Abstract
Intracellular recording of lumbosacral motoneurones in the decerebrate and partially spinalized cat injected with nialamide and L-dihydroxyphenylalanine (l-DOPA) was used to investigate the interneuronal convergence of two bulbospinal pathways and of the segmental pathways involved with the generation of extensor activities during locomotion.
Deiter's nucleus (DN) or the medial longitudinal fasciculus (MLF) was stimulated in alternation with, and in combination with, stimulation of group I afferents from extensor muscles or of contralateral flexor reflex afferents (coFRA). The evoked polysynaptic EPSPs were recorded in extensor motoneurones when long-latency, long-lasting discharges were evoked by the stimulation of coFRA and when the group I autogenetic inhibition in extensors was reversed to polysynaptic excitation. Spatial facilitation was inferred when the amplitude of the EPSPs evoked by the combined stimuli was notably larger than the algebraic sum of the EPSPs evoked by individual stimulation.
Both DN (16 motoneurones) and MLF inputs (8 motoneurones) showed spatial facilitation when preceded by coFRA stimuli and both could reset the rhythm of fictive stepping by triggering a precocious extensor phase. MLF showed spatial facilitation with extensor group I inputs in 69 % of trials but DN failed to show spatial facilitation in any cells.
These results indicate that DN and MLF project to the coFRA pathways of the extensor half-centre for locomotion and MLF, but not DN, converge on segmental interneurones of the extensor group I pathways. The implications of such convergence patterns on the functional organization of the extensor half-centre are discussed.
In the frame of a study on the intrinsic organization of the neuronal networks comprising the rhythm generator, we investigated the role of two of the major bulbospinal signals involved in the initiation and control of locomotion: the vestibulospinal and the reticulospinal pathways. We focussed our investigation on the interaction between each of these two descending pathways with segmental pathways which are thought to be sharing interneurones with the rhythm generators in the spinal cord: the pathways transmitting load signals from ankle and knee extensor muscles through group I afferents fibres (referred to as ‘extensor group I’ in the text).
The extensor group I pathways excite extensor motoneurones to promote the thrust during the stance phase of walking (Duysens & Pearson, 1980; cf. Pearson et al. 1998). During fictive locomotion, stimulation of extensor nerves at group I strength can reset the locomotor rhythm, e.g. by interrupting the burst activity in flexor nerves and simultaneously triggering activity in extensor nerves (Conway et al. 1987; Gossard & Hultborn, 1991; Gossard et al. 1994). Moreover, repetitive stimulation of extensor group I fibres was found to entrain the stepping rhythm (Conway et al. 1987; Pearson et al. 1992). Further studies have revealed that both group Ia and Ib afferents from ankle and knee extensor muscles contribute to these effects by evoking di- and polysynaptic excitation to homonymous motoneurones instead of the classical autogenetic disynaptic inhibition observed in anaesthetized cats (Gossard et al. 1994; Guertin et al. 1995; McCrea et al. 1995). These findings have led to the hypothesis that extensor group I pathways may share some interneurones with the extensor half-centre (cf. Gossard & Hultborn, 1991). Thus, determining which supraspinal inputs converge onto these pathways should help fulfilling the functional identification of such interneurones during locomotion (Hultborn et al. 1998) which in turn may lead to a better understanding of the intrinsic connectivity of the central pattern generator (CPG).
Our hypothesis is that descending and segmental pathways capable of resetting the locomotor rhythm do so via convergence onto common spinal interneuronal pathways. Convergence between descending and segmental inputs was tested using the spatial facilitation technique (Lundberg, 1975; Baldissera et al. 1981; Gossard et al. 1996; Burke, 1999) in lumbosacral extensor motoneurones recorded intracellularly in partially spinalized cats injected with nialamide and L-dihydroxyphenylalanine (l-DOPA; see Russel & Zajac, 1979; Gossard et al. 1994). Our results showed that vestibulospinal pathways originating from the Deiter's nucleus (DN), some reticulospinal descending tracts in the medial longitudinal fasciculus (MLF) and extensor group I fibres all have the ability to reset the locomotor rhythm. However, there was a complete absence of spatial facilitation between inputs from DN and of extensor group I afferents indicating that their signals are transmitted through separate spinal pathways. On the other hand, spatial facilitation between MLF and extensor group I inputs was found in 69 % of the trials, indicating that both pathways share some spinal interneurones. Some preliminary results have been reported before (Leblond & Gossard, 1996; Leblond et al. 1997, 1998).
METHODS
Surgical procedures
The experiments were performed on 30 cats of either sex (2.5-4.3 kg). Vestibulospinal pathways were tested in 15 cats, MLF pathways were tested in 12 and both in 3 cats. All animal experiments were conducted according to the Guide for the Care and Use of Experimental Animals (Canada), using protocols approved by the Ethics Committee of the Université de Montréal. Anaesthesia was induced by inhalation of a mixture of halothane (2.0-3.0 %), oxygen (50 %) and nitrous oxide (50 %). The right common carotid artery was cannulated for monitoring blood pressure and the left one was ligated. The level of anaesthesia was monitored by checking for constricted pupils and by changes in heart rate or blood pressure. One jugular vein and one cephalic vein were cannulated for administration of fluids. All animals received an intravenous injection of prednisolone sodium succinate (Solu-delta-cortef, 1 mg kg−1) to minimize the shock and brainstem swelling following decerebration. Noradrenaline (norepinephrine) bitartrate (Levophed) was given when necessary to maintain blood pressure within physiological limits (80-120 mmHg). Atropine sulphate (0.4 mg s.c.) and furosemide (frusemide) (Lasix, 5 mg kg−1i.m.) were also administered to decrease excessive secretions and to improve diuresis, respectively.
The following flexor and extensor muscle nerves from the left hindlimb were dissected free, cut and mounted on bipolar silver chloride electrodes for recording (electroneurogram, ENG) and stimulation: posterior biceps-semitendinosus (PBSt), semimembranosus-anterior biceps (SmAB), lateral gastrocnemius-soleus (LGS), medial gastrocnemius (MG), plantaris (Pl), flexor digitorum longus and flexor hallucis longus together (FDHL), tibialis anterior (TA), extensor digitorum longus (EDL), superficial peroneal (SP) and the uncut sciatic (Sci) nerve just below PBSt nerve. The quadriceps (Quad) nerve was mounted in a polymer cuff electrode. In four experiments, three nerves were also mounted from the right (contralateral, co-) hindlimb: coPBSt, coSmAB and coSci.
After a laminectomy of the L4-L7 and T13 vertebrae, the cats were transferred to a stereotaxic frame and an extensive craniotomy was performed. The cat was then decerebrated by making a precollicular, postmammillary transection with a spatula (inclined at about 30 deg from the vertical plane) and removing the rostral nervous tissue by aspiration. Anaesthesia was then discontinued and the cats were paralysed with gallamine triethiodide (Flaxedil; 10 mg kg−1 supplemented every 45 min) and artificially ventilated maintaining the expired CO2 near 4 %. For all but two cats with spinal cord intact (one for DN and one for MLF experiments), the spinal cord was partially transected at T13 to eliminate as much as possible irrelevant descending pathways, leaving the appropriate spinal quadrant intact: only the ipsilateral (i) ventral quadrant for experiments with DN stimulation and both the i- and co-ventral quadrants for experiments with MLF stimulation. Pools were constructed using the skin flaps surrounding the spinal cord and the hindlimb nerves and were filled with warm mineral oil. Both rectal and pool temperature was kept near 38°C with radiant heat lamps. When necessary, a bilateral pneumothorax was performed to minimize respiratory movements and to stabilize intracellular recordings.
Recording and stimulation
The cord dorsum potential (CDP) was recorded with a silver chloride ball electrode located close to the dorsal root entrance at the L6-L7 border. Stimulus intensity for peripheral nerves was expressed in multiples of the threshold (T) for the most excitable fibres in the nerve as monitored from the CDP. The motoneurones were recorded intracellularly in L6-S1 spinal cord segments with glass micropipettes filled with 3 M potassium acetate or with 100 mM QX314 and 2 M potassium acetate to prevent sodium spikes. The impaled cells were identified by antidromic invasion from muscle nerve stimulation. FDL and FHL cells could be distinguished by their responses to SP stimulation and the phase of peak depolarization during fictive stepping (Burke, 1999). Only motoneurones with spikes larger than 45 mV, a membrane potential more negative than -40 mV and a stable DC signal were accepted for study. ENG activities of the different muscle nerves were recorded using differential AC amplifiers. The signals were filtered (10 Hz to 10 kHz) and visualized (Labview software: National Instruments) to monitor the locomotor activity.
