Abstract
Resistance of Salmonella to extended-spectrum cephalosporins (ESCs) is being reported with increasing frequency. In humans, infections with Salmonella resistant to ESCs threaten the efficacy of ceftriaxone, the drug of choice for treating salmonellosis in children. To determine the occurrence of resistance to ESCs, we examined 8426 strains isolated from food-producing animals in Canada in 1994–99 for reduced susceptibility or resistance to ceftriaxone. Of the 8 such strains identified (7 from turkeys and 1 from cattle), 5 had reduced susceptibility, and 3 were resistant; 2 were isolated in 1995, 1 was isolated in each of 1996 and 1997, and 4 were isolated in 1999. Isoelectric focusing showed that all 8 isolates produced a β-lactamase with a pI ≥ 9. The strains were resistant to cefoxitin and not inhibited by clavulanic acid. Primers specific for the Citrobacter freundii blaAmpC gene produced the expected product in the polymerase chain reaction. DNA sequencing showed that all isolates possessed the blaCMY-2 gene. Plasmid DNA from all 8 isolates transformed Escherichia coli DH10B, whereas only 1 isolate transferred blaCMY-2 conjugally. All transformants and the transconjugant were resistant to ampicillin, cefoxitin, ceftiofur, cephalothin, streptomycin, sulfisoxazole, and tetracycline. Southern blots of plasmids from the isolates, the transformants, and the transconjugant showed that blaCMY-2 was located on similar-sized plasmids (60 or 90 MDa) in the transformants and the transconjugant. In the S. Typhimurium DT104 and S. Ohio isolates, the floSt gene was found on the same plasmid. Class 1 integrons with the aadB gene cassette were detected in the S. Bredeney isolates but not in their transformants or the transconjugant. Pulsed-field gel electrophoresis and plasmid profiles indicated that both clonal dispersion and horizontal transfer of blaCMY-2 may have caused dissemination of the resistance determinant.
Introduction
Infection with non-typhoidal Salmonella serovars continues to be one of the leading causes of gastroenteritis in Canada (1) and the United States. In the United States, an estimated 1.4 million cases occur annually (2). Of these, 95% may have been acquired through foodborne transmission, most cases affecting children and the elderly (2). Although salmonellosis is usually self-limiting, antimicrobials may be prescribed to susceptible populations (the young, the elderly, and those compromised immunologically) and to individuals with severe or invasive infection. In adults, fluoroquinolones and trimethoprim-sulfamethoxazole are the drugs of choice. In children, the use of fluoroquinolones is contraindicated. For that reason, and because of their pharmacodynamic properties and the low incidence of resistance, extended-spectrum cephalosporins (ESCs), such as ceftriaxone, are commonly used in children with systemic salmonellosis (3).
In recent years, however, reports of Salmonella isolates resistant to ESCs have increased. The majority of such isolates produce extended-spectrum β-lactamases (ESBLs) (4). These enzymes hydrolyze oxyimino-cephalosporins and monobactams but not cephamycins, such as cefoxitin and cefotetan. Generally, ESBLs are TEM or SHV β-lactamase derivatives or members of other β-lactamase families, such as CTX-M1, and are inhibited by clavulanic acid (5). Recently, however, a 2nd mechanism of resistance to ESCs in Salmonella has emerged, and strains producing AmpC-like cephalosporinases have been isolated. In the majority of isolates a plasmidic CMY-2 β-lactamase has been identified; these β-lactamases belong to a family of AmpC-like β-lactamases (BIL-1, CMY2b, LAT-1) (6,7) and share homology with the chromosomal ampC gene of Citrobacter freundii. These enzymes (blaAmpC) are similar to ESBLs in that they hydrolyze ESCs, but they differ in clinically significant ways. First, unlike ESBLs, these cephalosporinases hydrolyze cephamycins, such as cefoxitin. Second, clavulanic acid does not inhibit these β-lactamases.
The first Salmonella with resistance mediated by CMY-2 β-lactamase was isolated in Algeria (8). Horton, Sing and Jenkins (9) were the first to report a Salmonella producing an AmpC-like β-lactamase in the United States. Since then, there have been several reports of Salmonella producing plasmid-mediated CMY-2 β-lactamase (3,4,10,11). In England and Wales, although the incidence of resistance to ESCs is low (< 0.1% at 1 μg of ceftriaxone and cefotaxime per milliliter among the 1998 and 1999 isolates), resistance to ESCs is considered to be an emerging phenomenon (12).
