted 1/100, Boehringer Mannheim) and a secondary anti-mouse F(ab)2 fragment labelled with tetramethylrhodamine isothiocyanate (TRITC) (Boehringer Mannheim, diluted 1/10).
Results are presented as means ±s.e.m. (n is the number of observations). Statistical significance was assessed using Student's two-tailed paired t test. P is considered significant at < 0.05.
RESULTS
The oxygen-sensitive background K+ current in type-I cells is insensitive to TEA and 4-AP and shows only weak outward rectification under physiological conditions (i.e. 4.5 mM extracellular K+) with no time-dependent activation or inactivation (Buckler, 1997). These data suggested that the oxygen-sensitive background K+ current may lack intrinsic voltage sensitivity. To confirm this we re-investigated the voltage dependence of this current in a symmetrical potassium ion gradient (in the presence of TEA and 4-AP). Under these conditions it is notable that while the total whole cell current displays some outward rectification, the oxygen-sensitive current has a near-linear dependence on voltage (Fig. 1a and B). The reversal potential of this oxygen-sensitive current was close to 0 mV (-2.5 ± 1.25 mV; n = 9) as expected for a K+ current under these conditions.
To identify the channels responsible for this background K+ current we employed cell-attached patch recordings. At holding potentials equivalent to membrane potentials of -60 to -80 mV we observed single channel activity in about half of the patches (both in the presence, and in the absence of TEA, e.g. Fig. 1C). In excised inside-out patches the reversal potential of single channel currents was dependent upon bath/cytosolic potassium concentration and was close to the potassium equilibrium potential (EK), indicating that these channels were selective for potassium (data not shown). Channel openings were typically brief and very flickery (e.g. Fig. 1C) with one main conductance level of approximately 14 pS (determined by a linear regression over the range of pipette potentials 120 to -160 mV) and, in most patches, additional conductance levels of approximately double or other multiples of this (Fig. 1C). Higher conductance levels were less well resolved due to the brief open times making it difficult to ascertain whether these represent different conductance states of the same channel or the simultaneous opening of more than one channel. In the presence of 4-AP and TEA open probability (Popen) changed little over a wide range of negative potentials but at very positive potentials there appeared to be a 2- to 4-fold increase in channel activity (Fig. 1G). Outward channel currents at positive potentials were poorly defined, however, and often accompanied by an increase in baseline noise which made estimation of Popen at positive potentials difficult. These observations suggest either that Popen is weakly voltage dependent and/or that patches were contaminated with additional voltage-activated K+ channels.
In cell-attached patches hypoxia reduced single channel activity by 35 ± 5 % (Fig. 1D, E and F). This degree of inhibition is similar to that estimated for the inhibition of whole cell conductance (40 ± 6 %) by hypoxia in high K+ solutions at negative potentials (Fig. 1a). Hypoxic inhibition of background K+ channels was lost upon excision of the patches in the inside-out configuration (Fig. 1F, channel activity (number of active channels in a patch × open probability; NPopen): control, 0.192 ± 0.06; hypoxia, 0.186 ± 0.07; n = 10, at -100 mV membrane potential).
