Abstract
In order to further understand the role of voltage- and ligand-gated ryanodine receptors in the control of intracellular Ca2+ signalling during myogenesis, changes in cytosolic free calcium concentration ([Ca2+]i) were investigated by fura-2 videoimaging in C2C12 mouse myotubes developing in vitro.
A synchronous [Ca2+]i increase was observed after depolarisation with high [K+], while the Ca2+ response propagated as a wave following caffeine administration. Application of the two stimuli to the same myotube often revealed the existence of cellular zones that were responsive to depolarisation but not to caffeine.
Focal application of high [K+] promoted a [Ca2+]i response detectable only in the cellular areas close to the pipette tip, while focal application of caffeine elicited a [Ca2+]i increase which spread as a Ca2+ wave. Buffering of [Ca2+]i by BAPTA did not affect the pattern of the depolarisation-induced [Ca2+]i transient but abolished the Ca2+ waves elicited by caffeine.
When high [K+] and caffeine were applied in sequence, reciprocal inhibition of the [Ca2+]i responses was observed.
Our results suggest that the different spatial patterns of [Ca2+]i responses are due to uneven distribution of voltage- and ligand-gated ryanodine receptors within the myotube. These two types of receptor control two functionally distinct Ca2+ pools which are part of a common intracellular compartment. Finally, the two differently operated ryanodine receptor channels appear to be independently activated, so that a mechanism of Ca2+-induced Ca2+ release is not required to sustain the global response in C2C12 myotubes.
In skeletal muscle, excitation-contraction (EC) coupling is based on the ‘mechanical’ link between L-type voltage-operated calcium channels (VOCCs) arranged in tetrads on the transverse tubule, and ryanodine receptors (RyRs) inserted in the junctional regions of the sarco-endoplasmic reticulum (SER; reviewed by Ríos et al. 1991; Melzer et al. 1995). Since skeletal muscle contraction does not require extracellular Ca2+ (Armstrong et al. 1972), opening of the junctional type 1 RyRs (RyR1s) is controlled not by Ca2+ entry but by conformational changes in the VOCCs upon depolarisation (Ríos & Brum, 1987; Adams et al. 1990; for review see Ríos & Pizarro, 1991; Melzer et al. 1995; Ríos & Stern, 1997). For this reason, RyR1s coupled to VOCCs are also referred to as ‘voltage-gated RyR1s’ (Klein et al. 1996).
Ultrastructural studies have revealed that RyR1s are inserted in the SER membrane facing the transverse tubule and that VOCC tetrads are located opposite every second RyR1 molecule (for review see Franzini-Armstrong & Jorgensen, 1994). As a consequence, only one out of two junctional RyR1s are gated in a voltage-dependent manner. How the remaining junctional RyR1s are activated is still a matter of debate. According to the ‘stochastic-gating theory’ (Klein et al. 1996), the Ca2+ released by voltage-gated RyR1s is capable of activating nearby ligand-gated RyR1s by a Ca2+-induced Ca2+ release (CICR) mechanism (reviewed by Ríos & Stern, 1997). As an alternative, it has recently been proposed that all junctional RyR1s are mechanically coupled and thereby open/close in a concerted way (Marx et al. 1998). Thus, Ca2+ would not need to co-ordinate the opening of the junctional RyR1s, since activation of the voltage-gated RyR1s would automatically promote activation of the surrounding ‘mechanically gated RyR1s’ as well (see also Shirokova et al. 1998). Even less well defined is the role of RyRs that are located in the extrajunctional domains of the SER. They are generally assumed to participate, via a CICR mechanism, in the amplification of the Ca2+ signals generated at the level of the junctional regions (Takeshima et al. 1995). Accordingly, they are called ‘extrajunctional ligand-gated RyRs’. On the whole, the large uncertainty about the way these mechanisms can operate mainly stems from the incomplete knowledge of the pharmacological properties of RyRs and from the transient expression of different isoforms of the RyRs during EC maturation (Tarroni et al. 1997).
Recently, the fact that the SER as a unitary structure can be organised into distinct compartments that function as discrete units has been accredited to its immense complexity (Golovina & Blaustein, 1997; for review see Meldolesi & Pozzan, 1998). The terminal cisternae are a clear example of domains specialised in sequestering and rapidly releasing Ca2+, but other SER regions may well contribute to Ca2+ regulation (for reviews see Flucher, 1992; Franzini-Armstrong & Jorgensen, 1994).
In the present study, we investigated the spatiotemporal patterns of [Ca2+]i transients following either voltage or ligand activation of the RyRs in C2C12 cells. This cell line not only develops a mature skeletal-type EC coupling (Györke & Györke, 1996; Lorenzon et al. 1997) but also expresses RyR3s, an isoform that is thought to play an important role in the diversification of EC coupling apparatus (Tarroni et al. 1997) and in the voltage-independent control of Ca2+ homeostasis during myogenesis (Shirokova et al. 1999).
Based on our results, we propose that voltage- and ligand-gated RyRs control two distinct, independent Ca2+-releasing pathways. The presence of functionally separated compartments and their possible significance in the control of intracellular Ca2+ signalling is discussed.
Some of these results have been briefly presented previously (Lorenzon & Ruzzier, 1997).
