Abstract
The aim of this study was to determine the effect of glucose supplementation on leucine turnover during and after exercise and whether variation in the previous dietary protein content modulated this effect.
Postabsorptive subjects received a primed constant [1-13C, 15N]leucine infusion for 6 h, after previous consumption of a high (1.8 g kg−1 day−1, HP, n = 16) or low (0.7 g kg−1 day−1, LP, n = 16) protein diet for 7 days. The subjects were studied at rest; during 2 h of exercise, during which half of the subjects from each dietary protocol received 0.75 g kg−1 h−1 glucose (HP + G, LP + G) and the other half received water (HP + W, LP + W); then again for 2 h of rest.
Glucose supplementation suppressed leucine oxidation (P < 0.01) by 20 % in subjects consuming the high protein diet (58.2 ± 2.8 μmol kg−1 h−1, HP + G; 72.4 ± 3.9 μmol kg−1 h−1, HP + W) but not the low protein diet (51.1 ± 5.9 μmol kg−1 h−1, LP + G; 51.7 ± 5.5 μmol kg−1 h−1, LP + W), with no difference in skeletal muscle branched-chain 2-oxo acid dehydrogenase (BCOADH) activity between groups. Glucose supplementation did not alter the rate of whole-body protein synthesis or breakdown.
The sparing effect of glucose on leucine oxidation appears only to occur if previous protein intake was high. It was not mediated by a suppression of BCOADH fractional activity but may be due to reduced substrate availability.
Amino acid oxidation is increased during moderate intensity exercise (Rennie et al. 1981; Knapik et al. 1991), but does not contribute significantly to energy provision in comparison to fat and carbohydrate oxidation (Ahlborg et al. 1974). It has been suggested that habitual endurance exercisers may have an increased dietary protein requirement as a result of increased amino acid oxidation during exercise (Meredith et al. 1989). However, El-Khoury et al. (1997) recently demonstrated that the completion of two 90 min bouts of exercise per day at 43–54 %VO2,max (maximum rate of O2 uptake) did not cause a significant deterioration in 24 h leucine homeostasis in physically active men, when consuming 1 g protein kg−1 day−1.
Branched-chain amino acid (BCAA) oxidation involves two steps: first, reversible transamination of BCAA to form branched-chain 2-oxo acids (BCOA) with 2-oxoglutarate acting as an amino group acceptor; and second, irreversible decarboxylation of BCOA. Decarboxylation is thought to be the rate-limiting step (Harper et al. 1984) and is catalysed by the mitochondrial branched-chain 2-oxo acid dehydrogenase complex (BCOADH). BCOADH is present in an active dephosphorylated form and an inactive phosphorylated form, interconversion being controlled by a specific kinase-phosphatase system (for review see Harper et al. 1984). During exercise there is an increase in both the percentage activation of the skeletal muscle BCOADH complex (Wagenmakers et al. 1989; Bowtell et al. 1998) and amino acid oxidation (Rennie et al. 1981; Bowtell et al. 1998).
Most research on the interaction of nutrition and exercise has concentrated on the carbohydrate content of athletes’ diets since, during endurance exercise, fatigue is associated with the depletion of muscle glycogen stores (Bergstrom et al. 1967). Several tactics are commonly employed by athletes in order to maintain glycogen stores during exercise. One of these is carbohydrate loading, which involves the consumption of a high carbohydrate diet and the tapering of training activities in the days preceding an event to ensure a high basal muscle glycogen concentration (Bosch et al. 1993). Another tactic involves consumption of carbohydrate drinks during exercise in the hope of delaying fatigue (Widrick et al. 1993) either by sparing muscle glycogen (Yaspelkis et al. 1993), or by maintaining plasma glucose concentration (Bosch et al. 1994), which will facilitate continued glucose supply to the muscle. It has been suggested that glucose supplementation not only delays the onset of fatigue but also suppresses the exercise-induced increase in leucine oxidation (Davies et al. 1982). Wagenmakers et al. (1991) demonstrated that the combination of prior carbohydrate loading and glucose supplementation during exercise suppressed the exercise-induced increase in BCOADH activation. The first aim of this study was therefore to investigate the effect of glucose supplementation on leucine oxidation during exercise and to determine whether this could be attributed to altered skeletal muscle BCOADH activity.
Consumption of a high protein diet has been shown to stimulate amino acid oxidation both at rest and during exercise (Bowtell et al. 1998). This effect appears to be mediated not by increased skeletal muscle BCOADH activity, at least in the postabsorptive condition, but rather via increased flux through the oxidative pathway. There is some evidence that dietary protein may modulate insulin sensitivity.
Consumption of low protein diets stimulated increased sympathetic nervous system activation in rats and mice (Young et al. 1985) and high protein diets resulted in reduced noradrenaline turnover, which is indicative of reduced sympathetic nervous system activation (Johnston & Balachandran, 1987). Lembo et al. (1994) found that an acute noradrenergic activation caused resistance to the effects of insulin on carbohydrate metabolism. Consumption of high protein diets may therefore enhance insulin sensitivity. Indeed, Tse et al. (1995) found that weanling obese Zucker (fa/fa) rats fed a low protein diet for 3 weeks suffered a deterioration in glycaemic control, presumably due to increased peripheral insulin resistance. In contrast, however, Escriva et al. (1991) found that lean rats fed a low protein diet demonstrated increased insulin sensitivity. The second aim of the present study was, therefore, to determine whether previous dietary protein intake would influence the effect of glucose supplementation on leucine oxidation and skeletal muscle BCOADH activity.
Results from in vitro studies suggest that insulin inhibits protein breakdown and stimulates protein synthesis (Jefferson et al. 1977). However, it has proved difficult to reproduce the stimulation of protein synthesis in the hyperinsulinaemic state in vivo in humans (Heslin et al. 1992). It has been suggested that amino acid availability may be limiting for protein synthesis. During hyperinsulinaemia, the suppression of protein breakdown acts to reduce amino acid supply, thus limiting any increase in whole-body protein synthesis. The final aim of this study was, therefore, to determine whether glucose supplementation and the resultant elevation in insulin concentration modulates the endurance exercise-induced changes in whole-body protein turnover, and whether previous dietary protein intake and hence amino acid availability influences this effect.
METHODS
Subjects consumed either a high protein (1.8 g protein kg−1 day−1, HP, n = 16) or low protein diet (0.7 g protein kg−1 day−1, LP, n = 16) for 7 days. On day 8 of this period, whole-body leucine kinetics were traced before, during and after walking on a treadmill for 2 h at 60 %VO2,max. During exercise, half of the subjects from each dietary protein group received water (HP + W, LP + W) and the remainder received glucose (HP + G, LP + G). Four subjects (3 male and 1 female) participated in all four trials, separated by at least 4 weeks; diets were allocated by systematic rotation. A further group of 16 subjects (14 male and 2 female) participated in one trial only, being allocated by systematic rotation to one of the protocols (n = 4 in each group). This second group of subjects was recruited in order to obtain muscle data since it was not ethically sound to take 12 biopsies from each subject. The subject details are given in Table 1. An incremental exercise test on a motorised treadmill, using the criteria of Taylor et al. (1955) was adopted for the measurement of VO2,max using on-line gas analysis. The study was approved by the local Tayside Ethics Committee and was carried out in accordance with the Declaration of Helsinki. All subjects gave their written informed consent.