A tungsten electrode was placed stereotaxically in the chosen supraspinal structure: DN (15 cats), DN and coMLF (3 cats), coMLF (8 cats), iMLF (3 cats), i- and coMLF 1 cat). Then the position of the electrode was adjusted in order to obtain a maximal antidromic volley from the T13 ventral quadrant stimulated with a bipolar tungsten electrode which was removed following optimal positioning. A final adjustment was made to ensure that stimulation at the supraspinal site evoked a maximal descending volley in the spinal lumbar segments. Figure 1 illustrates, for two different cats, examples of extracellular field potentials recorded in these steps. In Fig. 1a, the stimulation of T13 ventral quadrant (1 pulse, 100 μA) evoked a clear antidromic potential recorded in DN (on left) and coMLF (on right). The axons being stimulated at T13 evoked short-latency, sharp responses in both regions. Figure 1b shows the CDPs evoked by the supraspinal stimulation of DN (1 pulse, 125 μA, 300 Hz; left) and of coMLF (1 pulse, 75 μA; right). The responses evoked in these cases occurred with a slightly longer latency than in Fig. 1a because of the distance between the stimulation site and the recording site (CDP) is longer. At the end of the experiment, an electrolytic lesion (500 mA for 30 s) was made at the site of stimulation, the animal was killed by an i.v. injection of an overdose of sodium pentobarbitone (Somnotol), perfused with formalin (10 %) and the brainstem was removed for routine histology to ascertain electrode positioning. Figure 1C shows the position of large electrolytic lesions in the middle of the DN (left) and coMLF (right). The same procedure was performed for all 30 experiments and excellent positioning was confirmed in all of them.
Figure 1. Positioning of the stimulating electrode in the brainstem.

A, mean (n = 20) antidromic volleys (Supra) recorded in Deiter's nucleus (DN; left) and the contralateral medial longitudinal fasciculus (coMLF; right) evoked by the stimulation of the T13 ventral quadrant (1 pulse, 100 μA). B, mean (n = 20) cord dorsum potentials (CDP) at the L6 segment of the spinal cord evoked by the stimulation of DN (1 pulse, 125 μA; left) and coMLF (1 pulse, 75 μA; right). C, photomicrograph of the electrolytic lesion in DN (left) and MLF (right).
Spatial facilitation testing
For testing spatial facilitation, it was preferable to have a quiescent background in motoneurones without the presence of locomotor-drive potential (LDP) related to fictive stepping (see Gossard et al. 1994). Thus, L-DOPA (20-50 mg kg−1) was slowly injected after nialamide (a monoamine oxidase inhibitor, 10–50 mg kg−1) and the injection was stopped before the appearance of rhythmic oscillations in motoneurones, i.e. (1) when the disynaptic ‘autogenetic inhibition’ evoked by the stimulation of extensor group I fibres was replaced by polysynaptic excitation in homonymous motoneurones (see Gossard et al. 1994) and (2) when long latency and long-lasting reflex discharges were evoked by stimulation of the flexor reflex afferents (FRAs) (see Conway et al. 1987). Under these conditions, transmission into the locomotor-related group I pathways was possible but LDPs in motoneurones, rhythmic activities in ENGs and oscillations in dorsal roots (Dubuc et al. 1988) were absent. In most experiments, further injection of L-DOPA (up to 150 mg kg−1) led to the full development of fictive stepping. Under these conditions, we tested to find if supraspinal structures could affect (reset) the locomotor rhythm (e.g. Fig. 2) and examined the phasic modulation of the evoked excitatory post-synaptic potentials (EPSPs) during fictive stepping (e.g. Fig. 8). In some cats, the initial injection of L-DOPA led to irregular episodes of ENG bursts alternating with episodes of quiescence (up to a few minutes). We believe that these conditions were appropriate to test spatial facilitation by rejecting trials occurring during the active episodes.
Figure 2. Resetting of the locomotor rhythm by bulbospinal inputs.

A train of stimuli (indicated by the vertical line) in either DN (50 pulses, 75 μA; A) or iMLF (20 pulses, 80 μA; B) is able to reset the fictive step cycle by interrupting the flexor phase and evoking a precocious extensor phase. C, stimulation of iMLF (50 pulses, 80 μA) during the extensor phase resets the rhythm by delaying the ongoing bursts of activity in extensors. The continuous markers before the stimulation show the onset of the LGS bursts of activity whereas the dotted markers after the stimulation indicate the expected onset of the LGS bursts had the rhythm not been perturbed. PBSt: posterior biceps-semitendinosus; TA, tibialis anterior; SmAB, semimembranosus-anterior biceps; LGS, lateral gastrocnemius-soleus; MG, medial gastrocnemius; Pl, plantaris; FDHL, flexor digitorum longus and flexor hallucis longus together.
Figure 8. Modulation of the amplitude of the bulbospinal EPSPs during fictive locomotion.

A, tilted vertically are the high gain intracellular responses in a Sci extensor motoneurone evoked by stimulation of coMLF (3 pulses, 120 μA) above a low gain intracellular record (IC) of the same cell displaying its locomotor drive potential. B, superimposition of the mean EPSPs evoked by the stimulation of coMLF (3 pulses, 50 μA) given during (black trace; n = 20) and in between (grey trace; n = 20) the depolarized phases of the locomotor drive potentials in a MG motoneurone. C, superimposition of mean EPSPs evoked by the stimulation of DN (3 pulses, 100 μA) given during (black trace; n = 25) and in-between (grey trace; n = 25) the depolarized phases in another MG motoneurone.
For spatial facilitation, EPSPs in motoneurones were evoked by the electrical stimulation of: (1) ankle or knee extensor group I fibres (3-8 pulses, 1.1-2.0T, 300 Hz); (2) the supraspinal structure (DN: 1–6 pulses, 50–200 μA, 300 Hz or i- or coMLF: 3–8 pulses, 10–120 μA, 300 Hz) and (3) both together with different intensities of stimulation and different coupling intervals (e.g. Figs 6 and 7). We also tested the convergence between supraspinal pathways and coFRA (coPBSt, coSmAB, coSci; 50–100 pulses, 20-50T, 300 Hz) using the same stimulation protocol (e.g. Figs 4 and 5). In the majority of cases, individual and combined stimuli were interleaved. The intensity of supraspinal stimulation was always below 200 μA to minimize excitation spread to other supraspinal regions (Grillner et al. 1971). The coupling interval between two inputs was chosen to either synchronize: (1) the arrival of the peripheral and descending volleys at the spinal cord level (CDPs) or (2) the onset of the evoked EPSPs (Gossard et al. 1996; Leblond & Gossard, 1997; Leblond et al. 1998). Only cells showing a clear polysynaptic excitation (latency ≥ 3.5 ms, Gossard et al. 1994) were analysed for spatial facilitation. If the amplitude of the averaged EPSPs from the combined stimulation was notably larger (≥ 0.25 mV) than the algebraic sum of individual responses recruited from the subliminal fringe, it was taken as evidence for spatial facilitation, i.e. probable convergence at a interneuronal level (Lundberg, 1975; Baldissera et al. 1981; Burke, 1999).
Figure 6. Interactions between DN, MLF and extensor group I inputs.

A, C and E, superimposition of the mean EPSPs (n >= 25) evoked by extensor group I fibres (grey trace) and by supraspinal site (black trace) stimulated individually. B, D and F, superimposition of the mean EPSPs (n >= 25) evoked by the combined stimulation (black trace) over the algebraic sum of the two individual responses (dotted line). A and B, in a MG motoneurone (same as in Fig. 4): stimuli from DN (3 pulses, 70 μA) and Pl (3 pulses, 1.8T). Calibration pulse in A and B equals 1 mV. C and D, in a Pl motoneurone, stimuli from DN (6 pulses, 100 μA) and LGS (6 pulses, 1.8T). E and F, in a MG motoneurone (from the same track as Pl in C and D), stimuli from coMLF (3 pulses, 50 μA) and LGS (6 pulses, 1.2T). Vertical scale bar equals 0.5 mV for C and D; 2 mV for E and F.
Figure 7. Spatial facilitation between MLF and extensor group I inputs in a non-spinal cat without L-DOPA.

A, superimposition of the mean EPSPs in a SmAB motoneurone evoked by the stimilation of coMLF (8 pulses, 20 μA; black trace; n = 25) and of GS (4 pulses, 1.8T; grey trace; n = 25) with a coupling interval of 10 ms. B, the mean EPSPs in the same motoneurone evoked by the combined stimulation of coMLF and LGS (black trace; n = 25) has a larger amplitude than the superimposed algebraic sum of the two individual responses (dotted line).
Figure 4. Spatial facilitation between DN and coFRA inputs.
