Abstract
We studied the effect of the continuous vibration of symmetrical dorsal neck muscles in seven normal subjects during (a) quiet standing, (b) stepping in place movements and (c) walking on the treadmill. The experiments were performed in a darkened room and the subjects were given the instruction not to resist the applied perturbation. In one condition the velocity of the treadmill was controlled by feedback from the subject's current position. Head, trunk and leg motion were recorded at 100 Hz.
In normal standing, neck vibration elicited a prominent forward body sway. During stepping in place, neck vibration produced an involuntary forward stepping at about 0.3 m s−1 without modifying the stepping frequency. If the head was turned horizontally 45 and 90 deg to the right or to the left, neck muscle vibration caused stepping approximately in the direction of the head naso-occipital axis. For lateral eye deviations, the direction of stepping was roughly aligned with gaze direction.
In treadmill locomotion, neck vibration produced an involuntary step-like increase of walking speed (by 0.1–0.6 m s−1), independent of the initial walking speed. During backward locomotion, the walking speed tended to decrease during neck vibration.
Thus, continuous neck vibration evokes changes in the postural reference during quiet standing and in the walking speed during locomotion. The results suggest that the proprioceptive input from the neck is integrated in the control of human posture and locomotion and is processed in the context of a viewer-centred reference frame.
Proprioceptive inputs are normally interpreted in frames of reference (such as viewer centred or body centred) derived from multisensory integration (cutaneous, visual and vestibular signals, in addition to muscle proprioception), giving rise to a coherent body scheme for posture (including equilibrium maintenance) as well as for movement (see Massion, 1992; Soechting & Flanders, 1992; Gurfinkel, 1994; Lacquaniti, 1997; Mergner et al. 1997; Gandevia & Phegan, 1999). One approach to probe the role of proprioception in posture and movement consists of applying vibrations to muscles or tendons (thereby activating mainly muscle spindle afferents; Bianconi & van der Meulen, 1963; Goodwin et al. 1972; Burke et al. 1976). When applied to a standing human subject, muscle vibration induces several effects (from illusions of ego- or exo-motion to actual body sway) that depend on the muscle vibrated, the sensory context and the task (Eklund, 1972; Lackner & Levine, 1979; Roll et al. 1989, 1998; Quoniam et al. 1990; Smetanin et al. 1993; Ivanenko et al. 1999b; Kavounoudias et al. 1999; Popov et al. 1999).
The system of postural regulation is considered to consist of numerous different sensorimotor loops (proprioceptive, vestibular, visual) whose interaction maintains the body position and restores lost balance. However, the question of how this interaction is organised remains one of the main problems in studies of the mechanisms of postural regulation. In the context of multisensory control of balance, we earlier studied the effect of neck muscle vibration on human posture during different head-on-feet and eye-in-orbit orientations. The symmetrical vibration of neck dorsal muscles elicited whole-body sway in the direction of the head naso-occipital axis when the eyes were aligned with it, and in the direction of gaze for lateral eye deviations (Ivanenko et al. 1999a). Therefore, we concluded that a viewer-centred frame of reference is important for processing multisensory information.
Based on the postural studies, one might expect that the vibration of neck muscles could also affect locomotion since posture and locomotion share the requirement of controlling the position of the centre of body mass to maintain the dynamic body balance. In the present study we have investigated the effects of applying continuous mechanical vibrations to neck dorsal muscles in healthy humans who performed the following three tasks: quiet standing, stepping in place, and walking on a treadmill at different speeds. The findings reveal that tonic proprioceptive signals evoke involuntary responses in all conditions studied: changes in the reference position during quiet standing, forward translation during stepping in place and changes in the walking speed during locomotion. Moreover, as in the case of quiet standing, the direction of body progression during stepping in place movements was systematically biased by gaze orientation. Thus, the reference frame for the locomotor adjustments in response to neck vibration also seems to be viewer centred.
METHODS
Experimental set-up
The experiments were carried out either on the ground or on a treadmill (Woodway XELG 70, Germany). Horizontal body displacement and orientation of body segments relative to the vertical in the sagittal plane were monitored by means of the ELITE system. Kinematic data were digitised at 100 Hz and filtered with an optimal low-pass FIR filter with automatic band-width selection. The general procedures have been described previously (Borghese et al. 1996; Bianchi et al. 1998). Four 100 Hz TV-cameras were spaced on the recording side of the treadmill to enhance spatial accuracy. After three-dimensional (3-D) calibration, the spatial accuracy of the system was better than 1.5 mm (root mean square). The position of selected points on the right side of the body was recorded by attaching infrared reflective markers to the skin overlying the following bony landmarks (Fig. 1B): gleno-humeral joint (GH), anterior superior iliac spine (ASIS) and posterior superior iliac spine (PSIS), greater trochanter (GT), lateral femur epicondyle (LE), lateral malleolus (LM) and fifth metatarso-phalangeal joint (VM). ASIS and PSIS co-ordinates were averaged to obtain ilium (IL) position.
Figure 1. Experimental set-up.
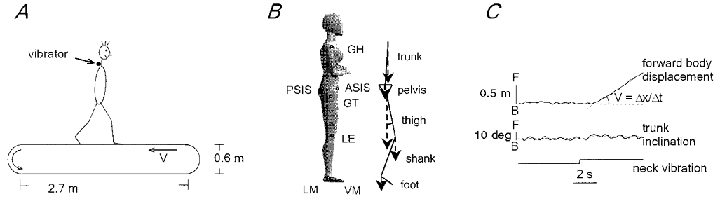
A, schematic view of the treadmill and position of the vibrator. V, treadmill velocity. B, schematic illustration of the body geometry. Markers placed on the right side of the subject were monitored by the ELITE system. From top to bottom: gleno-humeral joint (GH), anterior superior iliac spine (ASIS) and posterior superior iliac spine (PSIS), greater trochanter (GT), lateral femur epicondyle (LE), lateral malleolus (LM) and fifth metatarso-phalangeal joint (VM). ASIS and PSIS co-ordinates were averaged to obtain ilium (IL) position. Trunk, pelvis, thigh, shank and foot are the limb segments identified by these markers. Elevation angles were computed relative to the vertical (dashed line). C, recording of IL displacement (x) and upper trunk inclination induced by neck muscle vibration while walking on the treadmill (1 m s−1). F, forward; B, backward.
Head and shoulder orientation in the horizontal plane were monitored by the Optotrak system (Northern Digital, Waterloo, Ontario, Canada) at 100 Hz (see Ivanenko et al. 1999a).