Table 1.
Subject characteristics
| Dietary protocol | Age (years) | VO2,max (ml kg−1 min−1) | Weight (kg) | Body fat (%) |
|---|---|---|---|---|
| HP + W | 21.9 ± 0.6 | 45.8 ± 1.1 | 74.5 ± 3.4 | 12.4 ± 2.1 |
| LP + W | 22.0 ± 0.6 | 45.0 ± 1.2 | 71.9 ± 4.6 | 14.1 ± 2.2 |
| HP + G | 22.9 ± 0.7 | 44.5 ± 1.2 | 74.8 ± 3.6 | 14.4 ± 3.1 |
| LP + G | 22.8 ± 0.5 | 45.0 ± 1.7 | 72.8 ± 3.9 | 13.5 ± 2.4 |
Data are presented as means ± S. E. M. (n = 8)
Diets were designed to supply each subject's normal diurnal energy requirement (by consideration of average values for subject age, weight and activity level (WHO technical report (1985). Energy and Protein Requirements. Report of a Joint Expert Consultation. Who technical report series no. 724. World Health Organization, Geneva), corroborated by a 1 day food diary). Diet sheets were prepared prescribing all foods to be consumed, which provided most of the energy intake (82 ± 5 % of 3152 ± 178 kcal) and 0.7 g protein kg−1 day−1; subjects marked the sheets to show compliance. Subjects allocated to the high protein diet group (n = 16) consumed, in addition, a whey protein supplement (Maxipro, Scientific Hospital Supplies Ltd, Liverpool, UK) supplying 1.1 g protein kg−1 day−1; subjects allocated to the low protein diet group (n = 16) consumed an isoenergetic peanut oil supplement (Calogen, Scientific Hospital Supplies Ltd, Liverpool, UK). The supplements were prepared daily in liquid form and the subjects were supervised as they drank them; the diets were consumed for a period of 7 days. Subjects were instructed to maintain normal activity patterns but to refrain from exercise on day 7, the day before the exercise trial.
After 7 days of dietary equilibration, subjects (n = 8 in each dietary protocol) were studied during and after 2 h of treadmill exercise at 60 %VO2,max (Fig. 1). The subjects arrived at the laboratory after an overnight fast and cannulae were inserted into the antecubital veins of both arms. One cannula was used for withdrawal of mixed venous blood samples and one for delivery of the leucine isotope. Basal blood and breath samples were taken and the subjects then received a primed, constant (0.8 mg kg−1, 1 mg kg−1 h−1) infusion of L-[1-13C, 15N]leucine for 6 h. Subjects rested for the first 2 h, then walked on a treadmill at a speed and gradient designed to elicit an oxygen uptake of 60 % of their VO2,max for 2 h and then rested for a further 2 h. Half of the subjects from each dietary protein group (high or low protein) received 5 ml water kg−1 h−1 (HP + W, LP + W, n = 8) and the remaining eight subjects from each dietary protein group received 0.75 g glucose kg−1 h−1 (15 % w:v, dextrose monohydrate, potato starch, AVEBE Ltd, Veendam, The Netherlands; HP + G, LP + G, n = 8). The drinks were provided in six equal aliquots during exercise, one immediately before exercise and then five more at 20 min intervals. The δ13C of the potato starch-derived glucose is low (-26 ± 2 per thousand in comparison to the International Standard Pee Dee Belemnite (PDB)) and close to that of plasma glucose (-28 ± 2 per thousand vs. PDB); it therefore does not interfere with the recovery of 13CO2 derived from the infused L-[1-13C, 15N]leucine.
Figure 1.
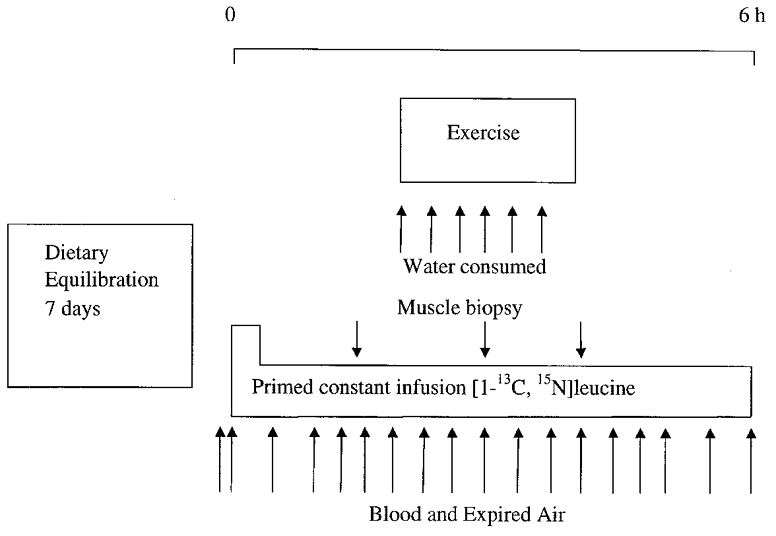
Schematic diagram of the study protocol.
Blood and breath samples were taken every 30 min for the first and last hours of the infusion and every 20 min at all other times. In half of the subjects (n = 4 in each dietary protocol), muscle biopsies were taken from quadriceps femoris using conchotome forceps (Dietrichson et al. 1987). A basal biopsy was taken in the 30 min preceding exercise and two further biopsies were taken after the first and second hours of exercise.
Blood was stored on ice until the end of the infusion period and then centrifuged at 1500 g for 20 min at 4°C. The resultant plasma was stored at -70°C until analysis could be performed. Plasma was analysed for insulin (radio-immunoassay kit, Amersham UK Ltd), glucose and urea (both kits from Sigma). To determine 13C and 15N labelling, plasma leucine and α-ketoisocaproate (KIC) were analysed as their tertiary butyldimethylsilyl (t-BDMS) (Biermann et al. 1986) and t-BDMS-quinoxalinol derivatives (Rocchicciolo et al. 1981), respectively, using gas-chromatograph mass spectrometry. Leucine and KIC concentrations were obtained using deuterated internal standards. Whole-body leucine oxidation, transamination, reamination, protein breakdown and protein synthesis were calculated using the model described previously (Matthews et al. 1981). Factors to account for the retention of 13C label in the bicarbonate pool were incorporated into the calculation of leucine oxidation. These factors were determined by measuring 13C recovery during a primed constant NaH13CO3 infusion, in conditions identical to the experimental protocol employed for the current study (Bowtell et al. 1994). Fractional leucine transamination, in other words the proportion of leucine nitrogen that was transaminated, was calculated as transamination divided by leucine nitrogen flux. Similarly, fractional oxidation of KIC, or the proportion of KIC formed which was oxidised, was calculated as oxidation divided by transamination.