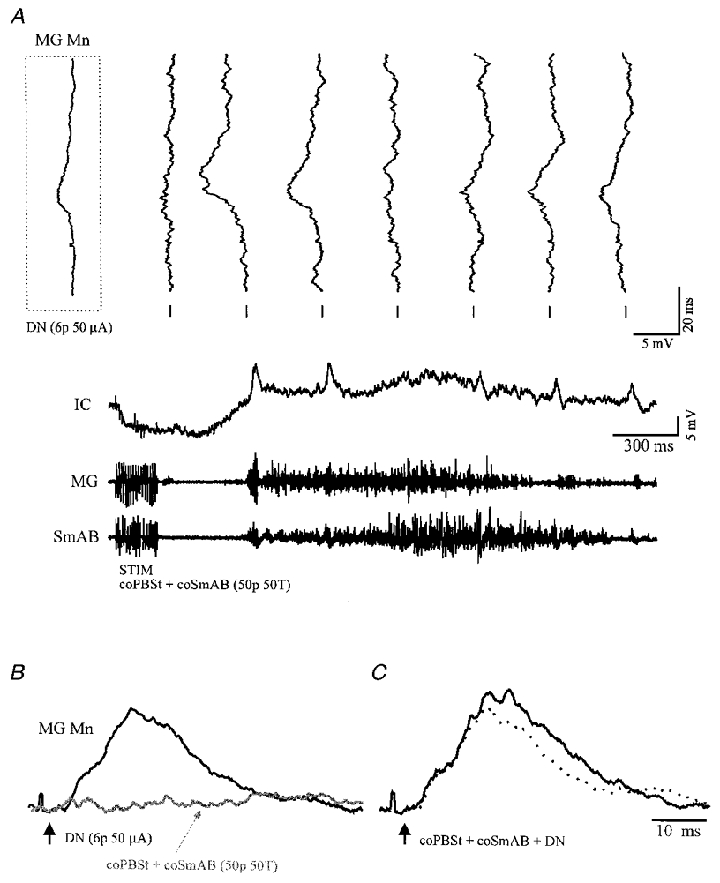
A, high gain intracellular responses in a MG motoneurone evoked by stimulation of DN (6 pulses, 50 μA) are tilted vertically over a low gain intracellular record (IC) of the same MG cell displaying a late, long-lasting EPSP evoked by stimulation of coPBSt + coSmAB (50 pulses, 50T). The first tilted trace in the inset is the mean EPSP (n = 20) evoked by the same DN stimulation in the absence of coFRA stimulation while the seven other tilted traces are individual responses to the DN stimulation given three times per second. Underneath are the evoked ENG activities of the MG and SmAB nerves. B and C, spatial facilitation test between DN and coFRA in the same MG motoneurone. B, superimposition of the mean EPSPs evoked by the separate stimulation of DN (6 pulses, 50 μA; black trace; n = 25) and of coPBSt + coSmAB (50 pulses, 50T; grey trace pointed by the thin arrow; n = 5). Note that the latter trace is only the portion of the coFRA depolarisation used for spatial facilitation. C, the mean EPSPs (n = 25) evoked by the combined stimulation (black trace) of DN and of coPBSt + coSmAB is larger than the algebraic sum of the two individual responses (dotted trace), thus indicating spatial facilitation. The vertical black arrows indicate the onset of the DN stimulation. Calibration pulse in B equals 1 mV.
Figure 5. Spatial facilitation between MLF and coFRA inputs.

A, superimposition of the mean EPSPs evoked in a SmAB motoneurone by the separate stimilation of coMLF (6 pulses, 70 μA; black trace; n = 25) and of coSci (100 pulses, 20T; grey trace indicated by the thin arrow; n = 8). Note that the latter trace is only the portion of the coFRA depolarisation used for spatial facilitation. B, the mean EPSPs in the same motoneurone evoked by the combined stimulation (black trace; n = 25) of coMLF and of coSci is larger than the algebraic sum of the two individual responses (dotted line), showing spatial facilitation. C, superimposition of the mean EPSPs evoked in a Pl motoneurone by the separate stimilation of coMLF (6 pulses, 100 μA; black trace; n = 25) and of coSci (100 pulses, 20T; grey trace indicated by the thin arrow; n = 5). As in A, the latter trace is only the portion of the coFRA depolarisation used for spatial facilitation. D, the mean EPSPs in this Pl motoneurone evoked by the combined stimulation (black trace; n = 25) of coMLF and of coSci which is much larger than the algebraic sum of the two individual responses (dotted line), thus indicating spatial facilitation. The vertical black arrows indicate the onset of the coMLF stimulation. Calibration pulses equal 1 mV.
All signals were recorded on videotape after digitization (Vetter 4000A; 15 channels with 200 μs risetime per channel). Tapes were played back off-line on an electrostatic printer (Gould ES-1000) and sections of data suited for analysis were digitized and analysed with interactive custom-made software (Spinal Cord Research Centre, University of Manitoba, Winnipeg).
RESULTS
Resetting of locomotor rhythm by DN and MLF stimulation
As mentioned in Methods, when the fictive stepping fully developed after administration of nialamide and L-DOPA, we studied the effects of the chosen supraspinal input on the locomotor rhythmicity. As described previously (Conway et al. 1987; Gossard & Hultborn, 1991; Gossard et al. 1994; Leblond & Gossard, 1997), a reset of the locomotor rhythm occurs when the stimulation is able to disrupt the rhythmic activities in all muscles of the hindlimb and to shift in time the onset of all following step cycles. Two patterns can be easily recognized: (1) the stimulation can interrupt the ongoing activities (e.g. flexor phase) and reinitiate precociously the bursts of activity in all antagonist muscles (e.g. extensor phase) or (2) the stimulation can prolong the ongoing activities (extensor phase) and delay the onset of activity in all antagonist muscles (e.g. flexor phase).
The stimulation of DN promotes the generation of the extensor phase of the fictive step cycle
The stimulation of DN had the same effect on the fictive stepping rhythm in all trials (n = 39, 11 cats). As illustrated in Fig. 2a, stimulation of DN (50 pulses, 75 μA, 300 Hz) during the flexor phase interrupted the flexor activities (represented by TA ENG) and initiated an extensor phase precociously. This can be appreciated by comparing the onsets of the LGS (or MG) ENG bursts with the dotted markers which indicate the expected onsets of extensor bursts had the rhythm been unperturbed. In this particular experiment, the coPBSt nerve was recorded (3rd trace) and its bursts of activity occurred in alternation with iPBSt and in synchrony with the ipsilateral extensor nerves given the alternating ipsi- and contralateral activities in stepping. It can also be seen that the stimulation of DN induced a precocious phase of activity in coPBSt ENG simultaneous to the ipsilateral extensor nerves. On the other hand, if the DN stimulation was given during the extensor phase, it was possible to prolong the extensor phase and delay the onset of the following flexor phase (not shown, see Leblond & Gossard, 1997). Moreover, repetitive stimulation of DN was found to entrain the locomotor rhythm (not shown).
The effect of stimulating MLF on the locomotor rhythm depends on the phase of stimulation
Overall, descending volleys evoked by stimulation of i- or coMLF had mixed effects on the timing of fictive locomotor bursts of activity. In 12 cats, we examined the effects of MLF stimulated during the flexor phase. The most common action of MLF is illustrated in Fig. 2B. A train of stimuli in iMLF (20 pulses, 80 μA, 300 Hz) stopped the flexor phase (TA ENG) and evoked a premature onset of the extensor phase (LGS, MG ENGs). Note that in the particular case of Fig. 2b, the onset of the extensor phase was first preceded by the activation of a short burst of activity in coPBSt (see arrow in 3rd trace). This pattern of resetting was observed both by iMLF (n = 2) or coMLF (n = 1) stimulation in 3 out of 4 experiments where co-nerves were recorded. It is not known, given the bifunctional nature of PBSt, if the evoked short burst of activity represents flexor or extensor-related activity (Perret & Cabelguen, 1980). Such pattern of resetting was never observed with the stimulation of DN. Also it was noted that, during the train of stimuli, there were short-latency excitatory responses in many nerves as may be seen in Fig. 2b (see Floeter et al. 1993). Overall, this pattern of resetting was observed in 58 % of the trials where i- or coMLF was stimulated during the flexor phase. In other cases, the stimulation of MLF interrupted (33 % of trials) or prolonged (9 % of trials) the activity in flexor nerves without evoking a clear reset in rhythmicity.
If the train of stimuli in MLF was given during the extensor phase (tested in 9 cats), the most common response was to temporarily promote flexor activities. Indeed, as illustrated in Fig. 2C, the stimulation of iMLF (50 pulses, 80 μA, 300 Hz) evoked responses in ipsilateral flexors (TA ENGs) and coPBSt during the train but also a short burst of activity following it. Whether the bursts of activity in extensor nerves were temporarily interrupted during this response or entirely reinitiated (LGS, MG ENGs) is undetermined. It is clear, however, that the locomotor rhythm was reset because all the following cycles were delayed by the stimulation. Overall, this pattern of resetting was observed in 56 % of the trials where MLF was stimulated during the extensor phase. In other cases, the stimulation of MLF interrupted (33 % of trials) or increased (11 %) the bursts of extensor activity without a clear reset.
The effects of stimulating group I afferents from extensors on the fictive locomotor rhythm was also tested in some experiments (not shown). Group I stimulation reset the rhythm by promoting the generation of the extensor phase as described previously in similar preparations (Conway et al. 1987; Gossard et al. 1994).
