Abstract
Pacemaker cells, known as interstitial cells of Cajal (ICC), generate electrical rhythmicity in the gastrointestinal tract. Pacemaker currents in ICC result from the activation of a voltage-independent, non-selective cation conductance, but the timing mechanism responsible for periodic activation of the pacemaker current is unknown.
Previous studies suggest that pacemaking in ICC is dependent upon metabolic activity 1y1yand1 Ca2+ release from intracellular stores. We tested the hypothesis that mitochondrial Ca2+ handling may underlie the dependence of gastrointestinal pacemaking on oxidative metabolism.
Pacemaker currents occurred spontaneously in cultured ICC and were associated with mitochondrial Ca2+ transients.
Inhibition of the electrochemical gradient across the inner mitochondrial membrane blocked Ca2+ uptake and pacemaker currents in cultured ICC and blocked slow wave activity in intact gastrointestinal muscles from mouse, dog and guinea-pig.
Pacemaker currents and rhythmic mitochondrial Ca2+ uptake in ICC were also blocked by inhibitors of IP3-dependent release of Ca2+ from the endoplasmic reticulum and by inhibitors of endoplasmic reticulum Ca2+ reuptake.
Our data suggest that integrated Ca2+ handling by endoplasmic reticulum and mitochondria is a prerequisite of electrical pacemaking in the gastrointestinal tract.
Phasic gastrointestinal (GI) muscles contain pacemaker mechanisms for autorhythmicity that generate electrical slow waves in the absence of external stimuli from nerves, hormones or paracrine substances (e.g. Szurszewski, 1987). It is now recognized that a special group of cells within the tunica muscularis, known as interstitial cells of Cajal (ICC), are responsible for the origination of slow waves (Langton et al. 1989; Ward et al. 1994; Huizinga et al. 1995). Recent studies, using cultured ICC that retain the phenotype of autorhythmicity, have demonstrated that pacemaker currents generated by ICC result primarily from the activation of a non-specific cation conductance (Koh et al. 1998; Thomsen et al. 1998). Preliminary studies suggest that the pacemaker conductance is voltage independent and may conduct Na+ and Ca2+ in physiological ionic gradients (Koh et al. 1998). At present the intracellular signals that regulate the opening of pacemaker current channels are unknown.
The mechanism that initiates slow wave activity has been a subject of investigation for several decades. ICC contain an abundance of mitochondria (e.g. Faussone-Pellegrini, 1985), but the significance of these organelles to pacemaker function has not been determined. Slow waves may be tied to metabolic activity of ICC, and some investigators have proposed that the ionic mechanism for pacemaker current is regulated metabolically (Connor et al. 1976; Prosser & Mangel, 1982; Nakayama et al. 1997). Others have suggested that pacemaker activity depends upon Ca2+ release from intracellular stores (Liu et al. 1995). The link between Ca2+ release, metabolic activity and current induction in the plasma membrane, however, remains a mystery. We studied the relationships between Ca2+ release from IP3 receptor-operated stores, Ca2+ uptake by mitochondria and initiation of pacemaker currents in cultured ICC from the murine small bowel and in intact GI smooth muscles. These experiments indicate that integrated Ca2+ handling by endoplasmic reticulum (ER) and mitochondria is a prerequisite of electrical pacemaking in the GI tract and provide a novel concept of how pacemaker currents are initiated in ICC.
METHODS
The use and treatment of animals was approved by the Institutional Animal Use and Care Committee at the University of Nevada. Cells were dispersed from murine jejunal muscles and cultured for 1–3 days as described previously (Koh et al. 1998). The mice were rendered unconcious with carbon dioxide and killed by cervical dislocation. ICC were identified by live immunostaining for Kit protein (Ward et al. 1994; Huizinga et al. 1995; Koh et al. 1998) using a monoclonal Kit antibody (ACK2) labelled with Alexa Fluor 488 (Molecular Probes, Eugene, OR, USA). Patch clamp experiments were performed as previously described (Koh et al. 1998), except that the equilibrium potential for chloride ions was adjusted to −80 mV.
To monitor mitochondrial [Ca2+], cells were loaded with reduced rhod-2 AM (4.4 μM; 50 min at 37°C) prepared according to the manufacturer's recommendations (Molecular Probes) and cultured for an additional 12–18 h to enhance specific loading into mitochondria (Hajnóczky et al. 1995). Some cultures were co-loaded with MitoTracker Green FM (Molecular Probes; 100 nM), a dye which binds covalently to the inner mitochondrial membrane and fluoresces independently of mitochondrial [Ca2+] and membrane potential thus providing an independent test of mitochondrial localization and specificity of rhod-2 fluorescence.
