Abstract
Whole-cell patch-clamp recordings were made from subthalamic nucleus (STN) neurones in brain slices from rats. Stimulation with bipolar electrodes evoked synaptic currents mediated by glutamate (EPSCs) and GABAA (IPSCs) receptors.
Dopamine reversibly reduced the amplitude of GABAA IPSCs by up to 48% with an IC50 value of 3.4 ± 0.8μm. The dopamine D2 receptor agonist quinpirole, but not the D1 receptor agonist SKF 82958, also inhibited GABAA IPSCs. This effect was completely reversed by the D2 receptor antagonist sulpiride but not by SCH 23390, a D1 antagonist.
Muscarine reversibly reduced the amplitude of GABAA IPSCs by up to 70 % with an IC50 value of 0.6 ± 0.1μm. Inhibition of IPSCs by muscarine was completely blocked by scopolamine (10 μm), a muscarinic receptor antagonist. The M3 muscarinic receptor antagonist 4-DAMP effectively reversed muscarine-induced inhibition of IPSCs with an IC50 of 0.11 ± 0.03μm. Although the M1 receptor antagonist pirenzepine also reversed the inhibition of IPSCs by muscarine, this effect was only observed at relatively high concentrations (IC50=21.7 ± 9.4μm).
Dopamine and muscarine both increased the paired-pulse ratio of GABAA IPSCs. Neither agent produced sustained changes in postsynaptic holding current.
Glutamate EPSCs were also inhibited reversibly by dopamine (by up to 29 %; IC50= 16 ± 3 μm) and muscarine (by up to 41 %; IC50=1.0 ± 0.4μm). However, both agents were more potent and efficacious for reducing GABA IPSCs compared with glutamate EPSCs.
These results suggest that the most significant effect of dopamine and muscarine in the STN is to reduce inhibitory synaptic input by acting at presynaptic dopamine D2 and muscarinic M3 receptors, respectively.
The subthalamic nucleus (STN) is composed of glutamate-containing neurones that project to the major output nuclei of the basal ganglia: substantia nigra reticulata and globus pallidus interna (Albin et al. 1995). Due to its excitatory influence on these structures, the STN plays a major role in regulating muscle tone and the dynamics of movement. As an example of the importance of the STN, it has long been known that a stroke to the STN produces hemiballismus. Other hyperkinetic movements such as chorea and athetosis are associated with reductions in STN neuronal activity (Crossman, 1987; Albin, 1995). In contrast, high levels of neuronal activity in the STN are associated with the rigidity, tremor and bradykinesia that are typical of Parkinson's disease. The central role played by the STN in controlling movement is supported further by studies that show that inactivation of the STN, either by surgical lesion or by high frequency electrical stimulation, is highly effective in obviating most of the signs of parkinsonism in patients with Parkinson's disease and in animals with chemically induced parkinsonism (Bergman et al. 1990; Krack et al. 1998).
Because Parkinson's disease is known to be produced by loss of dopamine-containing neurones in the substantia nigra, it is not surprising that dopamine replacement therapy (with levodopa) and treatment with dopamine agonist drugs effectively reduce symptoms of this disease. However, before levodopa was used clinically, anticholinergic agents were known to be effective and are still used widely in the treatment of Parkinson's disease. Although the striatum is generally thought to be an important site of action for the antiparkinsonian effects of dopaminergic and anticholinergic drugs, one must also consider the STN – which is now recognized as having a major role in the regulation of movements – as a possible site of action of antiparkinsonian drugs.
The STN is innervated by dopamine-containing pathways that arise from the substantia nigra zona compacta and the ventral tegmental area (Hassani et al. 1997). Moreover, dopamine D1- and D2-like receptor subtypes are both expressed in the STN (Flores et al. 1999). Cholinergic input to the STN arises from neurones in the pedunculopontine nucleus (Bevan & Bolam, 1995), and muscarinic receptors – especially the M3 receptor subtype – are prominently expressed in the STN (Levey et al. 1994). Therefore, the STN receives the anatomic connections and expresses the neurotransmitter receptors that would be required in order for dopamine and acetylcholine to play significant roles in regulating neuronal activity in the STN.
In the present study, we used whole-cell patch-clamp recording to characterize the actions of dopamine and muscarine on synaptic transmission in slices of STN prepared from rat brain. We found that dopamine and muscarine, acting at presynaptic D2 and M3 receptors, respectively, are both potent inhibitors of inhibitory transmission mediated by GABAA receptors.
METHODS
Tissue preparation
Methods used for preparing STN brain slices were virtually identical to those reported previously for preparing slices of midbrain (Shen & Johnson, 1997). Briefly, adult male Sprague-Dawley rats (120-300 g; Bantin & Kingman, Seattle, WA, USA) were anaesthetized with halothane and killed by severing major thoracic vessels, in accordance with national guidelines. The brain was rapidly removed and horizontal slices (300 μm) containing caudal diencephalon and rostral midbrain were cut in cold physiological saline by a vibratome. After a slice containing the STN was placed on a supporting net in a recording chamber (volume, 500 μl), it was immersed and superfused (1.5 ml min−1) with artificial spinal fluid which contained (mM): NaCl, 126; KCl, 2.5; CaCl2, 2.4; MgCl2, 1.2; NaH2PO4, 1.2; NaHCO3, 19; glucose, 11. This solution was saturated with 95 % O2 and 5 % CO2, and had a pH of 7.35 at 35–37°C. Using a dissection microscope for visual guidance, the STN was located as grey matter approximately 2.7 mm lateral to the midline and 2 mm rostral to the centre of the substantia nigra reticulata (Paxinos & Watson, 1986).