The instantaneous velocity of the treadmill was recorded via an optical encoder (resolution, 0.005 m s−1) and controlled by a computer at a frequency of approximately 30 Hz. We used two different treadmill control modes: (1) constant velocity and (2) variable position-related velocity. In the latter mode the velocity of the treadmill was controlled by a computer using feedback from the subject's position in such a way that forward displacements from the initial position increased the treadmill velocity while backward displacements decreased it proportionally (Fig. 2A–C). To measure the subject's position, a light-weight stiff thread was attached to the subject (at the level of the waist). The thread was kept in tension by a constant force (about 2 N) produced by a torque-motor (type JR24M4CH, ServoDisc, PMI; Commack, NY, USA). A linear potentiometer on the motor shaft measured the changes in the subject's position (when the velocity of the subject differed from that of the treadmill belt). Position was sampled at 30 Hz with an accuracy of about 2 mm. The subject's initial position was in general set in the middle of the treadmill belt. In order to avoid jerks, the treadmill velocity was low-pass filtered (cut-off frequency, 2 Hz); the transition from one velocity to another was automatically programmed with a ramp profile of 1 m s−2 acceleration. This allowed the damping of fast changes in treadmill velocity due to the natural horizontal oscillations of the body (of about ±5 cm; see Thorstensson et al. 1984) during locomotion. The servo provided an efficient and stable control of the treadmill speed in the frequency range 0–2 Hz. Safety circuits were incorporated into the system. In addition, either the experimental subject or the experimenter could stop the treadmill at any time.
Figure 2. Position-related feedback for the control of treadmill velocity.
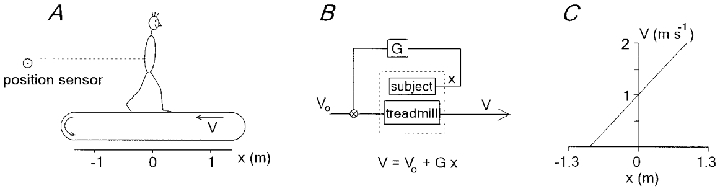
A, schematic view of the treadmill. V, treadmill velocity. B, block diagram of the position-related velocity controller. V0, initial velocity; x, position of the subject; G, gain. C, the law of the controller: the treadmill velocity changed in proportion to the horizontal displacement of the subject relative to the starting position (x= 0) as measured by a position sensor: a 1 m forward body displacement produced a 1.1 m s−1 increase of speed (G= 1.1). The subject started to walk from the central position.
Subjects
It is known that some subjects do not show behavioural effects upon muscle vibration (Eklund & Hagbarth, 1966; Gurfinkel et al. 1998). Thus, in a preliminary session we tested the body sway response to dorsal neck muscle vibration during quiet standing with the eyes open. Out of 10 healthy subjects tested, 7 turned out to be sensitive to vibration from the very first application. These subjects (aged 22–39 years, 4 males and 3 females) participated in the full series of experiments on locomotion. None of the subjects had any history of neurological disease or vestibular impairment. Written informed consent was obtained from all participants after the experimental procedure had been fully explained. The study was performed according to the Declaration of Helsinki, and the procedures were approved by the Ethics Committee of the Santa Lucia Institute.
Parameters of vibration
Stimulation of neck muscle proprioceptors (0.8 mm, 80 Hz sinusoid) was carried out by means of a custom-designed electromechanical vibrator (DC motor, equipped with small eccentric rotating masses; see Ivanenko et al. 1999a). The vibrator was fixed to the back of the neck (trapezius and splenius tendons, at the level between the fifth and seventh vertebrae) by means of an elastic shoulder girdle. Care was taken to place the vibrator in a symmetrical position with respect to the cervical spine in all tested head orientations, since if vibration is applied asymmetrically it may induce lateral displacements during standing (Smetanin et al. 1993).
Because this vibration could be mechanically transmitted to the vestibular apparatus in the inner ear, in four subjects we tested whether the direct vibration of the skull applied to the occipital pole induced the same effect as neck muscle vibration during quiet standing and stepping in place. Skull vibration could cause a small unbalance; however, the direction of sway was inconsistent and its amplitude was much smaller than during dorsal neck muscle vibration.
Protocol
Quiet standing and stepping in place movements were performed by the subjects in a darkened room with the eyes either open or closed. Subjects walked on the treadmill in a darkened room with their eyes open only (since they did not feel comfortable with their eyes closed for more than a few steps). In order to record the markers located on the right side of the body, the subjects were asked to keep their arms folded on their chest. In all experiments subjects were instructed not to resist the applied perturbation.
Experiments were performed in the following four conditions.
Posture
Subjects stood on a force platform (Kistler 9281B) which was used to measure the displacement of the centre of pressure in the sagittal and frontal directions. The centres of the heels were placed on marks 12 cm apart and the feet were splayed out at approximately 30 deg. After 5 s of quiet standing, a 6–8 s period of muscle vibration was applied.
We studied whether the direction of body sway induced by neck muscle vibration could be biased by horizontal head-on-feet and eye-in-orbit orientation (see Ivanenko et al. 1999a). To study the effect of head-on-feet orientation, before each trial the participant was asked to orient the head towards one of the following directions: straight ahead, 45 deg left, 45 deg right, 90 deg left, 90 deg right. Because 90 deg head rotations were at the end of the physiological range, subjects were allowed to accompany the head motion with a small rotation of the shoulders relative to the feet; this was measured to be 12 ± 6 deg (mean ±s.d.) (we evaluated the orientation by applying two markers to the shoulders). The subjects were instructed to look straight ahead (with respect to head orientation). To study the effect of eye-in-orbit position, the subjects were instructed to take different head-on-feet orientations (0 deg, 45 deg to the right and 45 deg to the left) and then different horizontal eye-in-orbit positions by fixating a visual target placed at the sightline height either straight ahead, or 30 deg to the right or 30 deg to the left relative to the required head position.
Stepping in place
Subjects were asked to step in place. After about 5–7 s of stepping movements at a comfortable cadence (about 0.5-1 Hz), muscle vibration was delivered (for 7–10 s). We studied whether the direction of body progression induced by neck muscle vibration could be biased by head-on-feet and eye-in-orbit orientation (same protocol as for posture).
Treadmill locomotion at constant belt speed
After 5–7 s of ‘steady state’ 1 m s−1 locomotion on the treadmill, muscle vibration was applied. Changes in walking speed were measured by computing the slope of the regression line fitting the displacement of the ilium (Fig. 1C).
Treadmill locomotion at variable position-controlled belt speed
To study the time course of speed changes we set up a walking condition where the subjects were free to change their current speed upon the application of vibration without the risk of reaching the belt limits. The experiment was performed in the following way: the subject was placed at the centre of the treadmill belt, with the initial speed set at 1 m s−1 (in some experiments the initial speed was set at 0.5-1.5 m s−1 in 0.25 m s−1 increments), and began walking (Fig. 2A); then the treadmill velocity was made to vary in proportion to the change in the subject's position relative to the starting point (by 1.1 m s−1 per 1 m displacement; Fig. 2C).
The change of walking speed induced by 10–15 s muscle vibration was calculated as the difference between the mean treadmill velocity (as derived from the optical encoder) during the last 5 s of muscle vibration and the mean treadmill velocity during the 5 s before vibration started.
Data analysis
The body was modelled as an interconnected chain of rigid segments: GH-IL for the trunk, IL-GT for the pelvis, GT-LE for the thigh, LE-LM for the shank and LM-VM for the foot (Fig. 1B). The main limb axis was defined as GT-LM. The elevation angle of each segment in the sagittal plane corresponds to the angle between the projected segment and the vertical. The gait cycle duration (T) was defined as the time between two successive maxima of the elevation angle of the main limb axis (Borghese et al. 1996). The stride length during walking on the treadmill was estimated as the treadmill speed multiplied by T averaged over several step cycles.
