Abstract
Embryonated chicken eggs (ECE) and the Madin-Darby canine kidney (MDCK) cell line were compared for isolation of swine influenza virus (SIV) from nasal swabs and tissue samples. Samples originated from 30 pigs experimentally inoculated with 2 × 106 to 2 × 107 embryo infectious dose 50% (EID50)/mL of swine influenza strain A/Swine/Indiana/1726/88 (H1N1). The results were analyzed with McNemar's chi-squared test for symmetry. The results indicated that more samples were SIV-positive with ECE than with tissue culture (P ≤ 0.001), suggesting that ECE remains the system of choice for isolation of SIV. It is recommend that routine use of both SIV isolation systems will increase the sensitivity of detection of virus shedding by considering the differences in growth and tropism of diverse SIV strains.
Influenza virus is an enveloped RNA virus with a helical nucleocapsid with 8 segments of single-stranded negative-sense RNA. The envelope contains hemagglutinin (H) and neuraminidase (N) proteins, of which there are 15 H and 9 N subtypes (1). Influenza A viruses are widespread in nature and are highly infectious respiratory pathogens of pigs, horses, humans, and birds (2,3). Swine influenza virus (SIV) has been detected in swine populations of North America since 1930 (4). Infection of pigs with SIV leads to weight loss or retarded weight gain and, in some cases, to death. Clinical disease is marked by a sudden onset of anorexia and lethargy, with labored breathing, especially when animals are forced to move. Other clinical signs include rhinitis, nasal discharge, sneezing, and coughing. Morbidity is high, but mortality is low, unless there are concurrent infections or the pigs are very young. Reproductive problems have also been associated with outbreaks of swine influenza (5).
The objective of this study was to compare 2 systems for the isolation of SIV. The SIV strain A/Swine/Indiana/1726/88 (H1N1) for this study was kindly provided by Dr. Christopher Olsen of the University of Wisconsin in Madison, Wisconsin, USA. The virus was passed once in 9-day-old chicken embryos and the allantoic fluid was harvested and stored at −70°C until used for the inoculation experiment. Infectivity titer of the virus preparation was determined in 9-day-old chicken embryos according to standard methods (6). A group of 40 apparently healthy, crossbred, 10-week-old pigs, without history of swine influenza or vaccination against the disease, were selected and were acclimatized for 7 d prior to the start of the experiment. All the pigs were confirmed to be serologically negative for SIV antibodies by competitive enzyme-linked immunosorbent assay (c-ELISA) (7), the hemagglutination inhibition (HI) test (8), and the agar gel immunodiffusion (AGID) test. They were fed a commercial, non-medicated, age-appropriate ration, ad libitum, and had full access to water. All laboratory and animal experiments were conducted in facilities operated under biosafety level 3 agriculture containment. A veterinarian observed the pigs daily for any clinical signs of disease. The local animal care committee approved all animal procedures prior to initiation of the study.
Thirty pigs were challenged with a virus dose equivalent to 2 ×106 to 2 × 107 embryo infectious dose 50% (EID50)/mL diluted in 0.01 M phosphate-buffered saline (PBS). Ten negative control pigs were inoculated with sterile allantoic fluid diluted in 0.01 M PBS. Each pig was inoculated with a syringe or by aerosolization with an atomizer. All the pigs were anesthetized with a short-acting injectable anesthetic before administration of the challenge inoculum or the placebo. Nasal swabs were collected prior to inoculation and on days 1, 3, 5, 7, 9, and 11 postinoculation. The swabs were immediately placed into transport medium [1% bovine serum albumin (BSA) in brain heart infusion (BHI) containing 1 mg/mL of streptomycin, 20 μg/mL of vancomycin, 500 μg/mL of gentamicin, and 50 units of nystatin]. On days 7 and 13 postchallenge, a blood sample was collected from the pigs for detection of serum antibodies to SIV, using C-ELISA (7) and the HI test. Antibodies against SIV were detected in 26 out of 30 pigs at 7 d postinoculation with both tests. All the animals had seroconverted by 13 d postchallenge. The antibody titers between 7 and 13 d postinoculation were increased approximately 30% in the C-ELISA and 4-fold in the HI test.
