Abstract
Target-specific expression of pre- and postsynaptic mechanisms of synaptic transmission has been shown in a variety of central neurons by a number of laboratories. These data have demonstrated that synaptic transmission between single axons diverging onto distinct target neurons can behave independently, differentially influencing activity in the target neuron. Similarly, single neurons are capable of manufacturing molecularly distinct ligand-gated receptors and targeting them to synapses innervated by distinct converging afferent projections. A picture is emerging consistent with a role for both pre- and postsynaptic mechanisms in influencing the target-specific nature of transmission at numerous diverse synapses throughout the mammalian CNS. This target specificity adds another level of complexity in unravelling the roles played by individual neurons within a computational network. To begin to understand the coordinated activity of large ensembles of neurons it is becoming clear that the nature of transmission between individual pre- and postsynaptic elements within a circuit must first be understood for each and every neural element involved.
New cellular processes do not extend randomly. Instead, they may be oriented along major pathways or even in the direction of intracellular associations mediating repeated solicitations of the will.…we saw that mechanism underlying the growth of new axonal branches may be attributed to chemotactic influences.Ramón y Cajal (1898)
Ramón y Cajal was among the first to propose an explanation of how the high degree of specificity among different types of neurons within the mammalian central nervous system developed. The works of Golgi (1884), Ramón y Cajal (1898) and Lorente de Nó (1934) provided the framework for the first classification of the basic cell types that make up the nervous system: the ‘principal cells’ and cells with ‘small axons’ which are are today commonly referred to as ‘local circuit interneurons’. Furthermore, their work suggested that the highly specific arborization patterns of both dendritic and axonal morphologies were based on the capacity of neurons to select their postsynaptic targets. Surprisingly, the concept of ‘synaptic targeting’ was born before the existence of different types of neurotransmitters, intracellular proteins, receptors and receptor subunits was known.
Until only recently, it was generally accepted that each presynaptic axon influenced all of its postsynaptic targets in a similar way, regardless of the neuroanatomical/neurochemical identity of the postsynaptic target. Such a configuration would ensure that activity coursing down an axon would be transmitted equivalently to all of the synaptic targets. The first clues that this was too simplified a notion were provided from work at the neuromuscular junction. In the lobster proximal accessory flexor muscle, transmission to different muscle fibres was facilitated to markedly different degrees (Frank, 1973). However, the stimulation of synapses on any single fibre evoked postsynaptic potentials with highly similar facilitation properties. Therefore it was concluded that the presynaptic nerve terminal and the single muscle fibre have ‘matching facilitation characteristics’. In the somatogastric system of the lobster two muscles, gm8 and gm9, are innervated by the same motor neuron. The synaptic specializations formed by this common axon were physiologically and anatomically distinct; gm8 muscle contracts slowly and maintains contracture, while gm9 contracts rapidly and then relaxes (Katz et al. 1993). Moreover, gm8 was shown to exhibit facilitation of excitatory junction potentials but gm9 did not. At the ultrastructural level, gm9 was shown to have larger and more numerous presynaptic bars than gm8. This higher density of release sites suggested a higher output of neurotransmitter, more rapid depletion of vesicles, and consequently the lack of synaptic facilitation on gm9 synapses. Electrophysiological studies in other invertebrate tissue also demonstrated that a single motor neuron axon could have terminals that exhibited different physiological attributes depending on the nature of the target tissue (Atwood & Bittner, 1971; Parnas, 1972; Muller & Nicholls, 1974; Gardner, 1991; Laurent & Sivaramakrishnan, 1992; Davis & Murphey, 1993). These seminal investigations paved the way for our current understanding of the nature of target-specific expression of both pre- and postsynaptic mechanisms. The present review attempts to bring together recent work from a variety of laboratories concerned with such issues. The review is divided into two parts: the first deals with target-specific mechanisms arising from diverging single axon projections; the second will discuss recent observations that single cells can target molecularly distinct receptor subunits across the somato-dendritic axis.
I. Target cell-specific mechanisms of synaptic transmission
In central neurons it had been assumed that the probability of neurotransmitter release (Pr) was identical at all terminals for a given synaptic type. The first evidence that this was not the case came from studying the Pr of synapses formed between cultured hippocampal pyramidal neurons and their targets. Rosenmund et al. (1993) demonstrated that the Pr of synapses from a single axon were non-uniform and showed considerable range (0.09-0.54). Although the exact mechanisms responsible for the differential properties of hippocampal synapses were not determined, this study provided the first clue that synapses from a single class of central neuron axon were not all functionally equivalent.
The question of whether axons in the mammalian central nervous system exhibit target-specific differences in the mechanism of synaptic transmission has recently been addressed by several laboratories. The first direct anatomical evidence for the existence of target-specific differentiation of a single central axon came from the laboratory of Peter Somogyi. In an elegant study, Shigemoto et al. (1996) demonstrated that axon terminals of hippocampal CA3 pyramidal cells innervating metabotropic glutamate receptor subtype 1α (mGluR1α)-expressing interneurons possessed a >10-fold higher level of presynaptic mGluR7 than axon terminals making contacts with mGluR1α-negative principal cells. The specificity of matching of pre- and postsynaptic receptor composition was extremely high; even a single terminal forming two active zones with different postsynaptic targets formed one mGluR7-containing and one mGluR7-negative synapse (Fig. 1). In the data of Shigemoto et al. (1996) the presence or absence of mGluR1α in the postsynaptic cell was not a determining factor since the pattern of mGluR7 expression was identical in genetically altered mice lacking postsynaptic mGluR1α expression. This raised the possibility that presynaptic neurons could regulate the probability of transmitter release at individual synapses according to their postsynaptic target.
Figure 1. Selective targeting of presynaptic mGluR7 receptors to synapses formed onto mGluR1α-expressing interneurons.
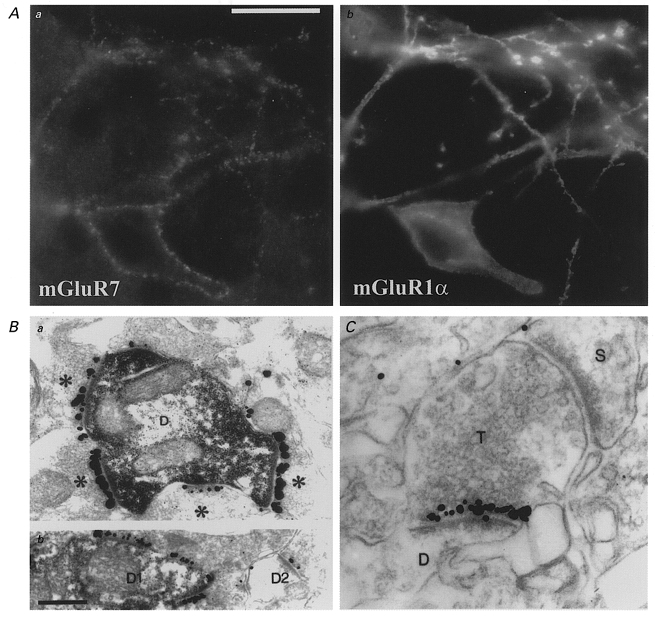
A and B, correlated distribution of immunoreactivity for mGluR7 and mGluR1α. mGluR7 positive terminals (Aa) outline the somas and dendrites of mGluR1α-positive interneurons in the stratum oriens/alveus of the rat hippocampus (Ab). Scale bar, 20 μm, applies to all panels. B, mGluR7 expression in the presynaptic grid at the electron microscopic level. Synaptic junctions terminating on a mGluR1α-positive dendrite (D) are heavily labelled for mGluR7 (Ba), while those on a mGluR1α-negative dendrite (D2) are labelled only weakly for mGluR7 (Bb). C, mGluR7 is differentially expressed at two synapses of the same terminal (T). The presynaptic specialization facing a dendrite (D) typical of mGluR1α-positive interneurons is heavily labelled for mGluR7, while the other synapse terminating on a pyramidal cell spine (S) lacks mGluR7 immunolabelling, demonstrating the exquisite selectivity of mGluR7 targeting. From Shigemoto et al. (1997). Data used by kind permission of the authors and reproduced with permission from Nature.
