Abstract
Arterial stiffness is an important determinant of cardiovascular risk. Augmentation index (AIx) is a measure of systemic arterial stiffness derived from the ascending aortic pressure waveform. The aim of the present study was to assess the effect of heart rate on AIx. We elected to use cardiac pacing rather than chronotropic drugs to minimize confounding effects on the systemic circulation and myocardial contractility.
Twenty-two subjects (13 male) with a mean age of 63 years and permanent cardiac pacemakers in situ were studied. Pulse wave analysis was used to determine central arterial pressure waveforms, non-invasively, during incremental pacing (from 60 to 110 beats min−1), from which AIx and central blood pressure were calculated. Peripheral blood pressure was recorded non-invasively from the brachial artery.
There was a significant, inverse, linear relationship between AIx and heart rate (r =−0.76; P < 0.001). For a 10 beats min−1 increment, AIx fell by around 4 %. Ejection duration and heart rate were also inversely related (r =−0.51; P < 0.001).
Peripheral systolic, diastolic and mean arterial pressure increased significantly during incremental pacing. Although central diastolic pressure increased significantly with pacing, central systolic pressure did not. There was a significant increase in the ratio of peripheral to central pulse pressure (P < 0.001), which was accounted for by the observed change in central pressure augmentation.
These results demonstrate an inverse, linear relationship between AIx and heart rate. This is likely to be due to alterations in the timing of the reflected pressure wave, produced by changes in the absolute duration of systole. Consideration of wave reflection and aortic pressure augmentation may explain the lack of rise in central systolic pressure during incremental pacing despite an increase in peripheral pressure.
The arterial pressure waveform, and systolic pressure in particular, varies throughout the arterial tree (Latham et al. 1985). This is due to differences in vessel compliance and the phenomenon of wave reflection (Nichols & O'Rourke, 1998). Indeed, the pressure waveform at any point is a composite of the forward-going and reflected wave. As a consequence, aortic systolic pressure may differ from brachial artery systolic pressure by more than 20 mmHg (Pauca et al. 1992) and, importantly, the relationship between the two is not fixed (Kelly et al. 1989a; Nichols & O'Rourke, 1998). Such differences may be clinically important because it is central aortic, and not brachial, pressure that determines left ventricular workload (Westerhof & O'Rourke, 1995). Indeed, left ventricular mass, an important and independent predictor of all-cause mortality, correlates well with the shape of the central waveform in normotensive (Saba et al. 1993) and hypertensive individuals (Lakatta, 1991). Moreover, the shape of the pressure waveform in the carotid artery, a common site for symptomatic atheroma formation, is much closer to the ascending aortic waveform than that recorded in the brachial artery (Nichols & O'Rourke, 1998). Therefore, measurement of central aortic, rather that brachial artery, pressure may provide a better prediction of cardiovascular risk (Wilkinson et al. 1999).
Although measurement of pulse pressure alone has been used to assess arterial stiffness and cardiovascular risk (O'Rourke, 1990; Benetos et al. 1997; Franklin et al. 1999), important additional information is contained within the pressure waveform (O'Rourke & Gallagher, 1996). Mahomed recognized the usefulness of the pressure waveform as a diagnostic aid over 100 years ago (Mahomed, 1872), and since then various devices have been employed to record and analyse the pressure waveform (Lax et al. 1956). However, until recently, pulse wave analysis (PWA) was confined to the peripheral waveform. O'Rourke and colleagues have further developed the technique of PWA, making non-invasive derivation of central pressure waveforms possible (O'Rourke & Gallagher, 1996). This PWA technique uses applanation tonometry to record pressure waves from the radial artery accurately (Kelly et al. 1989b), and a validated generalized transfer factor (Karamanoglu et al. 1993; Takazawa et al. 1996; Chen et al. 1997; Soderstrom et al. 1998; Fetics et al. 1999) is then used to generate the corresponding central arterial waveform. From this, the augmentation index (AIx) – a measure of systemic arterial stiffness – and central pressure can be determined non-invasively and reproducibly (Wilkinson et al. 1998) (Fig. 1).
Figure 1. A central aortic pressure waveform.
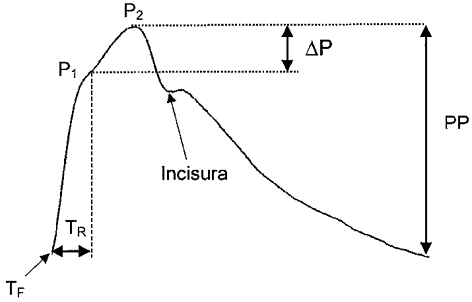
Ascending aortic pressure waveform from a 60-year-old man. Two systolic peaks are seen, P1 and P2, the latter becoming more prominent after the age of 35 years. The augmentation index is calculated as the difference between P2 and P1 (ΔP), expressed as a percentage of the pulse pressure (PP). Ejection duration was calculated as the time between the foot of the wave (TF) and the incisura, and TR is defined as the time between TF and the inflection point.
