Abstract
A prerequisite for the understanding of how a cortical column functions is a description of small and defined neuronal circuits consisting of only a few identified neurones. Here we summarise, with particular reference to the barrel cortex, the morphological and physiological properties of two synaptic connections, namely those between pairs of spiny neurones in layer 4 and pairs of pyramidal cells in layer 5. While layer 4 spiny neurones are the cortical input neurones that amplify and relay incoming excitation from the periphery, layer 5 pyramidal cells integrate neuronal activity both within and across cortical columns and subsequently distribute it to both cortical and subcortical brain regions.
A cortical column represents a distinct functional module that processes electrical signals arriving from sensory inputs (Mountcastle, 1997). To understand how patterns of electrical activity in representational areas of the cortex are generated, it is necessary to unravel the anatomical and functional wiring of neurones within a column. In simplified terms, the flow of excitation in sensory cortices can be summarised as follows: afferent fibres originating from the appropriate thalamic relay nuclei terminate predominantly in layer 4 (Hubel & Wiesel, 1962; McGuire et al. 1984; for review see Murray Sherman & Guillery, 1996). Here they contact excitatory spiny neurones that represent the main type of neurone for receiving inputs to the cortex (Mountcastle & Powell, 1959; Hubel & Wiesel, 1962; LeVay, 1973; Martin & Whitteridge, 1984; McGuire et al. 1984; Douglas & Martin, 1991; for review see Lund, 1984; McCormick, 1992). Most of these neurones are spiny stellate cells (∼80 %) while a smaller fraction have been described as star pyramidal neurones (Ahmed et al. 1994; Hirsch, 1995; Feldmeyer et al. 1999a). Both these classes of layer 4 spiny neurones relay excitation to pyramidal cells in layer 2/3. Within layer 2/3, excitation is distributed laterally and vertically to other cortical layers, in particular to layer 5. The output from the cortex is relayed from layer 5 to subcortical brain regions, mostly via the axons of large thick tufted pyramidal cells (Armstrong-James et al. 1992).
A first step towards understanding how a cortical column functions is to describe both the morphology and the physiology of small, well-defined neuronal circuits consisting of only a few identified neurones within a column. This approach was greatly facilitated by simultaneous multiple recordings from neurones in neocortical brain slices under visual control. These new techniques (Stuart et al. 1993) made it possible to study, in a quantitative way, the morphological and functional properties of small excitatory and inhibitory circuits in the neocortex. Here we review both the morphological and functional properties of excitatory synaptic connections made between the principal cells of layer 4, the main input layer, and layer 5, the main output layer of the neocortex with particular reference to the barrel cortex. A description of the properties of these synaptic connections is a first step towards understanding how a cortical column functions; however, for full understanding emerging data on other neuronal connections have to be taken into account.
Principal neurones in both layers are polarised into dendritic and axonal arbor compartments. With particular reference to the somatosensory cortex, we compared the morphological and functional properties of axonal and dendritic arbors to elucidate how differences in arborisation and in the number and the location of synaptic contacts contribute to the efficacy of synaptic transmission of small circuits.
Results
Morphology of dendritic and axonal arbors of layer 4 and layer 5 neurones obtained from ‘pair’ recordings
The dendritic arbor of spiny stellate cells in layer 4 (Fig. 1, left panel, red) is characterized by a comparatively small horizontal field span (< 200 μm) and remains restricted within layer 4. In the somatosensory cortex, the dendritic arbor of spiny stellate cells is asymmetrical and preferentially oriented towards the centre of a cortical barrel (Woolsey et al. 1975; Harris & Woolsey, 1981; Simons & Woolsey, 1984; Feldmeyer et al. 1999a). The layer 4 spiny cell dendrites receive inputs from thalamic afferents (in the case of the barrel cortex from the ventroposterior medial nucleus; Keller et al. 1985; Benshalom & White, 1986) and from the axon collaterals of other layer 4 spiny stellate neurones located in the same barrel (Feldmeyer et al. 1999a).
Figure 1. Dendritic arbor (red) and axonal projection (blue) of a layer 4 spiny stellate cell (left) and a layer 5 pyramidal cell of a postnatal day 14 (P14) rat.