Electrophysiological recordings
Whole-cell tight-seal recordings were made with pipettes containing (mM): potassium gluconate, 130; MgCl2, 2; CaCl2, 1; EGTA, 11; Hepes, 10; ATP, 1.5; GTP, 0.3. The pH of the internal solution was adjusted to 7.3-7.4 with KOH. Membrane currents were recorded under voltage clamp (-70 mV holding potential) and amplified with an Axopatch-1D amplifier. Data were acquired and analysed using pCLAMP software, a Digidata analog/digital interface (Axon Instruments, Foster City, CA, USA) and an IBM-compatible personal computer. Holding currents were recorded continuously using a MacLab analog/digital interface, Chart software (AD Instruments, Castle Hill, Australia) and a Macintosh IIVX computer. The voltage dependence of membrane currents was measured by delivering a series of hyperpolarizing and depolarizing voltage steps (400 ms duration) from a holding potential of −70 mV. Chord conductance was measured as the linear slope of the current-voltage relationship. Series resistance was electronically compensated by 50–80 % to 10–30 MΩ. Membrane potentials have been corrected for the liquid junction potential (10 mV).
Synaptic currents
Bipolar stimulation electrodes (tip separation, 300–500 μm) were made from electrolytically sharpened tungsten wire, and their tips were placed in the slice 300 μm rostral to the STN. A single rectangular pulse (0.1 ms duration) of constant current was used to evoke a synaptic current every 10 s. All synaptic currents shown in the figures are the average of three responses. An IPSC mediated by GABAA receptors was isolated pharmacologically by recording in the presence of (±)-2-amino-5-phosphonopentanoic acid (AP5; 50 μM) and 6-cyano-7-nitro-quinoxalone (CNQX; 10 μM) in order to block NMDA and non-NMDA receptors, respectively. Studies on EPSCs were done in the presence of bicuculline (30 μM) to block GABAA receptors.
Drugs
All drugs were dissolved in aqueous stock solutions with the exception of CNQX, sulpiride and 4-diphenylacetoxy-N-methylpiperidine methiodide (4-DAMP), which were dissolved in dimethyl sulfoxide. A stock solution of dopamine hydrochloride was made daily and kept on ice to retard oxidation. Each stock solution was diluted at least 1:1000 in perfusate immediately prior to its use. Dimethyl sulfoxide, diluted 1:1000 in artificial spinal fluid, had no effect on either holding current or synaptic currents. Approximately 30 s was required for the drug solution to enter the recording chamber; this delay was due to passage of the perfusate through a heat exchanger. Bicuculline methiodide, dopamine, muscarine and scopolamine were obtained from Sigma Chemical Co. (St Louis, MO, USA). Sulpiride, SKF 82958, quinpirole, SCH 23390, 4-DAMP, pirenzepine, AP5 and CNQX were obtained from Research Biochemicals, Inc. (Natick, MA, USA).
Data analysis
Numerical data in the text and error bars in the figures are expressed as means ±s.e.m. Two-way analysis of variance (ANOVA) with repeated measures was used to test for significant differences between concentration-response curves (SigmaStat software, Jandel Scientific, San Rafael, CA, USA). Student's two-tailed t tests were used to test for significant differences between chord conductances and holding currents. In evaluating concentration-dependent drug effects, an IC50 was calculated for the responses from each neurone using the KaleidaGraph curve-fitting program (Synergy Software, Reading, PA, USA) on a Macintosh computer. Concentration-response data were fitted to the Hill-Langmuir equation: y = ax/(x+b), where y is the magnitude of effect, a is maximum effect, x is the drug concentration and b is the concentration that inhibits the effect by 50 % (IC50). The mean IC50 and s.e.m. were calculated by averaging all IC50 values. An apparent dissociation constant for an antagonist (Kb) was calculated for the mean population using the Cheng-Prusoff equation: Kb = IC50/(1 +A/A50), where IC50 is the concentration of the antagonist that causes a 50 % maximum reversal of an agonist effect, A is the concentration of agonist used and A50 is the concentration of agonist that produces a 50 % maximum effect (Cheng & Prusoff, 1973).
RESULTS
Membrane properties and synaptic currents
The vast majority of neurones recorded in the STN fired action potentials (or currents) spontaneously (data not shown). When recording under current clamp (with zero holding current), STN neurones fired action potentials at a rate of 9.6 ± 1.9 Hz (range, 5–18 Hz; n = 7 cells); this rate is consistent with previous reports (Nakanishi et al. 1987; Overton & Greenfield, 1995). Each action potential lasted about 1 ms and was followed by significant after-hyperpolarization. When recording under voltage clamp (with zero holding current), the mean membrane potential was −59 ± 1 mV (range, −48 to −74 mV; n = 83). Voltage-clamp recordings made during a series of 400 ms hyperpolarizing voltage steps (0 to −70 mV from a holding potential of −70 mV) showed an ‘instantaneous’ inward current (Ii), a time-dependent inward current which slowly reached steady state (Iss) and an inward tail current (Itail) (see Fig. 1A). Current-voltage plots for Ii and Iss, shown in Fig. 1B, demonstrated prominent inward rectification. Chord conductance for Ii was 1.2 ± 0.2 nS when measured between −70 and −80 mV, but it increased to 8.7 ± 0.7 nS between −130 and −140 mV (n = 29). Inward rectification was reduced by barium chloride (300 μM, n = 5), as shown in Fig. 1C. The difference between Iss and Ii was −96 ± 5 pA (range, −26 to −211 pA) when measured during the voltage step from −70 to −140 mV (n = 81). This time-dependent hyperpolarization-activated inward current was completely blocked by caesium chloride (3 mM, n = 5), which is typical for the mixed cationic current Ih (Halliwell & Adams, 1982). Figure 1D shows that depolarizing voltage steps (to −60 and −50 mV) evoked inward currents that demonstrated little inactivation. These membrane properties of STN neurones are consistent with reports by others (Nakanishi et al. 1987; Bevan & Wilson, 1999).