The biopsied muscle tissue was placed in ice-cold buffer within 120 s of the cessation of exercise, to minimise any change in fractional activation, and assayed for branched-chain oxo acid dehydrogenase activity as described previously (Wagenmakers et al. 1989).
The breath samples were collected in 50 l Douglas bags for 4 min at rest and 2 min during exercise, and analysed for percentage content of oxygen (Taylor Servomex, Sybron Corporation) and carbon dioxide (Grubb Parsons, Newcastle, UK). Oxygen consumption, carbon dioxide production and respiratory quotient were calculated by standard methods. Following thorough mixing, small aliquots of each breath sample were transferred into 10 ml evacuated tubes and later analysed for 13CO2 enrichment using isotope ratio mass spectrometry (IRMS) on the ANCA 20–20 (Europa Scientific Ltd, Crewe, UK).
The plasma [13C]KIC enrichment, rather than plasma [13C]leucine enrichment, was used to calculate whole-body leucine carbon flux and oxidation; whole-body protein synthesis was calculated as the net difference between these two variables (Matthews et al. 1981). KIC is produced intracellularly via the transamination of leucine and it has therefore been suggested that plasma KIC enrichment is a better predictor of intracellular leucine enrichment (Watt et al. 1991).
Statistics
All data are presented as means ±s.e.m. The Mann-Whitney U test was used to compare the BCOADH data, due to the small sample size. A three-way ANOVA (dietary protein (high vs. low) by fluid supplementation (glucose vs. water) by time (pre-exercise, during hour 1, during hour 2 and post-exercise)) was used to analyse the remaining variables. A dependent analysis (all factors within subjects) was carried out on the data derived from the four subjects who completed all four trials. An independent analysis (two factors between and one factor within subjects) was employed for the data derived from the subjects who completed only one trial. Provided that the direction of change in the variable was the same for the two analyses, the P values were combined using meta analysis (Fisher, 1950). A posthoc Tukey test was used to identify the site of the difference where appropriate and the P values combined in the same way. The effect of dietary protein manipulation and exercise upon whole-body leucine metabolism and BCOADH activation has been described in a previous paper (Bowtell et al. 1998). In this paper we will examine the effect of glucose supplementation during exercise upon whole-body leucine metabolism and BCOADH activation.
RESULTS
Plasma glucose concentration did not change significantly for the water control subjects throughout the 6 h protocol (Fig. 2). Plasma glucose concentration was increased by glucose supplementation for HP + G subjects relative to controls (HP + W), during and in recovery from exercise (P < 0.01). However, plasma glucose concentration was significantly increased, relative to water controls, only in the post-exercise period for LP subjects (P < 0.01).
Figure 2. Effect of glucose supplementation on plasma glucose concentration at rest and during exercise, after consumption of a high or low protein diet.
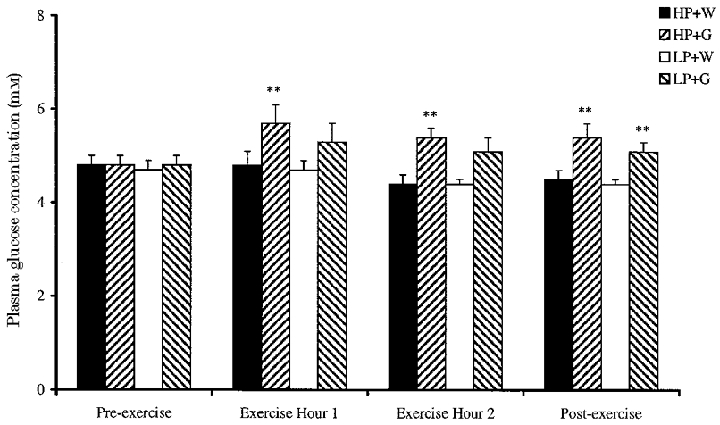
Values are means ±s.e.m. (n = 8). ** Significantly different from respective control values (P < 0.01).
Whole-body leucine oxidation was higher for the postabsorptive subjects consuming the high rather than the low protein diet (P < 0.01, Fig. 3). Leucine oxidation was increased approximately 3-fold during exercise, for all dietary protocols (P < 0.01). There was a main effect of glucose supplementation upon leucine oxidation (P < 0.01): leucine oxidation was reduced by 20 %vs. controls in the first hour of exercise for HP + G subjects (58.2 ± 2.8 vs. 72.4 ± 3.9 μmol kg−1 h−1), but there was no effect in the second hour of exercise (57.5 ± 4.2 vs. 56.9 ± 5.1 μmol kg−1 h−1), and leucine oxidation was not suppressed in LP + G subjects vs. controls.
Figure 3. Effect of glucose supplementation on whole-body leucine oxidation at rest and during exercise, after consumption of a high or low protein diet.
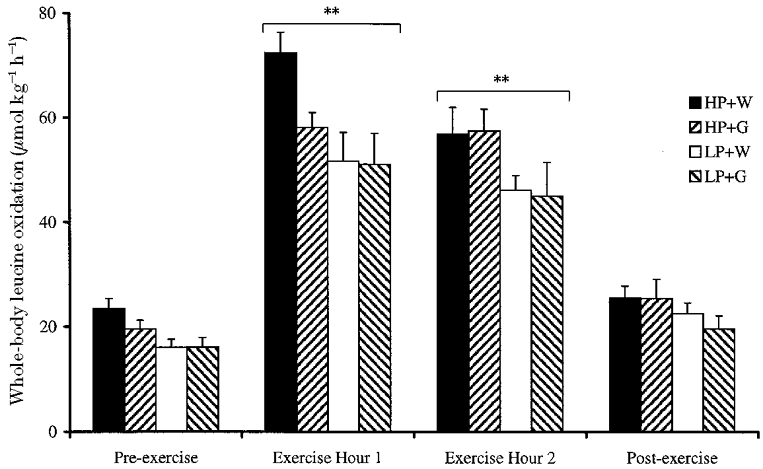
Means ±s.e.m. (n = 8). There was a main effect of glucose supplementation (P < 0.01), and results were significantly different from Pre-exercise values (** P < 0.01).
The tendency was for glucose supplementation to reduce transamination and reamination relative to controls in the subjects previously consuming a high protein diet, but to increase transamination and reamination relative to controls in the subjects previously consuming a low protein diet (Table 2). The suppression of transamination for glucose-supplemented subjects achieved significance for the analysis of independent data; however, due to missing data points from the dependent data set, it was not possible to test the statistical significance of the data as a whole.
Table 2.