To date, no data exists on the prevalence of ESC-resistant Salmonella in food-producing animals in Canada. The goal of our study was to assess the occurrence of reduced sensitivity to ESCs among Salmonella strains isolated from food-producing animals in Canada in 1994–99. Molecular and classical typing methods were used to examine the nature of the resistance determinant and the clonality of isolates belonging to the same serovar.
Materials and methods
Bacterial strains
During the period 1994–99, a total of 19 774 Salmonella strains isolated from food-producing animals, food products, and the animal environment in locations across Canada were submitted to our laboratory for serotyping. All isolates with a different serovar or phagetype or from a different source and every 4th isolate within a submission were examined for antimicrobial resistance, resulting in 8426 isolates. Any isolate that showed either reduced susceptibility (8 μg/mL) or resistance (64 μg/mL) to ceftriaxone was considered to be a potential producer of ESBL or an AmpC-like β-lactamase.
Antimicrobial resistance (AMR) testing
Antimicrobial resistance was determined by the agar dilution method, in accordance with the guidelines of the National Committee for Clinical Laboratory Standards (NCCLS) (13). Antimicrobials in powder form were purchased from Sigma Chemical Company (St. Louis, Missouri, USA). All isolates displaying reduced susceptibility or resistance to ceftriaxone were screened for susceptibility to amikacin (16 and 64 μg/mL), ampicillin (32 and 64 μg/mL), apramycin (32 μg/mL), carbadox (30 μg/mL), cefotaxime (64 μg/mL), cefoxitin (32 μg/mL), ceftiofur (8 μg/mL), ceftriaxone (8 and 64 μg/mL), cephalothin (32 μg/mL), chloramphenicol (32 μg/mL), ciprofloxacin (0.125, 1, and 4 μg/mL), cotrimoxazole (80 μg/mL: sulfamethoxazole and trimethoprim at 76 and 4 μg/mL, respectively), florfenicol (16 and 32 μg/mL), gentamicin (16 μg/mL), kanamycin (64 μg/mL), nalidixic acid (32 μg/mL), neomycin (16 μg/mL), nitrofurantoin (64 μg/mL), spectinomycin (64 μg/mL), streptomycin (64 μg/mL), sulfisoxazole (512 μg/mL), tetracycline (16 μg/mL), and tobramycin (8 μg/mL). Salmonella showing resistance to ESCs were also tested for resistance to ceftriaxone at 16 and 32 μg/mL. Resistance to ceftazidime, ceftazidime with clavulanic acid, and amoxicillin with clavulanic acid was tested with the Etest Antimicrobial Susceptibility Test (AB-BIODISK, Solna, Sweden). Aztreonam susceptibility was assessed with a disk diffusion assay (Difco Laboratories, Detroit, Michigan, USA), according to NCCLS guidelines (14). To determine resistance to florfenicol, Aquaflor (Schering Plough, Animal Health, Point Claire, Quebec), containing 50% florfenicol, was dissolved in dimethylformamide. Ceftiofur was purchased from Upjohn Pharmacia and Animal Health (Orangeville, Ontario).
Isoelectric focusing
A control strain producing CMY-2 β-lactamase was kindly provided by Dr. Paul Fey (University of Nebraska Medical Center, Omaha, Nebraska, USA). The strain produced both the TEM-1 (pI ≥ 5.4) and CMY-2 (pI ≥ 9.0) β-lactamases (3). Isolates were subjected to 5 freeze–thaw cycles. Reverse isoelectric focusing (IEF) was performed using precast vertical IEF gels (pH 3 to 10) (Novex, Scarborough, Ontario). Briefly, anode buffer was used in the upper chamber, cathode buffer was used in the lower chamber, and the electrodes were reversed. Samples were electrophoresed for 45 min at 100 V, 15 min at 200 V, and 15 min at 450 V. Nitrocefin (Oxoid, Nepean, Ontario) was used to visualize the β-lactamase bands (15).