Figure 1. Electrophysiological properties of STN neurones.

A, membrane currents recorded under voltage clamp during a series of hyperpolarizing voltage steps. Current traces show instantaneous current (Ii), steady-state current (Iss) and inward tail current (Itail). B, current-voltage plots for Ii (□) and Iss (▪) measured at times indicated by the arrows in A. Each data point is the mean of 29 cells. C, barium (300 μM, •) reduces the inward rectification of Ii compared with control values (○). Each data point is the mean of 5 cells. D, depolarizing voltage steps evoke inward currents.
Bipolar stimulation electrodes placed in the rostral region of the slice were used to evoke synaptic currents at a holding potential of −70 mV. As seen in Fig. 2A, a single stimulus evoked an inward synaptic current when recorded in the presence of bicuculline (30 μM). Although the late component of the EPSC was slightly reduced by the NMDA receptor antagonist AP5 (50 μM), the EPSC was completely blocked by combined application of CNQX (10 μM) and AP5 (50 μM). This suggests that the EPSC is largely mediated by non-NMDA receptors. When recordings were made in the presence of CNQX (10 μM) and AP5 (50 μM), focal stimulation evoked a GABAA receptor-mediated outward current that was completely blocked by bicuculline (30 μM; Fig. 2B). Thus, focal electrical stimulation of the brain slice can evoke synaptic currents mediated by GABA and glutamate.
Figure 2. Synaptic currents are mediated by GABA and glutamate.

A, superimposed EPSCs recorded in the presence of bicuculline (30 μM). Although the late component of the EPSC was slightly reduced by AP5 (50 μM), the EPSC was completely blocked by combined application of CNQX (10 μM) and AP5 (50 μM). B, superimposed IPSCs recorded in the presence of AP5 (50 μM) and CNQX (10 μM). The GABAA receptor-mediated outward synaptic current was completely blocked by bicuculline (30 μM).
Inhibition of synaptic currents by dopamine
As seen in Fig. 3A and B, dopamine produced reversible and concentration-dependent reductions in the amplitude of IPSCs (recorded in 10 μM CNQX and 50 μM AP5) and EPSCs (recorded in 30 μM bicuculline). The actions of dopamine began within 2 min of the start of the perfusion, and its effects reversed 10–15 min after washout (Fig. 3C). As seen in the concentration-response curves in Fig. 3D, dopamine reduced the amplitude of GABAA receptor-mediated IPSCs with an IC50 of 3.4 ± 0.8 μM (n = 5); the highest concentration of dopamine tested (100 μM) reduced GABAA IPSCs by 48 ± 5 % (n = 16). In contrast, dopamine reduced the amplitude of glutamate EPSCs with an IC50 of 16 ± 3 μM (n = 6), and the highest concentration of dopamine tested (100 μM) reduced glutamate EPSCs by 29 ± 6 % (n = 8). Thus, dopamine was significantly more potent for reducing IPSCs compared with EPSCs (P < 0.001).
Figure 3. Effects of dopamine on synaptic currents recorded in STN neurones.

A and B, dopamine produces a reversible and concentration-dependent reduction in amplitude of IPSCs (A) and EPSCs (B). IPSCs were recorded in AP5 (50 μM) and CNQX (10 μM), whereas EPSCs were recorded in bicuculline (30 μM). C, time-dependent reduction of IPSCs (•) and EPSCs (○) by dopamine (100 μM). Each data point is either the mean of 8 (EPSCs) or 16 (IPSCs) cells. D, concentration-response curves show that dopamine is more potent for reducing the amplitude of IPSCs (•) compared with EPSCs (○). Each point represents the mean of 5–6 cells.
Dopamine receptor pharmacology
Because dopamine was more potent for inhibiting GABA-mediated IPSCs compared with glutamate-mediated EPSCs, we focused further studies on the effects of dopamine on IPSCs. As seen in the superimposed current traces in Fig. 4A, the reduction in IPSC amplitude caused by dopamine (100 μM) was completely blocked by concurrent treatment with sulpiride (1 μM), which is an antagonist at D2-like dopamine receptors. Figure 4B shows similar findings obtained from six STN neurones; although dopamine (100 μM) reduced the amplitude of IPSCs to 63 ± 6 % of control (at t = 6.5 min), addition of sulpiride (1 μM) to the perfusate increased the amplitude of IPSCs to 105 ± 10 % of control (at t = 14 min). No enhancement of IPSC amplitude was produced by sulpiride alone, nor was there any effect of sulpiride on the holding current (n = 6).
Figure 4. Antagonism of dopamine-induced inhibition of GABAA IPSCs by sulpiride.

A, superimposed IPSCs show that the dopamine D2 receptor antagonist sulpiride (1 μM) blocks the inhibitory effect of dopamine (100 μM) on the GABAA receptor-mediated synaptic current. B, averaged data from 6 cells showing that sulpiride (1 μM) reverses the reduction in IPSC amplitude produced by dopamine (100 μM). Data were normalized to the amplitude of the first synaptic current evoked in each cell.
Quinpirole (10 μM), an agonist at D2-like receptors, mimicked the effect of dopamine by causing a reversible 38 ± 4 % reduction in the amplitude of GABAA IPSCs (n = 8), as shown in Fig. 5A. Quinpirole reached its peak effect within 3 min, and its effect reversed 15–20 min after washout. In contrast, an agonist at D1-like receptors, SKF 82958 (10 μM), failed to affect the amplitude of GABAA IPSCs (n = 8; Fig. 5B). Furthermore, an antagonist at D1-like receptors, SCH 23390 (10 μM), had no effect on dopamine-induced inhibition of GABAA IPSCs (n = 4; data not shown). Neither quinpirole (10 μM) nor SKF 82958 (10 μM) had a consistent effect on holding current.