METHODS
Cell culture
The C2C12 cells (Yaffe & Saxel, 1977) were prepared and cultured, according to the procedures previously described (Lorenzon et al. 1997), in a growth medium consisting of Dulbecco's modified Eagle's medium (DMEM) supplemented with 20 % heat-inactivated fetal calf serum, 4 mM L-glutamine, 100 i.u. ml−1 penicillin and 100 μg ml−1 streptomycin. Cell differentiation and myoblast fusion were obtained by switching to DMEM supplemented with 2 % heat-inactivated horse serum, 4 mM glutamine, 100 i.u. ml−1 penicillin and 100 μg ml−1 streptomycin (fusion medium). Experiments were performed on C2C12 myotubes from the third day of culture in fusion medium, i.e. when it is possible to select myotubes with mature skeletal-type EC coupling (see Results).
Fura-2 loading
C2C12 cells were first incubated for 30 min at 37°C in normal external solution (NES, see below) containing 10 mg ml−1 bovine serum albumin and 5 μM of the acetoxymethyl ester (AM) form of fura-2 and then washed with NES for 15 min at 37°C to allow de-esterification of the dye. The homogeneous distribution of the fluorescent dye was carefully checked before each experiment. Fura-2 compartmentalisation was never observed.
BAPTA loading
The loading of the Ca2+ chelator was performed by incubating cells for 3–4 min at 20°C in NES containing 10 mg ml−1 bovine serum albumin and 7 μM BAPTA AM. Afterwards, cells were carefully rinsed with NES.
Videoimaging
The videomicroscopy system was built around a Zeiss (Oberkochen, Germany) Axiovert 135 light microscope as previously described (Lorenzon et al. 1997). Briefly, excitation light at 340 and 380 nm was provided by a modified dual wavelength microfluorimeter (Jasco CAM-230, Tokyo, Japan). Fluorescence images were collected by an intensified CCD camera (Hamamatsu Photonics, Hamamatsu, Japan) and the analog output was digitised and integrated in real time by an image processor (typically 4 frames s−1 acquisition). The ratio of corresponding images at 340 and 380 nm was performed off-line (Grynkiewicz et al. 1985). Experiments were performed in a temperature-controlled microincubator chamber (Medical System Corporation, Greenvale, NY, USA) so that the temperature could be maintained at either 37 or 20°C.
Temporal plots analysis
The temporal plots, i.e. the variations in the mean value of fluorescence intensity, were calculated from ratio images in areas of interest, and analysed using pCLAMP 6.0 Clampfit software (Axon Instruments Inc., Foster City, CA, USA). Peak values and areas subtended by the [Ca2+]i transients were estimated by manually setting the baseline before the onset of each spike and by calculating the maximal value and the integral under the spike. Variations in peak and area values are expressed as a percentage. Data are given as means ±s.e.m.
Solutions and drug application
NES had the following composition (mM): 140 NaCl, 2.8 KCl, 2 CaCl2, 2 MgCl2, 10 glucose, 10 N-(2-hydroxyethyl)piperazine-N′-(2-ethanesulphonic acid) (Hepes)-NaOH (pH 7.3). In the Ca2+-free solution CaCl2 was replaced by 2 mM MgCl2 and 2 mM EGTA was added to chelate residual Ca2+. Drugs were applied either by rapidly changing the whole bath composition (Grohovaz et al. 1991) or by perfusion (1 ml min−1 of solutions administered by switching among lines controlled by pinch-valves) through a plastic pipette (700 μm inner diameter) positioned in close proximity to the cell. Focal application of substances was performed by approaching the cell membrane (∼5 μm distance) with a glass micropipette (3-5 μm inner diameter) containing the stimulating agent and then applying a positive pressure pulse (3-15 mmHg; 1–3 s).
Materials
Dulbecco's modified Eagle's medium, fetal calf serum, horse serum, antibiotics and glutamine were purchased from ICN Biomedicals (Costa Mesa, CA, USA); all other chemicals were from Sigma (St Louis, MO, USA).
RESULTS
Spatial organisation of the [Ca2+]i transients generated by caffeine or high [K+] depolarisation
In the majority of developing C2C12 myotubes, Ca2+ responses can be elicited by administration of caffeine and depolarisation with high [K+] in Ca2+-free solution (Lorenzon et al. 1997). The appearance of these two properties marks the establishment of a mature skeletal-type EC coupling, at which point it becomes possible to activate the RyR channels, with ensuing release of Ca2+ from intracellular compartments, in a ligand- and a voltage-dependent manner. Only C2C12 myotubes which exhibited this capability were used for [Ca2+]i measurements.
Ca2+ release from intracellular stores was studied by fura-2 videomicroscopy after bath application of caffeine or high [K+] in Ca2+-free solution (2 mM EGTA excess); experiments were performed at 20°C to slow down [Ca2+]i changes and better highlight the differences in the patterns of Ca2+ redistribution (Lorenzon et al. 1995). Under these experimental conditions, a clear correlation was observed between the spatiotemporal pattern of the Ca2+ response to caffeine and the concentration of the drug. This is illustrated in Fig. 1 where, within the same myotube, the lowest concentration (2 mM caffeine) promoted a [Ca2+]i elevation that was strictly confined to a discrete region of the cytoplasm (Fig. 1A), while increasing doses caused the localised Ca2+ response to spread over a wider area (Fig. 1B). At the maximal concentration (20 mM caffeine), the Ca2+ response usually invaded the whole myotube (40/47), often as a Ca2+ wave spreading throughout the cytoplasm (18/47; Figs 1C and 2Aa); in a minority of myotubes (7/47), the [Ca2+]i increases remained confined to a subcellular region (see Fig. 2Ba). On the contrary, the spatial evolution of [Ca2+]i changes upon depolarisation appeared to obey more an ‘all or none’ law. In fact, provided that extracellular [K+] was high enough to elicit an appreciable response (typically ≥15 mM), the spatial pattern of Ca2+ signals was invariably synchronous and homogeneous throughout the myotubes (global response; n = 60) and Ca2+ waves were never observed.