The observation that the oxygen-sensitive background K+ current is largely voltage independent suggests that it is unlikely to be mediated by a classical delayed-rectifier or inward rectifier K+ channel. The tandem-P-domain K+ channels, however, display weak, or no voltage dependence and are typically insensitive to the K+ channel blockers TEA and 4-AP (Fink et al. 1996, 1998; Duprat et al. 1997; Reyes et al. 1998; Leonoudakis et al. 1998; Kim et al. 1998). Of these TASK-1 displays a linear whole cell current- voltage relationship in a symmetrical K+ gradient and weak Goldman-Hodgkin-Katz-type outward rectification in a physiological K+ gradient (Duprat et al. 1997). TASK-1 also has nearly identical single channel conductances of 14 pS, 12 pS and 16 pS for rat (Leonoudakis et al. 1998), human (Patel et al. 1999) and mouse TASK-1/cTBAK-1 (Kim et al. 1998), to that of the background K+ channel in the type-I cell (14 pS), as well as other subconductance states (Kim et al. 1998). We therefore sought to compare the pharmacology of the type-I cell background K+ channel/current with that of TASK-1. Although no selective inhibitors or activators are available for TASK-1 it has a distinct pharmacological profile relative to other tandem-P-domain K+ channels. TASK-1 can be inhibited by barium (in mM concentrations), zinc, local anaesthetics and quinidine (Duprat et al. 1997; Leonoudakis et al. 1998; Kindler et al. 1999), and stimulated by some general anaesthetics (Patel et al. 1999). Figure 2 demonstrates that the oxygen-sensitive background K+ current in type-I cells (measured at a [K+]o of 4.5 mM) is also inhibited by 10 mM barium (57 % inhibition), 200 μM zinc (53 % inhibition), 200 μM bupivacaine (55 % inhibition) and 1 mM quinidine (105 % inhibition). In cell-attached patches TEA (in the pipette) had no effect upon single channel conductance (control: 13.7 ± 0.4 pS, n = 4; + 10 mM TEA: 13.8 ± 0.4 pS, n = 21), or kinetics (not shown). In inside-out patches, 500 μM bupivacaine (applied intracellularly) inhibited background K+ channel activity (reducing NPopen by 36 ± 8 %; n = 8). Difficulties in obtaining stable, single channel, outside-out patches precluded us from testing the effects of Ba2+ and Zn2+ on single channel activity. The general anaesthetic halothane (1.5 % in gas mixture) increased both whole cell conductance (to 170 % of control, Fig. 3a) and the oxygen-sensitive K+ current (to 176 % of control, Fig. 3b) in type-I cells. Similarly, in inside-out patch recordings background K+ channel activity in the type-I cell was markedly stimulated by 3 mM halothane (NPopen increased to 240 % of control, Fig. 3C and D). In contrast to the effects of halothane, which activates both TASK-1 and TREK (another 2-P-domain K+ channel), chloroform is reported to only activate TREK (Patel et al. 1999). Chloroform had no effect on background K+ channel activity in inside-out patches (Fig. 3D). The pharmacological properties of the type-I cell background K+ channel are therefore strikingly similar to those of TASK-1.
Figure 2. Pharmacology of background K+ channels.
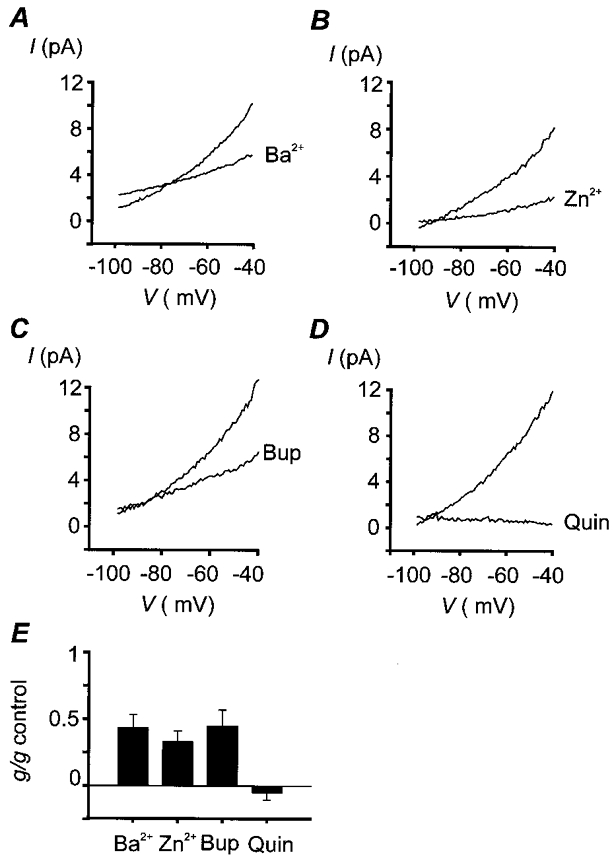
