Abstract
Evidence for the existence of sympathetic vasodilator nerves in human skeletal muscle is controversial. Manoeuvres such as contralateral ischaemic handgripping to fatigue that cause vasoconstriction in the resting forearm evoke vasodilatation after local α-adrenergic receptor blockade, raising the possibility that both constrictor and dilator fibres are present. The purpose of this study was to determine whether this dilatation is neurally mediated.
Ten subjects (3 women, 7 men) performed ischaemic handgripping to fatigue before and after acute local anaesthetic block of the sympathetic nerves (stellate ganglion) innervating the contralateral (resting) upper extremity. Forearm blood flow was measured with venous occlusion plethysmography in the resting forearm.
In control studies there was forearm vasoconstriction during contralateral handgripping to fatigue. During contralateral handgripping after stellate block, blood flow in the resting forearm increased from 6.1 ± 0.7 to 18.7 ± 2.2 ml dl−1 min−1 (P < 0.05). Mean arterial pressure measured concurrently increased from ≈90 to 130 mmHg and estimated vascular conductance rose from 6.5 ± 0.7 to 14.0 ± 1.5 units, indicating that most of the rise in forearm blood flow was due to vasodilatation.
Brachial artery administration of β-blockers (propranolol) and the nitric oxide (NO) synthase inhibitor NG-monomethyl-L-arginine (L-NMMA) after stellate block virtually eliminated all of the vasodilatation to contralateral handgrip.
Since vasodilatation was seen after stellate block, our data suggest that sympathetic dilator nerves are not responsible for limb vasodilatation seen during sympathoexcitation evoked by contralateral ischaemic handgripping to fatigue. The results obtained with propranolol and L-NMMA suggest that β-adrenergic mechanisms and local NO release contribute to the dilatation.
The existence of sympathetic cholinergic vasodilator nerves in human skeletal muscles remains controversial. Studies in some animal species clearly demonstrate the existence of sympathetic cholinergic vasodilator nerves to skeletal muscle (Bulbring & Burn, 1935; Uvnäs, 1966). These nerves are active during the defence response (Abrahams et al. 1964). Additionally, sympathetic cholinergic skeletal muscle vasodilatation is seen in cats when sympathetic nerves are stimulated following reserpine administration to eliminate noradrenaline release and vasoconstriction (Folkow et al. 1948). The existence of these dilator nerves has been confirmed histochemically (Bolme & Fuxe, 1970). More recently, NO has been implicated in the vasodilatation seen in cat and rat skeletal muscle either as a result of cholinergic stimulation of the vascular endothelium or via neural release of nitrosyl factors (Matsukawa et al. 1993; Davisson et al. 1994).
In humans, it has generally been assumed that if sympathetic vasodilator nerves to muscle exist they are activated during mental or emotional stress, states which can evoke physiological changes similar to the defence response (Roddie, 1977). Evidence to support this assumption comes from four observations. First, there can be marked skeletal muscle vasodilatation in the human forearm during mental stress and fainting (Barcroft & Edholm, 1945; Blair et al. 1959). Second, this dilatation is absent months and years after surgical sympathectomy (Barcroft & Edholm, 1945; Blair et al. 1959). Third, as in the animal studies, selective administration of drugs that eliminate α-adrenergic vasoconstriction leads to marked forearm vasodilatation during commonly used sympathoexcitatory manoeuvres such as contralateral ischaemic handgripping or the cold pressor test (Abboud & Eckstein, 1966; Dietz et al. 1997). Fourth, the dilatation can be partially blocked by atropine, suggesting a cholinergic component which also appears to be associated with NO release from the vascular endothelium (Blair et al. 1959; Abboud & Eckstein, 1966; Dietz et al. 1994, 1997).
By contrast, recent human studies raise the possibility that forearm muscle vasodilatation seen during mental stress or other sympathoexcitatory manoeuvres may be caused by local and/or circulating factors. First, during mental stress there is a reduction in sympathetic vasoconstrictor nerve traffic in subjects who show forearm vasodilatation (Halliwill et al. 1997). This reduction in constrictor nerve traffic is also seen in cats during the defence response (Dean & Coote, 1986). However, in cats there is also increased activity of sympathetic dilator fibres during the defence response (Dean & Coote, 1986). Such increased vasodilator activity is not seen in humans during mental stress (Halliwill et al. 1997). Second, mental stress still evokes forearm muscle vasodilatation when the sympathetic nerves to the upper extremity are blocked by local anaesthetic injection at the brachial plexus or stellate ganglion (Lindqvist et al. 1996; Halliwill et al. 1997). Additionally, histochemical approaches have not been able to demonstrate that humans possess sympathetic cholinergic nerves in their skeletal muscle (Bolme & Fuxe, 1970).
Along these lines, alternative mechanisms have emerged that could explain the forearm vasodilatation seen in humans during mental stress and during sympathoexcitatory manoeuvres after elimination of α-adrenergic vasoconstriction in the forearm. These mechanisms include stimulation of β2-adrenergic receptors by circulating catecholamines, NO release associated with mechanical stimulation of the vascular endothelium, and the activation of local cholinergic mechanisms (Lindqvist et al. 1996; Halliwill et al. 1997). Finally, chronic sympathectomy in animals has recently been shown to reduce the level of endothelial NO synthase (Aliev et al. 1996). This observation could explain the absence of the vasodilator response in older studies performed in patients after surgical sympathectomy (Barcroft & Edholm, 1945; Blair et al. 1959).
With this information as a background, the purpose of this study was to further test the hypothesis that human forearm muscle is innervated by sympathetic vasodilator nerves. To this end, we had subjects perform ischaemic handgrip exercise to fatigue before and after local anaesthetic block of the stellate ganglion innervating the contralateral (resting) upper extremity. We chose handgripping to fatigue for two reasons. First, it is associated with large and consistent increases in sympathetic traffic to muscle (Mark et al. 1985; Seals & Enoka, 1989). Second, it evokes vasodilatation in the resting forearm after local α-adrenergic receptor blockade (Dietz et al. 1997). We reasoned that if the dilatation was absent or blunted after stellate block, this would indicate that sympathetic vasodilator nerves contributed to it. Alternatively, if forearm muscle vasodilatation was still observed during handgrip trials following stellate block, it would support a role for circulating and/or local factors in mediating the vasodilator response. We tested this alternative hypothesis by brachial artery infusions of the NO synthase inhibitor L-NMMA and the β-blocker propranolol during handgrip trials following stellate block. With this study design we attempted to parallel the classic work of Folkow and colleagues (1948) which clearly demonstrated the existence of sympathetic vasodilator nerves in cat hindlimb muscles.