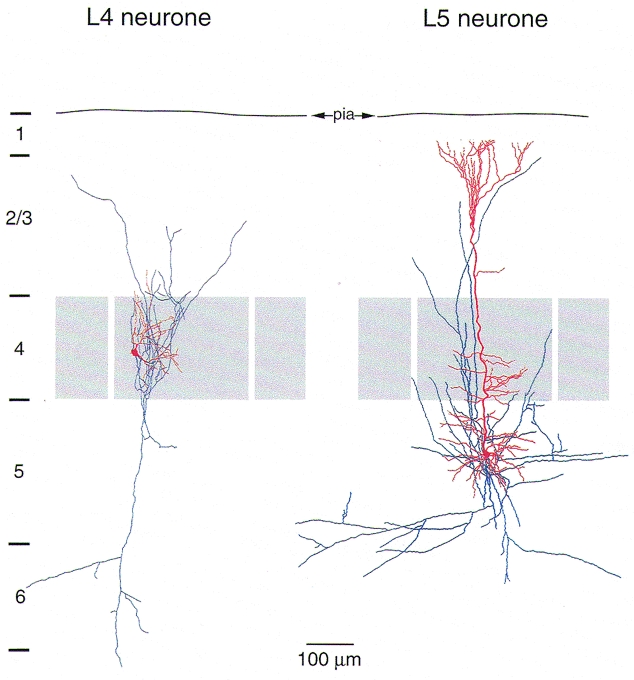
The dendrites of the spiny stellate cell are confined to layer 4 (grey). In rodent barrel cortex dendrites remain exclusively within the cortical barrel in which the soma is located and often show a highly asymmetrical orientation towards the centre of the barrel. The spiny stellate axon collaterals are predominantly vertically oriented. The axonal arbor spans the entire cortex, projecting to layer 1 and to the white matter. Most boutons are on axon collaterals within layer 4 and layer 2/3 (Lübke et al. 2000). The prominent apical dendrite of layer 5 pyramidal cells extends into layer 1 in a terminal tuft. The basal dendrites extend within layers 5 and 6. The axon is long (1-4 mm) and collaterals are mainly horizontally oriented. Fewer vertical collaterals project to layer 1 where they may form synapses with the terminal tufts of other pyramidal neurones.
The dendritic arbor of pyramidal cells in layer 5 (Fig. 1, right panel, red) is characterised by a long apical dendrite from which numerous oblique dendrites branch off. It extends to layer 1 where it branches profusely into multiple distal dendrites forming a terminal tuft. Layer 5 pyramidal cells receive synaptic input from other cortical areas via associational fibres, from axon collaterals of layer 2/3 neurones and also via the long vertical and horizontal axon collaterals from other layer 5 pyramidal cells. The soma of layer 5 pyramidal cells gives rise to numerous basal dendrites that have a predominantly but not exclusively horizontal orientation and receive afferent excitatory input from other layer 5 neurones (Markram et al. 1997a) and from layer 2/3 pyramidal cells (Reyes & Sakmann, 1999). The horizontal field span of the dendritic arbor is 300 μm (Markram et al. 1997a).
The morphology of axonal arbors of the principal neurones in layer 4 and layer 5 is also very different. The axonal arbor of spiny stellate neurones (in the barrel cortex) is oriented almost exclusively vertically and spans all cortical layers up to layer 1 and down to layer 6 and to the white matter. Axon collaterals of spiny stellate and star pyramidal neurones primarily form contacts with the dendrites of other layer 4 spiny neurones and layer 2/3 pyramidal cells, respectively (Stratford et al. 1996; Feldmeyer et al. 1999a,b; Lübke et al. 2000).
The axonal arbor of layer 5 pyramidal cells can be up to several millimetres long with both vertical and horizontal collaterals. Locally the axonal arbor of pyramidal cells has a more widespread and clustered arborisation than that of layer 4 neurones, extending mainly in the horizontal direction of layer 5. Vertical axon collaterals form connections between neurones of different layers, while horizontal axon collaterals establish synaptic contacts between neurones in the vicinity of cell bodies and also with neurones in different cortical columns (Gilbert & Wiesel, 1979; Thomson & Deuchars, 1994). The axon collaterals may span up to several millimetres in the adult. Thus the axon may extend across several cortical columns and serve to distribute excitation to both cortical as well as subcortical areas (Jones, 1984; Wang & McCormick, 1993; Kasper et al. 1994).
Morphology of synaptic connections between the principal neurones within layer 4 and layer 5
The properties of synaptic transmission between pairs of neurones within a layer are determined by the number and the dendritic location of synaptic contacts and the axonal distance of synaptic boutons. Figure 2 illustrates part of the local axonal arbor of the presynaptic neurone (blue), the dendritic arbor of the postsynaptic neurone (red) and the location of synaptic contacts made by the presynaptic cell with the dendrites of the postsynaptic neurone (blue dots). For the connections between layer 4 spiny neurones (Fig. 2, upper panels), synaptic contacts were found exclusively in layer 4 as the dendritic arbor is restricted to this layer. The mean number of contacts was 3.4 ± 0.2 (range 2-5; Feldmeyer et al. 1999a). In the barrel cortex, contacts were restricted to that barrel in which the soma of the presynaptic neurone was located and had an axon distance between 50 and 200 μm from the soma. Contacts were preferentially established on secondary to quaternary dendritic branches at an average geometric distance of 69 ± 37 μm (range 33–168 μm) from the soma (see also Fig. 4a).