Figure 5. The dopamine D2 receptor agonist quinpirole mimics the inhibitory effect of dopamine on GABAA IPSCs.

A, quinpirole (10 μM) reversibly reduces the amplitude of GABAA IPSCs. Each point represents the mean ±s.e.m. of 8 cells. B, the dopamine D1 receptor agonist SKF 82958 (10 μM) has no effect on the amplitude of GABAA IPSCs. Each point represents the mean ±s.e.m. of 8 cells.
Effects of dopamine receptor stimulation on paired IPSCs
In order to examine the site of action, the effects of dopamine were measured on IPSCs evoked by pairs of stimuli. In this paired-pulse protocol (Davies et al. 1990), the interval between pulses (30-50 ms) was adjusted such that the second IPSC was smaller than the first (Fig. 6A). Under control conditions, the paired-pulse ratio of IPSC amplitudes was 0.73 ± 0.05 (n = 5). As illustrated in Fig. 6B, dopamine (100 μM) significantly increased the IPSC paired-pulse ratio to 0.97 ± 0.03 (P < 0.001). Figure 6C shows that the dopamine-induced increase in the paired-pulse ratio occurred within 1 min and was readily reversible after washout. These data are consistent with the hypothesis that dopamine acts presynaptically to inhibit GABA release.
Figure 6. Dopamine reduces paired-pulse depression of IPSCs.

A, superimposed IPSCs evoked by pairs of stimuli (35 ms apart) in the absence (Control and Wash) and presence of dopamine (100 μM). B, traces are the same as those in A, except that the amplitude of the first IPSC recorded in dopamine has been normalized to match the amplitude of the first control IPSC. Note that in the presence of dopamine, the amplitude of the second IPSC is reduced very little compared with the first IPSC. C, averaged results from 5 cells show that dopamine (100 μM) increases the paired-pulse ratio of IPSCs (ratio of the second IPSC amplitude divided by the first). D, quinpirole (10 μM) increases the paired-pulse ratio of GABAA IPSCs, suggesting a presynaptic site of action. Each data point is the averaged response from 6 cells. E, SKF 82958 (10 μM), a D1 receptor agonist, has no effect on the paired-pulse ratio of IPSCs. These results are the means from 5 cells.
Quinpirole (10 μM) mimicked dopamine by increasing the IPSC paired-pulse ratio from 0.80 ± 0.04 to 0.99 ± 0.03 (n = 6; Fig. 6D). In contrast, Fig. 6E shows that SKF 82958 (10 μM) failed to affect the paired-pulse ratio (n = 5). However, sulpiride (1 μM) completely blocked the ability of dopamine to increase the IPSC paired-pulse ratio (data not shown). These results suggest that the presynaptic action of dopamine to inhibit GABA release is mediated by a member of the D2 receptor family.
Muscarine evokes an inward current
Muscarine (10 μM) produced a small inward current which reached a peak within 1 min (19 ± 2 pA, n = 18) and gradually diminished with time despite continued perfusion (data not shown). This inward current reversed polarity at −118 ± 5 mV (which is near the equilibrium potential for K+) and was accompanied by a small decrease in membrane conductance (0.65 ± 0.16 nS, n = 6). Subsequent applications of muscarine produced little or no current. These findings suggest that (1) inward current evoked by muscarine is caused by a reduction in a resting K+ conductance, and (2) acute tolerance to this effect of muscarine develops rapidly.
Inhibition of synaptic currents by muscarine
Muscarine reduced the amplitude of synaptic currents within 3 min of the start of perfusion. These effects were sustained during 30 min of continuous drug application even though the inward shift in holding current produced by muscarine returned to control within a few minutes. The effects of muscarine on synaptic currents reversed within 15 min after washout. Although low concentrations of muscarine (0.1-3 μM) produced reproducible reductions in synaptic currents, application of the highest concentrations of muscarine tested (10-30 μM) generally caused less inhibition of synaptic currents when perfused a second time. For example, the first application of 10 μM muscarine reduced the amplitude of IPSCs by 72 ± 6 % (n = 5), whereas the second application reduced IPSCs by only 43 ± 2 % in the same neurones. Consequently, 10 or 30 μM muscarine was applied only once to each brain slice.
Muscarine produced reversible and concentration-dependent reductions in the amplitudes of GABAA receptor-mediated IPSCs (Fig. 7A) and glutamate-mediated EPSCs (Fig. 7B). As seen in Fig. 7C, muscarine reduced the amplitude of GABAA IPSCs with an IC50 of 0.6 ± 0.1 μM (n = 6), and the highest concentration of muscarine tested (10 μM) reduced IPSCs by 70 ± 3 % (n = 6). In contrast, muscarine reduced glutamate EPSCs with an IC50 of 1.0 ± 0.4 μM (n = 5), and 10 μM muscarine reduced glutamate EPSCs by only 41 ± 5 % (n = 5). These data show that muscarine was significantly more potent for reducing IPSCs compared with EPSCs (P < 0.001).
Figure 7. Effects of muscarine on synaptic currents.

A and B, current traces show that muscarine produces a concentration-dependent and reversible reduction in the amplitude of GABAA IPSCs (A) and glutamate EPSCs (B). IPSCs were recorded in AP5 (50 μM) and CNQX (10 μM), whereas EPSCs were recorded in bicuculline (30 μM). C, concentration-response curves for percentage inhibition of synaptic current amplitude by muscarine. Muscarine is significantly more potent for inhibiting GABAA IPSCs (•) compared with glutamate EPSCs (○). Each point represents the mean ±s.e.m. of 5–6 cells.