Functional evidence that differential presynaptic mGluR expression endowed pyramidal cell axons with distinct mechanisms of synaptic transmission came from Scanziani et al. (1998). In this study, activation of presynaptic Group III mGluRs decreased EPSC amplitude only at pyramidal neuron axon terminals contacting inhibitory interneurons but not other pyramidal cells, even when recordings were made to show the same presynaptic cell contacting two distinct postsynaptic elements. A differential distribution of other members of the mGluR class of glutamate receptors has also been shown in the mossy fibre axons of dentate gyrus granule cells (Shigemoto et al. 1997) suggesting that targeting of mGluRs to specific axonal terminals may be a common feature of cortical synapses.
In cortical and hippocampal circuits, repetitive activation of afferents either progressively increases (facilitation) or decreases (depression) the amplitudes of excitatory synaptic events. Thus synapses can be ‘facilitating’ or ‘depressing’ depending on whether subsequent excitatory events are potentiated or depressed, respectively. Cells expressing either depressing or facilitating postsynaptic responses are thought to play distinct roles in a given neuronal network. Facilitation may act to summate, bringing second and subsequent postsynaptic responses closer to threshold for the generation of a postsynaptic action potential. Such a mechanism would increase the reliability of transmission at a given synaptic contact. In contrast, depression could weaken the influence of subsequent responses from the same input at higher firing rates. The depression or facilitation of synaptic transmission in response to a train of stimuli appears to be dictated in part by the target neuron. The use of paired recordings from connected neurons in the in vitro hippocampal or cortical slice preparation has generated an extended list of the depressing or facilitating properties of numerous synaptic connections. Consideration of this ever-growing list of synaptic properties suggests that few ‘hard and fast’ rules exist but serves to underscore that each synaptic connection must be considered a unique entity.
One of the first demonstrations that synaptic transmission from presynaptic cortical pyramidal cells was target specific came from pioneering work from the laboratory of Alex Thomson. By studying connected pairs of neocortical pyramidal neurons-pyramidal neurons and pyramidal neurons-inhibitory interneurons throughout all cortical layers, they demonstrated that pyramidal cell connections could demonstrate either paired pulse depression or paired pulse facilitation at connections with other pyramidal cells. In contrast, pyramidal neuron synapses terminating on two classes of interneuron only showed frequency-dependent facilitation (Thomson, 1997). Similarly, in the hippocampus, CA1 pyramidal neuron inputs onto stratum oriens/alveus interneurons showed facilitatory synaptic responses, while CA1 pyramidal inputs onto basket cells and bistratified cells demonstrated synaptic depression (Ali et al. 1998; Ali & Thomson, 1998). Furthermore, Scanziani et al. (1998) demonstrated that short-term transmission between a common CA3 pyramidal neuron and a sequentially recorded CA3 pyramidal neuron and a stratum oriens inhibitory interneuron in organotypic culture was fundamentally different. Unitary EPSCs onto stratum oriens interneurons typically had a greater paired pulse ratio in response to two action potentials than at connections between two pyramidal cells.
Differences not only in short-term, but also in long-term plasticity of synaptic transmission have been observed between two different postsynaptic targets innervated by the same input pathway. Single granule cell axons have specialized terminals including large mossy boutons, small en passant terminals and filopodial extensions of the mossy boutons (Fig. 2a). The interneuron targets of granule cells are preferentially innervated by filopodial extensions and small en passant terminals. In contrast, pyramidal neurons are innervated only by the more complex, large diameter mossy boutons and are spared from innervation by the smaller bouton types (Acsády et al. 1998). These anatomical specializations were also shown to be functionally distinct. Mossy fibre axons of the dentate granule cells exhibit a well described form of NMDA-independent, cAMP-dependent long-term potentiation (LTP) at synapses onto CA3 pyramidal cells. Maccaferri et al. (1998) demonstrated that an induction protocol that induced LTP at mossy fibre synapses onto CA3 pyramidal cells, induced either long-term depression (LTD) or no change in synaptic strength when the postsynaptic targets were interneurons (Fig. 2B–D). Similarly, elevation of cAMP levels by forskolin induced LTP at synapses onto pyramidal cells but failed to alter transmission at synapses onto interneurons. These data demonstrate that different mossy fibre terminals are functionally distinct, and that synaptic terminals arising from a common axon do not behave as a single compartment but are specialized depending on their postsynaptic target. The precise mechanisms underlying this lack of presynaptic mossy fibre plasticity at mossy fibre-interneuron synapses are unclear at this time. It is possible that an essential component of the cAMP-dependent cascade is absent from the pre- or postsynaptic terminals of interneurons.
Figure 2. Target-specific expression of mossy fibre long-term potentiation.
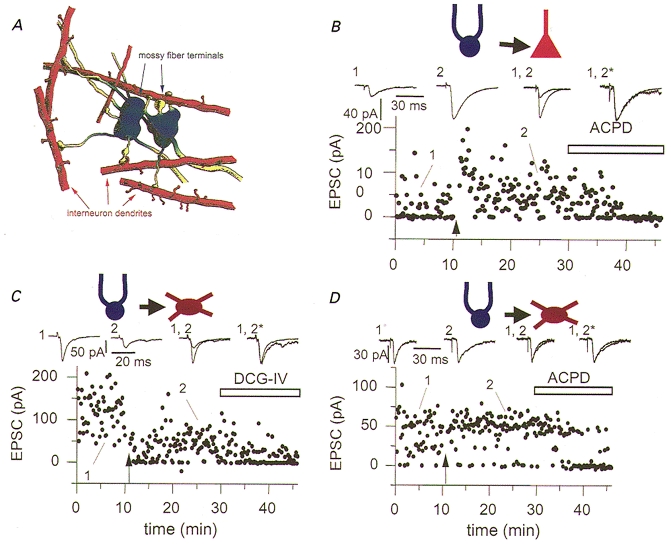
A, filopodial extensions (yellow) of large mossy fibre terminals (blue) are specialized to innervate GABAergic cells. Artistic impression of two mossy fibre terminals each with four filopodial extensions. All filopodia contact the dendrites or spines of interneurons. B-D, differential synaptic plasticity at mossy fibre-pyramidal cell (B) and mossy fibre-interneuron synapses (C and D). Synapses made by filopodial extensions onto interneurons lack a cAMP-dependent form of long-term potentiation (LTP, C and D) common to mossy fibre synapses onto CA3 pyramidal neurons (B). B, LTP at synapses onto pyramidal neurons was induced by tetanic stimulation (4 × 100 Hz, 1 s duration, indicated by arrow). Following tetanic stimulation neither short- or long-term potentiation was observed at mossy fibre synapses onto interneurons, instead interneuron long-term depression (iLTD) or no change in synaptic strength was observed. In all experiments activation of Group II metabotropic glutamate receptors (by either ACPD or DCG-IV) blocked synaptic transmission at synapses onto both pyramidal neurons and interneurons confirming their identity as mossy fibres. From Acsady et al. (1998) (A) and Maccaferri et al. (1998) (B–D). Data used by kind permission of the authors and reproduced with permission from Journal of Neuroscience (for A) and Science (copyright 1998 American Association for the Advancement of Science; for B–D).