Theoretically, augmentation of central systolic pressure should be influenced by heart rate, which may potentially limit the usefulness of AIx as a measure of systemic arterial stiffness. We hypothesized that AIx would be inversely, and linearly, related to heart rate because an increase in heart rate will decrease the absolute duration of systole, effectively shifting the reflected wave into diastole, thereby reducing AIx. Indeed, Stefanadis et al. (1998) have previously demonstrated an inverse but non-linear relationship between heart rate and AIx. However, they recorded pressure waveforms from the mid-thoracic rather than the ascending aorta, and used invasive ventricular pacing in subjects undergoing cardiac catheterization. The aim of the present study was to explore further the relationship between heart rate and the shape of the ascending aortic waveform non-invasively, using PWA, over a physiological range of heart rates.
METHODS
Twenty-two subjects (13 male) with a mean age of 63 years (range, 21–84 years), and permanent atrial or dual chamber cardiac pacemakers in situ, were recruited from the pacemaker clinic at the Royal Infirmary of Edinburgh. Subjects with a history of ventricular dysrhythmias, unstable angina pectoris, or neurological disease were excluded. Those receiving cardioactive medication, or with a history of cardiovascular disease, were not excluded in order to provide a realistic spectrum of patients that might be encountered in future clinical applications of PWA. Baseline characteristics are provided in Table 1. Approval for the study was obtained from the local Research Ethics Committee, and informed consent was obtained from each participant. The investigation conformed to the principles outlined in the Declaration of Helsinki.
Table 1.
Subject characteristics
| Subject | Sex | Age (years) | Pacemaker | Indication | IHD | Heart failure | Drugs |
|---|---|---|---|---|---|---|---|
| 1 | Female | 72 | AAI | Bradycardia | No | No | Nifedipine, bumetanide |
| 2 | Male | 73 | AAI | Stokes Adams attacks* | Yes | No | Aspirin |
| 3 | Female | 62 | AAI | Stokes Adams attacks* | Yes | No | Aspirin |
| 4 | Female | 64 | DDD | Second degree heart block | No | No | — |
| 5 | Male | 74 | DDD | Second degree heart block | No | No | — |
| 6 | Female | 54 | DDD | Stokes Adams attacks* | Yes | No | Aspirin |
| 7 | Male | 77 | DDD | Second degree heart block | Yes | No | Aspirin, frusemide, atenolol |
| 8 | Female | 71 | AAI | Stokes Adams attacks* | No | No | — |
| 9 | Female | 84 | AAI | Stokes Adams attacks | No | Yes | Lisinopril, bendrofluazide |
| 10 | Female | 77 | AAI | Stokes Adams attacks* | No | No | — |
| 11 | Male | 83 | AAI | Stokes Adams attacks* | Yes | No | Aspirin |
| 12 | Male | 77 | DDD | Syncope | Yes | Yes | Aspirin, enalapril |
| 13 | Male | 73 | DDD | Stokes Adams attacks* | No | No | — |
| 14 | Male | 73 | DDD | Stokes Adams attacks* | No | No | Bendrofluazide |
| 15 | Female | 65 | AAI | Stokes Adams attacks* | No | No | Bendrofluazide |
| 16 | Male | 54 | DDD | Complete heart block | No | No | — |
| 17 | Male | 51 | DDD | Syncope | No | No | — |
| 18 | Male | 59 | AAI | Stokes Adams attacks* | No | No | — |
| 19 | Female | 36 | DDD | Complete heart block | No | No | — |
| 20 | Male | 37 | AAI | Syncope | No | No | — |
| 21 | Male | 44 | DDD | Complete heart block | No | No | — |
| 22 | Male | 21 | AAI | Syncope | No | No | — |
AAI, atrial sensing and atrial pacing pacemaker. DDD, dual chamber pacemaker. IHD, ischaemic heart disease.
Documented sinus arrest.
Blood pressure measurement
Blood pressure was measured in duplicate in the brachial artery of the non-dominant arm using the validated Omron HEM-705CP oscillometric sphygmomanometer (O'Brien et al. 1996). Peripheral mean arterial pressure was calculated as the diastolic pressure plus one-third of the pulse pressure.
Pulse wave analysis
AIx and ascending aortic pressure were determined by PWA (O'Rourke & Gallagher, 1996), using the SphygmoCor apparatus (SCOR; PWV Medical, Sydney, Australia). A high fidelity micromanometer (SPC-301; Millar Instruments, TX, USA) was used to flatten the radial artery in the non-dominant hand at the wrist using gentle pressure. Data were collected directly into a portable computer and, after 20 sequential waveforms had been acquired, the integral software was used to generate an averaged peripheral and corresponding central waveform, which was then subjected to further analysis to determine AIx and ascending aortic pressure. The augmentation index was defined as the difference between the first (P1) and second (P2) peaks of the central arterial waveform, expressed as a percentage of the pulse pressure, and ejection duration as the time from the foot of the pressure wave to the incisura (Fig. 1). The aortic pulse wave velocity was estimated by calculating the time between the foot of the pressure wave and the inflection point, which provides a measure of the timing of the reflected wave, as described previously (Murgo et al. 1980; Marchais et al. 1993).