Whole-body leucine transamination and KIC reamination rates
| Protocol | Pre-exercise | Exercise hour 1 | Exercise hour 2 | Post-exercise |
|---|---|---|---|---|
| Transamination (μmol kg−1 h−1) | ||||
| HP + W | 118.0 ± 26.9 | 157.8 ± 20.8 | 127.6 ± 18.2 | 100.0 ± 16.1 |
| HP + G | 85.8 ± 13.3 | 118.4 ± 19.7 | 91.9 ± 8.8 | 81.8 ± 14.0 |
| LP + W | 74.4 ± 15.0 | 99.0 ± 12.9 | 96.8 ± 12.1 | 83.6 ± 11.6 |
| LP + G | 79 ± 13.1 | 110.3 ± 4.0 | 107.3 ± 2.8 | 84.8 ± 11.5 |
| Reamination (μmol kg−1 h−1) | ||||
| HP + W | 94.6 ± 26.4 | 83.0 ± 19.1 | 75.6 ± 17.5 | 74.3 ± 15.2 |
| HP + G | 66.7 ± 14.5 | 63.2 ± 17.2 | 35.6 ± 7.8 | 55.5 ± 15.8 |
| LP + W | 58.6 ± 13.9 | 50.1 ± 12.2 | 46.4 ± 11.5 | 61.8 ± 11.1 |
| LP + G | 64.6 ± 9.1 | 57.3 ± 5.1 | 56.6 ± 6.2 | 64.6 ± 12.3 |
Data are presented as means ± S. E. M. (HP+G, LP+W and LP+G, n = 8; HP+W, n = 7).
Due to missing data from the dependent data set, it was also not possible to test the statistical significance of the fractional transamination data as a whole. However, there was a tendency for an exercise-induced increase in fractional transamination for all subjects, which was considerably lower for HP + G subjects (30 %vs. 50–60 % for HP + W, LP + W and LP + G subjects; Fig. 4). The fractional transamination of leucine during exercise tended to be higher for LP + G subjects than for their controls, but fractional transamination tended to be lower for HP + G subjects than in their controls. The glucose effect achieved significance for the independent data set (P < 0.05).
Figure 4. Effect of glucose supplementation on fractional leucine transamination at rest and during exercise, after consumption of a high or low protein diet.
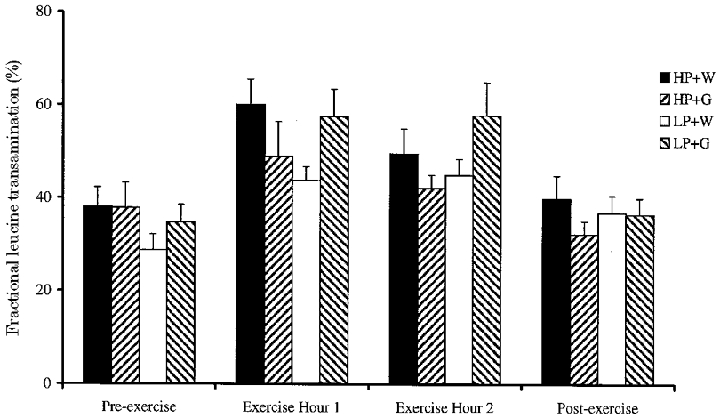
Means ±s.e.m. (n = 7).
Total skeletal muscle BCOADH activity was not altered by the different dietary protocols or by exercise (Table 3). The proportion of active skeletal muscle BCOADH complex was increased 2-fold during exercise; however, there were no statistically significant differences between experimental groups at any time point, although there was a tendency for the skeletal muscle BCOADH activation state to be lower for LP + G than for control subjects during exercise.
Table 3.
Skeletal muscle BCOADH activity
| Protocol | Pre-exercise | Exercise hour 1 | Exercise hour 2 |
|---|---|---|---|
| Total activity (nmol g−1 min−1) | |||
| HP + W | 5.6 ± 0.9 | 5.3 ± 0.2 | 4.2 ± 0.3 |
| HP + G | 5.2 ± 0.2 | 6.8 ± 0.5 | 5.6 ± 0.3 |
| LP + W | 5.5 ± 0.4 | 4.9 ± 0.6 | 5.9 ± 0.4 |
| LP + G | 4.6 ± 0.3 | 5.0 ± 0.2 | 4.2 ± 0.1 |
| Percentage activation | |||
| HP + W | 6.7 ± 2.1 | 10.6 ± 2.0* | 14.8 ± 3.0* |
| HP + G | 8.5 ± 1.8 | 14.2 ± 1.3* | 16.9 ± 0.9* |
| LP + W | 7.9 ± 0.8 | 21.8 ± 5.1* | 20.5 ± 5.8* |
| LP + G | 6.9 ± 0.5 | 10.3 ± 0.6* | 18.2 ± 1.1* |
Data are presented as means ± S. E. M. (n = 4).
Significantly different from pre-exercise values (P < 0.05).
Plasma leucine concentration changed significantly over time (P < 0.01, Fig. 5). Glucose supplementation tended to reduce plasma leucine concentration during the first hour of exercise for HP + G subjects and in the second hour of exercise for HP + G and LP + G subjects (P < 0.01). In the period following exercise, plasma leucine concentration was reduced relative to pre-exercise values (-25 %, HP + G; -22 %, LP + G; -8 %, HP + W; and -6 % LP + W, P < 0.01). Plasma KIC concentration data (not shown) were qualitatively identical to the plasma leucine concentration data.
Figure 5. Effect of glucose supplementation on plasma leucine concentration at rest and during exercise, after consumption of a high or low protein diet.
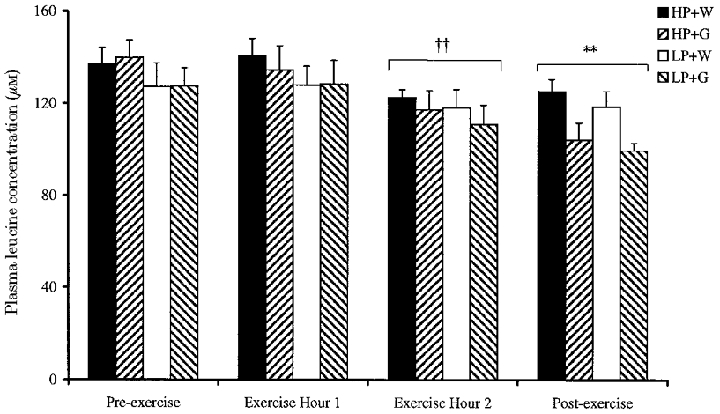
Means ±s.e.m. (n = 8). ** Significant difference from Pre-exercise values (P < 0.01); †† significant difference from Exercise hour 1 values (P < 0.01).
At rest before exercise, plasma insulin concentration was higher for subjects on the high protein diet (18.7 ± 1.3 μunits ml−1) than for those on the low protein diet (12.8 ± 1.1 μunits ml−1). For HP + W subjects, the plasma insulin concentration fell during exercise to LP + W levels, which remained relatively constant. In the subjects previously consuming a high protein diet, glucose supplementation prevented the exercise-induced decrease in plasma insulin concentration, but there was no significant difference between HP + G and control values at any time point. There was no significant difference in plasma insulin concentration between HP + G and LP + G during or after exercise. The insulin response to the glucose load, measured as the percentage difference between plasma insulin concentration in glucose-supplemented and control subjects, was 2-fold higher during the first 80 min of exercise in the subjects previously consuming a low protein diet (Table 4). The plasma insulin concentration tended to be higher for the glucose-supplemented groups than for the controls during recovery from exercise, but this did not reach significance.