On days 7 and 14 postchallenge, 20 pigs were euthanized, and tracheobronchial lymph nodes, lung, tonsil, and other affected tissues were sampled for virus isolation (lung was the only tissue with macroscopic lesions). Samples were collected aseptically, and a new set of instruments was used for collecting each organ sample. Tissues were placed in sterile plastic bags. Samples and swabs were processed immediately after collection or were stored at −70°C until processed for virus isolation. All carcasses were disposed of according to protocols of the Canadian Food Inspection Agency.
Tissue samples were weighed in a sterile glass vial and were emulsified with heart infusion broth to make a 10% w/v suspension using a Tenbroek tissue grinder. Larger pieces of tissue were removed by centrifugation at 2000 × g at 4°C for 20 min, and the supernatant was harvested. Antibiotic solution was added (1 mg/mL of streptomycin, 20 μg/mL of vancomycin, 500 μg/mL of gentamicin, and 50 units of nystatin), the mixture was incubated at room temperature for 1 h, and the suspension was centrifuged at 2000 × g at 4°C for 20 min. The supernatant was harvested and considered as the undiluted sample. A portion of the original tissue was retained and kept frozen at −70°C. Swabs were transferred to tubes with transport medium, were vortexed vigorously, and were then removed from the tube with sterile forceps. The absorbed medium was squeezed out of the swab, either by gentle pressure of the swab against the tube wall or with sterile forceps. The sample was processed as described for the tissue samples.
Five 9-day-old specific pathogen-free (SPF) embryonated chicken eggs (ECE) were inoculated via the allantoic sac with 0.2 mL of 10% tissue suspension or processed swab. Eggs were incubated in a stationary incubator at 37°C with 55% relative humidity and were candled daily for 7 d for embryo viability. Embryos that died within 24 h were discarded. Allantoic fluid from embryos that died after 24 h was collected aseptically and tested for hemagglutinating virus by the hemagglutination (HA) test. If no deaths occurred in the eggs after 6 d, all of the eggs were opened aseptically. The allantoic fluids were pooled and inoculated into 5 more embryonated eggs. If no deaths occurred after 7 d in the eggs of the second passage, all the eggs were opened and the allantoic fluid of each egg was tested for hemagglutinating activity.
If deaths occurred in the eggs and the material had a positive HA test, samples were considered positive, and this result was confirmed with the HI test. Allantoic fluids from embryos showing early mortality after inoculation were tested for bacterial contamination according to routine procedures.
For isolation of SIV in tissue culture, Madin-Darby canine kidney (MDCK) continuous cell line was used in 24 well plates. Cells were seeded at a concentration of 8 × 104 cells/cm2 in MEM-α, 5% v/v fetal bovine serum (FBS), 100 IU/mL of penicillin and 100 μg/mL of streptomycin. Prior to inoculation, the medium from the seeded wells was removed by gentle inversion and washed 3 times with MEM-α. MEM-α containing trypsin (0.3 mL), to a final concentration of 4 μg/mL, was added to each well in all 24 well plates. For each sample, 0.2 mL of the swab preparation or 10% w/v tissue emulsion was inoculated onto 3 wells of MDCK cells. The inoculum was allowed to absorb at 37°C for 60 min in a 5% CO2 humidified incubator and then 0.5 mL of MEM-α containing 4 μg/mL of trypsin was added to all wells. The cells were incubated at 37°C and 5% CO2 for 3 to 6 d. The plates were observed daily for cytopathic effect (CPE). After 3 to 6 d, media were