Muscarine receptor pharmacology
Because muscarine was more potent for inhibiting GABA-mediated IPSCs compared with glutamate-mediated EPSCs, further studies were focused on the effects of muscarine on IPSCs. As seen in Fig. 8, the muscarine-induced inhibition of GABAA IPSCs was completely reversed by the non-selective muscarine receptor antagonist scopolamine (10 μM). Although muscarine (10 μM) reduced the amplitude of IPSCs by 60 ± 5 %, addition of scopolamine (10 μM) to the superfusate reversed the inhibition and returned the amplitude of the IPSCs to 102 ± 5 % of control (n = 7).
Figure 8. Effects of muscarine on GABAA IPSCs.

Amplitude of GABAA IPSCs recorded over time showing that the reduction in IPSC amplitude by muscarine (10 μM) is reversed by scopolamine (10 μM). Each point represents the mean ±s.e.m. of 7 cells.
Receptor pharmacology was investigated further by testing the effects of the muscarinic antagonists 4-DAMP, which binds with high affinity to M3 and M1 receptor subtypes (but not to M2), and pirenzepine, which is more selective for M1 and M4 receptors (Bonner et al. 1987; Zubieta & Frey, 1993). In experiments with 4-DAMP and pirenzepine, concentrations were increased cumulatively because these antagonists were slow to wash out. Although Fig. 9A and B shows that both antagonists could block the inhibition of IPSCs by muscarine (30 μM), 4-DAMP was much more potent (Fig. 9C). By fitting the data in Fig. 9C to the Hill equation, the IC50 for 4-DAMP was calculated to be 110 ± 30 nM (n = 6), whereas the pirenzepine IC50 was 21.7 ± 9.4 μM (n = 4). Furthermore, the apparent Kb for 4-DAMP (2.1 ± 0.5 nM, n = 6) was much lower than that for pirenzepine (411 ± 178 nM, n = 4). At the highest concentration tested (3 μM), 4-DAMP reversed the muscarine-induced inhibition by 94 ± 1 % (n = 6), whereas 300 μM pirenzepine was required to reduce the effect of muscarine by a similar extent (90 ± 3 %, n = 4). Although these results show that these antagonists are equally efficacious, 4-DAMP is clearly more potent (by approximately 200-fold) than pirenzepine for blocking the effect of muscarine on GABAA IPSCs. Because 4-DAMP has a much higher affinity for the M3 receptor compared with pirenzepine (Zubieta & Frey, 1993), these results indicate that inhibition of the GABAA IPSC is mediated by the M3 receptor subtype.
Figure 9. Muscarine acts at the M3 receptor to inhibit GABAA IPSCs.

A, superimposed traces show that 4-DAMP reverses muscarine-induced inhibition of a GABAA IPSC in a concentration-dependent manner. B, superimposed traces show that pirenzepine also reduces the muscarine-induced inhibition of a GABAA IPSC, but that the concentrations are much higher than those required for 4-DAMP. C, concentration-inhibition curves show reversal of muscarine (30 μM)-induced depression of IPSC amplitude by muscarine antagonists. The greater potency of 4-DAMP compared with pirenzepine suggests that M3 receptors mediate the inhibitory effect of muscarine on GABAA IPSCs. Results are means ±s.e.m. of 6 cells for 4-DAMP and 4 cells for pirenzepine.
Effects of muscarine on paired IPSCs
In order to investigate the site of action of muscarine, we tested the effect of muscarine on IPSCs evoked by pairs of stimuli. Following the same protocol as that used above for studying dopamine, IPSCs were evoked by pairs of stimuli delivered 30–50 ms apart while recording in AP5 (50 μM) and CNQX (10 μM) (see Fig. 10A and B). In the absence of muscarine, the ratio of the amplitude of the second IPSC divided by the first was 0.75 ± 0.08 (n = 4). However, in the presence of muscarine (10 μM), this IPSC paired-pulse ratio increased to 1.11 ± 0.18 in the same neurones (P < 0.01). Moreover, this increase in the paired-pulse ratio was reversed by the addition of scopolamine (10 μM) to the superfusate (Fig. 10C). These data support the conclusion that muscarine acts presynaptically to inhibit GABA release.
Figure 10. Muscarine reduces paired-pulse depression of IPSCs.
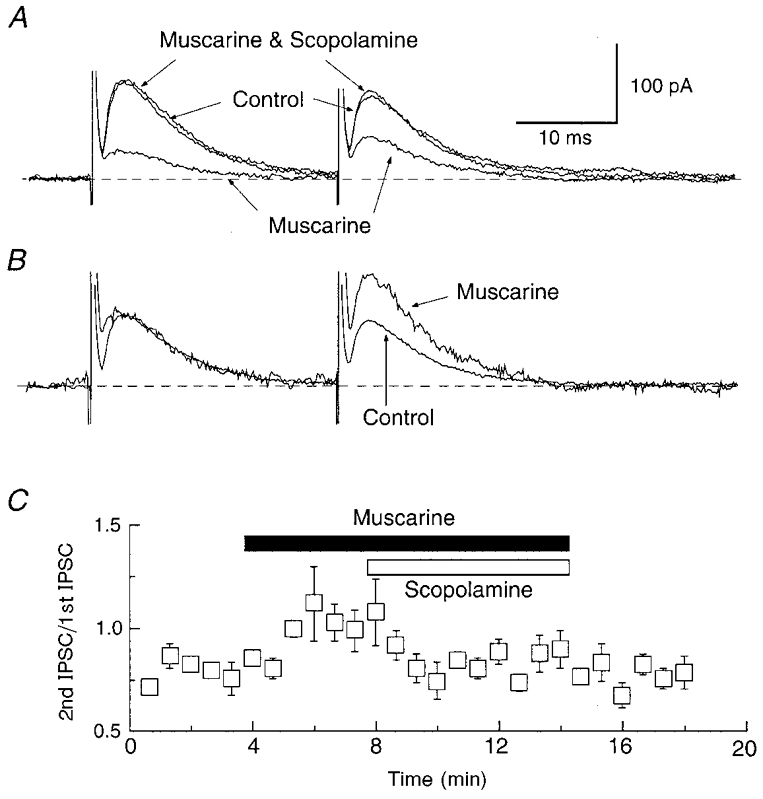
A, superimposed IPSCs evoked by pairs of stimuli in the absence (Control) and presence of muscarine (10 μM), and during combined application of muscarine plus scopolamine (10 μM). Note that the first IPSC is smaller in the presence of muscarine, but that the second IPSC is larger than the first. Scopolamine reverses the effect of muscarine. B, traces are the same as those in A, except that the amplitude of the first IPSC recorded in muscarine has been normalized to the first IPSC recorded under control conditions. C, the muscarine (10 μM)-induced increase in the IPSC paired-pulse ratio is reversed by scopolamine (10 μM). These results are the means from 4 cells.