Amplification, sequencing, and hybridization with blaCMY-2
The polymerase chain reaction (PCR) was used to screen strains for the presence of the C. freundii blaAmpC gene. Primers described by M'Zali and colleagues (16) were used to amplify 631 bp of the blaAmpC gene. Template DNA was prepared as follows. First, 3 mL of Luria–Bertani broth, Miller (LB broth; Becton Dickinson, Sparks, Maryland, USA) was inoculated and grown overnight. Then, a 1.5-mL aliquot was centrifuged for 1 min at 14 000 rpm, washed with 500 μL of FA buffer (Becton Dickinson), resuspended in 500 μL of water, and subjected to boiling water for 10 min. After centrifugation, the supernatant was decanted and subsequently used for PCR. Reaction mixtures (50 μL) contained 1 X Expand High Fidelity PCR Buffer, 25 pM of each primer, 0.2 mM of deoxynucleotide triphosphates, 5 μL of template DNA, and 1 U of Expand High Fidelity PCR system polymerase (Roche, Laval, Quebec). The mixture was cycled as follows: an initial denaturation step at 96°C for 15 s, 25 cycles at 96°C for 15 s, 60°C for 30 s, and 72°C for 2 min, followed by a final extension step at 72°C for 7 min. Reaction mixtures were cleaned prior to DNA sequencing, using the MinElute PCR Purification Kit (Qiagen, Mississauga, Ontario). DNA sequences were determined with the ABI PRISM dye terminator cycle- sequencing ready-reaction kit and an ABI 377 automated DNA sequencer (Perkin-Elmer; Applied Biosystems, Foster City, California, USA).
For hybridization studies, a digoxigenin (DIG)-labeled blaCMY-2 probe was generated with the PCR DIG Probe Synthesis Kit (Roche). With nylon membranes and the DIG DNA Labelling and Detection Kit (Roche), used according to the manufacturer's recommended directions, Southern blots were performed on plasmids separated by agarose gel electrophoresis to determine the location and size of the plasmid encoding blaCMY-2. Hybridizations were carried out with the donors, a transconjugant, and transformants.
Amplification and location of the floSt gene
A DIG-labeled floSt probe was generated with the use of the primers of Khan and associates (17) to determine the location of the florfenicol-resistance gene (floSt) in florfenicol-resistant strains and in their transformants.
Preparation of plasmid DNA
Plasmid DNA preparations were made by the method of Portnoy and White, as cited by Crosa and Falkow (18), with some modifications. Isolates were grown overnight on Luria–Bertani agar, Miller (LB agar) (Becton Dickinson), scraped off the surface of the agar with a sterile toothpick, and suspended in alkaline lysis buffer (pH 12.4). Plasmid DNA was subjected to electrophoresis in a horizontal 0.7% agarose gel in Tris-acetate buffer and stained with ethidium bromide (19). Plasmids used as molecular mass standards were: pSLT2, 60 MDa (20); pDT285, 96 MDa; and pDT369, 23 MDa (21); and the 8 plasmids of Escherichia coli V517 with molecular masses of 1.4 to 35.8 MDa (22).
Conjugation of strains
To determine the transmissibility of the resistance determinant, strains were conjugated with E. coli C600N, as described by Provence and Curtiss (23). Briefly, the donor Salmonella strain and the recipient E. coli C600N strain were grown at 37°C to a density of approximately 2 × 108 cells/mL. The recipient strain was grown for 15 to 20 min at 45°C prior to mating. The donor and recipient cells were conjugated for 60 min, then 250 μL of the mating mixture was transferred to 2.25 mL of prewarmed LB broth containing 50 μg/mL of nalidixic acid, incubated at 37°C for 30 min, and plated onto LB agar containing 50 μg/mL of each of cefoxitin and nalidixic acid. Plasmid DNA was electrophoresed and Southern blots were hybridized as described earlier.
Transformation of strains
Plasmid DNA, prepared as described earlier, was used to transform E. coli DH10B (Gibco BRL, Burlington, Ontario) by electroporation. Standard conditions (2500 kV, 200 ohms, and 25 μF) were used, and transformants were selected on LB agar plates containing 50 μg/mL cefoxitin. Plasmids were electrophoresed and Southern blots were hybridized as described earlier.
Integron analysis
The Int1 primers and conditions described by Sandvang, Aarestrup and Jensen (24) were used to detect class 1 integrons. DNA sequencing of the PCR products was performed to confirm the identity of gene cassettes inserted within the class 1 integrons.