Study protocol
Brachial artery pressure and radial pressure waveforms were recorded in duplicate after the subjects had rested for 30 min in a supine position in a quiet, temperature-conditioned room (22-24°C). Subjects were then paced at 60 beats min−1 and, after 3 min, measurements were repeated. The pacing rate was then increased from 60 to 110 beats min−1 in 10 beat increments and all measurements were repeated after 3 min at each step. Atrial pacing was used for subjects with AAI pacemakers (atrial sensing and atrial pacing). Patients with dual chamber models underwent sequential atrio-ventricular pacing, with an atrio-ventricular delay of 120 ms. Holter monitoring was used continuously throughout the study to ensure that heart rate accorded with the programmed rate. The original pacemaker settings were then restored. Due to interference between the pacemaker controller and the transthoracic bioimpedance system it was not possible to measure cardiac index or peripheral vascular resistance non-invasively during the study.
Data analysis
All data were analysed as changes from values at 60 beats min−1, using analysis of variance (ANOVA). Comparisons between data were made using ANOVA or Student's t tests, as appropriate. All values are reported as means ±s.e.m., unless otherwise stated, and a P value < 0.05 was considered as statistically significant.
In order to investigate the effect of age on the response to pacing, the subjects were split into two groups: group 1, < 60 years; group 2, ≥ 60 years. Division at 60 years was chosen to provide two reasonably sized groups with significantly different mean ages. Comparisons of the haemodynamic effects were also made between those receiving atrial and those receiving sequential atrio-ventricular pacing.
RESULTS
Baseline variables
Subject characteristics are shown in Table 1. The mean heart rate of the 22 subjects after 30 min of supine rest was 65 ± 2 beats min−1, brachial artery systolic blood pressure was 136 ± 6 mmHg (range, 97–203 mmHg), and diastolic pressure was 82 ± 3 mmHg (59-101 mmHg). Resting AIx was 24.5 ± 3.0 % (-14 to +45 %), central systolic blood pressure was 129 ± 6 mmHg (86-196 mmHg), and central diastolic pressure was 84 ± 3 mmHg (60-102 mmHg).
Haemodynamic changes during pacing
The mode of pacing, atrial versus sequential atrio-ventricular, did not significantly affect the haemodynamic responses during incremental pacing (data not shown). Therefore, the data were treated as homogeneous, and analysed as a single group.
Incremental pacing from 60 to 110 beats min−1 resulted in a significant increase in peripheral systolic, peripheral diastolic and central diastolic blood pressure; but no change in central systolic pressure occurred (Table 2). AIx declined linearly with increasing heart rate (linear regression analysis: r =−0.76; P < 0.001). The slope of the regression line was -0.39, indicating a reduction in AIx of 3.9 % for each 10 beats min−1 increase in heart rate (Fig. 2a). Defining negative values of AIx as zero did not significantly affect the relationship between AIx and heart rate. Ejection duration also fell linearly with increasing heart rate (Fig. 2b), but there was no significant change in the timing of the reflected wave (Table 2).
Table 2.
Effect of increasing heart rate on peripheral and central haemodynamics
| HR (beats min−1) | Aix (%) | PSBP (mmHg) | PDBP (mmHg) | PMAP (mmHg) | CSBP (mmHg) | CDBP (mmHg) | CMAP (mmHg) | PPP-CPP ratio | P1 (mmHg) | P1-CDBP (mmHg) | PPP:(P1-CDBP) ratio | TR (ms) | ED (ms) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 60 | 24.1 ± 2.9 | 135 ± 6 | 80 ± 2 | 98 ± 3 | 126 ± 6 | 82 ± 3 | 96 ± 3 | 1.28 ± 0.03 | 114 ± 4 | 30 ± 2 | 1.71 ± 0.03 | 137 ± 4 | 323 ± 5 |
| 70 | 21.3 ± 2.9 | 137 ± 6 | 84 ± 3 | 102 ± 4 | 128 ± 6 | 86 ± 3 | 100 ± 4 | 1.35 ± 0.04 | 117 ± 4 | 35 ± 3 | 1.73 ± 0.03 | 140 ± 4 | 307 ± 4 |
| 80 | 15.2 ± 3.1 | 141 ± 5 | 88 ± 2 | 106 ± 3 | 128 ± 5 | 90 ± 2 | 102 ± 3 | 1.42 ± 0.04 | 120 ± 4 | 34 ± 2 | 1.69 ± 0.03 | 138 ± 3 | 295 ± 4 |
| 90 | 12.1 ± 3.1 | 142 ± 6 | 90 ± 3 | 108 ± 4 | 128 ± 6 | 92 ± 3 | 104 ± 4 | 1.50 ± 0.04 | 122 ± 4 | 32 ± 2 | 1.72 ± 0.03 | 137 ± 2 | 284 ± 4 |
| 100 | 8.8 ± 3.3 | 144 ± 6 | 93 ± 3 | 110 ± 3 | 129 ± 5 | 96 ± 3 | 107 ± 3 | 1.59 ± 0.04 | 124 ± 4 | 32 ± 2 | 1.76 ± 0.03 | 135 ± 3 | 272 ± 3 |
| 110 | 5.0 ± 3.2 | 145 ± 5 | 96 ± 3 | 112 ± 3 | 129 ± 5 | 99 ± 3 | 109 ± 3 | 1.73 ± 0.05 | 126 ± 4 | 30 ± 2 | 1.83 ± 0.03 | 134 ± 2 | 257 ± 2 |
| P | <0.001 | 0.001 | <0.001 | <0.001 | 0.89 | <0.001 | <0.001 | <0.001 | <0.001 | 0.52 | 0.34 | 0.09 | <0.001 |
HR, heart rate; AIx, augmentation index; PSBP, peripheral systolic blood pressure; PDBP, peripheral diastolic blood pressure; PMAP, peripheral mean arterial pressure; CSBP, central systolic blood pressure; CDBP, central diastolic blood pressure; CMAP, central mean arterial pressure; PPP, peripheral pulse pressure; CPP, central pulse pressure; P1, height of the first systolic peak of the central waveform; TR, time to inflection point; ED, ejection duration. All values are quoted as means ± S. E. M. Significance was determined using ANOVA, and data were analysed as the change from values at a heart rate of 60 beats min−1.