NMDA-dependent LTP observed between Schaffer collateral axons and CA1 pyramidal neurons is similarly absent at the same synapses onto many inhibitory neurons (Ouardouz & Lacaille, 1995; Maccaferri & McBain, 1995, 1996; McMahon & Kauer, 1997). The lack of NMDA-dependent LTP is thought to result from the absence of calcium-calmodulin kinase II expression in inhibitory interneurons (Liu & Jones, 1996; Sík et al. 1998; see review by McBain et al. (1999) for further discussion).
Taken together, all of the above data strongly indicate that the synaptic properties of axons belonging to the same type of neuron are influenced by the nature of their postsynaptic targets.
The first direct demonstration that transmitter release could be differentially and simultaneously modulated at individual terminals from the same axon came with a technically demanding series of experiments using triple and quadruple recordings of connected neurons from the laboratories of Bert Sakmann (Reyes et al. 1998) and Henry Markram (Markram et al. 1998). By simultaneously recording from one presynaptic neocortical pyramidal cell and two postsynaptic cells, one pyramidal, the other interneuronal, Markram et al. (1998) showed that the very same presynaptic burst evoked a facilitating response at the interneuron synapse and a depressing response at a synapse onto the pyramidal cell (Fig. 3a). In another series of experiments, presynaptic bursts from three pyramidal cells, all converging onto the same interneuron, showed qualitatively similar facilitating and depressing postsynaptic responses (Markram et al. 1998). The time course of facilitation and depression, however, differed for each of these convergent connections, suggesting that different pre- to postsynaptic interactions underlie quantitative differences in synaptic properties.
Figure 3. Differential synaptic signalling via the same axon innervating two distinct targets.
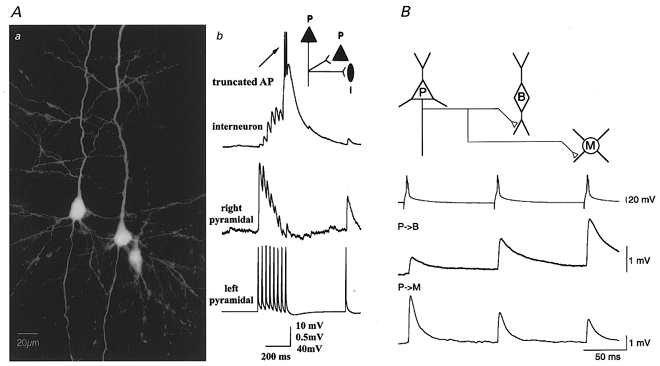
Aa, light microscopic image of three biocytin filled neurons. The pyramidal cell on the left innervated the pyramidal cell on the right and the bipolar interneuron on the right. Ab, single trial responses (30 Hz) to the same action potential train from the pyramidal cell on the left evoked a facilitating response in the interneuron and a depressing response in the pyramidal cell. B, triple recordings reveal differential short-term plasticity in two classes of interneurons innervated by a single cortical layer 2/3 pyramidal neuron. When the pyramidal cell was fired at 10 Hz (upper trace) the amplitude of unitary EPSPs evoked successively in the bitufted cell increased (middle trace), whereas the amplitude of those EPSPs evoked simultaneously in the multipolar cell decreased (lower trace). From Markram et al. (1998) (A) and Reyes et al. (1998) (B). Data used by kind permission of the authors and reproduced with permission from Proceedings of the National Academy of Sciences of the USA (copyright 1998 National Academy of Sciences, USA; for A) and Nature Neuroscience (for B).
Further evidence that the nature of the postsynaptic target alone (i.e. principal neuron versus inhibitory interneuron) did not determine the pattern of postsynaptic activity was demonstrated by Reyes et al. (1998), who demonstrated that synaptic transmission via a single pyramidal cell axon generated different responses in two distinct inhibitory interneuron classes. Simultaneous recording from a layer 2/3 pyramidal cell connected to a bitufted inhibitory cell and a multipolar inhibitory cell demonstrated that the same train of presynaptic action potentials resulted in facilitation at synapses onto bitufted cells, while synaptic depression was observed at multipolar cells (Figure 3b).
Evidence that depressing or facilitating synapses may reflect a developmental process was established by Bolshakov & Siegelbaum (1995) who demonstrated that the probability of release and the incidence of long-term potentiation were developmentally regulated. In young animals (< 8 days postnatal) the probability of release at Schaffer collaterals synapses onto CA1 hippocampal neurons is initially high (∼0.9), and at this stage normally occludes the expression of LTP. In contrast by 2–3 weeks postnatal the Pr fell to ∼0.5 and LTP was associated with an increase in Pr from a single synaptic site.
Further evidence for a developmental process underlying transmitter release was shown in recordings made between layers 2/3 and 5 of the rat sensorimotor cortex (Reyes & Sakmann, 1999). In the immature cortex (postnatal day (P)14), EPSPs evoked in all connected cells, whether between layers or within layers, showed depression in response to a short train of stimuli. The degree of depression was determined by the identity of the presynaptic neuron; responses evoked by stimulation of layer 5 neurons depressed significantly more than those evoked from layer 2/3 neurons. In the mature cortex (P28), however, the EPSPs evoked in connected cells were facilitated to a comparable degree, regardless of the layer in which the pre- or postsynaptic cell was located.
Whether a synapse facilitates or depresses in response to a short train of presynaptic stimuli is based on changes in the probability of evoked transmitter release (for review see Zucker, 1989, 1999). Analysis of the frequency-dependent changes in synaptic transmission at various cortical synapses has revealed complex heterogeneity of the synaptic transfer function. Markram et al. (1998) have suggested that such heterogeneity allows multiple synaptic representations of the same presynaptic action potential train, which depends in part on the precise synaptic parameters and the history of presynaptic action potential activity. Target-specific facilitation or depression could, in part, be due to the combined processes of Ca2+ dynamics in the distinct presynaptic terminals (i.e. the type of voltage-gated calcium channels expressed, mechanisms of Ca2+ buffering, differences in the Ca2+ domains in the active zone), magnitude and time course of presynaptic action potentials, differential expression of presynaptic voltage-gated potassium channels, the depletability of the readily releasable neurotransmitter pool, presynaptic autoreceptors, neurotransmitter concentration versus time profile, degree of transmitter spillover, density of neurotransmitter uptake transporters, geometry of synaptic cleft. Postsynaptic mechanisms such as receptor desensitization may also play a role (however see Markram et al. 1998; Reyes & Sakmann, 1999). Whether any or all of these mechanisms are involved will need to be determined one synapse at a time.