Time series experiments were performed using a video fluorescence imaging system (Ionoptix Corp., Milton, MA, USA) coupled to a Nikon Diaphot inverted microscope equipped with a × 40, 1.3 NA oil-immersion objective and filter sets appropriate for rhod-2 and Alexa Fluor 488 fluorescence (XF34 and XF23, respectively; Omega Optical, Inc., Brattleboro, VT, USA). Dye-loaded cells were excited with a xenon lamp and an intensified CCD camera collected fluorescence. Video signals were digitized at 5–20 Hz and analysed by IonWizard (version 4.4; Ionoptix) and smoothed using Microcal Origin 4.1 (Microcal Software, Inc., Northampton, MA, USA). Patch clamp and imaging experiments were performed at 30°C.
Confocal imaging was performed with a Bio-Rad MRC 600 (Hercules, CA, USA) coupled to a Nikon Diaphot inverted microscope. Images were acquired with a Nikon PlanApo × 60, 1.4 NA or a Nikon Fluor × 100, 1.3 NA oil-immersion objective using excitation wavelengths of 488 nm (for MitoTracker Green and Alexa Fluor 488) and 568 nm (for rhod-2). Confocal micrographs, constructed with CoMOS software (version 7.0a; Bio-Rad), are digital composites of Z-series scans of 8–12 optical sections through a depth of 2.8-3.6 μm using a confocal aperture 20 % of maximum. Confocal time series experiments were performed using the line scan option of the Bio-Rad MRC 600 (acquisition rate, 4 Hz) and traces were smoothed as described above. All imaging experiments were performed in the presence of 3 μM nicardipine. In most pharmacological experiments the excitation light source was turned off for 10 min after application of drugs to avoid photobleaching and to reduce phototoxicity.
Intracellular electrophysiological experiments were performed on intact muscles from the murine jejunum, guinea-pig antrum and canine proximal colon. Guinea-pigs were killed by the same method as mice, and dogs were killed by a lethal overdose of pentobarbital sodium (100 mg kg−1i.v.). Strips of jejunum and colon were prepared as previously described (Smith et al. 1987; Koh et al. 1998). Sheets of antral muscle (1 cm × 1 cm) from the guinea-pig stomach were pinned in a recording chamber after removal of the mucosa. Impalements of circular muscle cells were made near the submucosal surface of all preparations.
For statistical analyses, various parameters of rhythmic currents, slow waves or mitochondrial Ca2+ transients were expressed as a percentage of control. Comparisons were made using Mann-Whitney rank sum test or Kruskal-Wallis one-way ANOVA on ranks followed by multiple comparisons (Dunn's test for comparisons vs. control and Dunnett's test for all-pairwise comparisons). P < 0.05 was used as the cut-off for statistical significance.
Heparin was obtained from Sigma (St Louis, MO, USA). Antimycin, carbonyl cyanide p-(trifluoromethoxy)phenylhydrazone (FCCP), oligomycin and nicardipine (all from Sigma) were dissolved in absolute ethanol (1-50 mM) and further diluted in the superfusion buffer (see Koh et al. 1998) to the concentrations indicated in the text. Stock solutions (1-10 mM) of RU-360, rotenone and xestospongin C (from Calbiochem-Novabiochem Corp., La Jolla, CA, USA) and thapsigargin and carbonyl cyanide m-chlorophenylhydrazone (CCCP; from Sigma) were prepared in DMSO.
RESULTS
Small networks and single ICC cultured from the murine small intestine were identified with fluorescent Kit antibody (ACK2-Alexa Fluor 488; see Koh et al. 1998). Under voltage clamp, ICC networks displayed spontaneous inward currents averaging −595 ± 37 pA (holding potential, −60 mV) in amplitude and 13.7 ± 0.5 cycles min−1 (n = 20). The inward currents were associated with voltage oscillations under current clamp, averaging 22 ± 1.4 mV in amplitude. Spontaneous voltage oscillations in cultured ICC were similar to the electrical slow waves recorded from intact intestinal muscles (Koh et al. 1998). Individual ICC also generated spontaneous inward currents, at 13.7 ± 1.0 cycles min−1 and averaging −112.7 ± 32.8 pA in amplitude. As previously reported, pacemaker currents were insensitive to nicardipine (3 μM) and blocked by gadolinium (10 μM) (Koh et al. 1998; data not shown).
The relationship between pacemaker activity and mitochondrial Ca2+ uptake was investigated in Kit-positive ICC loaded with dihydro-rhod-2. Mitochondrial localization of rhod-2 was verified by co-labelling with MitoTracker Green FM (Fig. 1A–F). Ca2+-dependent fluorescence of rhod-2 was monitored by video or confocal microscopy and revealed rhythmic oscillations in mitochondrial [Ca2+] ([Ca2+]m) at a frequency of 13.2 ± 0.5 cycles min−1 (n = 12; Fig. 1G–J). [Ca2+]m oscillations were insensitive to 3 μM nicardipine. These properties are similar to the spontaneous membrane currents recorded by patch clamp.