The intersegmental co-ordination was evaluated in signal space as previously described (Borghese et al. 1996). Briefly, the changes of the elevation angles of the thigh, shank and foot covary linearly throughout the gait cycle. The thigh-shank-foot 3-D loops describe paths that can be fitted by a plane, which can be computed by means of orthogonal linear regression. The orientation of the plane relative to the three axes was measured as the direction cosine of the normal to the plane (i.e. the third eigenvector of the covariance matrix) and quantifies the temporal coupling among the three limb segments (Bianchi et al. 1998).
The change of trunk, shank and thigh orientation and centre-of-pressure displacement induced by 6–8 s muscle vibration during quiet standing were calculated as the difference between segment orientation during the last 5 s of muscle vibration and that in the 5 s period before vibration. Statistical analysis (Student's paired t test, within-subjects ANOVA) was performed on the increment in the walking speed evoked by muscle vibration. P < 0.05 was considered significant.
RESULTS
Posture
Neck muscle vibration during quiet standing evoked typical prominent forward body sway; the change in the centre of pressure in the sagittal direction was 5.6 ± 2.8 cm (mean ±s.d.). Spontaneous oscillations of the body in quiet standing mainly involve a rotation at the ankle joint and the body can be considered to a first approximation as a single segment (see Gurfinkel et al. 1994). During muscle vibration, trunk inclination generally resulted from simultaneous rotation at the pelvis, hip and knee, in addition to the ankle. The change in trunk inclination was 9.1 ± 4.0 deg, the change in thigh inclination was 3.9 ± 2.8 deg and the change in shank inclination was 3.8 ± 1.6 deg (positive values denote forward tilt). Therefore, the mean change of the trunk inclination was larger than that of the thigh and shank.
Stepping in place
When neck muscles were vibrated during stepping in place, subjects started moving forward. This is illustrated in Fig. 3A. Forward displacement was characterised by changes of the phase relation among adjacent lower limb segments. This can be appreciated in the 3-D position space (Fig. 3, bottom). Before vibration, the loops representing intersegmental co-ordination were close to a straight line due to the fact that the phase shift between adjacent segments was either about 0 or 180 deg. Application of muscle vibration did not increase the stepping frequency (stepping frequency was 0.79 ± 0.11 and 0.79 ± 0.10 cycles s−1 before and during neck vibration, respectively). Instead, it gave rise to a phase shift between thigh and shank segments and a forward translation of the centre of body mass. The thigh-shank- foot 3-D loops during forward translation describe paths that can be fitted by a planar surface (99 % of variance was explained by the planar regression; see Borghese et al. 1996). However, motion did not result from a transition to a natural locomotor pattern, as reflected by the differences in the patterns of co-ordination described by the 3-D gait loops of Fig. 3. Instead, subjects continued stepping in place but the swinging foot systematically landed in front of the standing foot.
Figure 3. Changes in kinematics evoked by neck muscle vibration during stepping in place (A) and walking on the treadmill with position-related belt speed (B), in the same subject.
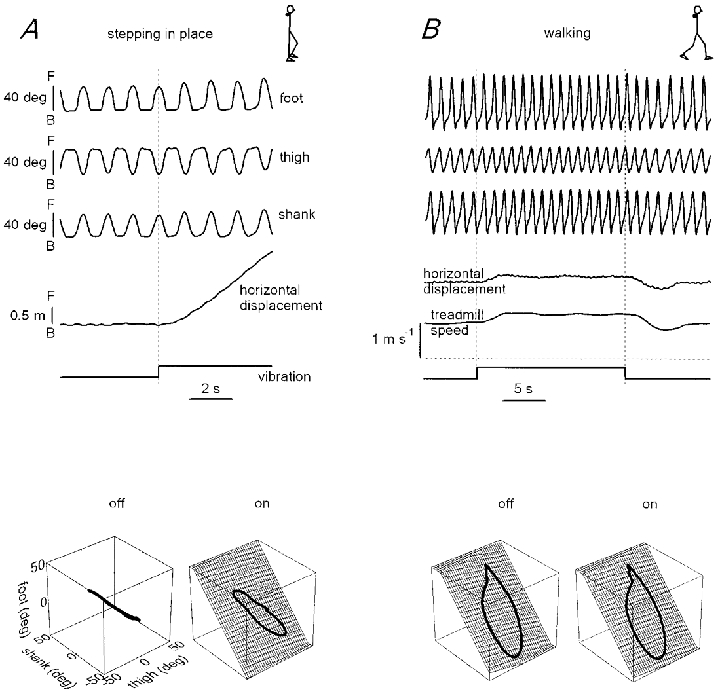
Labels and calibrations given in A also apply to B. F, forward inclination (corresponds to the clockwise rotation of segments on the top schematic drawings); B, backward inclination. Bottom 3-D plots represent the average thigh-shank-foot loops before (off) and during (on) vibration. During stepping in place without vibration, the three elevation angles covary linearly due to in- and opposite-phase changes in the waveforms. During vibration and during walking the elevation angles form a loop that evolves over a plane. However, the loop trajectory clearly differs between stepping in place and locomotion.
The mean speed of the evoked body displacement after neck muscle vibration was 0.24 ± 0.09 m s−1 (Fig. 4) while in the control (unperturbed) condition the speed of the spontaneous displacement of the subject during 10 s of stepping in place was 0.02 ± 0.02 m s−1.
Figure 4. Speed of forward progression during stepping in place and speed changes during walking on the treadmill evoked by continuous neck muscle vibration.
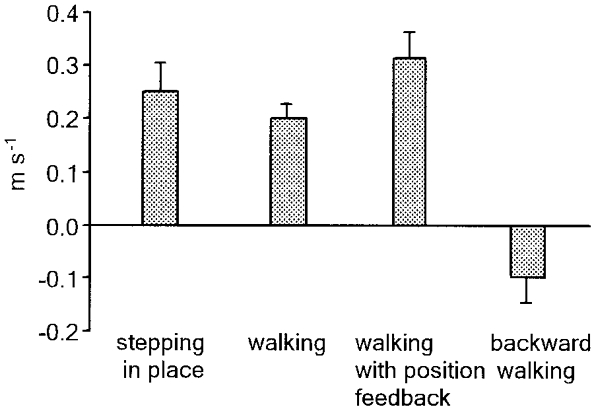
Walking on the treadmill was tested in three conditions: with a constant belt velocity (1 m s−1), with position-controlled belt velocity (the initial speed was about 1 m s−1), and in the backward direction (at about 0.5-0.75 m s−1). Bars represent means ±s.e.m. and were obtained by pooling the data from all subjects (n= 7); positive values indicate speed increments regardless of the walking direction.
Stepping in place per se (Table 1, Control) evoked some forward trunk inclination with respect to quiet standing (t(7) = 6.79, P < 0.0005, paired t test). Neck muscle vibration induced additional changes in trunk inclination; however, final trunk inclination was not significantly different from that induced in quiet standing (t(7) = 0.41, P= 0.70, paired t test).
Table 1.
Trunk orientation (deg) relative to the vertical in the unperturbed condition (Control) and during neck muscle vibration
| Control | Neck vibration | |
|---|---|---|
| Quiet standing | −4.0 ± 2.5 | 4.2 ± 4.4 |
| Stepping in place | 1.1 ± 3.3 | 4.1 ± 5.2 |
| Walking on the treadmill at 1 m s−1 | 0.5 ± 2.8 | 4.3 ± 5.3 |
Values are means ± S.D. (n= 7). Positive values denote forward tilt of the shoulders.