II. Afferent-specific innervation of molecularly distinct glutamate receptors
A large number of both voltage- and ligand-gated ion channels are polarized across the dendritic-somatic-axonal axis. The mechanisms underlying this polarization across neurons are poorly understood; however, distribution of membrane proteins is maintained by specialized domains situated at the axon initial segment of neurons, which prevents diffusion of proteins between axon and the somato-dendritic surface (Winckler et al. 1999). However, segregation of receptors is not only restricted to the axonal or somato-dendritic compartment. A single cell has several hundred dendritic branches that receive inputs from numerous distinct excitatory and inhibitory afferent pathways. Since Gray's pioneer work (1959) on the ultrastructure of the nervous system we know that two types of synapse exist, type I, or asymmetric, and type II, or symmetric. Later, type I synapses were shown to be excitatory, while type II were inhibitory in nature (Andersen et al. 1963; Eccles, 1964). At the neuromuscular junction, acetylcholine receptors are localized precisely on the opposite side of the presynaptic terminal (Fertuck & Salpeter, 1976). In the central nervous system, however, although many receptors are concentrated at synapses, in many cases they are also sparsely distributed at the non-synaptic membrane surfaces, i.e. extrasynaptic receptors (Richards et al. 1987; De Blas et al. 1988; Somogyi et al. 1989; Soltesz et al. 1990; Martin et al. 1993; Molnar et al. 1993; Baude et al. 1993, 1994; Nusser et al. 1995, 1998). The cellular and subcellular distribution of glutamate (Petralia & Wenthold, 1992; Martin et al. 1993; Molnar et al. 1993; Baude et al. 1993, 1994) and GABA receptors (Richards et al. 1987; De Blas et al. 1988; Somogyi et al. 1989; Soltesz et al. 1990; Nusser et al. 1995, 1996a, 1998) in fixed animal tissue showed that they are predominantly expressed in only one type of synapse; glutamate receptors in asymmetric synapses and GABA receptors in symmetric synapses (but see Nusser et al. 1996b who show α6 GABA subunits in glutamatergic synapses).
It is well established that many native ligand-gated receptors comprise several distinct subunits and that the subunit composition of a receptor can greatly influence the physiological parameters associated with that receptor. Since synaptic specializations exist to facilitate the precise transmission of signals between two adjacent neurons, the selective targeting of distinct receptor populations to synapses associated with a given set of afferents would be a powerful mechanism to increase the computational power of any given neuron. The question of whether single central neurons express receptors with different subunit composition on their surface, or are ‘committed’ to a certain subunit composition has only recently begun to be explored. Furthermore if a single neuron can express receptors with different molecular composition how are they distributed on the membrane surface? Is expression uniform or are certain receptor types facing different afferent inputs? The answers to these questions are of fundamental importance for our understanding of the computational power of a single neuron within a given network.
The first evidence that presynaptic innervation could influence synaptic receptor expression was provided by Sakmann & Brenner (Sakmann & Brenner, 1978; Brenner & Sakmann, 1983) working at the neuromuscular junction. During synapse development a neurally controlled conversion of ACh receptor channels occurs about 2–3 weeks after establishment of the nerve-muscle contact. Embryonic ACh synapses between nerve and muscle comprise α2βγδ subunits. Shortly after innervation, ε subunit mRNA expression is induced locally via a signal restricted to the end-plate region and dependent on the presence of the nerve only during a short period of early neuromuscular contact (Brenner et al. 1990). A concomitant reduction in the expression of the γ subunit is observed. Mature ACh receptors are then composed of α2βεδ subunits and are localized precisely on the opposite side of the presynaptic terminal (Fertuck & Salpeter, 1976). This developmental regulation of a synaptic receptor results in a reduction in the mean open time of ACh receptors, demonstrating that a change in gating behaviour accompanies the maturation of ACh receptors.
In frog sympathetic ganglion, B and C cells are innervated by two distinct classes of preganglionic axons (B and C fibres). Transmission at these two synapses differs 2-fold in kinetics, resulting from differences in the mean open time of the nicotinic ACh ion channels underlying each EPSC. By denervating B cells and then allowing them to become innervated solely by preganglionic C fibres, Marshall (1985) demonstrated that B cell EPSCs acquired kinetics normally characteristic of C cells, providing the first evidence that the kinetic properties of postsynaptic channels can be determined by the particular class of axon innervating them.
Examples of afferent-specific expression of molecularly distinct receptors in central neurons include expression of δ2 glutamate receptors in cerebellar Purkinje cells, the differential distribution of AMPA receptors in hippocampal interneurons and neurons of the cochlear nucleus, and compartmentalization of GABAA receptors on single hippocampal pyramidal neurons.
At the anatomical level, differential distribution of most glutamate receptor subunits has been described at the electron microscopic level. The so-called ‘orphan’ glutamate receptor subunit δ2 is prominently expressed in the cerebellum; however, δ2 subunits do not form functional channels alone nor have they been shown to modify the properties of other glutamate receptor subunit combinations. However, knockout of the δ2 subunit leads to a loss of activity-related depression of parallel fibre-Purkinje cell synapses highlighting a poorly understood but important role for the δ2 subunit (Kashiwabuchi et al. 1995). Electron microscopic investigation of δ2 glutamate receptor distribution demonstrated that in cerebellar Purkinje cells they are exclusively expressed at postsynaptic specializations of parallel fibre synapses and are absent from climbing fibre-Purkinje cell synapses (Landsend et al. 1997; Zhao et al. 1997). Even when both climbing fibre and parallel fibre synapses were identified on the same dendritic tree, δ2 expression was associated only with parallel fibre synapses. Similarly, parallel fibre-inhibitory interneuron synapses also lacked δ2 expression. These data would suggest that the expression pattern of δ2 glutamate receptors is controlled by the combined identity of both the pre- and postsynaptic targets.
The first functional demonstration of molecularly distinct AMPA receptor synapses on the same cell was demonstrated at mossy fibre versus CA3 commisural afferent inputs onto single interneurons of the CA3 hippocampus. Tóth & McBain (1998) demonstrated that mossy fibre synaptic inputs were blocked by philanthotoxin-433 (PhTx), a polyamine toxin that blocks GluR2-lacking AMPA receptors i.e. calcium-permeable AMPA receptors (Fig. 4) (Herlitze et al. 1993; Brackley et al. 1993; Isa et al. 1996; Washburn & Dingledine, 1996; Iino et al. 1996; Bähring & Mayer, 1998). In contrast synaptic transmission at CA3 pyramidal cell collaterals onto the same cell occurs through receptors composed of Ca2+-impermeable AMPA receptors. These data clearly demonstrate that afferent-specific innervation is also functionally distinct. At this time no data exist concerning the precise distribution of Ca2+-permeable and Ca2+-impermeable AMPA receptors on single hippocampal interneurons. It is unclear whether the two types of synaptic input innervate functionally distinct compartments of the interneurons or whether they are homogeneously distributed over the dendritic tree. The similarity in the rise times and decay time constants of the two EPSC populations, however, suggests overlapping electrotonic locations (authors’ unpublished observations).
Figure 4. Single hippocampal inhibitory interneurons express both calcium-permeable and calcium-impermeable AMPA receptors.