Oxygen-sensitive currents were determined by subtraction of currents obtained under hypoxic conditions from those obtained under normoxic conditions, both in the absence and in the presence of inhibitor. Experiments were performed in normal, physiological, bicarbonate-buffered saline, [K+]o= 4.5 mM; note that under these conditions the oxygen-sensitive current shows weak outward rectification. Data are mean I–V relationships for a number of cells. The degree of inhibition was determined by calculation of membrane conductance in the region -60 to -70 mV. A, effects of 10 mM Ba2+. Mean conductance: 161 ± 30 pS control, 61 ± 15 pS Ba2+, n = 9, P < 0.01 (Student's paired t test). Mean oxygen-sensitive current at -50 mV: 7.8 ± 1.0 pA control, 4.9 ± 0.8 pA Ba2+. B, effects of 200 μM Zn2+. Mean conductance: 160 ± 28 pS control, 47 ± 13 pS Zn2+, n = 6, P < 0.01. Mean oxygen-sensitive current at -50 mV: 6.1 ± 1.0 pA control, 2.0 ± 0.7 pA Zn2+. C, effects of 200 μM bupivacaine (Bup). Mean conductance: 218 ± 33 pS control, 93 ± 22 pS bupivacaine, n = 6, P < 0.005. Mean oxygen-sensitive current at -50 mV: 8.7 ± 1.6 pA control, 5.2 ± 0.6 pA bupivacaine. D, effects of 1 mM quinidine (Quin). Mean conductance: 219 ± 37 pS control, -11 ± 11 pS quinidine, n = 5, P < 0.005. Mean oxygen-sensitive current at -50 mV: 9.7 ± 1.5 pA control, 0.6 ± 0.1 pA quinidine. E, summary of effects of K+ channel inhibitors on oxygen-sensitive membrane conductance. Mean percentage reduction in conductance: Ba2+, 57 ± 10 %; Zn2+, 67 ± 8 %; bupivacaine, 55 ± 12 %; quinidine, 105 ± 5 %.
Figure 3. Effects of anaesthetics and acidosis on background K+ channels.
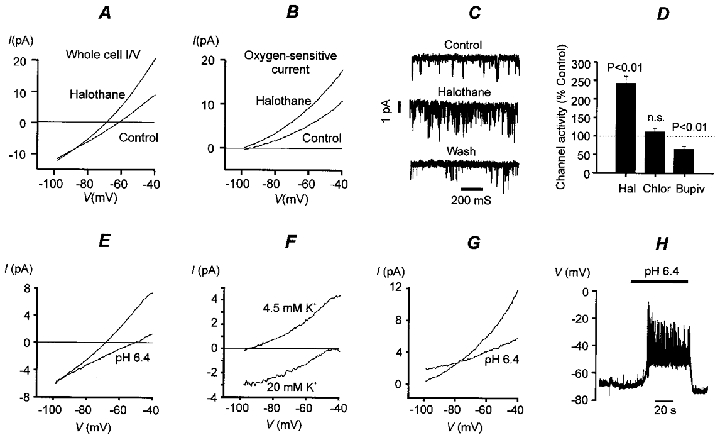
A, effect of halothane on whole cell conductance. Mean I–V plot from 9 cells. Mean conductance: 350 ± 36 pS control, 584 ± 68 pS halothane, n = 9, P < 0.001. Mean whole cell current at -50 mV: 4.9 ± 0.9 pA control, 12.6 ± 1.9 pA halothane. B, effects of halothane on mean oxygen-sensitive currents (determined by subtraction, control – hypoxia). Mean oxygen-sensitive conductance: 183 ± 33 pS control, 323 ± 64 pS halothane, n = 9, P < 0.02. Mean oxygen-sensitive current at -50 mV: 7.6 ± 1.4 pA control, 13.1 ± 2.6 pA halothane. Halothane was applied by gassing saline solutions with 1.5 % halothane-5 % CO2-20 % O2-73.5 % N2 (control) or 1.5 % halothane-5 % CO2-93.5 % N2 (hypoxia). C, 3 mM halothane applied intracellularly to inside-out patches increases channel activity. Membrane potential, -70 mV. D, comparison of effects of local and general anaesthetics on single channel activity in inside-out patches. Hal, 3 mM halothane; membrane potential -70 mV, n = 10. Chlor, 3 mM chloroform; membrane potential -70 mV, n = 7. Bupiv, 500 μM bupivacaine; membrane potential -100 mV, n = 8. E, effect of isocapnic acidosis (pH 6.4) on whole cell membrane conductance (mean I–V from 5 cells). Cells were exposed to acid solution for 20 s periods every 1–2 min. Note that acidosis reduces membrane conductance (control, 270 ± 22 pS; acidosis, 131 ± 19 pS; n = 5, P < 0.001) and shifts the zero current potential in a positive (depolarising) direction. Mean current at -50 