When stepping in place with the eyes closed, the effect of muscle vibration on the stepping speed increment (0.29 ± 0.15 m s−1) was not significantly different (F1,6= 4.0, P > 0.09, within-subjects ANOVA) from that observed with the eyes open in a darkened room (0.24 ± 0.09 m s−1).
Walking on the treadmill with a constant belt speed (1 m s−1)
In this protocol the subjects underwent vibration of the neck muscles while they were walking on the treadmill at 1 m s−1. Changes of walking speed upon stimulation were allowed within the limits of the treadmill length. Displacements tended to develop linearly with time; therefore, we measured the increment in walking speed as the slope of the regression line fitting the displacement of the pelvis point vs. time during the stimulus (Fig. 1C). The stimulation was switched off when the subject approached the end of the treadmill belt.
The relative effect of neck muscle vibration was roughly similar to that during stepping in place: vibration increased walking speed by about 0.2 m s−1 (that is, by about 20 % of the initial walking speed; Fig. 4). Trunk inclination induced by vibration was not significantly different from that induced in quiet standing or stepping in place (Table 1, right column).
Walking with constant and position-related treadmill velocity
While walking on the treadmill with position-related belt velocity (that is, when treadmill velocity was automatically adjusted to keep the subject's position constant), the speed increased often with a ramp-and-hold profile (Fig. 3B) and sometimes more progressively during muscle vibration. The relative magnitude of the effect of muscle vibration at steady state tended to be slightly larger than that during locomotion with a constant belt velocity (Fig. 4). At 1 m s−1 constant treadmill speed, neck muscle vibration increased walking speed by 0.19 ± 0.07 m s−1 (mean ±s.d.), whereas with position feedback the increase was 0.31 ± 0.15 m s−1.
The walking speed increment induced by neck vibration was accompanied by an increase in the stepping frequency and stride length. The stepping frequency was 0.84 ± 0.10 cycles s−1 before the stimulus and 0.96 ± 0.10 cycles s−1 during the stimulus, and the stride length was 1.26 ± 0.12 m before the stimulus and 1.30 ± 0.21 m during the stimulus.
We estimated whether the increments in speed were the result of changes to faster, but still ‘normal’ gait. The intersegmental co-ordination was evaluated in the 3-D position space (Fig. 3B, bottom) to quantify the temporal coupling among the three limb segments (Bianchi et al. 1998). During walking, the 3-D gait loops always evolved over a plane both with and without vibration (99 % of variance was explained by the planar regression). The orientation of the regression plane (as estimated by the third eigenvector of the thigh-shank-foot covariance matrix) changed upon vibration of the neck muscles (from 0.26 ± 0.11 to 0.22 ± 0.15; F1,6= 5.7, P= 0.05, within-subjects ANOVA) indicating a change in the phase relation among the three limb segments similar to what happens during natural speed increments (Bianchi et al. 1998).
The experiments with position-related treadmill velocity demonstrated that after the cessation of vibration the walking speed returned to the pre-stimulus level gradually or with some overshoot (Fig. 3B).
The effect of muscle vibration during backward locomotion was tested in the speed range 0.5-0.75 m s−1. Vibration of the neck muscles during backward walking caused different consequences as compared with forward walking (Fig. 4); vibration slowed locomotion down rather than speeding it up.
Influence of initial velocity
We tested whether the increment of walking speed evoked by neck vibration depends on the initial ‘background’ velocity. For this purpose we used the position-related mode of treadmill velocity control, starting with different initial speeds (in the range 0.5-1.5 m s−1). Figure 5 shows the results of such an experiment. The velocity increment due to muscle vibration tended to decrease slightly with increasing initial walking speed, although this trend was not statistically significant (F4,24= 1.47, P > 0.24, within-subject ANOVA).
Figure 5. Walking speed increment induced by neck muscle vibration while walking on the treadmill with different initial velocities (from 0.5 to 1.5 m s−1).
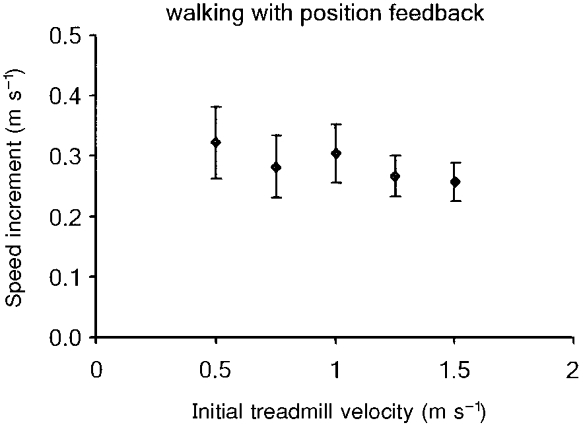
The treadmill velocity was controlled according to the subject's position. Values are means ±s.e.m.
It is worth noting that no subject showed a transition from walking to running. We tested in two subjects the effect of neck muscle vibration at an initial velocity (2 m s−1) close to or higher than the spontaneous velocity of the transition from walking to running (on average 1.9 m s−1; see Thorstensson & Roberthson, 1987). Vibration elicited a further increment of the walking speed (by 0.22 and 0.32 m s−1) but no transition to running. However, the effect of vibration was not confined to the walking mode; during running (at 2.3 m s−1), muscle vibration also elicited a speed increment in the same two subjects (by 0.30 and 0.27 m s−1).
Effect of head-on-feet and eye-in-orbit orientation during posture and stepping in place
Figure 6 shows how head-on-feet orientation biases the direction of the displacement of the centre of pressure induced by neck muscle vibration during quiet standing (A) and the direction of body translation during stepping in place (B), in a representative subject. When the head was rotated horizontally by 45 and 90 deg to the right or to the left, the direction of body displacement evoked by neck muscle vibration tended to align roughly with head orientation. The trunk did not rotate in the direction of the head and, thus, in the direction of the trajectory (as happens during natural curvilinear locomotion around a corner; see Grasso et al. 1998). Instead, the feet moved obliquely under the trunk and as a result stepping occurred in the lateral direction.
Figure 6. Dependence of the reaction to neck muscle vibration upon head-on-feet orientation (-90, -45, 0, 45, 90 deg).
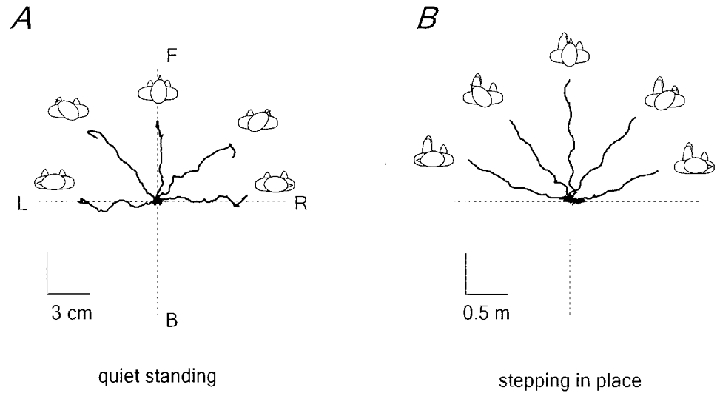
A, displacement of the centre of pressure during standing. B, displacement of the body (ilium) during stepping in place. The x–y plots show the data from the 8–10 s period covering the stimulus and 3 s before the stimulus. F, forward; B, backward; R, right; L, left. Note the difference in scale for A and B.