Figure 1. Ca2+ responses induced by caffeine in a developing C2C12 myotube.
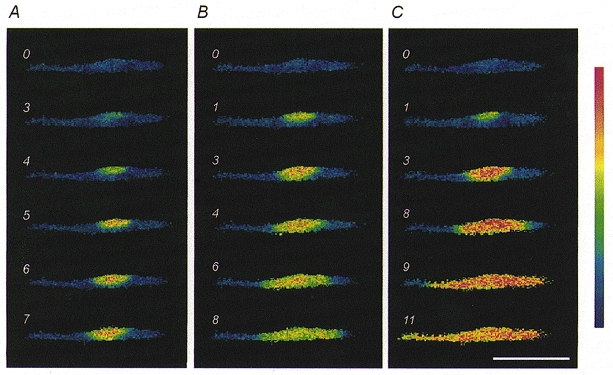
Here and in subsequent figures, the numbers indicate the time in seconds after administration of the stimulus (time 0), and the intensities of fluorescence ratio values are represented according to the pseudocolour calibration bar, where lowest values are coded blue. The same C2C12 myotube, bathed in the Ca2+-free solution, was stimulated by various caffeine concentrations: 2 mM in A; 8 mM in B; 20 mM in C. Note the Ca2+ wave spreading in a dose-dependent way (see text for further details). The C2C12 myotube was washed after each stimulation using the Ca2+-containing solution (NES) in order to reload the Ca2+ releasable pool. Scale bar, 200 μm.
Figure 2. Comparison of the [Ca2+]i changes induced by caffeine and high [K+] in individual myotubes.
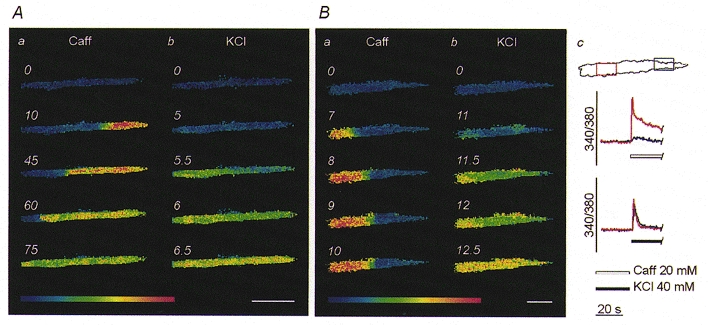
Generally, upon stimulation with 20 mM caffeine (Caff), [Ca2+]i increased in a discrete region before propagating as a Ca2+ wave along the myotube (Aa); in contrast, administration of 40 mM K+ elicited a synchronous [Ca2+]i elevation within the whole cytosol (Ab). On occasion, regions of the myotube were sensitive to high [K+] (Bb) but not to caffeine (Ba); this is further illustrated in Bc, where the red and blue traces represent the temporal plots of [Ca2+]i changes within the two regions marked with the corresponding colours on the upper cell profile. Notice that the red area was excited by both stimuli, while the blue area was insensitive to caffeine. Scale bars, 100 μm.
In light of the experimental variability, the Ca2+ responses elicited by 20 mM caffeine and 40 mM K+ were compared within individual C2C12 myotubes. In these experiments, after administration of the first stimulus, myotubes were extensively washed with Ca2+-containing solution (NES) to reload the intracellular releasable Ca2+ pool. Only myotubes showing a complete recovery of the resting [Ca2+]i levels were exposed to a second stimulus. The results confirmed that the patterns of Ca2+ responses were related to the type of stimulus, not to individual cell variability (Fig. 2A and B).
Interestingly, 40 mM K+ elicited a global response in the myotubes which displayed a localised transient even upon maximal caffeine stimulation (7/7; Fig. 2B). It should be pointed out that in these cases the inability of caffeine-induced [Ca2+]i transients to spread throughout the cytosol cannot be ascribed to weakness of the responses; on the contrary these responses displayed peak and area values which were higher than those induced by high [K+] (Δpeak, +20.88 ± 8.30 %; Δarea, +698.10 ± 66.93 %; n = 5). Finally, it was also possible to exclude an influence of the temperature on the spatial patterns since experiments performed at 37°C produced comparable results with only a moderate increase in the propagation rate of the caffeine-induced Ca2+ waves (data not shown).