Analysis of genetic relatedness
Pulsed-field gel electrophoresis (PFGE) patterns and plasmid profiles were compared to determine the genetic relatedness of isolates belonging to the same serovar. Whole-cell DNA for determination of PFGE patterns was prepared as described by the US Centers for Disease Control (25). DNA-containing slices of agarose plugs were digested overnight with 30 U of BlnI. The resulting fragments were separated by agarose gel electrophoresis in a 1% SeaKem GTG gel (FMC BioProducts, Rockland, Maine, USA), using the CHEF DR III system (BioRad, Hercules, California, USA) at 6 V/cm with 0.5X Tris borate/EDTA electrophoresis buffer. For S. Ohio isolates, thiourea (50 μM) was added to the running buffer. Pulse times of 2.2 to 64 s were applied for 20 h. Whole-cell DNA of S. Newport am01144 restricted with XbaI was used as a molecular size marker. The PFGE patterns were determined as described by Liebisch and Schwarz (26).
Results
Bacterial strains
Of the 8426 isolates screened for resistance to antimicrobials, 8 (0.1%) were resistant or had reduced susceptibility to ESCs; 2 were isolated in 1995, 1 was isolated in each of 1996 and 1997, and 4 were isolated in 1999. Of the isolates, 4 S. Bredeney and 3 S. Ohio were isolated from turkeys, 3 from yolk sacs, 3 from organs, and 1 from the intestines. The remaining isolate, S. Typhimurium DT104, was of bovine fecal origin. Of the 8 isolates, 6 were from geographically distinct regions in Alberta and Ontario. However, 2 of the S. Bredeney strains (SA992542 and SA993178) were isolated from the same location on 2 occasions, 78 d apart.
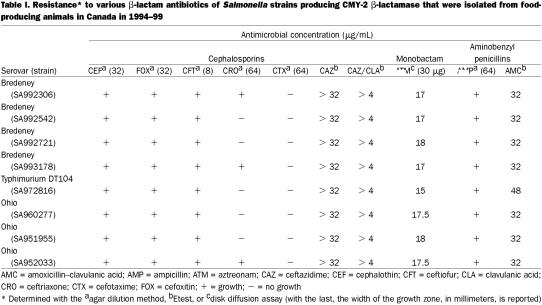
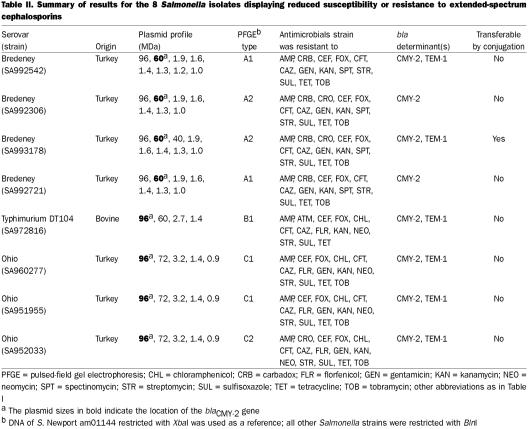
The 8 isolates were resistant to a number of antibiotics; their resistance to cephalosporins, a monobactam, and aminobenzyl penicillins is shown in Table I. All strains were resistant to cephalothin, cefoxitin, and ceftiofur. Although resistance to ceftriaxone was observed in only 3 strains, the remaining S. Bredeney and S. Ohio strains displayed reduced susceptibility at 32 μg/mL and the S. Typhimurium DT104 strain did so at 16 μg/mL. Despite the lack of resistance to cefotaxime, 5 of the 8 strains had reduced susceptibility at 32 μg/mL. All the S. Bredeney and S. Ohio strains were intermediately resistant (17-mm zone) to aztreonam, whereas the S. Typhimurium isolate was resistant (15-mm zone). Resistance to ceftazidime was not inhibited by clavulanic acid in any of the strains. Resistance to other classes of antibiotics is shown in Table II. All 8 strains were resistant to ampicillin, cefoxitin, ceftazidime, ceftiofur, cephalothin, kanamycin, streptomycin, sulfisoxazole, and tetracycline.
Table I.
Table II.
Isoelectric focusing
All strains produced a β-lactamase with a pI ≥ 9 that was similar to the CMY-2 β-lactamase produced by the control strain. Also, 2 of the S. Bredeney strains (SA992542 and SA993178), the S. Typhimurium strain, and all the S. Ohio strains produced a β-lactamase similar to that of the control TEM-1 (pI ≥ 5.4) β-lactamase (Table II).
PCR amplification and sequencing of blaCMY-2
All 8 strains were positive for the C. freundii blaAmpC PCR product. DNA sequencing of the 631-bp product revealed complete homology with a CMY-2 β-lactamase for all the isolates. The expected PCR product was also found in the transconjugant and the transformants.