mV: 4.6 ± 0.6 pA control, -0.6 ± 0.7 pA pH 6.4. F, mean acid-sensitive currents (control – acidosis; n = 6) determined at two different levels of extracellular K+ (acid stimuli applied for 1–2 min). Note leftward/downward shift of I–V in high K+ and depolarising shift in reversal potential. Mean acid-sensitive current at -50 mV: 4.6 ± 0.6 pA in 4.5 mM [K+]o, -0.4 ± 0.5 pA in 20 mM [K+]o. G, effects of acidosis (2 min) on oxygen-sensitive current. Acidosis reduced the oxygen-sensitive conductance from 203 ± 32 pS to 88 ± 21 pS (measured over voltage range -70 to -60 mV). Mean oxygen-sensitive current at -50 mV: 8.3 ± 1.3 pA control, 4.8 ± 1.0 pA pH 6.4. H, recording of membrane potential in a type-I cell during exposure to isocapnic acidosis. Experiments depicted in parts A, B, E, F, G and H were performed in a normal, physiological, bicarbonate-buffered saline, [K+]o= 4.5 mM. Inside-out patch recordings shown in C and D were performed in a Hepes-buffered saline with high [K+]o (see Methods).
One of the defining characteristics of TASK-1, relative to other tandem-P-domain channels thus far described, is that it is inhibited by external acidosis (Duprat et al. 1997; Leonoudakis, 1998). Figure 3E shows that brief periods of isocapnic acidosis (repeated 20 s exposures to a solution of pH 6.4) reduced membrane conductance in type-I cells by 52 %. The acid-sensitive current was found to reverse close to the predicted value for EK at superfusate potassium concentrations of 4.5 and 20 mM (Fig. 3F). Acidosis also reduced the oxygen-sensitive current by 56 % (Fig. 3G). In current clamp recordings isocapnic acidosis depolarised type-I cells (Fig. 3H) and evoked a rapid rise in intracellular calcium (not shown). These data indicate that acidosis also inhibits the oxygen-sensitive background K+ current in type-I cells.
Evidence for the expression of TASK-1 mRNA in isolated type-I cells was sought using in situ hybridisation with both anti-sense and sense probes (as a negative control). Cells were counter-stained with anti-tyrosine-hydroxylase to identify type-I cells. Anti-sense probes produced labelling of most cells in the preparation (no significant staining occurred with sense probes) including all type-I cells (Fig. 4). TASK-1 mRNA is therefore widely expressed in this tissue in both type-I cells and possibly other cell types as well.
Figure 4. In situ hybridisation for TASK-1.
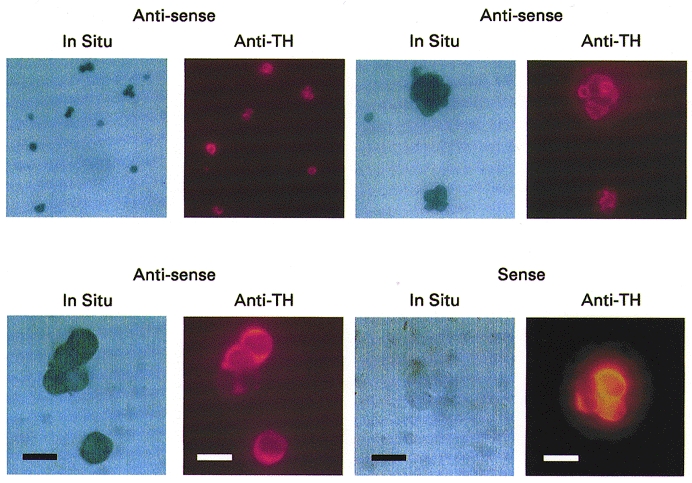
Staining for digoxigenin-labelled RNA probes with anti-digoxigenin-alkaline phosphatase (panels labelled In Situ). The same cells were also stained with anti-tyrosine hydroxylase (shown in adjacent panels labelled Anti-TH). Three examples of in situ hybridisation with anti-sense probes for TASK-1 are shown at increasing magnification (top row and bottom left), and one example of in situ hybridisation with sense probes (bottom right). Scale bar = 10 μm (bottom row only). Note that only anti-sense probes produced staining of cells, and that both type-I cells (positive for tyrosine hydroxylase) and possibly non-type-I cells (weak or no anti-tyrosine hydroxylase staining) express TASK-1 mRNA.