Role of CICR in the amplification of Ca2+ signals
In order to verify whether the Ca2+ released from intracellular stores is responsible for the spreading of Ca2+ signals, stimuli were locally applied via a glass micropipette to C2C12 myotubes bathed in Ca2+-free medium. When 40 mM K+ was administered to a discrete region of the cell surface, the [Ca2+]i increase occurred only in the area close to the pipette tip (5/5; Fig. 3A). In contrast, when caffeine was focally applied, the local [Ca2+]i increase was able to evolve into a Ca2+ wave, invading a large part of the cytosol (5/6; Fig. 3B). This issue was further addressed in a parallel series of experiments where stimuli were conventionally administered in the extracellular medium before and after loading the cell with BAPTA, a rapid Ca2+ chelator. Buffering of [Ca2+]i slightly affected the amplitude, but not the pattern of the Ca2+ responses induced by depolarisation (Fig. 4, upper panel). In contrast, the typical caffeine response, i.e. a local [Ca2+]i transient followed by spreading of Ca2+ waves, was virtually abolished by BAPTA, leaving just a moderate, synchronous elevation of [Ca2+]i within the whole myotube (Fig. 4, lower panel).
Figure 3. Spatial organisation of the [Ca2+]i transients elicited by focal application of caffeine and high [K+].
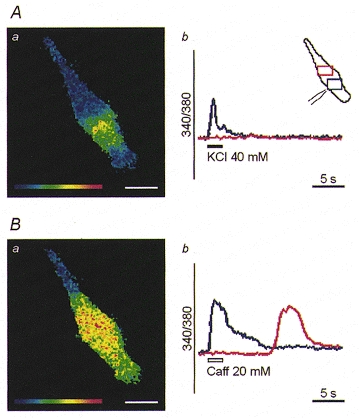
The red and blue traces in Ab and Bb show the [Ca2+]i changes measured in the two regions marked with the corresponding colours in the inset in Ab. The position of the stimulating pipette and recording windows is illustrated in the cell profile (see inset in Ab). The [Ca2+]i increase remained localised in the cellular region close to the pipette when 40 mM K+ was delivered (Aa), but spread into a wider area of the myotube upon focal application of caffeine (Caff) (Ba). Scale bars, 25 μm.
Figure 4. Effect of cytosolic Ca2+ buffering on the spatial organisation of the transients induced by high [K+] and caffeine.
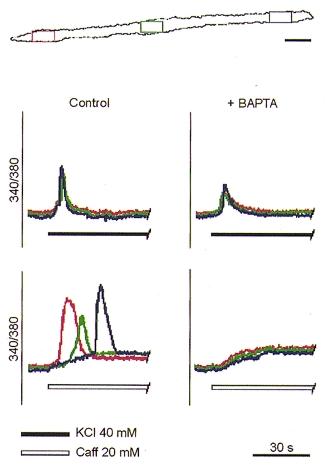
The red, green and blue traces show the [Ca2+]i changes measured in the regions marked with the corresponding colours on the cell profile at the top. Loading of myotubes with BAPTA reduced the amplitude of the Ca2+ response to 40 mM K+ but did not affect the spatial aspects. In contrast, Ca2+ buffering of the cytosol abolished the typical Ca2+ wave in response to caffeine (Caff); this was replaced by a synchronous and moderate elevation of [Ca2+]i. Scale bar, 25 μm.
Voltage- and ligand-induced Ca2+ releases are functionally related
Reloading of voltage- and ligand-releasable Ca2+ pools was established by administering paired stimuli to the bath (at 37°C) and by comparing peak and area values of the successive Ca2+ responses.
When a pair of caffeine pulses were timed so that [Ca2+]i returned to baseline before the second pulse, the responses were comparable provided that the extracellular Ca2+ concentration was in the physiological range (Fig. 5A). However, the second response was virtually abolished when the two caffeine pulses were delivered in Ca2+-free medium (Fig. 5B), thus suggesting that Ca2+ store replenishment requires Ca2+ influx. If the duration of the first pulse was limited to 20 s, i.e. a time insufficient to fully discharge intracellular Ca2+ pools, the response to the second caffeine pulse was only moderately affected (Δpeak, −17.26 ± 1.52 %; Δarea, −11.78 ± 2.22 %; n = 13; Fig. 5C)
Figure 5. Refilling of voltage- and ligand-releasable Ca2+ pools.
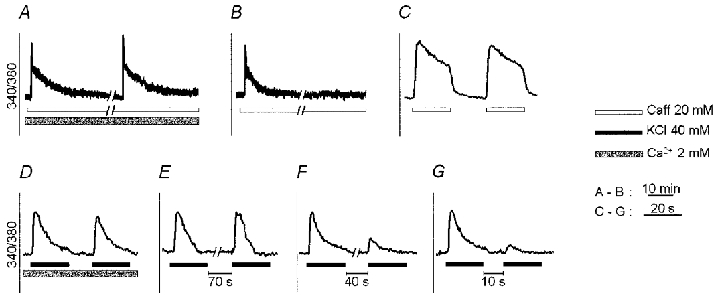
Two consecutive caffeine (Caff) applications (20 mM) were timed so that [Ca2+]i returned to baseline before the second pulse. They elicited similar responses when performed in the presence of extracellular Ca2+ (A), while in its absence, the second caffeine stimulation failed to increase [Ca2+]i (B). When the same experiment was performed in Ca2+-free solution and the duration of the caffeine pulse was reduced to 20 s, the response to a subsequent 20 mM caffeine pulse was virtually unaffected (C). Two consecutive 40 mM K+ pulses, administered in Ca2+-containing medium, also gave rise to comparable responses (D). When this protocol of stimulation was carried out in Ca2+-free medium, the second [Ca2+]i transient was comparable to the first provided that the time interval between the two pulses was >=70 s (E). After shorter intervals (40 s in F and 10 s in G), the response was progressively reduced in amplitude. In C–G the recordings were obtained from the same myotube. In A and B trace interruptions represent an interval of 10 min. Notice the significantly different time scale in A and B vs. C–G.