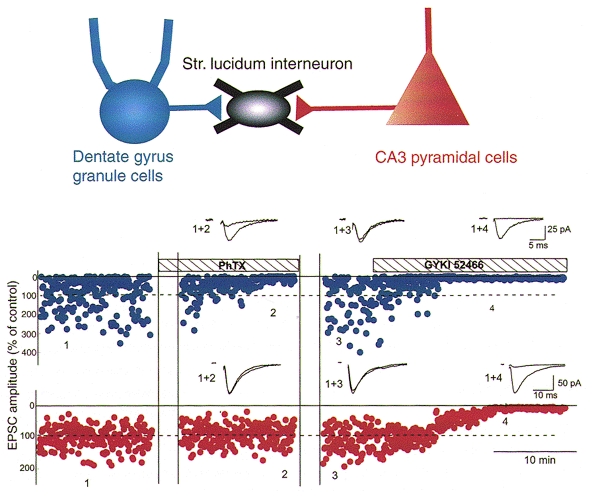
Upper panel, schematic diagram illustrating that a single stratum lucidum interneuron of the CA3 hippocampus receives afferent input from two distinct sources; the mossy fibre axons of dentate gyrus granule cells and the collaterals of CA3 pyramidal neurons. Lower traces, stimulating electrodes were positioned within the stratum radiatum and the dentate gyrus granule cell layer to stimulate either the CA3 pyramidal cell collaterals or the mossy fibres, respectively. A single experiment demonstrates that EPSCs evoked by alternate stimulation of mossy fibres (blue points) and CA3 collateral inputs (red points) can be detected on the same cell. On application of the polyamine toxin PhTX only the EPSC evoked by mossy fibre stimulation was blocked (traces 1 + 2); in contrast the CA3 recurrent collateral input remained unchanged. A 10 min portion of the initial exposure to PhTX was removed for clarity (first set of vertical lines). Following a 30 min washout period (second set of vertical lines) the mossy fibre EPSC recovered to control amplitude (trace 3). At the end of the experiment the selective AMPA receptor antagonist GYKI-52466 was added to the recording medium. Both mossy fibre- and CA3 collateral-evoked EPSCs were blocked (trace 4) confirming that they were generated by AMPA receptor activation. From Tóth & McBain (1998). Data reproduced with permission from Nature Neuroscience.
Evidence that particular glutamate receptor subunit expression can be polarized across the dendritic tree comes from analysis of different synaptic inputs to neurons of the dorsal cochlear nucleus. Fusiform cells of the dorsal cochlear nucleus receive excitatory input onto their apical dendrites from granule cell parallel fibres. Cochlear auditory nerve terminals synapse onto their basal dendrites. Several types of glutamate receptor subunits are found at both populations of synapses. However, the GluR4 AMPA receptor subunit, and the metabotropic receptor, mGluR1α, are both selectively targeted to basal dendrites and are associated only with synapses formed by the auditory nerve (Hunter et al. 1993; Rubio & Wenthold, 1997; Wang, 1998). AMPA receptors containing the GluR4 flop subunit gate rapidly (Mosbacher et al. 1994), which may be a receptor property essential in encoding timing information for proper sound localization (Raman et al. 1994; Trussell et al. 1994). Recently Gardner et al. (1999) demonstrated functional correlates of the molecular differences associated with inputs onto cochlear nuclear neurons. Miniature EPSCs arising from auditory nerve inputs possessed rapid kinetics and were blocked by PhTx. In contrast, mEPSCs associated with parallel fibres were resistant to PhTx and possessed slower kinetics. These data suggest that in addition to GluR4 segregation, GluR2, the AMPA receptor subunit responsible for conferring the presence or absence of calcium permeability, is also differentially targeted across the cochlear neuron somato-dendritic axis (however see Rubio & Wenthold, 1997).
A highly divergent population of inhibitory interneurons of the hippocampus and cortex possess axons that ramify extensively across different domains of both pyramidal neurons and inhibitory interneurons to strongly influence their activity (for review see Freund & Buzsaki, 1996). These cells, which use GABA as their neurotransmitter, activate GABAA receptors on their postsynaptic target to mediate fast inhibitory synaptic transmission. Evidence that GABAA receptors may also be segregated within individual neurons, similar to glutamate receptors, was provided by the laboratory of Pearce (1993), who demonstrated the presence of two anatomically segregated and pharmacologically distinct GABAA-mediated inhibitory postsynaptic currents (IPSCs) on single CA1 pyramidal neurons. IPSCs arising at the soma and presumably mediated by axo-axonic cells have a rapid time constant of decay (3-8 ms) and are blocked by furosemide (frusemide). In contrast an IPSC with a distal generation site possessed extremely slow kinetics (30-70 ms decay time constant) and was resistant to block by furosemide (Pearce, 1993; Banks et al. 1998). At this time the precise anatomical identity of the inhibitory interneurons generating these inhibitory synaptic events is unclear. Given the large number of anatomically distinct inhibitory interneurons that exist within the hippocampal formation it is likely that at least two inhibitory circuits exist to innervate pharmacologically distinct GABAA receptors on single pyramidal neurons.
How then do different inputs influence postsynaptic receptor distribution? As our understanding of the molecular machinery at synapses evolves it is apparent that multiple mechanisms are involved in the clustering of specific neurotransmitter receptor subunits at specific synapses. If one considers AMPA receptors alone, most mRNA for AMPA receptor subunits is largely confined to the cell soma (Craig et al. 1993; Benson, 1997) suggesting that the synaptic targeting of AMPA receptors requires both protein transport and local stabilization for precise positioning of each receptor form. Discussion of the machinery involved in synaptic targeting is out of the scope of the present review and the reader is referred to several excellent reviews for further reading (Sheng & Kim, 1996; Kennedy, 1997, 1998; O'Brien et al. 1998; Hsueh & Sheng, 1998). It is worthwhile pointing out that while these previous investigations have considered questions related to targeting and receptor sorting they provide little insight into how specific inputs control receptor distribution in a single cell.
Conclusions
The issues discussed in this review illustrate our increased understanding of the complexities of mechanisms of synaptic transmission via either a single axon diverging onto two distinct postsynaptic targets or two distinct afferent projections converging onto a common postsynaptic cell. Our understanding of how pre- and postsynaptic specializations target specific cellular elements essential for precise encoding of information at individual synapses is in its infancy. The identities of proteins known to bind to glutamate receptors are only beginning to be described. These proteins are important not only for receptor targeting and clustering but also for modulation of receptor activity and activation of appropriate signalling cascades. How these proteins are targeted at specific sites across the myriad spines and pre- and postsynaptic specializations remains a challenge for neurobiologists well into the next millenium.
Acknowledgments
The authors would like to thank Dr Josh Lawrence for his helpful comments on the manuscript.