Figure 2. The effect of heart rate on augmentation index and ejection duration.
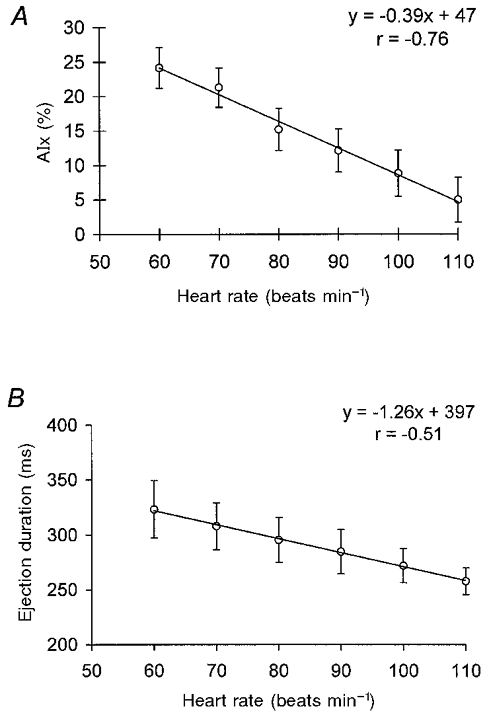
A, the relationship between augmentation index (AIx) and heart rate. B, the relationship between ejection duration and heart rate. Values represent means ±s.e.m. (n = 22). A linear regression line is shown (P < 0.001, ANOVA).
The responses of peripheral and central diastolic pressure to pacing did not differ significantly (P = 0.50), but the effect on systolic pressures did (P < 0.001) as can be seen in Fig. 3a and B. There was an increase in arterial pressure amplification from the aorta to the brachial artery, as defined by the ratio of peripheral to central pulse pressure, during pacing (Table 2). However, when the ratio of peripheral pulse pressure to non-augmented central pulse pressure (P1– central diastolic pressure) was calculated, there was no significant change.
Figure 3. The effect of heart rate on peripheral and central blood pressure.
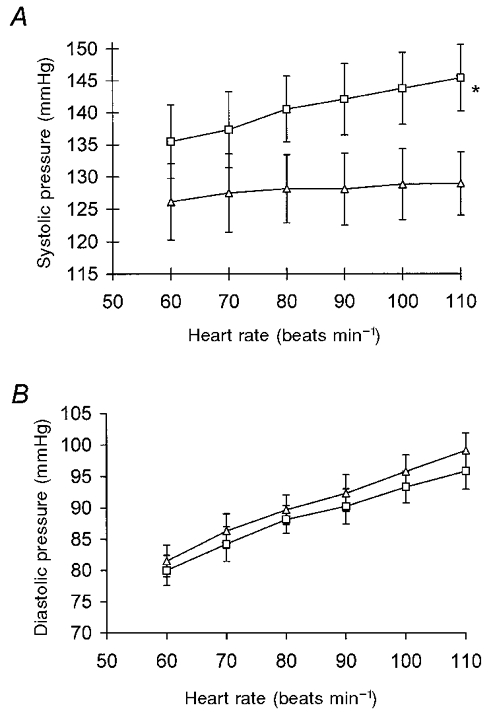
A, changes in systolic blood pressure with increasing heart rate. B, changes in diastolic blood pressure with increasing heart rate. □, peripheral (brachial) arterial pressure; ▵, central (ascending aortic) arterial pressure. Values represent means ±s.e.m. (n = 22). * P = 0.001 (ANOVA).
Influence of age on haemodynamic responses to pacing
The only parameter that differed significantly between the two groups at baseline, other than age, was AIx, which was lower in group 1 (Table 3). The effect of increasing heart rate on peripheral and central blood pressure (systolic and diastolic) did not differ between the groups, and in both groups AIx decreased with increasing heart rate. Although the slope of the regression lines (AIx against heart rate) was not significantly different (-0.39 in group 1 versus -0.40 in group 2; P = 0.90), the intercept with the y-axis was significantly lower in group 1 (+38 versus+53 %; P < 0.001), in keeping with the lower baseline AIx.
Table 3.
Baseline haemodynamics for groups 1 and 2
| Group 1 | Group 2 | |
|---|---|---|
| Number | 8 | 14 |
| Age (years) | 45±4 | 73±2 |
| AIx (%) | 15.8 ± 5.3 | 29.8 ± 2.9* |
| PSBP (mmHg) | 128 ± 7 | 145 ± 8 |
| PDBP (mmHg) | 80 ± 5 | 80 ± 3 |
| CSBP (mmHg) | 117 ± 7 | 136 ± 8 |
| CDBP (mmHg) | 82 ± 5 | 85 ± 3 |
Group 1, subjects under 60 years old; group 2, subjects over 60 years old. AIx, augmentation index; PSBP, peripheral systolic blood pressure; PDBP, peripheral diastolic blood pressure; CSBP, central systolic blood pressure; CDBP, central diastolic blood pressure. All values are quoted as means ± S. E. M. Comparisons between groups were made with unpaired Student's t tests. Unless indicated, there was no significant difference between parameters
P = 0.027.