Figure 1. Oscillations in [Ca2+] in mitochondria and pacemaker activity in ICC.
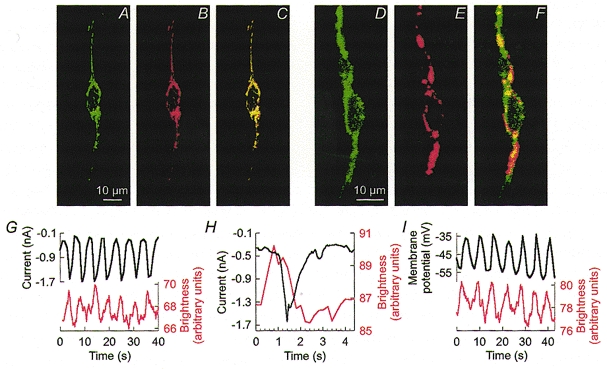
A–C, confocal images demonstrating mitochondrial localization of rhod-2. Dense clusters of mitochondria were labelled with MitoTracker Green FM (A) in the perinuclear region and within processes. Rhod-2 fluorescence (B) overlapped with MitoTracker Green FM as indicated by the yellow colour of the overlaid images (C). D–F, representative confocal images of an ICC labelled with an antibody for Kit protein (ACK2) conjugated with a fluorescent marker, Alexa Fluor 488 (D), and loaded with rhod-2 (E). F shows a composite image. Mitochondrial labelling in cells other than the Kit-positive cells was digitally removed in A–F. G–I, representative recordings illustrating the temporal relationship between [Ca2+]m oscillations and rhythmic inward currents (G and H) or membrane potential (I). Recordings in G and I were obtained from the same cell. Black traces, pacemaker currents recorded under voltage clamp (-60 mV holding potential) or membrane potential under current clamp (holding current = 0). Red traces, [Ca2+]m measured by rhod-2 fluorescence. Note that the increase in [Ca2+]m preceded the onset of the inward current (H).
The temporal relationship between [Ca2+]m oscillations and pacemaker activity was studied by monitoring rhod-2 fluorescence and membrane currents in eight voltage-clamped and four current-clamped ICC. There was a unitary relationship between [Ca2+]m oscillations and pacemaker currents, and the onset of the [Ca2+]m transients preceded inward currents and voltage transients (Fig. 1G–I).
We tested the relationship between mitochondrial respiration, [Ca2+]m oscillations and pacemaker currents. The mitochondrial uncouplers FCCP (1 μM; n = 5) and CCCP (0.5-50 μM; n = 10) initially reduced the frequency of pacemaker currents and then blocked these events (e.g. Fig. 2A). The respiratory chain (complex III) inhibitor antimycin (1-3 μM; n = 5; Fig. 2B) and complex I inhibitor rotenone (1-10 μM; n = 6; Fig. 2C) also inhibited pacemaker currents. It is unlikely that these effects were due to depletion of ATP because the cells were dialysed with 5 mM ATP, and oligomycin (5-10 μM), an inhibitor of the F1/F0 ATP synthase, failed to block pacemaker currents (n = 5; Fig. 2D). FCCP (1-10 μM; n = 6; Fig. 2E) and antimycin (10 μM; n = 6; Fig. 2F) also inhibited [Ca2+]m oscillations in cells loaded with rhod-2. Oligomycin (10 μM; n = 2) did not affect [Ca2+]m oscillations (not shown), suggesting that the effects of FCCP and antimycin on [Ca2+]m oscillations were not due to depletion of ATP.
Figure 2. Pacemaker currents in ICC are associated with mitochondrial Ca2+ uptake.
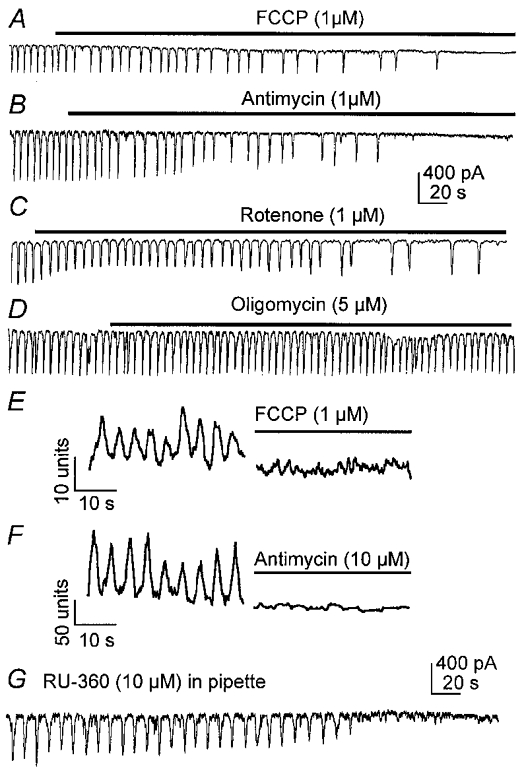
A–E show spontaneous currents recorded from ICC under voltage clamp conditions (holding potential, −60 mV). FCCP (1 μM, A), antimycin (1 μM, B) and rotenone (1 μM, C) slowed frequency and blocked pacemaker currents. Oligomycin (5 μM, D) had no effect on pacemaker currents. FCCP (E) and antimycin (F) also blocked oscillations in [Ca2+]m. Records of [Ca2+]m show oscillations before addition of drug and 10 min after application of the drug. RU-360 also inhibited spontaneous pacemaker currents in ICC (G).