Conjugations
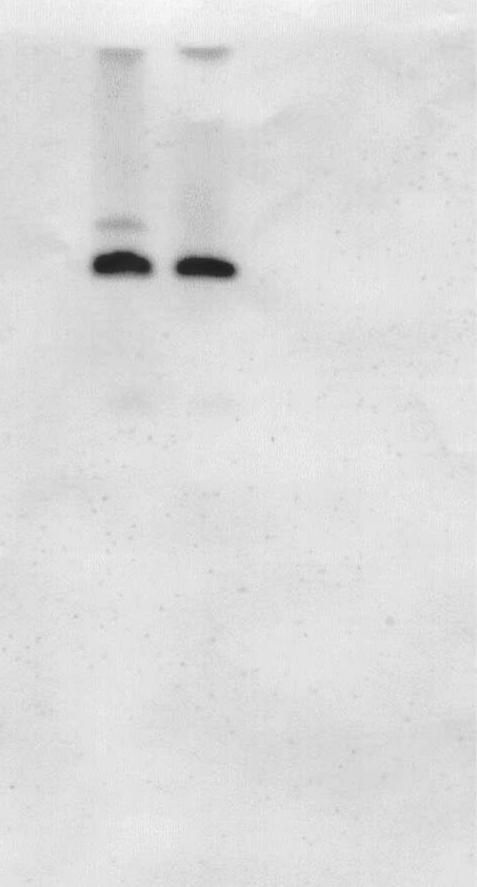
Despite numerous attempts, only 1 of the 8 strains (S. Bredeney SA993178) transferred reduced susceptibility to ceftriaxone by conjugation. Resistance to ampicillin, cefoxitin, ceftiofur, cephalothin, streptomycin, sulfisoxazole, and tetracycline was co-transferred, and the blaCMY-2 PCR product could be detected in the transconjugant. A DIG-labeled blaCMY-2 probe showed that the resistance determinant was located on a 60-MDa plasmid in both the donor and the transconjugant (Figure 1).
Figure 1. Southern blot hybridization of the Salmonella Bredeney SA993178 donor and the Escherichia coli C600N transconjugant using a digoxigenin-labeled blaCMY-2 probe. Lane 1 shows the S. Bredeney donor, lane 2 the cefoxitin-resistant E. coli transconjugant, and lane 3 the non-conjugated E. coli.
Transformation
To determine which plasmids encoded antimicrobial resistance, electrocompetent E. coli DH10B bacteria were transformed with plasmid DNA of the Salmonella strains. All donor strains transformed the E. coli recipient, and the transformants were resistant to ampicillin, cefoxitin, ceftiofur, cephalothin, streptomycin, sulfisoxazole, and tetracycline. The S. Ohio and S. Typhimurium isolates also transferred resistance to chloramphenicol, florfenicol, kanamycin, and neomycin. Two transformants, arising from the S. Ohio SA951955 and S. Typhimurium donors, were resistant to florfenicol at 32 μg/mL.
All transformants produced the expected blaCMY-2 PCR product. Hybridization using a DIG-labeled blaCMY-2 probe showed that the gene was located on plasmids of the same size (60 or 96 MDa) in both donors and transformants (Table II). Also, the DIG-labeled floSt probe showed that the floSt gene was located on the same plasmid as blaCMY-2 in both host and transformant.
Integron analysis
To determine whether the plasmid possessing the blaCMY-2 gene also harbored class 1 integrons, primers specific to the 5′ and 3′ conserved segments flanking the gene-cassette insertion site were used. The 4 S. Bredeney isolates were found to produce a PCR product of approximately 1.6 kb. This amplicon was not observed in any of the transformants and is therefore not located on the CMY-2 plasmid. Sequencing revealed the presence of the aadB gene cassette in all 4 isolates. None of the S. Ohio isolates or the S. Typhimurium isolate possessed class 1 integrons.