DISCUSSION
The present study describes the effect of incrementally increasing heart rate, via cardiac pacing, on peripheral arterial pressure, central arterial pressure and the ascending aortic waveform. AIx is a measure of the contribution made by the reflected pressure wave to the ascending aortic pressure waveform (O'Rourke & Kelly, 1993). The amplitude and speed of the reflected wave are dependent upon arterial stiffness and, thus, AIx provides a measure of systemic arterial stiffness (O'Rourke, 1990; Nichols & O'Rourke, 1998). We hypothesized that changing heart rate would alter the relative timing of the return of the reflected wave to the ascending aorta and, therefore, that AIx would be linearly and inversely related to heart rate.
Changes in AIx
As predicted, during incremental pacing from 60 to 110 beats min−1, we observed a significant and linear reduction in AIx (Fig. 2). This confirms the results obtained by Stefanadis et al. (1998), who employed invasive ventricular pacing in younger subjects undergoing cardiac catheterization. They reported an inverse relationship between heart rate and AIx, which appeared to be non-linear. However, non-linearity only became apparent as the heart rate increased above 120 beats min−1. The magnitude of the reduction in AIx with increasing heart rate was somewhat greater in the present study than reported by these authors. This important difference may relate to the mode of pacing used in the two studies (Stefanadis et al. employed right ventricular pacing) and variations in subject characteristics, particularly age and cardiac function, since such factors are known to influence the effect of pacing on cardiac output (Sowton, 1964; Clement et al. 1979). Moreover, Stefanadis et al. (1998) also calculated AIx from the waveform in the descending rather than the ascending aorta as in the present study.
Interestingly, in the present study, when subjects were divided into two groups according to age, there was no significant difference in the slope of the two regression lines. However, the intercept with the y-axis was significantly lower for group 1 (< 60 years), which is in keeping with the strong age dependency of AIx (Kelly et al. 1989a) and the lower baseline AIx of group 1 (Table 3). Although this suggests that age may not influence the effect of heart rate on AIx, data are required from a younger population to confirm this.
Observational studies imply a link between high resting heart rate and increased arterial stiffness (Sa Cunha et al. 1997), suggesting that arterial stiffness per se may be influenced by heart rate. Indeed, Stefanadis et al. (1998) reported reduced aortic stiffness and increased distensibility, assessed using ultrasound, during incremental ventricular pacing. We did not measure compliance directly in the present study, but did not observe any significant change in the timing of the reflected wave during pacing, which provides a measure of the aortic pulse wave velocity and, thus, aortic stiffness, as described previously (Murgo et al. 1980; Marchais et al. 1993). Our results, indicating a constant aortic pulse wave velocity across a range of heart rates, are in keeping with data from earlier studies in several species including man suggesting that large artery stiffness is not affected by changes in heart rate within the physiological range (Bergel, 1961, 1972; Noble et al. 1967; Nichols et al. 1977). Indeed, the constancy of vessel wall properties over a wide range of input frequencies provides the basis for steady-state analysis techniques of pressure- flow relationships (Bergel, 1972). Therefore, it is unlikely that changes in aortic stiffness account for the inverse relationship between heart rate and AIx noted in the present study. Indeed, it is more likely that this relationship is due to the reduction in ejection duration, associated with increasing heart rate (Lewis et al. 1977), causing a shift of the reflected wave into diastole.
Changes in peripheral pressure
During incremental pacing, peripheral systolic, diastolic and mean arterial pressure all increased significantly (Table 2). Previous studies, in healthy controls and patients with coronary artery disease, have shown similar changes in diastolic and mean arterial pressure with both atrial (Aborgast & Bourassa, 1973; Knoebel et al. 1973; Clement et al. 1979) and sequential atrio-ventricular pacing (Taylor et al. 1996). However, the effect on peripheral systolic pressure seems variable (Clement et al. 1979), but almost all studies, where data are reported, do show a trend for peripheral systolic pressure to rise.
We were unable to obtain reliable measures of cardiac index or peripheral vascular resistance, due to technical difficulties, and, therefore, cannot comment directly on mechanisms responsible for the observed rise in blood pressure. Previous studies have reported a rise in cardiac index with pacing and little change in peripheral vascular resistance (Bahler & Macleod, 1971; Clement et al. 1979), but this has not been confirmed by others (Parker et al. 1971; Knoebel et al. 1973). Again, differences in experimental methods and subject characteristics are likely to explain these discrepant findings (Clement et al. 1979). In particular, left ventricular function seems to be an important determinant of the haemodynamic response to pacing (Sowton, 1964). Indeed, Chandraratna et al. (1973) demonstrated that atrial pacing only increases cardiac index in patients with left ventricular dysfunction. However, as discussed in more detail below, changes in the reflected pressure wave will also influence arterial pressure, especially in the ascending aorta.