References
- Acsády L, Kamondi A, Sík A, Freund T, Buzsáki G. GABAergic cells are the major postsynaptic targets of mossy fibers in the rat hippocampus. Journal of Neuroscience. 1998;18:3386–3403. doi: 10.1523/JNEUROSCI.18-09-03386.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali AB, Deuchars J, Pawelzik H, Thomson AM. CA1 pyramidal to basket and bistratified cell EPSCs: dual intracellular recordings in rat hippocampal slices. The Journal of Physiology. 1998;507:201–217. doi: 10.1111/j.1469-7793.1998.201bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali AB, Thomson AM. Facilitating pyramid to horizontal oriens-alveus interneurone inputs: dual intracellular recordings in slices of rat hippocampus. The Journal of Physiology. 1998;507:185–199. doi: 10.1111/j.1469-7793.1998.185bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen P, Eccles JC, Loyning Y. Recurrent inhibition in the hippocampus with identification of the inhibitory cell and its synapse. Nature. 1963;198:540–542. doi: 10.1038/198540a0. [DOI] [PubMed] [Google Scholar]
- Atwood HL, Bitter GD. Matching of excitatory and inhibitory inputs to crustacean muscle fibers. Journal of Neurophysiology. 1971;34:157–170. doi: 10.1152/jn.1971.34.1.157. [DOI] [PubMed] [Google Scholar]
- Bähring R, Mayer ML. An analysis of philanthotoxin block for recombinant rat GluR6(Q) glutamate receptor channels. The Journal of Physiology. 1998;509:635–650. doi: 10.1111/j.1469-7793.1998.635bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks MI, Tong-Bin L, Pearce RA. The synaptic basis of GABAAslow. Journal of Neuroscience. 1998;18:1305–1317. doi: 10.1523/JNEUROSCI.18-04-01305.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baude A, Molnar E, Latawiec D, McIlhinney RA, Somogyi P. Cellular and subcellular localization of the GluR1 subunit of the AMPA type excitatory amino acid receptor in the rat cerebellum. Journal of Neuroscience. 1994;14:2830–2843. doi: 10.1523/JNEUROSCI.14-05-02830.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baude A, Nusser Z, Roberts JDB, Mulvihill E, McIlhinney RAJ, Somogyi P. The metabotropic glutamate receptor (mGluR1α) is concentrated at perisynaptic membrane of neuronal subpopulations as detected by immunogold reaction. Neuron. 1993;11:771–787. doi: 10.1016/0896-6273(93)90086-7. [DOI] [PubMed] [Google Scholar]
- Benson DL. Dendritic compartmentalization of NMDA receptor mRNA in cultured hippocampal neurons. NeuroReport. 1997;8:823–828. doi: 10.1097/00001756-199703030-00004. [DOI] [PubMed] [Google Scholar]
- Bolshakov VY, Siegelbaum SA. Regulation of hippocampal transmitter release during development and long term potentiation. Science. 1995;269:1730–1734. doi: 10.1126/science.7569903. [DOI] [PubMed] [Google Scholar]
- Brackley PT, Bell DR, Choi SK, Nakanishi K, Usherwood PN. Selective antagonism of native and cloned kainate and NMDA receptors by polyamine-containing toxins. Journal of Pharmacology and Experimental Therapeutics. 1993;266:1573–1580. [PubMed] [Google Scholar]
- Brenner HR, Sakmann B. Neurotrophic control of channel properties at neuromuscular synapses of the rat muscle. The Journal of Physiology. 1983;337:159–171. doi: 10.1113/jphysiol.1983.sp014617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner HR, Witzemann V, Sakmann B. Imprinting of acetylcholine receptor messenger RNA accumulation in mammalian neuromuscular synapses. Nature. 1990;344:544–547. doi: 10.1038/344544a0. [DOI] [PubMed] [Google Scholar]
- Craig AM, Blackstone CD, Huganir RL, Banker G. The distribution of glutamate receptors in cultured rat hippocampal neurons: postsynaptic clustering of AMPA-selective subunits. Neuron. 1993;10:1055–1068. doi: 10.1016/0896-6273(93)90054-u. [DOI] [PubMed] [Google Scholar]
- Craig AM, Blackstone CD, Huganir R, Banker G. Selective clustering of glutamate and γ-aminobutyric acid receptors opposite terminals releasing the corresponding neurotransmitters. Proceedings of the National Academy of Sciences of the USA. 1994;91:12373–12377. doi: 10.1073/pnas.91.26.12373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis GW, Murphey RK. A role for postsynaptic neurons in determining presynaptic release properties in the cricket CNS: Evidence for retrograde control of facilitation. Journal of Neuroscience. 1993;13:3827–3838. doi: 10.1523/JNEUROSCI.13-09-03827.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Blas AL, Vitorica J, Friedrich P. Localization of the GABAA receptor in the rat brain with a monoclonal antibody to the 57,000 Mr peptide of the GABAA receptor/benzodiazepine receptor/ Cl− channel complex. Journal of Neuroscience. 1988;8:602–614. doi: 10.1523/JNEUROSCI.08-02-00602.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JC. The Physiology of Synapses. Berlin: Springer-Verlag; 1964. [Google Scholar]
- Fertuck HC, Salpeter MM. Quantitation of junctional and extrajunctional acetylcholine receptors by electron microscope autoradiography after 125I-alpha-bungarotoxin binding at mouse neuromuscular junction. Journal of Cell Biology. 1976;69:144–158. doi: 10.1083/jcb.69.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank E. Matching of facilitation at the neuromuscular junction of the lobster: a possible case for influence of muscle nerve. The Journal of Physiology. 1973;233:635–658. doi: 10.1113/jphysiol.1973.sp010327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF, Buzsaki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Gardner D. Presynaptic transmitter release is specified by postsynaptic neurons of Aplysia buccal ganglia. Journal of Neurophysiology. 1991;66:2150–2154. doi: 10.1152/jn.1991.66.6.2150. [DOI] [PubMed] [Google Scholar]
- Gardner SM, Trussell LO, Oertel D. Time course and permeation of synaptic AMPA receptors in cochlear nuclear neurons correlate with input. Journal of Neuroscience. 1999;19:8721–8729. doi: 10.1523/JNEUROSCI.19-20-08721.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golgi C. Recherches sur I'histologie des centres nerveux. Archives Italiennes de Biologie. 1884;4:92–123. [Google Scholar]
- Gray EG. Axo-somatic and axo-dendritic synapses of the cerebral cortex: an electron microscope study. Journal of Anatomy. 1959;93:420–433. [PMC free article] [PubMed] [Google Scholar]
- Herlitze S, Raditsch M, Ruppersberg JP, Jahn W, Monyer H, Schoepfer R, Witzemann V. Argiotoxin detects molecular differences in AMPA receptor channels. Neuron. 1993;10:1131–1140. doi: 10.1016/0896-6273(93)90061-u. [DOI] [PubMed] [Google Scholar]
- Hsueh YP, Sheng M. Anchoring of glutamate receptors at the synapse. Progress in Brain Research. 1998;116:123–131. doi: 10.1016/s0079-6123(08)60434-3. [DOI] [PubMed] [Google Scholar]