METHODS
General methods
Subjects
Ten healthy, normotensive, non-smoking subjects (3 women, 7 men) between the ages of 18 and 40 years participated in the study. Participants were not taking medications. The study was approved by the Human Subjects Committee of the Mayo Clinic and was carried out in accordance with the Declaration of Helsinki. Each subject gave written informed consent. All female subjects had a negative serum pregnancy test within 12 h prior to participation.
Subject monitoring
During the study, heart rate was monitored using a five-lead electrocardiogram. Finger temperature was monitored in the hand of the non-exercising, left forearm with a thermocouple placed on the index finger. This measurement was used to help assess the efficacy of the stellate block. In some studies arterial pressure measurements and drug infusions were performed using a 5 cm, 20 gauge brachial artery catheter placed in the left arm using sterile techniques after local anaesthesia with 1–2 ml of 1 % lidocaine (lignocaine). A three-port connector was placed in series with a catheter-transducer system so that drugs could be infused and arterial pressure measured simultaneously (Dietz et al. 1994).
Forearm blood flow
Forearm blood flow was measured using venous occlusion plethysmography with mercury-in-Silastic strain gauges (Greenfield et al. 1963). During recordings, a wrist cuff was continuously inflated to suprasystolic pressure (250 mmHg) to occlude arterial blood flow to the hand, while a venous occlusion cuff around the upper arm was inflated to 50 mmHg for 7.5 s of every 15 s, providing one blood flow measurement every 15 s. Forearm blood flow values were expressed as millilitres per decilitre of tissue per minute (ml dl−1 min−1).
Skin blood flow
Cutaneous blood flow was measured with a laser Doppler probe (Perimed PF5001) placed over the midsection of the forearm, just distal to the mercury-in-Silastic strain gauge. This was done to evaluate the changes in skin blood flow when total forearm blood flow was changing.
Contralateral ischaemic handgrip exercise
To increase sympathetic outflow to skeletal muscle, subjects were instructed to rhythmically squeeze a handgripping device with the right hand to fatigue. Just prior to exercise, an arm cuff was inflated to suprasystolic pressure (250 mmHg). To ensure maximal effort, subjects were encouraged by the investigators to continue squeezing until they reached fatigue. The handgripping was followed by 1 min of post-exercise ischaemia. Handgripping to fatigue evokes a large increase in muscle sympathetic nerve activity (Mark et al. 1985). Additionally, the sympathetic responses to repeated bouts of fatiguing handgrip exercise are highly reproducible (Seals & Enoka, 1989).
Lower body suction
This technique was used to provide a standard stimulus for elevating sympathetic activity to the forearms. The lower body of the subject was sealed in an airtight box at the level of the iliac crests. Lower body suction was applied for 2 min at -30 mmHg to activate cardiovascular reflexes and induce forearm vasoconstriction. This permitted sympathetic vasoconstrictor responses in the upper extremity to be evaluated at various times during the study (Halliwill et al. 1997).
Stellate ganglion block
Bupivacaine HCl (6 ml of 0.25 %) was administered to produce local anaesthetic block of the left stellate ganglion at the sixth cervical vertebra. This technique temporarily (hours) eliminates sympathetic outflow to the face and left upper extremity (Joyner et al. 1992; Halliwill et al. 1997).
β-Adrenergic receptor blockade
Propranolol HCl was administered via the brachial artery catheter to block β-adrenergic receptors in the forearm. A 1 mg loading dose infused over 5 min was administered to all subjects and a 0.5 mg supplemental dose was given if propranolol was the first drug in the series. Doses of 0.5 mg have been shown to block the forearm vasodilator response to the β-adrenergic agonist isoproterenol (Johnsson, 1967; Eklund & Kaijser, 1976).
NO synthase inhibition
The NO synthase inhibitor L-NMMA was infused via the brachial artery catheter to inhibit NO production in the forearm. A total of 100 mg of L-NMMA was infused in divided doses, 80 mg of which was given as a loading dose and a maintenance infusion of 1 mg min−1 was used for the duration of the study (Dietz et al. 1994, 1997; Halliwill et al. 1997).
Specific experimental protocols
Protocol 1a: demonstration of forearm vasoconstriction during contralateral ischaemic handgrip exercise (Fig. 1)
Figure 1. Schematic representation of study protocols.
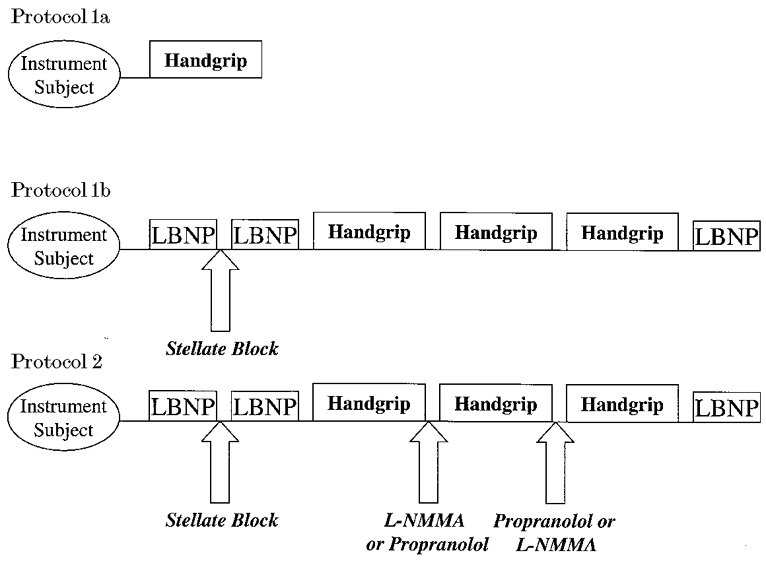
Protocol 1a: forearm blood flow responses at rest and during contralateral rhythmic handgripping were measured. The purpose of this protocol was to demonstrate that contralateral handgripping to fatigue normally causes vasoconstriction in the resting forearm. Three subjects were studied. Protocol 1b: the effects of an ipsilateral stellate ganglion block on the forearm blood flow response to contralateral ischaemic handgripping. Lower body suction (lower body negative pressure, LBNP) was used before and after the stellate block to ensure that vasoconstrictor outflow to the forearm was eliminated. Three repeated handgrips were then performed to determine whether the forearm blood flow responses to handgripping were consistent during repeated bouts. Three subjects were studied. Protocol 2: evaluation of the contribution of NO and β-adrenergic receptors to the vasodilatation observed during contralateral handgripping to fatigue after stellate ganglion block. Lower body suction (LBNP) was used before and after the stellate block to confirm that sympathetic vasoconstrictor nerves to the forearm had been anaesthetised. The first bout of handgripping was performed without drug administration (n = 10). Before the second bout of handgripping, half the subjects received L-NMMA or propranolol via a brachial artery catheter and handgripping was again performed. The opposite drug was then infused prior to the third bout of handgripping. Continued efficacy of the stellate block was assessed by a final LBNP test at the end of the trial.