Changes in central blood pressure
In contrast to the effect of pacing on peripheral pressure, central systolic pressure did not change with increasing heart rate. This reinforces the important contribution that the reflected pressure wave makes to central systolic pressure. In the periphery, systolic pressure is relatively independent of wave reflection but, after the age of 35 years, central systolic pressure is increasingly augmented by the reflected wave (Kelly et al. 1989a; Nichols & O'Rourke, 1998). Our study group had a mean age of 63 years, in keeping with the average age of patients undergoing permanent pacemaker implantation, and showed positive augmentation of central systolic pressure (mean AIx, 24.5 ± 3.0 %), indicating a dominant second systolic peak. Consequently, any rise in central systolic pressure produced by pacing would tend to be offset by a fall in AIx. The height of the first systolic peak is dependent on the amplitude of the forward-going wave (Nichols & O'Rourke, 1998), and consequently will vary with cardiac index. As expected, we observed a rise in P1 with increasing heart rate, in keeping with the observed change in peripheral systolic pressure. There was no significant change in the difference between P1 and central diastolic pressure (an index of peak ventricular ejection velocity, assuming constant aortic root diameter) with increasing heart rate, which is in line with previous studies demonstrating that the pattern of left ventricular ejection is relatively independent of heart rate within the physiological range (O'Rourke & Taylor, 1967). Thus, the observed rise in P1 is unlikely to be due to a change in the pattern of left ventricular ejection.
The height of P2 did not change significantly during the study, presumably because any potential increase, caused by a change in cardiac output or peripheral resistance, was counterbalanced by a shift of the reflected wave into diastole, as evidenced by the fall in AIx. The net effect, therefore, was no overall change in central systolic pressure, since P2 defined central systolic pressure for most subjects. The situation may be different in younger subjects, because baseline augmentation would be zero or negative. However, we were unable to address this issue because all but one of our subjects had positive augmentation at baseline.
Incremental pacing resulted in an identical rise in central and peripheral diastolic pressure (Fig. 3b). In addition to changes in diastolic pressure resulting from an alteration in cardiac index or peripheral resistance, increasing heart rate shortens the absolute duration of diastole, which serves to increase diastolic pressure by two mechanisms. Firstly, it reduces the time for diastolic decay and, secondly, it effectively shifts the reflected wave into diastole, due to a reduction in the absolute ejection duration, which will boost diastolic pressure. However, unlike systolic pressure, central and peripheral waveforms will be affected similarly.
The observed changes in peripheral and central pressure during pacing can also be expressed as a change in the amplification of pulse pressure – the peripheral to central pulse pressure ratio, which is usually greater than one and is frequency dependent (Nichols & O'Rourke, 1998). In the present study, there was an increase in pressure amplification with incremental pacing. However, the ratio of peripheral pulse pressure to non-augmented central pulse pressure (i.e. P1– central diastolic pressure) did not change significantly, indicating that the observed effect of heart rate on pressure amplification is likely to be a consequence of altered central pressure augmentation; this emphasizes the important contribution that wave reflection makes to the central pressure waveform.
Study limitations
In the present study incremental pacing was used to permit measurements to be made at a variety of heart rates, and a stabilization period of 3 min was observed before making any haemodynamic measurements (Sowton, 1964; Clement et al. 1979). We chose to use patients with permanent pacemakers in situ to allow us to employ a more physiological mode of pacing than that used by Stefanadis et al. (1998), and to avoid the need for any pre-medication, which most invasive studies have employed. The use of incremental pacing rather than chronotropic drugs to increase heart rate also minimizes any confounding effect that drugs may have on myocardial contractility, peripheral vascular resistance and arterial compliance. Since all the subjects studied had permanent cardiac pacemakers in situ, it must be assumed that they all had underlying SA node or conducting system disease. In addition, six members of our study group had documented ischaemic heart disease and two had heart failure or coronary artery disease, important determinants of the cardiovascular response to pacing. Healthy older subjects may exhibit different haemodynamic responses to pacing as discussed by Clement et al. (1979), as, indeed, may younger subjects, for the reasons outlined above.
Summary
In summary, an increase in heart rate, from 60 to 110 beats min−1, was associated with a rise in peripheral blood pressure and central diastolic pressure but no change in central systolic pressure. Over the same pacing interval there was a linear reduction in AIx. The difference between the effect on peripheral and central pressure, together with the response of AIx, can be explained by consideration of wave reflection. A better understanding of the interaction between heart rate and AIx may be of use in future studies employing PWA to assess systemic arterial stiffness.
Acknowledgments
We are grateful to the High Blood Pressure Foundation for their financial support for this project. Dr I. B. Wilkinson, Dr J. R. Cockcroft and Professor D. J. Webb are supported by a Biomedical Research Collaboration Grant from the Wellcome Trust (056223). Professor D. J. Webb is currently in receipt of a Research Leave Fellowship from the Wellcome Trust (052633).