removed with a pipette and pools of the sample were tested for HA activity. If the HA test was negative, the samples were passed in MDCK cells and after 6 d tested for the presence of SIV with the indirect immunoperoxidase assay (SIV-IPA). Briefly, cell culture plates were gently washed with 1 mL of warm (37°C) 0.01 M PBS (pH 7.2) and were incubated for 5 min at room temperature. The plate was blot dried and the cell monolayer fully dried with the aid of a hair dryer set on high heat and speed. The monolayer was fixed by adding 500 μL of cold (4°C) fixation fluid [20% acetone, 0.02% bovine serum albumin in PBS (0.01 M, pH 7.6)] to all wells; the cells were allowed to fix for 10 min at room temperature. The fixation fluid was removed by inverting the plate and blotting it dry. The microplate was further dried with a hair dryer as described above. After washing each well with 1 mL per well of PBS at 37°C, the plates were stained with monoclonal antibody (Mab) HB65 (IgG2a/ATCC H16-L10-4R5) (9), which is specific for nucleoprotein of influenza A. A 1/10 dilution of Mab (0.5 M NaCl, 0.05%; Tween 20 pH 7.6) was added to the fixed monolayer and then the plate was incubated at 37°C for 30 min and washed 3 times in 0.05% PBS-Tween-20 (PBS-T). Rabbit anti-mouse IgG horseradish peroxidase conjugate was added to all wells of the plate, which was incubated for 30 min at 37°C. The plate was washed as above and then 500 μL of the substrate working solution (3-amino-9-ethylcarbazole substrate; N,N-dimethylformamide; 3% v/v H2O2) was added to all wells. The reaction was allowed to occur for 15 min at room temperature. The substrate solution was discarded and the plate was dried. The reaction was read with an inverted light microscope. A positive reaction exhibited a red/brown cytoplasmic staining in response to the precipitating substrate.
The results were analyzed with the McNemar chi-squared test, and P ≤ 0.05 was considered statistically significant (10). During the study period, 240 nasal swabs were collected in total on different days postinfection and analyzed by virus isolation using ECE and MDCK tissue culture (Table I). A total of 92 (38%) samples were positive by either method. However, the isolation ratio at different days postinfection indicated that 19/80 samples (24%) collected between 0 and 1 d postinfection were positive and that 60/80 samples (75%) collected between 3 and 5 d postinfection were positive. In contrast, only 12/80 samples (15%) were positive on day 7 postinfection. In comparing the performance of the 2 SIV isolation methods, 90 (98%) and 67 (73%) of the 92 positive samples were positive by ECE and MDCK cell culture, respectively (Table II). Overall, more samples were positive by ECE than by MDCK cell culture (P ≤ 0.001). The isolation ratios for the 2 methods were significantly different only for samples collected from 0 to 1 d postinfection (P = 0.016) and from 7 to 11 d postinfection (P = 0.001) (Table II).
Table I.
Table II.
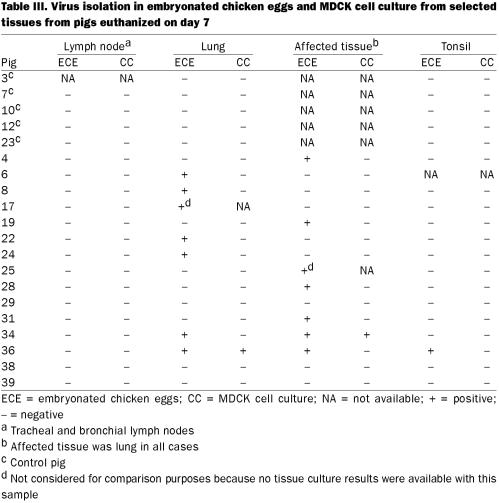
The proportions of virus positive samples were 67/240 (28%) and 90/240 (38%) by MDCK tissue culture and ECE, respectively, and the confidence interval for the difference was 10% ± 4. On day 7 postinfection, 13 of 69 tissues samples (18%) were positive by ECE and the MDCK method (Table III), whereas on day 14 postinfection no virus was isolated from 73 tissue samples. As expected, lung was the tissue of choice over tonsil for isolation of the virus. A total of 13 samples (19%) were positive by ECE, whereas only 2 (3%) were positive by tissue culture (P ≤ 0.001; confidence interval 16% ± 8, greater than found for nasal swabs).