The situation was somewhat different when C2C12 myotubes were exposed to pairs of high [K+] pulses. The Ca2+ responses were highly reproducible in the presence of extracellular Ca2+ (Fig. 5D) while in Ca2+-free medium the extent of the reduction in the response to the second pulse clearly depended on the time interval between the two treatments: the reduction was minimal after 70 s (Δpeak, −12.46 ± 2.44 %; Δarea, −17.07 % ± 3.09; n = 53; Fig. 5E), marked after 40 s (Δpeak, −32.21 % ± 4.73; Δarea, −36.55 % ± 4.97; n = 19; Fig. 5F) and even stronger after 10 s (Δpeak, −57.35 % ± 4.82; Δarea, −60.10 % ± 3.97; n = 19; Fig. 5G).
From the above results, it can be inferred that there are two distinct Ca2+ pools with different kinetics of release and refilling, even though refilling of both eventually depends on Ca2+ influx. In light of these considerations, and of the differences in the spatial patterns of the Ca2+ signals generated by caffeine and high [K+], experiments were designed to establish whether ligand and voltage activation promotes Ca2+ discharge from one single or two distinct intracellular stores. To this end, C2C12 myotubes were exposed in sequence to maximal concentrations of the two stimulants while bathed in a Ca2+-free solution to prevent store refilling between successive treatments.
Administration of high [K+] did not elicit [Ca2+]i transients in myotubes previously stimulated by 20 mM caffeine when return to resting [Ca2+]i was allowed before administering the second pulse (19/21; Fig. 6A). However, if the duration of the first caffeine pulse was no longer than 20 s, the response to the subsequent 40 mM K+ pulse was little affected (Δpeak, −14.27 % ± 7.35; Δarea, −12.35 % ± 6.73; n = 9; Fig. 6B).
Figure 6. Ca2+ responses to caffeine and high [K+] when administered in sequence in Ca2+-free medium.
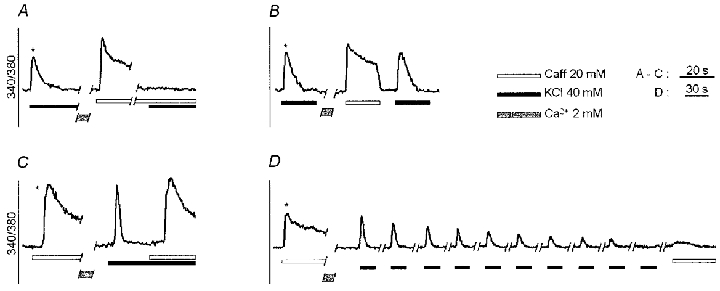
Control stimulations (*) were performed at the beginning of the experiments. When caffeine was maintained in the bath until [Ca2+]i subsided to basal values, the subsequent response to high [K+] was abolished (A). In contrast, in myotubes previously exposed to 20 mM caffeine, the response to 40 mM K+ was not significantly affected (compare with the control stimulation), provided that the duration of the first pulse was <= 20 s (B). When myotubes were first exposed to 40 mM K+, the caffeine (20 mM) response was comparable to the control stimulation (C). However, when a train of 40 mM K+ pulses was administered, the amplitude of the [Ca2+]i transients progressively decreased and, eventually, the caffeine response was abolished (D).
When myotubes were first stimulated by high [K+], the response to a subsequent caffeine pulse was not significantly affected in terms of spatial organisation (3/3; not shown), amplitude (Δpeak, −5.02 % ± 3.79, n = 13) and area of the transients (Δarea, −3.75 % ± 3.06, n = 13; Fig. 6C). However, when a train of high [K+] pulses was administered, the amplitude of the [Ca2+]i transients progressively decreased (Fig. 6D) and, as soon as high [K+] failed to promote [Ca2+]i transients, the caffeine response was also abolished (17/17).
These data suggest that voltage- and ligand-dischargeable Ca2+ pools, although functionally separated, are related.
DISCUSSION
In striated muscle fibres, RyRs are believed to mediate two distinct modes of Ca2+ release from the intracellular pools: depolarisation- and ligand-induced Ca2+ release. Our present study in developing C2C12 myotubes reveals that activation of the RyRs by these two different mechanisms leads to [Ca2+]i elevations that differ in spatial organisation and, possibly, in physiological significance.