DISCUSSION
Oxygen sensing in the type-I cell is thought to be mediated via membrane depolarisation, voltage-gated calcium entry and neurosecretion (Buckler & Vaughan-Jones, 1994a; Ureña et al. 1994; Montoro et al. 1996). In isolated rat type-I cells this depolarisation appears to be mediated largely through the inhibition of a background K+ channel (Buckler, 1997). In single channel recordings hypoxia reduced channel open probability in cell-attached patches but had no effect on channel activity in inside-out patches. This loss of oxygen sensitivity in excised patches is similar to that previously reported for oxygen-sensitive large conductance calcium-activated K+ channels in rat type-I cells (Wyatt & Peers, 1995) and suggests that some cytosolic messenger or co-factor is required to confer or maintain oxygen sensitivity in both of these K+ channels.
In this report we show that acidosis also inhibits the same background K+ current causing membrane depolarisation and voltage-gated calcium entry. Exposure of the cells to brief pulses of isocapnic acidosis for 20 s (Fig. 3E) caused a rapid reduction in membrane conductance suggesting that the effects of this acidosis were likely to be mediated by the fall in extracellular pH per se rather than a consequent, slower, fall in internal pH. In view of previous studies implicating changes of internal pH in acid sensing (Buckler et al. 1991; Buckler & Vaughan-Jones, 1993), it now seems probable that both internal and external pH will play a role in mediating the response to acid stimuli.
The potential importance of this background K+ current in determining the response to hypoxic, acidic and metabolic (Buckler & Vaughan-Jones, 1998) stimuli makes a compelling case for striving to characterise the channel at the molecular level so that we may better understand the signalling pathways involved. Despite the potential importance of background or leak currents in defining resting membrane potentials, the molecular nature of such channels has remained largely unknown. The biophysical properties of some of the recently discovered twin pore loop K+ channels, however, appear to meet the necessary criteria for such background/leak K+ currents (Duprat et al. 1997). In this study we have shown that the biophysical and pharmacological properties of the background K+ channel in type-I cells are strikingly similar to those of TASK-1. These data make a persuasive case for the identification of the type-I cell background K+ channel as an endogenous TASK-1 channel. It should not pass without comment, however, that we clearly do not yet know how many more two pore domain channels remain to be discovered. So far only seven channels have been cloned from Mammalia (Lesage et al. 1996; Fink et al. 1996, 1998; Duprat et al. 1997; Reyes et al. 1998; Chavez et al. 1999; Pountney et al. 1999; Salinas et al. 1999) yet over 50 putative two-P-domain channels have been identified in Caenorhabditis elegans (Bargmann, 1998). Consequently the possible existence of another channel with nearly identical properties to TASK-1 cannot yet be excluded. Despite this caveat, the observation that TASK-1 mRNA is expressed in rat type-I cells suggests that TASK-1 is a prime candidate for mediating the oxygen- and acid-sensitive background K+ current.
Finally, in the course of our pharmacological characterisation of the background K+ current in type-I cells we have found that it is strongly stimulated by halothane. This observation is of additional physiological interest because (a) halothane reduces the sensitivity of peripheral chemoreceptors to hypoxia (Davies et al. 1982), which may be partially responsible for the suppression of hypoxic ventilatory drive under anaesthesia (Hirshman et al. 1977; Knill & Gelb, 1978; Ide et al. 1999) and (b) it suggests that endogenous mammalian background K+ channels could be important targets for general anaesthetics.