References
- Aborgast R, Bourassa MG. Myocardial function during atrial pacing in patients with angina pectoris and normal coronary arteriograms. American Journal of Cardiology. 1973;32:257–263. doi: 10.1016/s0002-9149(73)80130-4. [DOI] [PubMed] [Google Scholar]
- Bahler RC, Macleod CA. Atrial pacing and exercise in the evaluation of patients with angina pectoris. Circulation. 1971;43:407–419. doi: 10.1161/01.cir.43.3.407. [DOI] [PubMed] [Google Scholar]
- Benetos A, Safar M, Rudnichi A, Smulyan H, Richard J-L, Ducimetiere P, Guize L. Pulse pressure: a predictor of long-term cardiovascular mortality in a French male population. Hypertension. 1997;30:1410–1415. doi: 10.1161/01.hyp.30.6.1410. [DOI] [PubMed] [Google Scholar]
- Bergel DH. The dynamic elastic properties of the arterial wall. The Journal of Physiology. 1961;156:458–469. doi: 10.1113/jphysiol.1961.sp006687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergel DH. Cardiovascular Fluid Dynamics. London: Academic Press; 1972. [Google Scholar]
- Chandraratna PAN, Shah PM, Kramer DH, Dacis RJ, Schreiner BF. Spectrum of haemodynamic responses to atrial pacing in coronary artery disease. British Heart Journal. 1973;35:1033–1040. doi: 10.1136/hrt.35.10.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C-H, Nevo E, Fetics B, Pak PH, Yin FCP, Maughan WL, Kass DA. Estimation of central aortic pressure waveform by mathematical transformation of radial tonometry pressure. Circulation. 1997;95:1827–1836. doi: 10.1161/01.cir.95.7.1827. [DOI] [PubMed] [Google Scholar]
- Clement D, Ector H, Piessens J, Kesteloot H, De Geest H. Left ventricular systolic time intervals and cardiac haemodynamics during atrial pacing in normal subjects and patients with coronary artery disease. Acta Cardiologica. 1979;34:233–258. [PubMed] [Google Scholar]
- Fetics B, Nevo E, Chen C-H, Kass DA. Parametric model derivation of transfer function for non-invasive estimation of aortic pressure by radial tonometry. IEEE Transactions on Biomedical Engineering. 1999;46:698–706. doi: 10.1109/10.764946. [DOI] [PubMed] [Google Scholar]
- Franklin SS, Khan SA, Wong ND, Larson MG, Levy D. Is pulse pressure useful in predicting risk for coronary heart disease? The Framingham heart study. Circulation. 1999;100:354–360. doi: 10.1161/01.cir.100.4.354. [DOI] [PubMed] [Google Scholar]
- Karamanoglu M, O'Rourke MF, Avolio AP, Kelly RP. An analysis of the relationship between central aortic and peripheral upper limb pressure waves in man. European Heart Journal. 1993;14:160–167. doi: 10.1093/eurheartj/14.2.160. [DOI] [PubMed] [Google Scholar]
- Kelly RP, Hayward C, Avolio AP, O'Rourke MF. Non-invasive determination of age-related changes in the human arterial pulse. Circulation. 1989a;80:1652–1659. doi: 10.1161/01.cir.80.6.1652. [DOI] [PubMed] [Google Scholar]
- Kelly RP, Hayward CS, Ganis J, Daley JM, Avolio AP, O'Rourke MF. Non-invasive registration of the arterial pulse waveform using high fidelity applanation tonometry. Journal of Vascular and Medical Biology. 1989b;1:142–149. [Google Scholar]
- Knoebel SB, McHenry PL, Bonner AJ, Phillips JF. Myocardial blood flow in coronary artery disease: effects of right atrial pacing and nitroglycerine. Circulation. 1973;47:690–696. doi: 10.1161/01.cir.47.4.690. [DOI] [PubMed] [Google Scholar]
- Lakatta EG. Similar myocardial effects of aging and hypertension. European Heart Journal. 1991;11:29–38. doi: 10.1093/eurheartj/11.suppl_g.29. [DOI] [PubMed] [Google Scholar]
- Latham RD, Westerhof N, Sipkema P, Rubal BJ, Reuderink P, Murgo J. Regional wave travel and reflections along the human aorta: a study with six simultaneous micromanometric pressures. Circulation. 1985;72:1257–1269. doi: 10.1161/01.cir.72.6.1257. [DOI] [PubMed] [Google Scholar]
- Lax H, Feinberg AW, Cohen BM. Studies of the arterial pulse wave. Journal of Chronic Diseases. 1956;3:618–631. doi: 10.1016/0021-9681(56)90158-8. [DOI] [PubMed] [Google Scholar]
- Lewis RP, Rittgers SE, Forester WF, Boudoulas H. A critical review of the systolic time intervals. Circulation. 1977;56:146–158. doi: 10.1161/01.cir.56.2.146. [DOI] [PubMed] [Google Scholar]
- Mahomed FA. The physiological and clinical use of the sphygmograph. Medical Times Gazette. 1872;1:62–64. [Google Scholar]
- Marchais SJ, Guerin AP, Pannier BM, Levy BI, Safar M, London GM. Wave reflections and cardiac hypertrophy in chronic uremia. Hypertension. 1993;22:876–883. doi: 10.1161/01.hyp.22.6.876. [DOI] [PubMed] [Google Scholar]
- Murgo JP, Westerhof N, Giolma JP, Altobelli SA. Aortic input impedance in normal man: relationship to pressure waveforms. Circulation. 1980;62:105–116. doi: 10.1161/01.cir.62.1.105. [DOI] [PubMed] [Google Scholar]