- Hunter C, Petralia RS, Vu T, Wenthold RJ. Expression of AMPA-selective glutamate receptor subunits in morphologically defined neurons of the mammalian cochlear nucleus. Journal of Neuroscience. 1993;13:1932–1946. doi: 10.1523/JNEUROSCI.13-05-01932.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino M, Koike M, Isa T, Ozawa S. Voltage-dependent blockage of Ca2+-permeable AMPA receptors by joro spider toxin in cultured rat hippocampal neurones. The Journal of Physiology. 1996;496:431–437. doi: 10.1113/jphysiol.1996.sp021696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isa T, Iino M, Ozawa S. Spermine blocks synaptic transmission mediated by Ca2+-permeable AMPA receptors. NeuroReport. 1996;29:689–692. doi: 10.1097/00001756-199602290-00002. [DOI] [PubMed] [Google Scholar]
- Kashiwabuchi N, Ikeda K, Araki K, Hirano T, Shibuki K, Takayama C, Inoue Y, Kutsuwada T, Yagi T, Kang Y, Aizawa S, Mishina M. Impairment of motor coordination, Purkinje cell synapse formation, and cerebellar long-term depression in GluR delta 2 mutant mice. Cell. 1995;81:245–252. doi: 10.1016/0092-8674(95)90334-8. [DOI] [PubMed] [Google Scholar]
- Katz PS, Kirk MD, Govind CK. Facilitation and depression at different branches of the same motor axon: Evidence for presynaptic differences in release. Journal of Neuroscience. 1993;13:3075–3089. doi: 10.1523/JNEUROSCI.13-07-03075.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy MB. The postsynaptic density at glutamatergic synapses. Trends in Neurosciences. 1997;20:264–268. doi: 10.1016/s0166-2236(96)01033-8. [DOI] [PubMed] [Google Scholar]
- Kennedy MB. Signal transduction molecules at the glutamatergic postsynaptic membrane. Brain Research Reviews. 1998;26:243–257. doi: 10.1016/s0165-0173(97)00043-x. [DOI] [PubMed] [Google Scholar]
- Landsend AS, Amiry-Moghaddam M, Matsubara A, Bergensen L, Usami S-I, Wenthold RJ, Ottersen OP. Differential localization of δ glutamate receptors in the rat cerebellum: Coexpression with AMPA receptors in parallel fiber-spine synapses and absence from climbing fiber-spine synapses. Journal of Neuroscience. 1997;17:834–842. doi: 10.1523/JNEUROSCI.17-02-00834.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent G, Sivaramakrishnan A. Single local interneurons in the locust make central synapses with different properties of transmitter release on distinct postsynaptic neurons. Journal of Neuroscience. 1992;12:2370–2380. doi: 10.1523/JNEUROSCI.12-06-02370.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XB, Jones EG. Localization of alpha type II calcium calmodulin-dependent protein kinase at glutamatergic but not gamma-aminobutyric acid (GABAergic) synapses in thalamus and cerebral cortex. Proceedings of the National Academy of Sciences of the USA. 1996;93:7332–7336. doi: 10.1073/pnas.93.14.7332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorente de Nó R. Studies on the structure of the cerebral cortex. Journal fur Psychologie und Neurologie. 1934;46:113–177. [Google Scholar]
- McBain CJ, Freund TF, Mody I. Glutamatergic synapses onto hippocampal interneurons: precision timing without lasting plasticity. Trends in Neurosciences. 1999;22:228–235. doi: 10.1016/s0166-2236(98)01347-2. [DOI] [PubMed] [Google Scholar]
- Maccaferri G, McBain CJ. Passive propagation of LTD to stratum oriens-alveus inhibitory neurons modulates the temporoammonic input to the hippocampal CA1 region. Neuron. 1995;15:137–145. doi: 10.1016/0896-6273(95)90071-3. [DOI] [PubMed] [Google Scholar]
- Maccaferri G, McBain CJ. Long-term potentiation in distinct subtypes of hippocampal nonpyramidal neurons. Journal of Neuroscience. 1996;16:5334–5343. doi: 10.1523/JNEUROSCI.16-17-05334.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccaferri G, Tóth K, McBain CJ. Target-specific expression of presynaptic mossy fiber plasticity. Science. 1998;279:1368–1370. doi: 10.1126/science.279.5355.1368. [DOI] [PubMed] [Google Scholar]
- McMahon LL, Kauer JA. Hippocampal interneurons express a novel form of synaptic plasticity. Neuron. 1997;18:295–305. doi: 10.1016/s0896-6273(00)80269-x. [DOI] [PubMed] [Google Scholar]
- Markram H, Wang Y, Tsodyks M. Differential signaling via the same axon of neocortical pyramidal neurons. Proceedings of the National Academy of Sciences of the USA. 1998;95:5323–5328. doi: 10.1073/pnas.95.9.5323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall LM. Presynaptic control of sympathetic channel kinetics in sympathetic neurones. Nature. 1985;317:621–623. doi: 10.1038/317621a0. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Blackstone CD, Levey AI, Huganir RL, Price DL. AMPA glutamate receptor subunits are differentially distributed in rat brain. Neuroscience. 1993;53:327–358. doi: 10.1016/0306-4522(93)90199-p. [DOI] [PubMed] [Google Scholar]
- Molnar E, Baude A, Richmond SA, Patel PB, Somogyi P, McIlhinney RAJ. Biochemical and immunocytochemical characterization of antipeptide antibodies to a cloned GluR1 glutamate receptor subunit: cellular and subcellular distribution in the rat forebrain. Neuroscience. 1993;53:307–326. doi: 10.1016/0306-4522(93)90198-o. [DOI] [PubMed] [Google Scholar]
- Mosbacher J, Schoepfer R, Monyer H, Burnashev N, Seeburg PH, Ruppersberg JP. A molecular determinant for submillisecond desensitization in glutamate receptors. Science. 1994;266:1059–1062. doi: 10.1126/science.7973663. [DOI] [PubMed] [Google Scholar]
- Muller KJ, Nicholls JG. Different properties of synapses between a single sensory neurone and two different motor cells in the leech C.N.S. The Journal of Physiology. 1974;238:357–369. doi: 10.1113/jphysiol.1974.sp010529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusser Z, Roberts JDB, Baude A, Richards JG, Somogyi P. Relative densities of synaptic and extrasynaptic GABAA receptors on cerebellar granule cells as determined by a quantitative immunogold method. Journal of Neuroscience. 1995;15:2948–2960. doi: 10.1523/JNEUROSCI.15-04-02948.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Benke D, Fritschy J-M, Somogyi P. Differential synaptic localization of two major γ-aminobutyric acid type A receptor α subunits on hippocampal pyramidal cells. Proceedings of the National Academy of Sciences of the USA. 1996a;93:11939–11944. doi: 10.1073/pnas.93.21.11939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Somogyi P. Segregation of different GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. Journal of Neuroscience. 1998;18:1693–1703. doi: 10.1523/JNEUROSCI.18-05-01693.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Stephenson FA, Somogyi P. The alpha 6 subunit of the GABAA receptor is concentrated in both inhibitory and excitatory synapses on cerebellar granule cells. Journal of Neuroscience. 1996b;16:103–114. doi: 10.1523/JNEUROSCI.16-01-00103.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien RJ, Lau LF, Huganir RL. Molecular mechanism of glutamate receptor clustering at excitatory synapses. Current Opinion in Neurobiology. 1998;8:364–369. doi: 10.1016/s0959-4388(98)80062-7. [DOI] [PubMed] [Google Scholar]