Voltage-activated RyRs are not activated by caffeine
It is well known that after maturation of the skeletal-type EC coupling, RyR1s acquire the capability of being activated by plasmalemmal depolarisation. This phenomenon is accompanied by changes in the properties of the RyRs that acquire different sensitivity to intracellular ions (Kramer & Corbett, 1996) and drugs (Takeshima et al. 1995; Oba, 1997). In line with these observations, the diverse spatial patterns of the [Ca2+]i transients observed after caffeine and high [K+] stimulation, could be ascribed to the uneven distribution of RyRs with different pharmacology. Indeed, large regions of cells were sensitive to high [K+] but not to caffeine (e.g. Fig. 2B) thus revealing the presence of RyRs that were voltage gated but not operated by the alkaloid. Since RyR1 is the only isoform that is directly coupled to L-type VOCCs we should infer that, at least in our experimental cell model, RyR1 is not operated by caffeine. This does not imply that the response to high [K+] was sustained solely by voltage-gated RyR1s but leaves open the possibility that surrounding RyR1s are mechanically gated (as proposed by Marx et al. 1998), thereby reinforcing Ca2+ release from inner stores. In developing C2C12 myotubes, RyR3s are also expressed (Tarroni et al. 1997). This isoform is generally thought to be the least sensitive to caffeine (Takeshima et al. 1995, 1996; Murayama & Ogawa, 1997); however, at the caffeine concentration we employed, it should be fully activated (Takeshima et al. 1994) so that the possibility that RyR3s are also resident in the cellular regions insensitive to caffeine appears unlikely.
We propose that the distinct patterns of Ca2+ signalling are triggered by different types of RyRs (possibly RyR1s and −3s) which are unequally distributed in the myotube and that only a subset of receptors (not voltage gated) shows a prominent responsiveness to caffeine.
EC coupling does not require CICR participation
There is general agreement that depolarisation initiates the intracellular Ca2+ response via activation of the VOCC-coupled RyR1s (Ríos & Pizarro, 1991), but whether CICR is required to release Ca2+ effectively from intracellular stores is still a matter of debate. The stochastic gating theory of the EC coupling in skeletal muscle fibres (reviewed by Ríos & Stern, 1997) proposes that membrane depolarisation is sensed by the voltage-gated RyR1 channels which then release an amount of Ca2+ high enough to activate adjacent RyRs by a CICR mechanism. According to this view, a synchronous and global Ca2+ response is the result of two sequential steps: depolarisation provides the trigger while CICR is responsible for the amplification of the initial signal. This mechanism is unlikely to operate in developing C2C12 myotubes, since focal application of high [K+] gave rise to [Ca2+]i transients that were unable to propagate into the neighbouring cellular regions. Likewise, increased buffering of [Ca2+]i by BAPTA did not modify the spatiotemporal patterns of the [Ca2+]i transients induced by depolarisation, but abolished the Ca2+ waves triggered by caffeine.
Our proposal that activation of voltage-gated RyR1 channels does not require the participation of the CICR mechanism to produce a global and synchronous [Ca2+]i variation, is supported by experimental evidence reported by other authors. A study in frog skeletal muscle reported that variations in the membrane voltage influenced neither amplitude nor spatiotemporal aspects of Ca2+ sparks (Lacampagne et al. 1996), the elementary events of Ca2+ signalling in striated muscle cells (Berridge, 1998). As a result, the global Ca2+ response that occurs after a large depolarisation was proposed to be the result of an increase in the probability of Ca2+ spark occurrence (i.e. a diffuse summation of sparks), rather than a spatial amplification of Ca2+ elementary events via CICR activation of the adjacent RyRs (Klein et al. 1996).
Our data, while inconsistent with the stochastic gating theory, do not exclude the possibility that a ‘coordinated gating’ mechanism of activation of the junctional RyRs (Marx et al. 1998; Shirokova et al. 1998) may also operate in C2C12. On the whole, we confirm that ligand-gated RyRs (RyR1s and/or RyR3s) are present in many extrajunctional regions but, on the other hand, we propose that these receptors are too far away to respond to Ca2+ signals upon depolarisation (Takeshima et al. 1995). Their concentration in the extrajunctional regions would, however, confer to these areas the CICR competence (Hirose & Iino, 1994) and thus the potential of sustaining propagation of Ca2+ signals as waves throughout the cytosol.
Voltage- and ligand-releasable Ca2+ pools are parts of the same intracellular Ca2+ store
The spatial organisation of the [Ca2+]i transients generated by high [K+] and caffeine indicates that voltage- and ligand-gated RyRs can coexist in subcellular regions. The cross-inhibition of the Ca2+ responses that is observed when the two stimuli are applied in sequence demonstrates the existence of a unique exchanging Ca2+ store that can be depleted upon either ligand or (repetitive) voltage activation of the RyRs. Our results also suggest that this common store consists of two Ca2+ pools of different size and with some degree of functional independence. In fact, when the two pools were discharged in sequence, an inverse correlation was observed between the extent of depletion of the first and the capability of the second to sustain further Ca2+ release. As a consequence, the smaller pool, i.e. the voltage-activated one, was rapidly exhausted but could be refilled at the expense of the other, provided that enough time for the intraluminal redistribution of [Ca2+] was ensured (see Figs 5E–G and 6D). This body of evidence would suggest a dynamic equilibrium between the two Ca2+ pools that is maintained by slow translocation of Ca2+. While the ‘communication’ between the two Ca2+ pools can represent a safety factor for the cell, their relative independence can be important to sustain distinct spatial patterns upon different conditions of stimulation.
In conclusion, we suggest that two functionally segregated Ca2+ pools, voltage and ligand releasable, are present in developing C2C12 myotubes. This seems not to be a peculiarity of the C2C12 cell line since preliminary experiments performed on developing murine satellite cells (P. Lorenzon & F. Ruzzier, unpublished observations) show similar segregation of the two Ca2+ pools in primary cells. Whether these different spatial patterns reflect a phase of the process of myogenesis or represent a hallmark of the mature EC coupling, remains unclear. Here we can report that once a fully functional EC coupling mechanism was established, the segregation of the Ca2+ pools was invariably observed in C2C12 cells. It is known that morphological differentiation of the EC coupling apparatus requires about 2 weeks in developing mammalian skeletal muscle (Flucher, 1992). In particular, the sarcoplasmic reticulum originates from the progressive transformation of subcompartments of the endoplasmic reticulum that might temporarily maintain some degree of independence.