Analysis of genetic relatedness
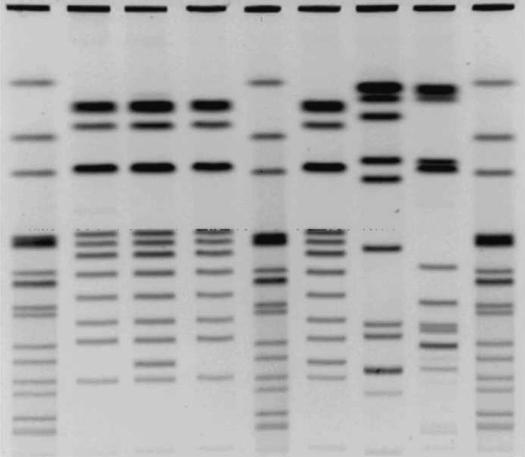
To determine whether clonal dispersion or horizontal transfer plays a role in the dissemination of blaCMY-2, plasmid and PFGE profiles were used to compare the relatedness of isolates belonging to the same serovar. All 4 of the S. Bredeney isolates had similar plasmid and PFGE profiles but were distinguishable (Figure 2, Table I). Two of the isolates (SA992542 and SA993178) were from the same geographic location but had been isolated 78 d apart; they had dissimilar plasmid and PFGE profiles, which suggests that a reservoir of blaCMY-2 may have been maintained and transferred through horizontal means. Two of the 3 S. Ohio had identical plasmid and PFGE profiles. They were from different geographic locations, which suggests clonal dissemination.
Figure 2. Pulsed-field gel electrophoresis patterns of Salmonella isolates possessing the blaCMY-2 gene. Lanes 1, 5, and 9 are S. Newport am01144 digested with XbaI; lanes 2, 3, 4, and 6 are S. Bredeney SA993178, SA992542, SA992721, and SA992306, respectively; lane 7 is S. Typhimurium DT104 (SA972816); and lane 8 is the S. Typhimurium var. Copenhagen strain provided by Dr. Paul Fey. All isolates were digested with BlnI (AvrII).
Discussion
CMY-2-mediated resistance to ESCs among Salmonella isolates from food-producing animals in Canada, although infrequent (found in 0.1% of isolates in 1994–99), is of concern. The increased emergence and possible spread of the blaCMY-2 gene may pose a serious threat to public health. The isolation of 2 resistant S. Bredeney strains from the same animal species at the same premises, 78 d apart, suggests that the blaCMY-2 determinant may have been maintained and possibly transferred through horizontal means within a resident microbial population in turkeys. The 2 isolates had dissimilar plasmid and PFGE patterns and, therefore, were not clonal. Also, finding the blaCMY-2 determinant in 3 different Salmonella serovars suggests that horizontal transfer may be responsible for its increasing prevalence.
Only the S. Bredeney strain SA993178 transferred the resistance determinant conjugally. We do not know the reason for the lack of transfer by conjugation. We have not examined whether the strains that do not transfer the blaCMY-2 gene lack or have mutated tra genes or lack other genes related to conjugal transfer of plasmids. Despite the nonconjugative properties, all plasmids encoding the CMY-2 β-lactamase were transferable by electroporation. A similar lack of conjugative mobility and transferability was found by M'Zali and colleagues (16), who reported that none of 5 E. coli isolates possessing the blaAmpC gene transferred it by conjugation. Winokur and co-workers (4) also found that 80% of blaCMY-2- producing isolates in their study possessed a nonconjugative but transferable plasmid of approximately 75 kb. Fey and associates (3) reported conjugative transfer of a 160-kb Salmonella plasmid possessing the blaCMY-2 determinant in a strain isolated from cattle and a child. Dunne et al (10) reported that 5 of 13 Salmonella isolates (38.5%) transferred ceftriaxone resistance by conjugation.
The reason for the recent emergence of resistance to ESCs mediated by blaCMY-2 is not known. It has been hypothesized that therapeutic use of ceftiofur, a 3rd-generation cephalosporin, in food-producing animals may be responsible for the emergence of ceftriaxone resistance by in vivo selection of strains that hyperproduce CMY-2 β-lactamase (4,11,27). In Canada ceftiofur is approved for therapeutic parenteral use in cattle, dogs, horses, swine, and turkeys. In turkeys, day-old poults may be injected subcutaneously with ceftiofur to control colibacillosis. Considering that 7 of the 8 isolates resistant to ESCs in this study were of turkey origin, this practice may be selecting for CMY-2-producing salmonellae. Similarly, in the United States, where ceftiofur is approved for use in domestic animals (3), 2 of 98 S. Typhimurium isolates (2%) from cattle and chickens at abattoirs were resistant to ceftriaxone (28). Also, Winokur and co-workers (4) found that 6% of veterinary isolates were resistant to ceftriaxone. These data suggest that microbial reservoirs of blaCMY-2 may be selected and maintained through ceftiofur use. In this study, however, it was not possible to determine whether ceftiofur use or other selection pressures selected for the resistance.