It is well known that ischaemic handgrip exercise evokes a progressive rise in muscle sympathetic nerve activity (Mark et al. 1985). We wanted to confirm in our laboratory that this manoeuvre evoked vasoconstriction in the resting forearm. Three subjects participated in this protocol. Subjects were placed in the supine position and both arms were instrumented to measure forearm blood flow. The left arm was also instrumented to monitor skin blood flow. One ischaemic handgrip trial was performed without stellate ganglion block or drug infusion. Blood flow measurements in the non-dominant resting forearm were made throughout the handgrip manoeuvres. These subjects also participated in protocol 1b and protocol 2 on separate days.
Protocol 1b: repeatability of forearm blood flow response during contralateral ischaemic handgrip after stellate ganglion block (Fig. 1)
To establish that the blood flow responses in the resting forearm to contralateral ischaemic handgripping were repeatable after stellate ganglion block, three subjects performed three bouts of handgripping after receiving a stellate block. Subjects were placed in a supine position in the lower body suction box and were instrumented to measure forearm blood flow and skin blood flow on the left side. The left hand was also instrumented to measure finger temperature. Baseline blood flow was recorded and -30 mmHg lower body suction was performed, followed by stellate ganglion blockade. After the stellate block, lower body suction was repeated to assess the efficacy of the stellate block. This was followed by three bouts of ischaemic handgripping to fatigue and post-exercise ischaemia maintained for 1 min. Approximately 10 min of recovery was allowed between each of these bouts. Lower body suction was also performed at the end of the study to confirm that the level of sympathectomy provided by the stellate block had been maintained through the course of the experiment.
Protocol 2: mechanisms of vasodilatation during contralateral ischaemic handgrip exercise (Fig. 1)
The purpose of this study was to determine whether contralateral ischaemic handgripping to fatigue evokes vasodilatation in the resting forearm after stellate block, and subsequently to determine the potential role of β-adrenergic receptors and NO as causes of the vasodilatation. Ten subjects took part in this protocol. Subjects were instrumented as in protocol 1b with the addition of a brachial artery catheter in the resting (left) arm. Baseline blood flow was recorded and -30 mmHg lower body suction applied, followed by stellate ganglion blockade. After stellate block, lower body suction was again performed to assess the efficacy of the stellate block, followed by the first ischaemic handgrip trial. Blood flow measurements in the non-dominant resting forearm were made throughout the handgrip manoeuvre. After recovery (10 min), either L-NMMA (5 subjects) or propranolol (5 subjects) was infused into the brachial artery catheter. Subjects performed a second handgrip trial to fatigue, followed by 10 min of recovery. The other drug (propranolol or L-NMMA) was then administered to the appropriate subjects and the third handgrip bout was performed. The lower body suction trial was then repeated to confirm maintenance of the stellate block over the course of the experiment.
Data analysis
Data were digitised at 200 Hz and stored on computer. Data were analysed off-line with signal processing software (Windaq; Dataq Instruments, Akron, OH, USA). Heart rate was derived from the electrocardiogram waveform. Mean arterial pressure was derived from the arterial pressure waveform. To assess vasoconstrictor and vasodilator responses, forearm vascular conductance (FVC) was calculated as (forearm blood flow)/(mean arterial pressure) and expressed as arbitrary ‘units’ (i.e. ml (dl tissue)−1 (100 mmHg)−1 min−1). These units were used since they are quantitatively similar to the standard units for forearm blood flow. Previously we have expressed FVC per millimetre of Hg of arterial pressure (Dietz et al. 1997).
Statistics
All values are reported as means ±s.e.m. Student's paired t tests were used to compare each subject's baseline and peak responses during handgripping, and peak responses during handgripping across stellate and drug infusion conditions. Significance was set at P < 0.05.
RESULTS
Protocol 1a: contralateral ischaemic handgrip exercise causes vasoconstriction in the resting forearm
Figure 2 shows the results of the contralateral ischaemic handgripping performed without intervention. During handgripping, heart rate increased from 67 ± 3 to 95 ± 4 beats min−1. Forearm blood flow averaged 4.6 ± 1.0 ml dl−1 min−1 at rest and a slight decrease was seen in all three subjects at the end of handgripping. In protocol 2 when blood pressure was measured in these subjects using a brachial catheter, resting mean arterial pressure was 96 ± 5 mmHg at rest and rose to 136 ± 6 mmHg at the end of handgripping. Since the arterial pressure responses to repeated bouts of handgripping to fatigue were extremely reproducible, these values were used to estimate forearm vascular conductance, which decreased by about one-third at the end of handgripping.
Figure 2. Forearm blood flow (FBF) and estimated forearm vascular conductance (FVC) responses to contralateral ischaemic handgripping to fatigue prior to stellate ganglion block (protocol 1a).
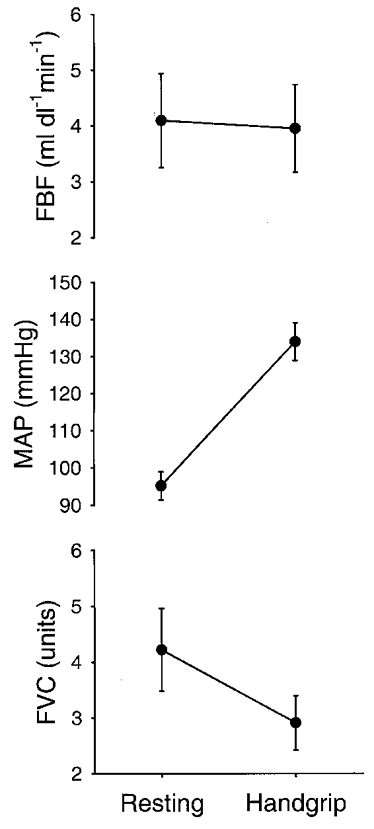
Resting values were obtained prior to contralateral ischaemic handgrip. Handgrip values are from the last measurement prior to cessation of contralateral ischaemic handgripping. Mean arterial pressure (MAP) values are from trials these subjects completed during protocol 2. Data are expressed as means ±s.e.m.