Consistent with blocking of pacemaker currents, mitochondrial uncouplers (FCCP and CCCP) and respiratory chain inhibitors (antimycin) also inhibited voltage oscillations in current-clamped ICC (not shown) and in intact muscles from murine small intestine, guinea-pig stomach and canine proximal colon. Intracellular microelectrode recording from circular muscle cells showed that intact muscles generated slow waves at average frequencies of 38.3 ± 3 cycles min−1 (jejunum; n = 15 muscles), 4.6 ± 0.8 cycles min−1 (stomach; n = 3) and 4.9 ± 1.6 cycles min−1 (colon; n = 15). Addition of FCCP (1 μM), CCCP (1 μM) and antimycin (10 μM; with or without oligomycin) inhibited slow wave activity (Fig. 3). In most cases the loss in slow wave activity was accompanied by a small depolarization of resting membrane potential. For example, canine colon depolarized from a control level of −77 ± 1 to −67 ± 1.6 mV (n = 15) and murine jejunal muscles depolarized from −70 ± 2 to −62 ± 2 mV (n = 15) in response to FCCP. This level of depolarization has been shown not to block slow wave activity in these muscles (Ward et al. 1994). Oligomycin had no effect on slow waves, suggesting the effects of FCCP and antimycin were not simply due to depletion of ATP.
Figure 3. Intracellular recordings showing effects of FCCP (1 μM) on slow wave activity from murine small intestine (A), guinea-pig stomach (B) and canine proximal colon (C).
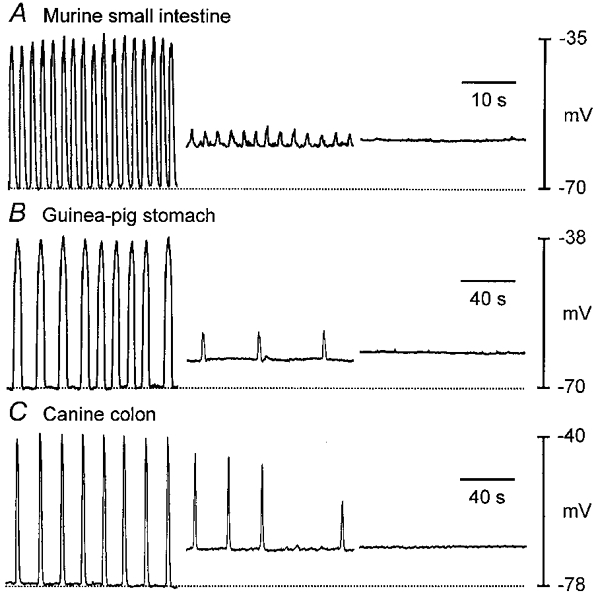
FCCP inhibited slow waves in all three models of GI electrical rhythmicity. Loss of rhythmicity was associated with small depolarizations in resting potential (dotted lines denote most polarized level between slow waves). Traces are excerpts of continuous recordings from the same cells, with the second and third portions beginning 6 and 12 min in A, 7 and 9 min in B, and 6 and 8 min in C after addition of FCCP to the perfusion buffer.
The mitochondrial uncouplers FCCP and CCCP are protonophores that reduce mitochondrial transmembrane potential (Ψm; Farkas et al. 1989). Similar effects on Ψm have been reported for respiratory chain inhibitors (Schinder et al. 1999; Tinel et al. 1999). These drugs would therefore reduce the driving force for Ca2+ uptake into mitochondria. A link between mitochondrial Ca2+ uptake and pacemaker currents was demonstrated by blocking the mitochondrial uniporter with RU-360 (Matlib et al. 1998). Dialysis of cells with RU-360 (1-10 μM) blocked spontaneous inward currents and slow waves (under current clamp) within 2–5 min of cell membrane rupture (Fig. 2G; n = 4). Taken together, these data suggest that mitochondrial Ca2+ uptake, but not increased ATP production that might result from Ca2+ uptake (McCormack et al. 1990; Robb-Gaspers et al. 1998), is necessary for activation of pacemaker currents.