Figure 2. Location of synaptic contacts in connections between pairs of layer 4 spiny stellate cells and layer 5 pyramidal cells.
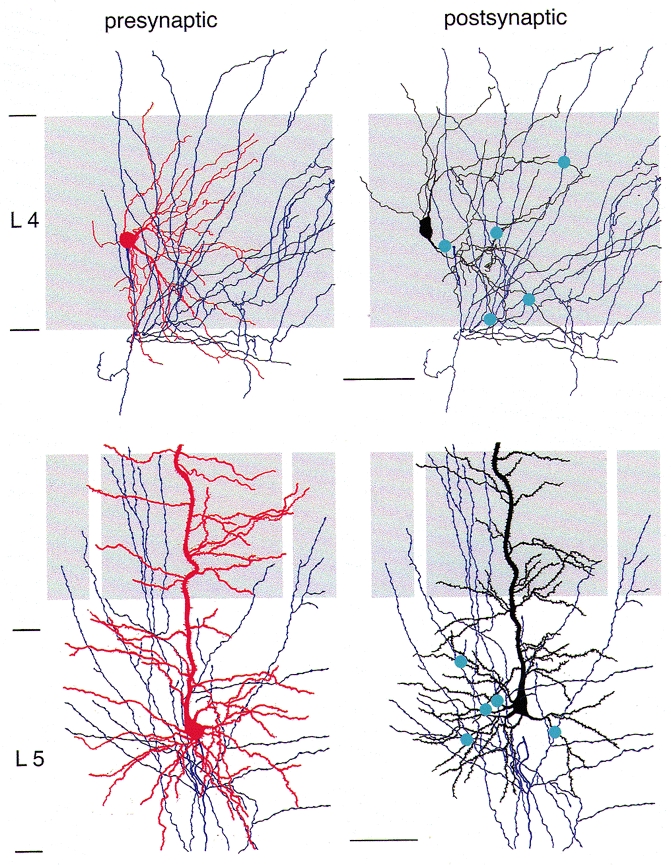
Left panels, presynaptic spiny stellate cell (top) and pyramidal cell in layer 5 (bottom). The cortical layer (L) is given on the left. The barrel structure is indicated by light grey shading. Dendritic arbor is red, axon arbor is blue. Right panels, axon of presynaptic (blue) and dendritic arbor of postsynaptic neurone (black) of a pair of spiny stellate cells (top) and a pair of layer 5 pyramidal cells (bottom), respectively. Blue circles indicate synaptic contacts between the presynaptic axon and the postsynaptic dendrites. Scale bar: 50 μm for top panels, 100 μm for bottom panels.
Figure 4. Morphology of synaptic connections and synaptic efficacy.
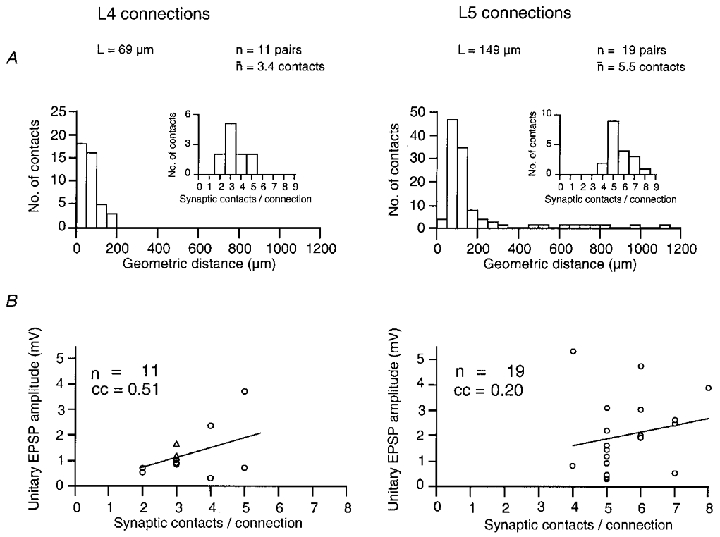
A, morphology: histogram of geometric distances from the soma of putative synaptic contacts in 11 pairs of layer 4 spiny neurones (left) and 19 pairs of layer 5 pyramidal cells (right). Note that synaptic contacts of layer 4 spiny neurones fall within 200 μm from the soma while in layer 5 pyramidal cells contacts can be as distal as 1000 μm from the soma. Insets, distribution of the number of synaptic contacts per connection. The mean number of synaptic contacts was 3.4 for layer 4 spiny neurones and 5.5 for layer 5 pyramidal cells. B, morphology and efficacy: relationship between unitary EPSP amplitude and the number of synaptic contacts per connection. The correlation coefficient (cc) obtained was 0.51 for layer 4 spiny neurones and 0.2 for layer 5 pyramidal cells. For both types of connections, there was no significant correlation between EPSP amplitude and contact number. Adapted from Markram et al. (1997a);Feldmeyer et al. (1999a).