Protocol 1b: contralateral ischaemic handgripping evokes marked forearm vasodilatation after stellate ganglion block
Figure 3 shows the results of three repeated handgripping trials performed after stellate block but without other interventions in the three control subjects. In these subjects, stellate block caused resting forearm blood flow to nearly double and finger temperature to increase from 32.7 ± 0.5 to 35.6 ± 0.5°C, and virtually eliminated the forearm vasoconstrictor response to lower body suction. Blood flow increased 3- to 4-fold in the resting forearm during handgripping, and similar increases in flow were seen in all three subjects across all three trials. During all three trials heart rate increased from ∼65 to ∼100 beats min−1 and in the subsequent study (protocol 2) with a brachial catheter these subjects showed consistent and significant increases in mean arterial pressure, from 96 ± 5 to 136 ± 6 mmHg. These values were used to estimate forearm vascular conductance, and these estimated forearm vascular conductance responses were consistent in all three subjects across all three trials. Since these responses clearly demonstrated consistent forearm muscle vasodilatation after stellate block, we embarked on protocol 2 to confirm this observation and to determine its cause.
Figure 3. Forearm blood flow and forearm vascular conductance responses to repeated bouts of contralateral ischaemic handgripping to fatigue after stellate ganglion block (protocol 1b).
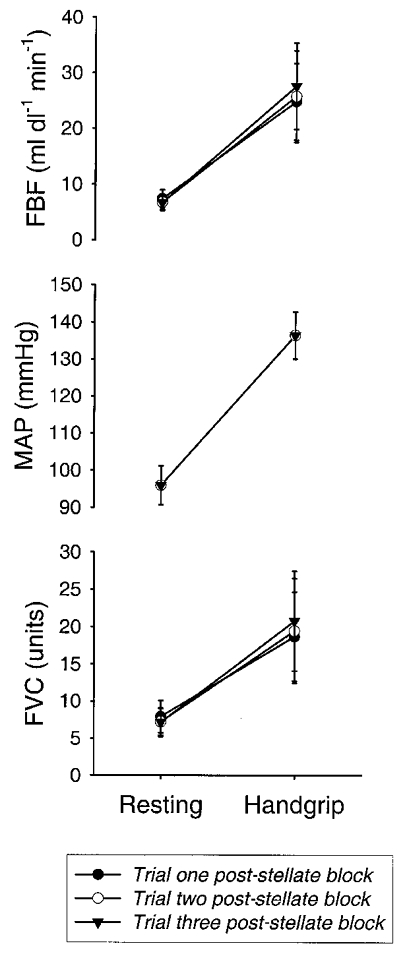
Resting values were obtained prior to contralateral ischaemic handgrip. Handgrip values are from the last measurement prior to cessation of contralateral ischaemic handgripping. After stellate ganglion block there was a marked increase in forearm blood flow during handgripping. Mean arterial pressure is from trials these subjects completed during protocol 2. Data are expressed as means ±s.e.m.
Protocol 2: vasodilatation elicited after stellate ganglion block is blunted by propranolol and L-NMMA
All ten subjects participating in protocol 2 demonstrated a dilated pupil, ptosis and nasal congestion on the ipsilateral side of the face after stellate block. In addition, finger temperature rose from 29.1 ± 1.3 to 35.1 ± 0.3°C (P < 0.05). Resting forearm blood flow on the blocked side rose from 3.2 ± 0.4 to 6.1 ± 0.7 ml dl−1 min−1 (P < 0.05). Lower body suction of -30 mmHg caused FVC in the control arm to fall from 2.6 ± 0.4 to 1.5 ± 0.2 units (P < 0.05). In the stellate-blocked forearm, FVC was 6.1 ± 0.7 units and remained nearly constant at 5.8 ± 0.7 units during lower body suction (P > 0.05). These findings are all consistent with a profound forearm sympathectomy. During all three bouts of handgripping, heart rate increased by ∼20-30 beats min−1 and mean arterial pressure increased by ∼30-40 mmHg. The increases in heart rate and arterial pressure were all significant and also similar across the three trials.
Figure 4 shows individual data for protocol 2. In the trial conducted after stellate block but before either propranolol or L-NMMA was given, contralateral ischaemic handgripping caused forearm blood flow to rise from 7 to 16 ml dl−1 min−1. During the second bout of handgripping, propranolol had no effect on baseline forearm blood flow, but markedly blunted the rise in flow with handgripping. During the third bout of handgripping, L-NMMA reduced the baseline flow and eliminated the rise in flow associated with handgripping.
Figure 4. Individual responses of one subject to protocol 2.
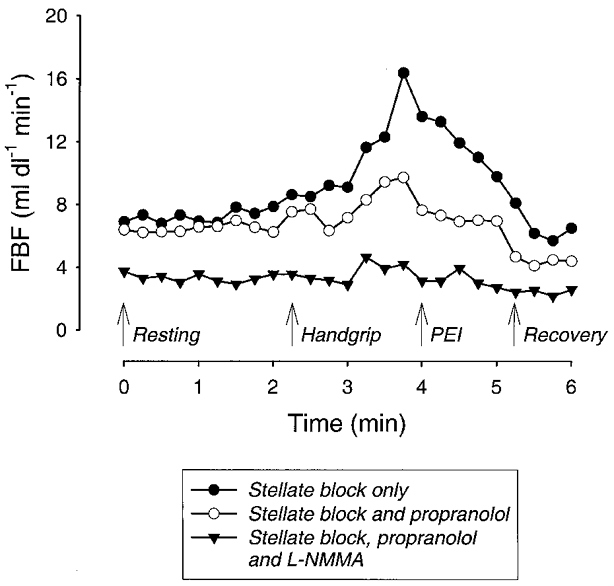
After stellate ganglion block, handgripping to fatigue caused forearm blood flow to increase 2.5-fold. Administration of propranolol via a brachial artery catheter had no effect on baseline blood flow after stellate block but blunted the increase in flow during handgripping and post-exercise ischaemia (PEI). L-NMMA given prior to the third bout of exercise caused a marked reduction in resting forearm blood flow and eliminated any changes in forearm blood flow during the final bout of handgripping.