IP3 receptor-operated channels in ER are in close apposition to mitochondria, and Ca2+ release from IP3 receptors can trigger mitochondrial Ca2+ uptake (Sparagna et al. 1995; Litsky & Pfeiffer, 1997; Rizzuto et al. 1998; Csordás et al. 1999). Ca2+ uptake into mitochondria occurs via facilitated diffusion through the mitochondrial Ca2+ uniporter down a large transmembrane potential gradient (Ψm), and some investigators have presented evidence that the uniporter is gated by cytoplasmic Ca2+ (Sparagna et al. 1995; Litsky & Pfeiffer, 1997). We tested the relationship between Ca2+ release from stores and oscillations in [Ca2+]m. Xestospongin C (1-3 μM), a membrane-permeable blocker of IP3 receptors (Gafni et al. 1997), reversibly inhibited [Ca2+]m oscillations (n = 10; Fig. 4A). Xestospongin C also inhibited spontaneous pacemaker currents (n = 3; Fig. 4B). The involvement of IP3 receptor-operated stores was also tested with intracellular heparin. When cells were dialysed with pipette solutions containing heparin (250 u ml−1), pacemaker currents and voltage oscillations resulting from pacemaker activity, which were apparent immediately after cell membrane rupture, decreased in frequency and ceased within 2–5 min of breaking into the cells (n = 4; Fig. 4C). Before cessation of [Ca2+]m oscillations and pacemaker currents, there was a slowing of frequency with IP3 receptor blockers. In contrast to the dependence of [Ca2+]m oscillations and pacemaker currents on IP3-dependent Ca2+ release, Ca2+ release via ryanodine receptors did not appear to participate in the regulation of pacemaker currents. Pacemaker activity was unaffected by ryanodine (50 μM, 20 min, n = 6 cells; 10 μM, n = 6 intact muscles; not shown). Treatment with thapsigargin (0.5-1 μM), an inhibitor of Ca2+ uptake by the ER, also blocked pacemaker currents (n = 4; Fig. 4D). These data demonstrate an important role for Ca2+ release from IP3 receptor-operated stores in initiating Ca2+ uptake by mitochondria and activation of pacemaker currents.
Figure 4. Relationship between Ca2+ stores and oscillations in [Ca2+]m and pacemaker currents.
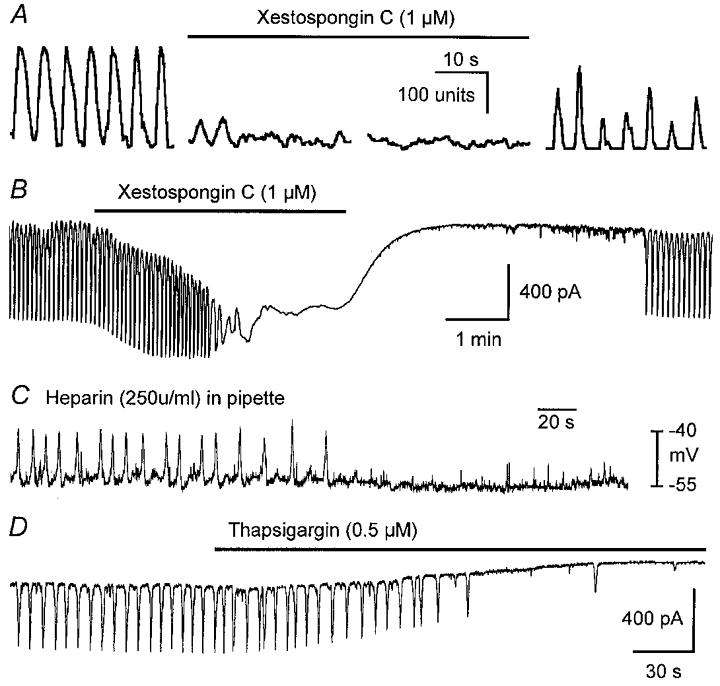
A shows oscillations in [Ca2+]m before (left trace), during (middle two traces) and after (right trace) exposure to xestospongin C (1 μM). B shows that pacemaker currents were also reversibly inhibited by xestospongin C (1 μM). C shows pacemaker activity (recorded under current clamp) inhibited by dialysis with heparin (250 u ml−1). Thapsigargin (0.5 μM) also inhibited pacemaker currents (D).