Vestibulo- and reticulospinal excitation of extensor motoneurones following L-DOPA administration
It has been described that the injection of L-DOPA in spinal cats induced a ‘reflex reversal’ from group Ib afferents of extensors: the ‘classical’ group Ib autogenetic disynaptic inhibition observed in anaesthetized cats was replaced by an polysynaptic excitation (Gossard et al. 1994). In the present study, it was possible to follow the changes in postsynaptic potentials (PSPs) in three extensor motoneurones in response to supraspinal inputs (DN, iMLF, coMLF) with the progressive injection of L-DOPA. In all cases, the amplitude of polysynaptic EPSPs in extensor motoneurones increased following L-DOPA injection. Figure 3a shows mean EPSPs from an MG motoneurone in response to a train of stimuli in DN (3 pulses, 100 μA, 300 Hz) recorded at 8, 15 and 55 min of slow injection of L-DOPA. It is obvious that the amplitude of EPSPs grows as the injection of L-DOPA progresses. Note that the short-latency EPSPs did not change notably while the late component (polysynaptic) clearly increased.
Figure 3. The amplitude of polysynaptic EPSPs from DN and MLF increases with the administration of L-DOPA.

In A and B the upper traces are superimposed mean intracellular recordings (IC) and the lower traces are mean cord dorsum potentials (CDP). A, mean responses (n >= 18) of a MG motoneurone to stimulation in DN (3 pulses, 100 μA) 8, 15 and 55 min after injection of L-DOPA. B, mean responses (n >= 25) of a Pl motoneurone to stimulation in coMLF (3 pulses, 50 μA) 1.5, 6.5 and 25.7 min after injection of L-DOPA.
Figure 3b illustrates, in another experiment, the averaged EPSPs in a Pl motoneurone in response to a train of stimuli in coMLF (3 pulses, 50 μA, 300 Hz) at 1.5, 6.5 and 25.7 min after the injection of L-DOPA. Again, it is clear that the amplitude of the late components of the EPSPs increased with the L-DOPA injection. The short-latency EPSPs appeared to change, if anything, in the opposite direction. Similar increases in late EPSPs were found in one other extensor motoneurone in response to the stimulation of iMLF.
Interactions between bulbospinal and segmental inputs
Convergence between bulbospinal and coFRA pathways
The emergence of DN, MLF and group I polysynaptic excitation with L-DOPA described in the previous section was observed in parallel with the appearance of long-lasting, late reflexes in response to FRA stimulation (‘Late DOPA reflexes’; Anden et al. 1966; Jankowska et al. 1967a,b). The late DOPA reflexes are believed to be mediated by interneuronal networks which are involved in the generation of the swing and stance phases of stepping (Jankowska et al. 1967a,b; Lundberg, 1969). The group I afferents from extensor muscles have already been described as sharing some spinal interneurones with the coFRA pathways (Conway et al. 1987; Gossard et al. 1994). It is thus of interest to evaluate whether DN or MLF pathways also share the FRA interneurones mediating the late DOPA reflexes.
First, it is important to mention that, in partially spinalized cats, late DOPA reflexes often showed a shortened duration and faster onset than those seen in complete spinalized cats (Jankowska et al. 1967a,b; Conway et al. 1987; Gossard et al. 1994). Actually, the long-lasting depolarization evoked in extensor motoneurones in our preparations had a time course similar to that of the depolarized phase of LDPs. It is likely that the remaining descending pathways passing through the intact quadrant of the spinal cord were the cause of this difference by their influence on the coFRA circuitry (Engberg et al. 1968a,b). Notwithstanding this difference, we tested for convergence with the spatial facilitation technique (see Methods) between coFRA and DN inputs in 16 motoneurones (1 LGS, 8 MG, 1 SmAB, 1 FHL and 5 QUAD) in 5 cats and between coFRA and MLF inputs in 8 motoneurones (2 FHL, 2 MG, 1 LGS, 2 Pl, 1 SmAB) in 4 cats.
A representative example of spatial facilitation between coFRA and DN is illustrated in Fig. 4. Figure 4a illustrates a typical long-lasting depolarization in a MG motoneurone in response to coFRA stimuli (coPBSt + coSmAB, 50 pulses, 50T, 300 Hz) together with the excitatory responses in two extensor nerves, MG and SmAB. During this coFRA excitation, DN was stimulated (6 pulses, 50 μA, 300 Hz) three times per second in order to have different conditioning-test intervals (top tilted traces). The EPSP in the inset on the left of Fig. 4a is the mean DN response (n = 20) without coFRA conditioning. The DN-EPSPs were clearly enhanced on the rising phase of the depolarization (2nd and 3rd traces) and became much smaller when the extensor activity was maximal (occlusion, see Discussion). Figure 4b gives the averaged EPSPs in response to DN (black trace; 6 pulses, 50 μA, 300 Hz) and to coFRA stimuli (grey trace; coPBSt + coSmAB, 50 pulses, 50T, 300 Hz) given independently. In Fig. 4C, the dotted trace is the algebraic sum of the individual responses while the black trace is the averaged DN-EPSPs occurring during the 1st third (before occlusion) of coFRA-evoked depolarization (n = 7) in that cell. It is clear that the response to DN stimulation is much larger when preceded by coFRA, i.e. there is spatial facilitation. Note that an equivalent DN stimulation in the same motoneurone failed to facilitate the EPSPs from extensor group I fibres (Fig. 6a and B, see below). Similar facilitation between coFRA and DN inputs was observed in all the 16 extensor motoneurones tested (see also Ten Bruggencate et al. 1969; Ten Bruggencate & Lundberg, 1974).
Figure 5 shows two different examples of spatial facilitation between MLF and coFRA. Figure 5a shows that the amplitude of the averaged EPSPs in a SmAB motoneurone evoked by the coMLF (6 pulses, 70 μA, 300 Hz) and of coFRA pathways (coSci 100 pulses, 20T, 300 Hz) stimulated individually. Figure 5b shows that the response to the combined stimulation (black trace) was much larger than the sum of the two individual responses (dotted line), indicating spatial facilitation. Another example of spatial facilitation between coMLF and coFRA pathways in a Pl motoneurone is illustrated in Fig. 5C and D with the same outline. Again, the amplitude of the averaged EPSPs evoked by the combined stimulation of MLF (6 pulses, 100 μA, 300 Hz) and coFRA pathways (coSci 100 pulses, 20T, 300 Hz; black trace in Fig. 5D) is much larger than the sum of the two individual responses (dotted line). In this particular example, it is interesting to note that the combined stimulation not only increased the EPSP amplitude but also appears to reduce its latency. Spatial facilitation was observed in all eight motoneurones tested between MLF and coPBSt, coSci or co-dorsal rootlets, as a coFRA source.
Interactions between bulbospinal pathways and group I afferents from extensor muscles
In the previous sections, we demonstrated that vestibulo- and MLF volleys could reset the stepping rhythm as it was described for group I afferents from extensor muscles. Moreover, we showed that there was spatial facilitation between the supraspinal and the coFRA inputs. These two lines of evidence suggest that the two supraspinal inputs may converge on spinal interneurones comprised in the group I pathways as well. Using again the spatial facilitation technique, we tested this premotoneuronal convergence in extensor motoneurones.
The convergence between DN and extensor group I inputs was tested in 46 trials (some including several tests with different coupling intervals) in 37 extensor motoneurones (4 SmAB, 3 Sci, 3 LGS, 16 MG, 2 Pl, 4 FHL, 5 QUAD). Figure 6a and B gives an example of the interaction between extensor group I and DN inputs in a MG motoneurone. In Fig. 6a, the EPSPs were evoked by the stimulation of DN (3 pulses, 70 μA, 300 Hz; black trace) and of extensor group I fibres from Pl (3 pulses, 1.8T, 300 Hz; grey trace) given separately. The response to the combined stimulation of DN and of extensor group I fibres (black trace) shown in Fig. 6b was actually smaller than the sum of the two individual responses (dotted line), showing that there was no spatial facilitation. Lower intensity of stimulation led to the same results (not shown; see Leblond & Gossard, 1997). This was a surprising result because a clear spatial facilitation was seen in the same MG motoneurone using DN and coFRA stimuli (see Fig. 4b). Overall, a convincing absence of spatial facilitation was observed in all 46 trials, including those using low or high intensities of stimulation, different number of pulses in the train and different coupling intervals. Another example of absence of spatial facilitation between extensor group I and DN inputs in a Pl motoneurone is shown in Fig. 6C and D, using a longer train of stimuli. Figure 6C depicts the individual responses to the stimulation of DN (6 pulses, 100 μA, 300 Hz; black trace) and of LGS (6 pulses, 1.8T, 300 Hz; grey trace). The absence of spatial facilitation is obvious in Fig. 6D; the averaged EPSP evoked by the combined stimulation (black trace) being, if anything, smaller than the summation of the two individual responses (dotted line).
We also tested spatial facilitation between extensor group I and MLF inputs. A typical example is depicted in Fig. 6E and F from a MG motoneurone recorded in the same micropipette track where the Pl motoneurone of Fig. 6C and D was found. The individual responses to coMLF (3 pulses, 50 μA, 300 Hz; black trace) and to LGS (6 pulses, 1.2T, 300 Hz; grey trace) stimuli are shown in Fig. 6E. It is clear that in Fig. 6F the response to the combined stimulation (black trace) was larger than the sum of the two individual responses (dotted trace), a sign of spatial facilitation. In the 61 extensor motoneurones tested (4 Sci, 14 MG, 12 LGS, 1 Pl, 15 FHL, 15 SmAB), spatial facilitation was observed in 69 % of the 83 trials (including 244 different combinations) in experiments where MLF stimulation promoted extensor activities (see above). Similar level of spatial facilitation was obtained in different cats whether we stimulated iMLF, coMLF, or both in the same cat. As explained in Methods, convergence was not tested when the MLF stimulation did not evoke a clear polysynaptic excitation in extensor motoneurones.