- Nichols WW, Conti R, Walker WE, Milnor WR. Input impedance of the systemic circulation in man. Circulation Research. 1977;40:451–458. doi: 10.1161/01.res.40.5.451. [DOI] [PubMed] [Google Scholar]
- Nichols WW, O'Rourke MF. McDonald's Blood Flow in Arteries: Theoretical, Experimental and Clinical Principles. London: Arnold; 1998. [Google Scholar]
- Noble MIM, Gabe IT, Trenchard D, Guz A. Blood pressure and flow in the ascending aorta of conscious dogs. Cardiovascular Research. 1967;1:9–20. doi: 10.1093/cvr/1.1.9. [DOI] [PubMed] [Google Scholar]
- O'Brien E, Mee F, Atkins N, Thomas M. Evaluation of three devices for self measurement of blood pressure according to the revised British Hypertension Society Protocol: the Omron HEM-705CP, Philips HP5332, and Nissei DS-175. Blood Pressure Monitoring. 1996;1:55–61. [PubMed] [Google Scholar]
- O'Rourke MF. Arterial stiffness, systolic blood pressure, and logical treatment of arterial hypertension. Hypertension. 1990;15:339–347. doi: 10.1161/01.hyp.15.4.339. [DOI] [PubMed] [Google Scholar]
- O'Rourke MF, Gallagher DE. Pulse wave analysis. Journal of Hypertension. 1996;14:147–157. [PubMed] [Google Scholar]
- O'Rourke MF, Kelly RP. Wave reflection in the systemic circulation and its implications in ventricular function. Journal of Hypertension. 1993;11:327–337. doi: 10.1097/00004872-199304000-00001. [DOI] [PubMed] [Google Scholar]
- O'Rourke MF, Taylor MG. Input impedance of the systemic circulation. Circulation Research. 1967;20:365–380. doi: 10.1161/01.res.20.4.365. [DOI] [PubMed] [Google Scholar]
- Parker JO, Khaja F, Case RB. Analysis of left ventricular function by atrial pacing. Circulation. 1971;43:241–252. doi: 10.1161/01.cir.43.2.241. [DOI] [PubMed] [Google Scholar]
- Pauca AL, Wallenhaupt ST, Kon ND, Tucker WY. Does radial artery pressure accurately reflect aortic pressure? Chest. 1992;102:1193–1198. doi: 10.1378/chest.102.4.1193. [DOI] [PubMed] [Google Scholar]
- Saba PS, Roman MJ, Pini R, Spitzer M, Ganau A, Devereux RB. Relation of arterial pressure waveform to left ventricular and carotid anatomy in normotensive subjects. Journal of the American College of Cardiology. 1993;22:1873–1880. doi: 10.1016/0735-1097(93)90772-s. [DOI] [PubMed] [Google Scholar]
- Sa Cunha R, Pannier B, Benetos A, Siche J-P, London GM, Mallion JM, Safar ME. Association between high heart rate and high arterial rigidity in normotensive and hypertensive subjects. Journal of Hypertension. 1997;15:1423–1430. doi: 10.1097/00004872-199715120-00009. [DOI] [PubMed] [Google Scholar]
- Soderstrom S, Nyberg G, Ponten J, Sellgren J, O'Rourke M. Substantial equivalence between ascending aortic pressure waveforms and waveforms derived from the radial pulse using a generalized transfer function? FASEB Journal. 1998;A712:4131. [Google Scholar]
- Sowton E. Haemodynamic studies in patients with artificial pacemakers. British Heart Journal. 1964;26:737–746. doi: 10.1136/hrt.26.6.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanadis C, Dernellis J, Vavuranakis M, Tsiamis E, Vlachopoulos C, Toutouzas K, Diamandopoulos L, Pitsavos C, Toutouzas P. Effects of ventricular pacing-induced tachycardia on aortic mechanics in man. Cardiovascular Research. 1998;39:506–514. doi: 10.1016/s0008-6363(98)00115-1. [DOI] [PubMed] [Google Scholar]
- Takazawa K, O'Rourke M, Fujita M. Estimation of ascending aortic pressure from radial arterial pressure using a generalized transfer function. Zeitschrift fur Kardiologie. 1996;85:137–139. [PubMed] [Google Scholar]
- Taylor JA, Morillo CA, Eckberg DL, Ellenbogen KA. Higher sympathetic nerve activity during ventricular (VVI) than during dual-chamber (DDD) pacing. Journal of the American College of Cardiology. 1996;28:1753–1758. doi: 10.1016/s0735-1097(96)00389-0. [DOI] [PubMed] [Google Scholar]
- Westerhof N, O'Rourke MF. Haemodynamic basis for the development of left ventricular failure in systolic hypertension and for its logical treatment. Journal of Hypertension. 1995;13:943–952. doi: 10.1097/00004872-199509000-00002. [DOI] [PubMed] [Google Scholar]
- Wilkinson IB, Cockcroft JR, Webb DJ. Pulse wave analysis and arterial stiffness. Journal of Cardiovascular Pharmacology. 1999;32:33–37. [PubMed] [Google Scholar]
- Wilkinson IB, Fuchs SA, Jansen IM, Spratt JC, Murray GD, Cockcroft JR, Webb DJ. The reproducibility of pulse wave velocity and augmentation index measured by pulse wave analysis. Journal of Hypertension. 1998;16:2079–2084. doi: 10.1097/00004872-199816121-00033. [DOI] [PubMed] [Google Scholar]