DISCUSSION
Our experiments suggest that Ca2+ release from Ca2+ stores in ICC drives the initiation of pacemaker currents. Using drugs to block specific Ca2+ release channels, we have provided data that Ca2+ release from IP3 receptor-operated stores (and not ryanodine receptor-operated stores) is linked to initiation of pacemaker current. It is well known that Ca2+ release from ER is capable of oscillatory and even regenerative activity (e.g. Berridge, 1993), and factors such as Ca2+ pump (SERCA pump) activity and lumenal ER Ca2+ regulate the frequency of Ca2+ release events (Petersen et al. 1993; Koizumi et al. 1999). We also found that blockers of Ca2+ uptake into the ER (i.e. SERCA pump inhibitors) inhibited spontaneous pacemaker currents suggesting that cycling of Ca2+ into and out of the ER may be the primary oscillator regulating pacemaker activity.
A second major finding of this study was the requisite integration between ER Ca2+ release and mitochondrial function in the initiation of pacemaker activity. Several drugs known to inhibit mitochondrial respiration or to collapse Ψm inhibited spontaneous pacemaker currents. Treatment with mitochondrial uncouplers and respiratory chain inhibitors has been shown to decrease Ψm and Ca2+ uptake (Farkas et al. 1989; Schinder et al. 1999; Tinel et al. 1999). We found that both classes of drugs blocked the spontaneous oscillations in [Ca2+]m that were associated with pacemaker currents. Inhibition of ATP production was apparently not responsible for inhibition of pacemaker activity because: (i) cells dialysed with 5 mM ATP were not protected from inhibition of pacemaker activity by mitochondrial uncouplers and respiratory chain inhibitors; (ii) oligomycin did not affect spontaneous pacemaker currents; (iii) glycolytic pathways were left intact when cells were treated with mitochondrial uncouplers and respiratory chain inhibitors; and (iv) there was no hyperpolarization or outward current noted in ICC due to activation of ATP-dependent K+ channels (present in ICC and activated by reduced cytoplasmic ATP; S. D. Koh & K. M. Sanders, unpublished observations).
Reduction of the electrochemical gradient across the mitochondrial membrane affects Ca2+ uptake. Imaging of mitochondrial rhod-2 fluorescence showed oscillations in [Ca2+]m at the same frequency as pacemaker currents. In experiments in which [Ca2+]m was monitored in voltage-clamped cells we found that the [Ca+]m oscillations preceded the onset of pacemaker currents. We also found that dialysis of cells with RU-360, an inhibitor of the Ca2+ uniporter, inhibited pacemaker currents. These data suggest that Ca2+ uptake by mitochondria may be an important link in the initiation of pacemaker currents. How mitochondrial Ca2+ uptake is coupled to activation of non-selective cation channels in the plasma membrane is not understood at the present time, but dynamic changes in Ca2+ in microdomains near ER, mitochondria and the plasma membrane could be important.
The importance of mitochondrial function in electrical slow wave generation has been considered in previous studies of intact GI muscles. Prosser & Mangel (1982) proposed an elaborate model for slow waves in which mitochondrial ATP production was regulated by mitochondrial Ca2+ uptake. These authors believed that oscillations in Na+ pump activity were the mechanism for slow waves (see Connor et al. 1974), and oscillations in ATP production were considered as a means of driving Na+ pump oscillations. Tomita and coworkers showed that cyanide inhibited slow wave activity in guinea-pig stomach muscles (Huang et al. 1993), and iodoacetate, an inhibitor of glycolysis, also inhibited slow waves (Nakayama et al. 1997). In a more recent study they concluded that the inhibitory effect of iodoacetate may have been due to accumulation of metabolites rather than block of ATP production (Ogino et al. 1999). All of these studies suggested that ATP was the major link between mitochondrial function and slow waves. The present study suggests that ATP (while obviously important for restoration of ionic gradients and resequestration of Ca2+ into the ER) is not the primary link between mitochondrial function and pacemaker activity. Our data are more compatible with the idea that localized changes in Ca2+ concentration may be important in regulating pacemaker current channels. Future studies will be required to more clearly characterize the channels responsible for pacemaker currents and to discover the intracellular messenger(s) that regulate these channels before it will be possible to determine the link between mitochondrial Ca2+ uptake and pacemaker currents.
In summary, ICC generate pacemaker currents in GI muscles. We propose the following model for activation of the non-selective cationic conductance responsible for pacemaker currents. Activation of pacemaker currents depends upon periodic release of Ca2+ from IP3 receptors. Ca2+ release from IP3 receptors triggers Ca2+ uptake by mitochondria, possibly by gating the rapid uptake mode of the uniporter (Sparagna et al. 1995; Litsky & Pfeiffer, 1997). Mitochondrial Ca2+ uptake is linked in an unknown way to activation of pacemaker currents. The pacemaker cycle may be completed and reset by uptake of Ca2+ into the ER (i.e. via a thapsigargin-sensitive Ca2+ pump). Uptake and periodic release of Ca2+ from IP3 receptor-operated stores appears to be the main oscillatory process responsible for GI autorhythmicity. Thus the pacemaker mechanism is a highly integrated process requiring physical proximity and coordination between ion channels and transport proteins in the ER, mitochondria and plasma membrane. Uncouplers of mitochondrial proton transport and respiratory chain inhibitors blocked slow waves in several intact GI muscles suggesting this mechanism may be commonly employed to generate pacemaker activity in visceral smooth muscles.