DISCUSSION
Although our studies show that dopamine and muscarine both inhibit inotropic glutamate and GABAA synaptic currents, our most important finding is that both ligands are more potent for inhibiting currents mediated by GABAA receptors. Results from paired-pulse studies on IPSCs suggest that dopamine and muscarine both act presynaptically to inhibit GABA release from nerve terminals. Furthermore, our pharmacological studies using relatively selective agonist and antagonist ligands indicate that the presynaptic effect of dopamine is mediated by D2-like receptors whereas that of muscarine is mediated by M3 receptors. Taken together, these data suggest that D2 and M3 receptors may regulate the excitability of STN neurones by acting presynaptically to alter GABA release.
By reducing the amplitude of synaptic currents mediated by GABA, one would expect dopamine to disinhibit STN neurones and thereby increase their firing rate. In agreement with this hypothesis, Mintz et al. (1986) reported that dopamine, applied locally to STN neurones by microiontophoresis, acted via a member of the D2 receptor family to increase the spontaneous firing rate of STN neurones recorded extracellularly in rats in vivo. However, others reported that this excitatory effect of dopamine was mimicked by the D1– rather than the D2– family of receptors (Kreiss et al. 1996, 1997). In contrast, other studies found an inhibitory effect of dopamine on the spontaneous firing rates of STN neurones (Campbell et al. 1985; Hassani & Féger, 1999). An inhibitory role for dopamine is also supported by a report by Parry et al. (1994), which showed that microinjection of apomorphine into the STN evoked oral dyskinesia in rats. Thus, there clearly is no consensus in the literature concerning the direct pharmacological actions of dopamine on the excitability of STN neurones.
Despite this lack of consensus, a considerable amount of data from patients with Parkinson's disease and in animals with chemically induced parkinsonism support an inhibitory role for dopamine on STN neuronal activity. For example, several studies have shown that chronic dopamine depletion increases the firing rate and/or increases the incidence of burst firing in STN neurones in laboratory animals (Hollerman & Grace, 1992; Hassani et al. 1996). Experimentally induced parkinsonism also increases neuronal activity in substantia nigra reticulata and globus pallidus interna, which is caused by an increase in excitatory output from the STN (Robledo & Féger, 1990; Burbaud et al. 1995). Furthermore, disabling the STN with either high frequency electrical stimuli or surgical lesions is as effective as levodopa in alleviating most symptoms of Parkinson's disease (Limousin et al. 1998; Moro et al. 1999). Therefore, our finding that dopamine is most potent for inhibiting GABA IPSCs is at odds with the above studies which suggest that dopamine exerts an inhibitory influence on STN neurones. This is because inhibition of GABA-mediated transmission by dopamine, as we report, would be expected to increase the output from STN neurones and thereby worsen symptoms of parkinsonism.
Similar to our observations with dopamine, we found that muscarine was also more potent for inhibiting GABAA receptor-mediated IPSCs compared with glutamate-mediated EPSCs. As a consequence, we predict that muscarinic agents should disinhibit STN neurones and thereby increase STN output. This expectation is consistent with results from two studies in which the muscarinic agonist carbachol was shown to excite STN neuronal activity (Flores et al. 1996) and increase output from the STN to the substantia nigra reticulata (Rosales et al. 1994). Furthermore, the study by Flores et al. (1996) found that the carbachol-induced increase in spontaneous firing rate was most consistent with activation of the M3 receptor subtype. Thus, these results from others are consistent with our hypothesis that muscarinic agonist ligands, which bind to presynaptic M3 receptors, will stimulate STN neuronal activity by reducing the tonic inhibitory influence of GABA. Moreover, this indirect effect would tend to reinforce a direct depolarizing excitatory influence of muscarinic ligands.
Our results suggest that muscarinic antagonists would prevent endogenous acetylcholine from inhibiting GABA synaptic transmission. In the presence of tonic background stimulation of muscarinic receptors, an anticholinergic agent would be expected to reduce cholinergic-dependent inhibition of GABA release which would thereby reduce the output from the STN. Because a reduction in STN neuronal activity fits with the prevailing theory of basal ganglia function (DeLong, 1990), this action of anticholinergic drugs would be consistent with their clinical efficacy in the treatment of Parkinson's disease. There is one problem, however, in that clinically useful anticholinergic drugs are thought to bind preferentially to M4 rather than to M3 receptors (Ciliax et al. 1997; Purkerson & Potter, 1998), but the selectivity of these agents used clinically is doubtful. Nevertheless, our data certainly support the conclusion that the STN may be an important site of action for anticholinergic medication in Parkinson's disease. It may be useful to explore the clinical effectiveness of a selective M3 receptor antagonist in the treatment of this disease.
It would be useful to know the origin of GABA-containing neurones that express D2 and/or M3 receptors and project to the STN. Both of these types of receptor are expressed by STN neurones (Camps et al. 1989; Levey et al. 1994), but these neurones do not contain GABA. Globus pallidus (externa), which provides the vast majority of GABA input to the STN, reportedly expresses little or no message for the dopamine D2 receptor (Hurd et al. 1994; Ciliax et al. 1997), and expression of mRNA for the M3 receptor is also quite weak (Weiner et al. 1990; Levey et al. 1994). Because the STN does not receive appreciable GABA-containing inputs from other sources, the globus pallidus is still the most likely source of this input.