In one experiment, we tested the interaction between MLF and extensor group I inputs in a non-spinal cat without the injection of L-DOPA. Low levels of tonic activities in ENGs were alternating with episodes of irregular bursts of activity. In periods showing no motoneuronal oscillations, individual and combined stimuli were interleaved (see Methods) and only complete trials showing clear polysynaptic excitation from extensor group I fibres were kept for analysis. A representative result is given in Fig. 7. Figure 7a shows the averaged EPSP evoked in a SmAB motoneurone by the stimulation of coMLF (8 pulses, 20 μA, 300 Hz; black trace) and of LGS plus MG (GS; 4 pulses, 1.8T, 300 Hz, 10 ms interval; grey trace) separately. Even though the spinal quadrants were intact, we believe that the descending volleys were only from reticulospinal axons as recruited by this low stimulation intensity in MLF (20 μA). In Fig. 7b, it is clear that the responses evoked by the combined stimulation (black trace) was larger than the sum of the two individual responses (dotted line). Thus, even without L-DOPA, it is possible to observe a clear facilitation between MLF and extensor group I inputs.
Modulation of bulbospinal responses during fictive locomotion
Gossard et al. (1994) reported that the amplitude of group I polysynaptic EPSP in extensor motoneurones was minimal during the extensor phase and maximal during the flexor phase. The presence of spatial facilitation between MLF and extensor group I inputs suggests that MLF polysynaptic EPSPs may have a similar pattern of modulation in amplitude during fictive locomotion. Figure 8 illustrates such modulation. High-gain intracellular recordings of the responses to a train of stimuli in coMLF (3 pulses, 120 μA, 300 Hz) are tilted vertically over a low-gain intracellular recording of the LDP in an Sci motoneurone in which depolarization was synchronous with the activity in extensor ENGs. The first three traces show large EPSPs in response to coMLF stimulation. The third one, which occurs at the onset of the depolarized phase of the LDP, reached the firing threshold (arrow). This is not uncommon with QX314 in the micropipette in similar conditions (Brownstone et al. 1994). The next three responses (asterisks) which occurred at the top of the depolarized phase are greatly reduced in amplitude. Soon after the onset of the hyperpolarized phase, the excitatory responses resume (last tilted trace).
In contrast to this modulation pattern, Floeter et al. (1993) observed that the amplitude of the disynaptic MLF EPSP was maximal during the depolarized phase of the LDP in many motoneurones including extensors. We thus investigated whether there was a difference in the modulation of the amplitude of di- vs. polysynaptic components of the MLF EPSPs during fictive locomotion in four different extensor motoneurones (4 cats). EPSPs occurring at the top of the depolarized phase of the LDP were averaged together and compared with those occurring in the hyperpolarized phase. A representative example is given in Fig. 8b where the black trace is the averaged EPSP in a MG motoneurone evoked by coMLF stimulation (3 pulses, 50 μA, 300 Hz) at the top of the depolarized phase and the grey trace is the evoked EPSPs during the hyperpolarized phase. Note that the disynaptic EPSP (arrow) was larger when the motoneurone was depolarized. It is clear, however, that the amplitude of MLF EPSPs of longer latency was much smaller when the motoneurone was depolarized. The same result was obtained with the stimulation of DN during fictive locomotion in the two extensor motoneurones (2 cats) as depicted in Fig. 8C. The averaged EPSPs from this MG motoneurone show clearly that the amplitude of the EPSPs of long latency evoked by the stimulation of DN (3 pulses, 100 μA, 300 Hz) is much smaller at the top of the depolarized phase (black trace) than during the hyperpolarized phase of the LDP (grey trace). There was a much smaller modulation of the disynaptic component in this motoneurone with a small unexpected (Gossard et al. 1996) increase during the hyperpolarized phase.
DISCUSSION
In this paper, a variety of protocols have been used to investigate the interactions between two bulbospinal systems, the vestibulo- and reticulospinal pathways included in the MLF, and spinal cord networks which are believed to be involved in the generation of extensor activities during locomotion. A great level of similarity was found between the actions of these two systems and of the group I signals from extensor muscles on the locomotor rhythm and on coFRA networks. However, a striking and unexpected difference was seen in the convergence patterns for the two descending systems.
We will argue, as stated in our initial hypothesis (see Introduction), that the similarity between reticulospinal system and extensor group I fibres in resetting the locomotor rhythm, their convergence on coFRA networks and the convergence between them suggest strongly that they share common spinal interneurones during fictive locomotion. Furthermore, we will argue that the similarity between vestibulospinal system and extensor group I fibres in resetting action and convergence on coFRA networks suggest strongly that they are acting on spinal cord networks generating extensor activities. However, the resolute absence of convergence between them suggests that their actions are mediated through separate spinal cord pathways. This in turn suggests that there are at least two, and possibly more, separate spinal pathways converging on extensor motoneurones to generate locomotor activities.
The extensor half-centre and the vestibulo- and reticulospinal pathways
In early studies using L-DOPA treated acute spinal cats, it was reported that single trains to the FRAs could evoke brief periods of alternating activity in flexor and extensor nerves (Lunberg, 1979). These alternating bursts of activity were believed to be forerunners of locomotion and it was thus proposed that the pathways mediating the FRA responses involved the same neuronal networks as the rhythm generator of locomotion (see Lunberg, 1979; Gossard & Hultborn, 1991). In the half-centre model, the iFRAs induce activity in the flexor motoneurones and coFRAs in extensor motoneurones and these two centres have reciprocal inhibitory connections (Jankowska et al. 1967a,b; Lundberg, 1969; Gossard & Hultborn, 1991). This is schematically illustrated in Fig. 9 as boxes F and E, respectively, with the coFRAs projecting onto the extensor half-centre (E box). However, in the light of these studies, it could be argued that the FRA interneuronal pathways could be located between the rhythm generator and motoneurones and not be intrinsic components of rhythmogenesis (see Hultborn et al. 1998). This possibility has been ruled out recently by Schomburg and collaborators (1998) who reported that short trains of FRA inputs induced a clear resetting of the fictive locomotor rhythm in high-spinal curarized cats. They concluded that the FRA afferent system had access to the spinal locomotor rhythm generators, and that at least part of the ‘late’ flexor reflexes seen after L-DOPA injection were indeed mediated via the rhythm-generating interneurones. Following similar reasoning, we can extrapolate that a convergence between a given afferent system (segmental or supraspinal) and the FRA pathways may be taken as evidence that the given afferent system also shares interneurones with the rhythm generator for locomotion.
Figure 9. Schematic diagram showing interactions between extensor group I afferents, coFRA, DN and MLF onto the di- and polysynaptic pathways to motoneurones.

The rhythm generator is represented by a flexor (F) and an extensor (E) half-centre with reciprocal inhibitory connections. The MLF, coFRA and extensor group I fibres all converge onto common interneurones comprised in the extensor half-centre. DN and extensor group I fibres converge onto common interneurones within the extensor half-centre. The two circles in the E box represents two separate interneuronal populations. Also represented are the projections from DN and MLF onto interneurones of the disynaptic pathways to motoneurones. Monosynaptic connections are not illustrated. Ext Gr I, extensor group I inputs; Ext Mn, extensor motoneurones; In, interneurones.
Spatial facilitation between each of the two bulbospinal systems and the coFRA was found in 100 % of the trials and strongly suggests that both vestibulo- and reticulospinal pathways project onto interneurones of the coFRA circuitry. Previously, a convergence between vestibulospinal disynaptic responses and coFRA excitation had been reported in chloralose anaesthetized cats (Ten Bruggencate et al. 1969; Ten Bruggencate & Lundberg, 1974). It was shown that stimulation of the DN, which previously had no detectable effect on an extensor motoneurone, if preceded by a conditioned volley in coFRA, evoked an appreciable EPSP with a disynaptic central latency. This was later confirmed by Ten Bruggencate & Lundberg (1974) and they also mentioned briefly that facilitation was also observed with vestibulospinal responses of longer latencies but did not further investigate this issue. Our results did not reveal a consistent facilitation of disynaptic DN-EPSPs by coFRA stimulation and this was not investigated further. There was, however, a clear spatial facilitation by coFRA excitation of DN-EPSPs occurring at long latency (> 3.5 ms; Gossard et al. 1994) consistent with a polysynaptic pathway to motoneurones.