Acknowledgments
This work was supported by grants from the NIDDK: DK41315 to K.M.S., S.M.W. and S.D.K. and DK40569 to K.M.S., S.M.W. and T.O. J.Y.J. was supported by Chosun University (Republic of Korea) and KOSEF postdoctoral fellowship programme. The authors are grateful to Drs James Kenyon, Andrew Thomas, Burt Horowitz and Terence Smith for valuable comments about the experiments and manuscript and to Nancy Horowitz for preparation of the cell cultures.
S. M. Ward, T. Ördög and S. D. Koh contributed equally to this study.
References
- Berridge MJ. Inositol trisphosphate and calcium signalling. Nature. 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Connor JA, Kreulen DL, Prosser CL. Relation between oxidative metabolism and slow rhythmic potentials in mammalian intestinal muscle. Proceedings of the National Academy of Sciences of the USA. 1976;73:4239–4243. doi: 10.1073/pnas.73.11.4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor JA, Prosser CL, Weems WA. A study of pace-maker activity in intestinal smooth muscle. The Journal of Physiology. 1974;240:671–701. doi: 10.1113/jphysiol.1974.sp010629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csordás G, Thomas AP, Hajnóczky G. Quasi-synaptic calcium signal transmission between endoplasmic reticulum and mitochondria. EMBO Journal. 1999;18:96–108. doi: 10.1093/emboj/18.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas DL, Wei M-D, Febbroriello P, Carson JH, Loew LM. Simultaneous imaging of cell and mitochondrial membrane potential. Biophysical Journal. 1989;56:1053–1069. doi: 10.1016/S0006-3495(89)82754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faussone-Pellegrini MS. Cytodifferentiation of the interstitial cells of Cajal related to the myenteric plexus of mouse intestinal muscle coat. An E.M. study from foetal to adult life. Anatomy and Embryology. 1985;171:163–169. doi: 10.1007/BF00341410. [DOI] [PubMed] [Google Scholar]
- Gafni J, Munsch JA, Lam TH, Catlin MC, Costa L G, Molinski TF, Pessah IN. Xestospongins: potent membrane permeable blockers of the inositol 1,4,5-trisphosphate receptor. Neuron. 1997;19:723–733. doi: 10.1016/s0896-6273(00)80384-0. [DOI] [PubMed] [Google Scholar]
- Hajnóczky G, Robb-Gaspers LD, Seitz MB, Thomas AP. Decoding of cytosolic calcium oscillations in the mitochondria. Cell. 1995;82:415–424. doi: 10.1016/0092-8674(95)90430-1. [DOI] [PubMed] [Google Scholar]
- Huang SM, Chowdhury JU, Kobayashi K, Tomita T. Inhibitory effects of cyanide on mechanical and electrical activities in the circular muscle of gastric antrum of guinea-pig stomach. Japanese Journal of Physiology. 1993;43:229–238. doi: 10.2170/jjphysiol.43.229. [DOI] [PubMed] [Google Scholar]
- Huizinga JD, Thuneberg L, Klüppel M, Malysz J, Mikkelsen HB, Bernstein A. W/kit gene required for intestinal pacemaker activity. Nature. 1995;373:347–349. doi: 10.1038/373347a0. [DOI] [PubMed] [Google Scholar]
- Koh SD, Sanders KM, Ward SM. Spontaneous electrical rhythmicity in cultured interstitial cells of Cajal from the murine small intestine. The Journal of Physiology. 1998;513:203–213. doi: 10.1111/j.1469-7793.1998.203by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi S, Lipp P, Berridge MJ, Bootman MD. Regulation of ryanodine receptor opening by lumenal Ca2+ underlies quantal Ca2+ release in PC12 cells. Journal of Biological Chemistry. 1999;274:33327–33333. doi: 10.1074/jbc.274.47.33327. [DOI] [PubMed] [Google Scholar]
- Langton P, Ward SM, Carl A, Norell MA, Sanders KM. Spontaneous electrical activity of interstitial cells of Cajal isolated from canine proximal colon. Proceedings of the National Academy of Sciences of the USA. 1989;86:7280–7284. doi: 10.1073/pnas.86.18.7280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu LW, Thuneberg L, Huizinga JD. Cyclopiazonic acid, inhibiting the endoplasmic reticulum calcium pump, reduces the canine colonic pacemaker frequency. Journal of Pharmacology and Experimental Therapeutics. 1995;275:1058–1068. [PubMed] [Google Scholar]