Table 4.
Percentage difference in plasma insulin concentration between glucose-supplemented and control subjects: HP + G vs. HP+W; LP+G vs. LP+W
| Insulin HP + G (μunits ml−1) | Insulin HP + W (μunits ml−1) | Differences (%) | Insulin LP + G (μunits ml−1) | Insulin LP + W (μunits ml−1) | Differences (%) | |
|---|---|---|---|---|---|---|
| Rest | 18.4 ± 1.9 | 19.2 ± 2.0 | −4.2 | 14.2 ± 2.1 | 11.4 ± 0.7 | 24.6 |
| Exercise 40 min | 20.1 ± 2.7 | 14.8 ± 2.6 | 35.8 | 20.4 ± 3.8 | 11.0 ± 0.9 | 85.5 |
| Exercise 80 min | 17.8 ± 2.7 | 14.5 ± 2.6 | 22.8 | 14.8 ± 1.6 | 9.9 ± 1.0 | 49.5 |
| Exercise 120 min | 19.0 ± 4.5 | 13.5 ± 2.0 | 40.7 | 15.8 ± 2.4 | 13.2 ± 1.8 | 19.7 |
| Post-exercise | 17.9 ± 4.2 | 14.7 ± 2.3 | 21.8 | 15.5 ± 2.3 | 12.5 ± 1.4 | 24.0 |
Plasma insulin concentrations are presented as means ± S. E. M. (n = 8).
Indirect calorimetry was used to calculate the proportion of energy derived from carbohydrate and fat (Frayn, 1983), after protein oxidation had been deducted assuming leucine oxidation represented 8 % of total protein oxidised. There were no statistically significant differences between experimental groups, but carbohydrate oxidation tended to be higher for the glucose-supplemented subjects relative to controls throughout exercise in the subjects who had previously consumed a high protein diet. However, carbohydrate oxidation did not appear to increase above control values until the second hour of exercise for LP + G subjects (Fig. 6).
Figure 6. Effect of glucose supplementation on whole-body carbohydrate oxidation at rest and during exercise, after consumption of a high or low protein diet.
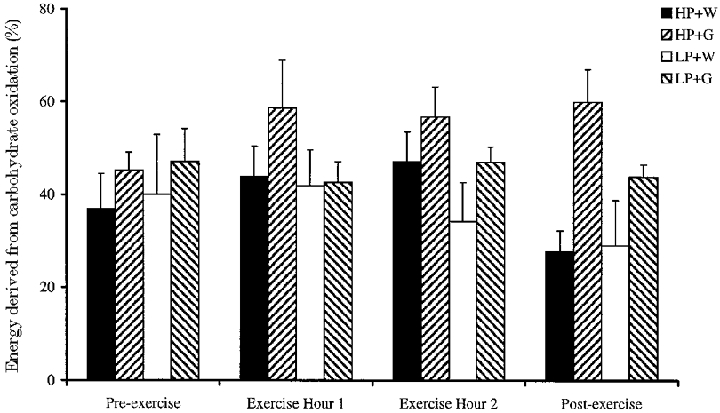
Means ±s.e.m. (n = 8).
Leucine carbon flux, which is an index of whole-body protein breakdown, did not vary between dietary protocols but there was a significant change over time (P < 0.05, Table 5). The rate of protein breakdown was significantly different during the post-exercise period from values during the first hour of exercise for all subjects (P < 0.01). Protein breakdown tended to decrease relative to pre-exercise values for HP + W, LP + W and HP + G subjects but increase for LP + G subjects during recovery. Non-oxidative leucine disposal, which is an index of whole-body protein synthesis, was suppressed during exercise for all subjects (P < 0.01, Table 5). Although protein synthesis increased during recovery relative to values during the first hour of exercise (P < 0.01), protein synthesis remained depressed relative to pre-exercise values (P < 0.01).
Table 5.
Rate of whole-body protein synthesis and breakdown
| Protocol | Pre-exercise | Exercise hour 1 | Exercise hour 2 | Post-exercise |
|---|---|---|---|---|
| Protein breakdown (μmol kg−1 h−1) | † | |||
| HP + W | 145.6 ± 7.5 | 151.5 ± 8.7 | 141.7 ± 3.9 | 130.7 ± 2.7 |
| HP + G | 142.1 ± 9.7 | 152.2 ± 11.1 | 145.9 ± 9.2 | 132.4 ± 7.8 |
| LP + W | 144.4 ± 6.6 | 139.2 ± 4.0 | 133.1 ± 5.1 | 132.6 ± 5.8 |
| LP + G | 139.5 ± 8.4 | 140.5 ± 5.7 | 135.1 ± 5.4 | 148.2 ± 10.9 |
| Protein synthesis (μmol kg−1 h−1) | * | * | *† | |
| HP + W | 123.8 ± 6.6 | 85.2 ± 24.3 | 84.1 ± 6.3 | 105.1 ± 2.8 |
| HP + G | 130.4 ± 7.6 | 77.8 ± 10.5 | 76.5 ± 9.5 | 110.8 ± 6.2 |
| LP + W | 128.5 ± 6.1 | 90.0 ± 5.7 | 89.3 ± 5.1 | 110.9 ± 6.5 |
| LP + G | 124.7 ± 7.7 | 82.4 ± 5.4 | 84.0 ± 3.8 | 111.5 ± 5.9 |
Data are presented as means ± S. E. M. (n = 8). There was a main effect of time:
significantly different from Pre-exercise values (P < 0.01)
significantly different from Exercise hour 1 values (P < 0.01).
Leucine nitrogen flux was suppressed by glucose supplementation for both levels of dietary protein intake (201.5 ± 6.3 vs. 242.3 ± 7.9 μmol kg−1 h−1, P < 0.01).
DISCUSSION
The increase in leucine oxidation observed during exercise in this and other studies (Rennie et al. 1981; Knapik et al. 1991; Bowtell et al. 1998) is thought to be related to the concurrent increase in the fractional activity of the skeletal muscle BCOADH complex (Wagenmakers et al. 1989; Bowtell et al. 1998). This exercise-induced increase in leucine oxidation is enhanced in glycogen-depleted subjects (Lemon & Mullin, 1980), and van Hall et al. (1995) found that the exercise-induced increase in BCOADH fractional activity was also enhanced during one-leg exercise in muscle with a reduced rather than a normal glycogen content. Previous work suggests that glucose supplementation during exercise reduces leucine oxidation (Davies et al. 1982); it seemed likely, therefore, that this may be related to changes in the fractional activity of the BCOADH complex. However, in the present study, glucose supplementation suppressed leucine oxidation but did not alter skeletal muscle BCOADH activation.