There was also clear spatial facilitation between coFRA and the MLF inputs. To our knowledge this convergence has never been reported in L-DOPA treated spinal cats before. The MLF carries axons from the brainstem which project to various locations of the spinal cord including the lumbosacral spinal cord. Stimulating MLF is a convenient way to specifically activate reticulospinal fibres which project to the lumbar spinal cord since large fibres from other systems like the medial vestibulospinal, tectospinal and interstitiospinal pathways, which also have axons passing in the MLF, terminate in the cervical and the thoracic level of the spinal cord (Floeter et al. 1993; Gossard et al. 1996). Notably, included in the iMLF are the axons from the larger cells located in the pontine gigantocellular tegmental field whereas larger cells of the bulbar gigantocellular tegmental field send their axons through the coMLF (see Mitani et al. 1988). The mesencephalic locomotor region (MLR), which is often used to initiate locomotion in decerebrate cat (Shik & Orlovsky, 1976; Jordan et al. 1979; Shefchyk & Jordan, 1985) do not send their axons directly to the lumbar spinal cord but rather terminate on neurons of various regions in the brainstem which project to the spinal cord through the ventral funiculus (Steeves & Jordan, 1984). Two targets in the brainstem for MLR cells are the gigantocellular and the magnocellular tegmental field of the reticular formation (Garcia-Rill et al. 1983) which send their projections into the ventral funiculus (Basbaum et al. 1978) via the MLF (Mitani et al. 1988). Thus, some of these axons must have been stimulated in our experiments and participate in the excitatory actions to motoneurones. The minimum linkage between MLR and hindlimb motoneurones has been determined to be a trisynaptic pathway, with the first contact in the reticular formation (Shefchyk & Jordan, 1985) and the second contact on spinal interneurones. If this pathway is included in our MLF stimulation then it indicates indirectly that MLR commands also converge on the FRA networks.
Previous studies have shown that the FRA networks are under the control of two inhibitory systems from the reticular formation (reviewed in Jankowska, 1992): (1) the dorsal reticulospinal system sends descending axons into the dorsal part of the lateral funiculus and inhibits FRA pathways to motoneurones and to primary afferents (Engberg et al. 1968a,b). The section of the spinal cord at T13 performed in our experiments probably destroyed most of this tract; (2) the ventral reticulospinal system which sends axons into the ventral part of the lateral funiculus and/or into the ventral funiculus could have been activated by our MLF stimuli. The stimulation of this system causes a collapse of the decerebrate rigidity and suppression of stretch reflexes (see Baldissera et al. 1981). The facilitation of the activity of this system by coFRA stimuli as seen in this study could occur in two different ways. First, if the ventral reticulospinal system remains inhibitory after injection of L-DOPA, then its facilitation with coFRA excitation could be mediated through an inhibition of the flexor half-centre. Indeed, because of the reciprocal inhibitory contacts between the two half-centres, a reticulospinal depression of the flexor half-centre would liberate the extensor half-centre from inhibition and facilitate its transmission. On the other hand, if the action of the ventral reticulospinal system is reversed to excitatory, concomitantly with the group I pathways from extensors, then the reticulospinal input may be redirected directly through the extensor half-centre and thus converge on coFRA interneurones. There is no direct evidence indicating whether the ventral reticulospinal system remains inhibitory or reverses to excitatory after L-DOPA injection. We like to interpret the progressive increase in MLF excitation with L-DOPA injection (Fig. 3) as being due, in part, to the reversal of inhibitory action of reticulospinal pathways and/or to the facilitation of reticulospinal excitatory pathways. Finally, there is a possibility that the injection of L-DOPA which is known to boost the release of noradrenaline and serotonin from descending axons in spinal cats (Anden et al. 1966) could have increased the transmitter release directly from reticulospinal terminals and participate in the facilitation. However, convergence between MLF (or DN) and extensor group I fibres was also obtained in a non-spinal decerebrate cat which did not receive L-DOPA.
Another, more obvious, observation suggesting that a given afferent pathway shares interneurones with rhythm generators is its ability to reset or entrain the locomotor rhythm (Conway et al. 1987; Pearson et al. 1992; Gossard et al. 1994; Schomburg et al. 1998; Hultborn et al. 1998). Effects of vestibulospinal and reticulospinal stimuli on the locomotor rhythm have been investigated before. Orlovsky (1972) reported that MLF stimulation during walking in the decerebrate cat increased the amplitude of ipsilateral flexor muscles activity when applied during the period of natural activity. Since no effect on the timing of the step cycle was seen, it was concluded that the function of reticulospinal pathways was to control the level of activity of muscles whereas the timing of the step cycle was dependent on spinal mechanisms. Later, Drew & Rossignol, (1984) showed that longer trains of stimuli in the medullary reticular formation (MRF) during walking in the decerebrate cat not only regulated the amplitude of activity in ipsilateral muscles but also affected the timing of the step cycle. Furthermore, Drew (1991) showed that stimulation of different loci in the reticular formation or of the MLF in the intact cat walking on a treadmill gave qualitatively the same results. Resetting of the fictive rhythm with MLF in L-DOPA partially spinalized cats was first reported by Russel & Zajac (1979). They showed that stimulation of the ‘flexor’ region of the MLF affected the timing of the step cycle by increasing the duration of bursts of activity in flexors. They also reported that DN stimulation, if given during the extensor phase, could prolong the latter and delay the onset of the next flexor phase of the step cycle, inducing a reset of the fictive locomotor rhythm. Also, Perreault and collaborators (1994) showed that stimulation of different loci in the MRF during fictive locomotion produced a clear reset in the ENGs of the forelimbs and hindlimbs of the decerebrate cat. Our results confirmed that both DN and MLF could dramatically alter the locomotor rhythm (e.g. Fig. 2). The ENG patterns following a reset by DN stimuli resembled the patterns obtained with stimulation of extensor group I fibres (Conway et al. 1987; Gossard et al. 1994) while the reset patterns obtained with the MLF stimuli were more complex and depended on the phase of the step cycle as well as the precise position of the electrode within MLF. Because reticulospinal pathways have wide and complex projections to the spinal cord enabling them to control interlimb co-ordination (see Drew, 1991), it was expected that MLF stimuli could affect the locomotor rhythm in several different ways. One of the reasons for testing the resetting ability of MLF in our experiments was to select axons which were able to promote extensor activities. A detailed analysis of how reticulospinal pathways may affect the fictive locomotor rhythm will require further investigation.
Bulbospinal inputs converge on interneurones of the polysynaptic Group I pathways
There are several lines of evidence indicating a great similarity of action between each of the tested bulbospinal systems and of the extensor group I pathways. Firstly, with the progressive injection of L-DOPA, there are simultaneously: an emergence of late DOPA reflexes, a reversal of group I autogenetic inhibition to excitation (Gossard et al. 1994) and a clear enhancement of polysynaptic EPSPs by vestibulospinal and MLF inputs (Fig. 3). This may suggest that there are parallel changes in polysynaptic excitatory pathways from all these sources in response to L-DOPA injection. Secondly, these three afferent systems converge on coFRA interneurones (Conway et al. 1987; Gossard et al. 1994). Thirdly, vestibulospinal and MLF volleys are able to reset the fictive step cycle by promoting extensor activities (e.g. Fig. 2) as described for extensor group I fibres (Conway et al. 1987) and coFRA pathways (Schomburg et al. 1998). Finally, the amplitude of polysynaptic bulbospinal EPSPs is modulated during the fictive step cycle (see next section) and this modulation has a pattern which is very similar to the one observed for extensor group I-evoked EPSPs (Gossard et al. 1994). Overall, there is thus compelling evidence indicating that descending reticulospinal and vestibulospinal pathways interact directly with interneurones included in extensor group I pathways.
Spatial facilitation between MLF and extensor group I fibres was found in 69 % of the trials suggesting a definite interneuronal convergence for the two inputs. This is schematically illustrated in Fig. 9 where the MLF inputs are projecting to a population of coFRA interneurones which are also contacted by extensor group I inputs. Given the similarity between these two systems as described above, a convergence of their inputs at the spinal cord level was expected. On the other hand, there was a complete absence of spatial facilitation between vestibulospinal and extensor group I pathways which strongly suggests that these two pathways do not converge on common interneurones but only meet at the motoneuronal level. This is a striking and unexpected result considering the similarity between these pathways in regard to their convergence on coFRA pathways and resetting abilities. These latter two characteristics clearly indicate that vestibulospinal input is mediated through the extensor half-centre. However, because extensor group I input also have these same characteristics, the easiest interpretation is that the coFRA interneurones within the extensor half-centre are divided into (at least) two sub-populations; one contacted by extensor group I fibres and the other by descending vestibulospinal pathways. This is schematically illustrated in Fig. 9 where DN projects to one population of coFRA interneurones in the extensor half-centre (E box) which is positioned next to another population of coFRA interneurones receiving extensor group I input. Such organization would provide independent ways to enhance excitability in the networks controlling the rhythmic activities of extensor muscles during locomotion. An absence of spatial facilitation between DN and MLF disynaptic excitation in motoneurones in the decerebrate cat was also taken as evidence for the existence of two completely separate interneuronal pathways for the transmission of excitation (Gossard et al. 1996). This is schematically illustrated in Fig. 9 where the DN and MLF pathways project to separate interneurones outside (below) the rhythm generator. The results reported here extend the independence between the DN and MLF control of motoneurones to the polysynaptic pathways which are parts of the locomotor networks.