In addition, the direction of body displacement evoked by neck muscle vibration was significantly biased by the horizontal eye-in-orbit position (Fig. 7B). In every combination of eye (0, -30 and 30 deg) and head (0, -45 and 45 deg) deviation, the body displacement induced by neck vibration during stepping in place movements tended to be directed towards the visual target, not towards the direction of the head or the trunk. The effect of gaze was highly significant: the mean gaze-induced difference in the direction of body displacement between two conditions, when fixating targets 30 deg to the left and 30 deg to the right of the head (data from all head orientations, -45, 0, 45 deg, were pooled together) was 50 ± 15 deg (the nominal angle between the two gaze directions was 60 deg). Visual target fixation was not crucial. Similar directional gaze-dependent body displacements in response to neck muscle vibration were observed with the eyes closed when the subjects were asked to orient their eyes towards the position of an imaginary target located about 30 deg to the left or to the right relative to the head. Thus, the direction of the body displacement tended to align with gaze orientation, as is the case for the body sway induced by neck vibration in the standing posture (Fig. 7A; see also Ivanenko et al. 1999a; Grasso et al. 1999).
Figure 7. Dependence of the reaction to neck muscle vibration upon gaze orientation.
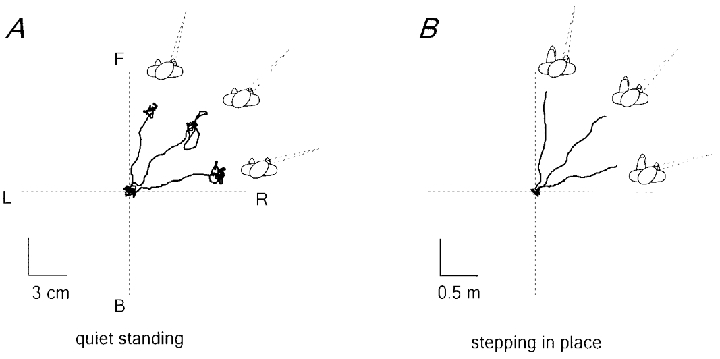
Three eye-in-orbit orientations are shown (-30, 0, 30 deg) when the head was rotated 45 deg to the right. A, displacement of the centre of pressure during quiet standing. B, displacement of the body (ilium) during stepping in place. The x–y plots show the data from the 8–10 s period covering the stimulus and 3 s before the stimulus. F, forward; B, backward; R, right; L, left.
Perceptual effects
Subjects were aware of the body sway and the walking speed increment induced by neck muscle vibration in quiet standing, stepping in place with the eyes open or closed and walking on the treadmill with the eyes open. In the no-intervention task, all subjects reported that they increased their speed as if ‘something was pushing me forward’. If so required, the subjects could make an effort to resist the imposed perturbation; this resulted in a reduction or suppression of the walking speed increment induced by neck muscle vibration.
DISCUSSION
The results show that a continuous vibration of neck muscles induces an involuntary forward displacement during stepping in place or an involuntary increase in the speed of progression during walking. The proprioceptive input evoked by muscle vibration seems to act as a sort of peripheral ‘push-button’ which can vary the activity of a hypothetical speed regulator without causing a transition from step-in-place to walk patterns, or from walk to run patterns. Furthermore, the changes in limb kinematics (Fig. 3) seem to be virtually indistinguishable from those during a natural voluntary increment in speed (Bianchi et al. 1998). The same stimulus, when applied in quiet upright standing, induces forward body sway. The head-on-feet and eyes-in-head directions summed together in biasing the direction of inclination during standing and the direction of progression during stepping in place. We argue below that these phenomena can be explained by the mechanisms whereby body configuration and movement are represented.
Local versus global effect of proprioceptive stimulation
It is worth emphasising that, in the standing posture, muscle vibration induces not local but global reactions related to the change of whole-body orientation relative to the vertical. The transition from local muscle reactions (in sitting posture, for example) to a whole-body sway was observed during stimulation of different muscles: shin, hand, neck and even eye muscles (Eklund, 1972; Lackner & Levine, 1979; Lund, 1980; Roll et al. 1989, 1998; Quoniam et al. 1990; Smetanin et al. 1993; Ivanenko et al. 1999b;Kavounoudias et al. 1999). For instance, in contrast to the sitting posture, neck muscle vibration during standing does not evoke changes in head-on-trunk orientation; instead, it elicits whole-body inclination. These findings stimulated the elaboration of the concept of postural body scheme (see Massion, 1992; Gurfinkel, 1994) and led to the conclusion that proprioceptive signals in the standing posture are interpreted as signals linked to the change in whole-body orientation.
Neck tonic effects, together with vestibular effects, were designated as some of the most important reflexes ensuring the maintenance of posture. Both neck proprioceptive and vestibular influences are related to the stability of the current posture (Britton et al. 1993; Fitzpatrick et al. 1994; Day et al. 1997; Ivanenko et al. 1999b). For example, the short- and middle-latency responses of leg muscles to galvanic vestibular stimulation were increased during standing on an unstable platform relative to normal standing and, in contrast, they disappeared altogether during upright standing with the trunk fixed (Fitzpatrick et al. 1994). During standing on an unstable movable support, neck muscle vibration elicits a quick initial body sway which is absent during quiet standing (Ivanenko et al. 1999b). In the sitting position, neither galvanic vestibular stimulation nor neck muscle vibration evokes responses in leg muscles (Smetanin et al. 1993; Fitzpatrick et al. 1994). It is worth noting the absence of postural responses to neck muscle vibration in bilateral labyrinthine-defective patients (Lekhel et al. 1997). As far as it concerns locomotion, not only neck muscle vibration (Fig. 4) but also galvanic vestibular stimulation has a prominent influence on walking trajectory (Fitzpatrick et al. 1999). Thus, vibration-evoked responses from neck muscles might reflect the presence of vestibular influences on the control of body orientation.
In general, controlling movement implies a simultaneous control of posture; when movement is planned, its postural consequences must be included in the motor programme (Mori, 1987; Massion, 1992; Jankowska & Edgley, 1993; Grasso et al. 2000): ‘posture follows movement like a shadow’ as Sherrington (1910) realised at the beginning of the last century. In fact, the interesting finding of our study was that neck muscle vibration not only elicited a change in body configuration relative to the vertical, as happens during quiet standing, but it also varied the speed of body progression.
Walking speed increment induced by neck muscle vibration
Since during quiet standing there was a forward shift of the body centre of mass and a resultant increment of the torque in the ankle joint, one could expect a similar compensatory effect during stepping in place and locomotion: a forward shift of the foot pressure (‘stepping on the toes’) but not a forward body progression. However, neck muscle vibration seemed to evoke different reactions depending on the ‘state’ of the system: changes in the reference position in a postural context, and changes in the speed of body progression in a locomotor context.