Acknowledgments
We would like to thank Professor Michel Lazdunski for providing sense and anti-sense probes for TASK-1 and for advice and support in the completion of this study. We would also like to thank Gisèle Jarretou for technical assistance with the in situ hybridisation. This work was funded by the British Heart Foundation, the Medical Research Council (UK) and the Centre National de la Recherche Scientifique.
References
- Bargmann CI. Neurobiology of the Caenorhabditis elegans genome. Science. 1998;282:2028–2033. doi: 10.1126/science.282.5396.2028. [DOI] [PubMed] [Google Scholar]
- Buckler KJ. A novel oxygen-sensitive potassium current in rat carotid body type I cells. The Journal of Physiology. 1997;498:649–662. doi: 10.1113/jphysiol.1997.sp021890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckler KJ, Vaughan-Jones RD. Effects of acidic stimuli on intracellular calcium in isolated type-I cells of the neonatal rat carotid body. Pflügers Archiv. 1993;425:22–27. doi: 10.1007/BF00374499. [DOI] [PubMed] [Google Scholar]
- Buckler KJ, Vaughan-Jones RD. Effects of hypoxia on membrane potential and intracellular calcium in rat neonatal carotid body type-I cells. The Journal of Physiology. 1994a;476:423–428. doi: 10.1113/jphysiol.1994.sp020143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckler KJ, Vaughan-Jones RD. Effects of hypercapnia on membrane potential and intracellular calcium in rat carotid body type-I cells. The Journal of Physiology. 1994b;478:157–171. doi: 10.1113/jphysiol.1994.sp020239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckler KJ, Vaughan-Jones RD. Effects of mitochondrial uncouplers on intracellular calcium, pH and membrane potential in rat carotid body type I cells. The Journal of Physiology. 1998;513:819–833. doi: 10.1111/j.1469-7793.1998.819ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckler KJ, Vaughan-Jones RD, Peers C, Lagadic-Gossmann D, Nye PCG. Effects of extracellular pH, pCO2 and HCO3− on intracellular pH in isolated type I cells of the neonatal rat carotid body. The Journal of Physiology. 1991;444:703–721. doi: 10.1113/jphysiol.1991.sp018902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez RA, Gray AT, Zhao BB, Kindler CH, Mazurek MJ, Mehta Y, Forsayeth JR, Yost CS. TWIK-2, a new weak inward rectifying member of the tandem pore domain potassium channel family. Journal of Biological Chemistry. 1999;274:7887–7892. doi: 10.1074/jbc.274.12.7887. [DOI] [PubMed] [Google Scholar]
- Davies RO, Edwards MW, Lahiri S. Halothane depresses the response of carotid body chemoreceptors to hypoxia and hypercapnia in the cat. Anesthesiology. 1982;57:153–159. doi: 10.1097/00000542-198209000-00002. [DOI] [PubMed] [Google Scholar]
- Duprat F, Lesage F, Fink M, Reyes R, Heurteaux C, Lazdunski M. TASK, a human background K+ channel to sense external pH variations near physiological pH. EMBO Journal. 1997;16:5464–5471. doi: 10.1093/emboj/16.17.5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink M, Duprat M, Lesage F, Reyes R, Romey G, Heurteaux C, Lazdunski M. Cloning, functional expression and brain localisation of a novel unconventional outward rectifier K+ channel. EMBO Journal. 1996;15:6854–6862. [PMC free article] [PubMed] [Google Scholar]
- Fink M, Lesage F, Duprat F, Heurteaux C, Reyes R, Fosset M, Lazdunski M. A neuronal two P domain K+ channel stimulated by arachidonic acid and polyunsaturated fatty acids. EMBO Journal. 1998;17:3297–3308. doi: 10.1093/emboj/17.12.3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshman CA, McCullough RE, Cohen PJ, Weil JV. Depression of hypoxic ventilatory response by halothane, enflurane and isoflurane in dogs. British Journal of Anaesthesia. 1977;49:957–963. doi: 10.1093/bja/49.10.957. [DOI] [PubMed] [Google Scholar]