Bulbospinal EPSPs during fictive locomotion
When the extensor half-centre is active, either spontaneously after decerebration, following a full dose of L-DOPA or because of MLR stimulation, we found that the amplitude of oligosynaptic EPSPs evoked by vestibulospinal and MLF inputs was modulated along the fictive step cycle. The amplitude of polysynaptic EPSPs evoked by MLF stimuli was decreased when given at the top of the depolarized phase of the LDP in extensor motoneurones (e.g. Fig. 8a and B). A very similar modulation pattern was previously reported for polysynaptic EPSPs evoked by extensor group I fibres (Gossard et al. 1994). Different modulation patterns during fictive locomotion would argue for separate interneuronal pathways being driven by the CPG (see Floeter et al. 1993; Gossard et al. 1996). The reduction of amplitude observed at the top of the depolarized phase is interpreted as being caused by occlusion of the pathways due to the already active interneurones of the extensor half-centre mediating the depolarized phase (see Gossard et al. 1994). A similar modulation pattern was seen for polysynaptic EPSPs evoked by DN stimuli, i.e. a reduction in amplitude when evoked at the top of the depolarized phase in the extensor motoneurones (e.g. Fig. 8C). The same reasoning may be applied as above regarding the occlusion of the pathways due to the activity in the extensor half-centre. Notice that the amplitude of bulbospinal responses is similarly reduced at the top of the depolarized phase of the LDP as well as at the top of depolarization evoked by coFRA stimuli (compare Figs 4A and 8A) thus reinforcing the idea that late-DOPA reflexes are forerunners of the different phases of the step cycle. Finally, also note that disynaptic EPSPs may follow a different modulation pattern during fictive locomotion with an enhancement during the active phase in the motoneurones (e.g. Fig. 8b). This was shown in similar preparations for the disynaptic EPSPs evoked by MLF (Floeter et al. 1993) and for those evoked by extensor group I input (McCrea et al. 1995).
Functional considerations
Our results suggest that transmission of load signals from extensor muscles in spinal cord pathways regulating the duration and activities of the stance phase (cf. Pearson et al. 1998) is under the direct control of reticulospinal pathways. Also, because the output of the MLR may be mediated by this reticulospinal system, it may also be involved in the supraspinal initiation of stepping. In addition, the results further suggest that commands from DN may be able to control extensor activities independently of load signals. Note that DN have also mono- and disynaptic projections to motoneurones and may thus interact with load signals at the motoneuronal level. This organization assumes that there are redundant parallel spinal cord networks involved in the generation of locomotor activities in extensor motoneurones. The idea of redundancy in CPG circuitry is applicable in several systems (von Euler, 1983; Friesen, 1994).
Of course, under our experimental conditions, we have the opportunity to stimulate individual control systems and to study their specific pathways independently. However, during real movements, these pathways need to be co-ordinated. For example, one could imagine vestibular nuclei, in response to a postural perturbation, sending biased locomotor signals to motoneurones in conflict with signals from sensory-entrained locomotor networks. However, under normal circumstances, by way of vestibular projections to the reticular formation, the postural adjustments would be well co-ordinated with (or even precede) ongoing movements (see Mori, 1989). A preliminary study on compensatory responses to disturbances in sway and loading at the ankle during walking in humans found a co-contraction of soleus (Sol) and TA muscles which is not part of the normal stepping pattern (Stephens et al. 1998). The authors further showed that the two responses were independently controlled by two control systems. The Sol response was found to be mediated by load (group I) pathways from extensors while the TA response was associated with a postural compensatory system (possibly DN).
Acknowledgments
This work was funded by a grant awarded to J.-P. Gossard from the Medical Research Council of Canada. H. Leblond was supported in part by the ‘Fonds pour la formation de chercheur et l'aide à la recherche’ (FCAR) du Québec. A. Ménard was supported by the Natural Sciences and Engineering Council of Canada (NSERC). The authors wish to thank France Lebel for her excellent technical assistance.
References
- Anden N-E, Jukes MGM, Lundberg A. The effect of DOPA on the spinal cord. I. Influence on the transmission from primary afferents. Acta Physiologica Scandinavica. 1966;67:373–386. doi: 10.1111/j.1748-1716.1966.tb03324.x. [DOI] [PubMed] [Google Scholar]
- Baldissera F, Hultborn H, Illert M. Integration in spinal neuronal systems. In: Brooks VB, editor. Handbook of Physiology. The Nervous System, Motor Control. II. Bethesda: American Physiological Society; 1981. pp. 509–595. part I. [Google Scholar]
- Basbaum AI, Clanton CH, Fields HL. Three bulbospinal pathways from the rostral medulla of the cat: an autoradiographic study of pain modulating systems. Journal of Comparative Neurology. 1978;178:209–224. doi: 10.1002/cne.901780203. [DOI] [PubMed] [Google Scholar]
- Brownstone RM, Gossard J-P, Hultborn H. Voltage-dependent excitation of motoneurones from spinal locomotor centres in cat. Experimental Brain Research. 1994;102:34–44. doi: 10.1007/BF00232436. [DOI] [PubMed] [Google Scholar]
- Burke RE. The use of state-dependent modulation of spinal reflexes as a tool to investigate the organization of spinal interneurons. Experimental Brain Research. 1999;128:263–277. doi: 10.1007/s002210050847. [DOI] [PubMed] [Google Scholar]
- Conway BA, Hultborn H, Kiehn O. Proprioceptive input resets central locomotor rhythm in the spinal cat. Experimental Brain Research. 1987;68:643–656. doi: 10.1007/BF00249807. [DOI] [PubMed] [Google Scholar]
- Drew T. Functional organization within the medullary reticular formation of the intact unanesthetized cat. III. Microstimulation during locomotion. Journal of Neurophysiology. 1991;66:919–938. doi: 10.1152/jn.1991.66.3.919. [DOI] [PubMed] [Google Scholar]
- Drew T, Rossignol S. Phase-dependent responses evoked in limb muscles by stimulation of medullary reticular formation during locomotion in the thalamic cats. Journal of Neurosciences. 1984;52:653–675. doi: 10.1152/jn.1984.52.4.653. [DOI] [PubMed] [Google Scholar]
- Dubuc R, Cabelguen J-M, Rossignol S. Rhythmic fluctuations of dorsal root potentials and antidromic discharges of primary afferents during fictive locomotion in the cat. Journal of Neurophysiology. 1988;60:2014–2036. doi: 10.1152/jn.1988.60.6.2014. [DOI] [PubMed] [Google Scholar]
- Duysens J, Pearson KG. Inhibition of flexor burst generator by loading ankle extensor muscles in walking cats. Brain Research. 1980;187:321–332. doi: 10.1016/0006-8993(80)90206-1. [DOI] [PubMed] [Google Scholar]
- Engberg I, Lundberg A, Ryall RW. Reticulospinal inhibition of transmission in reflex pathways. The Journal of Physiology. 1968a;194:201–223. doi: 10.1113/jphysiol.1968.sp008402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engberg I, Lundberg A, Ryall RW. Reticulospinal inhibition of interneurones. The Journal of Physiology. 1968b;194:225–236. doi: 10.1113/jphysiol.1968.sp008403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floeter MK, Sholomenko GN, Gossard J-P, Burke RE. Disynaptic excitation from the medial longitudinal fasciculus to lombosacral motoneurons: modulation by repetitive activation, descending pathways, and locomotion. Experimental Brain Research. 1993;92:407–419. doi: 10.1007/BF00229029. [DOI] [PubMed] [Google Scholar]
- Friesen WO. Reciprocal inhibition: a mechanism underlying oscillatory animal movements. Neuroscience and Biobehavioral Reviews. 1994;18:547–553. doi: 10.1016/0149-7634(94)90010-8. [DOI] [PubMed] [Google Scholar]
- Garcia-Rill E, Skinner RD, Gillmore SA, Ownings R. Connections of the mesencephalic locomotor region (MLR). II. Afferents and efferents. Brain Research Bulletin. 1983;10:63–71. doi: 10.1016/0361-9230(83)90076-x. [DOI] [PubMed] [Google Scholar]
- Gossard J-P, Brownstone RM, Barajon I, Hultborn H. Transmission in a locomotor-related group Ib pathway from hindlimb extensor muscles in the cat. Experimental Brain Research. 1994;98:213–228. doi: 10.1007/BF00228410. [DOI] [PubMed] [Google Scholar]