What is the mechanism of the walking speed increment evoked by neck muscle vibration? One might hypothesise that continuous neck muscle vibration engages brain structures involved in the control of the locomotor rhythm. For example, it has been found that continuous vibration of leg muscles in humans can produce rhythmic locomotor-like movements of the suspended leg (Gurfinkel et al. 1998). However, the increment in the walking speed can hardly be explained by a non-specific excitation of the central pattern generator, since (i) during backward locomotion, the walking speed decreased (Fig. 4), (ii) during stepping in place movements, neck muscle vibration did not evoke an increase in the stepping frequency but rather caused a change in the phase shift among lower limb segments and a forward progression (Fig. 3A), and (iii) the direction of progression during stepping in place was related to head-on-feet and eye-in-orbit orientation (Figs 6 and 7).
The mechanism underlying the speed increment may in contrast be similar to that invoked in the postural changes. It is possible that the reaction to head-related sensory perturbation represents a whole-body adjustment due to an altered internal representation of body orientation. A recent positron emission tomography study has shown that the motor rather than somatosensory cortex seems to be mainly activated during the illusion of limb movements induced by muscle vibration (Naito et al. 1999). Taking into account the reports of the subjects (‘something was pushing me forward’), one cannot exclude the possibility that vibrating the back of the neck produces an illusion of forward movement of the head or forward sway, to which the appropriate response is to step forward or speed up forward gait. Yet, the direction of the actual body sway is known to be typically opposite to that of the illusory whole-body tilt (Lackner & Levine, 1979; Fitzpatrick et al. 1994, 1999).
Alternatively, neck proprioceptive influences might be well framed in the context of information coming from the other sensory modalities (vestibular and visual). As already proposed for the postural effect (Lekhel et al. 1997), neck muscle vibration may evoke a distorted internal representation of the body configuration in the gravity field. Proprioception signals muscle lengthening (Prochazka, 1996) and, hence, head-on-trunk flexion. However, because the vestibular (and visual) input is constant, the head might be interpreted as stationary and the trunk tilted backward with respect to the support surface (Fig. 8A). As a result, the internal representation of the body centre of mass would be displaced backward, behind the supporting foot. In order to restore the dynamic equilibrium in the context of this distorted representation, compensatory moments of force are generated resulting in a forward acceleration of the body centre of mass, a forward inclination of the trunk and the perception that ‘something was pushing me forward’. Since the trunk is interpreted as being tilted with respect to the head (or gaze) sagittal plane, postural reactions are biased by head-on-feet and eye-in-orbit orientation (Figs 6 and 7). This mechanism by no means contradicts explanations in terms of reflex arcs (for example, at the level of motoneurones: Conway & Rosenberg, 1984; or vestibular nuclei: Wilson, 1991) but it provides a basis for understanding the functional reorganisation of reflex arcs that occurs in different behavioural contexts.
Figure 8. Hypothetical explanation of the effects of neck muscle vibration.
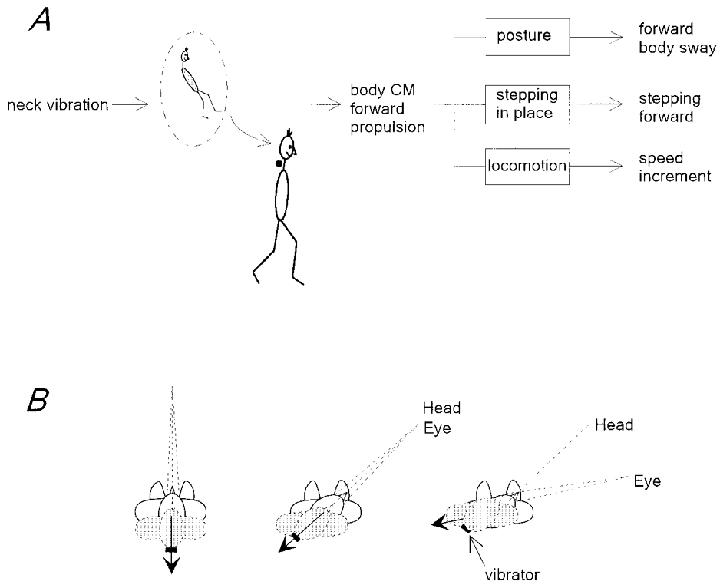
A, with a neutral head-on-trunk position, neck vibration induces a change in the internal representation of the body compatible with a backward displacement of the centre of mass (CM) relative to the support. A compensatory reaction propels the centre of mass forward. The final effect depends on the state of the system. B, gaze induces a redirection of the representation of the centre of mass displacement (arrow) evoked by neck muscle vibration. Three different conditions are shown: left panel, median head and eye position; middle panel, 45 deg head-on-feet and median eye-in-orbit orientation; and right panel, 45 deg head-on-feet and 30 deg eye-in-orbit orientation. The arrow is aligned with the direction of gaze and its direction is opposite to that of actual body inclination or displacement.
Viewer-centred frame of reference
The direction of the stepping in place movements in response to neck muscle vibration depends on head-on-feet (Fig. 6) and eye-in-orbit (Fig. 7) orientation and suggests that proprioceptive signals from the neck are interpreted in the context of both vestibular signals and gaze orientation. Thus, gaze effect is not confined to postural conditions (Ivanenko et al. 1999a), but is also present during stepping movements, highlighting the importance of a viewer-centred reference frame for processing multisensory information.
What is the mechanism by which gaze influences the direction of the neck vibration-induced reaction? One can hypothesise that postural and locomotor reactions are aimed at stabilising the visual input. Yet, the retinal slip does not seem to play a major role in determining evoked responses. Indeed, visual target fixation was not crucial. Similar directional gaze-dependent body displacements in response to neck muscle vibration were observed with the eyes closed. Alternatively, the results may arise from eye proprioception or the efferent control of gaze. The visual receptive fields of neurones in different cortical regions are modulated by the position of the eye in the orbit (see Andersen et al. 1997; Lacquaniti, 1997; Baker et al. 1999). For instance, the activity of many neurones from the dorsal premotor cortex reflects the direction of the subsequent limb movement but varies significantly with eye position (Boussaoud, 1995). The gaze signals also have a powerful control over the vestibular-ocular, vestibular-spinal and reticulospinal systems (Berthoz, 1988).
Vibration applied to the neck can activate not only neck muscle proprioception, but also to some extent skin mechanoreceptors and vestibular receptors. Nevertheless, whatever the relative contribution of all sensory inputs evoked by dorsal neck muscle vibration it is worth stressing that the observed effects (walking speed increment and gaze-dependent direction of the response) were consistent and should thus reflect some inherent properties of the interactions between posture and locomotion and the involvement of the viewer-centred reference frame for the processing of head-related sensory information.
The viewer-centred reference frame (eye-on-feet position) is important for the visual control of posture (Wolsley et al. 1996) and locomotion (Bardy et al. 1996; Rossignol, 1996). Due to the synergetic eye-head co-ordination mechanisms, the processing of neck proprioceptive information might occur in the context of the viewer-centred reference frame. It is known that vibration of neck muscles can induce a visual illusion of displacement of a small visual target viewed in the dark (Biguer et al. 1988; Roll et al. 1989; Smetanin et al. 1993) or of head rotation (Karnath et al. 1994). Interestingly, vibration of eye muscles per se can elicit a prominent body sway (Roll et al. 1989). Thus, extraocular proprioception may play an important part in the organisation of whole-body posture and in inter-relating body space with extrapersonal space.