- Ouardouz M, Lacaille JC. Mechanism of selective long-term potentiation of exctitatory synapses in stratum oriens/alveus interneurons of rat hippocampal slices. Journal of Neurophysiology. 1995;73:810–819. doi: 10.1152/jn.1995.73.2.810. [DOI] [PubMed] [Google Scholar]
- Parnas I. Differential block at high frequency of branches of a single axon innervating two muscles. Journal of Neurophysiology. 1972;35:903–914. doi: 10.1152/jn.1972.35.6.903. [DOI] [PubMed] [Google Scholar]
- Pearce RA. Physiological evidence for two distinct GABAA responses in rat hippocampus. Neuron. 1993;10:189–200. doi: 10.1016/0896-6273(93)90310-n. [DOI] [PubMed] [Google Scholar]
- Petralia RS, Wenthold RJ. Light and electron immunocytochemical localization of AMPA-selective glutamate receptors in the rat brain. Journal of Comparative Neurology. 1992;318:329–354. doi: 10.1002/cne.903180309. [DOI] [PubMed] [Google Scholar]
- Pritchett DR, Luddens H, Seeburg PH. Type I and Type II GABAA-benzodiazepine receptors produced in transfected cells. Science. 1989;245:1389–1392. doi: 10.1126/science.2551039. [DOI] [PubMed] [Google Scholar]
- Raman IM, Zhang S, Trussel LO. Pathway-specific variants of AMPA receptors and their contribution to neural signaling. Journal of Neuroscience. 1994;14:4998–5010. doi: 10.1523/JNEUROSCI.14-08-04998.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramón y Cajal S. Texture del Sistema Nervioso del Hombre y de los Vertebrados. Madrid: Moya; 1898. [Google Scholar]
- Reyes A, Lujan R, Rozov A, Burnashev N, Somogyi P, Sakmann B. Target-cell-specific facilitation and depression in neocortical circuits. Nature Neuroscience. 1998;1:279–285. doi: 10.1038/1092. [DOI] [PubMed] [Google Scholar]
- Reyes A, Sakmann B. Developmental switch in the short-term modification of unitary EPSPs evoked in layer 2/3 and layer 5 pyramidal neurons of rat neocortex. Journal of Neuroscience. 1999;19:3827–3835. doi: 10.1523/JNEUROSCI.19-10-03827.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JG, Scoch P, Haring P, Takacs B, Mohler H. Resolving GABAA/benzodiazepine receptors: cellular and subcellular localization in the CNS with monoclonal antibodies. Journal of Neuroscience. 1987;7:1866–1886. doi: 10.1523/JNEUROSCI.07-06-01866.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenmund C, Clements JD, Westbrook GL. Nonuniform probability of glutamate release at a hippocampal synapse. Science. 1993;262:754–757. doi: 10.1126/science.7901909. [DOI] [PubMed] [Google Scholar]
- Rubio ME, Wenthold RJ. Glutamate receptors are selectively targeted to postsynaptic sites in neurons. Neuron. 1997;18:939–950. doi: 10.1016/s0896-6273(00)80333-5. [DOI] [PubMed] [Google Scholar]
- Sakmann B, Brenner HR. Change in synaptic channel gating during neuromuscular development. Nature. 1978;276:401–402. doi: 10.1038/276401a0. [DOI] [PubMed] [Google Scholar]
- Scanziani M, Gähwiler BH, Charpak S. Target cell-specific modulation of transmitter release at terminals from a single axon. Proceedings of the National Academy of Sciences of the USA. 1998;95:12004–12009. doi: 10.1073/pnas.95.20.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M, Kim E. Ion channel associated proteins. Current Opinion in Neurobiology. 1996;6:602–608. doi: 10.1016/s0959-4388(96)80091-2. [DOI] [PubMed] [Google Scholar]
- Shigemoto R, Kinoshita A, Wada E, Nomura S, Ohishi H, Takada M, Flor PJ, Neki A, Abe T, Nakanishi S, Mizuno N. Differential presynaptic localization of metabotropic glutamate receptor subtypes in the rat hippocampus. Journal of Neuroscience. 1997;17:7503–7522. doi: 10.1523/JNEUROSCI.17-19-07503.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigemoto R, Kulik A, Roberts JDB, Ohishi H, Nusser Z, Kaneko T, Somogyi P. Target-cell-specific concentration of metabotropic glutamate receptors in the presynaptic zone. Nature. 1996;381:523–525. doi: 10.1038/381523a0. [DOI] [PubMed] [Google Scholar]
- Sík A, Freund TF. The absence of a major Ca2+-signaling pathway in GABAergic neurons of the hippocampus. Proceedings of the National Academy of Sciences of the USA. 1998;95:3245–3250. doi: 10.1073/pnas.95.6.3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltesz I, Roberts JDB, Takagi H, Richards JG, Mohler H, Somogyi P. Synaptic and nonsynaptic localization of benzodiazepine/GABAA receptor/Cl− channel complex using monoclonal antibodies in the dorsal lateral geniculate of the cat. European Journal of Neuroscience. 1990;2:414–429. doi: 10.1111/j.1460-9568.1990.tb00434.x. [DOI] [PubMed] [Google Scholar]
- Somogyi P, Takagi H, Richards JG, Mohler H. Subcellular localization of benzodiazepine/GABAA receptors in the cerebellum of rat, cat and monkey using monoclonal antibodies. Journal of Neuroscience. 1989;9:2197–2209. doi: 10.1523/JNEUROSCI.09-06-02197.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson AM. Activity-dependent properties of synaptic transmission at two classes of connections made by rat neocortical pyramidal axons in vitro. The Journal of Physiology. 1997;502:131–147. doi: 10.1111/j.1469-7793.1997.131bl.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tóth K, McBain CJ. Afferent-specific innervation of two distinct AMPA receptor subtypes on single hippocampal interneurons. Nature Neuroscience. 1998;1:572–578. doi: 10.1038/2807. [DOI] [PubMed] [Google Scholar]
- Trussell LO, Raman IM, Zhang S. AMPA receptors and the rapid synaptic transmission. Seminars in the Neurosciences. 1994;6:71–79. [Google Scholar]
- Wang YX, Wenthold RJ, Ottersen OP, Petralia RS. Endbulb synapses in the anteroventral cochlear nucleus express a specific subset of AMPA-type glutamate receptor subunits. Journal of Neuroscience. 1998;18:1148–1160. doi: 10.1523/JNEUROSCI.18-03-01148.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washburn MS, Dingledine R. Block of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors by polyamines and polyamine toxins. Journal of Pharmacology and Experimental Therapeutics. 1996;278:669–678. [PubMed] [Google Scholar]
- Winckler B, Forscher P, Mellman I. A diffusion barrier maintains distribution of membrane proteins in polarized neurons. Nature. 1999;397:698–701. doi: 10.1038/17806. [DOI] [PubMed] [Google Scholar]
- Zhao H-M, Wenthold RJ, Wang Y-X, Petralia RS. δ-Glutamate receptors are differentially distributed at parallel and climbing fiber synapses on Purkinje cells. Journal of Neurochemistry. 1997;68:1041–1052. doi: 10.1046/j.1471-4159.1997.68031041.x. [DOI] [PubMed] [Google Scholar]
- Zucker RS. Short-term synaptic plasticity. Annual Review of Neuroscience. 1989;12:13–31. doi: 10.1146/annurev.ne.12.030189.000305. [DOI] [PubMed] [Google Scholar]
- Zucker RS. Calcium- and activity-dependent synaptic plasticity. Current Opinion in Neurobiology. 1999;9:305–313. doi: 10.1016/s0959-4388(99)80045-2. [DOI] [PubMed] [Google Scholar]