In summary, our data clearly show that dopamine and muscarine both alter synaptic transmission in the STN. We suggest that dopamine and cholinergic inputs may play important roles in regulating the output from the STN.
Acknowledgments
This work was supported by USPHS grant NS38715.
References
- Albin RL. The pathophysiology of chorea/ballism and parkinsonism. Parkinsonism and Related Disorders. 1995;1:3–11. doi: 10.1016/1353-8020(95)00011-t. [DOI] [PubMed] [Google Scholar]
- Albin RL, Young AB, Penney JB., Jr The functional anatomy of disorders of the basal ganglia. Trends in Neurosciences. 1995;18:63–64. [PubMed] [Google Scholar]
- Bergman H, Wichmann T, DeLong MR. Reversal of experimental parkinsonism by lesions of the subthalamic nucleus. Science. 1990;249:1436–1438. doi: 10.1126/science.2402638. [DOI] [PubMed] [Google Scholar]
- Bevan MD, Bolam JP. Cholinergic, GABAergic, and glutamate-enriched inputs from the mesopontine tegmentum to the subthalamic nucleus in the rat. Journal of Neuroscience. 1995;15:7105–7120. doi: 10.1523/JNEUROSCI.15-11-07105.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan MD, Wilson CJ. Mechanisms underlying spontaneous oscillation and rhythmic firing in rat subthalamic neurons. Journal of Neuroscience. 1999;19:7617–7628. doi: 10.1523/JNEUROSCI.19-17-07617.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner TI, Buckley NJ, Young AC, Brann MR. Identification of a family of muscarinic acetylcholine receptor genes. Science. 1987;237:527–532. doi: 10.1126/science.3037705. [DOI] [PubMed] [Google Scholar]
- Burbaud P, Gross C, Benazzouz A, Coussemacq M, Bioulac B. Reduction of apomorphine-induced rotational behaviour by subthalamic lesion in 6-OHDA lesioned rats is associated with a normalization of firing rate and discharge pattern of pars reticulata neurons. Experimental Brain Research. 1995;105:48–58. doi: 10.1007/BF00242181. [DOI] [PubMed] [Google Scholar]
- Campbell GA, Eckardt MJ, Weight FF. Dopaminergic mechanisms in subthalamic nucleus of rat: Analysis using horseradish peroxidase and microiontophoresis. Brain Research. 1985;333:261–270. doi: 10.1016/0006-8993(85)91580-x. [DOI] [PubMed] [Google Scholar]
- Camps M, Cortes R, Gueye B, Probst A, Palacios JM. Dopamine receptors in human brain: Autoradiographic distribution of D2 sites. Neuroscience. 1989;28:275–290. doi: 10.1016/0306-4522(89)90179-6. [DOI] [PubMed] [Google Scholar]
- Cheng Y-C, Prusoff WH. Relationship between the inhibition constant (Ki) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochemical Pharmacology. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Ciliax BJ, Greenamyre JT, Levey AI. Functional biochemistry and molecular neuropharmacology of the basal ganglia and motor systems. In: Watts RL, Koller WC, editors. Movement Disorders: Neurologic Principles and Practice. New York: McGraw-Hill; 1997. pp. 99–116. [Google Scholar]
- Crossman AR. Primate models of dyskinesia: The experimental approach to the study of basal ganglia-related involuntary movement disorders. Neuroscience. 1987;21:1–40. doi: 10.1016/0306-4522(87)90322-8. [DOI] [PubMed] [Google Scholar]
- Davies CH, Davies SN, Collingridge GL. Paired-pulse depression of monosynaptic GABA-mediated inhibitory postsynaptic responses in rat hippocampus. The Journal of Physiology. 1990;424:513–531. doi: 10.1113/jphysiol.1990.sp018080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends in Neurosciences. 1990;13:281–285. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- Flores G, Hernández S, Rosales MG, Sierra A, Martínez-Fong D, Flores-Hernández J, Aceves J. M3 muscarinic receptors mediate cholinergic excitation of the spontaneous activity of subthalamic neurons in the rat. Neuroscience Letters. 1996;203:203–206. doi: 10.1016/0304-3940(95)12297-4. [DOI] [PubMed] [Google Scholar]
- Flores G, Liang JJ, Sierra A, Martínez-Fong D, Quirion R, Aceves J, Srivastava LK. Expression of dopamine receptors in the subthalamic nucleus of the rat: characterization using reverse transcriptase-polymerase chain reaction and autoradiography. Neuroscience. 1999;91:549–556. doi: 10.1016/s0306-4522(98)00633-2. [DOI] [PubMed] [Google Scholar]
- Halliwell JV, Adams PR. Voltage-clamp analysis of muscarinic excitation in hippocampal neurons. Brain Research. 1982;250:71–92. doi: 10.1016/0006-8993(82)90954-4. [DOI] [PubMed] [Google Scholar]
- Hassani O-K, Féger J. Effects of intrasubthalamic injection of dopamine receptor agonists on subthalamic neurons in normal and 6-hydroxydopamine-lesioned rats: an electrophysiological and c-Fos study. Neuroscience. 1999;92:533–543. doi: 10.1016/s0306-4522(98)00765-9. [DOI] [PubMed] [Google Scholar]
- Hassani O-K, François C, Yelnik J, Féger J. Evidence for a dopaminergic innervation of the subthalamic nucleus in the rat. Brain Research. 1997;749:88–94. doi: 10.1016/s0006-8993(96)01167-5. [DOI] [PubMed] [Google Scholar]