- Gossard J-P, Floeter M-K, Simon ES, Degtyarenko AM, Burke RE. Disynaptic vestibulospinal and reticulospinal excitation in cat lumbosacral motoneurones: modulation during fictive locomotion. Experimental Brain Research. 1996;109:277–288. doi: 10.1007/BF00231787. [DOI] [PubMed] [Google Scholar]
- Gossard J-P, Hultborn H. The organization of the spinal rhythm generation in locomotion. In: Wernig A, editor. Restorative Neurology. Vol. 5. Amsterdam: Elsevier; 1991. pp. 385–404. chap. 42. [Google Scholar]
- Grillner S, Hongo T, Lund S. Convergent effects on alpha motoneurones from vestibulospinal tract and a pathway descending in the medial longitudinal fasciculus. Experimental Brain Research. 1971;12:457–479. doi: 10.1007/BF00234243. [DOI] [PubMed] [Google Scholar]
- Guertin P, Angel M, Perreault M-C, McCrea DA. Ankle extensor group I afferents excite extensors throughout the hindlimb during fictive locomotion in the cat. The Journal of Physiology. 1995;487:197–209. doi: 10.1113/jphysiol.1995.sp020871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H, Conway BA, Gossard J-P, Brownstone R, Fedirchuk B, Schomburg ED, Enriquez-Denton M, Perreault MC. How do we approach the locomotor network in the mammalian spinal cord? In: Kiehn O, Harris-Warrick LM, Jordan LM, Hultborn H, Kudo N, editors. Annals of the New York Academy of Sciences. Neuronal Mechanism for Generating Locomotor Activity. Vol. 860. New York: The New York Academy of Sciences; 1998. pp. 70–82. [DOI] [PubMed] [Google Scholar]
- Jankowska E. Interneuronal relay in spinal pathways from proprioceptors. Progress in Neurobiology. 1992;38:335–378. doi: 10.1016/0301-0082(92)90024-9. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Jukes MG, Lund S, Lundberg A. The effect of DOPA on the spinal cord. 5. Reciprocal organization of pathways transmitting excitatory action to alpha motoneurones of flexors and extensors. Acta Physiologica Scandinavica. 1967a;70:369–388. doi: 10.1111/j.1748-1716.1967.tb03636.x. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Jukes MG, Lund S, Lundberg A. The effect of DOPA on the spinal cord. 6. Half-centre organization of interneurones transmitting effects from the flexor reflex afferents. Acta Physiologica Scandinavica. 1967b;70:389–402. doi: 10.1111/j.1748-1716.1967.tb03637.x. [DOI] [PubMed] [Google Scholar]
- Jordan LM, Pratt CA, Menzies JE. Locomotion evoked by brainstem stimulation: occurrence without phasic segmental afferent input. Brain Research. 1979;177:204–207. doi: 10.1016/0006-8993(79)90933-8. [DOI] [PubMed] [Google Scholar]
- Leblond H, Gossard J-P. Interaction between Deiters’ nucleus and Group I input from extensors muscles during fictive locomotion in the cat. Society for Neuroscience Abstracts. 1996;22:804.14. [Google Scholar]
- Leblond H, Gossard J-P. Supraspinal and segmental signals can be transmitted through separate spinal cord pathways to enhance locomotor activity in extensor muscles in the cat. Experimental Brain Research. 1997;114:188–192. doi: 10.1007/pl00005619. [DOI] [PubMed] [Google Scholar]
- Leblond H, Ménard A, Gossard J-P. Convergence between reticulospinal fibres and group I input from extensor muscles during fictive locomotion in the cat. Society for Neuroscience Abstracts. 1997;23:298.15. [Google Scholar]
- Leblond H, Ménard A, Gossard J-P. Vestibulo- and reticulospinal control of the extensor half-centre in locomotion. In: Kiehn O, Harris-Warrick LM, Jordan LM, Hultborn H, Kudo N, editors. Annals of the New York Academy of Sciences. Neuronal Mechanism for Generating Locomotor Activity. Vol. 860. New York: The New York Academy of Sciences; 1998. pp. 563–565. [DOI] [PubMed] [Google Scholar]
- Lundberg A. The Nansen Memorial Lecture V. Olso: Universitetsforlaget; 1969. Reflex Control of Stepping; pp. 5–42. [Google Scholar]
- Lundberg A. Control of spinal mechanisms from the brain. In: Tower DB, editor. The Nervous System, The Basic Neurosciences. I. New York: Raven Press; 1975. pp. 253–26. [Google Scholar]
- Lundberg A. Multisensory control of spinal reflex pathways. Progress in Brain Research. 1979;50:11–28. doi: 10.1016/S0079-6123(08)60803-1. [DOI] [PubMed] [Google Scholar]
- McCrea DA, Shefchyk SJ, Stephens MJ, Pearson KG. Disynaptic group I excitation of synergist ankle extensor motoneurones during fictive locomotion in the cat. The Journal of Physiology. 1995;487:527–539. doi: 10.1113/jphysiol.1995.sp020897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitani A, Ito K, Mitani Y, McCarley RW. Descending projections from the gigantocellular tegmental field in the cat: cells of origin and their brainstem and spinal cord trajectories. Journal of Comparative Neurology. 1988;268:546–565. doi: 10.1002/cne.902680406. [DOI] [PubMed] [Google Scholar]
- Mori S. Contribution of postural muscle tone to full expression of posture and locomotion movements: multi-faceted analysis of its setting brainstem-spinal cord mechanisms in the cat. Japanese Journal of Physiology. 1989;39:785–809. [PubMed] [Google Scholar]
- Orlovsky GN. The effect of different descending systems on flexor and extensor activity during locomotion. Brain Research. 1972;40:359–371. doi: 10.1016/0006-8993(72)90139-4. [DOI] [PubMed] [Google Scholar]
- Pearson KG, Misiaszek JE, Fouad K. Enhancement and resetting of locomotor activity by muscle afferents. In: Kiehn O, Harris-Warrick LM, Jordan LM, Hultborn H, Kudo N, editors. Annals of the New York Academy of Sciences. Neuronal Mechanism for Generating Locomotor Activity. Vol. 860. New York: The New York Academy of Sciences; 1998. pp. 203–215. [DOI] [PubMed] [Google Scholar]
- Pearson KG, Ramirez JM, Jiang W. Entrainment of the locomotor rhythm by group Ib afferents from ankle extensor muscles in spinal cats. Experimental Brain Research. 1992;90:557–566. doi: 10.1007/BF00230939. [DOI] [PubMed] [Google Scholar]
- Perreault M-C, Rossignol S, Drew T. Microstimulation of the medullary reticular formation during fictive locomotion. Journal of Neurophysiology. 1994;71:229–245. doi: 10.1152/jn.1994.71.1.229. [DOI] [PubMed] [Google Scholar]
- Perret C, Cabelguen J-M. Main characteristics of the hindlimb locomotor cycle in the decorticate cat with special reference to bifunctional muscles. Brain Research. 1980;187:333–352. doi: 10.1016/0006-8993(80)90207-3. [DOI] [PubMed] [Google Scholar]
- Russel DF, Zajac FE. Effects of stimulating Deiters’ nucleus and medial longitudinal fasciculus on the timing of the fictive locomotor rhythm induced in cats by DOPA. Brain Research. 1979;177:588–592. doi: 10.1016/0006-8993(79)90478-5. [DOI] [PubMed] [Google Scholar]
- Schomburg ED, Petersen N, Barajon I, Hultborn H. Flexor reflex afferents reset the step cycle during fictive locomotion in the cat. Experimental Brain Research. 1998;122:339–350. doi: 10.1007/s002210050522. [DOI] [PubMed] [Google Scholar]
- Shefchyk SJ, Jordan LM. Excitatory and inhibitory postsynaptic potentials in α-motoneurons produced during fictive locomotion by stimulation of the mesencephalic locomotor region. Journal of Neurophysiology. 1985;53:1345–1355. doi: 10.1152/jn.1985.53.6.1345. [DOI] [PubMed] [Google Scholar]
- Shik ML, Orlovsky GN. Neurophysiology of locomotor automatism. Physiological Reviews. 1976;56:465–501. doi: 10.1152/physrev.1976.56.3.465. [DOI] [PubMed] [Google Scholar]
- Steeves JD, Jordan LM. Autoradiographic demonstration of the projections from the mesencephalic locomotor region. Brain Research. 1984;307:263–276. doi: 10.1016/0006-8993(84)90480-3. [DOI] [PubMed] [Google Scholar]
- Stephens MJ, Misiaszek JE, Yang JF, Pearson KG. Compensatory reactions at the ankle to disturbances at the torso during walking in humans. Society for Neursocience Abstracts. 1998;24:838.13. [Google Scholar]
- Ten Bruggencate G, Burke R, Lundberg A, Udo M. Interaction between the vestibulospinal tract, contralateral flexor reflex afferents and Ia afferents. Brain Research. 1969;14:529–532. doi: 10.1016/0006-8993(69)90131-0. [DOI] [PubMed] [Google Scholar]
- Ten Bruggencate G, Lundberg A. Facilitatory interaction in transmission to motoneurones from vestibulospinal fibres and contralateral primary afferents. Experimental Brain Research. 1974;19:248–270. doi: 10.1007/BF00233233. [DOI] [PubMed] [Google Scholar]
- von Euler C. On the central pattern generator for the basic breathing rhythmicity. Journal of Applied Physiology: Respiratory, Environmental and Exercise Physiology (Bethesda) 1983;55:1647–1659. doi: 10.1152/jappl.1983.55.6.1647. [DOI] [PubMed] [Google Scholar]