In contrast, pairs of layer 5 pyramidal cells had on average more synaptic contacts (5.5 ± 1.1, range 4-8; Markram et al. 1997a) than pairs of layer 4 spiny neurones. A similar value (5; range 2-8) was also obtained for pyramidal cells in infragranular layers 5 and 6 (Deuchars et al. 1994). The average axonal distance was ∼380 μm from the soma, clearly larger than for layer 4 connections. Although synaptic contacts between layer 5 pyramidal cells were distributed over cortical layers 1-5, the majority of contacts were located on basal dendrites in layer 5 (63 %, mean dendritic distance: 82 ± 35 μm for the soma) with a smaller fraction on apical oblique dendrites (27 %, 145 ± 59 μm), and a small percentage on the tuft (10 %, 611 ± 174 μm) on tertiary dendritic branches (see also Fig. 4a).
Thus the morphology of axonal and dendritic arbors of connected neurones suggests that signal flow between spiny stellate cells in layer 4 is expected to be spatially more restricted and possibly less variable as compared with signal flow between layer 5 pyramidal cells. Functional connectivity in neuronal circuits is determined by the efficacy of synaptic transmission and its short term modulation when neurones are activated repetitively. Morphological parameters only partly govern functional connectivity of neuronal circuits. For a more complete understanding, the efficacy, reliability and time course of synaptic transmission must be determined.
Time course, efficacy and reliability of synaptic transmission between principal neurones within layer 4 and layer 5
The time course of unitary EPSPs is markedly different in layer 4 spiny neurones and layer 5 pyramidal cells. Figure 3a shows that the latency between the presynaptic action potential and EPSP is shorter in layer 4 spiny neurones (0.9 ± 0.4 ms; n = 131) than in connections between layer 5 pyramidal cells (1.7 ± 0.9 ms; n = 138) and the rise time and decay time constants are faster (1.5 ± 0.5 vs. 2.9 ± 2.3 ms and 18 ± 6vs. 40 ± 18 ms, respectively). These differences are probably due to differences in axonal and dendritic morphologies, and the differences in locations of synaptic contacts of layer 4 and layer 5 neurones as described above. However, rise and decay time may also be affected by the different NMDA/AMPA receptor ratios at these synapses (Deuchars et al. 1994; Markram et al. 1997a; Feldmeyer et al. 1999a).
Figure 3. Unitary EPSPs of layer 4 spiny stellate and layer 5 pyramidal cells.
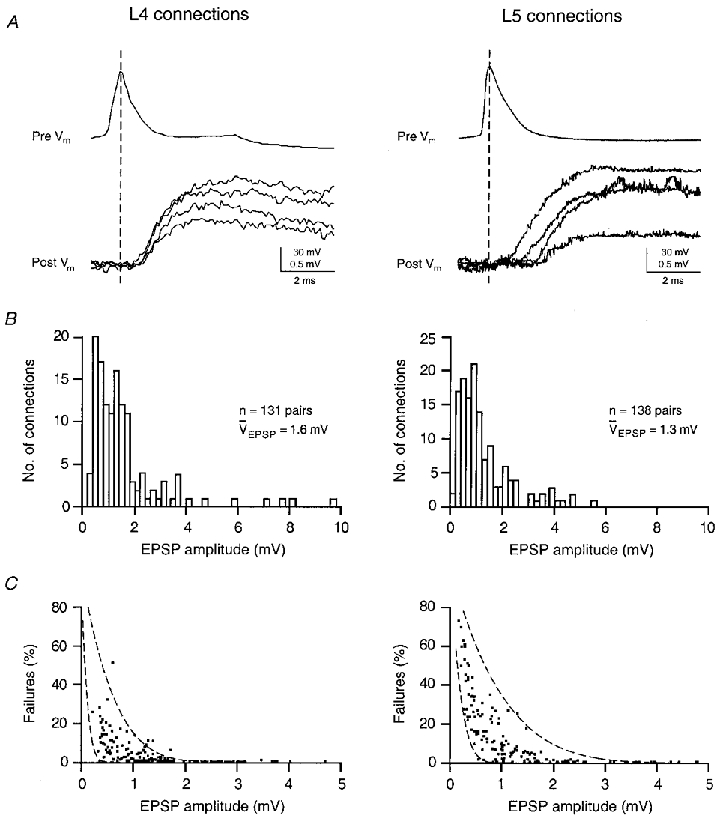
A, latency: presynaptic action potential and four unitary EPSPs in a layer 4 (left) and a layer 5 (right) synaptic connection. The top trace in each panel shows the presynaptic action potential (pre Vm) and the bottom traces the postsynaptic response (post Vm). The dashed line indicates the peak of the action potential. Note that the latency between presynaptic action potential and EPSPs is significantly shorter and fluctuates less in the layer 4 connection. B, efficacy: amplitude distribution of unitary EPSPs in 131 pairs of layer 4 spiny neurones (left) and 138 pairs of layer 5 pyramidal cells (right). The mean EPSP amplitude was 1.6 mV for layer 4 connections and 1.3 mV for layer 5 connections. C, reliability: percentage of failures plotted as a function of the mean peak amplitude of the unitary EPSPs of a connection. The two dashed lines in the two plots represent the predictions of binomial release statistics for the percentage of failures as a function of EPSP amplitude. For layer 4 connections the relevant parameters were nb = 4 and q = 0.15< mV (left) and q = 0.8< mV (right) as pr increases from 0.08 to 0.6 (left lines) and from 0.05 to 1.0 (right lines). For layer 5 connections the parameters were nb = 6 and q = 0.2< mV (left) and q = 1.0< mV (right) as pr increases from 0.125 to 0.9 (left lines) and from 0.025 to 0.9 (right lines), respectively. Adapted from Markram et al. (1997a);Feldmeyer et al. (1999a).