Visual, vestibular, proprioceptive and efference copy signals are centrally integrated to monitor head orientation in space. In these processes, gaze, as an important indicator of our attention, might represent an egocentric frame of reference for internal representations, interaction with the extrapersonal space and motion planning. Also, the most significant role played by vision, as well as by intention, may be in feed-forward control. For example, during locomotion we observed that gaze and head orientation constantly anticipate the walking trajectory and motor events (Grasso et al. 1998). We suggest that the use of a viewer-centred reference frame for processing head-related sensory information might have some advantages and is important for the visual control of balance and of steering of locomotion.
Acknowledgments
This work was supported in part by grants from the Italian Health Ministry, the Italian Space Agency, the Ministero della Universita e Ricerca Scientifica e Tecnologica, and Telethon-Italy. Dr Ivanenko is temporarily on leave from the Institute for Information Transmission Problems, Russian Academy of Sciences, Moscow, Russia, with a fellowship from the Italian Ministry of Health. The authors thank B. Bronzi and D. Prissinotti for their skilful technical help.
References
- Andersen RA, Snyder LH, Bradley DC, Xing J. Multimodal representation of space in the posterior parietal cortex and its use in planning movements. Annual Review of Neuroscience. 1997;20:303–330. doi: 10.1146/annurev.neuro.20.1.303. [DOI] [PubMed] [Google Scholar]
- Baker JT, Donoghue JP, Sanes JN. Gaze direction modulates finger movement activation patterns in human cerebral cortex. Journal of Neuroscience. 1999;19:10044–10052. doi: 10.1523/JNEUROSCI.19-22-10044.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardy BG, Warren WH, Jr, Kay BA. Motion parallax is used to control postural sway during walking. Experimental Brain Research. 1996;111:271–282. doi: 10.1007/BF00227304. [DOI] [PubMed] [Google Scholar]
- Berthoz A. The role of gaze in compensation of vestibular disfunction: the gaze substitution hypothesis. Progress in Brain Research. 1988;76:411–420. doi: 10.1016/s0079-6123(08)64528-8. [DOI] [PubMed] [Google Scholar]
- Bianchi L, Angelini D, Orani GP, Lacquaniti F. Kinematic coordination in human gait: relation to mechanical energy cost. Journal of Neurophysiology. 1998;79:2155–2170. doi: 10.1152/jn.1998.79.4.2155. [DOI] [PubMed] [Google Scholar]
- Bianconi R, van der Meulen JR. The response to vibration of the end organ of mammalian muscle spindles. Journal of Neurophysiology. 1963;26:177–190. doi: 10.1152/jn.1963.26.1.177. [DOI] [PubMed] [Google Scholar]
- Biguer B, Donaldson IM, Hein A, Jeannerod M. Neck muscle vibration modifies the representation of visual motion and direction in man. Brain. 1988;111:1405–1424. doi: 10.1093/brain/111.6.1405. [DOI] [PubMed] [Google Scholar]
- Borghese NA, Bianchi L, Lacquaniti F. Kinematic determinants of human locomotion. The Journal of Physiology. 1996;494:863–879. doi: 10.1113/jphysiol.1996.sp021539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boussaoud D. Primate premotor cortex: modulation of preparatory neuronal activity by gaze angle. Journal of Neurophysiology. 1995;73:886–890. doi: 10.1152/jn.1995.73.2.886. [DOI] [PubMed] [Google Scholar]
- Britton TC, Day BL, Brown P, Rothwell JC, Thompson PD, Marsden CD. Postural electromyographic responses in the arm and leg following galvanic vestibular stimulation in man. Experimental Brain Research. 1993;94:143–151. doi: 10.1007/BF00230477. [DOI] [PubMed] [Google Scholar]
- Burke D, Hagbarth KE, Lofstedt L, Wallin BG. The responses of human muscle spindle endings to vibration during isometric contraction. The Journal of Physiology. 1976;261:695–711. doi: 10.1113/jphysiol.1976.sp011581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway BA, Rosenberg JR. The participation of forelimb flexors in labyrinth and neck reflexes in the decerebrate cat. Brain Research. 1984;322:148–151. doi: 10.1016/0006-8993(84)91195-8. [DOI] [PubMed] [Google Scholar]
- Day BL, Severac Cauquil A, Bartolomei L, Pastor MA, Lyon IN. Human body-segment tilts induced by galvanic stimulation: a vestibularly driven balance protection mechanism. The Journal of Physiology. 1997;500:661–672. doi: 10.1113/jphysiol.1997.sp022051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund G. General features of vibration-induced effects on balance. Upsala Journal of Medical Sciences. 1972;77:112–124. doi: 10.1517/03009734000000016. [DOI] [PubMed] [Google Scholar]
- Eklund G, Hagbarth KE. Normal variability of tonic vibration reflexes in man. Experimental Neurology. 1966;16:80–92. doi: 10.1016/0014-4886(66)90088-4. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick R, Burke D, Gandevia SC. Task-dependent reflex responses and movement illusions evoked by galvanic vestibular stimulation in standing humans. The Journal of Physiology. 1994;478:363–372. doi: 10.1113/jphysiol.1994.sp020257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick RC, Wardman DL, Taylor JL. Effects of galvanic vestibular stimulation during human walking. The Journal of Physiology. 1999;517:931–939. doi: 10.1111/j.1469-7793.1999.0931s.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandevia SC, Phegan CM. Perceptual distortions of the human body image produced by local anaesthesia, pain and cutaneous stimulation. The Journal of Physiology. 1999;514:609–616. doi: 10.1111/j.1469-7793.1999.609ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin GM, McCloskey DI, Matthews PB. The contribution of muscle afferents to kinaesthesia shown by vibration induced illusions of movement and by the effects of paralysing joint afferents. Brain. 1972;95:705–748. doi: 10.1093/brain/95.4.705. [DOI] [PubMed] [Google Scholar]
- Grasso R, Ivanenko Y, Lacquaniti F. Time course of gaze influences on postural responses to neck proprioceptive and galvanic vestibular stimulation in humans. Neuroscience Letters. 1999;273:121–124. doi: 10.1016/s0304-3940(99)00658-8. [DOI] [PubMed] [Google Scholar]
- Grasso R, Prevost P, Ivanenko YP, Berthoz A. Eye-head coordination for the steering of locomotion in humans: an anticipatory synergy. Neuroscience Letters. 1998;253:115–118. doi: 10.1016/s0304-3940(98)00625-9. [DOI] [PubMed] [Google Scholar]
- Grasso R, Zago M, Lacquaniti F. Interactions between posture and locomotion: motor patterns in humans walking with bent posture versus erect posture. Journal of Neurophysiology. 2000;83:288–300. doi: 10.1152/jn.2000.83.1.288. [DOI] [PubMed] [Google Scholar]