In humans, the BCOADH complex is present in many tissues, including liver, kidney, brain and adipose tissue as well as skeletal muscle; however, at rest 54 % of the body's actual BCOADH activity is sited within skeletal muscle and only 13 % is located in the liver (Suryawan et al. 1998). The predominant site of leucine oxidation in humans, therefore, is within skeletal muscle, particularly during exercise, partly as a consequence of a further activation of skeletal muscle BCOADH and also due to the increase in blood flow to the exercising muscle. Even if hepatic leucine oxidation were entirely suppressed, it is extremely unlikely that altered hepatic BCOADH activity could account for the 20 % suppression in whole-body leucine oxidation that resulted from glucose supplementation in the high protein group.
The skeletal muscle BCOADH complex was activated to a similar extent by exercise for the two glucose-supplemented groups regardless of previous dietary protein intake. There were no significant differences between experimental groups in skeletal muscle BCOADH activation at any time point. However, there was a tendency for BCOADH activation to be reduced by glucose supplementation relative to controls in subjects who had previously consumed a low protein diet. Yet, in these subjects leucine oxidation was not suppressed by glucose supplementation. It appears, therefore, that the effect of glucose supplementation on leucine oxidation was independent of changes in skeletal muscle BCOADH activity. Initially this appeared surprising since Wagenmakers et al. (1991) found that the exercise-induced activation of skeletal muscle BCOADH was inhibited by prior carbohydrate loading and glucose supplementation during exercise when compared to BCOADH activation during exercise in glycogen-depleted muscle. Wagenmakers et al. (1991) concluded that there was an inverse correlation between the glycogen content of the muscle and BCOADH activation. In the present study, oral glucose was consumed during exercise but initial muscle glycogen content was not manipulated. Glucose supplementation can delay the onset of fatigue during endurance exercise (Coyle et al. 1983), but there is conflicting evidence in the literature as to whether this effect is mediated through a sparing of muscle glycogen (Hargreaves et al. 1984), or through maintenance of plasma glucose concentration and hence oxidation rates (Coyle et al. 1986). The absence of any effect on BCOADH activation suggests that sparing of muscle glycogen did not occur as a result of glucose supplementation in the present study. Instead, the suppression of leucine oxidation by glucose supplementation in subjects previously consuming a high protein diet, seems to be related to a reduction in flux through the BCOADH complex via changes in substrate delivery to the skeletal muscle complex.
In rat skeletal muscle, the activity of BCAA transaminase (BCAAT) is 30-fold higher than that of BCOADH, whereas hepatic BCOADH activity is 11-fold higher than hepatic BCAAT activity (Suryawan et al. 1998). The skeletal muscle therefore acts as a net exporter of KIC for uptake and decarboxylation in the liver. However, in humans, BCAAT activity is 24-fold higher than BCOADH activity in skeletal muscle and 20-fold higher than BCOADH activity in the liver (Suryawan et al. 1998). It is perhaps unsurprising, therefore, that there is little or no KIC release from human muscle at rest (Cheng et al. 1985) or during exercise (Fielding et al. 1986). This suggests that the KIC formed by transamination and not reaminated is decarboxylated within the mitochondria of the skeletal muscle cells. Indeed, the mitochondrial KIC concentration is thought to be 20–25 μM (Hutson & Harper, 1981) and the Km of the BCOADH complex is 15–50 μM (Harper et al. 1984). The complex is therefore working at or below its Km so any change in mitochondrial KIC concentration is likely to result in an equivalent alteration in leucine oxidation. Fractional transamination tended to be higher for LP + G compared to control subjects during exercise but tended to be lower for HP + G subjects than controls. This suggests that the amount of KIC presented to the BCOADH complex was increased for LP + G relative to LP + W subjects, and one would therefore expect flux through the complex to be stimulated. However, leucine oxidation was not increased, possibly due to the tendency for lower activation of the complex. One would expect mitochondrial KIC availability to be reduced for HP + G subjects relative to controls, since both fractional transamination and plasma leucine concentration tended to be reduced. This would tend to reduce flux through the BCOADH complex resulting in the observed reduction in leucine oxidation. This is indicative of an alteration in the partitioning of leucine carbon flux between oxidation and synthesis and appears to be independent of the small changes in BCOADH activation.
In this study, it was not possible to distinguish between a direct effect of an increase in plasma glucose on leucine oxidation and an indirect effect through the increase in plasma insulin in response to the glucose load, although the suppression of leucine oxidation in the hyperinsulinaemic state is, itself, probably an indirect effect mediated through the observed fall in plasma amino acids resulting from the insulin-induced suppression of protein breakdown (Tessari et al. 1987). Plasma glucose was elevated by the exogenous glucose throughout exercise for HP + G subjects, but plasma glucose did not increase significantly until the recovery period for LP + G subjects. This may in part be due to the extent of the increase in plasma insulin concentration in response to the glucose load. There was no difference in the plasma insulin concentration between glucose-supplemented groups; however, the increase in plasma insulin concentration in the glucose-supplemented subjects relative to controls was approximately 2-fold greater for LP + G subjects than HP + G subjects in the first 80 min of exercise (Table 4).
In vitro experiments have shown that the provision of alternative energy substrates such as fatty acids, ketone bodies and pyruvate inhibits flux through the BCOADH complex (Wagenmakers & Veerkamp, 1984), therefore oxidation of the exogenous glucose is likely to reduce amino acid oxidation. In the present study, whole-body carbohydrate oxidation was increased for HP + G vs. control subjects, especially during the first hour of exercise. However, carbohydrate oxidation in the LP + G subjects did not increase above control values until the second hour of exercise; presumably the exogenous glucose was directed towards storage rather than oxidation. There is no evidence to suggest that an alteration of dietary protein content would alter the rate of gastric emptying or absorption of glucose. This apparent difference in the fate of the exogenous glucose between high and low protein dietary groups may provide one explanation for the differential effect on leucine oxidation.
Whole-body leucine carbon flux, which is indicative of whole-body protein breakdown in the postabsorptive state, was not significantly altered by glucose supplementation in subjects from either dietary protein group. However, plasma leucine concentration was decreased during recovery from exercise, suggesting that the efflux of leucine into the plasma pool was reduced. In the absence of an increase in whole-body leucine oxidation this can only be attributed to a suppression of protein breakdown. One explanation may be that the whole-body constancy masks altered protein breakdown within individual tissues; the anti-proteolytic effect of insulin has been found to be more pronounced in skeletal muscle than in other tissues of the body (Heslin et al. 1992).
Whole-body protein synthesis in the glucose-supplemented subjects did not differ from that of control subjects in either dietary protein group. Again, it would have been expected that the increase in insulin associated with the glucose load would stimulate protein synthesis (Jefferson et al. 1977); however, this interpretation is complicated by the reduction in plasma amino acid concentration which may become limiting for synthesis. Certainly, in studies conducted where hyperinsulinaemic subjects were maintained at euaminoacidaemia or hyperaminoacidaemia, a stimulation of protein synthesis was demonstrated (Tessari et al. 1987). More recently, Biolo et al. (1995) demonstrated that local hyperinsulinaemia, which did not alter leg arterial amino acid delivery from the systemic circulation, increased leg muscle protein synthesis from 0.040 to 0.068 % per hour.