Table III.
Isolation of SIV is routinely performed in 10- to 11-day-old ECE from swab material or the supernatant of 10% lung suspension (11). However, some recent reports in which only a small number of samples (10) was used suggest that isolation of SIV can be improved by using MDCK cell culture (12).
Overall, it was found that nasal swabs remain the preferred sample over lung for virus isolation and that virus isolation is more effective with ECE than with cell culture. The choice of the MDCK cell line for isolation of SIV was based on a previous study (13), in which 3 different cell lines were compared for the isolation of influenza A virus. The sensitivity of the MDCK cell line was greater than the sensitivity of the Vero and MRC-5 cell lines and, as a result, the MDCK cell line was recommended for the isolation of influenza viruses. MDCK cells were first used in influenza A diagnosis in 1968 (14). In a recent study (15), primary fetal porcine kidney cells were evaluated for the isolation of SIV. Similar results were obtained when compared with isolation by inoculation of ECE.
From 1975 on, MDCK cells were more frequently used for isolation of influenza A viruses after it was found that adding trypsin to the culture stimulates the growth of influenza A viruses and enables many influenza virus strains to form plaques with high efficiency (16). In addition to embryonated eggs, MDCK cells in culture medium containing trypsin are now considered a valuable system for isolation of these viruses from clinical specimens (17,18).
Influenza A viruses have significantly different host properties (Webster, 1997) and possibly different in vitro growth properties, depending on the strain (19). These differences may be associated with the preferential cell receptor, which some influenza A virus strains need to induce infection (2). Some reports describe that some human and swine influenza isolates do not grow well in ECE and that cell culture provides better results for primary isolation (12,20). The H1N1 virus in this study was an egg adapted strain, which could explain the increased sensitivity of isolation in ECE over MDCK. However, the virus was passaged through pigs for the purpose of this study.
Although results indicate that ECE are more suitable for isolation of SIV, the high variability of these viruses and the evidence presented by other authors suggest that both ECE and cell culture need to be used for the primary isolation of SIV. Replacing ECE by cell culture in routine isolation of SIV will reduce the sensitivity of the isolation, as suggested previously (12).
Footnotes
Acknowledgments
We thank Kelly Richmond, Kevin Hills, Jose Riva, Halina Kobylecka, and the Animal Care Team of the National Centre for Foreign Animal Disease for their excellent technical assistance. This research was supported by Pfizer Inc. and the Canadian Food Inspection Agency.
Address correspondence and reprint requests to Dr. Alfonso Clavijo, tel: 204-789-2047, fax: 204-789-2038, e-mail: aclavijo@inspection.gc.ca
Received September 25, 2001. Accepted December 18, 2001.
References
- 1.Webster RG, Bean WJ, Gorman OT, Chambers TM, Kawaoka Y. Evolution and ecology of influenza A viruses. Microbiol Rev 1992;56:152–179. [DOI] [PMC free article] [PubMed]
- 2.Scholtissek C, Hinshaw VS, Olsen CW. Influenza in pigs and their role as the intermediate host. In: Nicholson KG, Webster RG, Hay AJ, eds. Textbook of Influenza. Oxford: Blackwell Science Ltd. UK, 1998:137–145.
- 3.Webster RG. Influenza virus: transmission between species and relevance to emergence of the next human pandemic. Arch Virol [Suppl] 1997;13:105–113. [DOI] [PubMed]
- 4.Schultz U, Fitch WM, Ludwig S, Mandler J, Scholtissek C. Evolution of pig influenza viruses. Virology 1999;183:61–73. [DOI] [PubMed]
- 5.Easterday BC, Hinshaw VC. Swine influenza. In: Leman AD, Straw BE, Mengeling WL, D'Allaire S, Taylor DJ, eds. Diseases of Swine. 7th ed, Ames: Iowa State University Press, 1992:349–357.