Future studies are required to more fully investigate the relationship between ceftiofur use and the potential for development and spread of CMY-2-mediated resistance. Also, as suggested by Winokur and co-workers (4), it is of utmost importance to understand whether the use of other antimicrobials, be it therapeutic or to promote growth, favors the maintenance and proliferation of AmpC-like resistance determinants. The multiresistant nature of these R-plasmids might imply that the use of any drug to which they are resistant would favor maintaining the plasmid.
The evidence presented here suggests that, despite the low occurrence of Salmonella resistant to ESCs in food-producing animals in Canada, it would be prudent to identify reasons for the emergence of these resistant bacteria. Reassessment of current veterinary and industry practices may aid in abating an emerging resistance. If practices do not change, the current situation may be exacerbated to the point of challenging the efficacy of ceftriaxone, ceftiofur, and other ESCs as therapeutic agents in humans and animals. A more detailed understanding of how these AmpC-like resistance determinants are maintained and transmitted in vivo is required. The extensive co-transfer of resistance in the transconjugant and transformants in this study illustrates how antimicrobial resistance presents a multifaceted problem. The emergence of resistance to 1 antimicrobial may be linked to the expression and, or, maintenance of other resistance determinants. Selection pressures, both perceived and not perceived, may be driving the evolution, dissemination, and maintenance of resistance determinants in microbial populations associated with food-producing animals.
Footnotes
Acknowledgments
We are very thankful to Laura Martin for technical assistance and for reviewing the manuscript. We thank Dr. Mohamed Karmali for valuable suggestions and comments. We also thank Ms. Linda Cole, Ms. Bettie Wilkie, and Mr. David Sturrock for serotyping the Salmonella isolates and Dr. Anne Muckle for phagetyping the S. Typhimurium strains.
Address correspondence and reprint requests to Dr. Cornelius Poppe, tel: 519-822-3300, fax: 519-822-2280, e-mail: Cornelius_Poppe@hc-sc.gc.ca
Received March 8, 2002. Accepted April 16, 2002.
References
- 1.Health Canada. Enteric Pathogens Identified in Canada — Annual Summary. Ottawa: Health Canada, 1996.
- 2.Mead PS, Slutsker L, Dietz V, et al. Food-related illness and death in the United States. Emerg Infect Dis 1999;5:607–625. [DOI] [PMC free article] [PubMed]
- 3.Fey PD, Safranek TJ, Rupp ME, et al. Ceftriaxone-resistant Salmonella infection acquired by a child from cattle. N Engl J Med 2000;342:1242–1249. [DOI] [PubMed]
- 4.Winokur PL, Brueggemann A, DeSalvo DL, et al. Animal and human multidrug-resistant, cephalosporin-resistant Salmonella isolates expressing a plasmid-mediated CMY-2 AmpC β-lactamase. Antimicrob Agents Chemother 2000;44:2777–2783. [DOI] [PMC free article] [PubMed]
- 5.Bush K, Jacoby GA, Medeiros AA. A functional classification scheme for β-lactamases and its correlation with molecular structure. Antimicrob Agents Chemother 1995;39: 1211–1233. [DOI] [PMC free article] [PubMed]
- 6.Barnaud G, Arlet G, Verdet C, Gaillot O, Lagrange PH, Philippon A. Salmonella Enteritidis: AmpC plasmid-mediated inducible β-lactamase (DHA-1) with an ampR gene from Morganella morganii. Antimicrob Agents Chemother 1998;42:2352–2358. [DOI] [PMC free article] [PubMed]
- 7.Bauernfeind A, Stemplinger I, Jungwirth R, Giamarellou H. Characterization of the plasmidic β-lactamase CMY-2, which is responsible for cephamycin resistance. Antimicrob Agents Chemother 1996;40:221–224. [DOI] [PMC free article] [PubMed]
- 8.Koeck JL, Arlet G, Philippon A, et al. A plasmid-mediated CMY-2 beta-lactamase from an Algerian clinical isolate of Salmonella Senftenberg. FEMS Microbiol Lett 1997;152: 255–260. [DOI] [PubMed]
- 9.Horton JM, Sing RF, Jenkins SG. Multidrug-resistant Salmonella associated with AmpC hyperproduction. Clin Infect Dis 1999;29: 1348. [DOI] [PubMed]
- 10.Dunne EF, Fey PD, Kludt P, et al. Emergence of domestically acquired ceftriaxone-resistant Salmonella infections associated with AmpC β-lactamase. JAMA 2000;284:3151–3156. [DOI] [PubMed]
- 11.Winokur PL, Vonstein DL, Hoffman LJ, Uhlenhopp EK, Doern GV. Evidence for transfer of CMY-2 AmpC β-lactamase plasmids between Escherichia coli and Salmonella isolates from food animals and humans. Antimicrob Agents Chemother 2001;45:2716–2722. [DOI] [PMC free article] [PubMed]
- 12.Threlfall EJ, Skinner JA, Graham A, Ward LR, Smith HR. Resistance to ceftriaxone and cefotaxime in non-typhoidal Salmonella enterica in England and Wales, 1998–1999. J Antimicrob Chemother 2000;46:860–862. [DOI] [PubMed]
- 13.National Committee for Clinical Laboratory Standards. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals: Approved Standard. NCCLS Document M31-A. Villanova, Pennsylvania: NCCLS, 1999.