Figures 5 and Figures 6 show the cumulative data for all ten subjects. During the first bout of handgripping in the stellate block only condition, forearm blood flow and forearm vascular conductance both increased significantly. During the second bout of handgripping in the five subjects who received propranolol first, forearm blood flow and vascular conductance both still increased relative to baseline, but the magnitude of the increase in both flow and conductance was blunted substantially (P < 0.05) by propranolol in comparison to stellate block alone (Fig. 6). Prior to the third bout of handgripping, these subjects received L-NMMA and supplemental doses of propranolol. L-NMMA caused resting forearm blood flow to fall to 2.8 ± 0.2 ml dl−1 min−1 (P < 0.05). During handgripping forearm blood flow rose modestly but significantly to 3.6 ± 0.3 ml dl−1 min−1 (P < 0.05), but forearm vascular conductance was unchanged at the end of handgripping (Fig. 5). The magnitude of the increase in forearm blood flow and vascular conductance after both propranolol and L-NMMA treatment in these subjects was significantly (P < 0.05) less than that seen during the first bout of handgripping (Fig. 6).
Figure 5. Forearm blood flow, mean arterial pressure and forearm vascular conductance responses for protocol 2.
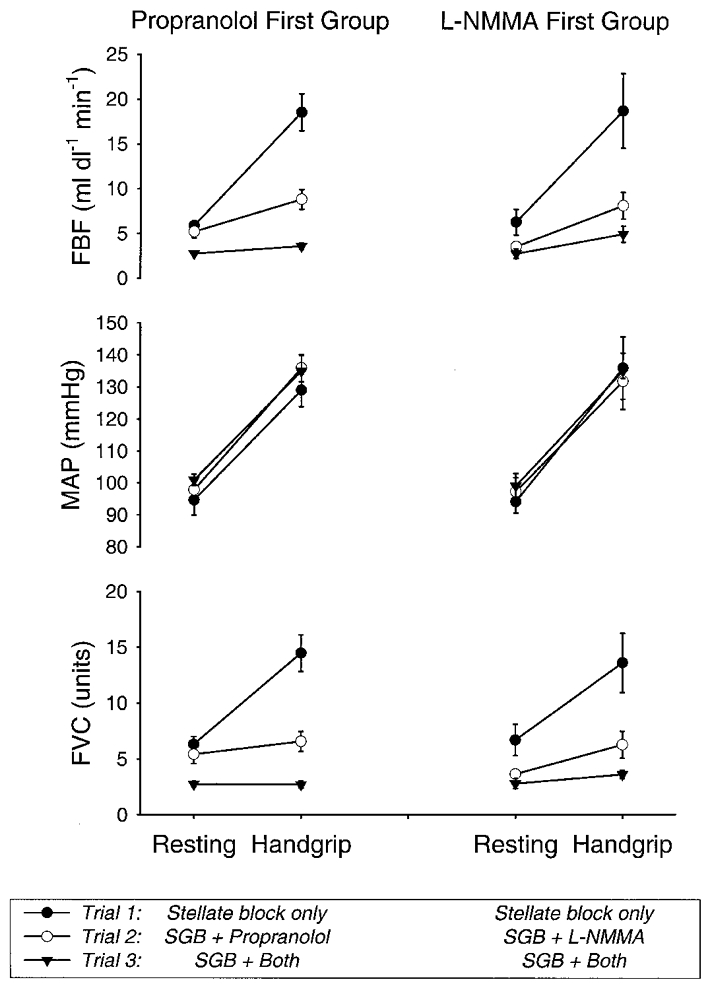
Resting values were obtained prior to contralateral ischaemic handgrip. Handgrip values are from the last measurement prior to cessation of contralateral ischaemic handgripping. Propranolol first group, 5 subjects. L-NMMA first group, 5 subjects. SGB, stellate ganglion blockade. Data are expressed as means ±s.e.m.
Figure 6. Changes in FVC during handgripping in protocol 2.
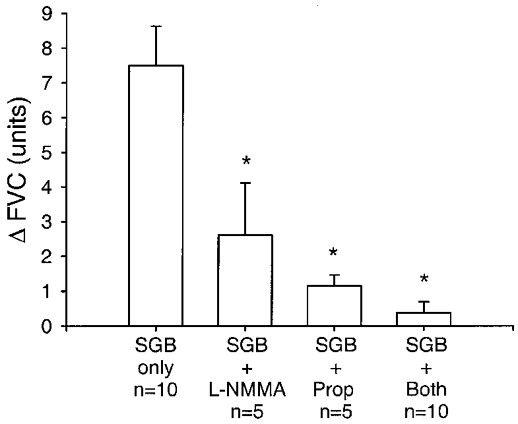
The change in FVC (Δ FVC) is the difference between FVC measurements taken prior to the onset of contralateral ischaemic handgrip and the final FVC measurement taken before cessation of contralateral ischaemic handgrip exercise. SGB, stellate ganglion blockade. Prop, propranolol. The number of subjects for each group (n) is shown. Data are expressed as means ±s.e.m.* Significantly different from SGB alone (P < 0.05).
In the five subjects who received L-NMMA first, resting forearm blood flow fell from 6.3 ± 1.4 to 3.5 ± 0.3 ml dl−1 min−1 (P < 0.05). During the second bout of handgripping, forearm blood flow and vascular conductance both rose modestly, but L-NMMA blunted the magnitude of the increase in flow and conductance (P < 0.05) in comparison to the first bout of handgripping after stellate block alone (Fig. 6). The subjects who were given L-NMMA first then received propranolol and a supplemental infusion of L-NMMA. During the third bout of handgripping, the magnitude of the increases in flow and conductance was significantly (P < 0.05) lower in comparison to the first bout (Fig. 6).