- Hassani O-K, Mouroux M, Féger J. Increased subthalamic neuronal activity after nigral dopaminergic lesion independent of disinhibition via the globus pallidus. Neuroscience. 1996;72:105–115. doi: 10.1016/0306-4522(95)00535-8. [DOI] [PubMed] [Google Scholar]
- Hollerman JR, Grace AA. Subthalamic nucleus cell firing in the 6-OHDA-treated rat: Basal activity and response to haloperidol. Brain Research. 1992;590:291–299. doi: 10.1016/0006-8993(92)91108-q. [DOI] [PubMed] [Google Scholar]
- Hurd YL, Pristupa ZB, Herman MM, Niznik HB, Kleinman JE. The dopamine transporter and dopamine D2 receptor messenger RNAs are differentially expressed in limbic- and motor-related subpopulations of human mesencephalic neurons. Neuroscience. 1994;63:357–362. doi: 10.1016/0306-4522(94)90535-5. [DOI] [PubMed] [Google Scholar]
- Krack P, Benazzouz A, Pollak P, Limousin P, Piallat B, Hoffmann D, Xie J, Benabid A-L. Treatment of tremor in Parkinson's disease by subthalamic nucleus stimulation. Movement Disorders. 1998;13:907–914. doi: 10.1002/mds.870130608. [DOI] [PubMed] [Google Scholar]
- Kreiss DS, Anderson LA, Walters JR. Apomorphine and dopamine D1 receptor agonists increase the firing rates of subthalamic nucleus neurons. Neuroscience. 1996;72:863–876. doi: 10.1016/0306-4522(95)00583-8. [DOI] [PubMed] [Google Scholar]
- Kreiss DS, Mastropietro CW, Rawji SS, Walters JR. The response of subthalamic nucleus neurons to dopamine receptor stimulation in a rodent model of Parkinson's disease. Journal of Neuroscience. 1997;17:6807–6819. doi: 10.1523/JNEUROSCI.17-17-06807.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levey AI, Edmunds SM, Heilman CJ, Desmond TJ, Frey KA. Localization of muscarinic m3 receptor protein and M3 receptor binding in rat brain. Neuroscience. 1994;63:207–221. doi: 10.1016/0306-4522(94)90017-5. [DOI] [PubMed] [Google Scholar]
- Limousin P, Krack P, Pollak P, Benazzouz A, Ardouin C, Hoffmann D, Benabid A-L. Electrical stimulation of the subthalamic nucleus in advanced Parkinson's disease. New England Journal of Medicine. 1998;339:1105–1111. doi: 10.1056/NEJM199810153391603. [DOI] [PubMed] [Google Scholar]
- Mintz I, Hammond C, Féger J. Excitatory effect of iontophoretically applied dopamine on identified neurons of the rat subthalamic nucleus. Brain Research. 1986;375:172–175. doi: 10.1016/0006-8993(86)90971-6. [DOI] [PubMed] [Google Scholar]
- Moro E, Scerrati M, Romito LMA, Roselli R, Tonali P, Albanese A. Chronic subthalamic nucleus stimulation reduces medication requirements in Parkinson's disease. Neurology. 1999;53:85–90. doi: 10.1212/wnl.53.1.85. [DOI] [PubMed] [Google Scholar]
- Nakanishi H, Kita H, Kitai ST. Electrical membrane properties of rat subthalamic neurons in an in vitro slice preparation. Brain Research. 1987;437:35–44. doi: 10.1016/0006-8993(87)91524-1. [DOI] [PubMed] [Google Scholar]
- Overton PG, Greenfield SA. Determinants of neuronal firing pattern in the guinea-pig subthalamic nucleus: An in vivo and in vitro comparison. Journal of Neural Transmission. Parkinson's Disease and Dementia Section. 1995;10:41–54. doi: 10.1007/BF02256628. [DOI] [PubMed] [Google Scholar]
- Parry TJ, Eberle-Wang K, Lucki I, Chesselet M-F. Dopaminergic stimulation of subthalamic nucleus elicits oral dyskinesia in rats. Experimental Neurology. 1994;128:181–190. doi: 10.1006/exnr.1994.1126. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic Press; 1986. [Google Scholar]
- Purkerson SL, Potter LT. Use of antimuscarinic toxins to facilitate studies of striatal m4 muscarinic receptors. Journal of Pharmacology and Experimental Therapeutics. 1998;284:707–713. [PubMed] [Google Scholar]
- Robledo P, Féger J. Excitatory influence of rat subthalamic nucleus to substantia nigra pars reticulata and the pallidal complex: electrophysiological data. Brain Research. 1990;518:47–54. doi: 10.1016/0006-8993(90)90952-8. [DOI] [PubMed] [Google Scholar]
- Rosales MG, Flores G, Hernández S, Martínez-Fong D, Aceves J. Activation of subthalamic neurons produces NMDA receptor-mediated dendritic dopamine release in substantia nigra pars reticulata: a microdialysis study in the rat. Brain Research. 1994;645:335–337. doi: 10.1016/0006-8993(94)91669-1. [DOI] [PubMed] [Google Scholar]
- Shen K-Z, Johnson SW. Presynaptic GABAB and adenosine A1 receptors regulate synaptic transmission to rat substantia nigra reticulata neurones. The Journal of Physiology. 1997;505:153–163. doi: 10.1111/j.1469-7793.1997.153bc.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner DM, Levey AI, Brann MR. Expression of muscarinic acetylcholine and dopamine receptor mRNAs in rat basal ganglia. Proceedings of the National Academy of Sciences of the USA. 1990;87:7050–7054. doi: 10.1073/pnas.87.18.7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubieta JK, Frey KA. Autoradiographic mapping of M3 muscarinic receptors in the rat brain. Journal of Pharmacology and Experimental Therapeutics. 1993;264:415–422. [PubMed] [Google Scholar]