- 14.National Committee for Clinical Laboratory Standards. Performance Standards for Antimicrobial Susceptibility Testing; 8th Informational Supplement. NCCLS Document M100-S8. Villanova, Pennsylvania: NCCLS, 1998.
- 15.Matthew M, Harris AM, Marshall MJ, Ross GW. The use of analytical isoelectric focusing for detection and identification of β-lactamases. J Gen Microbiol 1975;88:169–178. [DOI] [PubMed]
- 16.M'Zali FH, Heritage J, Gascoyne-Binzi DM, Denton M, Todd NJ, Hawkey PM. Transcontinental importation into the UK of Escherichia coli expressing a plasmid-mediated AmpC-type β-lactamase exposed during an outbreak of SHV-5 extended-spectrum β-lactamase in a Leeds hospital. J Antimicrob Chemother 1997;40:823–831. [DOI] [PubMed]
- 17.Khan AA, Nawaz MS, Khan S, Cerniglia CE. Detection of multidrug-resistant Salmonella Typhimurium DT104 by multiplex polymerase chain reaction. FEMS Microbiol Lett 2000;182:355–360. [DOI] [PubMed]
- 18.Crosa JH, Falkow S. Plasmids. In: Gerhardt P, ed. Manual of Methods for General Bacteriology. Washington, DC: Am Soc Microbiol, 1981:267–268.
- 19.Maniatis TE, Fritsch EF, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, New York, 1982.
- 20.Sanderson KE, Kadam SK, MacLauglan PR. Derepression of F factor function in Salmonella Typhimurium. Can J Microbiol 1983;29:1205–1212. [DOI] [PubMed]
- 21.Poppe C, McFadden KA, Demczuk WHB. Drug resistance, plasmids, biotypes and susceptibility to bacteriophages of Salmonella isolated from poultry in Canada. Int J Food Microbiol 1996;30:325–344. [DOI] [PubMed]
- 22.Macrina FL, Kopecko KJ, Jones KR, Ayers DJ, McCowen SM. A multiple plasmid-containing E. coli strain: convenient source of size reference plasmid molecules. Plasmid 1987;1:417–420. [DOI] [PubMed]
- 23.Provence DA, Curtiss R III. Gene transfer in gram-negative bacteria. In Gerhardt P, Murray RGE, Wood WA, Krieg NR, eds. Methods for General and Molecular Biology. Washington, DC: Am Soc Microbiol, 1994:337–345.
- 24.Sandvang D, Aarestrup FM, Jensen LB. Characterisation of integrons and antibiotic resistance genes in Danish multiresistant Salmonella enterica Typhimurium DT104. FEMS Microbiol Lett 1998;160:37–41. [DOI] [PubMed]
- 25.Centers for Disease Control. One-day (24–28 h) standardized laboratory protocol for molecular subtyping of non-typhoidal Salmonella by pulsed field gel electrophoresis. Atlanta, Georgia: CDC, 2001.
- 26.Liebisch B, Schwarz S. Molecular typing of Salmonella enterica subsp. enterica serovar Enteritidis isolates. J Med Microbiol 1996;44:52–59. [DOI] [PubMed]
- 27.Bradford PA, Petersen PJ, Fingerman IM, White DG. Characterization of expanded-spectrum cephalosporin resistance in E. coli isolates associated with bovine calf diarrhoeal disease. J Antimicrob Chemother 1999;44:607–610. [DOI] [PubMed]
- 28.Food and Drug Administration, US Department of Agriculture, Centers for Disease Control and Prevention. National Antimicrobial Resistance Monitoring Program: Enteric Pathogens. Rockville, Maryland: FDA, USDA, CDCP, 1998.