- Ide T, Sakurai Y, Aono M, Nishino T. Contribution of peripheral chemoreception to the depression of the hypoxic ventilatory response during halothane anesthesia in cats. Anesthesiology. 1999;90:1084–1091. doi: 10.1097/00000542-199904000-00023. [DOI] [PubMed] [Google Scholar]
- Kim D, Fujita A, Horio Y, Kurachi Y. Cloning and functional expression of a novel cardiac two-pore background K+ channel (cTBAK-1) Circulation Research. 1998;82:513–518. doi: 10.1161/01.res.82.4.513. [DOI] [PubMed] [Google Scholar]
- Kindler CH, Yost CS, Gray AT. Local anesthetic inhibition of baseline potassium channels with two pore domains in tandem. Anesthesiology. 1999;90:1092–1102. doi: 10.1097/00000542-199904000-00024. [DOI] [PubMed] [Google Scholar]
- Knill RL, Gelb AW. Ventilatory responses to hypoxia and hypercapnia during halothane sedation and anesthesia in man. Anesthesiology. 1978;49:244–251. doi: 10.1097/00000542-197810000-00004. [DOI] [PubMed] [Google Scholar]
- Leonoudakis D, Gray AT, Winegar BD, Kindler CH, Harada M, Taylor DM, Chavez RA, Forsayeth JR, Yost CS. An open rectifier potassium channel with two pore domains in tandem cloned from rat cerebellum. Journal of Neuroscience. 1998;18:868–877. doi: 10.1523/JNEUROSCI.18-03-00868.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesage F, Guillemare E, Fink M, Duprat F, Lazdunski M, Romey G, Barhanin J. TWIK-1, a ubiquitous human weakly inward rectifying K+ channel with a novel structure. EMBO Journal. 1996;15:1004–1011. [PMC free article] [PubMed] [Google Scholar]
- Montoro RJ, Ureña J, Fernández-Chacón R, Alvarez de Toledo G, López-Barneo J. Oxygen sensing by ion channels and chemotransduction in single glomus cells. Journal of General Physiology. 1996;107:133–143. doi: 10.1085/jgp.107.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AJ, Honoré E, Lesage F, Fink M, Romey G, Lazdunski M. Inhalational anaesthetics activate two-pore-domain background K+ channels. Nature Neuroscience. 1999;2:422–426. doi: 10.1038/8084. [DOI] [PubMed] [Google Scholar]
- Pountney DJ, Gulkarov I, Vega-Sanez de Miera E, Holmes D, Saganich M, Rudy B, Artman M, Coetzee WA. Identification and cloning of TWIK-originated similarity sequence (TOSS): a novel human 2-pore K+ channel principal subunit. FEBS Letters. 1999;450:191–196. doi: 10.1016/s0014-5793(99)00495-0. [DOI] [PubMed] [Google Scholar]
- Reyes R, Duprat F, Lesage F, Fink M, Salinas M, Farman N, Lazdunski M. Cloning and expression of a novel pH-sensitive two pore domain K+ channel from human kidney. Journal of Biological Chemistry. 1998;273:30863–30869. doi: 10.1074/jbc.273.47.30863. [DOI] [PubMed] [Google Scholar]
- Salinas M, Reyes R, Lesage F, Fosset M, Heurteaux C, Romey G, Lazdunski M. Cloning of a new mouse two-P domain channel subunit and a human homologue with a unique pore structure. Journal of Biological Chemistry. 1999;274:11751–11760. doi: 10.1074/jbc.274.17.11751. [DOI] [PubMed] [Google Scholar]
- Ureña J, Fernandez-Chacon R, Bentot AR, Alvarez de Toledo G, Lopez-Barneo J. Hypoxia induces voltage-dependent Ca2+-entry and quantal dopamine secretion in carotid body glomus cells. Proceedings of the National Academy of Sciences of the USA. 1994;91:10208–10211. doi: 10.1073/pnas.91.21.10208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt CN, Peers C. Ca2+-activated K+ channels in isolated type I cells of the neonatal rat carotid body. The Journal of Physiology. 1995;483:559–565. doi: 10.1113/jphysiol.1995.sp020606. [DOI] [PMC free article] [PubMed] [Google Scholar]