Efficacy of synaptic connections, measured by the average peak amplitude of unitary EPSPs, was higher in layer 4 spiny neurones (1.6 ± 1.5 mV; n = 131) than in layer 5 pyramidal cells (1.3 ± 1.1 mV; n = 138). Within each layer the efficacy of individual connections could vary about 20-fold. In some pairs of layer 4 spiny neurones the unitary EPSPs were sufficiently large to elicit action potentials in the target neurone. This high efficacy was never observed in layer 5 pyramidal cells although these neurones had on average a somewhat more depolarised resting potential than layer 4 spiny neurones (-60 ± 2 vs. -68 ± 3 mV, respectively).
The reliability of synaptic transmission as determined by the percentage of failures of a presynaptic action potential to elicit a unitary EPSP was, on average, higher in layer 4 spiny neurones (5.3 ± 7.8 % failures) than in layer 5 pyramidal cell pairs (14.3 ± 17.6 %). Accordingly, the coefficient of variation (CV) was larger in layer 5 connections (0.52 ± 0.37 vs. 0.37 ± 0.18, respectively). In cat visual cortex, an even lower CV for layer 4 connections was found (0.21; Tarczy-Hornoch et al. 1999).
The comparison of unitary EPSPs suggests that synaptic transmission between neurones in the cortical (input) layer 4 is more efficient and more reliable than that between neurones of the (output) layer 5. This is consistent with the idea that fewer neurones within a layer would have to be active to recruit additional neurones into an ensemble of synchronously active neurones, thought to represent functional columns.
Determinants of efficacy and reliability
To delineate anatomical and functional determinants of synaptic efficacy (unitary EPSP amplitude) and reliability (failure rate) in selected pairs of excitatory layer 4 and layer 5 neurones we compared how the synaptic efficacy of a connection is dependent on the number of synaptic contacts and their location. Figure 4a illustrates the distribution of the number of contacts and their geometric distances from the soma in a subset of paired recordings and subsequent morphological reconstructions. Spiny layer 4 neurones have a lower number of synaptic contacts that are located closer to the soma than in layer 5 pyramidal cells.
In both layer 4 and layer 5 connections, the unitary EPSP amplitude was only weakly correlated with the number of synaptic contacts (Fig. 4b). Similarly, in previous studies there was no significant correlation between the mean dendritic location and EPSP amplitude in both types of connection (Markram et al. 1997a; Feldmeyer et al. 1999a). It is therefore likely that the large differences in efficacy and reliability of connections between layer 4 spiny neurones and layer 5 pyramidal cells are determined to a significant degree by differences in the functional properties of the synapse such as the release probability pr and the quantal event q.
Conclusions
Polarisation of neurones
The morphology of the connections between excitatory spiny neurones located in layer 4 and pyramidal cells located in layer 5, respectively, suggests that they subserve different functions in cortical signal flow. Dendritic arbors of layer 4 neurones are asymmetrical and confined to barrel borders. They receive specific inputs from the thalamus and from neighbouring neurones in layer 4 confined to the same barrel. Their axonal arbor projects to layer 4 spiny neurones in the same barrel, and in the vertical direction specifically to layer 2/3 pyramidal cells of the same cortical column. Dendritic arbors of layer 5 pyramidal cells, on the other hand, receive input arriving from cortical layers 1-5, and their dendrites are not confined to barrel borders. The morphology of their axonal arbors suggests that layer 5 neurones are sending signals across several columns, mostly within layer 5.