In conclusion, neither whole-body protein synthesis nor whole-body protein breakdown was altered by glucose supplementation during exercise. Leucine oxidation was suppressed by the provision of exogenous glucose but only in those subjects who had previously consumed a high protein diet. This could not be attributed to a deactivation of the skeletal muscle BCOADH complex, but rather may be due to altered substrate delivery. The oxidation of the exogenous glucose in the subjects who had previously consumed a high protein diet, rather than storage of the exogenous glucose in the subjects who had previously consumed a low protein diet, appears to conserve leucine.
Acknowledgments
This work was supported by SmithKline Beecham Consumer Healthcare and the University of Dundee.
References
- Ahlborg G, Felig PE, Hagenfeldt L, Hendler R, Wahren J. Substrate turnover during prolonged exercise in man. Journal of Clinical Investigation. 1974;53:1080–1090. doi: 10.1172/JCI107645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergstrom J, Hermansen L, Hultman E, Saltin B. Diet, muscle glycogen and physical performance. Acta Physiologica Scandinavica. 1967;71:140–150. doi: 10.1111/j.1748-1716.1967.tb03720.x. [DOI] [PubMed] [Google Scholar]
- Biermann CJ, Kinoshita CM, Marlett JA, Steele D. Analysis of amino acids as tertiary butyldimethylsilyl derivatives by gas chromatography. Journal of Chromatography. 1986;357:330–334. doi: 10.1016/s0021-9673(01)95837-6. [DOI] [PubMed] [Google Scholar]
- Biolo GP, Fleming RYD, Wolfe RR. Physiological hyperinsulinaemia stimulates protein synthesis and enhances transport of selected amino acids in human skeletal muscle. Journal of Clinical Investigation. 1995;95:811–819. doi: 10.1172/JCI117731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch AN, Dennis SC, Noakes TD. Influence of carbohydrate loading on fuel substrate turnover and oxidation during prolonged exercise. Journal of Applied Physiology. 1993;74:1921–1927. doi: 10.1152/jappl.1993.74.4.1921. [DOI] [PubMed] [Google Scholar]
- Bosch AN, Dennis SC, Noakes TD. Influence of carbohydrate ingestion on fuel substrate turnover and oxidation during prolonged exercise. Journal of Applied Physiology. 1994;76:2364–2372. doi: 10.1152/jappl.1994.76.6.2364. [DOI] [PubMed] [Google Scholar]
- Bowtell JL, Leese GP, Smith K, Watt PW, Nevill A, Rooyackers O, Wagenmakers AJM, Rennie MJ. Modulation of whole-body protein metabolism, during and after exercise, by variation of dietary protein. Journal of Applied Physiology. 1998;85:1744–1752. doi: 10.1152/jappl.1998.85.5.1744. [DOI] [PubMed] [Google Scholar]
- Bowtell JL, Reynolds N, Rennie MJ. Differential modulation of 13C recovery during a 13C bicarbonate infusion, by dietary protein and glucose supplementation, at rest and during exercise. Clinical Science. 1994;87(suppl.):86. [Google Scholar]
- Cheng KN, Dworzak F, Ford GC, Rennie MJ, Halliday D. Direct determination of leucine metabolism and protein breakdown in humans using L-[1-13C,15N]-leucine and the forearm model. European Journal of Clinical Investigation. 1985;15:349–354. doi: 10.1111/j.1365-2362.1985.tb00283.x. [DOI] [PubMed] [Google Scholar]
- Coyle EF, Coggan AR, Hemmert MK, Ivy JL. Muscle glycogen utilisation during prolonged strenuous exercise when fed carbohydrate. Journal of Applied Physiology. 1986;6:165–172. doi: 10.1152/jappl.1986.61.1.165. [DOI] [PubMed] [Google Scholar]
- Coyle EF, Hagberg JM, Hurley BF, Martin WH, Ehsani AA, Holloszy JO. Carbohydrate feeding during prolonged strenuous exercise can delay fatigue. Journal of Applied Physiology. 1983;55:230–235. doi: 10.1152/jappl.1983.55.1.230. [DOI] [PubMed] [Google Scholar]
- Davies CTM, Halliday D, Millward DJ, Rennie MJ, Sutton JR. Glucose inhibits CO2 production from leucine during whole-body exercise in man. The Journal of Physiology. 1982;332:41–42P. [Google Scholar]
- Dietrichson P, Coakley J, Smith PEM, Griffiths RD, Helliwell TR, Edwards RHT. Conchotome and needle percutaneous biopsy of skeletal muscle. Journal of Neurology, Neurosurgery and Psychiatry. 1987;50:1461–1467. doi: 10.1136/jnnp.50.11.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Khoury AE, Forslund A, Olsson R, Branth S, Sjodin A, Andersson A, Atkinson A, Selvaraj A, Hambraeus LM, Young VR. Moderate exercise at energy balance does not affect 24-h leucine oxidation or nitrogen retention in healthy men. American Journal of Physiology. 1997;273:E394–407. doi: 10.1152/ajpendo.1997.273.2.E394. [DOI] [PubMed] [Google Scholar]
- Escriva F, Kergoat M, Bailbe D, Pascual-Leone AM, Portha B. Increased insulin action in the rat after protein malnutrition early in life. Diabetalogia. 1991;34:559–564. doi: 10.1007/BF00400273. [DOI] [PubMed] [Google Scholar]
- Fielding RA, Evans WJ, Hughes VA, Moldawer LL, Bistrian B. The effects of high intensity exercise on muscle and plasma levels of alpha-ketoisocaproate. European Journal of Applied Physiology. 1986;55:482–485. doi: 10.1007/BF00421641. [DOI] [PubMed] [Google Scholar]
- Fisher RA. Statistical Methods for Research Workers. Edinburgh: Oliver and Boyd; 1950. [Google Scholar]
- Frayn KN. Calculation of substrate oxidation rates in vivo. Journal of Applied Physiology. 1983;55:628–634. doi: 10.1152/jappl.1983.55.2.628. [DOI] [PubMed] [Google Scholar]
- Hargreaves M, Costill DL, Coggan A, Fink WJ, Nishibata I. Effect of carbohydrate feedings on muscle glycogen utilisation and exercise performance. Medicine and Science in Sports and Exercise. 1984;16:219–222. [PubMed] [Google Scholar]
- Harper AE, Miller RH, Block KP. Branched chain amino acid metabolism. Annual Review of Nutrition. 1984;4:409–454. doi: 10.1146/annurev.nu.04.070184.002205. [DOI] [PubMed] [Google Scholar]
- Heslin MJ, Newman E, Wolfe RF, Pisters PWR, Brennan MF. Effect of hyperinsulinaemia on whole body and skeletal muscle leucine carbon kinetics in humans. American Journal of Physiology. 1992;262:E911–918. doi: 10.1152/ajpendo.1992.262.6.E911. [DOI] [PubMed] [Google Scholar]