When data for all ten subjects were considered for the third bout of exercise after each subject had received both propranolol and L-NMMA, blood flow increased modestly from 2.8 ± 0.3 to 4.3 ± 0.5 ml dl−1 min−1 (P < 0.05). However, forearm vascular conductance did not change significantly, having a value of 2.8 ± 0.2 units at rest and 3.1 ± 0.3 units at the end of handgripping. Again, the magnitude of the increases in both forearm blood flow and vascular conductance was significantly lower after the third bout of handgripping than for the first bout of handgripping when all ten subjects were compared (Fig. 6).
Skin blood flow responses to protocol 2
Baseline skin blood flow was 25.4 ± 4.8 units and was unaffected by stellate ganglion block (P > 0.05). In the five subjects who received propranolol first, drug infusion did not alter resting skin blood flow (P > 0.05), while in the five subjects who received L-NMMA first, drug infusion reduced skin blood flow from 20.8 ± 4.6 to 9.9 ± 1.1 units (P < 0.05). Subsequent infusion of L-NMMA in the five subjects who received propranolol first also resulted in a reduction in skin blood flow, from 37.8 ± 4.8 to 22.5 ± 4.3 units (P < 0.05). No changes in skin vascular conductance were seen during handgripping after stellate block (P > 0.05), stellate block plus L-NMMA (P > 0.05) or stellate block plus L-NMMA plus propranolol (P > 0.05), but a modest decrease was observed under stellate block plus propranolol (resting baseline, 31.3 ± 0.05 units vs. handgrip, 26.1 ± 0.05 units, P < 0.05). These data confirm that changes in forearm vascular conductance observed during handgripping occurred almost exclusively in the forearm muscles.
Arterial catecholamine responses to handgripping
In order to assess the effect of handgripping on circulating levels of adrenaline, we measured arterial adrenaline at rest and at the end of handgripping in three subjects. These values rose from 42.1 ± 12.7 to 155.6 ± 28.0 pg ml−1, indicating that circulating adrenaline levels were substantially elevated by the time subjects stopped handgripping.
DISCUSSION
Overview
We have demonstrated that contralateral ischaemic handgripping to fatigue evokes profound skeletal muscle vasodilatation in the resting forearm after the sympathetic nerves to that forearm have been acutely blocked with local anaesthetics. Additionally, propranolol or L-NMMA given alone blunted this vasodilatation, and in combination virtually eliminated it. These vasodilator responses are similar in magnitude to our previous observations made during contralateral ischaemic handgripping to fatigue after local pharmacological sympathectomy of the resting forearm (Dietz et al. 1997), and these new findings indicate that sympathetic vasodilator nerves do not mediate this response. Taken together, our data suggest that circulating catecholamines operating through β2-adrenergic receptors and local NO release are the probable mechanisms of the forearm vasodilatation elicited during sympathoexcitatory manoeuvres in humans. In this context, we will discuss the limitations of our findings, and the potential mechanisms associated with them. We will also attempt to relate them to previous observations made concerning the forearm vasodilator response to mental stress.
Limitations
There are several potential limitations to the above conclusions. The first limitation concerns our level of certainty that local anaesthetic injection at the stellate ganglion blocked all the sympathetic fibres to the forearm, especially any vasodilator fibres. The increases in finger temperature and resting forearm blood flow, the absence of vasoconstrictor responses to lower body negative pressure, and our clinical observations are all consistent with the successful block of sympathetic vasoconstrictor fibres. Additionally, we injected the local anaesthetic in the same general anatomical area as that resected by surgeons to sympathectomize patients in the older studies (Barcroft & Edholm, 1945; Blair et al. 1959). However, this still does not eliminate the possibility that sympathetic cholinergic fibres to the forearm do not travel through the stellate ganglion and would not be blocked by local anaesthetic injection there. In this context, Lindqvist et al. (1996) have shown that the forearm vasodilator responses to mental stress are still present in the upper extremity of individuals who have undergone local anaesthetic block of the brachial plexus (i.e. motor, sensory and sympathetic) at the axilla. Taken together, these observations all argue against an unappreciated anatomical location of sympathetic vasodilator nerves and a failure to block them with the local anaesthetic as an explanation for our findings.
A second potential limitation to our findings is that contralateral handgripping can sometimes be associated with unintended contractions in the resting forearm (Cotzias & Marshall, 1993). However, in this study three subjects who performed contralateral ischaemic handgrip without intervention demonstrated forearm vasoconstriction. These same subjects demonstrated consistent, profound forearm vasodilatation during contralateral ischaemic handgrip following stellate ganglion blockade. In this context, it would seem unlikely that our subjects were consistently able to relax the resting forearm during contralateral ischaemic handgrip before stellate ganglion block but then consistently demonstrated inadvertent contraction on all subsequent trials. Furthermore, the magnitude of the increase in blood flow in the resting forearm during contralateral ischaemic handgripping to fatigue after stellate block exceeded that which might be caused by unintended contractions (Cotzias & Marshall, 1993). In fact, it was similar to that seen during mild and moderate rhythmic forearm handgripping (Joyner et al. 1992). Therefore, it would seem that unintended contractions probably do not explain a major portion of the vasodilator responses that we saw.
A third alternative explanation is that vasodilating nerves, local factors and circulating factors can all potentially cause forearm vasodilatation during sympathoexcitation. If sympathetic dilator nerves did contribute, we would expect the dilator response in the resting forearm during contralateral ischaemic handgripping to fatigue to be less than the dilator responses we previously observed using local pharmacological sympathectomy (Dietz et al. 1997). This expectation is based on the concept that during the study by Dietz et al. (1997) all three factors would have been contributing to the dilatation. By contrast, after stellate block, only the local and circulating factors would be available to contribute. However, the forearm dilatation we saw during contralateral ischaemic handgripping after stellate ganglion block was similar to or greater than that observed after pharmacological sympathectomy. This observation argues against a role for sympathetic vasodilator fibres in human forearm skeletal muscle.
Taken together, we believe that the above limitations, while conceptually possible, do not explain the findings of the current study. This leaves us with several questions including how the findings reported in the older studies in patients after chronic surgical sympathectomy can be explained, and what the local or circulating factor(s) responsible for the vasodilatation that we saw after nerve block are.