- 6.Payment P, Trudel M. Methods and Techniques in Virology. New York: Marcel Dekker, 1993:19–38.
- 7.Zhou EM, Chan M, Heckert RA, Riva J, Cantin MF. Evaluation of a competitive ELISA for detection of antibodies against avian influenza virus nucleoprotein. Avian Dis 1988;42:517–522. [PubMed]
- 8.Beard CW. Serological procedures. In: Purchase LH, Arp LH, Domermuth CH, Pearson JE, eds. A Laboratory Manual for the Isolation and Identification of Avian Pathogens. 3rd ed. Kennett Square, Pennsylvania: Am Assoc Avian Pathol, 1989: 192–194.
- 9.Yewdell JW, Frank E, Gerhardt W. Expression of influenza A virus internal antigens on the surface of infected P825 cells. J Immunol 1981;126:1814–1819. [PubMed]
- 10.Schönmann MJ, BonDurant RH, Gardner IA, Van Hoosear K, Baltzer W, Kachulis C. Comparison of sampling and culture methods for the diagnosis of Trichomonas foetus infection in bulls. Vet Rec 1994;134:620–622. [DOI] [PubMed]
- 11.Bachmann PA. Swine influenza. In: Pensaert MB, ed. Virus Infections of Pigs. London: Elsevier Science, 1989:193–207.
- 12.Erickson GA, Krauss S, Landgraf JG, Swenson SL, Greer-Graham SC. New approaches to swine influenza virus isolation. Proc 42nd Ann Meet Am Assoc Vet Lab Diagnos, 1999:45.
- 13.Reina J, Fernandez-Baca V, Blanco I, Munar M. Comparison of Madin-Darby canine kidney cells (MDCK) with a green monkey continuous cell line (Vero) and human lung embryonated cells (MRC-5) in the isolation of influenza A virus from nasopharyngeal aspirates by shell vial culture. J Clin Microbiol 1997;35: 1900–1901. [DOI] [PMC free article] [PubMed]
- 14.Gaush CR, Smith TF. Replication and plaque assay of influenza virus in an established line of canine kidney cells. Appl Microbiol 1968;16:588–594. [DOI] [PMC free article] [PubMed]
- 15.Swenson SL, Vincent LL, Lute BM, et al. A comparison of diagnostic assays for the detection of type A swine influenza virus from nasal swabs and lungs. J Vet Diag Invest 2001;13:36–42. [DOI] [PubMed]
- 16.Tobita K, Sugiura A, Enomote C, Furuyama M. Plaque assay and primary isolation of influenza A viruses in an established line of canine kidney cells (MDCK) in the presence of trypsin. Med Microbiol Immunol 1975;162:9–14. [DOI] [PubMed]
- 17.Brown IH, Done SH, Spencer YI, Cooley WA, Harris PA, Alexander DJ. Pathogenicity of a swine influenza H1N1 virus antigenically distinguishable from classical and European strains. Vet Rec 1993;132:598–602. [DOI] [PubMed]
- 18.Gourreau JM, Kaiser C, Valette M, Douglas AR, Labie J, Aymard M. Isolation of two H1N2 influenza viruses from swine in France. Arch Virol 1994;135:365–382. [DOI] [PubMed]
- 19.Shope RE. Swine influenza. III. Filtration experiments and aetiology. J Exp Med 1931;54:373–385. [DOI] [PMC free article] [PubMed]
- 20.Carman S, Stansfield C, Weber J, Bildfell R, Van Dreumel T. H3N2 influenza A virus recovered from a neonatal pig in Ontario — 1997. Can Vet J 1999;40:889–890. [PMC free article] [PubMed]