The functional properties of these connections suggest that synaptic transmission within layer 4 is more efficient and more reliable than it is in layer 5 (Markram et al. 1997a; Feldmeyer et al. 1999a). The difference in reliability is evident from the lower percentage of failures (5.3 vs. 14 %) of unitary EPSPs and the lower coefficient of variation (0.37 vs. 0.52, respectively).
Functional determinants of connectivity
How could differences in axonal and dendritic arbors and location of synaptic contacts contribute to the differences in connectivity? In making certain simplifying assumptions, the efficacy of a connection is given by ΔV = nbqpr, where ΔV is the mean amplitude of the unitary EPSP of a connection, nb is the number of functional release sites, q is the size of the quantal event, taking into account dendritic filtering, and pr is the release probability at each release site. The number of anatomically identified synaptic contacts between pairs of layer 4 neurones is smaller, implying a smaller value of nb. Axonal and dendritic distances between contacts of layer 4 connections are shorter, which may imply a larger quantal EPSP. The smaller number of putative synaptic contacts between layer 4 spiny neurones may be ‘compensated’ for by the more compact electrotonic structure of their dendritic arbors and by the fact that synaptic contacts are located closer to the neurones’ action potential initiation region, presumably located in the axon, causing EPSPs to be less attenuated by dendritic filtering. Finally, the reliability in layer 4 connections is higher, implying a higher release probability. It seems likely that an important factor that determines differences in the efficacy of connections in the two layers, and also the differences between different pairs in the same layer, is the difference in release probability.
Implications for network properties of the two layers
Spiny layer 4 neurones can function as strong amplifiers of even weak thalamic inputs as they are highly interconnected (Feldmeyer et al. 1999a). These neurones may boost the stream of excitatory synaptic activity from the thalamus causing widespread activation within a single barrel. This putative amplification of specific inputs contrasts with the integration function of layer 5 pyramidal cells. Because of the elongated morphology of their dendrites these neurones can integrate synaptic activity arriving from different cortical layers (Larkum et al. 1999), from different columns and presumably also from different cortical areas (Jones, 1984). The layer 5 pyramidal cells connect ensembles of neurones via their long horizontal axons projecting mostly within layer 5 (Markram et al. 1997a). Layer 5 pyramidal cells are the major cortical output neurones that extend to subcortical centres of the brain and to other cortical regions. Thus they can associate signals arriving from different cortical layers and distribute signals intracortically across several columns to generate synchronised output.
Long term synaptic efficacy
Repeated coincident activity can induce long term changes in efficacy although the effect is different in layers 4 and 5. The same pattern of coincident pre- and postsynaptic action potentials induces depression in synaptically connected layer 4 spiny neurones but potentiation between synaptically connected pyramidal cells in layer 5 or layer 2/3 (Markram et al. 1997b; Egger et al. 1999). However, the long term changes in efficacy, induced in an individual connection, are small compared with the average difference in efficacy in connections of different layers. Differences in release probability in different connections are large compared with short and long term changes induced by repetitive, coincident pre- and postsynaptic activity. Therefore it seems likely that these long lasting differences could be due to different mechanisms than those changes induced rapidly by coincident electrical activity.
Functional significance for columnar organisation
To understand how the network of cortical neurones ‘represents’ a sensory stimulus in the brain as a time- and space-dependent pattern of action potentials it is necessary to establish the morphological and functional basis of how electrical signals arriving from thalamic afferents propagate within and between layers. For this, it is particulary important to elucidate the factors which are responsible for the large differences in release probability in different connections as this is one of the key mechanisms contributing to dynamic changes in neuronal networks. Only once this has been clarified for all connections in a column might one be in a position to identify those mechanisms that are responsible for the organisation and reorganisation of cortical maps (i.e. the territories of particular whisker representations) observed during development or as a consequence of sensory input or the lack of it at a cellular and possibly at the molecular level.
The polarisation of neurones in dendritic and axonal arbors as well as connection-specific transmission properties seem to be a major determinant of the functional architecture of a column. The data discussed here provide ample evidence that the elucidation of mechanisms that determine the efficacy and reliability of synaptic connections as well as their long term changes (referred to as ‘plasticity’) need to be studied under conditions as close as possible to the physiological situation, in particular with an intact neuronal morphology.