- Gurfinkel VS. The mechanisms of postural regulation in man. In: Turpaev TM, editor. Soviet Scientific Reviews, section F, Physiology and General Biology Reviews. part 5. Vol. 7. Yverdon: Harwood Academic Publishers GmbH; 1994. pp. 59–89. [Google Scholar]
- Gurfinkel VS, Ivanenko YP, Levik YS. The contribution of foot deformation to the changes of muscular length and angle in the ankle joint during standing in man. Physiological Research. 1994;43:371–377. [PubMed] [Google Scholar]
- Gurfinkel VS, Levik YS, Kazennikov OV, Selionov VA. Locomotor-like movements evoked by leg muscle vibration in humans. European Journal of Neuroscience. 1998;10:1608–1612. doi: 10.1046/j.1460-9568.1998.00179.x. [DOI] [PubMed] [Google Scholar]
- Ivanenko YP, Grasso R, Lacquaniti F. Effect of gaze on postural responses to neck proprioceptive and vestibular stimulation in humans. The Journal of Physiology. 1999a;519:301–314. doi: 10.1111/j.1469-7793.1999.0301o.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanenko YP, Talis VL, Kazennikov OV. Support stability influences postural responses to muscle vibration in humans. European Journal of Neuroscience. 1999b;11:647–654. doi: 10.1046/j.1460-9568.1999.00471.x. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Edgley S. Interactions between pathways controlling posture and gait at the level of spinal interneurones in the cat. Progress in Brain Research. 1993;97:161–171. doi: 10.1016/s0079-6123(08)62274-8. [DOI] [PubMed] [Google Scholar]
- Karnath HO, Sievering D, Fetter M. The interactive contribution of neck muscle proprioception and vestibular stimulation to subjective ‘straight ahead’ orientation in man. Experimental Brain Research. 1994;101:140–146. doi: 10.1007/BF00243223. [DOI] [PubMed] [Google Scholar]
- Kavounoudias A, Gilhodes JC, Roll R, Roll JP. From balance regulation to body orientation: two goals for muscle proprioceptive information processing? Experimental Brain Research. 1999;124:80–88. doi: 10.1007/s002210050602. [DOI] [PubMed] [Google Scholar]
- Lackner JR, Levine MS. Changes in apparent body orientation and sensory localization induced by vibration of postural muscles: vibratory myesthetic illusions. Aviation Space and Environmental Medicine. 1979;50:346–354. [PubMed] [Google Scholar]
- Lacquaniti F. Frames of reference in sensorimotor coordination. In: Boller F, Grafman J, editors. Handbook of Neuropsychology. Amsterdam: Elsevier Science B.V.; 1997. pp. 27–64. [Google Scholar]
- Lekhel H, Popov K, Anastasopoulos D, Bronstein A, Bhatia K, Marsden CD, Gresty M. Postural responses to vibration of neck muscles in patients with idiopathic torticollis. Brain. 1997;120:583–591. doi: 10.1093/brain/120.4.583. [DOI] [PubMed] [Google Scholar]
- Lund S. Postural effects of neck muscle vibration in man. Experientia. 1980;36:1398. doi: 10.1007/BF01960120. [DOI] [PubMed] [Google Scholar]
- Massion J. Movement, posture and equilibrium: interaction and coordination. Progress in Neurobiology. 1992;38:35–56. doi: 10.1016/0301-0082(92)90034-c. [DOI] [PubMed] [Google Scholar]
- Mergner T, Huber W, Becker W. Vestibular-neck interaction and transformation of sensory coordinates. Journal of Vestibular Research. 1997;7:347–367. [PubMed] [Google Scholar]
- Mori S. Integration of posture and locomotion in acute decerebrate cats and in awake, freely moving cats. Progress in Neurobiology. 1987;28:161–195. doi: 10.1016/0301-0082(87)90010-4. [DOI] [PubMed] [Google Scholar]
- Naito E, Ehrsson HH, Geyer S, Zilles K, Roland PE. Illusory arm movements activate cortical motor areas: a positron emission tomography study. Journal of Neuroscience. 1999;19:6134–6144. doi: 10.1523/JNEUROSCI.19-14-06134.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popov KE, Kozhina GV, Smetanin BN, Shlikov VY. Postural responses to combined vestibular and hip proprioceptive stimulation in man. European Journal of Neuroscience. 1999;11:3307–3311. doi: 10.1046/j.1460-9568.1999.00733.x. [DOI] [PubMed] [Google Scholar]
- Prochazka A. Proprioceptive feedback and movement regulation. In: Rowell LB, Shepherd JT, editors. Handbook of Physiology, section 12, Exercise: Regulation and Integration of Multiple Systems. New York and Oxford: Oxford University Press; 1996. pp. 89–127. [Google Scholar]
- Quoniam C, Roll JP, Deat A, Massion J. Proprioceptive induced interactions between segmental and whole body posture. In: Brandt Th, Paulus W, Bles W, Dieterich M, Krafcyzk S, Straube A., editors. Disorders of Posture and Gait. New York: Georg Thieme Verlag Stuttgart; 1990. pp. 194–197. [Google Scholar]
- Roll JP, Vedel JP, Roll R. Eye, head and skeletal muscle spindle feedback in the elaboration of body references. Progress in Brain Research. 1989;80:113–123. doi: 10.1016/s0079-6123(08)62204-9. [DOI] [PubMed] [Google Scholar]
- Roll R, Gilhodes JC, Roll JP, Popov K, Charade O, Gurfinkel V. Proprioceptive information processing in weightlessness. Experimental Brain Research. 1998;122:393–402. doi: 10.1007/s002210050527. [DOI] [PubMed] [Google Scholar]
- Rossignol S. Visuomotor regulation of locomotion. Canadian The Journal of Physiology and Pharmacology. 1996;74:418–425. [PubMed] [Google Scholar]
- Sherrington CS. Flexion reflex on the limb, cross extension-reflex and reflex stepping and standing. The Journal of Physiology. 1910;40:28–121. doi: 10.1113/jphysiol.1910.sp001362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smetanin BN, Popov KE, Shlykov VY. Postural responses to vibrostimulation of neck muscle proprioceptors in humans. Neurophysiology. 1993;25:86–92. [Google Scholar]
- Soechting JF, Flanders M. Moving in three-dimensional space: frames of reference, vectors, and coordinate systems. Annual Review of Neuroscience. 1992;15:167–191. doi: 10.1146/annurev.ne.15.030192.001123. [DOI] [PubMed] [Google Scholar]
- Thorstensson A, Nilsson J, Carlson H, Zomlefer MR. Trunk movements in human locomotion. Acta Physiologica Scandinavica. 1984;121:9–22. doi: 10.1111/j.1748-1716.1984.tb10452.x. [DOI] [PubMed] [Google Scholar]
- Thorstensson A, Roberthson H. Adaptations to changing speed in human locomotion: speed of transition between walking and running. Acta Physiologica Scandinavica. 1987;131:211–214. doi: 10.1111/j.1748-1716.1987.tb08228.x. [DOI] [PubMed] [Google Scholar]
- Wilson VJ. Vestibulospinal and neck reflexes: interaction in the vestibular nuclei. Archives Italiennes de Biologie. 1991;129:43–52. [PubMed] [Google Scholar]
- Wolsley CJ, Sakellari V, Bronstein AM. Reorientation of visually evoked postural responses by different eye-in-orbit and head-on-trunk angular positions. Experimental Brain Research. 1996;111:283–288. doi: 10.1007/BF00227305. [DOI] [PubMed] [Google Scholar]