The separation of purely voltage-gated and ligand-gated RyRs can represent an advantage for both developing myotubes and mature muscle fibres. In fact, whereas activation of the voltage-dependent machinery (voltage-gated RyR1s together with mechanically linked RyR1s) provides a synchronous and global Ca2+ signal that assures an efficient contraction, the ligand-dependent machinery (caffeine-activated RyRs) might guarantee muscle cells the possibility of generating spatially defined [Ca2+]i transients that represent a parallel encoding for intracellular signalling with a high Ca2+ activation threshold (see also Shirokova et al. 1999).
Acknowledgments
The authors wish to thank Drs R. Giniatullin and A. Nistri for their valuable comments on the manuscript, V. Degasperi, A. Giovannelli and A. Acquavita for their help in some experiments, and Fondazione Callerio for providing some laboratory facilities. The financial support of Telethon-Italy (grant 785 to F.R.) is gratefully acknowledged. This work was also supported by grants to F.R. from MURST-Italy, C.N.R. and European Community (grants BMH4-CT97-276 and QLRT-1999-02034).
References
- Adams BA, Tanabe T, Mikami A, Numa S, Beam KG. Intramembrane charge movement restored in dysgenic skeletal muscle by injection of dihydropyridine receptor cDNAs. Nature. 1990;346:569–572. doi: 10.1038/346569a0. [DOI] [PubMed] [Google Scholar]
- Armstrong CM, Bezanilla F, Horowicz P. Twitches in the presence of ethylene glycol bis(α-aminoethylether)-N,N′-tetraacetic acid. Biochimica et Biophysica Acta. 1972;267:605–608. doi: 10.1016/0005-2728(72)90194-6. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Elementary and global aspects of calcium signalling. The Journal of Physiology. 1997;499:291–306. doi: 10.1113/jphysiol.1997.sp021927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonanno A, Fields RD. Gene regulation by patterned electrical activity during neural and skeletal muscle development. Current Opinion in Neurobiology. 1999;9:110–120. doi: 10.1016/s0959-4388(99)80014-2. [DOI] [PubMed] [Google Scholar]
- Flucher BE. Structural analysis of muscle development: transverse tubules, sarcoplasmic reticulum, and the triad. Developmental Biology. 1992;154:245–260. doi: 10.1016/0012-1606(92)90065-o. [DOI] [PubMed] [Google Scholar]
- Franzini-Armstrong C, Jorgensen O. Structure and development of e-c coupling units in skeletal muscle. Annual Review of Physiology. 1994;56:509–534. doi: 10.1146/annurev.ph.56.030194.002453. [DOI] [PubMed] [Google Scholar]
- Golovina VA, Blaustein MP. Spatially and functionally distinct Ca2+ stores in sarcoplasmic and endoplasmic reticulum. Science. 1997;275:1643–1648. doi: 10.1126/science.275.5306.1643. [DOI] [PubMed] [Google Scholar]
- Grohovaz F, Zacchetti D, Clementi E, Lorenzon P, Meldolesi J, Fumagalli G. [Ca2+]i imaging in PC12 cells: multiple response patterns to receptor activation reveal new aspects of transmembrane signaling. Journal of Cell Biology. 1991;113:1341–1350. doi: 10.1083/jcb.113.6.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. Journal of Biological Chemistry. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Györke I, Györke S. Adaptive control of intracellular Ca2+ release in C2C12 mouse myotubes. Pflügers Archiv. 1996;431:838–843. doi: 10.1007/s004240050075. [DOI] [PubMed] [Google Scholar]
- Hirose K, Iino M. Heterogeneity of channel density in inositol-1,4,5-trisphosphate-sensitive Ca2+ stores. Nature. 1994;372:791–794. doi: 10.1038/372791a0. [DOI] [PubMed] [Google Scholar]
- Klein MG, Cheng H, Santana LF, Jiang Y-H, Lederer WJ, Schneider MF. Two mechanisms of quantized calcium release in skeletal muscle. Nature. 1996;379:455–458. doi: 10.1038/379455a0. [DOI] [PubMed] [Google Scholar]
- Kramer JW, Corbett AM. Comparison of Ca2+ loading and retention in isolated skeletal muscle triads and terminal cisternae. American Journal of Physiology. 1996;270:C1602–1610. doi: 10.1152/ajpcell.1996.270.6.C1602. [DOI] [PubMed] [Google Scholar]
- Lacampagne A, Lederer WJ, Schneider MF, Klein MG. Repriming and activation alter the frequency of stereotyped discrete Ca2+ release events in frog skeletal muscle. The Journal of Physiology. 1996;497:581–588. doi: 10.1113/jphysiol.1996.sp021791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzon P, Giovannelli A, Ragozzino D, Eusebi F, Ruzzier F. Spontaneous and repetitive calcium transients in C2C12 mouse myotubes during in vitro myogenesis. European Journal of Neuroscience. 1997;9:800–808. doi: 10.1111/j.1460-9568.1997.tb01429.x. [DOI] [PubMed] [Google Scholar]