Acknowledgments
We would like to thank Drs Matthew Larkum, Carl Petersen, Nathan Urban and Dipl.-Phys. Arnd Roth for critically reading this manuscript.
References
- Ahmed B, Anderson JC, Douglas RJ, Martin KAC, Nelson JC. Polyneuronal innervation of spiny stellate neurons in cat visual cortex. Journal of Comparative Neurology. 1994;341:39–49. doi: 10.1002/cne.903410105. [DOI] [PubMed] [Google Scholar]
- Armstrong-James M, Fox K, Das-Gupta A. Flow of excitation within rat barrel cortex on striking a single vibrissa. Journal of Neurophysiology. 1992;68:1345–1358. doi: 10.1152/jn.1992.68.4.1345. [DOI] [PubMed] [Google Scholar]
- Benshalom G, White EL. Quantification of thalamocortical synapses with spiny stellate neurons in layer IV of mouse somatosensory cortex. Journal of Comparative Neurology. 1986;253:303–314. doi: 10.1002/cne.902530303. [DOI] [PubMed] [Google Scholar]
- Deuchars J, West DC, Thomson AM. Relationships between morphology and physiology of pyramid-pyramid single axon connections in rat neocortex in vitro. The Journal of Physiology. 1994;478:423–435. doi: 10.1113/jphysiol.1994.sp020262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas RJ, Martin KAC. A functional microcircuit for cat visual cortex. The Journal of Physiology. 1991;440:735–769. doi: 10.1113/jphysiol.1991.sp018733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger V, Feldmeyer D, Sakmann B. Coincidence detection and efficacy changes in synaptic connections between spiny stellate neurons of the rat barrel cortex. Nature Neuroscience. 1999;2:1098–1105. doi: 10.1038/16026. [DOI] [PubMed] [Google Scholar]
- Feldmeyer D, Egger V, Lübke J, Sakmann B. Synaptic connections between excitatory layer 4 neurones in the ‘barrel field’ of rat somatosensory cortex. The Journal of Physiology. 1999a;521:169–190. doi: 10.1111/j.1469-7793.1999.00169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmeyer D, Silver RA, Lübke J, Sakmann B. Synaptic connections between spiny layer 4 neurones and layer 2/3 pyramidal cells of rat barrel cortex. The Journal of Physiology. 1999b;518:141.P–142P. [Google Scholar]
- Gilbert CD, Wiesel TN. Morphology and intracortical projections of functionally characterised neurones in the cat visual cortex. Nature. 1979;280:120–125. doi: 10.1038/280120a0. [DOI] [PubMed] [Google Scholar]
- Harris RM, Woolsey TA. Dendritic plasticity in mouse barrel cortex following postnatal vibrissa follicle damage. Journal of Comparative Neurology. 1981;196:357–376. doi: 10.1002/cne.901960302. [DOI] [PubMed] [Google Scholar]
- Hirsch JA. Synaptic integration in layer 4 of the ferret striate cortex. The Journal of Physiology. 1995;483:183–199. doi: 10.1113/jphysiol.1995.sp020577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. The Journal of Physiology. 1962;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EG. Laminar distribution of cortical efferent cells. In: Peters A, Jones EG, editors. Cellular Components of the Cerebral Cortex. Vol. 1. New York: Plenum Press; 1984. pp. 521–556. [Google Scholar]
- Kasper EM, Lübke J, Larkman AU, Blakemore C. Pyramidal neurons in layer 5 of the rat visual cortex. III. Differential maturation of axonal targeting, dendritic morphology, and electrophysiological properties. Journal of Comparative Neurology. 1994;339:495–518. doi: 10.1002/cne.903390404. [DOI] [PubMed] [Google Scholar]
- Keller A, White EL, Cipolloni PB. The identification of thalamocortical axon terminals in barrels of mouse Sml cortex using immunohistochemistry of anterogradely transported lectin (Phaseolus vulgaris-leucoagglutinin) Brain Research. 1985;343:159–165. doi: 10.1016/0006-8993(85)91171-0. [DOI] [PubMed] [Google Scholar]
- Larkum ME, Zhu JJ, Sakmann B. A novel cellular mechanism for coupling inputs arriving at different cortical layers. Nature. 1999;398:338–341. doi: 10.1038/18686. [DOI] [PubMed] [Google Scholar]
- LeVay S. Synaptic patterns in the visual cortex of the cat and monkey. Electron microscopy of Golgi preparations. Journal of Comparative Neurology. 1973;150:53–86. doi: 10.1002/cne.901500104. [DOI] [PubMed] [Google Scholar]