- Litsky ML, Pfeiffer DR. Regulation of the mitochondrial Ca2+ uniporter by external adenine nucleotides: the uniporter behaves like a gated channel which is regulated by nucleotides and divalent cations. Biochemistry. 1997;36:7071–7080. doi: 10.1021/bi970180y. [DOI] [PubMed] [Google Scholar]
- McCormack JG, Halestrap AP, Denton RM. Role of calcium ions in regulation of mammalian intramitochondrial metabolism. Physiological Reviews. 1990;70:391–425. doi: 10.1152/physrev.1990.70.2.391. [DOI] [PubMed] [Google Scholar]
- Matlib MA, Zhou Z, Knight S, Ahmed S, Choi KM, Krause-Bauer J, Phillips R, Altschuld R, Katsube Y, Sperelakis N, Bers DM. Oxygen-bridged dinuclear ruthenium amine complex specifically inhibits Ca2+ uptake into mitochondria in vitro and in situ in single cardiac myocytes. Journal of Biological Chemistry. 1998;273:10223–10231. doi: 10.1074/jbc.273.17.10223. [DOI] [PubMed] [Google Scholar]
- Nakayama S, Chihara S, Clark JF, Huang SM, Horiuchi T, Tomita T. Consequences of metabolic inhibition in smooth muscle isolated from guinea-pig stomach. The Journal of Physiology. 1997;505:229–240. doi: 10.1111/j.1469-7793.1997.229bc.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogino K, Takai A, Ishida Y, Tomita T. Effects of iodoacetate on spontaneous electrical activity, slow wave, in the circular muscle of the guinea-pig gastric antrum. Japanese Journal of Physiology. 1999;49:521–526. doi: 10.2170/jjphysiol.49.521. [DOI] [PubMed] [Google Scholar]
- Petersen CC, Petersen OH, Berridge MJ. The role of endoplasmic reticulum calcium pumps during cytosolic calcium spiking in pancreatic acinar cells. Journal of Biological Chemistry. 1993;268:22262–22264. [PubMed] [Google Scholar]
- Prosser CL, Mangel AW. Cellular Pacemakers. New York: John Wiley & Sons; 1982. p. 288. [Google Scholar]
- Rizzuto R, Pinton P, Carrington W, Fay FS, Fogarty KE, Lifshitz LM, Tuft RA, Pozzan T. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science. 1998;280:1763–1766. doi: 10.1126/science.280.5370.1763. [DOI] [PubMed] [Google Scholar]
- Robb-Gaspers LD, Burnett P, Rutter GA, Denton RM, Rizzuto R, Thomas AP. Integrating cytosolic calcium signals into mitochondrial metabolic responses. EMBO Journal. 1998;17:4987–5000. doi: 10.1093/emboj/17.17.4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinder AF, Olson EC, Spitzer NC, Montal M. Mitochondrial dysfunction is a primary event in glutamate neurotoxicity. Journal of Neuroscience. 1999;16:6125–6133. doi: 10.1523/JNEUROSCI.16-19-06125.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TK, Reed JB, Sanders KM. Origin and propagation of electrical slow waves in circular muscle of canine proximal colon. American Journal of Physiology. 1987;252:C215–224. doi: 10.1152/ajpcell.1987.252.2.C215. [DOI] [PubMed] [Google Scholar]
- Sparagna GC, Gunter KK, Sheu SS, Gunter TE. Mitochondrial calcium uptake from physiological-type pulses of calcium. A description of the rapid uptake mode. Journal of Biological Chemistry. 1995;270:27510–27515. doi: 10.1074/jbc.270.46.27510. [DOI] [PubMed] [Google Scholar]
- Szurszewski JH. Electrical basis for gastrointestinal motility. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. 2. New York: Raven Press; 1987. pp. 383–422. [Google Scholar]
- Thomsen L, Robinson TL, Lee JC, Farraway LA, Hughes MJ, Andrews DW, Huizinga JD. Interstitial cells of Cajal generate a rhythmic pacemaker current. Nature Medicine. 1998;4:848–851. doi: 10.1038/nm0798-848. [DOI] [PubMed] [Google Scholar]
- Tinel H, Cancela JM, Mogami H, Gerasimenko JV, Gerasimenko OV, Tepikin AV, Petersen OH. Active mitochondria surrounding the pancreatic acinar granule region prevent spreading of inositol trisphosphate-evoked local cytosolic Ca2+ signals. EMBO Journal. 1999;18:4999–5008. doi: 10.1093/emboj/18.18.4999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SM, Burns AJ, Torihashi S, Sanders KM. Mutation of the proto-oncogene c-kit blocks development of interstitial cells and electrical rhythmicity in murine intestine. The Journal of Physiology. 1994;480:91–97. doi: 10.1113/jphysiol.1994.sp020343. [DOI] [PMC free article] [PubMed] [Google Scholar]