- Lorenzon P, Ruzzier F. Dual activation of the ryanodine sensitive calcium compartment in C2C12 myotubes. Trends in Neurosciences. 1997;20(Suppl.):50. abstract 76. [Google Scholar]
- Lorenzon P, Zacchetti D, Codazzi F, Fumagalli G, Meldolesi J, Grohovaz F. Ca2+ waves in PC12 neurites: a bidirectional, receptor-oriented form of Ca2+ signaling. Journal of Cell Biology. 1995;129:797–804. doi: 10.1083/jcb.129.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx SO, Ondrias O, Marks AR. Coupled gating between individual skeletal muscle Ca2+ release channels (ryanodine receptors) Science. 1998;281:818–821. doi: 10.1126/science.281.5378.818. [DOI] [PubMed] [Google Scholar]
- Meldolesi J, Pozzan T. The endoplasmic reticulum Ca2+ store: a view from the lumen. Trends in Biochemical Sciences. 1998;23:10–14. doi: 10.1016/s0968-0004(97)01143-2. [DOI] [PubMed] [Google Scholar]
- Melzer W, Herrmann-Frank A, Luttgau HC. The role of Ca2+ ions in excitation-contraction coupling of skeletal muscle fibres. Biochimica et Biophysica Acta. 1995;1241:59–116. doi: 10.1016/0304-4157(94)00014-5. [DOI] [PubMed] [Google Scholar]
- Murayama T, Ogawa Y. Characterization of type 3 ryanodine receptor (RyR3) of sarcoplasmic reticulum from rabbit skeletal muscles. Journal of Biological Chemistry. 1997;272:24030–24037. doi: 10.1074/jbc.272.38.24030. [DOI] [PubMed] [Google Scholar]
- Oba T. Niflumic acid differentially modulates two types of skeletal ryanodine-sensitive Ca2+-release channels. American Journal of Physiology. 1997;273:C1588–1595. doi: 10.1152/ajpcell.1997.273.5.C1588. [DOI] [PubMed] [Google Scholar]
- Ríos E, Brum G. Involvement of dihydropyridine receptors in excitation-contraction coupling in skeletal muscle. Nature. 1987;325:717–720. doi: 10.1038/325717a0. [DOI] [PubMed] [Google Scholar]
- Ríos E, Ma J, Gonzáles A. The mechanical hypothesis of excitation-contraction (EC) coupling in skeletal muscle. Journal of Muscle Research and Cell Motility. 1991;12:127–135. doi: 10.1007/BF01774031. [DOI] [PubMed] [Google Scholar]
- Ríos E, Pizarro G. Voltage sensor of excitation-contraction coupling in skeletal muscle. Physiological Reviews. 1991;71:849–908. doi: 10.1152/physrev.1991.71.3.849. [DOI] [PubMed] [Google Scholar]
- Ríos E, Stern MD. Calcium in close quarters: microdomain feedback in excitation-contraction coupling and other cell biological phenomena. Annual Review of Biophysics and Biomolecular Structure. 1997;26:47–82. doi: 10.1146/annurev.biophys.26.1.47. [DOI] [PubMed] [Google Scholar]
- Shirokova N, García J, Ríos E. Local calcium release in mammalian skeletal muscle. The Journal of Physiology. 1998;512:377–384. doi: 10.1111/j.1469-7793.1998.377be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirokova N, Shirokov R, Rossi D, Gonzáles A, Kirsch WG, García J, Sorrentino V, Ríos E. Spatially segregated control of Ca2+ release in developing skeletal muscle of mice. The Journal of Physiology. 1999;521:483–495. doi: 10.1111/j.1469-7793.1999.00483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshima H, Iino M, Takekura H, Nishi M, Kuno J, Minowa O, Takano H, Noda T. Excitation-contraction uncoupling and muscular degeneration in mice lacking functional skeletal muscle ryanodine-receptor gene. Nature. 1994;369:556–559. doi: 10.1038/369556a0. [DOI] [PubMed] [Google Scholar]
- Takeshima H, Ikemoto T, Nishi M, Nishiyama N, Shimuta M, Sugitani Y, Kuno J, Saito I, Saito H, Endo M, Iino M, Noda T. Generation and characterization of mutant mice lacking ryanodine receptor type 3. Journal of Biological Chemistry. 1996;271:19649–19652. doi: 10.1074/jbc.271.33.19649. [DOI] [PubMed] [Google Scholar]
- Takeshima H, Yamazawa T, Ikemoto T, Takekura H, Nishi M, Noda T, Iino M. Ca2+-induced Ca2+ release in myocytes from dyspedic mice lacking the type-1 ryanodine receptor. EMBO Journal. 1995;14:2999–3006. doi: 10.1002/j.1460-2075.1995.tb07302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarroni P, Rossi D, Conti A, Sorrentino V. Expression of the ryanodine receptor type 3 calcium release channel during development and differentiation of mammalian skeletal muscle cells. Journal of Biological Chemistry. 1997;272:19808–19813. doi: 10.1074/jbc.272.32.19808. [DOI] [PubMed] [Google Scholar]
- Yaffe D, Saxel O. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature. 1977;270:725–727. doi: 10.1038/270725a0. [DOI] [PubMed] [Google Scholar]