- Lübke J, Egger V, Sakmann B, Feldmeyer D. Columnar organization of dendrites and axons of synaptically coupled excitatory spiny neurons in layer 4 of the rat barrel cortex. Journal of Neuroscience. 2000 doi: 10.1523/JNEUROSCI.20-14-05300.2000. in the Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund JS. Spiny stellate neurons. In: Jones EG, Peters A, editors. The Cerebral Cortex. Vol. 1. New York, NY, USA: Plenum Press; 1984. pp. 255–308. chap. 7. [Google Scholar]
- McCormick DA. Neurotransmitter actions in the thalamus and cerebral cortex and their role in neuromodulation of thalamocortical activity. Progress in Neurobiology. 1992;29:337–388. doi: 10.1016/0301-0082(92)90012-4. [DOI] [PubMed] [Google Scholar]
- McGuire BA, Hornung JP, Gilbert CD, Wiesel TN. Patterns of synaptic input to layer 4 of cat striate cortex. Journal of Neuroscience. 1984;4:3021–3033. doi: 10.1523/JNEUROSCI.04-12-03021.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H, Lübke J, Frotscher M, Roth A, Sakmann B. Physiology and anatomy of synaptic connections between thick tufted pyramidal neurones in the developing rat neocortex. The Journal of Physiology. 1997a;500:409–440. doi: 10.1113/jphysiol.1997.sp022031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H, Lübke J, Frotscher M, Sakmann B. Regulation of synaptic efficacy by coincidence of postsynaptic APs and EPSPs. Science. 1997b;275:213–215. doi: 10.1126/science.275.5297.213. [DOI] [PubMed] [Google Scholar]
- Martin KAC, Whitteridge D. Form, function and intracortical projections of spiny neurones in the striate visual cortex of the cat. The Journal of Physiology. 1984;353:463–504. doi: 10.1113/jphysiol.1984.sp015347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountcastle VB. The columnar organisation of the neocortex. Brain. 1997;120:701–722. doi: 10.1093/brain/120.4.701. [DOI] [PubMed] [Google Scholar]
- Mountcastle VB, Powell PS. Neural mechanisms subserving cutaneous sensibility, with special reference to the role of afferent inhibition in sensory perception and discrimination. Bulletin of Johns Hopkins Hospital. 1959;105:201–232. [PubMed] [Google Scholar]
- Murray Sherman S, Guillery RW. Functional organization of thalamocortical relays. Journal of Neurophysiology. 1996;76:1367–1395. doi: 10.1152/jn.1996.76.3.1367. [DOI] [PubMed] [Google Scholar]
- Reyes A, Sakmann B. Developmental switch in the short-term modification of unitary EPSPs evoked in layer 2/3 and layer 5 pyramidal neurons of rat neocortex. Journal of Neuroscience. 1999;19:3827–3835. doi: 10.1523/JNEUROSCI.19-10-03827.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons DJ, Woolsey TA. Morphology of Golgi-Cox impregnated barrel neurons in rat Sm1 cortex. Journal of Comparative Neurology. 1984;230:119–132. doi: 10.1002/cne.902300111. [DOI] [PubMed] [Google Scholar]
- Stratford KJ, Tarczy-Hornoch K, Martin KAC, Bannister NJ, Jack JJB. Excitatory synaptic inputs to spiny stellate cells in cat visual cortex. Nature. 1996;382:258–261. doi: 10.1038/382258a0. [DOI] [PubMed] [Google Scholar]
- Stuart GJ, Dodt H-U, Sakmann B. Patch-clamp recordings from the soma and dendrites of neurons in brain slices using infrared video microscopy. Pflügers Archiv. 1993;423:511–518. doi: 10.1007/BF00374949. [DOI] [PubMed] [Google Scholar]
- Tarczy-Hornoch K, Martin KAC, Stratford KJ, Jack JJB. Intracortical excitation of spiny neurons in layer 4 of cat striate cortex in vitro. Cerebral Cortex. 1999;9:833–843. doi: 10.1093/cercor/9.8.833. [DOI] [PubMed] [Google Scholar]
- Thomson AM, Deuchars J. Temporal and spatial properties of local circuits in neocortex. Trends in Neurosciences. 1994;17:119–126. doi: 10.1016/0166-2236(94)90121-x. [DOI] [PubMed] [Google Scholar]
- Wang Z, McCormick DA. Control of firing mode of corticotectal and corticopontine layer V burst-generating neurons by norepinephrine, acetylcholine, and 1S, 3R-ACPD. Journal of Neuroscience. 1993;13:2199–2216. doi: 10.1523/JNEUROSCI.13-05-02199.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolsey TA, Dierker ML, Wann DF. Mouse SmI cortex: Qualitative and quantitative classification of Golgi-impregnated barrel neurons. Proceedings of the National Academy of Sciences of the USA. 1975;72:2165–2169. doi: 10.1073/pnas.72.6.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]