- Hutson SM, Harper AE. Blood and tissue branched chain amino and α-keto acid concentrations: effect of diet, starvation, and disease. American Journal of Clinical Nutrition. 1981;34:173–183. doi: 10.1093/ajcn/34.2.173. [DOI] [PubMed] [Google Scholar]
- Jefferson LS, Li JB, Rannels SR. Regulation by insulin of amino acid release and protein turnover in the perfused rat hemicorpus. Journal of Biological Chemistry. 1977;252:1476–1483. [PubMed] [Google Scholar]
- Johnston JL, Balachandran AV. Effects of dietary protein, energy and tyrosine on central and peripheral noradrenaline turnover in mice. Journal of Nutrition. 1987;117:2046–2053. doi: 10.1093/jn/117.12.2046. [DOI] [PubMed] [Google Scholar]
- Knapik J, Meredith C, Jones B, Fielding R, Young VR, Evans W. Leucine metabolism during fasting and exercise. Journal of Applied Physiology. 1991;70:43–47. doi: 10.1152/jappl.1991.70.1.43. [DOI] [PubMed] [Google Scholar]
- Lembo G, Capaldo B, Rendina V, Iaccarino G, Napoli R, Guida R, Trimarco B, Sacca L. Acute noradrenergic activation induces insulin resistance in human skeletal muscle. American Journal of Physiology. 1994;266:E242–247. doi: 10.1152/ajpendo.1994.266.2.E242. [DOI] [PubMed] [Google Scholar]
- Lemon PWR, Mullin JP. Effect of initial muscle glycogen levels on protein catabolism during exercise. Journal of Applied Physiology. 1980;48:624–629. doi: 10.1152/jappl.1980.48.4.624. [DOI] [PubMed] [Google Scholar]
- Matthews DE, Bier DM, Rennie MJ, Edwards RHT, Halliday D, Millward DJ, Clugston GA. Regulation of leucine metabolism in man: a stable isotope study. Science. 1981;214:1129–1131. doi: 10.1126/science.7302583. [DOI] [PubMed] [Google Scholar]
- Meredith CN, Zackin MJ, Frontera WR, Evans WJ. Dietary protein requirements and body protein metabolism in endurance-trained men. Journal of Applied Physiology. 1989;66:2850–2856. doi: 10.1152/jappl.1989.66.6.2850. [DOI] [PubMed] [Google Scholar]
- Rennie MJ, Edwards RHT, Krywawych S, Davies CTM. Effect of exercise on protein turnover in man. Clinical Science. 1981;61:627–639. doi: 10.1042/cs0610627. [DOI] [PubMed] [Google Scholar]
- Rocchicciolo F, Leroux JP, Cartier P. Quantitation of 2-ketoacids in biological fluids by gas chromatography chemical ionization mass spectrometry of o-trimethylsilyl-quinoxalinol derivatives. Biomedicine and Mass Spectrometry. 1981;8:160–164. doi: 10.1002/bms.1200080406. [DOI] [PubMed] [Google Scholar]
- Suryawan A, Hawes JW, Harris RA, Shimomura Y, Jenkins AE, Hutson SM. A molecular model of human branched-chain amino acid metabolism. American Journal of Clinical Nutrition. 1998;68:72–81. doi: 10.1093/ajcn/68.1.72. [DOI] [PubMed] [Google Scholar]
- Taylor HL, Buskirk E, Henschel A. Maximal oxygen intake as an objective measure of cardio-respiratory performance. Journal of Applied Physiology. 1955;8:73–80. doi: 10.1152/jappl.1955.8.1.73. [DOI] [PubMed] [Google Scholar]
- Tessari P, Inchiostro S, Biolo GP, Trevisan R, Fantin G, Marescotti MC, Iori E, Tiengo A, Crepaldi G. Differential effects of hyperinsulinemia and hyperaminoacidemia on leucine-carbon metabolism in vivo. Journal of Clinical Investigation. 1987;79:1062–1069. doi: 10.1172/JCI112919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse EO, Gregoire FM, Magrum LJ, Johnson PR, Stern JS. A low protein diet lowers islet insulin secretion but does not alter hyperinsulinaemia in obese Zucker (fa/fa) rats. Journal of Nutrition. 1995;125:1923–1929. doi: 10.1093/jn/125.7.1923. [DOI] [PubMed] [Google Scholar]
- van Hall G, Saltin B, van der Vusse GJ, Soderlund K, Wagenmakers AJM. Mechanisms of activation of muscle branched-chain α-keto acid dehydrogenase during exercise in man. The Journal of Physiology. 1995;489:251–261. doi: 10.1113/jphysiol.1996.sp021542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagenmakers AJM, Beckers EJ, Brouns F, Kuipers H, Soeters PB, van der Vusse GJ, Saris WHM. Carbohydrate supplementation, glycogen depletion and amino acid metabolism during exercise. American Journal of Physiology. 1991;260:E883–890. doi: 10.1152/ajpendo.1991.260.6.E883. [DOI] [PubMed] [Google Scholar]
- Wagenmakers AJM, Brookes JH, Coakley JH, Reilly T, Edwards RHT. Exercise-induced activation of the branched chain 2-oxoacid dehydrogenase in human muscle. European Journal of Applied Physiology. 1989;59:159–167. doi: 10.1007/BF02386181. [DOI] [PubMed] [Google Scholar]
- Wagenmakers AJM, Veerkamp JH. Interaction of various metabolites and agents with branched chain 2-oxo acid oxidation in rat and human muscle in vitro. International Journal of Biochemistry. 1984;16:971–976. doi: 10.1016/0020-711x(84)90113-7. [DOI] [PubMed] [Google Scholar]
- Watt PW, Lindsay Y, Scrimgeour CM, Chien PAF, Gibson JNA, Taylor DJ, Rennie MJ. Isolation of aminoacyl-tRNA and its labeling with stable-isotope tracers: use in studies of human tissue protein synthesis. Proceedings of the National Academy of Sciences of the USA. 1991;88:5892–5896. doi: 10.1073/pnas.88.13.5892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widrick JJ, Costill DL, Fink WJ, Hickey MS, McConell GK, Tanaka H. Carbohydrate feedings and exercise performance: effect of initial muscle glycogen concentration. Journal of Applied Physiology. 1993;74:2998–3005. doi: 10.1152/jappl.1993.74.6.2998. [DOI] [PubMed] [Google Scholar]
- Yaspelkis BB, Patterson JG, Anderla PA, Ding Z, Ivy JL. Carbohydrate supplementation spares muscle glycogen during variable intensity exercise. Journal of Applied Physiology. 1993;75:1477–1485. doi: 10.1152/jappl.1993.75.4.1477. [DOI] [PubMed] [Google Scholar]
- Young JB, Kaufman LN, Saville ME, Landsberg L. Increased sympathetic nervous system activity in rats fed a low protein diet. American Journal of Physiology. 1985;248:R627–637. doi: 10.1152/ajpregu.1985.248.5.R627. [DOI] [PubMed] [Google Scholar]