Possible mechanisms
As discussed in the Introduction, chronic surgical sympathectomy eliminates the forearm vasodilator responses to mental stress, suggesting that sympathetic dilator nerves are responsible (Blair et al. 1959; Roddie, 1977). However, in the current study we demonstrated that marked sympathoexcitation can cause vasodilatation in a resting forearm after stellate ganglion block. Similar observations have been made after acute neural blockade by local anaesthetic during mental stress (Lindqvist et al. 1996; Halliwill et al. 1997). Since at least a portion of the vasodilator responses to these manoeuvres is eliminated by NO synthase inhibition, β-adrenergic receptor blockade or atropine, it is possible that chronic changes in either the vascular endothelium or the vascular smooth muscle are seen with surgical sympathectomy. Along these lines, recent observations in animals indicate that chronic sympathectomy eliminates endothelial NO synthase expression in the vascular endothelium of the sympathectomized blood vessels (Aliev et al. 1996). This suggests that normal NO-dependent responses to local and circulating factors would be present following acute sympathectomy with local anaesthetics or drugs injected into the brachial artery, but that these responses would be absent in the months and years following surgical sympathectomy.
In view of the evidence discussed above, we believe that the most likely mechanisms involved in the forearm vasodilator responses to sympathoexcitatory manoeuvres after stellate block probably centre around β2-adrenergic receptor-mediated stimulation evoking vasodilatation via activation of receptors on the vascular smooth muscle. We also believe that substantial NO-dependent vasodilatation may be seen as a result of mechanical stimulation of the vascular endothelium and β2-adrenergic receptor-mediated stimulation of the vascular endothelium (Rubanyi et al. 1986; Dawes et al. 1997). Additionally, in previous studies we and others have demonstrated that at least some of these responses are atropine sensitive (Blair et al. 1959; Abboud & Eckstein, 1966; Dietz et al. 1994, 1997). Since we have argued that these responses are not due to sympathetic cholinergic nerves and others have failed to provide histochemical evidence for such nerves in human skeletal muscle, how might these responses be atropine sensitive? The best possible explanation is that a small fraction of the vascular endothelial cells may synthesise and release ACh (Milner et al. 1990).
In this context, mechanically stimulated NO release from the canine femoral artery (and subsequent vasodilatation) is absent when the femoral artery is treated with either atropine or exogenous acetylcholinesterase (Martin et al. 1996). These observations raise the possibility that there can be substantial local ACh release that might contribute to the vasodilatation seen during mental stress and other sympathoexcitatory manoeuvres in humans. A possible physiological role for this local vasodilator mechanism has been advanced by Pohl and colleagues, who have shown that when blood pressure and sympathetic vasoconstrictor traffic to skeletal muscle vessels are increased, the vasoconstriction associated with the myogenic response and the increased sympathetic traffic are partially offset by local NO release (Pohl & de Wit, 1996). Perhaps the dilatation seen during mental stress or contralateral ischaemic handgrip after stellate ganglion block is a response that normally opposes various constrictor mechanisms.
In summary, we have presented evidence that there can be marked vasodilatation in human muscle during sympathoexcitatory manoeuvres that is not neurally mediated. This vasodilatation instead appears to be caused by a combination of circulating and/or local mechanisms. The responses we have observed in this experiment are qualitatively similar to recent observations made by our group and others on the vasodilator responses to mental stress in humans (Dietz et al. 1994; Lindqvist et al. 1996; Halliwill et al. 1997). While there are a variety of other potential explanations for the vasodilatation seen in the current study, including the existence of neural pathways not blocked by local anaesthetics administered at the stellate ganglion, or the possibility of unintended contractions in the ‘resting’ forearm, we believe that these mechanisms are unlikely. Additionally, when the classic older studies in humans with surgically sympathectomized limbs are viewed in the context of emerging information, we believe it is possible to reconcile those observations with observations from this study and conclude that sympathetic cholinergic nerves are not responsible for the forearm vasodilator responses seen during sympathoexcitatory manoeuvres (or mental stress) in conscious human volunteers. A major new hypothesis generated by these observations concerns the long-term interactions between the sympathetic vasoconstrictor nerves to skeletal muscle and the integrity of endothelially dependent vasodilator systems.
Acknowledgments
The time and effort put forth by the subjects is greatly appreciated. The authors also appreciate the excellent administrative assistance and subject recruitment and scheduling by Karen Krucker, and the excellent secretarial assistance of Janet Beckman. The authors further thank the nursing staff of the Mayo General Clinical Research Center for their assistance with this project. This study was supported by NIH grants HL 46493, NS32352, NRSA HL 10123 (C.T.M.) and NRSA DK09826 (J.R.H.). Further support was provided by the Mayo General Clinical Research Center (grant RR00585). M.E.T. was supported by a postdoctoral fellowship funded by the Natural Sciences and Engineering Research Council of Canada. A.S.R. was supported by the Howard E. Burchill Scholarship of the American Heart Association.
References
- Abboud FM, Eckstein JW. Active reflex vasodilation in man. Federation Proceedings. 1966;25:1611–1617. [PubMed] [Google Scholar]
- Abrahams VC, Hilton SM, Zbrozyna AW. The role of active muscle vasodilation in the alerting stage of the defence reaction. The Journal of Physiology. 1964;171:189–202. doi: 10.1113/jphysiol.1964.sp007371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aliev G, Ralevic V, Burnstock G. Depression of endothelial nitric oxide synthase but increased expression of endothelin-1 immunoreactivity in rat thoracic aortic endothelium associated with long-term, but not short-term, sympathectomy. Circulation Research. 1996;79:317–323. doi: 10.1161/01.res.79.2.317. [DOI] [PubMed] [Google Scholar]
- Barcroft H, Edholm OG. On the vasodilation in human skeletal muscle during post-haemorrhagic fainting. The Journal of Physiology. 1945;104:161–175. doi: 10.1113/jphysiol.1945.sp004113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair DA, Glover WE, Greenfield ADM, Roddie IC. Excitation of cholinergic vasodilator nerves to human skeletal muscles during emotional stress. The Journal of Physiology. 1959;148:633–647. doi: 10.1113/jphysiol.1959.sp006312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolme P, Fuxe K. Adrenergic and cholinergic nerve terminals in skeletal muscle vessels. Acta Physiologica Scandinavica. 1970;78:52–59. doi: 10.1111/j.1748-1716.1970.tb04638.x. [DOI] [PubMed] [Google Scholar]
- Bulbring E, Burn JH. The sympathetic dilator fibres in the muscles of the cat and dog. The Journal of Physiology. 1935;83:483–501. doi: 10.1113/jphysiol.1935.sp003246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotzias C, Marshall JM. Vascular and electromyographic responses evoked in forearm muscle by isometric contraction of the contralateral forearm. Clinical Autonomic Research. 1993;3:21–30. doi: 10.1007/BF01819139. [DOI] [PubMed] [Google Scholar]
- Davisson RL, Johnson AK, Lewis SJ. Nitrosyl factors mediate active neurogenic hindquarter vasodilation in the conscious rat. Hypertension. 1994;23:962–966. doi: 10.1161/01.hyp.23.6.962. [DOI] [PubMed] [Google Scholar]
- Dawes M, Chowienczyk PJ, Ritter MM. Effects of inhibition of the L-arginine/nitric oxide pathway on vasodilation caused by β-adrenergic agonists in human forearm. Circulation. 1997;95:2293–2297. doi: 10.1161/01.cir.95.9.2293. [DOI] [PubMed] [Google Scholar]
- Dean C, Coote JH. Discharge patterns in postganglionic neurones to skeletal muscle and kidney during activation of the hypothalamic and midbrain defence areas in the cat. Brain Research. 1986;377:271–278. doi: 10.1016/0006-8993(86)90868-1. [DOI] [PubMed] [Google Scholar]
- Dietz NM, Engelke KA, Samuel TT, Fix RT, Joyner MJ. Evidence for nitric oxide-mediated sympathetic forearm vasodilatation in humans. The Journal of Physiology. 1997;498:531–540. doi: 10.1113/jphysiol.1997.sp021879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz NM, Rivera JM, Eggener SE, Fix RT, Warner DO, Joyner MJ. Nitric oxide contributes to the rise in forearm blood flow during mental stress in humans. The Journal of Physiology. 1994;480:361–368. doi: 10.1113/jphysiol.1994.sp020366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund B, Kaijser L. Effect of regional α- and β-adrenergic blockade on blood flow in the resting forearm during contralateral isometric handgrip. The Journal of Physiology. 1976;262:39–50. doi: 10.1113/jphysiol.1976.sp011584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkow B, Haeger K, Uvnäs B. Cholinergic vasodilator nerves in the sympathetic outflow to the muscles of the hind limbs of the cat. Acta Physiologica Scandinavica. 1948;15:401–411. doi: 10.1111/j.1748-1716.1948.tb00515.x. [DOI] [PubMed] [Google Scholar]
- Greenfield A D M, Whitney RJ, Mowbray JF. Methods for the investigation of peripheral blood flow. British Medical Bulletin. 1963;19:101–109. doi: 10.1093/oxfordjournals.bmb.a070026. [DOI] [PubMed] [Google Scholar]
- Halliwill JR, Lawler LA, Eickhoff TJ, Dietz NM, Nauss LA, Joyner MJ. Forearm sympathetic withdrawal and vasodilatation during mental stress in humans. The Journal of Physiology. 1997;504:211–220. doi: 10.1111/j.1469-7793.1997.211bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsson G. The effects of intra-arterially administered propranolol and H 56/28 on blood flow in the forearm – a comparative study of two β-adrenergic receptor antagonists. Acta Pharmacologica et Toxicologica. 1967;25:63–74. doi: 10.1111/j.1600-0773.1967.tb02997.x. [DOI] [PubMed] [Google Scholar]
- Joyner MJ, Nauss LA, Warner MA, Warner DO. Sympathetic modulation of blood flow and oxygen uptake in rhythmically contracting human forearm muscles. American Journal of Physiology. 1992;263:H1078–1083. doi: 10.1152/ajpheart.1992.263.4.H1078. [DOI] [PubMed] [Google Scholar]
- Lindqvist M, Davidsson S, Hjemdahl P, Melcher A. Sustained forearm vasodilation in humans during mental stress is not neurogenically mediated. Acta Physiologica Scandinavica. 1996;158:7–14. doi: 10.1046/j.1365-201X.1996.529288000.x. [DOI] [PubMed] [Google Scholar]
- Mark AL, Victor RG, Nerhed C, Wallin BG. Microneurographic studies of the mechanisms of sympathetic nerve responses to static exercise in humans. Circulation Research. 1985;57:461–469. doi: 10.1161/01.res.57.3.461. [DOI] [PubMed] [Google Scholar]
- Martin CM, Beltran-del-Rio A, Albrecht A, Lorenz RR, Joyner MJ. Local cholinergic mechanisms mediate nitric oxide-dependent, flow-induced vasorelaxation in vitro. American Journal of Physiology. 1996;270:H442–446. doi: 10.1152/ajpheart.1996.270.2.H442. [DOI] [PubMed] [Google Scholar]
- Matsukawa K, Shindo T, Shirai M, Ninomiya I. Nitric oxide mediates cat hindlimb cholinergic vasodilation induced by stimulation of posterior hypothalamus. Japanese The Journal of Physiology. 1993;43:473–483. doi: 10.2170/jjphysiol.43.473. [DOI] [PubMed] [Google Scholar]
- Milner P, Kirkpatrick KA, Ralevic V, Toothill V, Pearson J, Burnstock G. Endothelial cells cultured from human umbilical vein release ATP, substance P and acetylcholine in response to increased flow. Proceedings of the Royal Society. 1990;B 241:245–248. doi: 10.1098/rspb.1990.0092. [DOI] [PubMed] [Google Scholar]
- Pohl U, de Wit C. Interaction of nitric oxide with myogenic and adrenergic vasoconstrictor processes in the control of microcirculatory blood flow. Pflügers Archiv. 1996;432:R107–110. [PubMed] [Google Scholar]
- Roddie IC. Human responses to emotional stress. Irish Journal of Medical Science. 1977;146:395–417. doi: 10.1007/BF03030998. [DOI] [PubMed] [Google Scholar]
- Rubanyi GM, Romero JC, Vanhoutte PM. Flow-induced release of endothelium-derived relaxing factor. American Journal of Physiology. 1986;250:H1145–1149. doi: 10.1152/ajpheart.1986.250.6.H1145. [DOI] [PubMed] [Google Scholar]
- Seals DR, Enoka RM. Sympathetic activation is associated with increases in EMG during fatiguing exercise. Journal of Applied Physiology. 1989;66:88–95. doi: 10.1152/jappl.1989.66.1.88. [DOI] [PubMed] [Google Scholar]
- Uvnäs B. Cholinergic vasodilator nerves. Federation Proceedings. 1966;25:1618–1622. [PubMed] [Google Scholar]


